Modulation of Plasma Membrane Composition and Microdomain Organization Impairs Heat Shock Protein Expression in B16-F10 Mouse Melanoma Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cyclodextrin and Nystatin
2.2. Cell Culture
2.3. Analysis of HSP70 and HSP25 Expression Levels
2.4. Analysis of HSF1 Expression/Posttranslational Modification Levels
2.5. Analysis of Cholesterol Levels
2.6. Stress Survival Experiments
2.7. Image-Based Fluorescence Correlation Spectroscopy (ImFCS)
2.8. Lipidomics
2.9. Statistics
3. Results
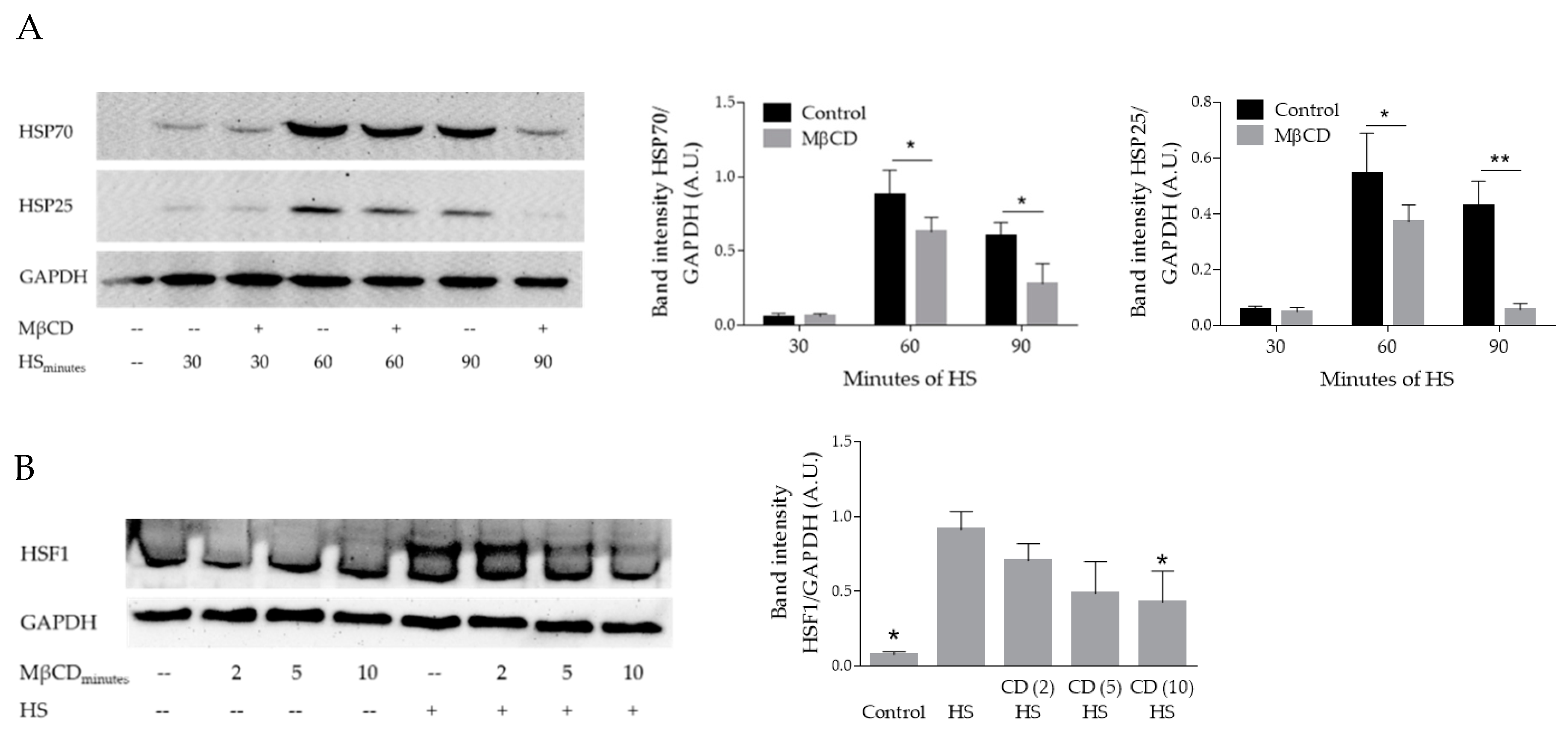
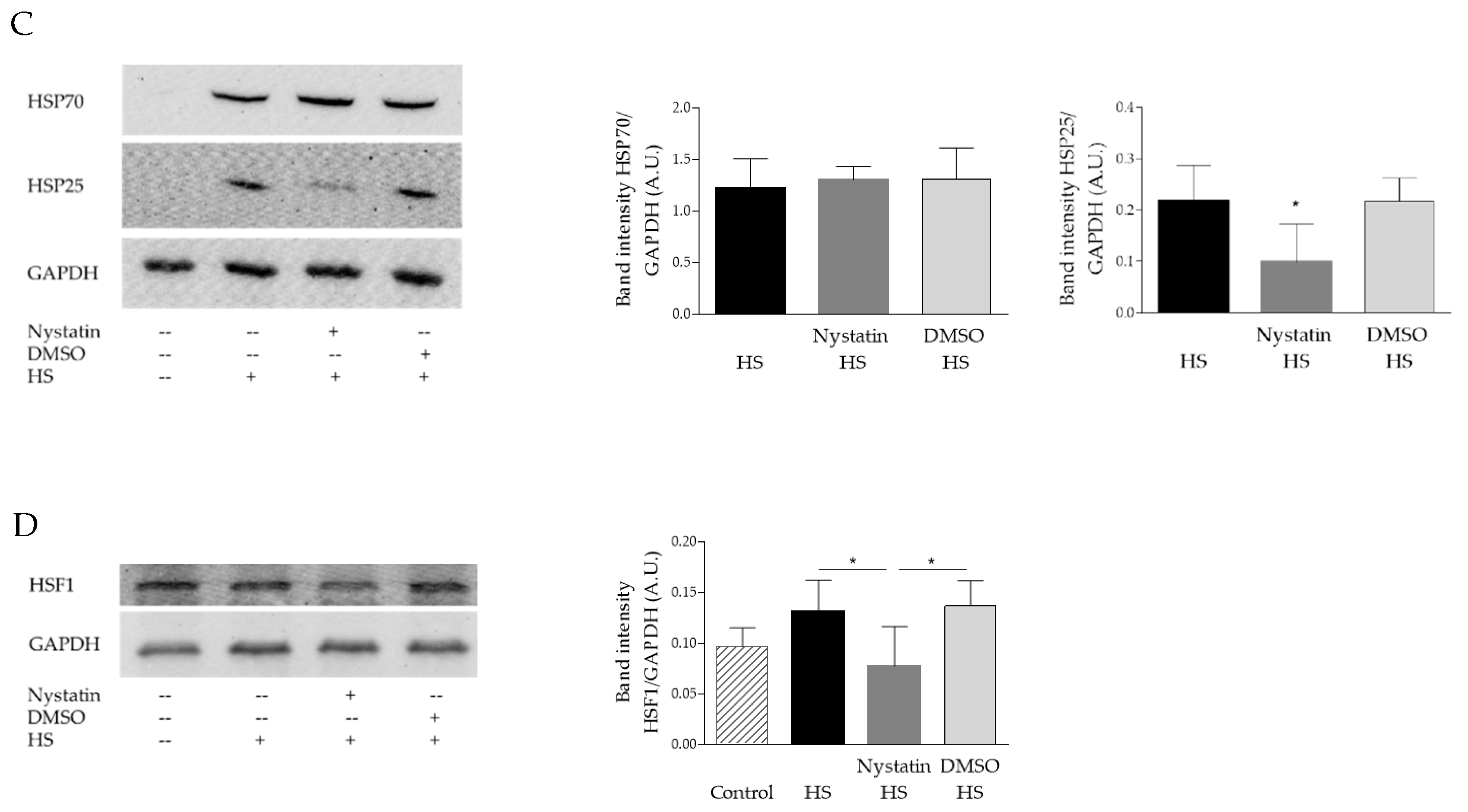
3.1. Plasma Membrane Modulations with Methyl-β-Cyclodextrin (MβCD) and Nystatin Impair the Heat-Induced Stress Response
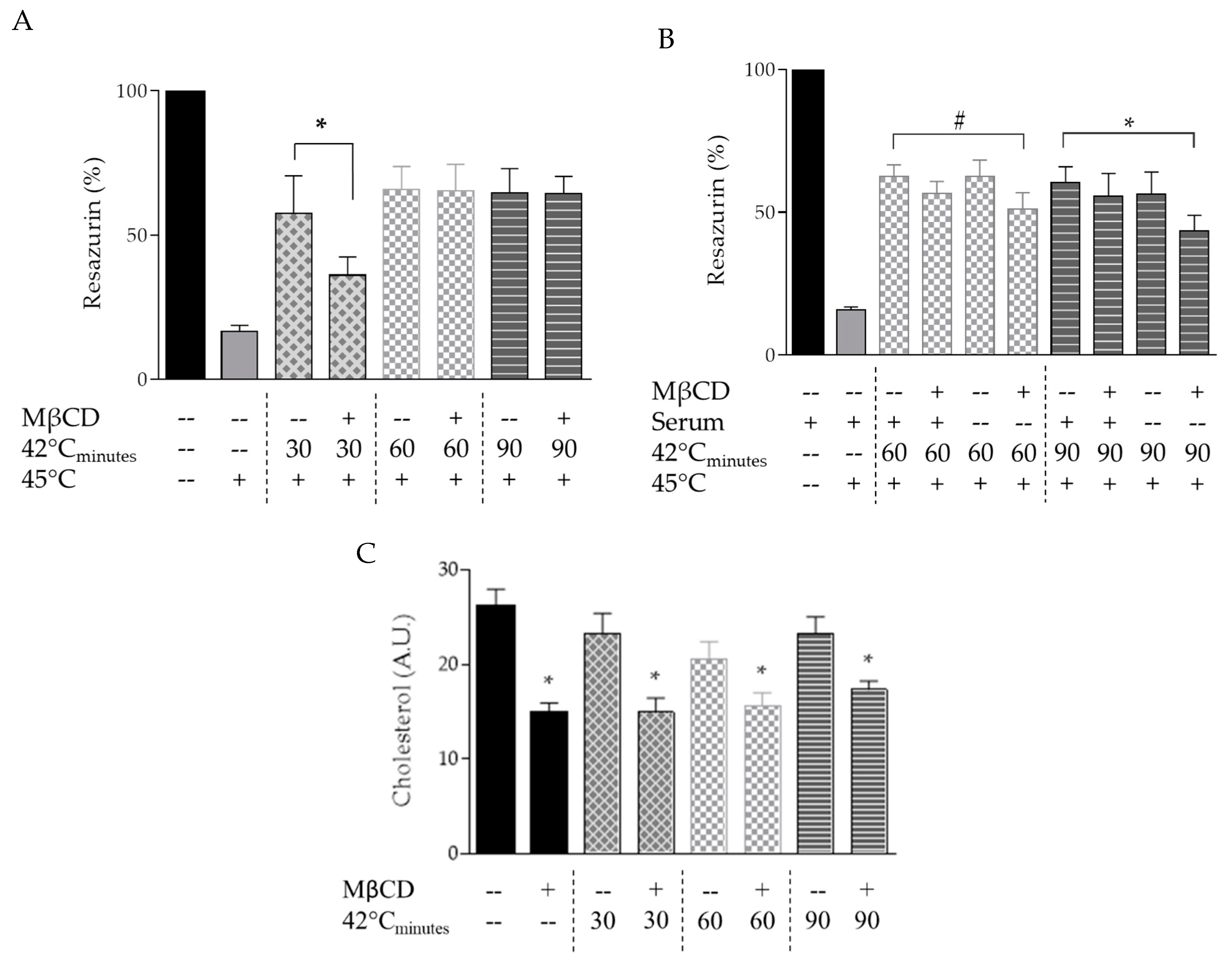
3.2. Plasma Membrane Modulation with MβCD Alters Acquired Thermotolerance of B16-F10 Cells
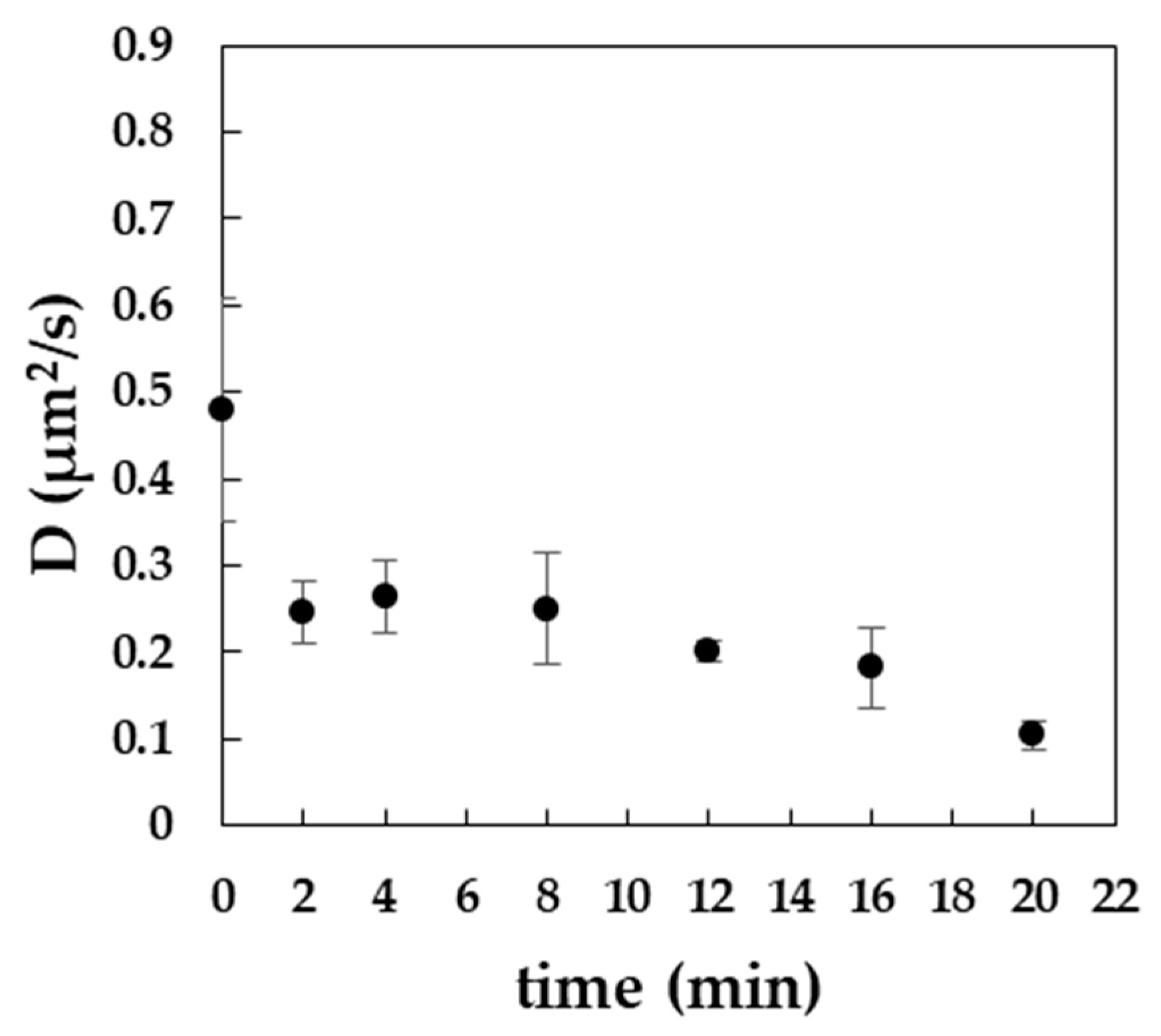
3.3. Exposure to MβCD Alters the Lateral Diffusion of Cholesterol in the Plasma Membrane
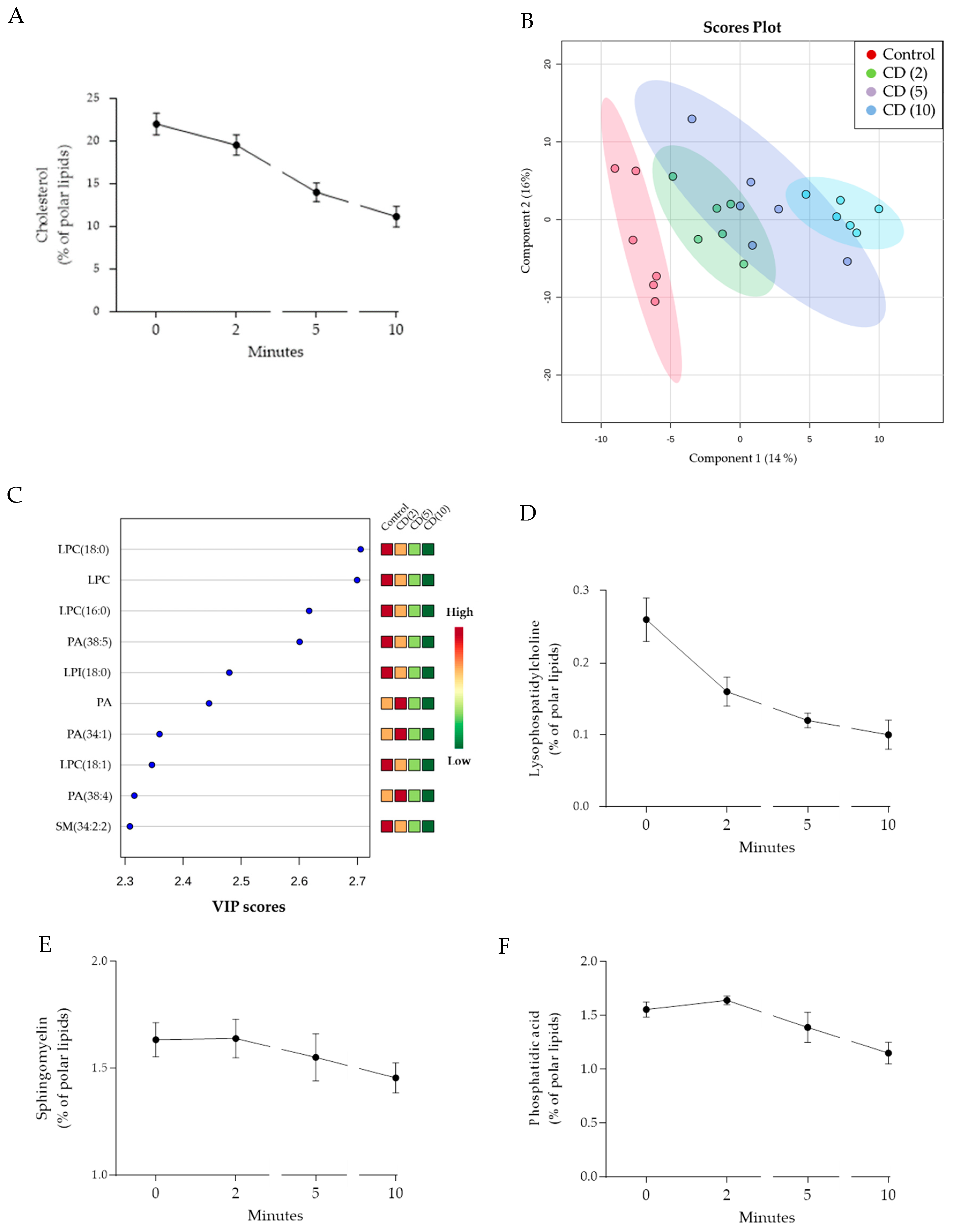
3.4. Lipidomics Analysis Indicates an Immediate and Extensive MβCD-Induced Lipidome Remodeling
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kültz, D. Molecular and evolutionary basis of the cellular stress response. Annu. Rev. Physiol. 2005, 67, 225–257. [Google Scholar] [CrossRef] [PubMed]
- Lindquist, S. The Heat-Shock Response. Annu. Rev. Biochem. 1986, 55, 1151–1191. [Google Scholar] [CrossRef] [PubMed]
- Kampinga, H.H.; Hageman, J.; Vos, M.J.; Kubota, H.; Tanguay, R.M.; Bruford, E.; Cheetham, M.E.; Chen, B.; Hightower, L.E. Guidelines for the nomenclature of the human heat shock proteins. Cell Stress Chaperones 2008, 14, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Anckar, J.; Sistonen, L. Regulation of H SF 1 Function in the Heat Stress Response: Implications in Aging and Disease. Annu. Rev. Biochem. 2011, 80, 1089–1115. [Google Scholar] [CrossRef]
- Horváth, I.; Glatz, A.; Varvasovszki, V.; Török, Z.; Páli, T.; Balogh, G.; Kovács, E.; Nádasdi, L.; Benkö, S.; Joó, F.; et al. Membrane physical state controls the signaling mechanism of the heat shock response in Synechocystis PCC 6803: Identification of hsp17 as a “fluidity gene”. Proc. Natl. Acad. Sci. USA 1998, 95, 3513–3518. [Google Scholar] [CrossRef]
- Horvàth, I.; Multhoff, G.; Sonnleitner, A.; Vígh, L. Membrane-associated stress proteins: More than simply chaperones. Biochim. et Biophys. Acta (BBA)-Biomembr. 2008, 1778, 1653–1664. [Google Scholar] [CrossRef]
- Török, Z.; Goloubinoff, P.; Horváth, I.; Tsvetkova, N.M.; Glatz, A.; Balogh, G.; Varvasovszki, V.; Los, D.A.; Vierling, E.; Crowe, J.H.; et al. Synechocystis HSP17 is an amphitropic protein that stabilizes heat-stressed membranes and binds denatured proteins for subsequent chaperone-mediated refolding. Proc. Natl. Acad. Sci. USA 2001, 98, 3098–3103. [Google Scholar] [CrossRef]
- Balogi, Z.; Cheregi, O.; Giese, K.C.; Juhász, K.; Vierling, E.; Vass, I.; Vígh, L.; Horváth, I. A mutant small heat shock protein with increased thylakoid association provides an elevated resistance against UV-B damage in synechocystis 6803. J. Boil. Chem. 2008, 283, 22983–22991. [Google Scholar] [CrossRef]
- Tsvetkova, N.M.; Horváth, I.; Török, Z.; Wolkers, W.F.; Balogi, Z.; Shigapova, N.; Crowe, L.M.; Tablin, F.; Vierling, E.; Crowe, J.H.; et al. Small heat-shock proteins regulate membrane lipid polymorphism. Proc. Natl. Acad. Sci. USA 2002, 99, 13504–13509. [Google Scholar] [CrossRef]
- Nagy, E.; Balogi, Z.; Gombos, I.; Åkerfelt, M.; Björkbom, A.; Balogh, G.; Török, Z.; Maslyanko, A.; Fiszer-Kierzkowska, A.; Lisowska, K.M.; et al. Hyperfluidization-coupled membrane microdomain reorganization is linked to activation of the heat shock response in a murine melanoma cell line. Proc. Natl. Acad. Sci. USA 2007, 104, 7945–7950. [Google Scholar] [CrossRef]
- Balogh, G.; Horvàth, I.; Nagy, E.; Hoyk, Z.; Benkő, S.; Bensaude, O.; Vígh, L. The hyperfluidization of mammalian cell membranes acts as a signal to initiate the heat shock protein response. FEBS J. 2005, 272, 6077–6086. [Google Scholar] [CrossRef] [PubMed]
- Balogh, G.; Maulucci, G.; Gombos, I.; Horváth, I.; Török, Z.; Péter, M.; Fodor, E.; Pali, T.; Benkő, S.; Parasassi, T.; et al. Heat Stress Causes Spatially-Distinct Membrane Re-Modelling in K562 Leukemia Cells. PLoS ONE 2011, 6, e21182. [Google Scholar] [CrossRef] [PubMed]
- Sezgin, E.; Levental, I.; Mayor, S.; Eggeling, C. The mystery of membrane organization: Composition, regulation and roles of lipid rafts. Nat. Rev. Mol. Cell Boil. 2017, 18, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Parton, R.G.; Tillu, V.; Collins, B.M. Caveolae. Curr. Boil. 2018, 28, R402–R405. [Google Scholar] [CrossRef]
- Balogh, G.; Péter, M.; Glatz, A.; Gombos, I.; Török, Z.; Horvàth, I.; Harwood, J.L.; Vígh, L. Key role of lipids in heat stress management. FEBS Lett. 2013, 587, 1970–1980. [Google Scholar] [CrossRef]
- Csoboz, B.; Balogh, G.E.; Kúsz, E.; Gombos, I.; Péter, M.; Crul, T.; Gungor, B.; Haracska, L.; Bogdanovics, G.; Török, Z.; et al. Membrane fluidity matters: Hyperthermia from the aspects of lipids and membranes. Int. J. Hyperth. 2013, 29, 491–499. [Google Scholar] [CrossRef]
- Kang, Y.-S.; Ko, Y.-G.; Seoab, J.-S. Caveolin Internalization by Heat Shock or Hyperosmotic Shock. Exp. Cell Res. 2000, 255, 221–228. [Google Scholar] [CrossRef]
- Zidovetzki, R.; Levitan, I. Use of cyclodextrins to manipulate plasma membrane cholesterol content: Evidence, misconceptions and control strategies. Biochim. et Biophys. Acta (BBA)-Biomembr. 2007, 1768, 1311–1324. [Google Scholar] [CrossRef]
- Coutinho, A.; Silva, L.; Fedorov, A.; Prieto, M. Cholesterol and Ergosterol Influence Nystatin Surface Aggregation: Relation to Pore Formation. Biophys. J. 2004, 87, 3264–3276. [Google Scholar] [CrossRef]
- Rothberg, K.G.; Heuser, J.E.; Donzell, W.C.; Ying, Y.-S.; Glenney, J.R.; Anderson, R.G. Caveolin, a protein component of caveolae membrane coats. Cell 1992, 68, 673–682. [Google Scholar] [CrossRef]
- Sankaran, J.; Shi, X.; Ho, L.Y.; Stelzer, E.H.K.; Wohland, T. ImFCS: A software for imaging FCS data analysis and visualization. Opt. Express 2010, 18, 25468–25481. [Google Scholar] [CrossRef] [PubMed]
- Ng, X.W.; Bag, N.; Wohland, T. Characterization of Lipid and Cell Membrane Organization by the Fluorescence Correlation Spectroscopy Diffusion Law. Chim. Int. J. Chem. 2015, 69, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Sankaran, J.; Bag, N.; Kraut, R.S.; Wohland, T. Accuracy and Precision in Camera-Based Fluorescence Correlation Spectroscopy Measurements. Anal. Chem. 2013, 85, 3948–3954. [Google Scholar] [CrossRef] [PubMed]
- Péter, M.; Glatz, A.; Gudmann, P.; Gombos, I.; Török, Z.; Horváth, I.; Vigh, L.; Balogh, G. Metabolic crosstalk between membrane and storage lipids facilitates heat stress management in Schizosaccharomyces pombe. PLoS ONE 2017, 12, e0173739. [Google Scholar] [CrossRef] [PubMed]
- Peksel, B.; Gombos, I.; Péter, M.; Vigh, L.; Tiszlavicz, Á.; Brameshuber, M.; Balogh, G.; Schütz, G.J.; Horváth, I.; Török, Z. Mild heat induces a distinct “eustress” response in Chinese Hamster Ovary cells but does not induce heat shock protein synthesis. Sci. Rep. 2017, 7, 15643. [Google Scholar] [CrossRef]
- Chong, J.; Soufan, O.; Li, C.; Caraus, I.; Li, S.; Bourque, G.; Wishart, D.S.; Xia, J. MetaboAnalyst 4.0: Towards more transparent and integrative metabolomics analysis. Nucleic Acids Res. 2018, 46, W486–W494. [Google Scholar] [CrossRef]
- Landry, J.; Chrétien, P.; Lambert, H.; Hickey, E.; Weber, L.A. Heat shock resistance conferred by expression of the human HSP27 gene in rodent cells. J. Cell Boil. 1989, 109, 7–15. [Google Scholar] [CrossRef]
- Wang, H.-X.; Yang, Y.; Guo, H.; Hou, D.-D.; Zheng, S.; Hong, Y.-X.; Cai, Y.-F.; Huo, W.; Qi, R.-Q.; Zhang, L.; et al. HSPB1 deficiency sensitizes melanoma cells to hyperthermia induced cell death. Oncotarget 2016, 7, 67449–67462. [Google Scholar] [CrossRef]
- Worley, B.; Powers, R. Multivariate Analysis in Metabolomics. Curr Metabolomics 2013, 1, 92–107. [Google Scholar]
- Vígh, L.; Nakamoto, H.; Landry, J.; Harwood, J.L.; Horvath, I.; Gomez-Muñoz, A. Membrane Regulation of the Stress Response from Prokaryotic Models to Mammalian Cells. Ann. New York Acad. Sci. 2007, 1113, 40–51. [Google Scholar] [CrossRef]
- Ciocca, D.R.; Cuello-Carrión, F.D.; Natoli, A.L.; Restall, C.; Anderson, R.L. Absence of caveolin-1 alters heat shock protein expression in spontaneous mammary tumors driven by Her-2/neu expression. Histochem. Cell Biol. 2012, 137, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Török, Z.; Crul, T.; Maresca, B.; Schütz, G.J.; Viana, F.; Dindia, L.; Piotto, S.; Brameshuber, M.; Balogh, G.; Péter, M.; et al. Plasma membranes as heat stress sensors: From lipid-controlled molecular switches to therapeutic applications. Biochim. et Biophys. Acta (BBA)-Biomembr. 2014, 1838, 1594–1618. [Google Scholar] [CrossRef] [PubMed]
- Black, A.T.; Hayden, P.J.; Casillas, R.P.; Heck, D.E.; Gerecke, N.R.; Sinko, P.J.; Laskin, D.L.; Laskin, J.D. Regulation of Hsp27 and Hsp70 expression in human and mouse skin construct models by caveolae following exposure to the model sulfur mustard vesicant, 2-chloroethyl ethyl sulfide. Toxicol. Appl. Pharmacol. 2011, 253, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Ito, H.; Kamei, K.; Iwamoto, I. Selective stimulation of Hsp27 and αB-crystallin but not Hsp70 expression by p38 MAP kinase activation. Cell Stress Chaperones 1999, 4, 94–101. [Google Scholar] [CrossRef]
- Vitiello, M.; Finamore, E.; Raieta, K.; Kampanaraki, A.; Mignogna, E.; Galdiero, E.; Galdiero, M. Cellular cholesterol involvement in Src, PKC, and p38/JNK transduction pathways by porins. J. Interf. Cytokine Res. 2009, 29, 791–799. [Google Scholar] [CrossRef]
- Ladjohounlou, R.; Lozza, C.; Pichard, A.; Constanzo, J.; Karam, J.; Le Fur, P.; Deshayes, E.; Boudousq, V.; Paillas, S.; Busson, M.; et al. Drugs that modify cholesterol metabolism alter the p38/JNK-mediated targetedand nontargeted response to alpha and auger radioimmunotherapy. Clin. Cancer Res. 2019, 25, 4775–4790. [Google Scholar] [CrossRef]
- Budzyński, M.A.; Puustinen, M.C.; Joutsen, J.; Sistonen, L. Uncoupling Stress-Inducible Phosphorylation of Heat Shock Factor 1 from Its Activation. Mol. Cell. Boil. 2015, 35, 2530–2540. [Google Scholar] [CrossRef]
- Björk, J.K.; Sistonen, L. Regulation of the members of the mammalian heat shock factor family. FEBS J. 2010, 277, 4126–4139. [Google Scholar] [CrossRef]
- Nefkens, I.; Negorev, D.G.; Ishov, A.; Michaelson, J.S.; Yeh, E.T.; Tanguay, R.M.; Müller, W.E.G.; Maul, G.G. Heat shock and Cd2+ exposure regulate PML and Daxx release from ND10 by independent mechanisms that modify the induction of heat-shock proteins 70 and 25 differently. J. Cell Sci. 2003, 116, 513–524. [Google Scholar] [CrossRef]
- Boellmann, F.; Guettouche, T.; Guo, Y.; Fenna, M.; Mnayer, L.; Voellmy, R. DAXX interacts with heat shock factor 1 during stress activation and enhances its transcriptional activity. Proc. Natl. Acad. Sci. USA 2004, 101, 4100–4105. [Google Scholar] [CrossRef]
- Sun, S.; Wen, J.; Qiu, F.; Yin, Y.; Xu, G.; Li, T.; Nie, J.; Xiong, G.; Zhang, C.; Liao, D.; et al. Identification of the C-terminal domain of Daxx acts as a potential regulator of intracellular cholesterol synthesis in HepG2 cells. Biochem. Biophys. Res. Commun. 2016, 480, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Oommen, D.; Giricz, Z.; Srinivas, U.K.; Samali, A. Atypical heat shock response and acquisition of thermotolerance in P388D1 cells. Biochem. Biophys. Res. Commun. 2013, 430, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Fisher, B.; Kraft, P.; Hahn, G.M.; Anderson, R.L. Thermotolerance in the absence of induced heat shock proteins in a murine lymphoma. Cancer Res. 1992, 52, 2854–2861. [Google Scholar] [PubMed]
- Pinkwart, K.; Schneider, F.; Lukoseviciute, M.; Sauka-Spengler, T.; Lyman, E.; Eggeling, C.; Sezgin, E. Nanoscale dynamics of cholesterol in the cell membrane. J. Boil. Chem. 2019, 294, 12599–12609. [Google Scholar] [CrossRef]
- Kern, R.; Joseleau-Petit, D.; Chattopadhyay, M.K.; Richarme, G. Chaperone-like Properties of Lysophospholipids. Biochem. Biophys. Res. Commun. 2001, 289, 1268–1274. [Google Scholar] [CrossRef]
- Chasserot-Golaz, S.; Coorssen, J.R.; Meunier, F.A.; Vitale, N. Lipid Dynamics in Exocytosis. Cell. Mol. Neurobiol. 2010, 30, 1335–1342. [Google Scholar] [CrossRef]
- Péter, M.; Balogh, G.; Gombos, I.; Liebisch, G.; Horvàth, I.; Török, Z.; Nagy, E.; Maslyanko, A.; Benkő, S.; Schmitz, G.; et al. Nutritional lipid supply can control the heat shock response of B16 melanoma cells in culture. Mol. Membr. Boil. 2012, 29, 274–289. [Google Scholar] [CrossRef]
- Balogi, Z.; Multhoff, G.; Jensen, T.K.; Lloyd-Evans, E.; Yamashima, T.; Jäättelä, M.; Harwood, J.L.; Vígh, L.; Multhoff, G. Hsp70 interactions with membrane lipids regulate cellular functions in health and disease. Prog. Lipid Res. 2019, 74, 18–30. [Google Scholar] [CrossRef]
- Poulcharidis, D.; Belfor, K.; Kros, A.; Van Kasteren, S.I. A flow cytometry assay to quantify intercellular exchange of membrane components. Chem. Sci. 2017, 8, 5585–5590. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crul, T.; Csoboz, B.; Gombos, I.; Marton, A.; Peter, M.; Balogh, G.; Vizler, C.; Szente, L.; Vigh, L. Modulation of Plasma Membrane Composition and Microdomain Organization Impairs Heat Shock Protein Expression in B16-F10 Mouse Melanoma Cells. Cells 2020, 9, 951. https://doi.org/10.3390/cells9040951
Crul T, Csoboz B, Gombos I, Marton A, Peter M, Balogh G, Vizler C, Szente L, Vigh L. Modulation of Plasma Membrane Composition and Microdomain Organization Impairs Heat Shock Protein Expression in B16-F10 Mouse Melanoma Cells. Cells. 2020; 9(4):951. https://doi.org/10.3390/cells9040951
Chicago/Turabian StyleCrul, Tim, Balint Csoboz, Imre Gombos, Annamaria Marton, Maria Peter, Gabor Balogh, Csaba Vizler, Lajos Szente, and Laszlo Vigh. 2020. "Modulation of Plasma Membrane Composition and Microdomain Organization Impairs Heat Shock Protein Expression in B16-F10 Mouse Melanoma Cells" Cells 9, no. 4: 951. https://doi.org/10.3390/cells9040951
APA StyleCrul, T., Csoboz, B., Gombos, I., Marton, A., Peter, M., Balogh, G., Vizler, C., Szente, L., & Vigh, L. (2020). Modulation of Plasma Membrane Composition and Microdomain Organization Impairs Heat Shock Protein Expression in B16-F10 Mouse Melanoma Cells. Cells, 9(4), 951. https://doi.org/10.3390/cells9040951





