Overexpression of GRP78/BiP in P-Glycoprotein-Positive L1210 Cells is Responsible for Altered Response of Cells to Tunicamycin as a Stressor of the Endoplasmic Reticulum
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells, Culture Conditions, and Transfections
2.2. Effect of Culturing in Media Containing Tunicamycin on the Viability of S, R and T Cells
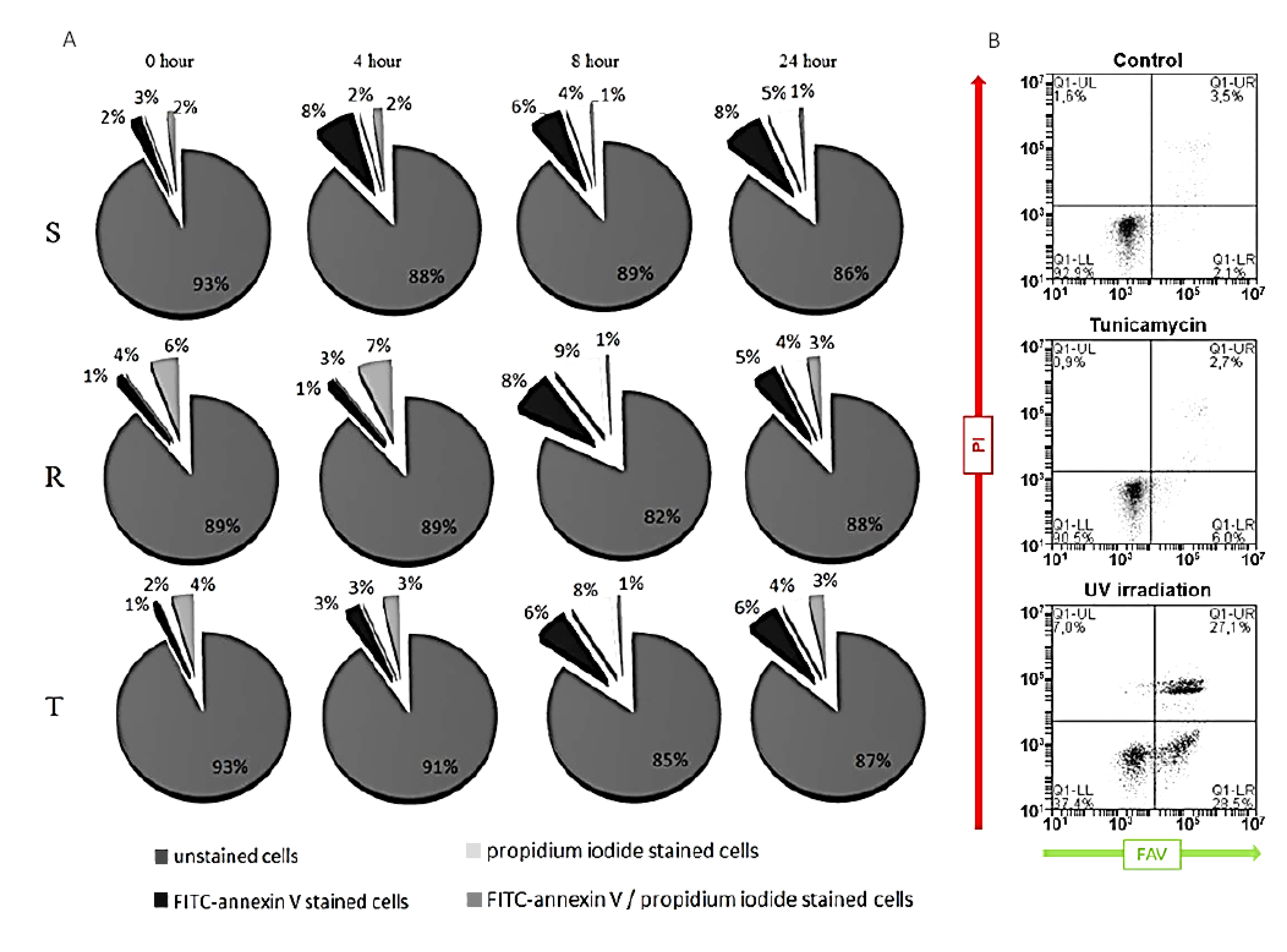
2.3. Detection of Tunicamycin-Induced Apoptosis and Necrosis in S, R, and T Cells
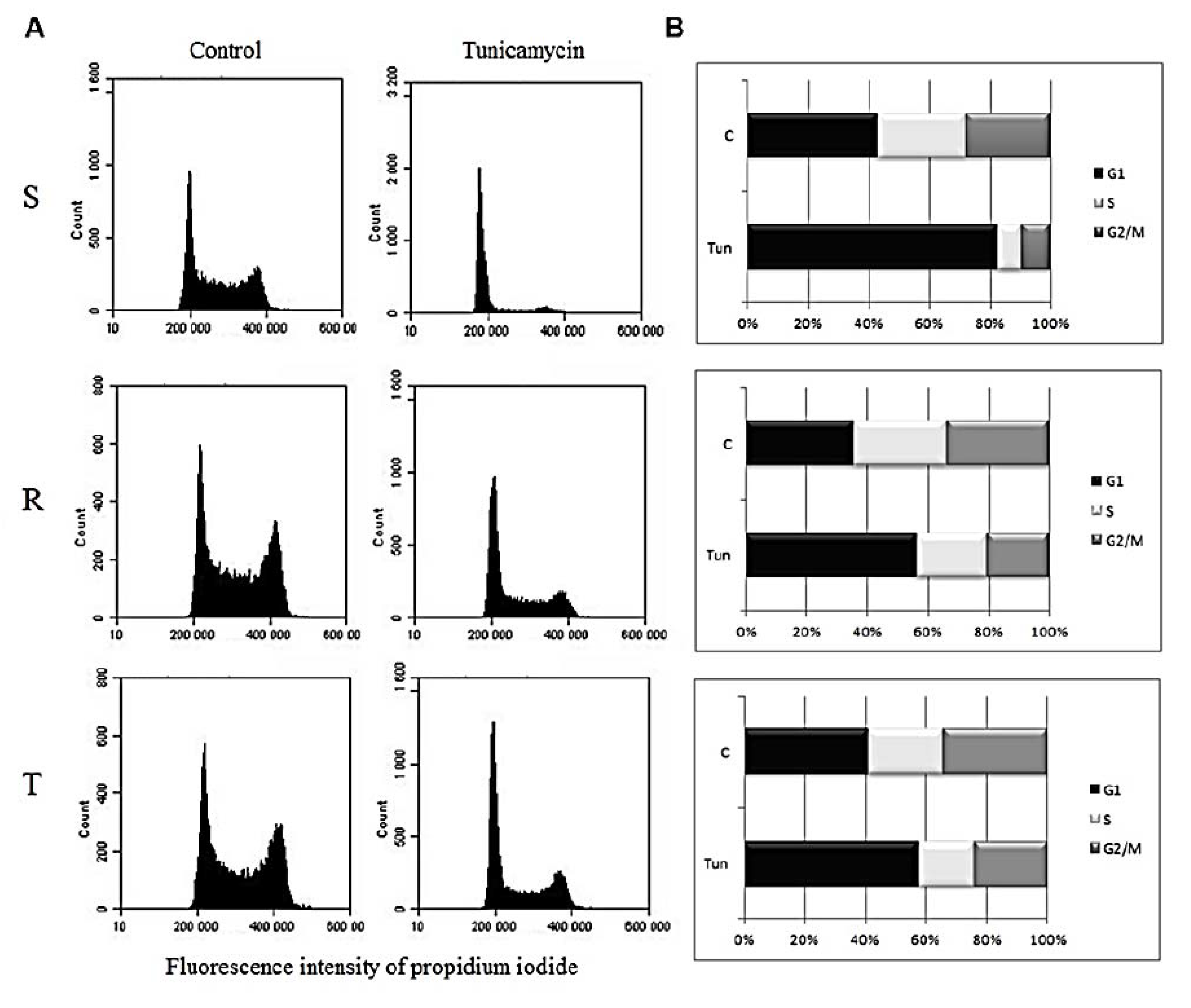
2.4. Monitoring cell cycle progression
2.5. Western Blotting
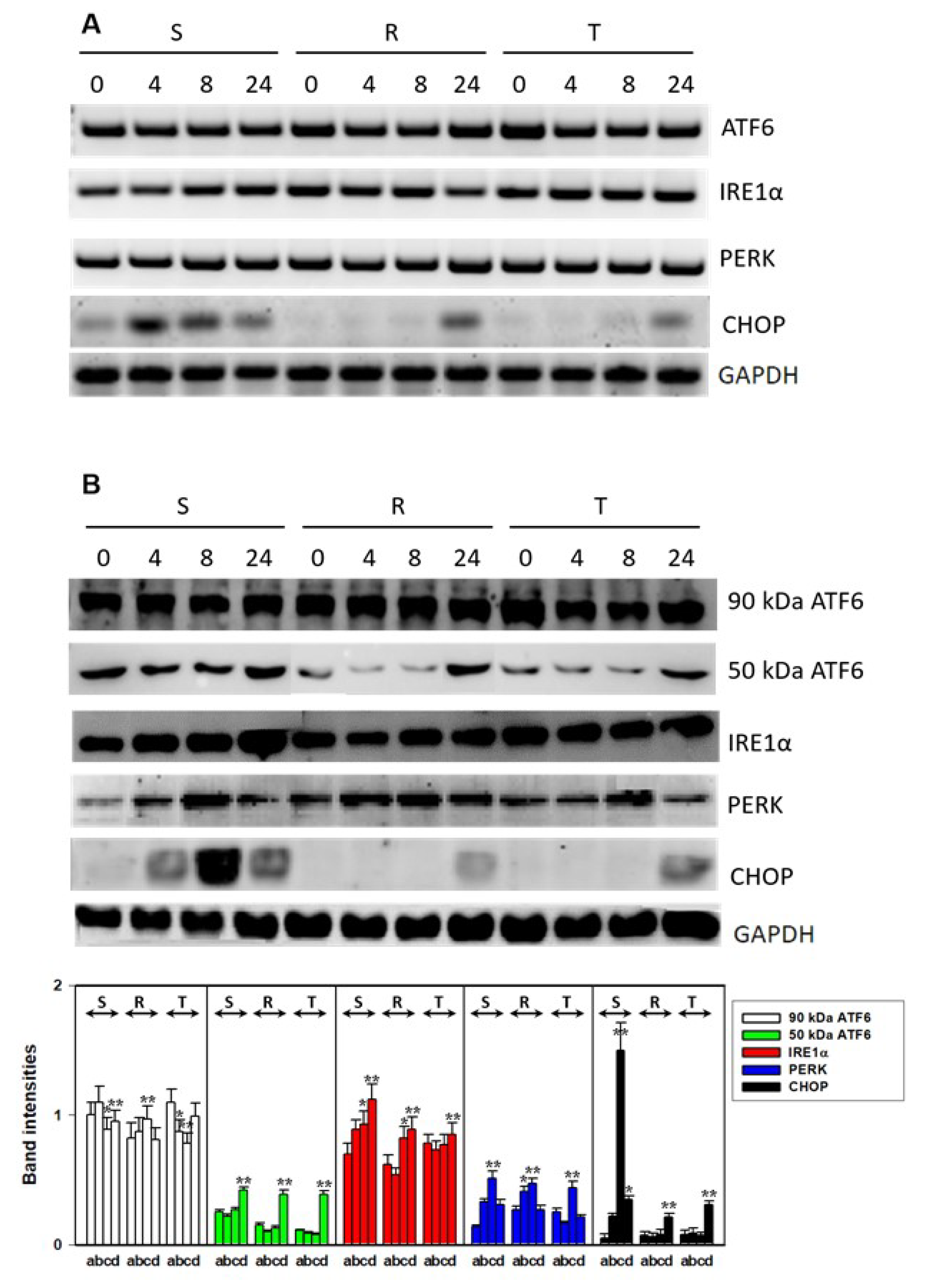
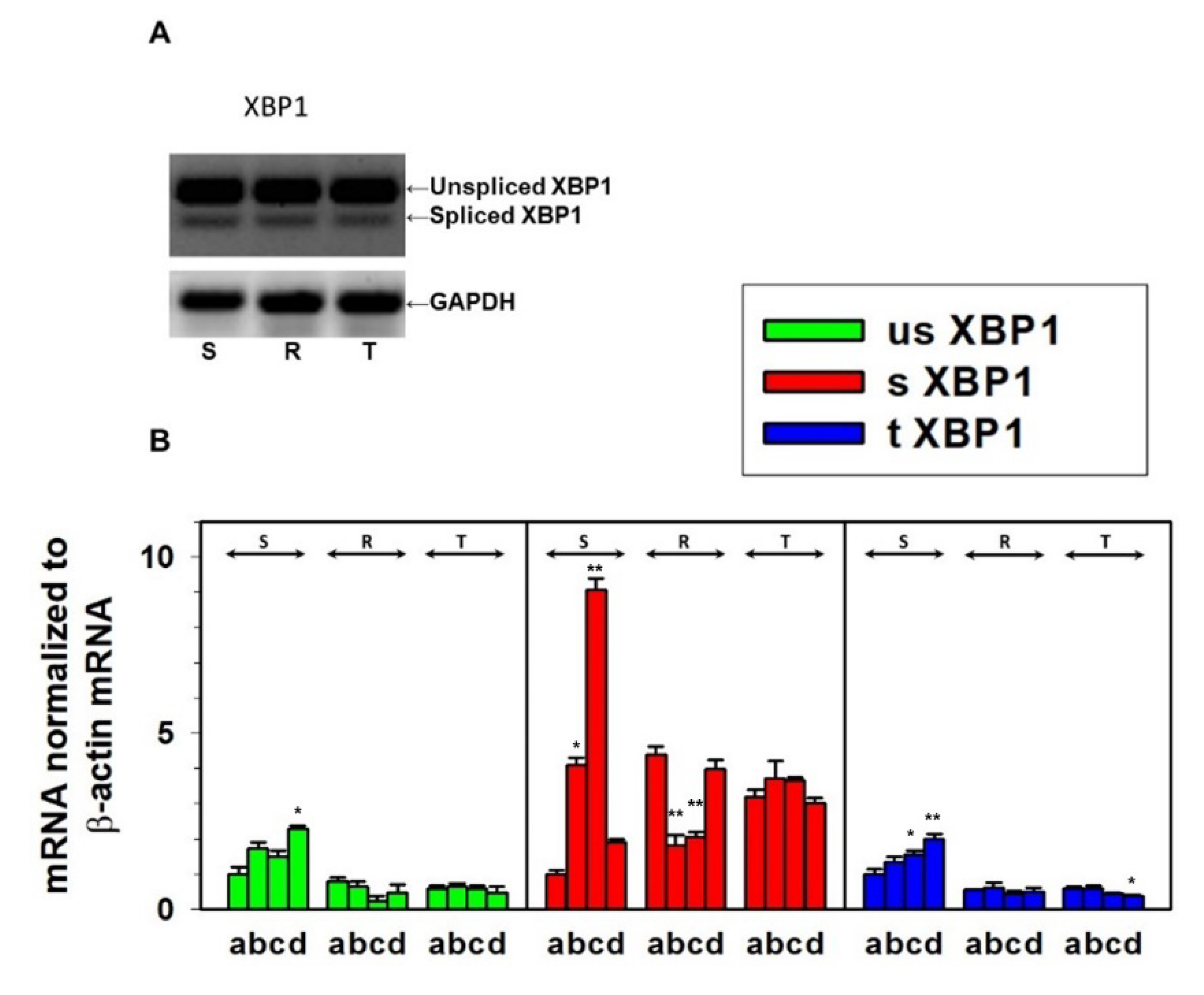
2.6. Determination of ER Stress-Induced Factors and Chaperones by RT-PCR
2.7. Determination of Stress Response Proteins by qPCR
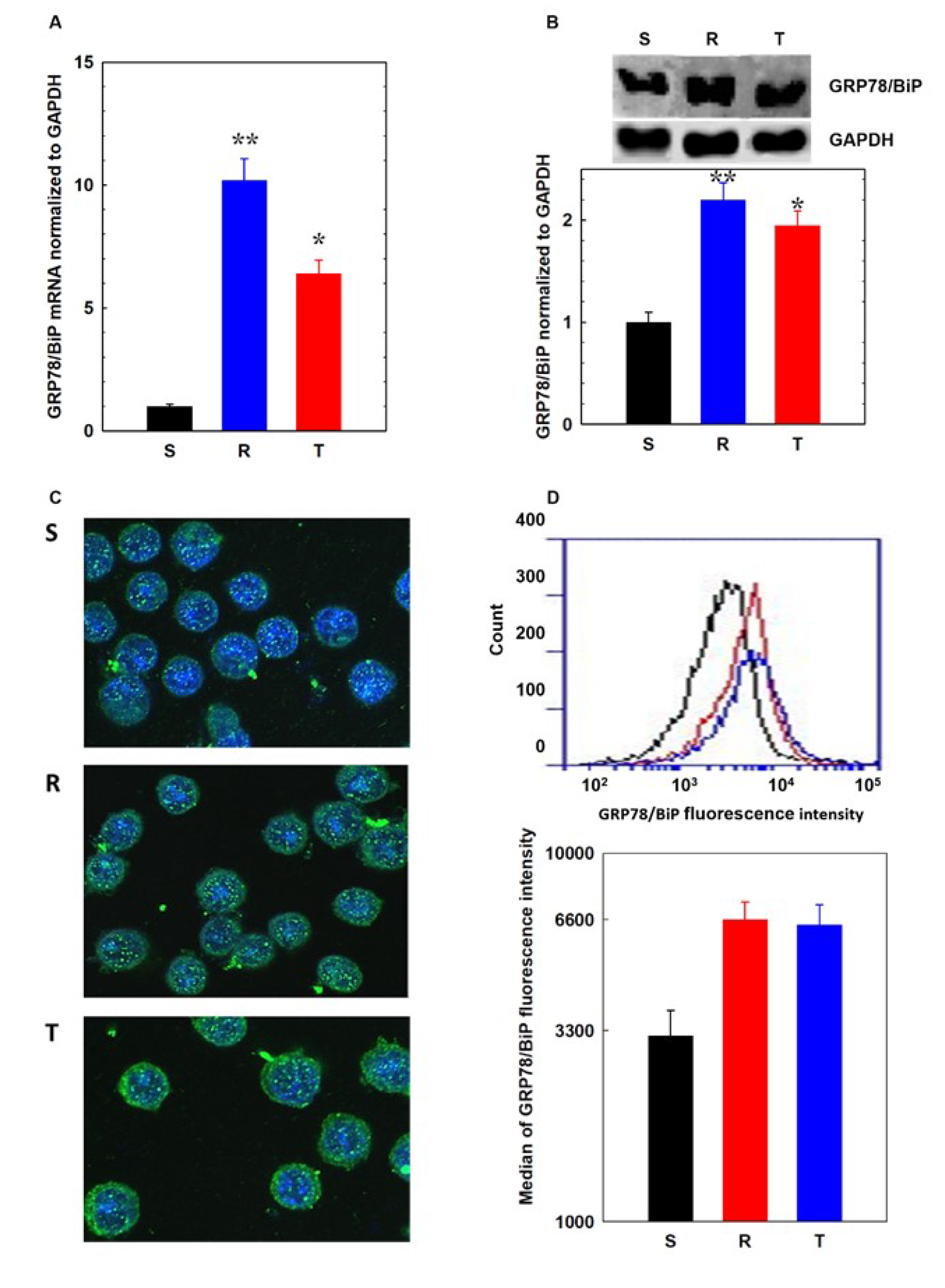
2.8. Visualization of GRP78/BiP in S, R, and T Cells Using Immunofluorescence Confocal Microscopy
2.9. Detection of GRP78/Bip on Cell Surface of L1210 cells by Flow Cytometry
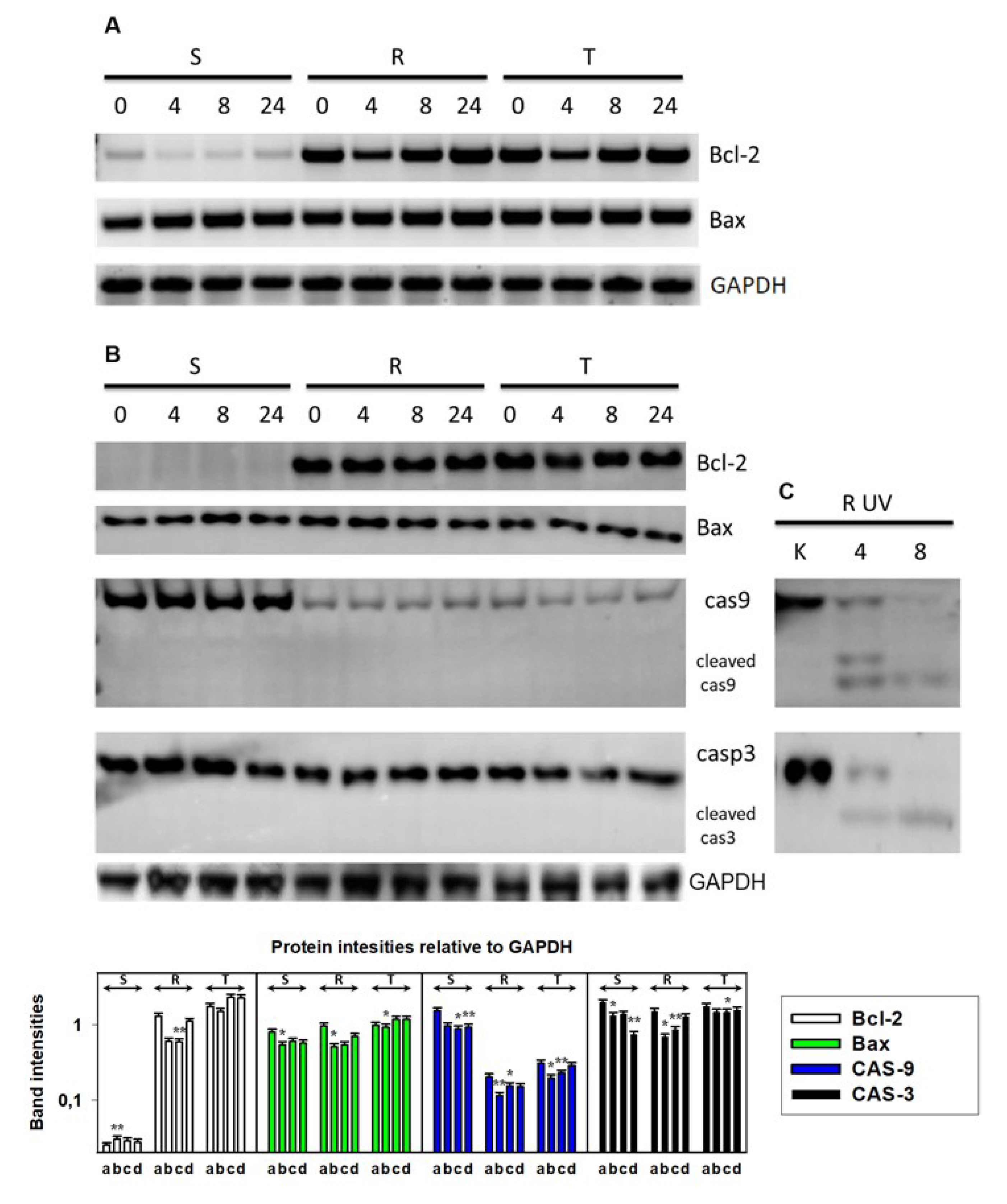
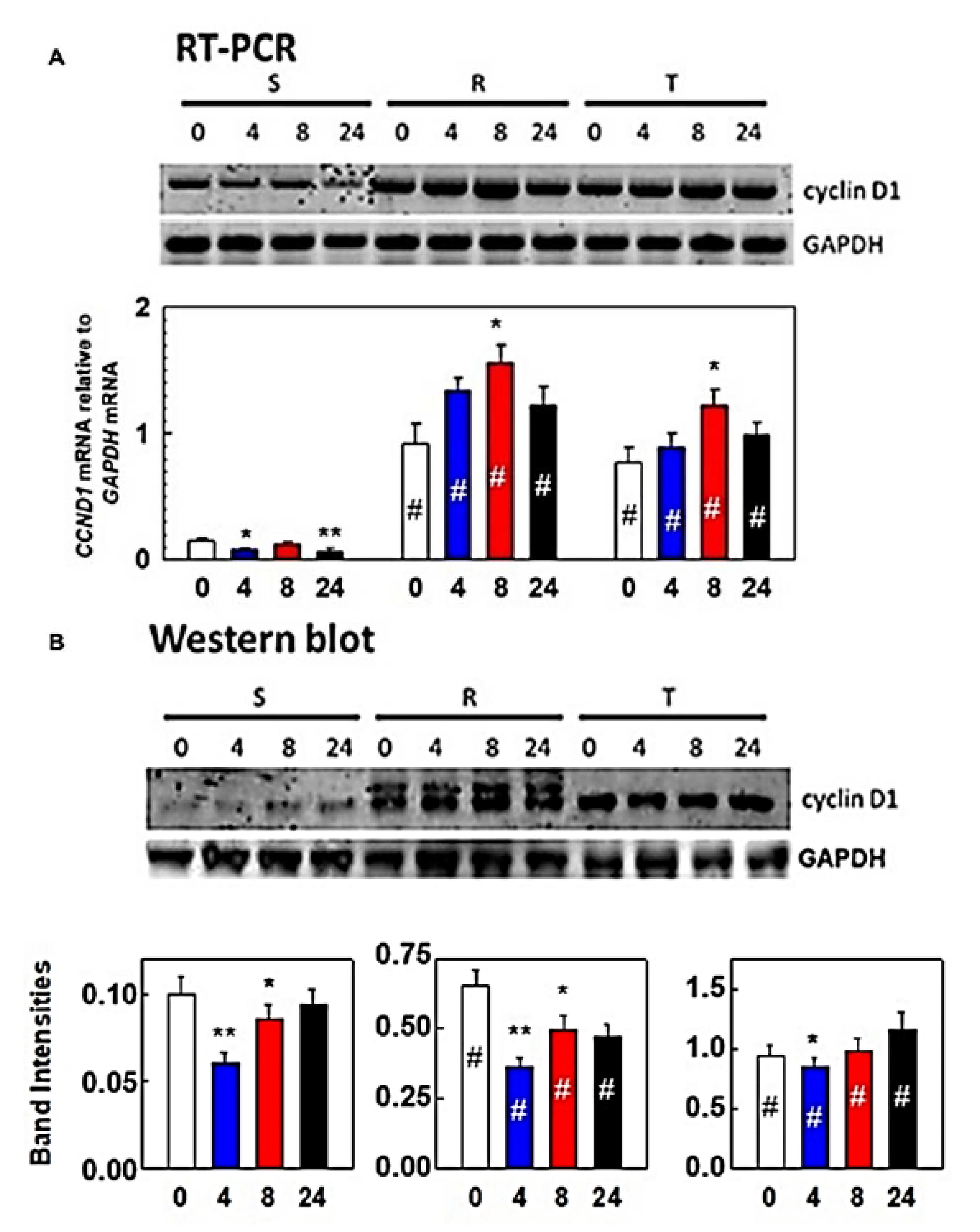
3. Results
3.1. Effect of Tunicamycin on S, R, and T Cell Cycle Progression
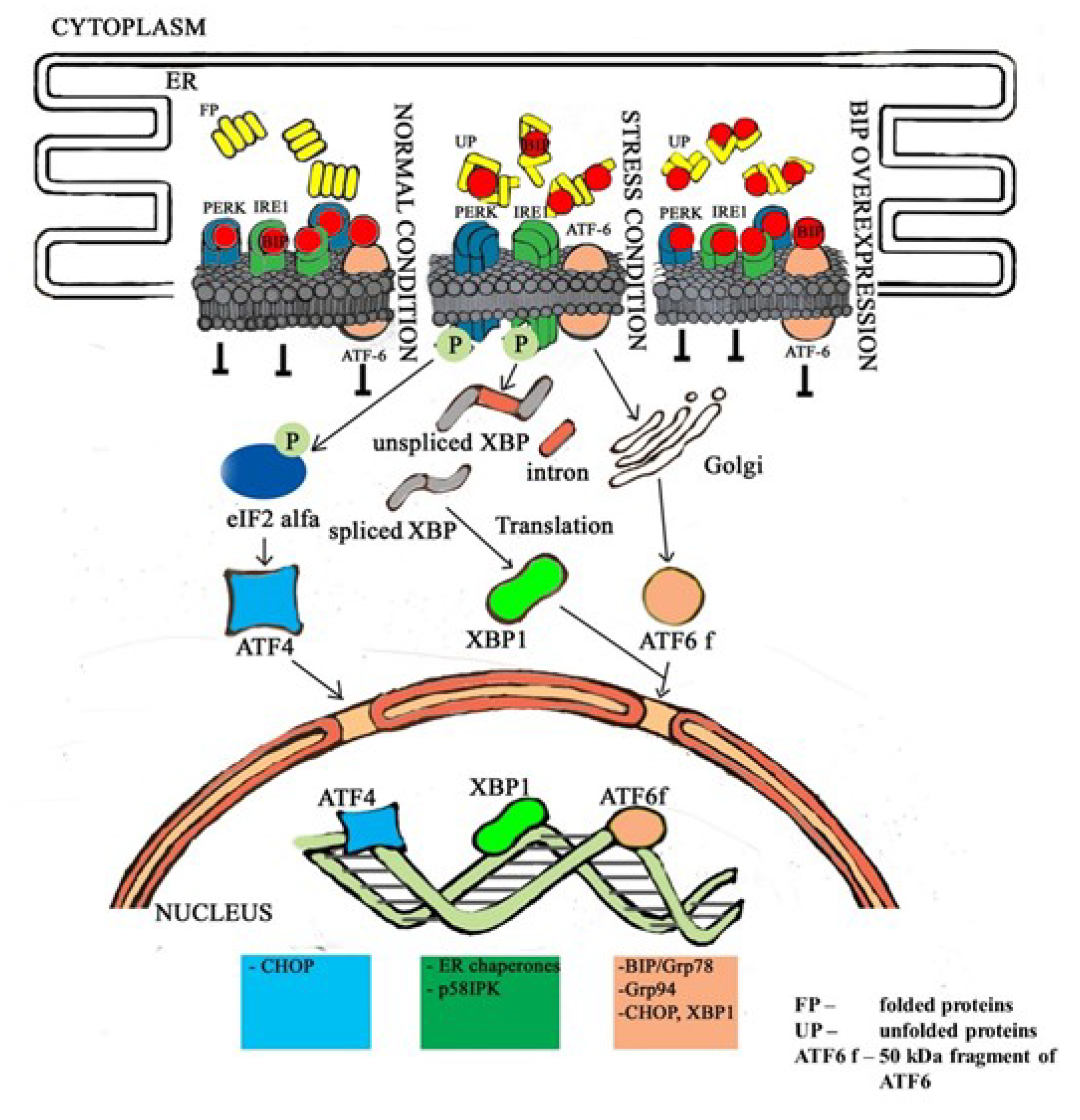
3.2. Endoplasmic Stress Receptors and Activation of the UPR
3.3. Downregulation of CHOP Expression After Transfection of S Cells With GRP78/BiP
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ABC | ATP-binding cassette |
| ALL | acute lymphoblasticleukemia |
| calcein AM | calcein acetoxymethyl |
| AML | Acute myeloid leukemia |
| ATF4 | activatingtranscription factor 4 |
| ATF6 | activating transcription factor 6 |
| ATF6/f | 50-kDa proteolytic fragment of ATF6 |
| Bax | Bcl-2-associated X protein |
| Bcl-2 | B-cell lymphoma 2 protein |
| BSA | Bovine serum albumin |
| cAMP | Cyclic adenosine monophosphate |
| CDK4 | Cyclindependentkinase 4 |
| CHOP | C/EBP homologous protein |
| CNX | Calnexin |
| CRT | calreticulin |
| Ct value | cycle threshold value |
| DAPI | 4′-6-diamidino-2-phenylindole |
| DMBA | dimethylbenzanthracene |
| DSMZ | German Collection of Microorganisms and Cell Cultures |
| eIF2 | eukaryotic translation-initiation factor 2 |
| ER | endoplasmic reticulum |
| ERAD | Endoplasmic-reticulum-associated protein degradation |
| FAV | Fluorescein linked annexin V |
| FP | folded protein |
| FITC | Fluorescein isothiocyanate |
| GAPDH | Glyceraldehyde 3-phosphate dehydrogenase |
| GRP78/BiP | glucose-regulated protein 78/Binding immunoglobulin protein |
| GRP94 | glucose-regulated protein 94 |
| SIRE1 | inositol-requiring enzyme 1 |
| MDR | multidrug resistance |
| MTT | 3-(4,5-dimethyl-2-thiazolyl)-2, 5-diphenyl-2H-tetrazolium bromide |
| PBS | Phosphate-buffered saline |
| PCR | polymerasechain reaction |
| PERK | pancreatic ER kinase-like ER kinase |
| P-gp | P-glycoprotein |
| PI | propidium iodide |
| SDS-PAGE | sodium dodecyl sulfate–polyacrylamide gel electrophoresis |
| UPR | unfolded proteinresponse |
| UP | unfolded protein |
| UV | ultraviolet |
| s/us/t XBP1 | spliced/unspliced/total X-boxbinding protein 1 |
| qRT-PCR | quantitative RT-PCR |
| RPMI 1640 | Roswell Park MemorialInstitute 1640 medium |
| HRP | horseradish peroxidase |
| VCR | vincristine |
References
- Pahl, H.L. Signal transduction from the endoplasmic reticulum to the cell nucleus. Physiol. Rev. 1999, 79, 683–701. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Ng, D.T. Glycosylation-directed quality control of protein folding. Nat. Rev. Mol. Cell Biol. 2015, 16, 742–752. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.S.; Kaufman, R.J. Unfolded protein response. Curr. Biol. 2012, 22, R622–R626. [Google Scholar] [CrossRef] [PubMed]
- Rutkowski, D.T.; Kaufman, R.J. A trip to the er: Coping with stress. Trends Cell Biol. 2004, 14, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Schroder, M.; Kaufman, R.J. The mammalian unfolded protein response. Annu. Rev. Biochem. 2005, 74, 739–789. [Google Scholar] [CrossRef]
- Wang, M.; Wey, S.; Zhang, Y.; Ye, R.; Lee, A.S. Role of the unfolded protein response regulator grp78/bip in development, cancer, and neurological disorders. Antioxid. Redox Signal. 2009, 11, 2307–2316. [Google Scholar] [CrossRef]
- Shi, Y.; Vattem, K.M.; Sood, R.; An, J.; Liang, J.; Stramm, L.; Wek, R.C. Identification and characterization of pancreatic eukaryotic initiation factor 2 alpha-subunit kinase, pek, involved in translational control. Mol. Cell Biol. 1998, 18, 7499–7509. [Google Scholar] [CrossRef]
- Nishitoh, H. Chop is a multifunctional transcription factor in the er stress response. J. Biochem. 2012, 151, 217–219. [Google Scholar] [CrossRef]
- Klymenko, O.; Henneke, I.; Ruppert, C.; Mahavadi, P.; Seeger, W.; Guenther, A.; Korfei, M. Regulation and role of the pro-apoptotic transcription factor c/ebp homologous protein (chop) in type ii cells apoptosis and idiopathic pulmonary fibrosis (ipf). Am. J. Respir. Crit. Care Med. 2015, 191, A3439. [Google Scholar]
- Ye, J.; Rawson, R.B.; Komuro, R.; Chen, X.; Dave, U.P.; Prywes, R.; Brown, M.S.; Goldstein, J.L. Er stress induces cleavage of membrane-bound atf6 by the same proteases that process srebps. Mol. Cell 2000, 6, 1355–1364. [Google Scholar] [CrossRef]
- Park, H.R.; Oh, R.; Wagner, P.; Panganiban, R.; Lu, Q. New insights into cellular stress responses to environmental metal toxicants. Int. Rev. Cell Mol. Biol. 2017, 331, 55–82. [Google Scholar] [PubMed]
- Harrington, P.E.; Biswas, K.; Malwitz, D.; Tasker, A.S.; Mohr, C.; Andrews, K.L.; Dellamaggiore, K.; Kendall, R.; Beckmann, H.; Jaeckel, P.; et al. Unfolded protein response in cancer: Ire1alpha inhibition by selective kinase ligands does not impair tumor cell viability. ACS Med. Chem. Lett. 2014, 6, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Hirota, M.; Kitagaki, M.; Itagaki, H.; Aiba, S. Quantitative measurement of spliced xbp1 mrna as an indicator of endoplasmic reticulum stress. J. Toxicol. Sci. 2006, 31, 149–156. [Google Scholar] [CrossRef]
- Wiersma, V.R.; Michalak, M.; Abdullah, T.M.; Bremer, E.; Eggleton, P. Mechanisms of translocation of er chaperones to the cell surface and immunomodulatory roles in cancer and autoimmunity. Front. Oncol. 2015, 5, 7. [Google Scholar] [CrossRef] [PubMed]
- Reddy, R.K.; Dubeau, L.; Kleiner, H.; Parr, T.; Nichols, P.; Ko, B.; Dong, D.; Ko, H.; Mao, C.; DiGiovanni, J.; et al. Cancer-inducible transgene expression by the grp94 promoter: Spontaneous activation in tumors of various origins and cancer-associated macrophages. Cancer Res. 2002, 62, 7207–7212. [Google Scholar]
- Wang, Q.; Groenendyk, J.; Michalak, M. Glycoprotein quality control and endoplasmic reticulum stress. Molecules 2015, 20, 13689–13704. [Google Scholar] [CrossRef]
- Heifetz, A.; Keenan, R.W.; Elbein, A.D. Mechanism of action of tunicamycin on the udp-glcnac:Dolichyl-phosphate glc-nac-1-phosphate transferase. Biochemistry 1979, 18, 2186–2192. [Google Scholar] [CrossRef]
- Morin, M.J.; Bernacki, R.J. Biochemical effects and therapeutic potential of tunicamycin in murine l1210 leukemia. Cancer Res. 1983, 43, 1669–1674. [Google Scholar]
- Hemming, F. Glycosyl phosphopolyprenols. In Glycolipids; Wiegandt, H., Ed.; Elsevier Biomedical Press: Amsterdam, The Netherlands, 1985; pp. 261–305. [Google Scholar]
- You, S.; Li, W.; Guan, Y. Tunicamycin inhibits colon carcinoma growth and aggressiveness via modulation of the erk-jnk-mediated akt/mtor signaling pathway. Mol. Med. Rep. 2018, 17, 4203–4212. [Google Scholar] [CrossRef]
- Han, C.; Jin, L.; Mei, Y.; Wu, M. Endoplasmic reticulum stress inhibits cell cycle progression via induction of p27 in melanoma cells. Cell Signal. 2013, 25, 144–149. [Google Scholar] [CrossRef]
- Wang, X.; Xiong, W.; Tang, Y. Tunicamycin suppresses breast cancer cell growth and metastasis via regulation of the protein kinase b/nuclear factor-kappab signaling pathway. Oncol. Lett. 2018, 15, 4137–4142. [Google Scholar] [PubMed]
- Qi, W.; Zeng, S.; Liu, M.; Yang, S.; Tan, X.; Yu, B. Tunicamycin induces apoptosis in non-small cell lung cancer cells through c/ebp homologous protein activation-mediated endoplasmic reticulum stress. Int. J. Clin. Exp. Med. 2018, 11, 5310–5322. [Google Scholar]
- da Silva, D.C.; Valentao, P.; Andrade, P.B.; Pereira, D.M. Endoplasmic reticulum stress signaling in cancer and neurodegenerative disorders: Tools and strategies to understand its complexity. Pharmacol. Res. 2020, 155, 104702. [Google Scholar] [CrossRef] [PubMed]
- Kelly, G.L.; Strasser, A. The essential role of evasion from cell death in cancer. Adv. Cancer Res. 2011, 111, 39–96. [Google Scholar]
- Bublik, D.R.; Bursac, S.; Sheffer, M.; Orsolic, I.; Shalit, T.; Tarcic, O.; Kotler, E.; Mouhadeb, O.; Hoffman, Y.; Fuchs, G.; et al. Regulatory module involving fgf13, mir-504, and p53 regulates ribosomal biogenesis and supports cancer cell survival. Proc. Natl. Acad. Sci. USA 2017, 114, E496–E505. [Google Scholar] [CrossRef]
- Jang, H.H. Regulation of protein degradation by proteasomes in cancer. J. Cancer Prev. 2018, 23, 153–161. [Google Scholar] [CrossRef]
- Sonneveld, P. Multidrug resistance in haematological malignancies. J. Intern. Med. 2000, 247, 521–534. [Google Scholar] [CrossRef]
- Breier, A.; Barancik, M.; Sulova, Z.; Uhrik, B. P-glycoprotein--implications of metabolism of neoplastic cells and cancer therapy. Curr. Cancer Drug Targets 2005, 5, 457–468. [Google Scholar] [CrossRef]
- Breier, A.; Gibalova, L.; Seres, M.; Barancik, M.; Sulova, Z. New insight into p-glycoprotein as a drug target. Anticancer Agents Med. Chem. 2013, 13, 159–170. [Google Scholar] [CrossRef]
- Juliano, R.L.; Ling, V. A surface glycoprotein modulating drug permeability in chinese hamster ovary cell mutants. Biochim. Biophys. Acta 1976, 455, 152–162. [Google Scholar] [CrossRef]
- Greer, D.A.; Ivey, S. Distinct n-glycan glycosylation of p-glycoprotein isolated from the human uterine sarcoma cell line mes-sa/dx5. Biochim. Biophys. Acta 2007, 1770, 1275–1282. [Google Scholar] [CrossRef] [PubMed]
- Seres, M.; Cholujova, D.; Bubencikova, T.; Breier, A.; Sulova, Z. Tunicamycin depresses p-glycoprotein glycosylation without an effect on its membrane localization and drug efflux activity in l1210 cells. Int. J. Mol. Sci. 2011, 12, 7772–7784. [Google Scholar] [CrossRef] [PubMed]
- Breier, A.; Stetka, J.; Bohacova, V.; Macejova, D.; Brtko, J.; Sulova, Z. Effect of 9-cis retinoic acid and all-trans retinoic acid in combination with verapamil on p-glycoprotein expression in l1210 cells. Neoplasma 2014, 61, 553–565. [Google Scholar] [CrossRef] [PubMed]
- Pavlikova, L.; Seres, M.; Imrichova, D.; Hano, M.; Rusnak, A.; Zamorova, M.; Katrlik, J.; Breier, A.; Sulova, Z. The expression of p-gp in leukemia cells is associated with cross-resistance to protein n-glycosylation inhibitor tunicamycin. Gen. Physiol. Biophys. 2016, 35, 497–510. [Google Scholar] [CrossRef] [PubMed]
- Hano, M.; Tomasova, L.; Seres, M.; Pavlikova, L.; Breier, A.; Sulova, Z. Interplay between p-glycoprotein expression and resistance to endoplasmic reticulum stressors. Molecules 2018, 23, 337. [Google Scholar] [CrossRef]
- Polekova, L.; Barancik, M.; Mrazova, T.; Pirker, R.; Wallner, J.; Sulova, Z.; Breier, A. Adaptation of mouse leukemia cells l1210 to vincristine. Evidence for expression of p-glycoprotein. Neoplasma 1992, 39, 73–77. [Google Scholar]
- Sulova, Z.; Ditte, P.; Kurucova, T.; Polakova, E.; Rogozanova, K.; Gibalova, L.; Seres, M.; Skvarkova, L.; Sedlak, J.; Pastorek, J.; et al. The presence of p-glycoprotein in l1210 cells directly induces down-regulation of cell surface saccharide targets of concanavalin a. Anticancer Res. 2010, 30, 3661–3668. [Google Scholar]
- Pastan, I.; Gottesman, M.M.; Ueda, K.; Lovelace, E.; Rutherford, A.V.; Willingham, M.C. A retrovirus carrying an mdr1 cdna confers multidrug resistance and polarized expression of p-glycoprotein in mdck cells. Proc. Natl. Acad. Sci. USA 1988, 85, 4486–4490. [Google Scholar] [CrossRef]
- Werstuck, G.H.; Lentz, S.R.; Dayal, S.; Hossain, G.S.; Sood, S.K.; Shi, Y.Y.; Zhou, J.; Maeda, N.; Krisans, S.K.; Malinow, M.R.; et al. Homocysteine-induced endoplasmic reticulum stress causes dysregulation of the cholesterol and triglyceride biosynthetic pathways. J. Clin. Investig. 2001, 107, 1263–1273. [Google Scholar] [CrossRef]
- Zeng, Y.; Wagner, E.J.; Cullen, B.R. Both natural and designed micro rnas can inhibit the expression of cognate mrnas when expressed in human cells. Mol. Cell 2002, 9, 1327–1333. [Google Scholar] [CrossRef]
- Samali, A.; Fitzgerald, U.; Deegan, S.; Gupta, S. Methods for monitoring endoplasmic reticulum stress and the unfolded protein response. Int. J. Cell Biol. 2010, 2010, 830307. [Google Scholar] [CrossRef] [PubMed]
- Oslowski, C.M.; Urano, F. Measuring er stress and the unfolded protein response using mammalian tissue culture system. Methods Enzymol. 2011, 490, 71–92. [Google Scholar] [PubMed]
- Rastogi, S.; Boylan, M.; Wright, E.G.; Coates, P.J. Interactions of apoptotic cells with macrophages in radiation-induced bystander signaling. Radiat. Res. 2013, 179, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Prischi, F.; Nowak, P.R.; Carrara, M.; Ali, M.M. Phosphoregulation of ire1 rnase splicing activity. Nat. Commun. 2014, 5, 3554. [Google Scholar] [CrossRef]
- Lin, J.A.; Fang, S.U.; Su, C.L.; Hsiao, C.J.; Chang, C.C.; Lin, Y.F.; Cheng, C.W. Silencing glucose-regulated protein 78 induced renal cell carcinoma cell line g1 cell-cycle arrest and resistance to conventional chemotherapy. Urol. Oncol. 2014, 32, 29.e1–29.e11. [Google Scholar] [CrossRef]
- Guha, P.; Kaptan, E.; Gade, P.; Kalvakolanu, D.V.; Ahmed, H. Tunicamycin induced endoplasmic reticulum stress promotes apoptosis of prostate cancer cells by activating mtorc1. Oncotarget 2017, 8, 68191–68207. [Google Scholar] [CrossRef]
- Ji, Y.; Luo, X.; Yang, Y.; Dai, Z.; Wu, G.; Wu, Z. Endoplasmic reticulum stress-induced apoptosis in intestinal epithelial cells: A feed-back regulation by mechanistic target of rapamycin complex 1 (mtorc1). J. Anim. Sci. Biotechnol. 2018, 9, 38. [Google Scholar] [CrossRef]
- Oda, T.; Kosuge, Y.; Arakawa, M.; Ishige, K.; Ito, Y. Distinct mechanism of cell death is responsible for tunicamycin-induced er stress in sk-n-sh and sh-sy5y cells. Neurosci. Res. 2008, 60, 29–39. [Google Scholar] [CrossRef]
- Pavlikova, L.; Seres, M.; Hano, M.; Bohacova, V.; Sevcikova, I.; Kyca, T.; Breier, A.; Sulova, Z. L1210 cells overexpressing abcb1 drug transporters are resistant to inhibitors of the n- and o-glycosylation of proteins. Molecules 2017, 22, 1104. [Google Scholar] [CrossRef]
- Seres, M.; Pavliková, L.; Sulova, Z.; Breier, A. Lectin detection of cell surface saccharides remodeling induced by development of p-glycoprotein mediated multidrug resistance phenotype in l1210 leukemia cells. Acta Chim. Slovaca 2014, 7, 52–56. [Google Scholar] [CrossRef][Green Version]
- Del Principe, M.I.; Del Poeta, G.; Maurillo, L.; Buccisano, F.; Venditti, A.; Tamburini, A.; Bruno, A.; Cox, M.C.; Suppo, G.; Tendas, A.; et al. P-glycoprotein and bcl-2 levels predict outcome in adult acute lymphoblastic leukaemia. Br. J. Haematol. 2003, 121, 730–738. [Google Scholar] [CrossRef] [PubMed]
- Kasimir-Bauer, S.; Beelen, D.; Flasshove, M.; Noppeney, R.; Seeber, S.; Scheulen, M.E. Impact of the expression of p glycoprotein, the multidrug resistance-related protein, bcl-2, mutant p53, and heat shock protein 27 on response to induction therapy and long-term survival in patients with de novo acute myeloid leukemia. Exp. Hematol. 2002, 30, 1302–1308. [Google Scholar] [CrossRef]
- Lee, J.S.; Jung, W.K.; Jeong, M.H.; Yoon, T.R.; Kim, H.K. Sanguinarine induces apoptosis of ht-29 human colon cancer cells via the regulation of bax/bcl-2 ratio and caspase-9-dependent pathway. Int. J. Toxicol. 2012, 31, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Karpel-Massler, G.; Ishida, C.T.; Zhang, Y.; Halatsch, M.E.; Westhoff, M.A.; Siegelin, M.D. Targeting intrinsic apoptosis and other forms of cell death by bh3-mimetics in glioblastoma. Expert Opin. Drug Discov. 2017, 12, 1031–1040. [Google Scholar] [CrossRef]
- Hsu, J.L.; Chiang, P.C.; Guh, J.H. Tunicamycin induces resistance to camptothecin and etoposide in human hepatocellular carcinoma cells: Role of cell-cycle arrest and grp78. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2009, 380, 373–382. [Google Scholar] [CrossRef]
- Du, Z.; Tong, X.; Ye, X. Cyclin d1 promotes cell cycle progression through enhancing ndr1/2 kinase activity independent of cyclin-dependent kinase 4. J. Biol. Chem. 2013, 288, 26678–26687. [Google Scholar] [CrossRef]
- Cooley, A.; Zelivianski, S.; Jeruss, J.S. Impact of cyclin e overexpression on smad3 activity in breast cancer cell lines. Cell Cycle 2010, 9, 4900–4907. [Google Scholar] [CrossRef]
- Darzynkiewicz, Z.; Zhao, H.; Zhang, S.; Lee, M.Y.; Lee, E.Y.; Zhang, Z. Initiation and termination of DNA replication during s phase in relation to cyclins d1, e and a, p21waf1, cdt1 and the p12 subunit of DNA polymerase delta revealed in individual cells by cytometry. Oncotarget 2015, 6, 11735–11750. [Google Scholar] [CrossRef]
- Xie, X.; Lin, W.; Zheng, W.; Chen, T.; Yang, H.; Sun, L.; Huang, F.; Wang, Z.; Lin, H.; Chen, L.; et al. Downregulation of g2/mitotic-specific cyclinb1 triggers autophagy via ampk-ulk1-dependent signal pathway in nasopharyngeal carcinoma cells. Cell Death Dis. 2019, 10, 94. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, R.; Ni, M.; Gill, P.; Lee, A.S. Cell surface relocalization of the endoplasmic reticulum chaperone and unfolded protein response regulator grp78/bip. J. Biol. Chem. 2010, 285, 15065–15075. [Google Scholar] [CrossRef]
- Sulova, Z.; Seres, M.; Barancik, M.; Gibalova, L.; Uhrik, B.; Polekova, L.; Breier, A. Does any relationship exist between p-glycoprotein-mediated multidrug resistance and intracellular calcium homeostasis. Gen. Physiol. Biophys. 2009, 28, F89–F95. [Google Scholar] [PubMed]
- Seres, M.; Polakova, E.; Krizanova, O.; Hudecova, S.; Klymenko, S.V.; Breier, A.; Sulova, Z. Overexpression of p-glycoprotein in l1210/vcr cells is associated with changes in several endoplasmic reticulum proteins that may be partially responsible for the lack of thapsigargin sensitivity. Gen. Physiol. Biophys. 2008, 27, 211–221. [Google Scholar] [PubMed]
- Bubencikova, T.; Cholujova, D.; Messingerova, L.; Mislovicova, D.; Seres, M.; Breier, A.; Sulova, Z. Detection of glycomic alterations induced by overexpression of p-glycoprotein on the surfaces of l1210 cells using sialic acid binding lectins. Int. J. Mol. Sci. 2012, 13, 15177–15192. [Google Scholar] [CrossRef] [PubMed]
- Sulova, Z.; Mislovicova, D.; Gibalova, L.; Vajcnerova, Z.; Polakova, E.; Uhrik, B.; Tylkova, L.; Kovarova, A.; Sedlak, J.; Breier, A. Vincristine-induced overexpression of p-glycoprotein in l1210 cells is associated with remodeling of cell surface saccharides. J. Proteome Res. 2009, 8, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Xia, Y.; Li, J.; Zhang, C.; Zhang, H.; Ma, T.; Yang, L.; Kong, L. Grp78 inhibition enhances atf4-induced cell death by the deubiquitination and stabilization of chop in human osteosarcoma. Cancer Lett. 2017, 410, 112–123. [Google Scholar] [CrossRef] [PubMed]

| Gene | Forward Primer | Revers Primer | bp |
|---|---|---|---|
| GAPDH b | 5′-TAT GTC GTG GAG TCT ACT GGT GTC-3′ | 5′-GTC ATC ATA CTT GGC AGG TTT CTC-3′ | 492 |
| IRE1α b | 5′-AAC ACA CCG ACC ACC GTA TC-3′ | 5′-AGG GTC CTG GGT AAG GTC TC-3′ | 282 |
| PERK b | 5′-GCC GAC GAT CAA ATG GAA GC-3′ | 5′-GTG GGG CTG AGG ATG GAA AA-3′ | 370 |
| ATF6 b | 5′-TGG AAG TGG GAA GAT CGG GA-3′ | 5′-AGC CAC AGG TCC TCT TTA GG-3′ | 312 |
| XBP1 a | 5′-GAA CCA GGA GTT AAG AAC ACG-3′ | 5′-AGG CAA CAG TGT CAG AGT CC-3′ | us 205 s 179 |
| CHOP b | 5′-GGA ACC TGA GGA GAG GTG TTC-3′ | 5′-TGC AGA TCC TCA TAC CAG G-3′ | 162 |
| GRP94 b | 5′-GGG GAG GTC ACC TTC AAG TC-3′ | 5′-TGA GGG GGA GAT CAT CGG AA-3′ | 199 |
| Cnx b | 5′-AGT GGG AAG TAG ATG AGA TGA AGG-3′ | 5′-ATA CAC CTG TCT TGG GAT TTT TGT-3′ | 333 |
| cyclin D1 b | 5′-TCA CCC TGA GAG TAG GGA GC-3′ | 5′-GGC CTT CAG GCA AAA ACC AG-3′ | 592 |
| cyclin A b | 5′-AGC AGA ACT CAT TCG GCT CT-3′ | 5′-CAA GGG AAA AGG AAG AAG AAG AGA A-3′ | 297 |
| cyclin E b | 5′-ATG TTA CAG ATG GCG CTT GC-3′ | 5′-GAG GAC ACC ATA AGG AAA TCT GA-3′ | 254 |
| cyclin B1 b | 5′-CAG TTG TGT GCC CAA GAA GA-3′ | 5′-CTA CGG AGG AAG TGC AGA GG-3′ | 216 |
| Bcl-2 b | 5′-GGC TGG GGA TGA CTT CTC TC-3′ | 5′-GCA TGC TGG GGC CAT ATA GTT-3‘ | 323 |
| Bax b | 5′-ATC CAA GAC CAG GGT GGC T-3′ | 5′-CTT CCC CCA TTC ATC CCA GG-3′ | 197 |
| Gene | Forward Primer | Revers Primer |
|---|---|---|
| sXBP1 c,s | 5′-GTC CAT GGG AAG ATG TTC TGG-3′ | 5′-CTG AGT CCG AAT CAG GTG CAG-3′ |
| usXBP1 c, us | 5′-GTC CAT GGG AAG ATG TTC TGG-3′ | 5′-CAG CAC TCA GAC TAT GTG CA-3′ |
| totalXBP1 c,t | 5′-GTC CAT GGG AAG ATG TTC TGG-3′ | 5′-TGG CCG GGT CTG CTG AGT CCG-3′ |
| CHOP c | 5′-AGG TGA AAG GCA GGG ACT CA-3′ | 5′-CCA CCA CAC CTG AAA GCA GAA-3′ |
| GRP78/BiP c | 5′-TTT TCT GAT GTA TCC TCT TCA CCA GT-3′ | 5′-TTC AGC CAA TTA TCA GCA AAC TCT-3′ |
| GRP94 c | 5′-CAA ATG GAG AAG ATT CCG CC-3′ | 5′-AAG AAT GAA GGA AAA ACA GGA CAA AA-3′ |
| β-actin c | 5′-TGT CCA CCT TCC AGC AGA T-3′ | 5′-AGC TCA GTA ACA GTC CGC C-3′ |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šereš, M.; Pavlíková, L.; Boháčová, V.; Kyca, T.; Borovská, I.; Lakatoš, B.; Breier, A.; Sulová, Z. Overexpression of GRP78/BiP in P-Glycoprotein-Positive L1210 Cells is Responsible for Altered Response of Cells to Tunicamycin as a Stressor of the Endoplasmic Reticulum. Cells 2020, 9, 890. https://doi.org/10.3390/cells9040890
Šereš M, Pavlíková L, Boháčová V, Kyca T, Borovská I, Lakatoš B, Breier A, Sulová Z. Overexpression of GRP78/BiP in P-Glycoprotein-Positive L1210 Cells is Responsible for Altered Response of Cells to Tunicamycin as a Stressor of the Endoplasmic Reticulum. Cells. 2020; 9(4):890. https://doi.org/10.3390/cells9040890
Chicago/Turabian StyleŠereš, Mário, Lucia Pavlíková, Viera Boháčová, Tomáš Kyca, Ivana Borovská, Boris Lakatoš, Albert Breier, and Zdena Sulová. 2020. "Overexpression of GRP78/BiP in P-Glycoprotein-Positive L1210 Cells is Responsible for Altered Response of Cells to Tunicamycin as a Stressor of the Endoplasmic Reticulum" Cells 9, no. 4: 890. https://doi.org/10.3390/cells9040890
APA StyleŠereš, M., Pavlíková, L., Boháčová, V., Kyca, T., Borovská, I., Lakatoš, B., Breier, A., & Sulová, Z. (2020). Overexpression of GRP78/BiP in P-Glycoprotein-Positive L1210 Cells is Responsible for Altered Response of Cells to Tunicamycin as a Stressor of the Endoplasmic Reticulum. Cells, 9(4), 890. https://doi.org/10.3390/cells9040890





