Key Components of Human Myofibre Denervation and Neuromuscular Junction Stability are Modulated by Age and Exercise
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Muscle Biopsies
2.3. RNA Extraction
2.4. Real-Time RT-PCR
2.5. Immunohistochemistry
2.6. Microscopy
2.7. Immunocytochemistry
2.8. Statistics
3. Results
3.1. Subject Characteristics
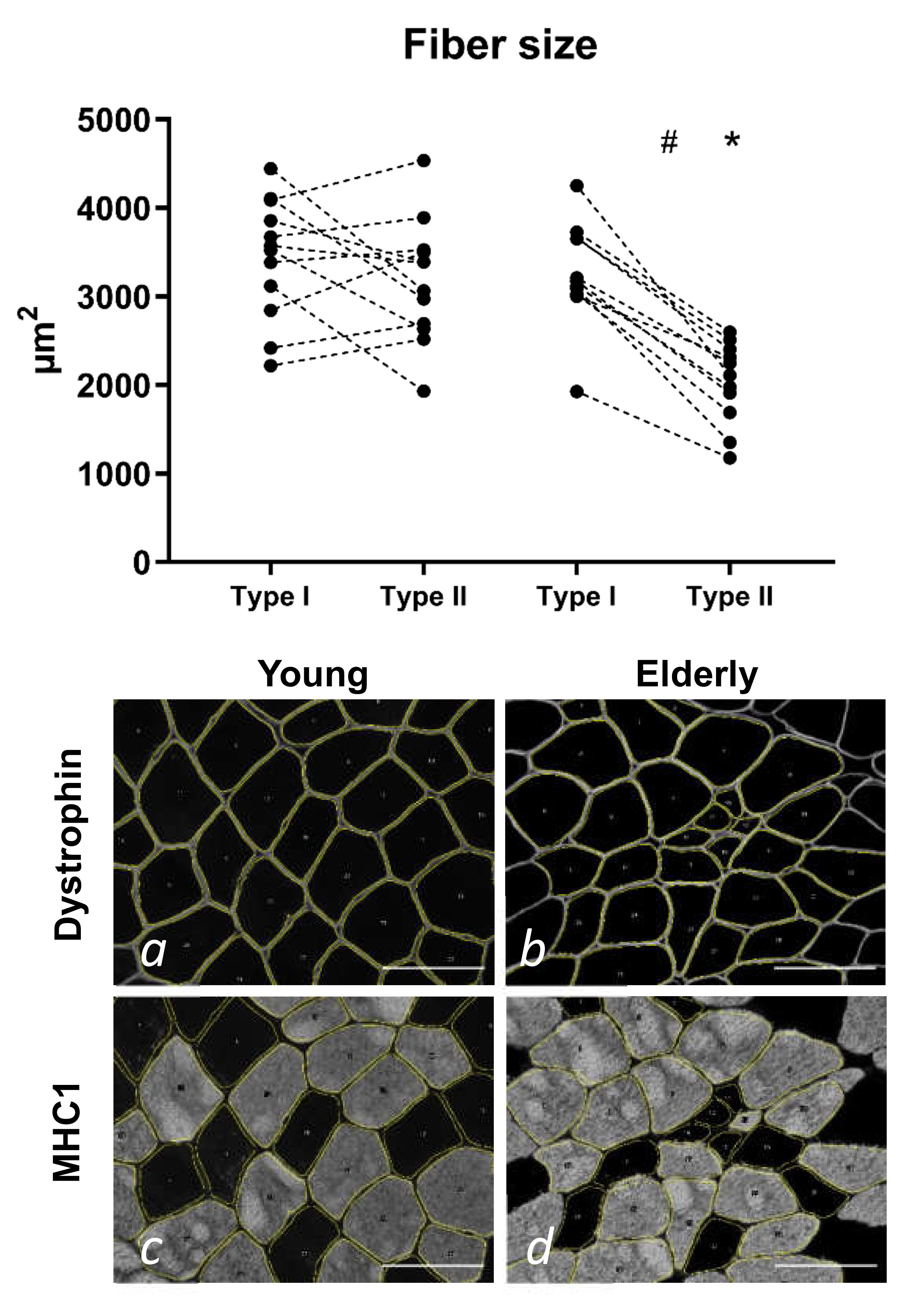
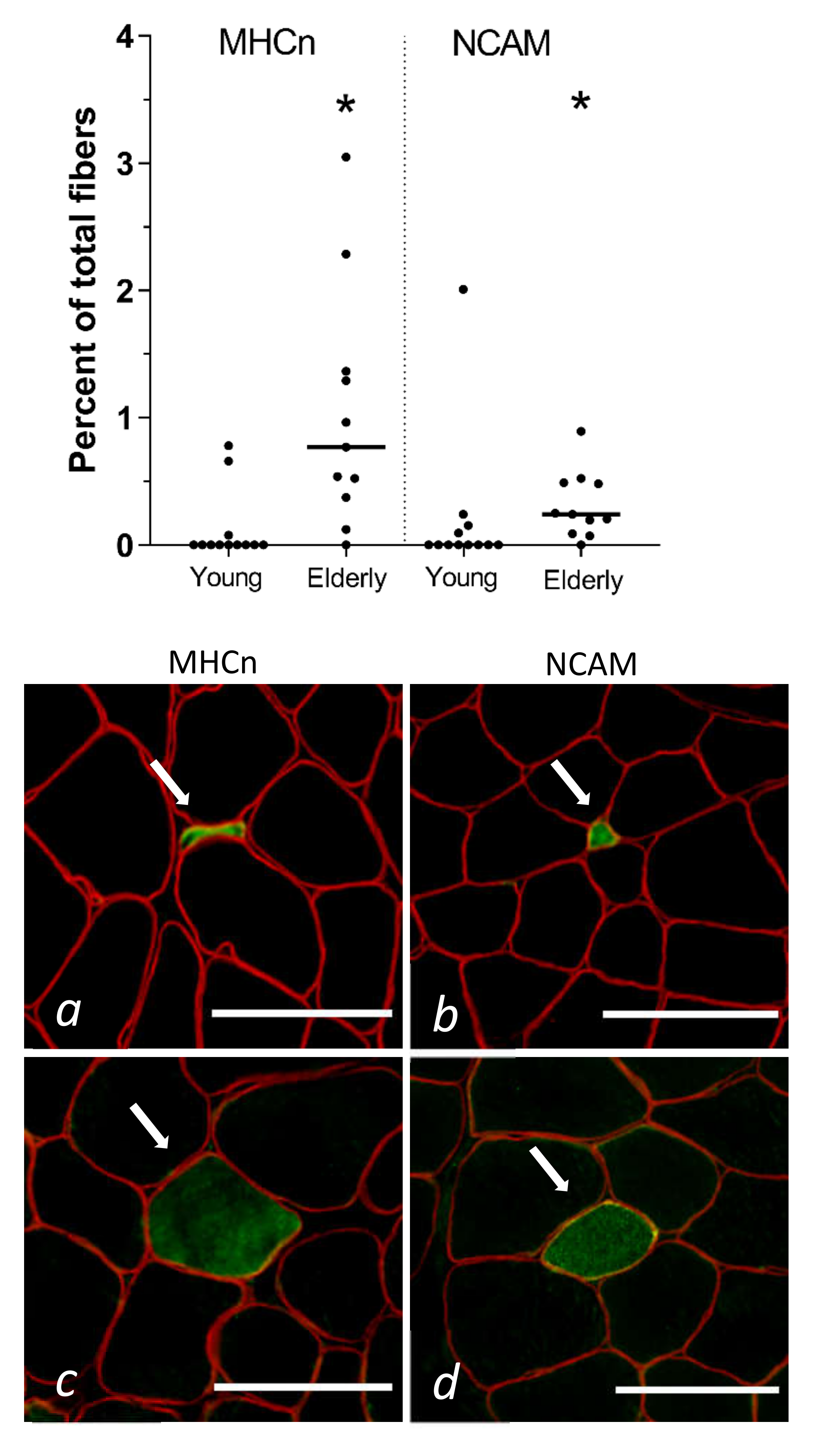
3.2. Tissue Immunohistochemistry at Baseline—Young and Elderly Women
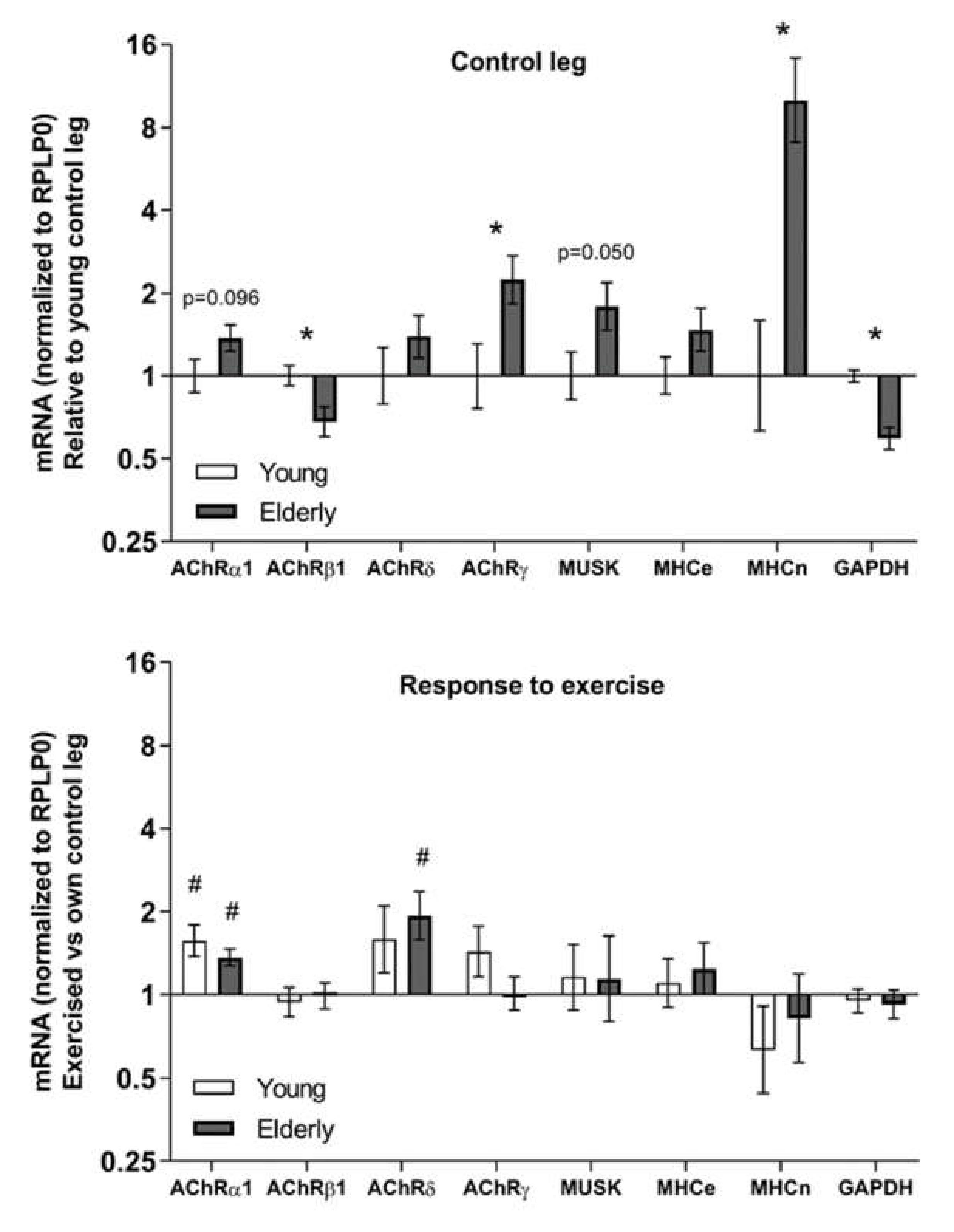
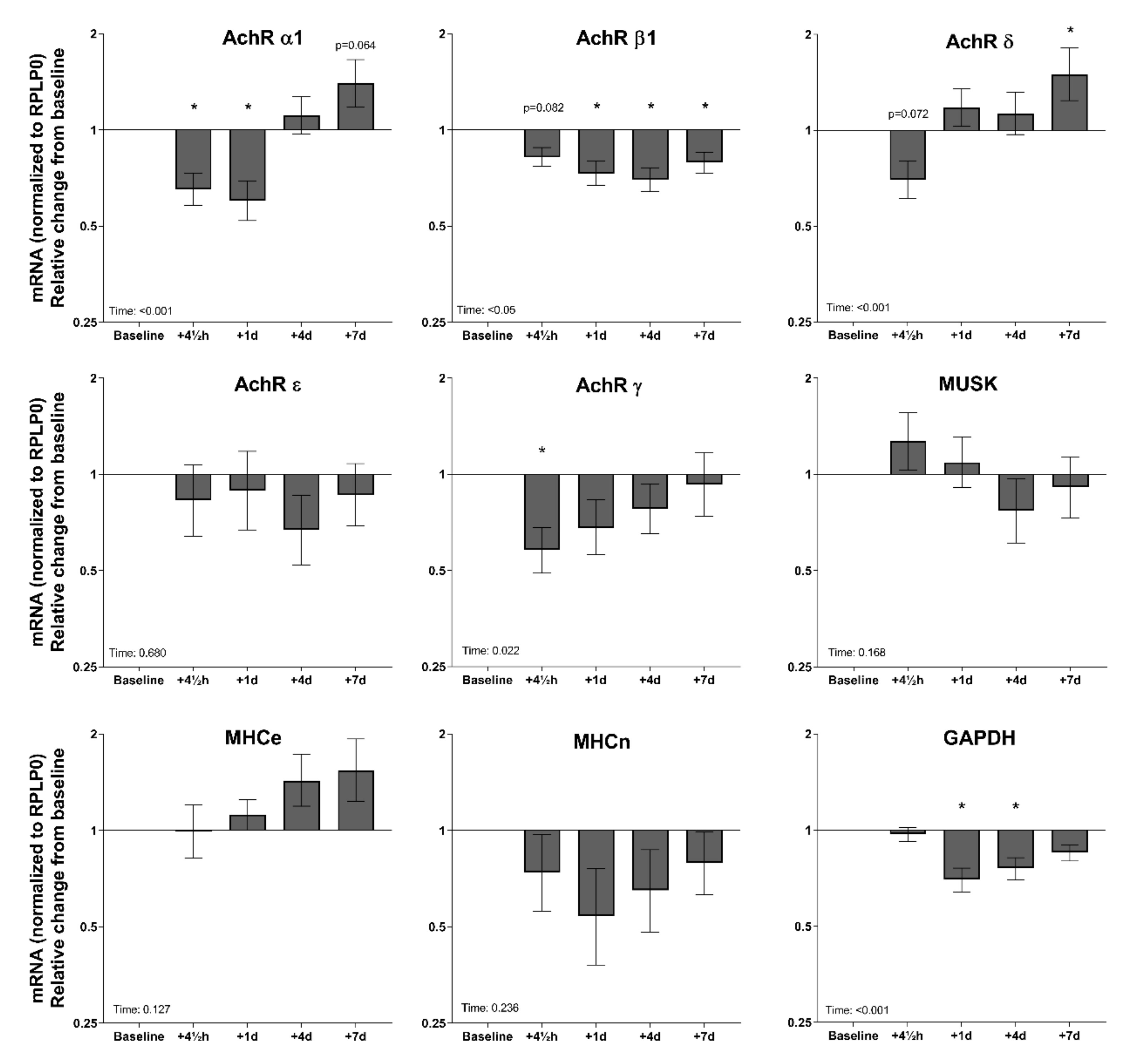
3.3. Tissue mRNA at Baseline and in Response to Exercise—Young and Elderly Women
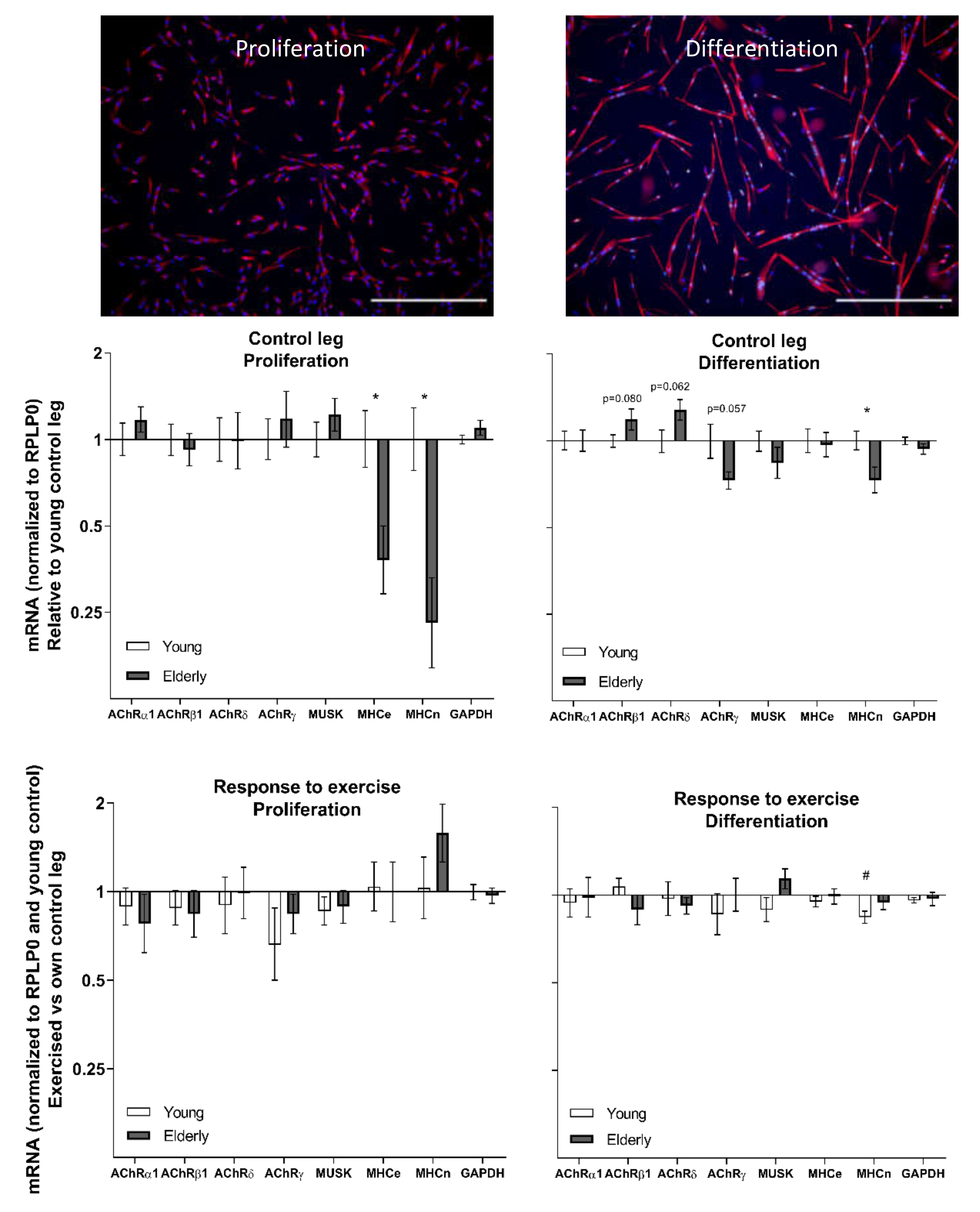
3.4. Cell Culture at Baseline and in Response to Exercise—Young and Elderly Women
3.5. Tissue mRNA in Response to Exercise—Elderly Men
4. Discussions
4.1. Muscle Fibre Denervation in Elderly Humans
4.2. Ageing and Exercise Alter Acetylcholine Receptor Gene Expression
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| MHCe | Embryonic myosin heavy chain |
| MHCn | Neonatal myosin heavy chain |
| NCAM | Neural cell adhesion molecule |
| MHC1 | Myosin heavy chain 1 |
| DYST | Dystrophin |
| AChR | Acetylcholine receptor |
| DAPI | 4′,6-Di-amidino-2-phenylindole |
| TBS | Tris-buffered saline |
| BSA | Bovine serum albumin |
| mTOR | Mammalian target of rapamycin |
References
- Lexell, J.; Taylor, C.C.; Sjöström, M. What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. J. Neurol. Sci. 1988, 84, 275–294. [Google Scholar] [CrossRef]
- Bean, J.F.; Kiely, D.K.; Herman, S.; Leveille, S.G.; Mizer, K.; Frontera, W.R.; Fielding, R.A. The Relationship Between Leg Power and Physical Performance in Mobility-Limited Older People. J. Am. Geriatr. Soc. 2002, 50, 461–467. [Google Scholar] [PubMed]
- Janssen, I.; Heymsfield, S.B.; Ross, R. Low Relative Skeletal Muscle Mass (Sarcopenia) in Older Persons Is Associated with Functional Impairment and Physical Disability. J. Am. Geriatr. Soc. 2002, 50, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Reid, K.F.; Naumova, E.N.; Carabello, R.J.; Phillips, E.M.; Fielding, R.A. Lower extremity muscle mass predicts functional performance in mobility-limited elders. J. Nutr. Health Aging 2008, 12, 493. [Google Scholar] [CrossRef] [PubMed]
- Porter, M.M.; Vandervoort, A.A.; Lexell, J. Aging of human muscle: Structure, function and adaptability. Scand. J. Med. Sci. Sports 1995, 5, 129–142. [Google Scholar] [CrossRef]
- Campbell, M.J.; McComas, A.J.; Petito, F. Physiological changes in ageing muscles. J. Neurol. Neurosurg. Psychiatry 1973, 36, 174–182. [Google Scholar] [CrossRef]
- Tomlinson, B.E.; Irving, D. The numbers of limb motor neurons in the human lumbosacral cord throughout life. J. Neurol. Sci. 1977, 34, 213–219. [Google Scholar] [CrossRef]
- Hepple, R.T.; Rice, C.L. Innervation and neuromuscular control in ageing skeletal muscle. J. Physiol. (Lond.) 2016, 594, 1965–1978. [Google Scholar] [CrossRef]
- Snijders, T.; Leenders, M.; de Groot, L.C.P.G.M.; van Loon, L.J.C.; Verdijk, L.B. Muscle mass and strength gains following 6 months of resistance type exercise training are only partly preserved within one year with autonomous exercise continuation in older adults. Exp. Gerontol. 2019, 121, 71–78. [Google Scholar] [CrossRef]
- Bechshøft, R.L.; Malmgaard-Clausen, N.M.; Gliese, B.; Beyer, N.; Mackey, A.L.; Andersen, J.L.; Kjær, M.; Holm, L. Improved skeletal muscle mass and strength after heavy strength training in very old individuals. Exp. Gerontol. 2017, 92, 96–105. [Google Scholar] [CrossRef]
- Nishimune, H.; Stanford, J.A.; Mori, Y. ROLE of exercise in maintaining the integrity of the neuromuscular junction: Invited Review: Exercise and NMJ. Muscle Nerve 2014, 49, 315–324. [Google Scholar] [CrossRef]
- Baehr, L.M.; West, D.W.D.; Marcotte, G.; Marshall, A.G.; De Sousa, L.G.; Baar, K.; Bodine, S.C. Age-related deficits in skeletal muscle recovery following disuse are associated with neuromuscular junction instability and ER stress, not impaired protein synthesis. Aging 2016, 8, 127–146. [Google Scholar] [CrossRef] [PubMed]
- Hughes, D.C.; Marcotte, G.R.; Marshall, A.G.; West, D.W.D.; Baehr, L.M.; Wallace, M.A.; Saleh, P.M.; Bodine, S.C.; Baar, K. Age-related Differences in Dystrophin: Impact on Force Transfer Proteins, Membrane Integrity, and Neuromuscular Junction Stability. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2016, 72, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Sonjak, V.; Jacob, K.; Morais, J.A.; Rivera-Zengotita, M.; Spendiff, S.; Spake, C.; Taivassalo, T.; Chevalier, S.; Hepple, R.T. Fidelity of muscle fibre reinnervation modulates ageing muscle impact in elderly women. J. Physiol. 2019, 597, 5009–5023. [Google Scholar] [CrossRef] [PubMed]
- Fambrough, D.M.; Drachman, D.B.; Satyamurti, S. Neuromuscular junction in myasthenia gravis: Decreased acetylcholine receptors. Science 1973, 182, 293–295. [Google Scholar] [CrossRef] [PubMed]
- Merlie, J.P.; Sanes, J.R. Concentration of acetylcholine receptor mRNA in synaptic regions of adult muscle fibres. Nature 1985, 317, 66–68. [Google Scholar] [CrossRef] [PubMed]
- Fambrough, D.M. Control of acetylcholine receptors in skeletal muscle. Physiol. Rev. 1979, 59, 165–227. [Google Scholar] [CrossRef] [PubMed]
- Gundersen, K.; Rabben, I.; Klocke, B.J.; Merlie, J.P. Overexpression of myogenin in muscles of transgenic mice: Interaction with Id-1, negative crossregulation of myogenic factors, and induction of extrasynaptic acetylcholine receptor expression. Mol. Cell. Biol. 1995, 15, 7127–7134. [Google Scholar] [CrossRef]
- Pestronk, A.; Drachman, D.B. Motor Nerve Sprouting and Acetylcholine Receptors. Science 1978, 199, 1223–1225. [Google Scholar] [CrossRef]
- Soendenbroe, C.; Heisterberg, M.F.; Schjerling, P.; Karlsen, A.; Kjaer, M.; Andersen, J.L.; Mackey, A.L. Molecular indicators of denervation in aging human skeletal muscle. Muscle Nerve 2019, 60, 453–463. [Google Scholar] [CrossRef]
- Sanes, J.R.; Lichtman, J.W. Induction, assembly, maturation and maintenance of a postsynaptic apparatus. Nat. Rev. Neurosci. 2001, 2, 791–805. [Google Scholar] [CrossRef] [PubMed]
- Reist, N.E.; Werle, M.J.; McMahan, U.J. Agrin released by motor neurons induces the aggregation of acetylcholine receptors at neuromuscular junctions. Neuron 1992, 8, 865–868. [Google Scholar] [CrossRef]
- Liu, W.; Klose, A.; Forman, S.; Paris, N.D.; Wei-LaPierre, L.; Cortés-Lopéz, M.; Tan, A.; Flaherty, M.; Miura, P.; Dirksen, R.T.; et al. Loss of adult skeletal muscle stem cells drives age-related neuromuscular junction degeneration. Elife 2017, 6, e26464. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wei-LaPierre, L.; Klose, A.; Dirksen, R.T.; Chakkalakal, J.V. Inducible depletion of adult skeletal muscle stem cells impairs the regeneration of neuromuscular junctions. Elife 2015, 4, e09221. [Google Scholar] [CrossRef] [PubMed]
- Bechshøft, C.J.L.; Jensen, S.M.; Schjerling, P.; Andersen, J.L.; Svensson, R.B.; Eriksen, C.S.; Mkumbuzi, N.S.; Kjaer, M.; Mackey, A.L. Age and prior exercise in vivo determine the subsequent in vitro molecular profile of myoblasts and nonmyogenic cells derived from human skeletal muscle. Am. J. Physiol. Cell Physiol. 2019, 316, C898–C912. [Google Scholar] [CrossRef] [PubMed]
- Heisterberg, M.F.; Andersen, J.L.; Schjerling, P.; Bülow, J.; Lauersen, J.B.; Roeber, H.L.; Kjaer, M.; Mackey, A.L. Effect of Losartan on the Acute Response of Human Elderly Skeletal Muscle to Exercise. Med. Sci. Sports Exerc. 2018, 50, 225–235. [Google Scholar] [CrossRef]
- Bergstrom, J. Percutaneous needle biopsy of skeletal muscle in physiological and clinical research. Scand. J. Clin. Lab. Investig. 1975, 35, 609–616. [Google Scholar] [CrossRef]
- Koch, M.; Schulze, J.; Hansen, U.; Ashwodt, T.; Keene, D.R.; Brunken, W.J.; Burgeson, R.E.; Bruckner, P.; Bruckner-Tuderman, L. A novel marker of tissue junctions, collagen XXII. J. Biol. Chem. 2004, 279, 22514–22521. [Google Scholar] [CrossRef]
- Jakobsen, J.R.; Mackey, A.L.; Knudsen, A.B.; Koch, M.; Kjaer, M.; Krogsgaard, M.R. Composition and adaptation of human myotendinous junction and neighboring muscle fibers to heavy resistance training. Scand. J. Med. Sci. Sports 2017, 27, 1547–1559. [Google Scholar] [CrossRef]
- Karlsen, A.; Bechshøft, R.L.; Malmgaard-Clausen, N.M.; Andersen, J.L.; Schjerling, P.; Kjaer, M.; Mackey, A.L. Lack of muscle fibre hypertrophy, myonuclear addition, and satellite cell pool expansion with resistance training in 83-94-year-old men and women. Acta Physiol. (Oxf.) 2019, 227, e13271. [Google Scholar] [CrossRef]
- Brown, M.C.; Holland, R.L.; Hopkins, W.G. Motor Nerve Sprouting. Annu. Rev. Neurosci. 1981, 4, 17–42. [Google Scholar] [CrossRef] [PubMed]
- Covault, J.; Sanes, J.R. Neural cell adhesion molecule (N-CAM) accumulates in denervated and paralyzed skeletal muscles. Proc. Natl. Acad. Sci. USA 1985, 82, 4544–4548. [Google Scholar] [CrossRef] [PubMed]
- Gillon, A.; Sheard, P. Elderly mouse skeletal muscle fibres have a diminished capacity to upregulate NCAM production in response to denervation. Biogerontology 2015, 16, 811–823. [Google Scholar] [CrossRef] [PubMed]
- Viguie, C.A.; Lu, D.-X.; Huang, S.-K.; Rengen, H.; Carlson, B.M. Quantitative study of the effects of long-term denervation on the extensor digitorum longus muscle of the rat. Anat. Rec. 1997, 248, 346–354. [Google Scholar] [CrossRef]
- Gosztonyi, G.; Naschold, U.; Grozdanovic, Z.; Stoltenburg-Didinger, G.; Gossrau, R. Expression of Leu-19 (CD56, N-CAM) and nitric oxide synthase (NOS) I in denervated and reinnervated human skeletal muscle. Microsc. Res. Tech. 2001, 55, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Doppler, K.; Mittelbronn, M.; Bornemann, A. Myogenesis in human denervated muscle biopsies. Muscle Nerve 2008, 37, 79–83. [Google Scholar] [CrossRef]
- Mishina, M.; Takai, T.; Imoto, K.; Noda, M.; Takahashi, T.; Numa, S.; Methfessel, C.; Sakmann, B. Molecular distinction between fetal and adult forms of muscle acetylcholine receptor. Nature 1986, 321, 406–411. [Google Scholar] [CrossRef]
- Gu, Y.; Hall, Z.W. Immunological evidence for a change in subunits of the acetylcholine receptor in developing and denervated rat muscle. Neuron 1988, 1, 117–125. [Google Scholar] [CrossRef]
- Missias, A.C.; Chu, G.C.; Klocke, B.J.; Sanes, J.R.; Merlie, J.P. Maturation of the acetylcholine receptor in skeletal muscle: Regulation of the AChR gamma-to-epsilon switch. Dev. Biol. 1996, 179, 223–238. [Google Scholar] [CrossRef]
- Goldman, D.; Staple, J. Spatial and temporal expression of acetylcholine receptor RNAs in innervated and denervated rat soleus muscle. Neuron 1989, 3, 219–228. [Google Scholar] [CrossRef]
- Witzemann, V.; Brenner, H.R.; Sakmann, B. Neural factors regulate AChR subunit mRNAs at rat neuromuscular synapses. J. Cell Biol. 1991, 114, 125–141. [Google Scholar] [CrossRef] [PubMed]
| mRNA | Genbank | Sense | Antisense |
|---|---|---|---|
| RPLP0 | NM_053275.3 | GGAAACTCTGCATTCTCGCTTCCT | CCAGGACTCGTTTGTACCCGTTG |
| AchRα1 | NM_000079.3 | GCAGAGACCATGAAGTCAGACCAGGAG | CCGATGATGCAAACAAGCATGAA |
| AchRβ1 | NM_000747.2 | TTCATCCGGAAGCCGCCAAG | CCGCAGATCAGGGGCAGACA |
| AchRδ | NM_000751.2 | CAGCTGTGGATGGGGCAAAC | GCCACTCGGTTCCAGCTGTCTT |
| AchRε | NM_000080.4 | TGGCAGAACTGTTCGCTTATTTTCC | TTGATGGTCTTGCCGTCGTTGT |
| AchRγ | NM_005199.4 | GCCTGCAACCTCATTGCCTGT | ACTCGGCCCACCAGGAACCAC |
| MuSK | NM_005592.3 | TCATGGCAGAATTTGACAACCCTAAC | GGCTTCCCGACAGCACACAC |
| MHCe | NM_002470.3 | CGGATATCGCAGAATCTCAAGTCAA | CTCCAGAAGGGCTGGCTCACTC |
| MHCn | NM_002472.2 | CGGAAACATGAGCGACGAGTAAAA | CAGCCTGAGAACATTCTTGCGATCTT |
| GAPDH | NM_002046.6 | GAGGGGCCATCCACAGTCTTCT | GACATGCCCAAGACCCAGAAGGA |
| Host | Antibody | Primary Antibody Company | Cat. no. | Concentration |
|---|---|---|---|---|
| Mouse | Dystrophin, IgG2b | Sigma-Aldrich | D8168 | 1:500 |
| Mouse | Myosin 1, IgG1 | Hybridoma Bank | A4.951 | 1:200 |
| Mouse | MHCe, IgG1 | Hybridoma Bank | F1.652 | 1:100 |
| Mouse | MHCn, IgG1 | Novocastra | NCL-MHCn | 1:100 |
| Mouse | NCAM, IgG1 | Becton Dickinson | 347740 | 1:50 |
| Rabbit | Desmin, IgG | Abcam | AB32362 | 1:1000 |
| Mouse | Myogenin, IgG1 | Hybridoma Bank | F5D-s | 1:50 |
| Host | Antibody | Secondary Antibody Company | Cat. no. | Concentration |
| Goat | 488, green, IgG1 | Invitrogen | A-21121 | 1:500 |
| Goat | 568, red, IgG2b | Invitrogen | A-21144 | 1:200 |
| Goat | 568, red, IgG | Invitrogen | A-11036 | 1:500 |
| Goat | 488, green, IgG | Invitrogen | A-11029 | 1:500 |
| Young Women | Old Women | Old Men | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n = 12 | n = 11 | n = 25 | ||||||||||
| Age (yr) | 23 | ± | 3 | 20–28 | 74 | ± | 3 | 71–78 | 70 | ± | 7 | 64–90 |
| Height (cm) | 168 | ± | 7 | 157–177 | 166 | ± | 3 | 162–169 | 180 | ± | 5 | 172–189 |
| Weight (kg) | 64 | ± | 8 | 53–75 | 69 | ± | 10 | 57–84 | 82 | ± | 10 | 67–98 |
| BMI (kg/m2) | 23 | ± | 2 | 19–26 | 25 | ± | 4 | 20–30 | 26 | ± | 3 | 21–31 |
| Knee extension 1RM (kg) | 39 | ± | 8 | 30–50 | 23 | ± | 5 | 12–28 | 56 | ± | 14 | 23–82 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soendenbroe, C.; Bechshøft, C.J.L.; Heisterberg, M.F.; Jensen, S.M.; Bomme, E.; Schjerling, P.; Karlsen, A.; Kjaer, M.; Andersen, J.L.; Mackey, A.L. Key Components of Human Myofibre Denervation and Neuromuscular Junction Stability are Modulated by Age and Exercise. Cells 2020, 9, 893. https://doi.org/10.3390/cells9040893
Soendenbroe C, Bechshøft CJL, Heisterberg MF, Jensen SM, Bomme E, Schjerling P, Karlsen A, Kjaer M, Andersen JL, Mackey AL. Key Components of Human Myofibre Denervation and Neuromuscular Junction Stability are Modulated by Age and Exercise. Cells. 2020; 9(4):893. https://doi.org/10.3390/cells9040893
Chicago/Turabian StyleSoendenbroe, Casper, Cecilie J. L. Bechshøft, Mette F. Heisterberg, Simon M. Jensen, Emma Bomme, Peter Schjerling, Anders Karlsen, Michael Kjaer, Jesper L. Andersen, and Abigail L. Mackey. 2020. "Key Components of Human Myofibre Denervation and Neuromuscular Junction Stability are Modulated by Age and Exercise" Cells 9, no. 4: 893. https://doi.org/10.3390/cells9040893
APA StyleSoendenbroe, C., Bechshøft, C. J. L., Heisterberg, M. F., Jensen, S. M., Bomme, E., Schjerling, P., Karlsen, A., Kjaer, M., Andersen, J. L., & Mackey, A. L. (2020). Key Components of Human Myofibre Denervation and Neuromuscular Junction Stability are Modulated by Age and Exercise. Cells, 9(4), 893. https://doi.org/10.3390/cells9040893





