A Potential Role of RUNX2- RUNT Domain in Modulating the Expression of Genes Involved in Bone Metastases: An In Vitro Study with Melanoma Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Cultures
2.2. Construction of RUNX-2 Expression Vector
2.3. Exogenous PTHrP Supplementation
2.4. AKT and ERK Inhibition
2.5. PCR Array
2.6. Real-Time RT-PCR
2.7. Western Blot Analysis
2.8. Immunofluorescence
2.9. Migration to Bone Ability
2.10. ELISA
2.11. Bioinformatics Analysis
2.12. Statistical Analysis
3. Results
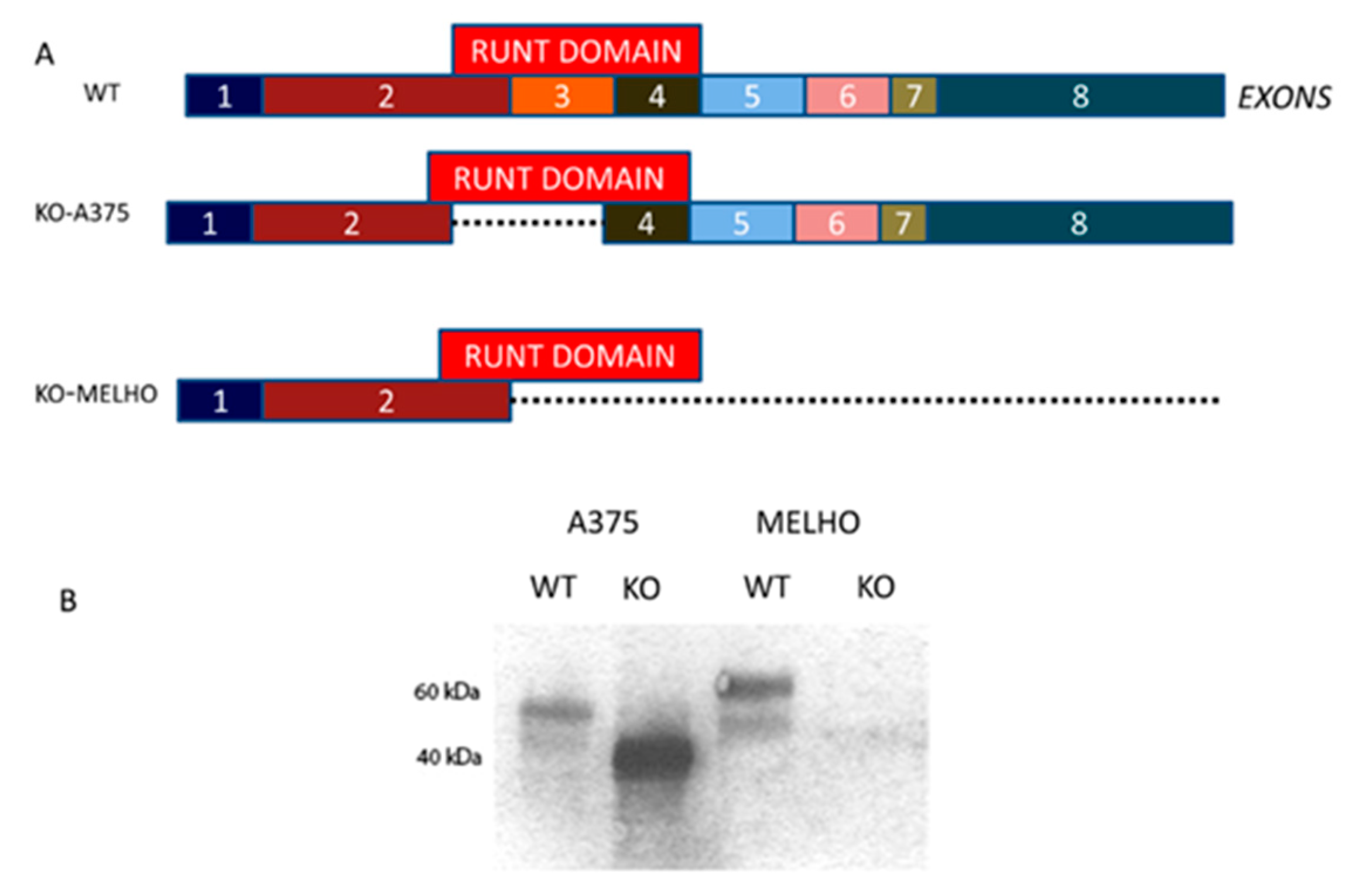
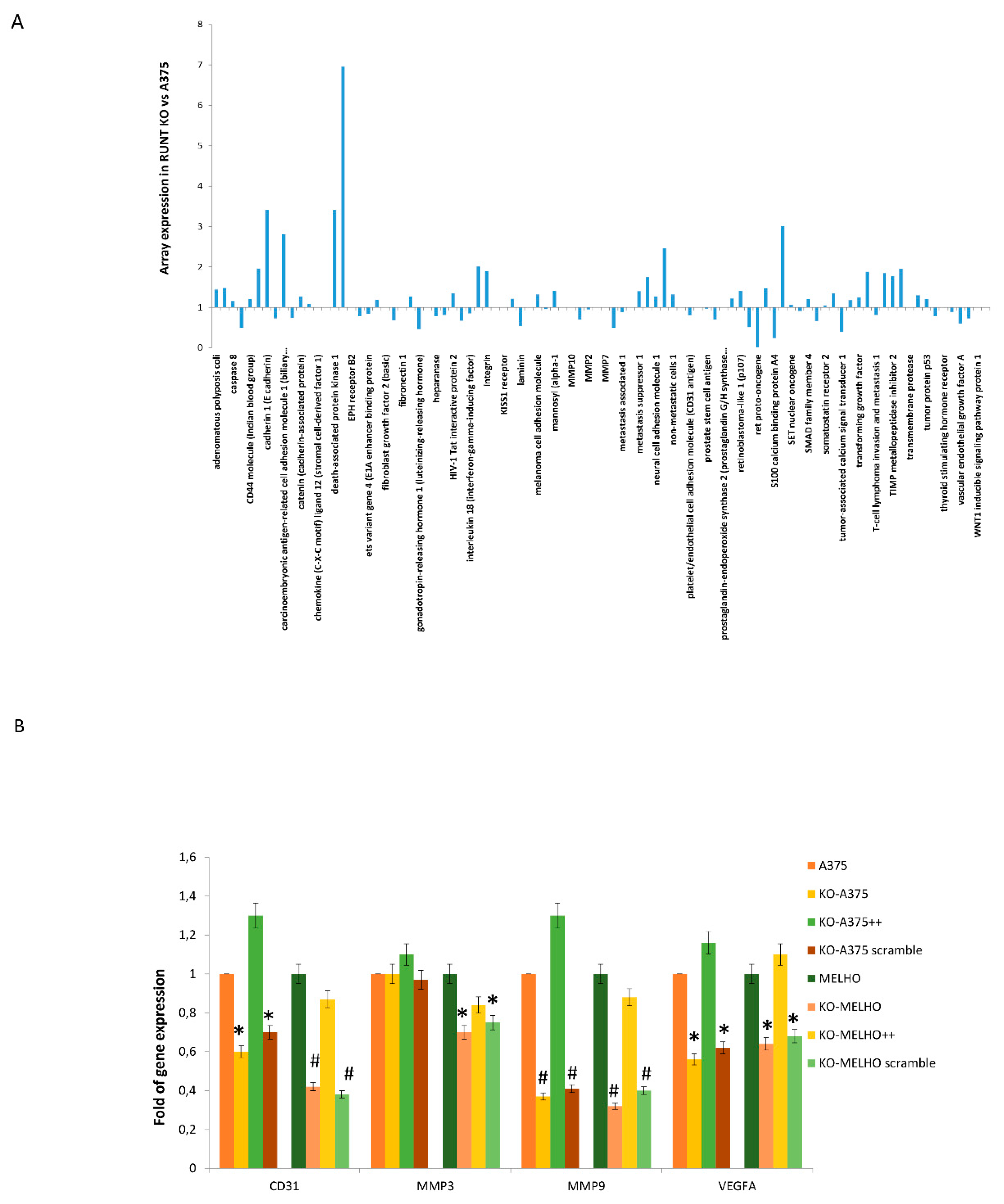
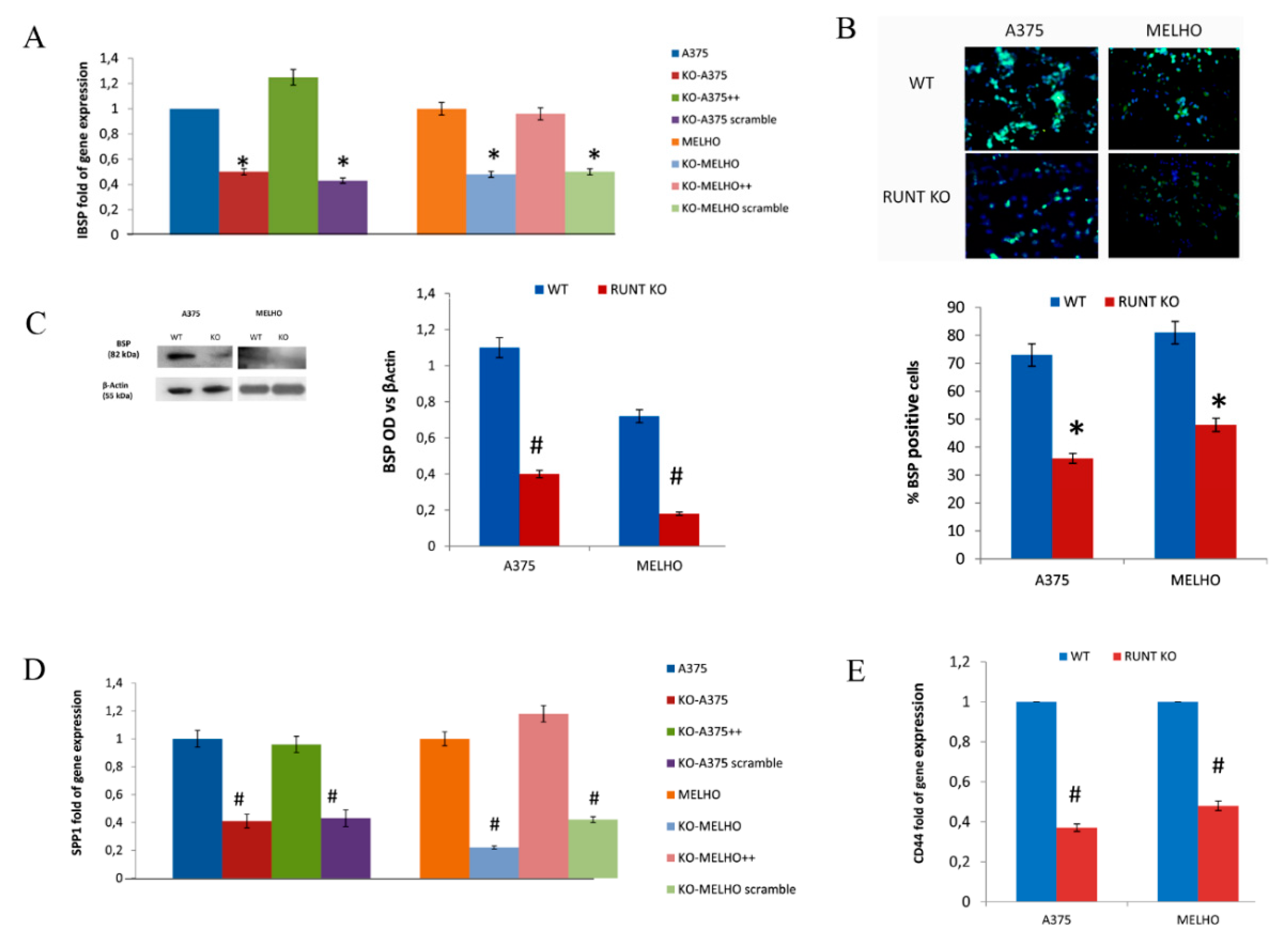
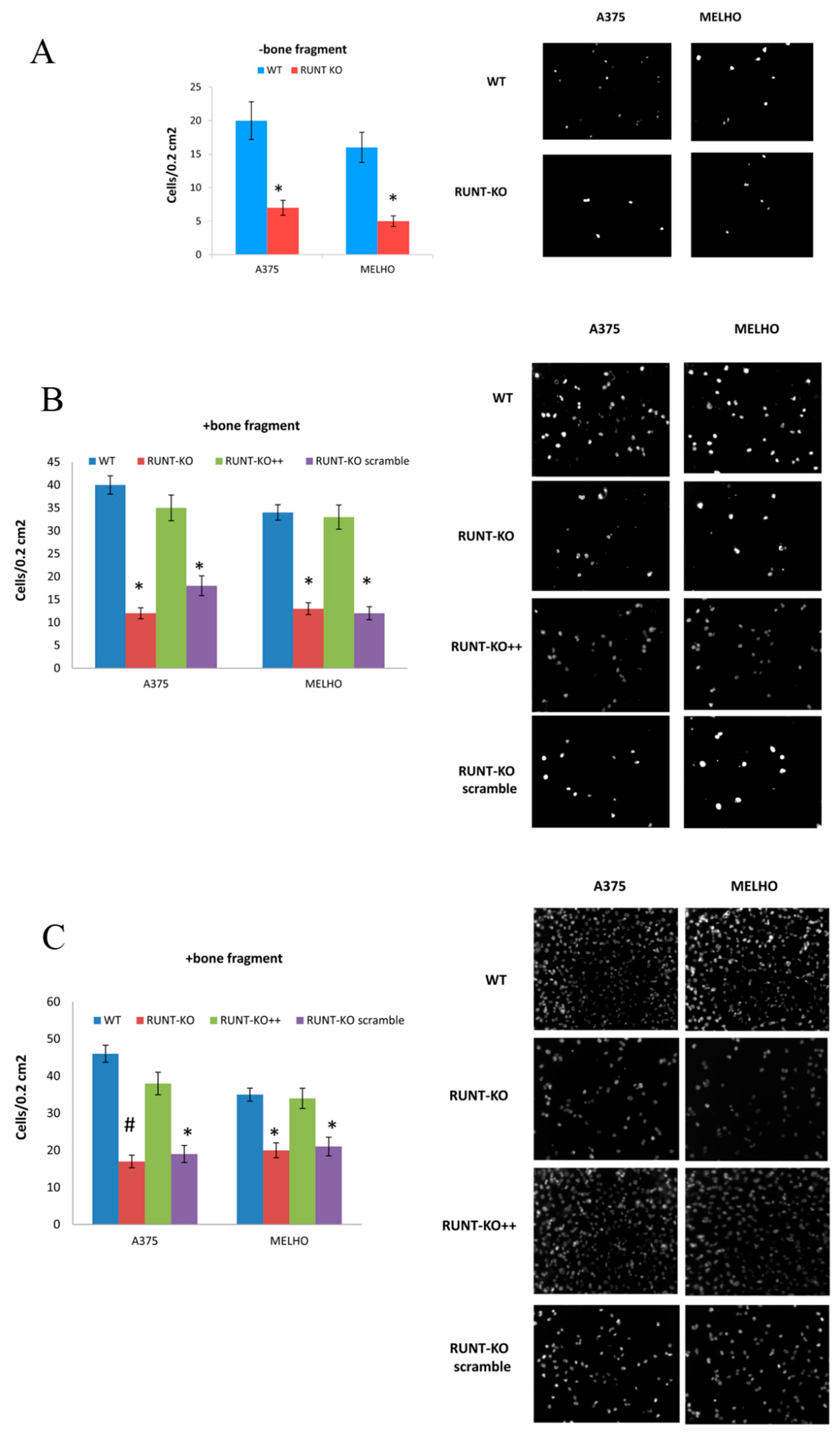
3.1. RUNX2 RUNT Domain Empowers RUNX2 Metastatic Ability in Melanoma Cells
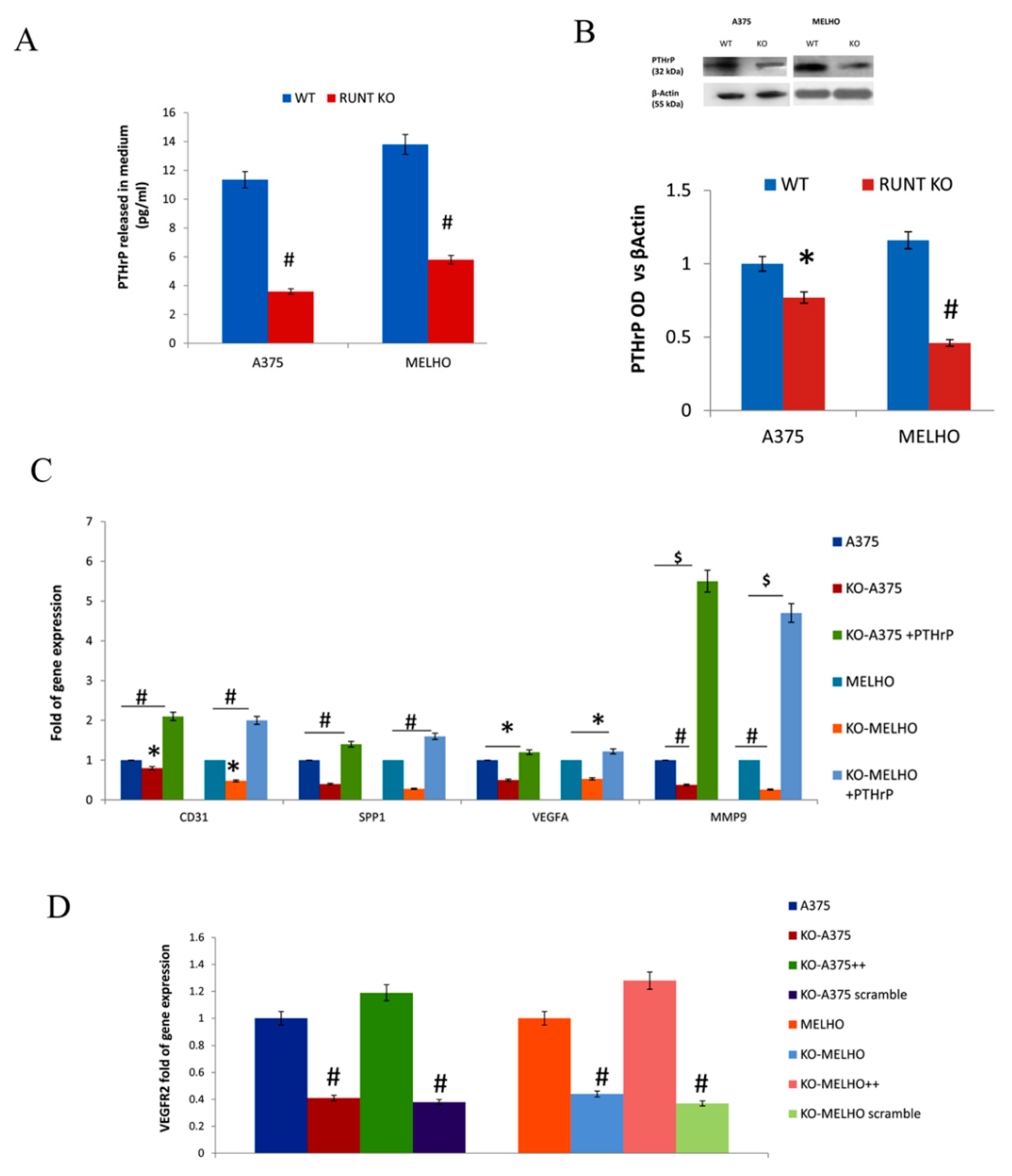
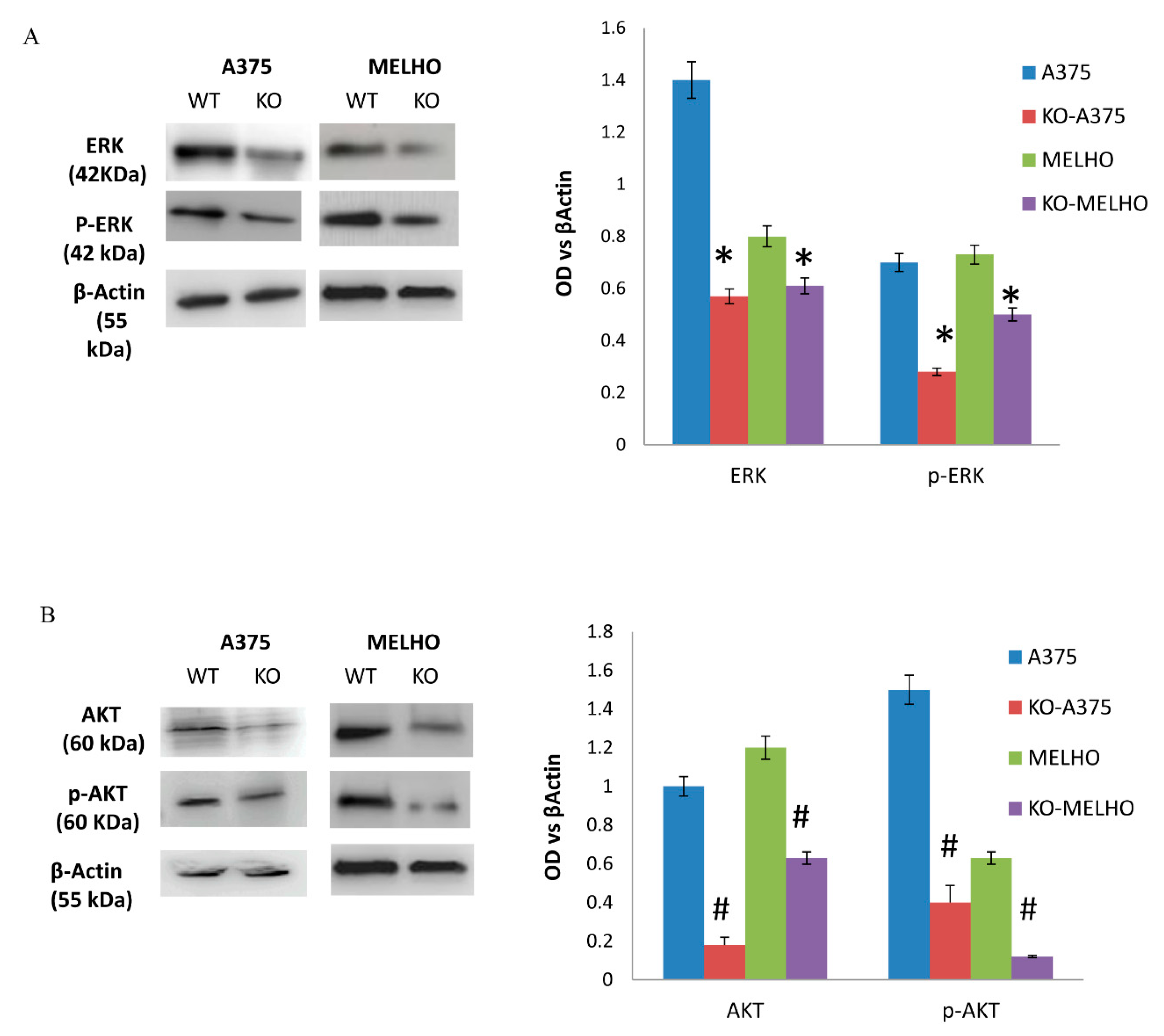
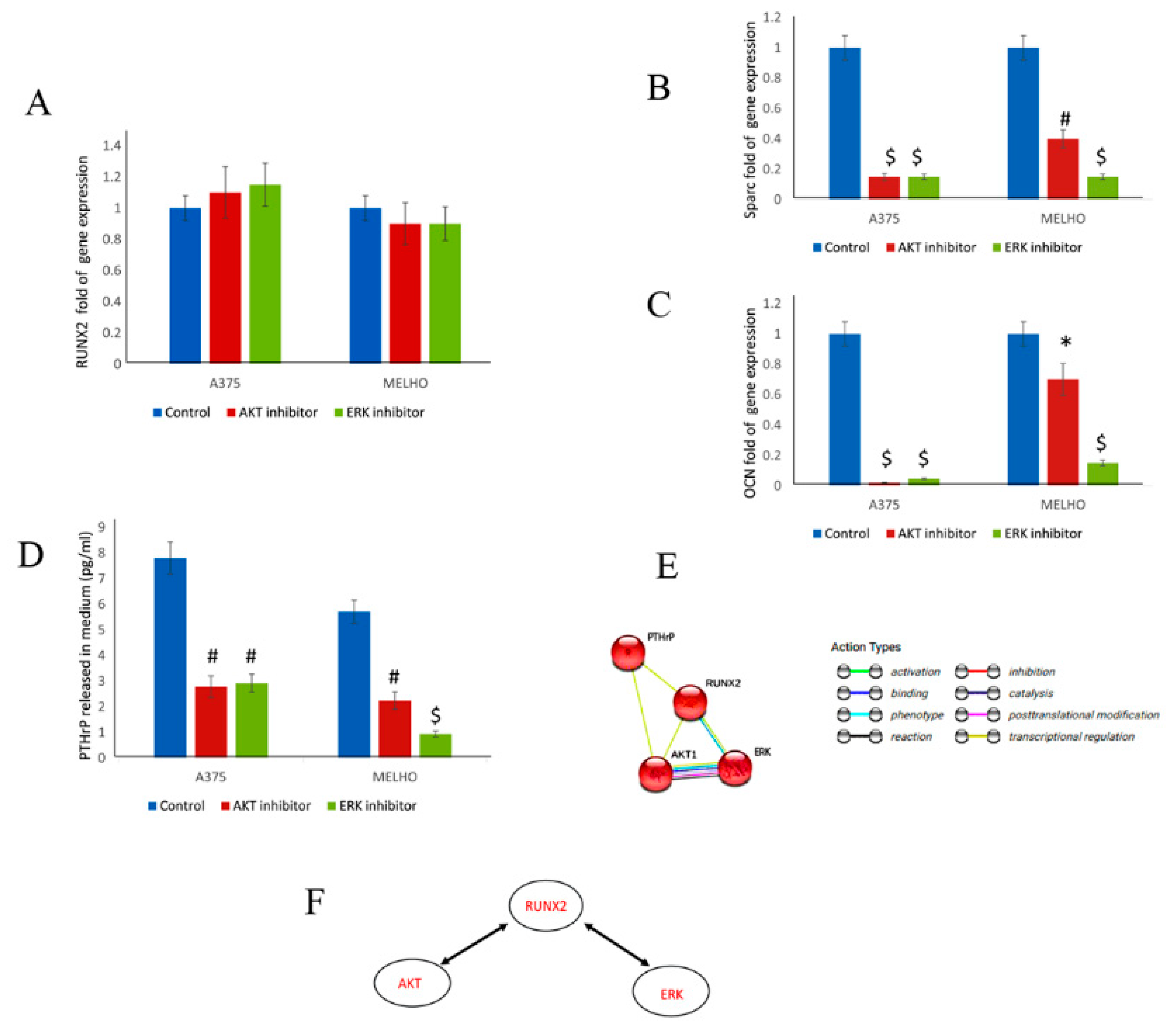
3.2. The RUNT Domain Increases PTHrP Levels in Melanoma and Activates AKT and ERK Pathways
3.3. RUNX2 Regulates AKT and ERK in a Reciprocal Way
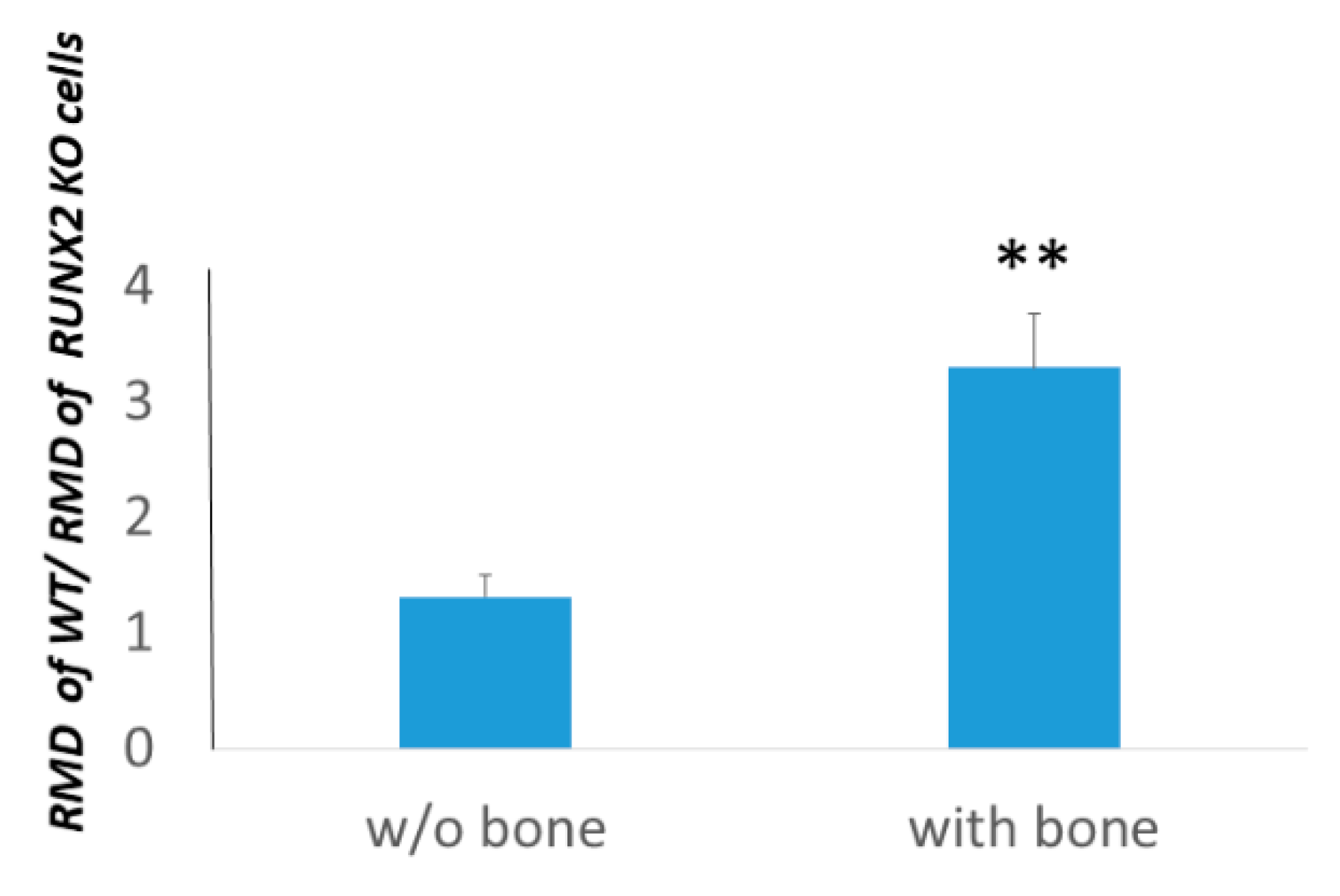
3.4. Osteotropism is Reduced in RUNT KO Melanoma Cells
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Aielli, F.; Ponzetti, M.; Rucci, N. Bone metastasis pain, from the bench to the bedside. Int. J. Mol. Sci. 2019, 20, 280. [Google Scholar] [CrossRef] [PubMed]
- Patten, R.M.; Shuman, W.P.; Teefey, S. Metastases from malignant melanoma to the axial skeleton: A CT study of frequency and appearance. AJR Am. J. Roentgenol. 1990, 155, 109–112. [Google Scholar] [CrossRef] [PubMed]
- Clauser, L.C.; Tieghi, R.; Galie, M.; Carinci, F. Structural fat grafting: Facial volumetric restoration in complex reconstructive surgery. J. Craniofac. Surg. 2011, 22, 1695–1701. [Google Scholar] [CrossRef] [PubMed]
- Rucci, N.; Teti, A. Osteomimicry: How the Seed Grows in the Soil. Calcif. Tissue Int. 2018, 102, 131–140. [Google Scholar] [CrossRef]
- Coleman, R.E. Impact of bone-targeted treatments on skeletal morbidity and survival in breast cancer. Oncology 2016, 30, 695–702. [Google Scholar]
- Damsky, W.E.; Bosenberg, M. Melanocytic nevi and melanoma: Unraveling a complex relationship. Oncogene 2017, 36, 5771–5792. [Google Scholar] [CrossRef]
- Cohen-Solal, K.A.; Boregowda, R.K.; Lasfar, A. RUNX2 and the PI3K/AKT axis reciprocal activation as a driving force for tumor progression. Mol. Cancer 2015, 14, 137. [Google Scholar] [CrossRef]
- Perduca, M.; Carbonare, L.D.; Bovi, M.; Innamorati, G.; Cheri, S.; Cavallini, C.; Scupoli, M.T.; Mori, A.; Valenti, M.T. Runx2 downregulation, migration and proliferation inhibition in melanoma cells treated with BEL beta-trefoil. Oncol. Rep. 2017, 37, 2209–2214. [Google Scholar] [CrossRef]
- Valenti, M.T.; Serafini, P.; Innamorati, G.; Gili, A.; Cheri, S.; Bassi, C.; Dalle Carbonare, L. Runx2 expression: A mesenchymal stem marker for cancer. Oncol. Lett. 2016, 12, 4167–4172. [Google Scholar] [CrossRef]
- Valenti, M.T.; Dalle Carbonare, L.; Mottes, M. Ectopic expression of the osteogenic master gene RUNX2 in melanoma. World J. Stem Cells 2018, 10, 78–81. [Google Scholar] [CrossRef]
- Dalle Carbonare, L.; Frigo, A.; Francia, G.; Davi, M.V.; Donatelli, L.; Stranieri, C.; Brazzarola, P.; Zatelli, M.C.; Menestrina, F.; Valenti, M.T. Runx2 mRNA expression in the tissue, serum, and circulating non-hematopoietic cells of patients with thyroid cancer. J. Clin. Endocrinol. Metab. 2012, 97, E1249–E1256. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Akech, J.; Browne, G.; Russell, S.; Wixted, J.J.; Stein, J.L.; Stein, G.; Lian, J. Runx2-Smad signaling impacts the progression of tumor-induced bone disease. Int. J. Cancer 2015, 136, 1321–1332. [Google Scholar] [CrossRef] [PubMed]
- Kruger, T.E.; Miller, A.H.; Godwin, A.K.; Wang, J. Bone sialoprotein and osteopontin in bone metastasis of osteotropic cancers. Crit. Rev. Oncol. Hematol. 2014, 89, 330–341. [Google Scholar] [CrossRef] [PubMed]
- Riminucci, M.; Corsi, A.; Peris, K.; Fisher, L.W.; Chimenti, S.; Bianco, P. Coexpression of bone sialoprotein (BSP) and the pivotal transcriptional regulator of osteogenesis, Cbfa1/Runx2, in malignant melanoma. Calcif. Tissue Int. 2003, 73, 28128–28129. [Google Scholar] [CrossRef]
- Ibrahim, T.; Leong, I.; Sanchez-Sweatman, O.; Khokha, R.; Sodek, J.; Tenenbaum, H.C.; Ganss, B.; Cheifetz, S. Expression of bone sialoprotein and osteopontin in breast cancer bone metastases. Clin. Exp. Metastasis 2000, 18, 253–260. [Google Scholar] [CrossRef]
- Nemoto, H.; Rittling, S.R.; Yoshitake, H.; Furuya, K.; Amagasa, T.; Tsuji, K.; Nifuji, A.; Denhardt, D.T.; Noda, M. Osteopontin deficiency reduces experimental tumor cell metastasis to bone and soft tissues. J. Bone Miner. Res. 2001, 16, 652–659. [Google Scholar] [CrossRef]
- Soki, F.N.; Park, S.I.; McCauley, L.K. The multifaceted actions of PTHrP in skeletal metastasis. Futur. Oncol. 2012, 8, 803–817. [Google Scholar] [CrossRef]
- Chang, W.M.; Lin, Y.F.; Su, C.Y.; Peng, H.Y.; Chang, Y.C.; Hsiao, J.R.; Chen, C.L.; Chang, J.Y.; Shieh, Y.S.; Hsiao, M.; et al. Parathyroid Hormone-Like Hormone is a Poor Prognosis Marker of Head and Neck Cancer and Promotes Cell Growth via RUNX2 Regulation. Sci. Rep. 2017, 7, 41131. [Google Scholar] [CrossRef]
- De Gortazar, A.R.; Alonso, V.; Alvarez-Arroyo, M.V.; Esbrit, P. Transient exposure to PTHrP (107–139) exerts anabolic effects through vascular endothelial growth factor receptor 2 in human osteoblastic cells in vitro. Calcif. Tissue Int. 2006, 79, 360–369. [Google Scholar] [CrossRef]
- Lu, H.; Jiang, T.; Ren, K.; Li, Z.L.; Ren, J.; Wu, G.; Han, X. RUNX2 plays an oncogenic role in esophageal carcinoma by activating the PI3K/AKT and ERK signaling pathways. Cell. Physiol. Biochem. 2018, 49, 217–225. [Google Scholar] [CrossRef]
- Deiana, M.; Dalle Carbonare, L.; Serena, M.; Cheri, S.; Parolini, F.; Gandini, A.; Marchetto, G.; Giulio, I.; Manfredi, M.; Marengo, E.; et al. New insights into the runt domain of RUNX2 in melanoma cell proliferation and migration. Cells 2018, 7, 220. [Google Scholar] [CrossRef] [PubMed]
- Romanelli, M.G.; Lorenzi, P.; Sangalli, A.; Diani, E.; Mottes, M. Characterization and functional analysis of cis-acting elements of the human farnesyl diphosphate synthetase (FDPS) gene 5’ flanking region. Genomics 2009, 93, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Park, E.; Gong, E.Y.; Romanelli, M.G.; Lee, K. Suppression of estrogen receptor-alpha transactivation by thyroid transcription factor-2 in breast cancer cells. Biochem. Biophys. Res. Commun. 2012, 421, 532–537. [Google Scholar] [CrossRef] [PubMed]
- Mannavola, F.; Tucci, M.; Felici, C.; Passarelli, A.; D’Oronzo, S.; Silvestris, F. Tumor-derived exosomes promote the in vitro osteotropism of melanoma cells by activating the SDF-1/CXCR4/CXCR7 axis. J. Transl. Med. 2019, 17, 230. [Google Scholar] [CrossRef]
- Sowder, M.E.; Johnson, R.W. Bone as a preferential site for metastasis. JBMR Plus 2019, 3, e10126. [Google Scholar] [CrossRef]
- Hofbauer, L.C.; Rachner, T.D.; Coleman, R.E.; Jakob, F. Endocrine aspects of bone metastases. Lancet Diabetes Endocrinol. 2014, 2, 500–512. [Google Scholar] [CrossRef]
- Karim, S.M.; Brown, J.; Zekri, J. Efficacy of bisphosphonates and other bone-targeted agents in metastatic bone disease from solid tumors other than breast and prostate cancers. Clin. Adv. Hematol. Oncol. 2013, 11, 281–287. [Google Scholar]
- Makhoul, I.; Montgomery, C.O.; Gaddy, D.; Suva, L.J. The best of both worlds - managing the cancer, saving the bone. Nat. Rev. Endocrinol. 2016, 12, 29–42. [Google Scholar] [CrossRef]
- Zekri, J.; Marples, M.; Taylor, D.; Kandukurti, K.; McParland, L.; Brown, J.E. Complications of bone metastases from malignant melanoma. J. Bone Oncol. 2017, 8, 13–17. [Google Scholar] [CrossRef]
- Gokaslan, Z.L.; Aladag, M.A.; Ellerhorst, J.A. Melanoma metastatic to the spine: A review of 133 cases. Melanoma Res. 2000, 10, 78–80. [Google Scholar] [CrossRef]
- Patel, J.K.; Didolkar, M.S.; Pickren, J.W.; Moore, R.H. Metastatic pattern of malignant melanoma. A study of 216 autopsy cases. Am. J. Surg. 1978, 135, 807–810. [Google Scholar] [CrossRef]
- Dalle Carbonare, L.; Innamorati, G.; Valenti, M.T. Transcription factor Runx2 and its Application to bone tissue engineering. Stem Cell Rev. Rep. 2012, 8, 891–897. [Google Scholar] [CrossRef] [PubMed]
- Boregowda, R.K.; Medina, D.J.; Markert, E.; Bryan, M.A.; Chen, W.; Chen, S.; Rabkin, A.; Vido, M.J.; Gunderson, S.I.; Chekmareva, M.; et al. The transcription factor RUNX2 regulates receptor tyrosine kinase expression in melanoma. Oncotarget 2016, 7, 29689–29707. [Google Scholar] [CrossRef] [PubMed]
- Boregowda, R.K.; Olabisi, O.O.; Abushahba, W.; Jeong, B.S.; Haenssen, K.K.; Chen, W.; Chekmareva, M.; Lasfar, A.; Foran, D.J.; Goydos, J.S.; et al. RUNX2 is overexpressed in melanoma cells and mediates their migration and invasion. Cancer Lett. 2014, 348, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Satelli, A.; Li, S. Vimentin in cancer and its potential as a molecular target for cancer therapy. Cell. Mol. Life Sci. 2011, 68, 3033–3046. [Google Scholar] [CrossRef]
- Bradner, J.E.; Hnisz, D.; Young, R.A. Transcriptional addiction in cancer. Cell 2017, 168, 629–643. [Google Scholar] [CrossRef] [PubMed]
- Sancisi, V.; Gandolfi, G.; Ambrosetti, D.C.; Ciarrocchi, A. Histone deacetylase inhibitors repress tumoral expression of the proinvasive factor RUNX2. Cancer Res. 2015, 75, 1868–1882. [Google Scholar] [CrossRef]
- Herreno, A.M.; Ramirez, A.C.; Chaparro, V.P.; Fernandez, M.J.; Canas, A.; Morantes, C.F.; Moreno, O.M.; Brugés, R.E.; Mejía, J.A.; Bustos, F.J.; et al. Role of RUNX2 transcription factor in epithelial mesenchymal transition in non-small cell lung cancer lung cancer: Epigenetic control of the RUNX2 P1 promoter. Tumour Biol. 2019, 41, 1010428319851014. [Google Scholar] [CrossRef]
- Senbanjo, L.T.; Chellaiah, M.A. CD44: A multifunctional cell surface adhesion receptor is a regulator of progression and metastasis of cancer cells. Front. Cell Dev. Biol. 2017, 5, 18. [Google Scholar] [CrossRef]
- Miletti-Gonzalez, K.E.; Murphy, K.; Kumaran, M.N.; Ravindranath, A.K.; Wernyj, R.P.; Kaur, S.; Miles, G.; Lim, E.T.; Chan, R.; Chekmareva, M.; et al. Identification of function for CD44 intracytoplasmic domain (CD44-ICD): Modulation of matrix metalloproteinase 9 (MMP-9) transcription via novel promoter response element. J Biol Chem. 2012, 287, 18995–19007. [Google Scholar] [CrossRef]
- Senbanjo, L.T.; AlJohani, H.; Majumdar, S.; Chellaiah, M.A. Characterization of CD44 intracellular domain interaction with RUNX2 in PC3 human prostate cancer cells. Cell Commun. Signal. 2019, 17, 80. [Google Scholar] [CrossRef]
- Zhao, H.; Chen, Q.; Alam, A.; Cui, J.; Suen, K.C.; Soo, A.P.; Eguchi, S.; Gu, J.; Ma, D. The role of osteopontin in the progression of solid organ tumour. Cell Death Dis. 2018, 9, 356. [Google Scholar] [CrossRef]
- Gupta, A.; Cao, W.; Chellaiah, M.A. Integrin alphavbeta3 and CD44 pathways in metastatic prostate cancer cells support osteoclastogenesis via a Runx2/Smad 5/receptor activator of NF-kappaB ligand signaling axis. Mol Cancer. 2012, 11, 66. [Google Scholar] [CrossRef]
- Templeton, Z.S.; Lie, W.R.; Wang, W.; Rosenberg-Hasson, Y.; Alluri, R.V.; Tamaresis, J.S.; Bachmann, M.H.; Lee, K.; William, J.; Maloney, W.J.; et al. Breast cancer cell colonization of the human bone marrow adipose tissue niche. Neoplasia 2015, 17, 849–861. [Google Scholar] [CrossRef]
- Allan, A.L.; Tuck, A.B.; Bramwell, V.H.C.; Vandenberg, T.A.; Winquist, E.W.; Chambers, A.F.; Singh, G. Contribution of Osteopontin to the Development of Bone Metastasis; Singh, G., Rabbani, S.A., Eds.; Humana Press: Totowa, NJ, USA, 2005. [Google Scholar]
- Wang, L.; Song, L.; Li, J.; Wang, Y.; Yang, C.; Kou, X.; Xiao, B.; Zhang, W.; Li, L.; Liu, S.; et al. Bone sialoprotein-alphavbeta3 integrin axis promotes breast cancer metastasis to the bone. Cancer Sci. 2019, 110, 3157–3172. [Google Scholar] [CrossRef]
- Shevde, L.A.; Samant, R.S. Role of osteopontin in the pathophysiology of cancer. Matrix Biol. 2014, 37, 131–141. [Google Scholar] [CrossRef]
| Antibody | Ab Dilution | Origin | Secondary Antibody |
|---|---|---|---|
| BSP (Bone Sialoprotein) II | 1:1000 | (Cell Signaling, 5486) | Anti-rabbit (Cell Signaling, 7074) |
| AKT (C67E7) | 1:1000 | (Cell Signaling, 4691) | Anti-rabbit (Cell Signaling, 7074) |
| p_AKT (193H12) | 1:1000 | (Cell Signaling, 4058) | Anti-rabbit (Cell Signaling, 7074) |
| ERK (13F5) | 1:1000 | (Cell Signaling, 4695) | Anti-rabbit (Cell Signaling, 7074) |
| p_ERK (D13.14.4E) | 1:2000 | (Cell Signaling, 4370) | Anti-rabbit (Cell Signaling, 7074) |
| PTHrP (1D1) | 1:1000 | (SantaCruz Biotech., Dallas, TX, USA) | Anti-mouse (Cell Signaling, 7076) |
| β ACTIN (BA3R) | 1:5000 | (Thermo Scientific) | Anti-mouse (Cell Signaling, 7076) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deiana, M.; Dalle Carbonare, L.; Serena, M.; Cheri, S.; Mutascio, S.; Gandini, A.; Innamorati, G.; Lorenzi, P.; Cumerlato, M.; Bertacco, J.; et al. A Potential Role of RUNX2- RUNT Domain in Modulating the Expression of Genes Involved in Bone Metastases: An In Vitro Study with Melanoma Cells. Cells 2020, 9, 751. https://doi.org/10.3390/cells9030751
Deiana M, Dalle Carbonare L, Serena M, Cheri S, Mutascio S, Gandini A, Innamorati G, Lorenzi P, Cumerlato M, Bertacco J, et al. A Potential Role of RUNX2- RUNT Domain in Modulating the Expression of Genes Involved in Bone Metastases: An In Vitro Study with Melanoma Cells. Cells. 2020; 9(3):751. https://doi.org/10.3390/cells9030751
Chicago/Turabian StyleDeiana, Michela, Luca Dalle Carbonare, Michela Serena, Samuele Cheri, Simona Mutascio, Alberto Gandini, Giulio Innamorati, Pamela Lorenzi, Michela Cumerlato, Jessica Bertacco, and et al. 2020. "A Potential Role of RUNX2- RUNT Domain in Modulating the Expression of Genes Involved in Bone Metastases: An In Vitro Study with Melanoma Cells" Cells 9, no. 3: 751. https://doi.org/10.3390/cells9030751
APA StyleDeiana, M., Dalle Carbonare, L., Serena, M., Cheri, S., Mutascio, S., Gandini, A., Innamorati, G., Lorenzi, P., Cumerlato, M., Bertacco, J., Antoniazzi, F., Romanelli, M. G., Mottes, M., Zipeto, D., & Valenti, M. T. (2020). A Potential Role of RUNX2- RUNT Domain in Modulating the Expression of Genes Involved in Bone Metastases: An In Vitro Study with Melanoma Cells. Cells, 9(3), 751. https://doi.org/10.3390/cells9030751










