Spermatogonial Stem Cells for In Vitro Spermatogenesis and In Vivo Restoration of Fertility
Abstract
1. Introduction
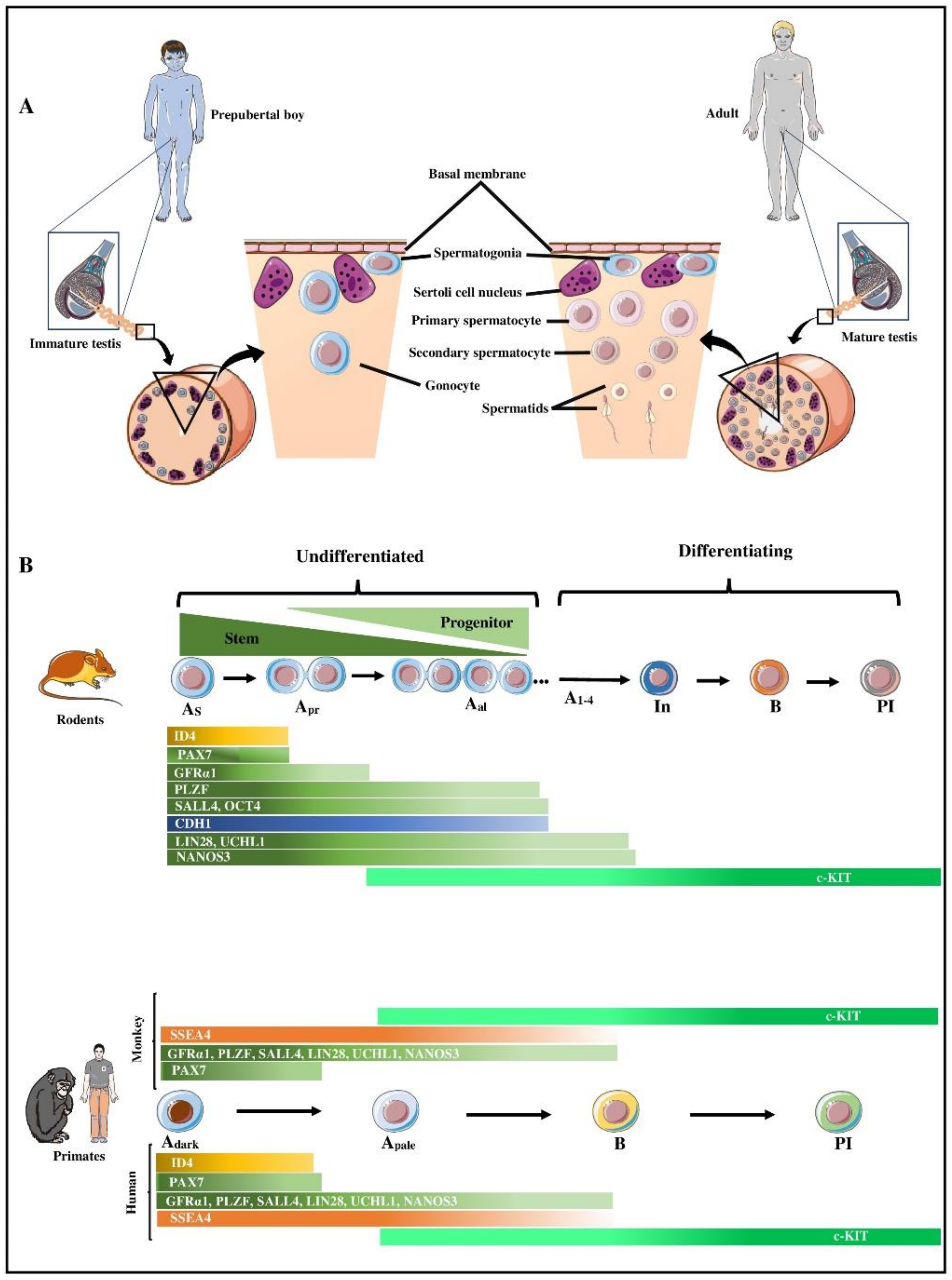
2. Spermatogonial Stem Cells (SSCs)
3. Characteristics of Spermatogonia in Primates
4. Isolation and Enrichment of SSCs in Primates
5. In Vitro Culture of Primate Gonocytes/Spermatogonia
6. In Vitro Models of SSC Differentiation
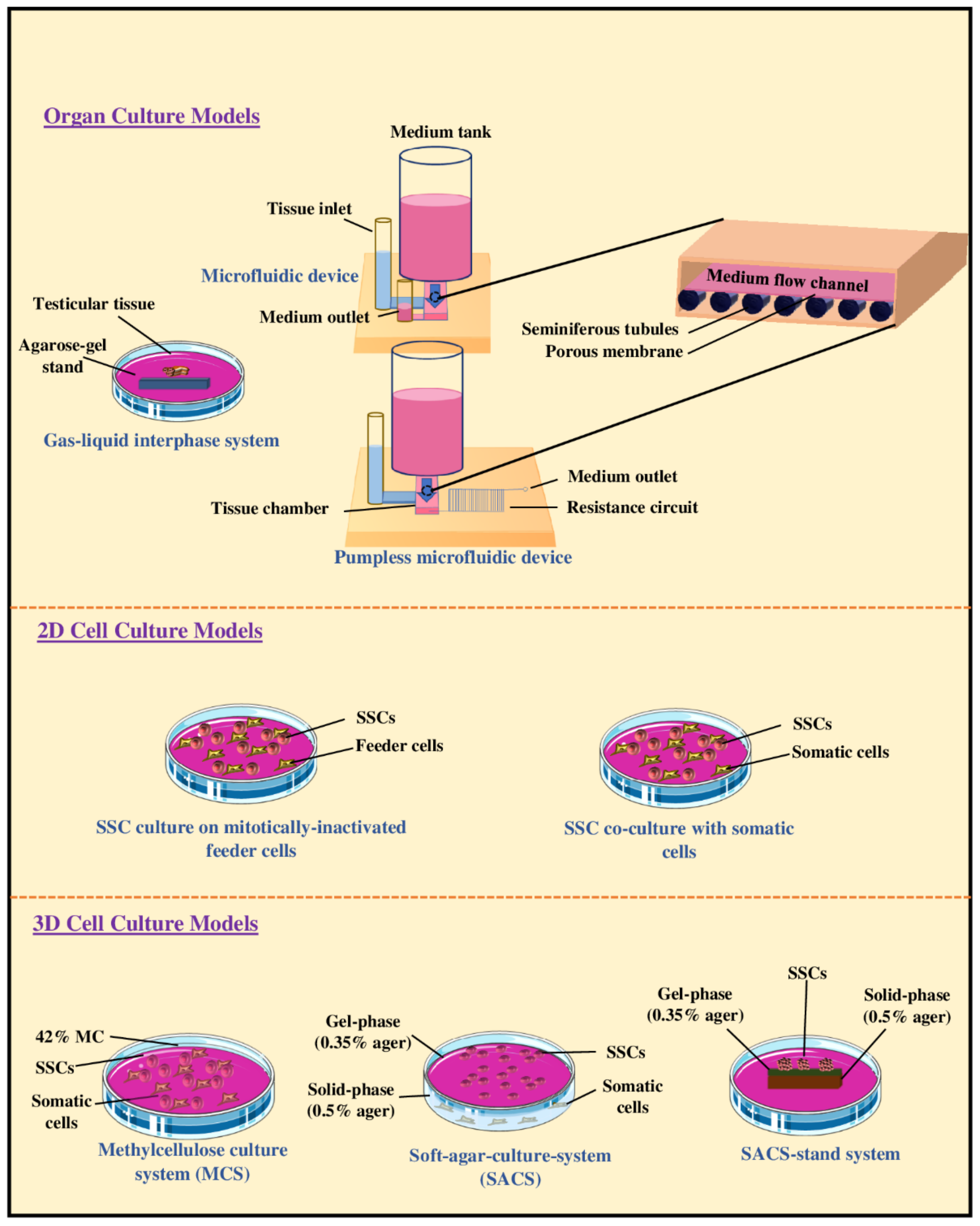
6.1. Organotypic Culture of Testicular Tissue Fragments
6.2. Two-Dimensional Culture of Testis Cell Suspensions
6.3. Three-Dimensional Culture of Testis Cell Suspensions
7. Progress of In Vitro Spermatogenesis in Primates
8. Limitations of In Vitro Spermatogenesis in Non-Rodents
9. Future Prospects of In Vitro Spermatogenesis for Fertility Preservation and Restoration
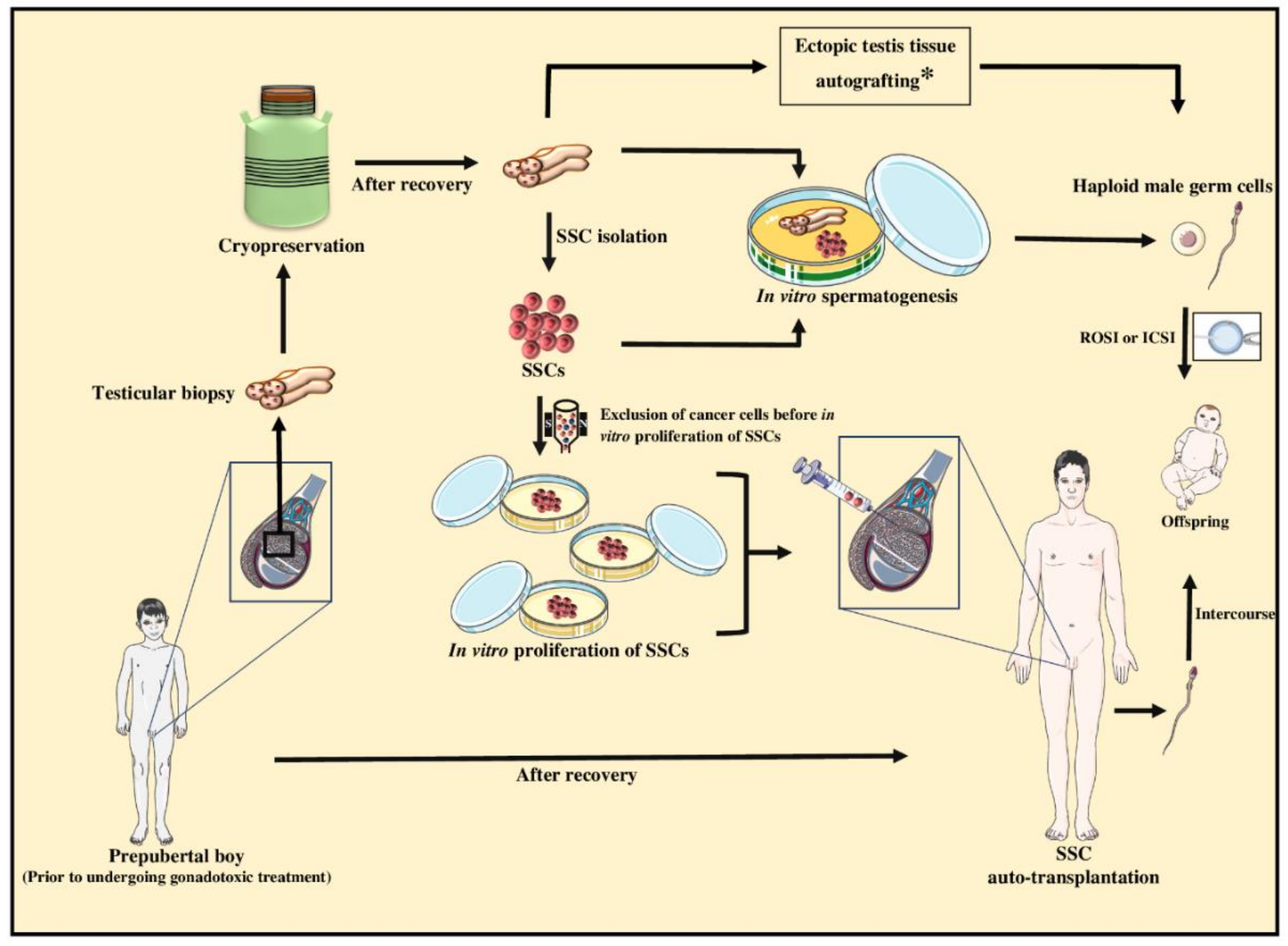
9.1. Fertility Preservation of Prepubertal Cancer Patients for Subsequent Fertility Restoration
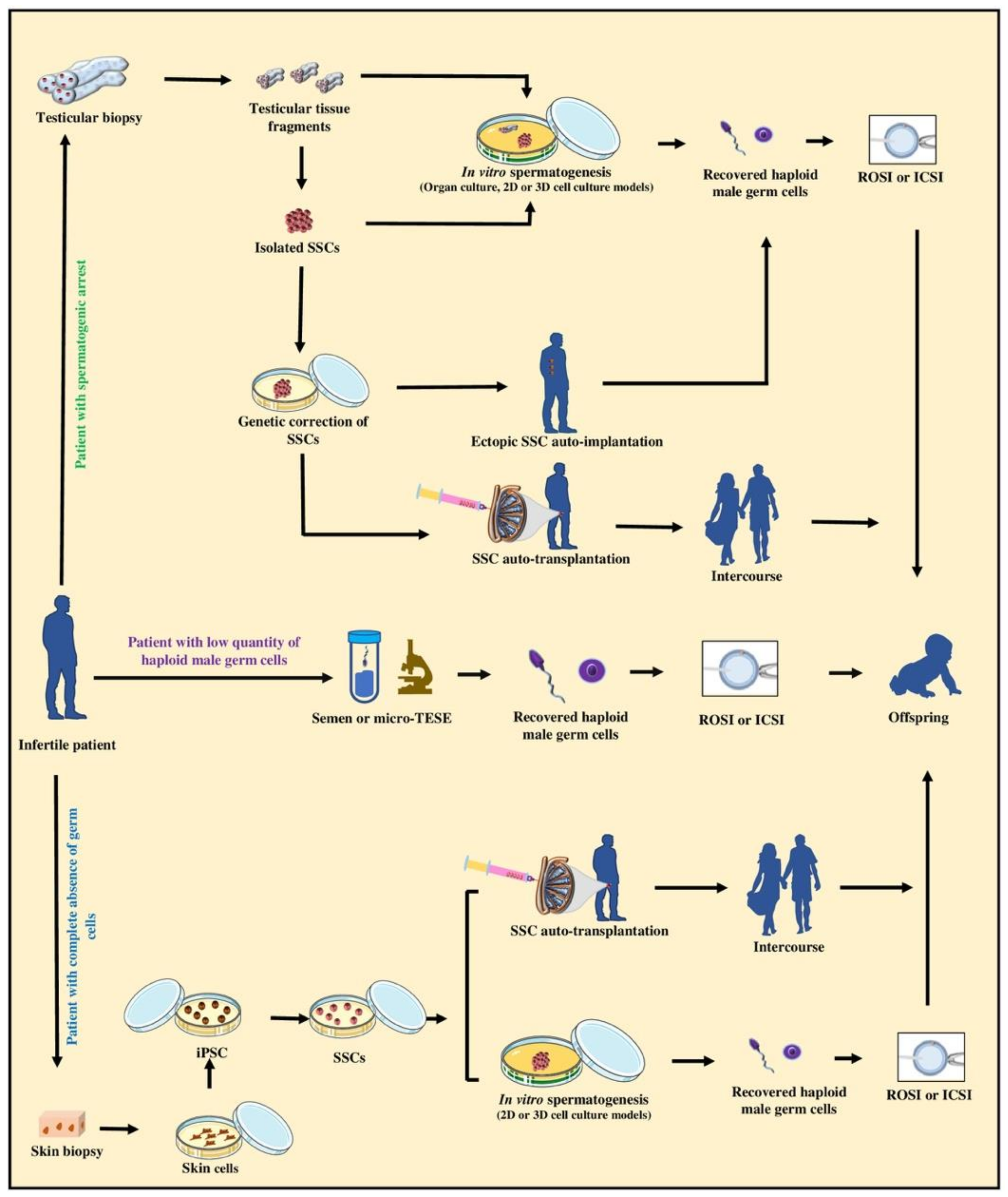
9.2. Upholding the Biological Fatherhood of Adult Infertile Patients
10. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Definition | Abbreviation |
| Artificial insemination | AI |
| Cadherin-1 | CDH1 |
| Casitas B-lineage lymphoma | CBL |
| Checkpoint kinase 2 | CHK2 |
| c-Kit ligand | KITL |
| Cluster of differentiation 9 | CD9 |
| Cluster of differentiation 90 | CD90 |
| Days post-coitum | dpc |
| Days post-partum | dpp |
| DEAD-box helicase 4 | DDX4 |
| Deleted in azoospermia-like | DAZL |
| Desmoglein 2 | DSG2 |
| Developmental pluripotency associated 4 | DPPA4 |
| Doublesex and mab-3 related transcription factor 1 | DMRT1 |
| Eagle’s minimum essential media | MEM |
| Embryonic stem | ES |
| Engineered blood-testis barrier | eBTB |
| Enolase 2 | ENO2 |
| Extracellular matrices | ECM |
| Fetal bovine serum | FBS |
| Fibroblast growth factor receptor 3 | FGFR3 |
| Fluorescence in situ hybridization | FISH |
| Fluorescence-activated cell sorting | FACS |
| GDNF family receptor alpha-1 | GFRα1 |
| G-protein coupled receptor 125 | GPR125 |
| In vitro fertilization | IVF |
| In vitro spermatogenesis | IVS |
| Induced pluripotent stem cells | iPSC |
| Intracytoplasmic sperm injection | ICSI |
| Knockout serum replacement | KSR |
| Lin-28 homology A | LIN28 |
| Magnetic-activated cell sorting | MACS |
| Melanoma-associated antigen 4 | MAGEA4 |
| Methylcellulose | MCS |
| Microscopic testicular sperm extraction | micro-TESE |
| Nanos C2HC-type zinc finger | NANOS |
| Neurogenin 3 | NGN3 |
| Octamer-binding transcription factor 4 | OCT4 |
| Paired box 7 | PAX7 |
| Peritubular factor that modulates Sertoli cell function | PModS |
| Phospholipid phosphatase related 3 | PLPPR3 |
| Piwi like RNA-mediated gene silencing 4 | PIWIL4 |
| POU class 5 homeobox 1 | POU5F1 |
| Primordial germ cells | PGCs |
| Prominin 1 (Cluster of differentiation 133) | PROM1 (CD133) |
| Promyelocytic leukemia zinc finger protein | PLZF |
| Ret proto-oncogene | RET |
| Retinoic acid | RA |
| RNA binding motif | RBM |
| Round-spermatid injection | ROSI |
| Sal-like protein 4 | SALL4 |
| Serum free medium | SFM |
| Soft-agar culture system | SACS |
| Spermatogonial stem cells | SSCs |
| SPOC domain containing 1 | SPOCD1 |
| Stage-specific embryonic antigen-4 | SSEA4 |
| Steel | SL |
| Stimulated by retinoic acid 8 | STRA8 |
| Synaptosome associated protein 91 | SNAP91 |
| Testis-specific Y-encoded protein | TSPY |
| Tetraspanin 33 | TSPAN33 |
| Three-dimensional | 3D |
| Three-layer gradient system | 3-LGS |
| Transformer-1 | TRA-1 |
| Two-dimensional | 2D |
| Ubiquitin C-terminal hydrolase L1 | UCHL1 |
| Undifferentiated embryonic cell transcription factor 1 | UTF1 |
| Vasa gene-encoded RNA binding protein | VASA |
| Zinc finger with KRAB and SCAN domains 2 | ZKSCAN2 |
References
- Russell, L.D.; Ettlin, R.A.; Hikim, A.P.S.; Clegg, E.D. Histological and Histopathological Evaluation of the Testis. Int. J. Androl. 1993, 16, 83. [Google Scholar] [CrossRef]
- Martin, L.A.; Seandel, M. Propagation of adult SSCs: From mouse to human. Biomed. Res. Int. 2013, 2013, 384734. [Google Scholar] [CrossRef]
- Oatley, J.M.; Brinster, R.L. The germline stem cell niche unit in mammalian testes. Physiol. Rev. 2012, 92, 577–595. [Google Scholar] [CrossRef]
- Kubota, H.; Brinster, R.L. Spermatogonial stem cells. Biol. Reprod. 2018, 99, 52–74. [Google Scholar] [CrossRef]
- Jan, S.Z.; Hamer, G.; Repping, S.; de Rooij, D.G.; Van Pelt, A.M.M.; Vormer, T.L. Molecular control of rodent spermatogenesis. Biochim. Biophys. Acta. 2012, 1822, 1838–1850. [Google Scholar] [CrossRef]
- Hutson, J.M.; Li, R.; Southwell, B.R.; Petersen, B.L.; Thorup, J.; Cortes, D. Germ cell development in the postnatal testis: The key to prevent malignancy in cryptorchidism? Front. Endocrinol. 2013, 3, 176. [Google Scholar] [CrossRef]
- Griswold, M.D. Spermatogenesis: The Commitment to Meiosis. Physiol. Rev. 2015, 96, 1–17. [Google Scholar] [CrossRef]
- Griswold, M.D.; Oatley, J.M. Concise review: Defining characteristics of mammalian spermatogenic stem cells. Stem Cells 2013, 31, 8–11. [Google Scholar] [CrossRef]
- Nagano, M.C.; Yeh, J.R. The identity and fate decision control of spermatogonial stem cells: Where is the point of no return? Curr. Top. Dev. Biol. 2013, 102, 61–95. [Google Scholar]
- De Rooij, D.G.; Russell, L.D. All you wanted to know about spermatogonia but were afraid to ask. J. Androl. 2000, 6, 776–798. [Google Scholar]
- Fayomi, A.P.; Orwig, K.E. Spermatogonial stem cells and spermatogenesis in mice, monkeys and men. Stem Cell Res. 2018, 29, 207–214. [Google Scholar] [CrossRef]
- Clermont, Y. Two classes of spermatogonial stem cells in the monkey (Cercopithecus aethiops). Am. J. Anat. 1969, 126, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Ibtisham, F.; Wu, J.; Xiao, M.; An, L.; Banker, Z.; Nawab, A.; Zhao, Y.; Li, G. Progress and future prospect of in vitro spermatogenesis. Oncotarget 2017, 8, 66709–66727. [Google Scholar] [CrossRef] [PubMed]
- Boitani, C.; Di Persio, S.; Esposito, V.; Vicini, E. Spermatogonial cells: Mouse, monkey and man comparison. Semin. Cell Dev. Biol. 2016, 59, 79–88. [Google Scholar] [CrossRef]
- Amann, R.P. The cycle of the seminiferous epithelium in humans: A need to revisit? J. Androl. 2008, 29, 469–487. [Google Scholar] [CrossRef]
- Lajtha, L. Stem cell concepts. Differentiation 1979, 14, 23–34. [Google Scholar] [CrossRef]
- Heller, C.G.; Clermont, Y. Spermatogenesis in man: An estimate of its duration. Science 1963, 140, 184–186. [Google Scholar] [CrossRef]
- De Rooij, D.G. The nature and dynamics of spermatogonial stem cells. Development 2017, 144, 3022–3030. [Google Scholar] [CrossRef]
- Brinster, R.L.; Zimmermann, J.W. Spermatogenesis following male germ-cell transplantation. Proc. Natl. Acad. Sci. 1994, 91, 11298–11302. [Google Scholar] [CrossRef]
- Nagano, M.; Brinster, R.L. Spermatogonial transplantation and reconstitution of donor cell spermatogenesis in recipient mice. APMIS 1998, 106, 47. [Google Scholar] [CrossRef]
- Honaramooz, A.; Yang, Y. Recent advances in application of male germ cell transplantation in farm animals. Vet. Med. Int. 2010, 2011, 1–9. [Google Scholar] [CrossRef]
- Zheng, K.; Wu, X.; Kaestner, K.H.; Wang, P.J. The pluripotency factor LIN28 marks undifferentiated spermatogonia in mouse. BMC Dev. Biol. 2009, 9, 38. [Google Scholar] [CrossRef]
- Aeckerle, N.; Eildermann, K.; Drummer, C.; Ehmcke, J.; Schweyer, S.; Lerchl, A.; Bergmann, M.; Kliesch, S.; Gromoll, J.; Schlatt, S.; et al. The pluripotency factor LIN28 in monkey and human testes: A marker for spermatogonial stem cells? Mol. Hum. Reprod. 2012, 18, 477–488. [Google Scholar] [CrossRef]
- Eildermann, K.; Gromoll, J.; Behr, R. Misleading and reliable markers to differentiate between primate testis-derived multipotent stromal cells and spermatogonia in culture. Hum. Reprod. 2012, 27, 1754–1767. [Google Scholar] [CrossRef]
- Hermann, B.P.; Sukhwani, M.; Lin, C.-C.; Sheng, Y.; Tomko, J.; Rodriguez, M.; Shuttleworth, J.J.; McFarland, D.; Hobbs, R.M.; Pandolfi, P.P.; et al. Characterization, cryopreservation, and ablation of spermatogonial stem cells in adult rhesus macaques. Stem Cells 2007, 25, 2330–2338. [Google Scholar] [CrossRef]
- Lin, Z.Y.-C.; Hirano, T.; Shibata, S.; Seki, N.M.; Kitajima, R.; Sedohara, A.; Siomi, M.C.; Sasaki, E.; Siomi, H.; Imamura, M.; et al. Gene expression ontogeny of spermatogenesis in the marmoset uncovers primate characteristics during testicular development. Dev. Biol. 2015, 400, 43–58. [Google Scholar] [CrossRef]
- Muller, T.; Eildermann, K.; Dhir, R.; Schlatt, S.; Behr, R. Glycan stem-cell markers are specifically expressed by spermatogonia in the adult non-human primate testis. Hum. Reprod. 2008, 23, 2292–2298. [Google Scholar] [CrossRef]
- Hermann, B.P.; Sukhwani, M.; Simorangkir, D.R.; Chu, T.; Plant, T.M.; Orwig, K.E. Molecular dissection of the male germ cell lineage identifies putative spermatogonial stem cells in rhesus macaques. Hum. Reprod. 2009, 24, 1704–1716. [Google Scholar] [CrossRef]
- Shinohara, T.; Avarbock, M.R.; Brinster, R.L. Beta1- and alpha6-integrin are surface markers on mouse spermatogonial stem cells. Proc. Natl. Acad. Sci. 1999, 96, 5504–5509. [Google Scholar] [CrossRef]
- Kubota, H.; Avarbock, M.R.; Brinster, R.L. Spermatogonial stem cells share some, but not all, phenotypic and functional characteristics with other stem cells. Proc. Natl. Acad. Sci. 2003, 100, 6487–6492. [Google Scholar] [CrossRef]
- Kanatsu-shinohara, M.; Toyokuni, S.; Shinohara, T. CD9 Is a Surface Marker on Mouse and Rat Male Germline Stem Cells. Biol. Reprod. 2004, 70, 70–75. [Google Scholar] [CrossRef]
- Conrad, S.; Renninger, M.; Hennenlotter, J.; Wiesner, T.; Just, L.; Bonin, M.; Aicher, W.; Buhring, H.-J.; Mattheus, U.; Mack, A.; et al. Generation of pluripotent stem cells from adult human testis. Nature 2008, 456, 344–349. [Google Scholar] [CrossRef]
- Sakashita, A.; Yeh, Y.-H.V.; Namekawa, S.H.; Lin, S.-P. Epigenomic and single-cell profiling of human spermatogonial stem cells. Stem Cell Investig. 2018, 5, 11. [Google Scholar] [CrossRef]
- Chan, F.; Oatley, M.J.; Kaucher, A.V.; Yang, Q.-E.; Bieberich, C.J.; Shashikant, C.S.; Oatley, J.M. Functional and molecular features of the Id4+ germline stem cell population in mouse testes. Genes Dev. 2014, 28, 1351–1362. [Google Scholar] [CrossRef]
- Seandel, M.; James, D.; Shmelkov, S.V.; Falciatori, I.; Kim, J.; Chavala, S.; Scherr, D.S.; Zhang, F.; Torres, R.; Gale, N.W.; et al. Generation of functional multipotent adult stem cells from GPR125+ germline progenitors. Nature 2007, 449, 346–350. [Google Scholar] [CrossRef]
- Sachs, C.; Robinson, B.D.; Andres Martin, L.; Webster, T.; Gilbert, M.; Lo, H.-Y.; Rafii, S.; Ng, C.K.; Seandel, M. Evaluation of candidate spermatogonial markers ID4 and GPR125 in testes of adult human cadaveric organ donors. Andrology 2014, 2, 607–614. [Google Scholar] [CrossRef]
- Naughton, C.K.; Jain, S.; Strickland, A.M.; Gupta, A.; Milbrandt, J. Glial cell-line derived neurotrophic factor-mediated RET signaling regulates spermatogonial stem cell fate. Biol. Reprod. 2006, 74, 314–321. [Google Scholar] [CrossRef]
- Giuili, G.; Tomljenovic, A.; Labrecque, N.; Oulad-Abdelghani, M.; Rassoulzadegan, M.; Cuzin, F. Murine spermatogonial stem cells: Targeted transgene expression and purification in an active state. EMBO Rep. 2002, 3, 753–759. [Google Scholar] [CrossRef]
- Tokuda, M.; Kadokawa, Y.; Kurahashi, H.; Marunouchi, T. CDH1 is a specific marker for undifferentiated spermatogonia in mouse testes. Biol. Reprod. 2007, 76, 130–141. [Google Scholar] [CrossRef]
- Makela, J.-A.; Hobbs, R.M. Molecular regulation of spermatogonial stem cell renewal and differentiation. Reproduction 2019, 158, R169–R187. [Google Scholar] [CrossRef]
- Honecker, F.; Stoop, H.; de Krijger, R.R.; Chris Lau, Y.-F.; Bokemeyer, C.; Looijenga, L.H.J. Pathobiological implications of the expression of markers of testicular carcinoma in situ by fetal germ cells. J. Pathol. 2004, 203, 849–857. [Google Scholar] [CrossRef]
- Kido, T.; Lau, Y.-F.C. The rat Tspy is preferentially expressed in elongated spermatids and interacts with the core histones. Biochem. Biophys. Res. Commun. 2006, 350, 56–67. [Google Scholar] [CrossRef]
- Bartkova, J.; Falck, J.; Rajpert-De Meyts, E.; Skakkebaek, N.E.; Lukas, J.; Bartek, J. Chk2 tumour suppressor protein in human spermatogenesis and testicular germ-cell tumours. Oncogene 2001, 20, 5897–5902. [Google Scholar] [CrossRef][Green Version]
- Maki, C.B.; Pacchiarotti, J.; Ramos, T.; Pascual, M.; Pham, J.; Kinjo, J.; Anorve, S.; Izadyar, F. Phenotypic and molecular characterization of spermatogonial stem cells in adult primate testes. Hum. Reprod. 2009, 24, 1480–1491. [Google Scholar] [CrossRef]
- Husen, B.; Giebel, J.; Rune, G. Expression of the integrin subunits alpha 5, alpha 6 and beta 1 in the testes of the common marmoset. Int. J. Androl. 1999, 22, 374–384. [Google Scholar] [CrossRef]
- Lim, J.J.; Sung, S.-Y.; Kim, H.J.; Song, S.-H.; Hong, J.Y.; Yoon, T.K.; Kim, J.K.; Kim, K.-S.; Lee, D.R. Long-term proliferation and characterization of human spermatogonial stem cells obtained from obstructive and non-obstructive azoospermia under exogenous feeder-free culture conditions. Cell Prolif. 2010, 43, 405–417. [Google Scholar] [CrossRef]
- Tadokoro, Y.; Yomogida, K.; Ohta, H.; Tohda, A.; Nishimune, Y. Homeostatic regulation of germinal stem cell proliferation by the GDNF/FSH pathway. Mech. Dev. 2002, 113, 29–39. [Google Scholar] [CrossRef]
- Maki, C.; Yuen, C.; Zhao, H.H.; Wong, J.; Pacchiarotti, J.; Howerton, K.; Chow, M.; Greilach, S.; Ramos, T.; Izadyar, F.; et al. Identification and characterization of repopulating spermatogonial stem cells from the adult human testis. Hum. Reprod. 2011, 26, 1296–1306. [Google Scholar]
- Lai, M.-S.; Wang, C.-Y.; Yang, S.-H.; Wu, C.-C.; Sun, H.S.; Tsai, S.-J.; Chuang, J.-I.; Chen, Y.-C.; Huang, B.-M. The expression profiles of fibroblast growth factor 9 and its receptors in developing mice testes. Organogenesis 2016, 12, 61–77. [Google Scholar] [CrossRef][Green Version]
- von Kopylow, K.; Kirchhoff, C.; Jezek, D.; Schulze, W.; Feig, C.; Primig, M.; Steinkraus, V.; Spiess, A.-N. Screening for biomarkers of spermatogonia within the human testis: A whole genome approach. Hum. Reprod. 2010, 25, 1104–1112. [Google Scholar] [CrossRef]
- Sohni, A.; Tan, K.; Song, H.-W.; Burow, D.; de Rooij, D.G.; Laurent, L.; Hsieh, T.-C.; Rabah, R.; Hammoud, S.S.; Vicini, E.; et al. The neonatal and adult human testis defined at the single-cell level. Cell Rep. 2019, 26, 1501–1517.e4. [Google Scholar] [CrossRef]
- Zohni, K.; Zhang, X.; Tan, S.L.; Chan, P.; Nagano, M. CD9 is expressed on human male germ cells that have a long-term repopulation potential after transplantation into mouse testes. Biol. Reprod. 2012, 87, 27. [Google Scholar] [CrossRef]
- Aloisio, G.M.; Nakada, Y.; Saatcioglu, H.D.; Pena, C.G.; Baker, M.D.; Tarnawa, E.D.; Mukherjee, J.; Manjunath, H.; Bugde, A.; Sengupta, A.L.; et al. PAX7 expression defines germline stem cells in the adult testis. J. Clin. Invest. 2014, 124, 3929–3944. [Google Scholar] [CrossRef]
- Sakai, Y.; Noce, T.; Yamashina, S. Cleavage-like cell division and explosive increase in cell number of neonatal gonocytes. Dev. Growth Differ. 2004, 46, 15–21. [Google Scholar] [CrossRef]
- Altman, E.; Yango, P.; Moustafa, R.; Smith, J.F.; Klatsky, P.C.; Tran, N.D. Characterization of human spermatogonial stem cell markers in fetal, pediatric, and adult testicular tissues. Reproduction 2014, 148, 417–427. [Google Scholar] [CrossRef]
- Mitchell, R.T.; Cowan, G.; Morris, K.D.; Anderson, R.A.; Fraser, H.M.; Mckenzie, K.J.; Wallace, W.H.B.; Kelnar, C.J.H.; Saunders, P.T.K.; Sharpe, R.M. Germ cell differentiation in the marmoset (Callithrix jacchus) during fetal and neonatal life closely parallels that in the human. Hum. Reprod. 2008, 23, 2755–2765. [Google Scholar] [CrossRef]
- Rajpert-De Meyts, E.; Jacobsen, G.K.; Bartkova, J.; Aubry, F.; Samson, M.; Bartek, J.; Skakkebaek, N.E. The immunohistochemical expression pattern of Chk2, p53, p19INK4d, MAGE-A4 and other selected antigens provides new evidence for the premeiotic origin of spermatocytic seminoma. Histopathology 2003, 42, 217–226. [Google Scholar] [CrossRef]
- Sada, A.; Suzuki, A.; Suzuki, H.; Saga, Y. The RNA-binding protein NANOS2 is required to maintain murine spermatogonial stem cells. Science 2009, 325, 1394–1398. [Google Scholar] [CrossRef]
- Yamauchi, K.; Hasegawa, K.; Chuma, S.; Nakatsuji, N.; Suemori, H. In vitro germ cell differentiation from cynomolgus monkey embryonic stem cells. PLoS ONE 2009, 4, e5338. [Google Scholar] [CrossRef]
- Jorgensen, A.; Nielsen, J.E.; Blomberg Jensen, M.; Graem, N.; Rajpert-De Meyts, E. Analysis of meiosis regulators in human gonads: A sexually dimorphic spatio-temporal expression pattern suggests involvement of DMRT1 in meiotic entry. Mol. Hum. Reprod. 2012, 18, 523–534. [Google Scholar] [CrossRef]
- Gassei, K.; Orwig, K.E. SALL4 expression in gonocytes and spermatogonial clones of postnatal mouse testes. PLoS ONE 2013, 8, e53976. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-L.; Liu, W.; Sun, Y.-J.; Kwon, J.; Setsuie, R.; Osaka, H.; Noda, M.; Aoki, S.; Yoshikawa, Y.; Wada, K. Overexpression of ubiquitin carboxyl-terminal hydrolase L1 arrests spermatogenesis in transgenic mice. Mol. Reprod. Dev. 2006, 73, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Buaas, F.W.; Kirsh, A.L.; Sharma, M.; McLean, D.J.; Morris, J.L.; Griswold, M.D.; de Rooij, D.G.; Braun, R.E. Plzf is required in adult male germ cells for stem cell self-renewal. Nat. Genet. 2004, 36, 647–652. [Google Scholar] [CrossRef]
- Pesce, M.; Wang, X.; Wolgemuth, D.J.; Scholer, H. Differential expression of the Oct-4 transcription factor during mouse germ cell differentiation. Mech. Dev. 1998, 71, 89–98. [Google Scholar] [CrossRef]
- Bhartiya, D.; Kasiviswanathan, S.; Unni, S.K.; Pethe, P.; Dhabalia J., V.; Patwardhan, S.; Tongaonkar, H.B. Newer insights into premeiotic development of germ cells in adult human testis using Oct-4 as a stem cell marker. J. Histochem. Cytochem. 2010, 58, 1093–1106. [Google Scholar] [CrossRef]
- Yoshida, S.; Takakura, A.; Ohbo, K.; Abe, K.; Wakabayashi, J.; Yamamoto, M.; Suda, T.; Nabeshima, Y.-I. Neurogenin3 delineates the earliest stages of spermatogenesis in the mouse testis. Dev. Biol. 2004, 269, 447–458. [Google Scholar] [CrossRef]
- Zhang, T.; Oatley, J.; Bardwell, V.J.; Zarkower, D. DMRT1 is required for mouse spermatogonial stem cell maintenance and replenishment. PLoS Genet. 2016, 12, e1006293. [Google Scholar] [CrossRef]
- Guo, J.; Grow, E.J.; Yi, C.; Mlcochova, H.; Maher, G.J.; Lindskog, C.; Murphy, P.J.; Wike, C.L.; Carrell, D.T.; Goriely, A.; et al. Chromatin and single-cell rna-seq profiling reveal dynamic signaling and metabolic transitions during human spermatogonial stem cell development. Cell Stem Cell 2017, 21, 533–546.e6. [Google Scholar] [CrossRef]
- van Bragt, M.P.A.; Roepers-Gajadien, H.L.; Korver, C.M.; Bogerd, J.; Okuda, A.; Eggen, B.J.L.; de Rooij, D.G.; van Pelt, A.M.M. Expression of the pluripotency marker UTF1 is restricted to a subpopulation of early A spermatogonia in rat testis. Reproduction 2008, 136, 33–40. [Google Scholar] [CrossRef]
- Valli, H.; Sukhwani, M.; Dovey, S.L.; Peters, K.A.; Donohue, J.; Castro, C.A.; Chu, T.; Marshall, G.R.; Orwig, K.E. Fluorescence- and magnetic-activated cell sorting strategies to isolate and enrich human spermatogonial stem cells. Fertil. Steril. 2014, 102, 566–580.e7. [Google Scholar] [CrossRef]
- Wang, M.; Liu, X.; Chang, G.; Chen, Y.; An, G.; Yan, L.; Gao, S.; Xu, Y.; Cui, Y.; Dong, J.; et al. Single-cell RNA sequencing analysis reveals sequential cell fate transition during human spermatogenesis. Cell Stem Cell 2018, 23, 599–614.e4. [Google Scholar] [CrossRef]
- Fayomi, A.P. Stem Cells and Spermatogenic Lineage Development in the Primate Testis; University of Pittsburgh: Pittsburgh, PA, USA, 2018; Available online: http://dscholarship.pitt.edu/34319/1/fayomiadetunjip_etd2018.pdf (accessed on 1 March 2020).
- Jarvis, S.; Elliott, D.J.; Morgan, D.; Winston, R.; Readhead, C. Molecular markers for the assessment of postnatal male germ cell development in the mouse. Hum. Reprod. 2005, 20, 108–116. [Google Scholar] [CrossRef]
- Elliott, D.J.; Millar, M.R.; Oghene, K.; Ross, A.; Kiesewetter, F.; Pryor, J.; McIntyre, M.; Hargreave, T.B.; Saunders, P.T.; Vogt, P.H.; et al. Expression of RBM in the nuclei of human germ cells is dependent on a critical region of the Y chromosome long arm. Proc. Natl. Acad. Sci. 1997, 94, 3848–3853. [Google Scholar] [CrossRef]
- Kossack, N.; Meneses, J.; Shefi, S.; Nguyen, H.N.; Chavez, S.; Nicholas, C.; Gromoll, J.; Turek, P.J.; Reijo-Pera, R.A. Isolation and characterization of pluripotent human spermatogonial stem cell-derived cells. Stem Cells 2009, 27, 138–149. [Google Scholar] [CrossRef]
- Yang, Y.; Yarahmadi, M.; Honaramooz, A. Development of novel strategies for the isolation of piglet testis cells with a high proportion of gonocytes. Reprod. Fertil. Dev. 2010, 22, 1057–1065. [Google Scholar] [CrossRef]
- Gassei, K.; Ehmcke, J.; Dhir, R.; Schlatt, S. Magnetic activated cell sorting allows isolation of spermatogonia from adult primate testes and reveals distinct GFRa1-positive subpopulations in men. J. Med. Primatol. 2010, 39, 83–91. [Google Scholar] [CrossRef]
- Nickkholgh, B.; Sefika Canan, M.; Korver, C.; van Saskia, K.D.; Andreas, M. Enrichment of spermatogonial stem cells from long-term cultured human testicular cells. Fertil. Steril. 2014, 102, 558–565. [Google Scholar] [CrossRef]
- Liu, S.; Tang, Z.; Xiong, T.; Tang, W. Isolation and characterization of human spermatogonial stem cells. Reprod. Biol. Endocrinol. 2011, 9, 141. [Google Scholar] [CrossRef]
- He, Y.; Chen, X.; Zhu, H.; Wang, D. Developments in techniques for the isolation, enrichment, main culture conditions and identification of spermatogonial stem cells. Cytotechnology 2015, 67, 921–930. [Google Scholar] [CrossRef]
- Ibtisham, F.; Zhao, Y.; Wu, J.; Nawab, A.; Mei, X.; Li, G.; Lilong, A. The optimized condition for the isolation and in vitro propagation of mouse spermatogonial stem cells. Biol. Futur. 2019, 70, 79–87. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, L.; Sun, M.; Hai, Y.; Li, Z.; He, Z. Expansion and long-term culture of human spermatogonial stem cells via the activation of SMAD3 and AKT pathways. Exp. Biol. Med. 2015, 240, 1112–1122. [Google Scholar] [CrossRef]
- Sadri-Ardekani, H.; Mizrak, S.C.; van Daalen, S.K.M.; Korver, C.M.; Roepers-Gajadien, H.L.; Koruji, M.; Hovingh, S.; de Reijke, T.M.; de la Rosette, J.J.M.C.H.; van der Veen, F.; et al. Propagation of human spermatogonial stem cells in vitro. JAMA 2009, 302, 2127–2134. [Google Scholar] [CrossRef]
- Kanatsu-Shinohara, M.; Miki, H.; Inoue, K.; Ogonuki, N.; Toyokuni, S.; Ogura, A.; Shinohara, T. Long-term culture of mouse male germline stem cells under serum-or feeder-free conditions. Biol. Reprod. 2005, 72, 985–991. [Google Scholar] [CrossRef]
- Kim, Y.-H.; Kang, H.-G.; Kim, B.-J.; Jung, S.-E.; Karmakar, P.C.; Kim, S.-M.; Hwang, S.; Ryu, B.-Y. Enrichment and In vitro culture of spermatogonial stem cells from pre-pubertal monkey testes. Tissue Eng. Regen. Med. 2017, 14, 557–566. [Google Scholar] [CrossRef]
- Trowell, O.A. The culture of mature organs in a synthetic medium. Exp. Cell Res. 1959, 16, 118–147. [Google Scholar] [CrossRef]
- Steinberger, E.; Steinberger, A.; Perluff, W.H. Initiation of spermatogenesis in vitro. Endocrinology 1964, 74, 788–792. [Google Scholar] [CrossRef]
- Steinberger, E.; Steinberger, A.; Perloff, W.H. Studies in growth in organ culture of testicular tissue from rats of various age. Anat. Rec. 1964, 148, 581–589. [Google Scholar] [CrossRef]
- Steinberger, A.; Steinberger, E. Stimulatory effect of vitamins and glutamine on the differentiation of germ cells in rat testes organ culture grown in chemically defined media. Exp. Cell Res. 1966, 44, 429–435. [Google Scholar] [CrossRef]
- Matte, R.; Sasaki, M. Autoradiographic evidence of human male germ-cell differentiation in vitro. Cytologia. 1971, 36, 298–303. [Google Scholar] [CrossRef]
- Boitani, C.; Politi, M.G.; Menna, T. Spermatogonial cell proliferation in organ culture of immature rat testis. Biol. Reprod. 1993, 48, 761–767. [Google Scholar] [CrossRef]
- Steinberger, A.; Steinberger, E.; Perloff, W.H. Mammalian testes in organ culture. Exp. Cell Res. 1964, 36, 19–27. [Google Scholar] [CrossRef]
- Suzuki, S.; Sato, K. The fertilising ability of spermatogenic cells derived from cultured mouse immature testicular tissue. Zygote 2003, 11, 307–316. [Google Scholar] [CrossRef]
- Sato, T.; Katagiri, K.; Gohbara, A.; Inoue, K.; Ogonuki, N.; Ogura, A.; Kubota, Y.; Ogawa, T. In vitro production of functional sperm in cultured neonatal mouse testes. Nature 2011, 471, 504–507. [Google Scholar] [CrossRef]
- Sato, T.; Yokonishi, T.; Komeya, M.; Katagiri, K.; Kubota, Y.; Matoba, S.; Ogonuki, N.; Ogura, A.; Yoshida, S.; Ogawa, T. Testis tissue explantation cures spermatogenic failure in c-Kit ligand mutant mice. Proc. Natl. Acad. Sci. 2012, 109, 16934–16938. [Google Scholar] [CrossRef]
- Yokonishi, T.; Sato, T.; Komeya, M.; Katagiri, K.; Kubota, Y.; Nakabayashi, K.; Hata, K.; Inoue, K.; Ogonuki, N.; Ogura, A.; et al. Offspring production with sperm grown in vitro from cryopreserved testis tissues. Nat. Commun. 2014, 5, 4320. [Google Scholar] [CrossRef]
- Sato, T.; Katagiri, K.; Kojima, K.; Komeya, M.; Yao, M.; Ogawa, T. In vitro spermatogenesis in explanted adult mouse testis tissues. PLoS ONE 2015, 10, e0130171. [Google Scholar] [CrossRef]
- Komeya, M.; Kimura, H.; Nakamura, H.; Yokonishi, T.; Sato, T.; Kojima, K.; Hayashi, K.; Katagiri, K.; Yamanaka, H.; Sanjo, H.; et al. Long-term ex vivo maintenance of testis tissues producing fertile sperm in a microfluidic device. Sci. Rep. 2016, 6, 21472. [Google Scholar] [CrossRef]
- Komeya, M.; Hayashi, K.; Nakamura, H.; Yamanaka, H.; Sanjo, H.; Kojima, K.; Sato, T.; Yao, M.; Kimura, H.; Fujii, T.; et al. Pumpless microfluidic system driven by hydrostatic pressure induces and maintains mouse spermatogenesis in vitro. Sci. Rep. 2017, 7, 15459. [Google Scholar] [CrossRef]
- Dietrich, A.J.; Scholten, R.; Vink, A.C.; Oud, J.L. Testicular cell suspensions of the mouse in vitro. Andrologia 1983, 15, 236–246. [Google Scholar] [CrossRef]
- Nagao, Y. Viability of meiotic prophase spermatocytes of rats is facilitated in primary culture of dispersed testicular cells on collagen gel by supplementing epinephrine or norepinephrine: Evidence that meiotic prophase spermatocytes complete meiotic divisions in vitro. Cell. Dev. Biol. 1989, 25, 1088–1098. [Google Scholar]
- Rassoulzadegan, M.; Paquis-Flucklinger, V.; Bertino, B.; Sage, J.; Jasin, M.; Miyagawa, K.; van Heyningen, V.; Besmer, P.; Cuzin, F. Transmeiotic differentiation of male germ cells in culture. Cell 1993, 75, 997–1006. [Google Scholar] [CrossRef]
- Hue, D.; Staub, C.; Perrard-Sapori, M.H.; Weiss, M.; Nicolle, J.C.; Vigier, M.; Durand, P. Meiotic differentiation of germinal cells in three-week cultures of whole cell population from rat seminiferous tubules. Biol. Reprod. 1998, 59, 379–387. [Google Scholar] [CrossRef]
- Staub, C.; Hue, D.; Nicolle, J.C.; Perrard-Sapori, M.H.; Segretain, D.; Durand, P. The whole meiotic process can occur in vitro in untransformed rat spermatogenic cells. Exp. Cell Res. 2000, 260, 85–95. [Google Scholar] [CrossRef]
- Marh, J.; Tres, L.L.; Yamazaki, Y.; Yanagimachi, R.; Kierszenbaum, A.L. Mouse round spermatids developed in vitro from preexisting spermatocytes can produce normal offspring by nuclear injection into in vivo-developed mature oocytes1. Biol. Reprod. 2003, 69, 169–176. [Google Scholar]
- Iwanami, Y.; Kobayashi, T.; Kato, M.; Hirabayashi, M.; Hochi, S. Characteristics of rat round spermatids differentiated from spermatogonial cells during co-culture with Sertoli cells, assessed by flow cytometry, microinsemination and RT-PCR. Theriogenology 2006, 65, 288–298. [Google Scholar]
- Wang, P.; Suo, L.-J.; Shang, H.; Li, Y.; Li, G.-X.; Li, Q.-W.; Hu, J.-H. Differentiation of spermatogonial stem cell-like cells from murine testicular tissue into haploid male germ cells in vitro. Cytotechnology 2014, 66, 365–372. [Google Scholar] [CrossRef]
- Kanatsu-Shinohara, M.; Ogonuki, N.; Inoue, K.; Miki, H.; Ogura, A.; Toyokuni, S.; Shinohara, T. Long-term proliferation in culture and germline transmission of mouse male germline stem cells. Biol. Reprod. 2003, 69, 612–616. [Google Scholar] [CrossRef]
- Kubota, H.; Avarbock, M.R.; Brinster, R.L. Growth factors essential for self-renewal and expansion of mouse spermatogonial stem cells. Proc. Natl. Acad. Sci. 2004, 101, 16489–16494. [Google Scholar] [CrossRef]
- Kanatsu-Shinohara, M.; Inoue, K.; Ogonuki, N.; Morimoto, H.; Ogura, A.; Shinohara, T. Serum- and Feeder-Free Culture of Mouse Germline Stem Cells1. Biol. Reprod. 2011, 84, 97–105. [Google Scholar] [CrossRef]
- Langenstroth, D.; Kossack, N.; Westernstroer, B.; Wistuba, J.; Behr, R.; Gromoll, J.; Schlatt, S. Separation of somatic and germ cells is required to establish primate spermatogonial cultures. Hum. Reprod. 2014, 29, 2018–2031. [Google Scholar]
- Kokkinaki, M.; Djourabtchi, A.; Golestaneh, N. Long-term culture of human ssea-4 positive spermatogonial stem cells (SSCs). J. Stem Cell Res. Ther. 2011, 2, 2488. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, H.J.; Kim, H.; Lee, S.J.; Gye, M.C. In vitro spermatogenesis by three-dimensional culture of rat testicular cells in collagen gel matrix. Biomaterials 2006, 27, 2845–2853. [Google Scholar] [CrossRef] [PubMed]
- Stukenborg, J.-B.; Wistuba, J.; Luetjens, C.M.; Elhija, M.A.; Huleihel, M.; Lunenfeld, E.; Gromoll, J.; Nieschlag, E.; Schlatt, S. Coculture of spermatogonia with somatic cells in a novel three-dimensional soft-agar-culture-system. J. Androl. 2008, 29, 312–329. [Google Scholar] [CrossRef] [PubMed]
- Abu Elhija, M.; Lunenfeld, E.; Schlatt, S.; Huleihel, M. Differentiation of murine male germ cells to spermatozoa in a soft agar culture system. Asian J. Androl. 2012, 14, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Huleihel, M.; Nourashrafeddin, S.; Plant, T.M. Application of three-dimensional culture systems to study mammalian spermatogenesis, with an emphasis on the rhesus monkey (Macaca mulatta). Asian J. Androl. 2015, 17, 972–980. [Google Scholar] [CrossRef] [PubMed]
- Stukenborg, J.-B.; Schlatt, S.; Simoni, M.; Yeung, C.-H.; Elhija, M.A.; Luetjens, C.M.; Huleihel, M.; Wistuba, J. New horizons for in vitro spermatogenesis? An update on novel three-dimensional culture systems as tools for meiotic and post-meiotic differentiation of testicular germ cells. Mol. Hum. Reprod. 2009, 15, 521–529. [Google Scholar] [CrossRef]
- Legendre, A.; Froment, P.; Desmots, S.; Lecomte, A.; Habert, R.; Lemazurier, E. An engineered 3D blood-testis barrier model for the assessment of reproductive toxicity potential. Biomaterials 2010, 31, 4492–4505. [Google Scholar] [CrossRef]
- Yokonishi, T.; Sato, T.; Katagiri, K.; Komeya, M.; Kubota, Y.; Ogawa, T. In vitro reconstruction of mouse seminiferous tubules supporting germ cell differentiation. Biol. Reprod. 2013, 89, 1–6. [Google Scholar] [CrossRef]
- Alves-Lopes, J.P.; Soder, O.; Stukenborg, J.-B. Testicular organoid generation by a novel in vitro three-layer gradient system. Biomaterials 2017, 130, 76–89. [Google Scholar] [CrossRef]
- Steinberger, A.; Steinberger, E. Factors affecting spermatogenesis in organ cultures of mammalian testes. J. Reprod. Fertil. 1967, (Suppl.), 117–124. [Google Scholar]
- Curtis, D. In vitro differentiation of diakinesis figures in human testis. Hum. Genet. 1981, 59, 406–411. [Google Scholar] [CrossRef]
- Tesarik, J.; Greco, E.; Rienzi, L.; Ubaldi, F.; Guido, M.; Cohen-Bacrie, P.; Mendoza, C. Differentiation of spermatogenic cells during in-vitro culture of testicular biopsy samples from patients with obstructive azoospermia: Effect of recombinant follicle stimulating hormone. Hum. Reprod. 1998, 13, 2772–2781. [Google Scholar] [CrossRef]
- Cremades, N.; Bernabeu, R.; Barros, A.; Sousa, M. In-vitro maturation of round spermatids using co-culture on Vero cells. Hum. Reprod. 1999, 14, 1287–1293. [Google Scholar] [CrossRef]
- Sousa, M.; Cremades, N.; Alves, C.; Silva, J.; Barros, A. Developmental potential of human spermatogenic cells co-cultured with Sertoli cells. Hum. Reprod. 2002, 17, 161–172. [Google Scholar] [CrossRef][Green Version]
- Tanaka, A.; Nagayoshi, M.; Awata, S.; Mawatari, Y.; Tanaka, I.; Kusunoki, H. Completion of meiosis in human primary spermatocytes through in vitro coculture with Vero cells. Fertil. Steril. 2003, 79 (Suppl. 1), 795–801. [Google Scholar] [CrossRef]
- Riboldi, M.; Rubio, C.; Pellicer, A.; Gil-Salom, M.; Simon, C. In vitro production of haploid cells after coculture of CD49f+ with Sertoli cells from testicular sperm extraction in nonobstructive azoospermic patients. Fertil. Steril. 2012, 98, 580–590.e4. [Google Scholar] [CrossRef]
- de Michele, F.; Poels, J.; Vermeulen, M.; Ambroise, J.; Gruson, D.; Guiot, Y.; Wyns, C. Haploid germ cells generated in organotypic culture of testicular tissue from prepubertal boys. Front. Physiol. 2018, 9, 1413. [Google Scholar] [CrossRef]
- Awang-Junaidi, A.H.; Honaramooz, A. Optimization of culture conditions for short-term maintenance, proliferation, and colony formation of porcine gonocytes. J. Anim. Sci. Biotechnol. 2018, 9, 8. [Google Scholar] [CrossRef]
- Awang-Junaidi, A.H.; Singh, J.; Honaramooz, A. Regeneration of testis tissue after ectopic implantation of porcine testis cell aggregates in mice: Improved consistency of outcomes and in situ monitoring. Reprod. Fertil. Dev. 2020. [Google Scholar] [CrossRef]
- Fayaz, M.A.; Awang-Junaidi, A.H.; Singh, J.; Honaramooz, A. Validation of ultrasound biomicroscopy for the assessment of xenogeneic testis tissue grafts and cell implants in recipient mice. Andrology 2020. [Google Scholar] [CrossRef]
- Ibtisham, F.; Awang-Junaidi, A.H.; Honaramooz, A. The study and manipulation of spermatogonial stem cells using animal models. Cell Tissue Res. 2020. [Google Scholar]
- Albrecht, M. Insights into the nature of human testicular peritubular cells. Ann. Anat. 2009, 191, 532–540. [Google Scholar] [CrossRef]
- Hudson, M.M. Reproductive outcomes for survivors of childhood cancer. Obstet. Gynecol. 2010, 116, 1171–1183. [Google Scholar] [CrossRef]
- Thomson, A.B.; Campbell, A.J.; Irvine, D.C.; Anderson, R.A.; Kelnar, C.J.H.; Wallace, W.H.B. Semen quality and spermatozoal DNA integrity in survivors of childhood cancer: A case-control study. Lancet 2002, 360, 361–367. [Google Scholar] [CrossRef]
- Goossens, E.; Van Saen, D.; Tournaye, H. Spermatogonial stem cell preservation and transplantation: From research to clinic. Hum. Reprod. 2013, 28, 897–907. [Google Scholar] [CrossRef]
- Gassei, K.; Orwig, K.E. Experimental methods to preserve male fertility and treat male factor infertility. Fertil. Steril. 2016, 105, 256–266. [Google Scholar] [CrossRef]
- Honaramooz, A.; Megee, S.O.; Dobrinski, I. Germ cell transplantation in pigs. Biol. Reprod. 2002, 66, 21–28. [Google Scholar] [CrossRef]
- Honaramooz, A.; Behboodi, E.; Blash, S.; Megee, S.O.; Dobrinski, I. Germ cell transplantation in goats. Mol. Reprod. Dev. 2003, 64, 422–428. [Google Scholar] [CrossRef]
- Wu, X.; Schmidt, J.A.; Avarbock, M.R.; Tobias, J.W.; Carlson, C.A.; Kolon, T.F.; Ginsberg, J.P.; Brinster, R.L. Prepubertal human spermatogonia and mouse gonocytes share conserved gene expression of germline stem cell regulatory molecules. Proc. Natl. Acad. Sci. 2009, 106, 21672–21677. [Google Scholar] [CrossRef]
- Patra, T.; Gupta, M.K. Cryopreservation of murine testicular Leydig cells by modified solid surface vitrification with supplementation of antioxidants. Cryobiology 2019, 88, 38–46. [Google Scholar] [CrossRef]
- Oblette, A.; Rondeaux, J.; Dumont, L.; Delessard, M.; Saulnier, J.; Rives, A.; Rives, N.; Rondanino, C. DNA methylation and histone post-translational modifications in the mouse germline following in-vitro maturation of fresh or cryopreserved prepubertal testicular tissue. Reprod. Biomed. Online 2019, 39, 383–401. [Google Scholar] [CrossRef]
- Weissbein, U.; Plotnik, O.; Vershkov, D.; Benvenisty, N. Culture-induced recurrent epigenetic aberrations in human pluripotent stem cells. PLoS Genet. 2017, 13, e1006979. [Google Scholar] [CrossRef]
- Wang, J.; Wu, Y.; Gao, W.; Li, F.; Bo, Y.; Zhu, M.; Fu, R.; Liu, Q.; Wen, S.; Wang, B. Identification and characterization of CD133(+)CD44(+) cancer stem cells from human laryngeal squamous cell carcinoma cell lines. J. Cancer 2017, 8, 497–506. [Google Scholar] [CrossRef]
- Lau, G.A.; Schaeffer, A.J. Current standing and future directions in pediatric oncofertility: A narrative review. Transl. Androl. Urol. 2018, 7, S276–S282. [Google Scholar] [CrossRef]
- Jahnukainen, K.; Ehmcke, J.; Nurmio, M.; Schlatt, S. Autologous ectopic grafting of cryopreserved testicular tissue preserves the fertility of prepubescent monkeys that receive sterilizing cytotoxic therapy. Cancer Res. 2012, 72, 5174–5178. [Google Scholar] [CrossRef] [PubMed]
- Fayomi, A.P.; Peters, K.; Sukhwani, M.; Valli-Pulaski, H.; Shetty, G.; Meistrich, M.L.; Houser, L.; Robertson, N.; Roberts, V.; Ramsey, C.; et al. Autologous grafting of cryopreserved prepubertal rhesus testis produces sperm and offspring. Science. 2019, 363, 1314–1319. [Google Scholar] [CrossRef]
- Honaramooz, A.; Snedaker, A.; Boiani, M.; Scholer, H.; Dobrinski, I.; Schlatt, S. Sperm from neonatal mammalian testes grafted in mice. Nature 2002, 418, 778–781. [Google Scholar] [CrossRef]
- Honaramooz, A. Potential and challenges of testis tissue xenografting from diverse ruminant species. Biosci. Proc. 2019, 8. [Google Scholar] [CrossRef]
- Honaramooz, A.; Li, M.-W.; Penedo, M.C.T.; Meyers, S.; Dobrinski, I. Accelerated maturation of primate testis by xenografting into mice. Biol. Reprod. 2004, 70, 1500–1503. [Google Scholar] [CrossRef]
- Schlatt, S.; Honaramooz, A.; Ehmcke, J.; Goebell, P.J.; Rübben, H.; Dhir, R.; Dobrinski, I.; Patrizio, P. Limited survival of adult human testicular tissue as ectopic xenograft. Hum. Reprod. 2006, 21, 384–389. [Google Scholar] [CrossRef]
- Van Saen, D.; Goossens, E.; Bourgain, C.; Ferster, A.; Tournaye, H. Meiotic activity in orthotopic xenografts derived from human postpubertal testicular tissue. Hum. Reprod. 2011, 26, 282–293. [Google Scholar] [CrossRef] [PubMed]
- de Michele, F.; Poels, J.; Weerens, L.; Petit, C.; Evrard, Z.; Ambroise, J.; Gruson, D.; Wyns, C. Preserved seminiferous tubule integrity with spermatogonial survival and induction of Sertoli and Leydig cell maturation after long-term organotypic culture of prepubertal human testicular tissue. Hum. Reprod. 2017, 32, 32–45. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Mulgund, A.; Hamada, A.; Chyatte, M.R. A unique view on male infertility around the globe. Reprod. Biol. Endocrinol. 2015, 13, 37. [Google Scholar] [CrossRef] [PubMed]
- Nordkap, L.; Joensen, U.N.; Blomberg Jensen, M.; Jorgensen, N. Regional differences and temporal trends in male reproductive health disorders: Semen quality may be a sensitive marker of environmental exposures. Mol. Cell. Endocrinol. 2012, 355, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Hamada, A.; Esteves, S.C.; Nizza, M.; Agarwal, A. Unexplained Male infertility: Diagnosis and Management. Int. Braz J. Urol 2012, 38, 576–594. [Google Scholar] [CrossRef] [PubMed]
- Honaramooz, A.; Megee, S.O.; Rathi, R.; Dobrinski, I. Building a testis: Formation of functional testis tissue after transplantation of isolated porcine (Sus scrofa) testis cells. Biol. Reprod. 2007, 76, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Yanagimachi, R. Development of normal mice from oocytes injected with secondary spermatocyte nuclei. Biol. Reprod. 1995, 53, 855–862. [Google Scholar] [CrossRef]
- Sofikitis, N.; Mantzavinos, T.; Loutradis, D.; Yamamoto, Y.; Tarlatzis, V.; Miyagawa, I. Ooplasmic injections of secondary spermatocytes for non-obstructive azoospermia. Lancet 1998, 351, 1177–1178. [Google Scholar] [CrossRef]
| Markers | Mouse | Monkey | Human |
|---|---|---|---|
| Cell Surface Markers | |||
| THY1 (CD90) | + [30] | + [44] | + [32] |
| β1-integrin (ITGB1, CD29) | + [29] | + [45] | + [46] |
| α6-Integrin (ITGA6, CD49f) | + [29] | + [44] | + [32] |
| PROM1 (CD133) | ? | ? | + [32] |
| GFRα1 | + [47] | + [28] | + [32] |
| CDH1 | + [39] | ? | ? |
| SSEA4 | ? | + [44] | + [48] |
| FGFR3 | + [49] | ? | + [50] |
| DSG2 | ? | ? | + [50] |
| LPPR3 | ? | ? | + [51] |
| TSPAN33 | ? | ? | + [51] |
| CD9 | + [31] | ? | + [52] |
| GPR125 | + [35] | ? | + [36] |
| Intracellular Markers | |||
| PAX7 | + [53] | + [53] | + [53] |
| DDX4 (VASA) | + [54] | + [25] | + [55] |
| DAZL | + [13] | + [25] | + [33] |
| CHK2 | ? | ? | + [43] |
| LIN28 | + [22] | + [23] | + [23] |
| MAGEA4 | ? | + [56] | + [57] |
| NANOS2 | + [58] | + [59] | + [60] |
| NANOS3 | + [60] | + [59] | + [60] |
| SALL4 | + [61] | + [27] | + [24] |
| UCHL1 (PGP9.5) | + [62] | + [24] | + [33] |
| PLZF | + [63] | + [25] | + [33] |
| POU5F1 (OCT4) | + [64] | + [56] | + [65] |
| ID4 | + [34] | ? | + [36] |
| NEUROG3 (NGN3) | + [66] | + [28] | ? |
| DMRT1 | + [67] | ? | + [68] |
| UTF1 | + [69] | + [23] | + [70,71] |
| TCF3 | ? | ? | + [33] |
| SPOCD1 | ? | ? | + [68] |
| ENO2 | ? | + [72] | + [70] |
| SNAP91 | ? | ? | + [50] |
| CBL | ? | ? | + [50] |
| PIWIL4 | ? | ? | + [51] |
| ZKSCAN2 | ? | ? | + [68] |
| RET | + [37] | ? | ? |
| STRA8 | + [38] | ? | ? |
| RBM | + [73] | ? | + [74] |
| TSPY | − [42] | ? | + [41] |
| Culture Conditions | Mouse | Monkey * | Human | ||||
|---|---|---|---|---|---|---|---|
| Donor age | Newborn | 4.5–7.5-day-old | 8-day-old | 3.7–4.6 years (average age) | NA | 14, 34, and 45 years | 22–35 years |
| SSC isolation method | Two-step enzymatic digestion | Two-step enzymatic digestion | Two-step enzymatic digestion | Two-step enzymatic digestion | Two-step enzymatic digestion | Two-step enzymatic digestion | Two-step enzymatic digestion |
| SSC enrichment method | Diff. plat. | MACS | MACS | Diff. plat./un enriched | Diff. plat. | Diff. plat. + MACS | Diff. plat. + MACS |
| Basic medium | StemPro-34 SFM | Alpha-MEM | StemPro-34 SFM | Alpha-MEM | StemPro-34 SFM | StemPro-34 SFM | StemPro-34 SFM |
| Additional supplements | StemPro supplement | Supplement | StemPro supplement | NA | StemPro supplement | StemPro supplement | StemPro supplement |
| Protein/serum source | FCS | BSA | Albumax II | FBS | FCS | BSA | FBS, BSA |
| Growth factors | GDNF, bFGF, EGF, LIF | GDNF, bFGF, GFRα1 | GDNF, bFGF, EGF | NA | GDNF, LIF, EGF, bFGF | GDNF, LIF, EGF, bFGF | GDNF, LIF, EGF, bFGF |
| Feeder cells | MEF | STO | NA | NA/Somatic cells | NA | NA | NA |
| Temperature | 37 oC | 37 oC | 37 oC | 35 oC | 37 oC | 37 oC | 37 oC |
| Subculture | 10–14 days | 5–7 days | 5–6 days | NA | 7–10 days | 10–15 days | NA |
| Max. culture duration | ∼160 days | ~180 days | ~178 days | ~11 days | ~196 days | ~120 days | ~60 days |
| Germ cell clump formation | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| SSC identification | Flow cytometry | Immunostaining | Flow cytometry | Immunostaining | Immunostaining | PCR | Immunostaining |
| Functional analyses | Yes | Yes | Yes | Yes | Yes | No | No |
| Reference | [108] | [109] | [110] | [111] | [83] | [112] | [82] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibtisham, F.; Honaramooz, A. Spermatogonial Stem Cells for In Vitro Spermatogenesis and In Vivo Restoration of Fertility. Cells 2020, 9, 745. https://doi.org/10.3390/cells9030745
Ibtisham F, Honaramooz A. Spermatogonial Stem Cells for In Vitro Spermatogenesis and In Vivo Restoration of Fertility. Cells. 2020; 9(3):745. https://doi.org/10.3390/cells9030745
Chicago/Turabian StyleIbtisham, Fahar, and Ali Honaramooz. 2020. "Spermatogonial Stem Cells for In Vitro Spermatogenesis and In Vivo Restoration of Fertility" Cells 9, no. 3: 745. https://doi.org/10.3390/cells9030745
APA StyleIbtisham, F., & Honaramooz, A. (2020). Spermatogonial Stem Cells for In Vitro Spermatogenesis and In Vivo Restoration of Fertility. Cells, 9(3), 745. https://doi.org/10.3390/cells9030745






