Revisiting the Interaction of γδ T-Cells and B-Cells
Abstract
1. Introduction
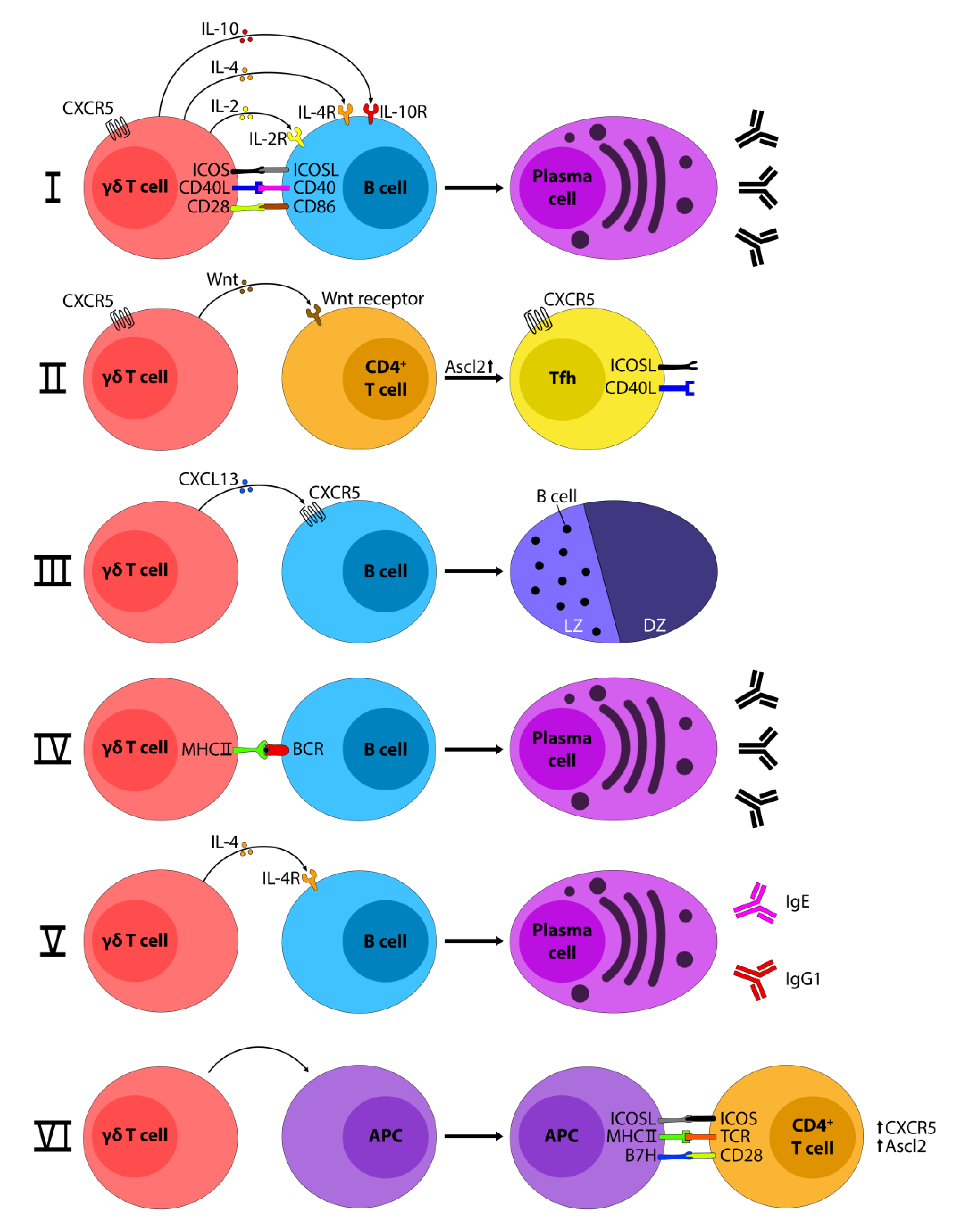
2. How Do γδ T-Cells Influence B-Cells?
3. How Do B-cells Influence γδ T-Cells?
4. γδ T-Cells and Germinal Centers
5. γδ T-cells and Autoimmune Disease
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chien, Y.; Becker, D.M.; Lindsten, T.; Okamura, M.; Cohen, D.I.; Davis, M.M. A third type of murine T-cell receptor gene. Nature 1984, 312, 31–35. [Google Scholar] [CrossRef]
- Saito, H.; Kranz, D.M.; Takagaki, Y.; Hayday, A.C.; Eisen, H.N.; Tonegawa, S. Complete primary structure of a heterodimeric T-cell receptor deduced from cDNA sequences. Nature 1984, 309, 757–762. [Google Scholar] [CrossRef] [PubMed]
- Hayday, A. Structure, organization, and somatic rearrangement of T cell gamma genes. Cell 1985, 40, 259–269. [Google Scholar] [CrossRef]
- Morita, C.T.; Mariuzza, R.A.; Brenner, M.B. Antigen recognition by human γδ T cells: Pattern recognition by the adaptive immune system. Springer Semin. Immunopathol. 2000, 22, 191–217. [Google Scholar] [CrossRef] [PubMed]
- Brenner, M.B.; McLean, J.; Dialynas, D.P.; Strominger, J.L.; Smith, J.A.; Owen, F.L.; Seidman, J.G.; Ip, S.; Rosen, F.; Krangel, M.S. Identification of a putative second T-cell receptor. Nature 1986, 322, 145–149. [Google Scholar] [CrossRef]
- Carding, S.R.; Egan, P.J. γδ T cells: Functional plasticity and heterogeneity. Nat. Rev. Immunol. 2002, 2, 336–345. [Google Scholar] [CrossRef]
- Ravens, S.; Schultze-Florey, C.; Raha, S.; Sandrock, I.; Drenker, M.; Oberdörfer, L.; Reinhardt, A.; Ravens, I.; Beck, M.; Geffers, R.; et al. Human γδ T cells are quickly reconstituted after stem-cell transplantation and show adaptive clonal expansion in response to viral infection. Nat. Immunol. 2017, 18, 393–401. [Google Scholar] [CrossRef]
- Vantourout, P.; Hayday, A. Six-of-the-best: Unique contributions of γδ T cells to immunology. Nat. Rev. Immunol. 2013, 13, 88–100. [Google Scholar] [CrossRef]
- Parker, M.E.; Ciofani, M. Regulation of γδ T Cell Effector Diversification in the Thymus. Front. Immunol. 2020, 11. [Google Scholar] [CrossRef]
- Silva-Santos, B. Lymphotoxin-Mediated Regulation of Cell Differentiation by T Cell Progenitors. Science 2005, 307, 925–928. [Google Scholar] [CrossRef]
- Pennington, D.J.; Silva-Santos, B.; Shires, J.; Theodoridis, E.; Pollitt, C.; Wise, E.L.; Tigelaar, R.E.; Owen, M.J.; Hayday, A.C. The inter-relatedness and interdependence of mouse T cell receptor γδ+ and αβ+ cells. Nat. Immunol. 2003, 4, 991–998. [Google Scholar] [CrossRef] [PubMed]
- Pennington, D.J.; Silva-Santos, B.; Silberzahn, T.; Escórcio-Correia, M.; Woodward, M.J.; Roberts, S.J.; Smith, A.L.; Dyson, P.J.; Hayday, A.C. Early events in the thymus affect the balance of effector and regulatory T cells. Nature 2006, 444, 1073–1077. [Google Scholar] [CrossRef] [PubMed]
- Phalke, S.P.; Huang, Y.; Rubtsova, K.; Getahun, A.; Sun, D.; Reinhardt, R.L.; O’Brien, R.L.; Born, W.K. γδ T cells shape memory-phenotype αβ T cell populations in non-immunized mice. PLoS ONE 2019, 14, e0218827. [Google Scholar] [CrossRef] [PubMed]
- Born, W.K.; Yin, Z.; Hahn, Y.-S.; Sun, D.; O’Brien, R.L. Analysis of γδ T Cell Functions in the Mouse. J. Immunol. 2010, 184, 4055–4061. [Google Scholar] [CrossRef] [PubMed]
- Gerber, D.J.; Azuara, V.; Levraud, J.P.; Huang, S.Y.; Lembezat, M.P.; Pereira, P. IL-4-producing gamma delta T cells that express a very restricted TCR repertoire are preferentially localized in liver and spleen. J. Immunol. 1999, 163, 3076–3082. [Google Scholar] [PubMed]
- Bonneville, M.; O’Brien, R.L.; Born, W.K. γδ T cell effector functions: A blend of innate programming and acquired plasticity. Nat. Rev. Immunol. 2010, 10, 467–478. [Google Scholar] [CrossRef]
- Roark, C.L.; French, J.D.; Taylor, M.A.; Bendele, A.M.; Born, W.K.; O’Brien, R.L. Exacerbation of Collagen-Induced Arthritis by Oligoclonal, IL-17-Producing γδ T Cells. J. Immunol. 2007, 179, 5576–5583. [Google Scholar] [CrossRef]
- Dodd, J.; Riffault, S.; Kodituwakku, J.S.; Hayday, A.C.; Openshaw, P.J.M. Pulmonary Vγ4+ γδ T Cells Have Proinflammatory and Antiviral Effects in Viral Lung Disease. J. Immunol. 2009, 182, 1174–1181. [Google Scholar] [CrossRef]
- Huber, S.A.; Graveline, D.; Newell, M.K.; Born, W.K.; O’Brien, R.L. Vγ1+ T Cells Suppress and Vγ4+ T Cells Promote Susceptibility to Coxsackievirus B3-Induced Myocarditis in Mice. J. Immunol. 2000, 165, 4174–4181. [Google Scholar] [CrossRef]
- Arden, B.; Clark, S.; Kabelitz, D.; Mak, T. Human T-cell receptor variable gene segment families. Immunogenetics 1995, 42, 455–500. [Google Scholar] [CrossRef]
- Pang, D.J.; Neves, J.F.; Sumaria, N.; Pennington, D.J. Understanding the complexity of γδ T-cell subsets in mouse and human. Immunology 2012, 136, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Morita, C.T.; Jin, C.; Sarikonda, G.; Wang, H. Nonpeptide antigens, presentation mechanisms, and immunological memory of human Vγ2Vδ2 T cells: Discriminating friend from foe through the recognition of prenyl pyrophosphate antigens. Immunol. Rev. 2007, 215, 59–76. [Google Scholar] [CrossRef] [PubMed]
- Davey, M.S.; Willcox, C.R.; Hunter, S.; Kasatskaya, S.A.; Remmerswaal, E.B.M.; Salim, M.; Mohammed, F.; Bemelman, F.J.; Chudakov, D.M.; Oo, Y.H.; et al. The human Vδ2+ T-cell compartment comprises distinct innate-like Vγ9+ and adaptive Vγ9− subsets. Nat. Commun. 2018, 9, 1760. [Google Scholar] [CrossRef] [PubMed]
- Born, W.K.; Huang, Y.; Reinhardt, R.L.; Huang, H.; Sun, D.; O’Brien, R.L. γδ T Cells and B Cells. In Advances in Immunology; Elsevier Inc.: Amsterdam, The Netherlands, 2017; Volume 134, pp. 1–45. ISBN 9780128124079. [Google Scholar]
- Caccamo, N.; Battistini, L.; Bonneville, M.; Poccia, F.; Fournié, J.J.; Meraviglia, S.; Borsellino, G.; Kroczek, R.A.; La Mendola, C.; Scotet, E.; et al. CXCR5 identifies a subset of Vgamma9Vdelta2 T cells which secrete IL-4 and IL-10 and help B cells for antibody production. J. Immunol. 2006, 177, 5290–5295. [Google Scholar] [CrossRef]
- Bansal, R.R.; Mackay, C.R.; Moser, B.; Eberl, M. IL-21 enhances the potential of human γδ T cells to provide B-cell help. Eur. J. Immunol. 2012, 42, 110–119. [Google Scholar] [CrossRef]
- Suárez-Fueyo, A.; Bradley, S.J.; Tsokos, G.C. T cells in Systemic Lupus Erythematosus. Curr. Opin. Immunol. 2016, 43, 32–38. [Google Scholar] [CrossRef]
- Huang, Y.; Getahun, A.; Heiser, R.A.; Detanico, T.O.; Aviszus, K.; Kirchenbaum, G.A.; Casper, T.L.; Huang, C.; Aydintug, M.K.; Carding, S.R.; et al. γδ T Cells Shape Preimmune Peripheral B Cell Populations. J. Immunol. 2015, 196, 217–231. [Google Scholar] [CrossRef]
- Breitfeld, D.; Ohl, L.; Kremmer, E.; Ellwart, J.; Sallusto, F.; Lipp, M.; Förster, R. Follicular B Helper T Cells Express Cxc Chemokine Receptor 5, Localize to B Cell Follicles, and Support Immunoglobulin Production. J. Exp. Med. 2000, 192, 1545–1552. [Google Scholar] [CrossRef]
- Crotty, S. T Follicular Helper Cell Differentiation, Function, and Roles in Disease. Immunity 2014, 41, 529–542. [Google Scholar] [CrossRef]
- Okada, T.; Miller, M.J.; Parker, I.; Krummel, M.F.; Neighbors, M.; Hartley, S.B.; O’Garra, A.; Cahalan, M.D.; Cyster, J.G. Antigen-engaged B cells undergo chemotaxis toward the T zone and form motile conjugates with helper T cells. PLoS Biol. 2005, 3, e150. [Google Scholar] [CrossRef]
- Qi, H.; Cannons, J.L.; Klauschen, F.; Schwartzberg, P.L.; Germain, R.N. SAP-controlled T–B cell interactions underlie germinal centre formation. Nature 2008, 455, 764–769. [Google Scholar] [CrossRef] [PubMed]
- Nurieva, R.I.; Chung, Y.; Hwang, D.; Yang, X.O.; Kang, H.S.; Ma, L.; Wang, Y.; Watowich, S.S.; Jetten, A.M.; Tian, Q.; et al. Generation of T Follicular Helper Cells Is Mediated by Interleukin-21 but Independent of T Helper 1, 2, or 17 Cell Lineages. Immunity 2008, 29, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Crotty, S. Follicular Helper CD4 T Cells (T FH). Annu. Rev. Immunol. 2011, 29, 621–663. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.S.; Deenick, E.K.; Batten, M.; Tangye, S.G. The origins, function, and regulation of T follicular helper cells. J. Exp. Med. 2012, 209, 1241–1253. [Google Scholar] [CrossRef] [PubMed]
- Victora, G.D.; Nussenzweig, M.C.; MacLennan, I.C.M. Germinal Centers. Annu. Rev. Immunol. 2012, 30, 429–457. [Google Scholar] [CrossRef] [PubMed]
- Horner, A.A.; Jabara, H.; Ramesh, N.; Geha, R.S. γ/δ T lymphocytes express CD40 ligand and induce isotype switching in B lymphocytes. J. Exp. Med. 1995, 181, 1239–1244. [Google Scholar] [CrossRef]
- Rezende, R.M.; Lanser, A.J.; Rubino, S.; Kuhn, C.; Skillin, N.; Moreira, T.G.; Liu, S.; Gabriely, G.; David, B.A.; Menezes, G.B.; et al. γδ T cells control humoral immune response by inducing T follicular helper cell differentiation. Nat. Commun. 2018, 9, 1–13. [Google Scholar] [CrossRef]
- Vermijlen, D.; Ellis, P.; Langford, C.; Klein, A.; Engel, R.; Willimann, K.; Jomaa, H.; Hayday, A.C.; Eberl, M. Distinct Cytokine-Driven Responses of Activated Blood γδ T Cells: Insights into Unconventional T Cell Pleiotropy. J. Immunol. 2007, 178, 4304–4314. [Google Scholar] [CrossRef]
- Wen, L.; Roberts, S.J.; Viney, J.L.; Wong, F.S.; Mallick, C.; Findly, R.C.; Peng, Q.; Craft, J.E.; Owen, M.J.; Mayday, A.C. Immunoglobulin synthesis and generalized autoimmunity in mice congenitally deficient in αβ(+) T cells. Nature 1994, 369, 654–658. [Google Scholar] [CrossRef]
- Bouteau, A.; Kervevan, J.; Su, Q.; Zurawski, S.M.; Contreras, V.; Dereuddre-Bosquet, N.; Le Grand, R.; Zurawski, G.; Cardinaud, S.; Levy, Y.; et al. DC subsets regulate humoral immune responses by supporting the differentiation of distinct TFH cells. Front. Immunol. 2019, 10, 1134. [Google Scholar] [CrossRef]
- Petrasca, A.; Melo, A.M.; Breen, E.P.; Doherty, D.G. Human Vδ3+ γδ T cells induce maturation and IgM secretion by B cells. Immunol. Lett. 2018, 196, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Brandes, M. Professional Antigen-Presentation Function by Human gd T Cells. Science 2005, 309, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Junttila, I.S. Tuning the cytokine responses: An update on interleukin (IL)-4 and IL-13 receptor complexes. Front. Immunol. 2018, 9, 888. [Google Scholar] [CrossRef] [PubMed]
- Gascan, H.; Gauchat, J.F.; Roncarolo, M.G.; Yssel, H.; Spits, H.; de Vries, J.E. Human B cell clones can be induced to proliferate and to switch to IgE and IgG4 synthesis by interleukin 4 and a signal provided by activated CD4+ T cell clones. J. Exp. Med. 1991, 173, 747–750. [Google Scholar] [CrossRef]
- Nelms, K.; Keegan, A.D.; Zamorano, J.; Ryan, J.J.; Paul, W.E. THE IL-4 RECEPTOR: Signaling Mechanisms and Biologic Functions. Annu. Rev. Immunol. 1999, 17, 701–738. [Google Scholar] [CrossRef]
- Huang, Y.; Heiser, R.A.; Detanico, T.O.; Getahun, A.; Kirchenbaum, G.A.; Casper, T.L.; Aydintug, M.K.; Carding, S.R.; Ikuta, K.; Huang, H.; et al. γδ T cells affect IL-4 production and B-cell tolerance. Proc. Natl. Acad. Sci. USA 2015, 112, E39–E48. [Google Scholar] [CrossRef]
- Crawford, G.; Hayes, M.D.; Seoane, R.C.; Ward, S.; Dalessandri, T.; Lai, C.; Healy, E.; Kipling, D.; Proby, C.; Moyes, C.; et al. Epithelial damage and tissue γδ T cells promote a unique tumor-protective IgE response. Nat. Immunol. 2018, 19, 859–870. [Google Scholar] [CrossRef]
- Wright, A.; Lee, J.E.; Link, M.P.; Smith, S.D.; Carroll, W.; Levy, R.; Clayberger, C.; Krensky, A.M. Cytotoxic T lymphocytes specific for self tumor immunoglobulin express T cell receptor delta chain. J. Exp. Med. 1989, 169, 1557–1564. [Google Scholar] [CrossRef]
- Bukowski, J.F.; Morita, C.T.; Tanaka, Y.; Bloom, B.R.; Brenner, M.B.; Band, H. V gamma 2V delta 2 TCR-dependent recognition of non-peptide antigens and Daudi cells analyzed by TCR gene transfer. J. Immunol. 1995, 154, 998–1006. [Google Scholar]
- Fisch, P.; Malkovsky, M.; Kovats, S.; Sturm, E.; Braakman, E.; Klein, B.; Voss, S.; Morrissey, L.; DeMars, R.; Welch, W.; et al. Recognition by human V gamma 9/V delta 2 T cells of a GroEL homolog on Daudi Burkitt’s lymphoma cells. Science 1990, 250, 1269–1273. [Google Scholar] [CrossRef]
- Fayen, J.D.; Tykocinski, M.L. Tykocinski The expansion of human γδ T cells in response to Daudi cells requires the participation of CD4+ T cells. Immunology 1999, 97, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Orsini, D.L.M.; van Gils, M.; Kooy, Y.M.C.; Struyk, L.; Klein, G.; Elsen, P.V.D.; Koning, F. Functional and molecular characterization of B cell-responsive Vδ1+ γδ T cells. Eur. J. Immunol. 1994, 24, 3199–3204. [Google Scholar] [CrossRef] [PubMed]
- Kierkels, G.J.J.; Scheper, W.; Meringa, A.D.; Johanna, I.; Beringer, D.X.; Janssen, A.; Schiffler, M.; Aarts-Riemens, T.; Kramer, L.; Straetemans, T.; et al. Identification of a tumor-specific allo-HLA–restricted gdTCR. Blood Adv. 2019, 3, 2870–2882. [Google Scholar] [CrossRef]
- Vermijlen, D.; Gatti, D.; Kouzeli, A.; Rus, T.; Eberl, M. γδ T cell responses: How many ligands will it take till we know? Semin. Cell Dev. Biol. 2018, 84, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, D.A.; Reith, W.; Trowsdale, J. Regulation of Immunity by Butyrophilins. Annu. Rev. Immunol. 2016, 34, 151–172. [Google Scholar] [CrossRef]
- Boyden, L.M.; Lewis, J.M.; Barbee, S.D.; Bas, A.; Girardi, M.; Hayday, A.C.; Tigelaar, R.E.; Lifton, R.P. Skint1, the prototype of a newly identified immunoglobulin superfamily gene cluster, positively selects epidermal γδ T cells. Nat. Genet. 2008, 40, 656–662. [Google Scholar] [CrossRef]
- Di Marco Barros, R.; Roberts, N.A.; Dart, R.J.; Vantourout, P.; Jandke, A.; Nussbaumer, O.; Deban, L.; Cipolat, S.; Hart, R.; Iannitto, M.L.; et al. Epithelia Use Butyrophilin-like Molecules to Shape Organ-Specific γδ T Cell Compartments. Cell 2016, 167, 203–218. [Google Scholar] [CrossRef]
- Rigau, M.; Ostrouska, S.; Fulford, T.S.; Johnson, D.N.; Woods, K.; Ruan, Z.; McWilliam, H.E.G.; Hudson, C.; Tutuka, C.; Wheatley, A.K.; et al. Butyrophilin 2A1 is essential for phosphoantigen reactivity by γδ T cells. Science 2020. [Google Scholar] [CrossRef]
- Harly, C.; Guillaume, Y.; Nedellec, S.; Peigné, C.M.; Mönkkönen, H.; Mönkkönen, J.; Li, J.; Kuball, J.; Adams, E.J.; Netzer, S.; et al. Key implication of CD277/butyrophilin-3 (BTN3A) in cellular stress sensing by a major human γδ T-cell subset. Blood 2012, 120, 2269–2279. [Google Scholar] [CrossRef]
- Wang, H.; Henry, O.; Distefano, M.D.; Wang, Y.-C.; Räikkönen, J.; Mönkkönen, J.; Tanaka, Y.; Morita, C.T. Butyrophilin 3A1 Plays an Essential Role in Prenyl Pyrophosphate Stimulation of Human Vγ2Vδ2 T Cells. J. Immunol. 2013, 191, 1029–1042. [Google Scholar] [CrossRef]
- Willcox, C.R.; Vantourout, P.; Salim, M.; Zlatareva, I.; Melandri, D.; Zanardo, L.; George, R.; Kjaer, S.; Jeeves, M.; Mohammed, F.; et al. Butyrophilin-like 3 Directly Binds a Human Vγ4+ T Cell Receptor Using a Modality Distinct from Clonally-Restricted Antigen. Immunity 2019, 51, 813–825. [Google Scholar] [CrossRef] [PubMed]
- Smith, I.A.; Knezevic, B.R.; Ammann, J.U.; Rhodes, D.A.; Aw, D.; Palmer, D.B.; Mather, I.H.; Trowsdale, J. BTN1A1, the Mammary Gland Butyrophilin, and BTN2A2 Are Both Inhibitors of T Cell Activation. J. Immunol. 2010, 184, 3514–3525. [Google Scholar] [CrossRef] [PubMed]
- Goodman, T.; Lefrancois, L. Intraepithelial lymphocytes. Anatomical site, not T cell receptor form, dictates phenotype and function. J. Exp. Med. 1989, 170, 1569–1581. [Google Scholar] [CrossRef] [PubMed]
- Houlden, B.A.; Matis, L.A.; Cron, R.Q.; Widacki, S.M.; Brown, G.D.; Pampeno, C.; Meruelo, D.; Bluestone, J.A. A TCR Cell Recognizing a Novel TL-encoded Gene Product. Cold Spring Harb. Symp. Quant. Biol. 1989, 54, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Koenecke, C.; Chennupati, V.; Schmitz, S.; Malissen, B.; Fo, R.; Prinz, I. In vivo application of mAb directed against the cd TCR does not deplete but generates “invisible” cd T cells. Eur. J. Immunol. 2009, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Itohara, S.; Mombaerts, P.; Lafaille, J.; Iacomini, J.; Nelson, A.; Clarke, A.R.; Hooper, M.L.; Farr, A.; Tonegawa, S. T cell receptor δ gene mutant mice: Independent generation of αβ T cells and programmed rearrangements of γδ TCR genes. Cell 1993, 72, 337–348. [Google Scholar] [CrossRef]
- Mombaerts, P.; Arnoldi, J.; Russ, F.; Tonegawa, S.; Kaufmann, S.H.E. Different roles of αβ and γδ T cells in immunity against an intracellular bacterial pathogen. Nature 1993, 365, 53–56. [Google Scholar] [CrossRef]
- Ramsburg, E.; Tigelaar, R.; Craft, J.; Hayday, A. Age-dependent Requirement for γδ T Cells in the Primary but Not Secondary Protective Immune Response against an Intestinal Parasite. J. Exp. Med. 2003, 198, 1403–1414. [Google Scholar] [CrossRef]
- Jameson, J.M.; Cauvi, G.; Witherden, D.A.; Havran, W.L. A Keratinocyte-Responsive γδ TCR Is Necessary for Dendritic Epidermal T Cell Activation by Damaged Keratinocytes and Maintenance in the Epidermis. J. Immunol. 2004, 172, 3573–3579. [Google Scholar] [CrossRef]
- Sandrock, I.; Reinhardt, A.; Ravens, S.; Binz, C.; Wilharm, A.; Martins, J.; Oberdörfer, L.; Tan, L.; Lienenklaus, S.; Zhang, B.; et al. Genetic models reveal origin, persistence and non-redundant functions of IL-17–producing γδ T cells. J. Exp. Med. 2018, 215, 3006–3018. [Google Scholar] [CrossRef]
- Stebegg, M.; Kumar, S.D.; Silva-Cayetano, A.; Fonseca, V.R.; Linterman, M.A.; Graca, L. Regulation of the Germinal Center Response. Front. Immunol. 2018, 9, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.D.C.; Okada, T.; Cyster, J.G. Germinal-Center Organization and Cellular Dynamics. Immunity 2007, 27, 190–202. [Google Scholar] [CrossRef] [PubMed]
- De Silva, N.S.; Klein, U. Dynamics of B cells in germinal centres. Nat. Rev. Immunol. 2015, 15, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Reboldi, A.; Cyster, J.G. Peyer’s patches: Organizing B-cell responses at the intestinal frontier. Immunol. Rev. 2016, 271, 230–245. [Google Scholar] [CrossRef]
- Mackay, C.R.; Hein, W.R. A large proportion of bovine T cells express the γδ T cell receptor and show a distinct tissue distribution and surface phenotype. Int. Immunol. 1989, 1, 540–545. [Google Scholar] [CrossRef]
- Groh, V.; Porcelli, S.; Fabbi, M.; Lanier, L.L.; Picker, L.J.; Anderson, T.; Warnke, R.A.; Bhan, A.K.; Strominger, J.L.; Brenner, M.B. Human lymphocytes bearing T cell receptor gamma/delta are phenotypically diverse and evenly distributed throughout the lymphoid system. J. Exp. Med. 1989, 169, 1277–1294. [Google Scholar] [CrossRef]
- Brandes, M.M.; Willimann, K.; Lang, A.B.; Nam, K.-H.; Jin, C.; Brenner, M.B.; Morita, C.T.; Moser, B. Flexible migration program regulates γδ T-cell involvement in humoral immunity. Blood 2003, 102, 3693–3701. [Google Scholar] [CrossRef]
- Pao, W.; Wen, L.; Smith, A.L.; Gulbranson-Judge, A.; Zheng, B.; Kelsoe, G.; MacLennan, I.C.M.; Owen, M.J.; Hayday, A.C. γδ T cell help of B cells is induced by repeated parasitic infection, in the absence of other T cells. Curr. Biol. 1996, 6, 1317–1325. [Google Scholar] [CrossRef]
- Wen, L.; Pao, W.; Wong, F.S.; Peng, Q.; Craft, J.; Zheng, B.; Kelsoe, G.; Dianda, L.; Owen, M.J.; Hayday, A.C. Germinal center formation, immunoglobulin class switching, and autoantibody production driven by “non alpha/beta” T cells. J. Exp. Med. 1996, 183, 2271–2282. [Google Scholar] [CrossRef]
- Born, W.K.; Huang, Y.; Jin, N.; Huang, H.; O’Brien, R.L. Balanced approach of γδ T cells to type 2 immunity. Immunol. Cell Biol. 2010, 88, 269–274. [Google Scholar] [CrossRef]
- Bannard, O.; Horton, R.M.; Allen, C.D.C.; An, J.; Nagasawa, T.; Cyster, J.G. Germinal Center Centroblasts Transition to a Centrocyte Phenotype According to a Timed Program and Depend on the Dark Zone for Effective Selection. Immunity 2013, 39, 912–924. [Google Scholar] [CrossRef] [PubMed]
- Cyster, J.G.; Ansel, K.M.; Reif, K.; Ekland, E.H.; Hyman, P.L.; Tang, H.L.; Luther, S.A.; Ngo, V.N. Follicular stromal cells and lymphocyte homing to follicles. Immunol. Rev. 2000, 176, 181–193. [Google Scholar] [PubMed]
- Maloy, K.J.; Odermatt, B.; Hengartner, H.; Zinkernagel, R.M. Interferon γ-producing γδ T cell-dependent antibody isotype switching in the absence of germinal center formation during virus infection. Proc. Natl. Acad. Sci. USA 1998, 95, 1160–1165. [Google Scholar] [CrossRef] [PubMed]
- Shilpi, S.P.; Lal, G. Role of gamma-delta (γδ) T cells in autoimmunity. J. Leukoc. Biol. 2015, 97, 259–271. [Google Scholar]
- Hayday, A. γδ cells regulate autoimmunity. Curr. Opin. Immunol. 1997, 9, 884–889. [Google Scholar] [CrossRef]
- Wen, L.; Hayday, A.C. γδ T-cell help in responses to pathogens and in the development of systemic autoimmunity. Immunol. Res. 1997, 16, 229–241. [Google Scholar] [CrossRef]
- Granato, A.; Hayashi, E.A.; Baptista, B.J.A.; Bellio, M.; Nobrega, A. IL-4 Regulates Bim Expression and Promotes B Cell Maturation in Synergy with BAFF Conferring Resistance to Cell Death at Negative Selection Checkpoints. J. Immunol. 2014, 192, 5761–5775. [Google Scholar] [CrossRef]
- Illera, V.A.; Perandones, C.E.; Stunz, L.L.; Mower, D.A.; Ashman, R.F. Apoptosis in splenic B lymphocytes. Regulation by protein kinase C and IL-4. J. Immunol. 1993, 151, 2965–2973. [Google Scholar]
- Bilancio, A.; Okkenhaug, K.; Camps, M.; Emery, J.L.; Ruckle, T.; Rommel, C.; Vanhaesebroeck, B. Key role of the p110δ isoform of PI3K in B-cell antigen and IL-4 receptor signaling: Comparative analysis of genetic and pharmacologic interference with p110δ function in B cells. Blood 2006, 107, 642–650. [Google Scholar] [CrossRef]
- Wu, M.; Yang, J.; Li, X.; Chen, J. The Role of γδ T Cells in Systemic Lupus Erythematosus. J. Immunol. Res. 2016, 2016. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rampoldi, F.; Ullrich, L.; Prinz, I. Revisiting the Interaction of γδ T-Cells and B-Cells. Cells 2020, 9, 743. https://doi.org/10.3390/cells9030743
Rampoldi F, Ullrich L, Prinz I. Revisiting the Interaction of γδ T-Cells and B-Cells. Cells. 2020; 9(3):743. https://doi.org/10.3390/cells9030743
Chicago/Turabian StyleRampoldi, Francesca, Leon Ullrich, and Immo Prinz. 2020. "Revisiting the Interaction of γδ T-Cells and B-Cells" Cells 9, no. 3: 743. https://doi.org/10.3390/cells9030743
APA StyleRampoldi, F., Ullrich, L., & Prinz, I. (2020). Revisiting the Interaction of γδ T-Cells and B-Cells. Cells, 9(3), 743. https://doi.org/10.3390/cells9030743