Molecular Regulations and Functions of the Transient Receptor Potential Channels of the Islets of Langerhans and Insulinoma Cells
Abstract
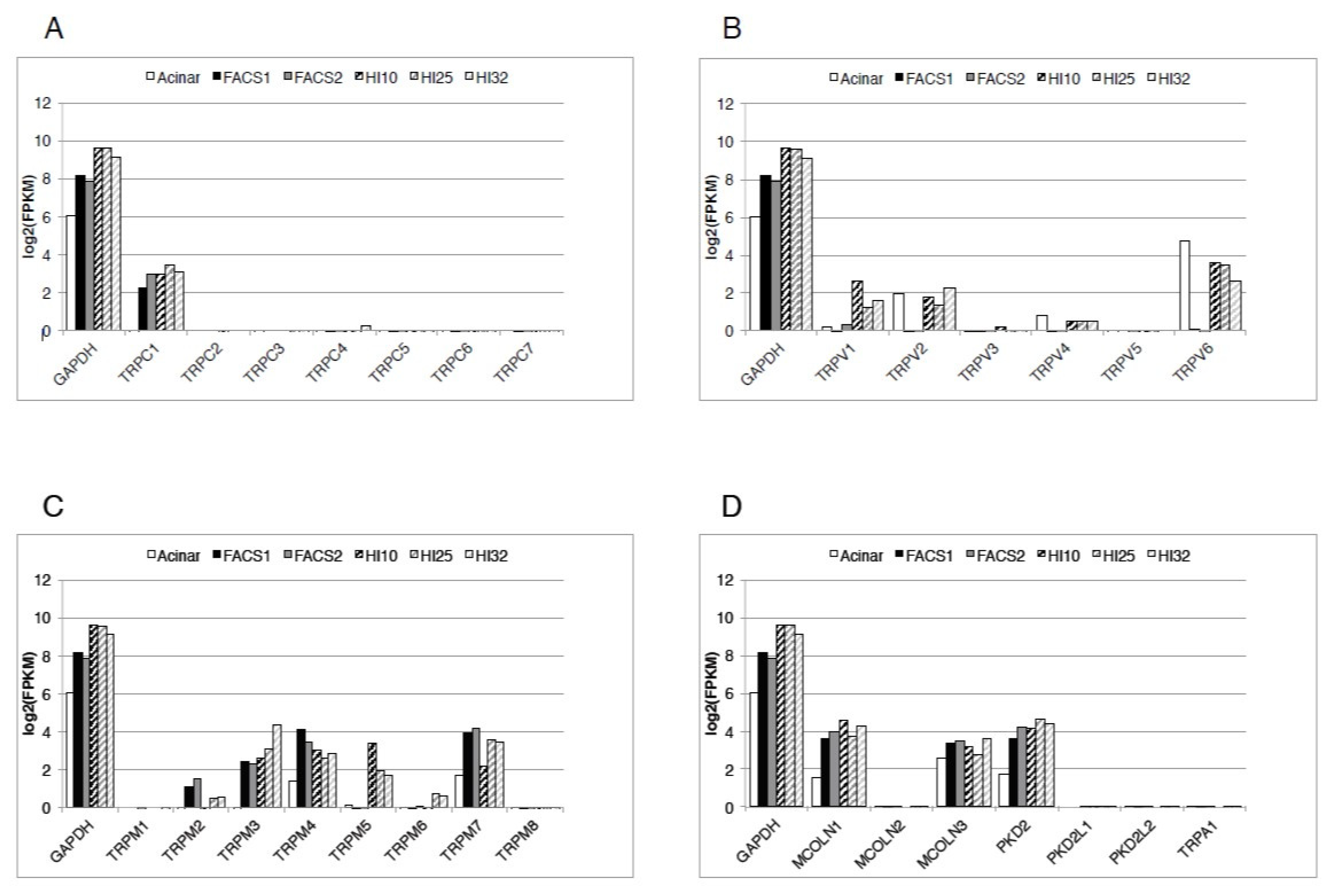
1. Introduction
2. TRPC1
3. TRPC2, TRPC3, TRPC4, TRPC5 and TRPC6
4. TRPM2
4.1. Role of the TRPM2 Channel in Stimulus-Secretion Coupling
4.2. Heat as a Regulator of TRPM2
4.3. TRPM2 and β-Cell Death
4.4. TRPM2 Channels Located on the Intracellular Membranes
5. TRPM3
6. TRPM4
7. TRPM5
8. TRPM6 and TRPM7
9. TRPV1
10. TRPV2
11. TRPV3 and TRPV4
12. TRPV5 and TRPV6
13. TRPML
14. TRPP
15. TRPA1
16. Conclusions
| Channel | Cell Type | Method | References |
|---|---|---|---|
| TRPC1 | human β-cell | RNA sequencing | [7] |
| INS-1 cells, rat β-cell, rat islet | RT-PCR, WB | [10,11,12] | |
| MIN6 cells, mouse islet | RT-PCR, NB | [8,9] | |
| TRPC2 | MIN6 cells | RT-PCR | [8] |
| TRPC3 | rat β-cells | EP | [20] |
| mouse β-cells | pharmacological tools | [20] | |
| mouse and rat islets | RT-PCR pharmacological tools, microarray | [8,20,21] | |
| TRPC4 | mouse β-cell, INS-1 cell | EP | [22,23,24] |
| MIN6, βTC3, INS-1, rat β-cell | RT-PCR, NB | [8,11] | |
| TRPC5 | βTC3 | RT-PCR | [8] |
| TRPC6 | MIN6 | RT-PCR | [8] |
| rat islet | microarray | [21] | |
| INS-1E | WB | [21] | |
| TRPM2 | human β-cell | RNA sequencing | [7] |
| human islet | RT-PCR, WB | [29,35] | |
| INS-1E | EP | [29,34,44] | |
| RIN-5F | EP, IF, WB, RT-PCR | [30,31,32,62] | |
| CRI-G1 | EP, RT-PCR | [28] | |
| HIT-T15 | IF, EP | [33] | |
| mouse β-cell | IF, EP | [31,34,50,54] | |
| rat β-cell | Ca2+ imaging | [31] | |
| TRPM3 | human β-cell | RNA sequencing | [7] |
| INS-1, mouse islet | EP, RT-PCR, NB, WB, shRNA, KO mice | [71,72,73] | |
| mouse β-cell | EP | [71] | |
| TRPM4 | human β-cell | RNA sequencing, IF, EP | [7,84,91,95] |
| INS-1, RINm5F, HIT-T15, MIN-6, βTC3 | RT-PCR, WB, EP | [84,91,93,153] | |
| rat islet | pharmacological tool | [94] | |
| mouse islet | KO mice, EP | [95] | |
| αTC1-6, INR1G9 | EP | [91,92] | |
| CRI-G1 | EP | [82] | |
| TRPM5 | MIN6, INS-1, human islet | RT-PCR, RNA sequencing | [7,98,107,153] |
| Mouse β-cell | RT-PCR, IF, KO, EP, Ca2+, insulin secretion | [95,98,102,104] | |
| rat islet | pharmacological tool | [154] | |
| TRPM6 | mouse islet | RT-PCR | [110] |
| TRPM7 | human β-cell | RNA-sequencing | [7] |
| INS-1 | RT-PCR, SiRNA | [110] | |
| mouse islet | RT-PCR | [110] | |
| TRPV1 | human islet | RNA-sequencing, WB | [7,116] |
| INS-1 | WB, pharmacological tool, RT-PCR, EP | [116,121] | |
| mouse islet | KO mice | [122] | |
| rat islet | RT-PCR | [121] | |
| RINm5F | RT-PCR | [121] | |
| TRPV2 | mouse islet | RT-PCR, WB, IF | [130] |
| human islet | RNA-sequencing | [7] | |
| MIN6 | RT-PCR, WB | [131,132,133] | |
| mouse β-cell | IF | [132] | |
| TRPV4 | MIN6 | RT-PCR, Ca2+ imaging | [135] |
| INS-1E, rat islet | RT-PCR, WB, Ca2+ imaging | [136,137] | |
| TRPV6 | INS-1E | RT-PCR, WB, IF | [138] |
| rat islet, rat β-cell | RT-PCR, WB, IF | [138] | |
| human islet | RNA sequencing | [7] | |
| mouse α-cells | IF | [130] | |
| TRPML1 (MCOLN1) | human β-cell | RNA sequencing | [7] |
| human islet | RNA sequencing | [7] | |
| TRPML3 (MCOLN3) | human β-cell | RNA sequencing | [7] |
| human islets | RNA sequencing | [7] | |
| TRPP1 (PKD2) | human β-cell | RNA sequencing | [7] |
| human islet | RNA sequencing | [7] | |
| TRPA1 | INS-1 | pharmacological tool shRNA | [147,151] |
| mouse islet | pharmacological tool | [147] | |
| RINm5F, rat islet | RT-PCR, WB, siRNA | [32,146] | |
| rat β-cell | IF, EP | [146] | |
| rat islet | pharmacological tool, WB, RT-PCR, EP | [150,151] |
Funding
Acknowledgments
Conflicts of Interest
References
- Islam, M.S.; Gustafsson, A.J. Islets of Langerhans: Cellular structure and physiology. In Chronic Allograft Failure: Natural History, Pathogenesis, Diagnosis and Management; Ahsan, N., Ed.; Landes Bioscience: Austin, TX, USA, 2007; pp. 229–232. [Google Scholar]
- Islam, M.S. Stimulus-Secretion Coupling in Beta-Cells: From Basic to Bedside. Adv. Exp Med. Biol. 2020, 1131, 943–963. [Google Scholar] [PubMed]
- Islam, M.S. Calcium Signaling in the Islets. In Islets of Langerhans, 2nd ed.; Islam, M.S., Ed.; Springer: Dordrecht, The Netherlands, 2014; pp. 605–632. [Google Scholar]
- Drews, G.; Krippeit-Drews, P.; Düfer, M. Electrophysiology of Islet Cells. In Islets of Langerhans, 2nd ed.; Islam, M.S., Ed.; Springer: Dordrecht, The Netherlands, 2015; Volume 1, pp. 249–303. [Google Scholar]
- Philippaert, K.; Vennekens, R. The Role of TRP Channels in the Pancreatic Beta-Cell. In Neurobiology of TRP Channels, 2nd ed.; Emir, T.L.R., Ed.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2017; pp. 229–250. [Google Scholar]
- Islam, M.S. TRP channels of islets. Adv. Exp. Med. Biol. 2011, 704, 811–830. [Google Scholar] [PubMed]
- Marabita, F.; Islam, M.S. Expression of Transient Receptor Potential Channels in the Purified Human Pancreatic beta-Cells. Pancreas 2017, 46, 97–101. [Google Scholar] [CrossRef]
- Roe, M.W.; Worley, J.F., 3rd; Qian, F.; Tamarina, N.; Mittal, A.A.; Dralyuk, F.; Blair, N.T.; Mertz, R.J.; Philipson, L.H.; Dukes, I.D. Characterization of a Ca2+ release-activated nonselective cation current regulating membrane potential and [Ca2+]i oscillations in transgenically derived beta-cells. J. Biol. Chem. 1998, 273, 10402–10410. [Google Scholar] [CrossRef]
- Sakura, H.; Ashcroft, F.M. Identification of four trp1 gene variants murine pancreatic beta-cells. Diabetologia 1997, 40, 528–532. [Google Scholar] [CrossRef] [PubMed]
- Sabourin, J.; Le Gal, L.; Saurwein, L.; Haefliger, J.A.; Raddatz, E.; Allagnat, F. Store-operated Ca2+ Entry Mediated by Orai1 and TRPC1 Participates to Insulin Secretion in Rat beta-Cells. J. Biol. Chem. 2015, 290, 30530–30539. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zhang, Z.M. Comparative identification of Ca2+ channel expression in INS-1 and rat pancreatic beta cells. World J. Gastroenterol. 2009, 15, 3046–3050. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, W.; Cui, W.; Shi, B.; Wang, H. PKCα promotes insulin secretion via TRPC1 phosphorylation in INS-1E cells. Biosci. Biotechnol. Biochem. 2019, 83, 1676–1682. [Google Scholar] [CrossRef]
- Sabourin, J.; Allagnat, F. Store-operated Ca2+ entry: A key component of the insulin secretion machinery. J. Mol. Endocrinol. 2016, 57, F35–F39. [Google Scholar] [CrossRef]
- Ambudkar, I.S.; de Souza, L.B.; Ong, H.L. TRPC1, Orai1, and STIM1 in SOCE: Friends in tight spaces. Cell Calcium 2017, 63, 33–39. [Google Scholar] [CrossRef]
- Zeng, W.; Yuan, J.P.; Kim, M.S.; Choi, Y.J.; Huang, G.N.; Worley, P.F.; Muallem, S. STIM1 gates TRPC channels, but not Orai1, by electrostatic interaction. Mol. Cell 2008, 32, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Kono, T.; Tong, X.; Taleb, S.; Bone, R.N.; Iida, H.; Lee, C.C.; Sohn, P.; Gilon, P.; Roe, M.W.; Evans-Molina, C. Impaired Store-Operated Calcium Entry and STIM1 Loss Lead to Reduced Insulin Secretion and Increased Endoplasmic Reticulum Stress in the Diabetic beta-Cell. Diabetes 2018, 67, 2293–2304. [Google Scholar] [CrossRef] [PubMed]
- Mori, Y.; Otabe, S.; Dina, C.; Yasuda, K.; Populaire, C.; Lecoeur, C.; Vatin, V.; Durand, E.; Hara, K.; Okada, T.; et al. Genome-wide search for type 2 diabetes in Japanese affected sib-pairs confirms susceptibility genes on 3q, 15q, and 20q and identifies two new candidate Loci on 7p and 11p. Diabetes 2002, 51, 1247–1255. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, F.; Ochiai, Y.; Serizawa, M.; Yanai, K.; Kuzuya, N.; Kajio, H.; Honjo, S.; Takeda, N.; Kaburagi, Y.; Yasuda, K.; et al. Search for type 2 diabetes susceptibility genes on chromosomes 1q, 3q and 12q. J. Hum. Genet. 2008, 53, 314–324. [Google Scholar] [CrossRef]
- Chen, K.; Jin, X.; Li, Q.; Wang, W.; Wang, Y.; Zhang, J. Association of TRPC1 gene polymorphisms with type 2 diabetes and diabetic nephropathy in Han Chinese population. Endocr. Res. 2013, 38, 59–68. [Google Scholar] [CrossRef]
- Yamada, H.; Yoshida, M.; Ito, K.; Dezaki, K.; Yada, T.; Ishikawa, S.; Kakei, M. Potentiation of Glucose-stimulated Insulin Secretion by the GPR40-PLC-TRPC Pathway in Pancreatic beta-Cells. Sci. Rep. 2016, 6, 9. [Google Scholar] [CrossRef]
- Hayes, H.L.; Moss, L.G.; Schisler, J.C.; Haldeman, J.M.; Zhang, Z.S.; Rosenberg, P.B.; Newgard, C.B.; Hohmeier, H.E. Pdx-1 Activates Islet alpha- and beta-Cell Proliferation via a Mechanism Regulated by Transient Receptor Potential Cation Channels 3 and 6 and Extracellular Signal-Regulated Kinases 1 and 2. Mol. Cell. Biol. 2013, 33, 4017–4029. [Google Scholar] [CrossRef]
- Park, S.H.; Ryu, S.Y.; Yu, W.J.; Han, Y.E.; Ji, Y.S.; Oh, K.; Sohn, J.W.; Lim, A.; Jeon, J.P.; Lee, H.; et al. Leptin promotes K-ATP channel trafficking by AMPK signaling in pancreatic beta-cells. Proc. Natl. Acad. Sci. USA 2013, 110, 12673–12678. [Google Scholar] [CrossRef]
- Srivastava, S.; Li, Z.; Soomro, I.; Sun, Y.; Wang, J.; Bao, L.; Coetzee, W.A.; Stanley, C.A.; Li, C.; Skolnik, E.Y. Regulation of KATP Channel Trafficking in Pancreatic beta-Cells by Protein Histidine Phosphorylation. Diabetes 2018, 67, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Wie, J.; Kim, J.; Ha, K.; Zhang, Y.H.; Jeon, J.H.; So, I. Dexamethasone activates transient receptor potential canonical 4 (TRPC4) channels via Rasd1 small GTPase pathway. Pflug. Arch. 2015, 467, 2081–2091. [Google Scholar] [CrossRef]
- Reale, V.; Hales, C.N.; Ashford, M.L. The effects of pyridine nucleotides on the activity of a calcium-activated nonselective cation channel in the rat insulinoma cell line, CRI-G1. J. Membr Biol 1994, 142, 299–307. [Google Scholar] [CrossRef]
- Herson, P.S.; Ashford, M.L. Activation of a novel non-selective cation channel by alloxan and H2O2 in the rat insulin-secreting cell line CRI-G1. J. Physiol. 1997, 501, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Sumoza-Toledo, A.; Penner, R. TRPM2: A multifunctional ion channel for calcium signalling. J. Physiol.-Lond. 2011, 589, 1515–1525. [Google Scholar] [CrossRef] [PubMed]
- Inamura, K.; Sano, Y.; Mochizuki, S.; Yokoi, H.; Miyake, A.; Nozawa, K.; Kitada, C.; Matsushime, H.; Furuichi, K. Response to ADP-ribose by activation of TRPM2 in the CRI-G1 insulinoma cell line. J. Membr. Biol. 2003, 191, 201–207. [Google Scholar] [CrossRef]
- Bari, M.R.; Akbar, S.; Eweida, M.; Kuhn, F.J.; Gustafsson, A.J.; Luckhoff, A.; Islam, M.S. H2O2-induced Ca2+ influx and its inhibition by N-(p-amylcinnamoyl) anthranilic acid in the beta-cells: Involvement of TRPM2 channels. J. Cell Mol. Med. 2009, 13, 3260–3267. [Google Scholar] [CrossRef]
- Hara, Y.; Wakamori, M.; Ishii, M.; Maeno, E.; Nishida, M.; Yoshida, T.; Yamada, H.; Shimizu, S.; Mori, E.; Kudoh, J.; et al. LTRPC2 Ca2+-permeable channel activated by changes in redox status confers susceptibility to cell death. Mol. Cell 2002, 9, 163–173. [Google Scholar] [CrossRef]
- Togashi, K.; Hara, Y.; Tominaga, T.; Higashi, T.; Konishi, Y.; Mori, Y.; Tominaga, M. TRPM2 activation by cyclic ADP-ribose at body temperature is involved in insulin secretion. Embo J. 2006, 25, 1804–1815. [Google Scholar] [CrossRef] [PubMed]
- Numazawa, S.; Takase, M.; Ahiko, T.; Ishii, M.; Shimizu, S.; Yoshida, T. Possible Involvement of Transient Receptor Potential Channels in Electrophile-Induced Insulin Secretion from RINm5F Cells. Biol. Pharm. Bull. 2012, 35, 346–354. [Google Scholar] [CrossRef][Green Version]
- Du, J.; Xie, J.; Yue, L. Intracellular calcium activates TRPM2 and its alternative spliced isoforms. Proc. Natl. Acad. Sci. USA 2009, 106, 7239–7244. [Google Scholar] [CrossRef]
- Lange, I.; Yamamoto, S.; Partida-Sanchez, S.; Mori, Y.; Fleig, A.; Penner, R. TRPM2 functions as a lysosomal Ca2+-release channel in beta cells. Sci. Signal. 2009, 2, ra23. [Google Scholar] [CrossRef]
- Qian, F.; Huang, P.; Ma, L.; Kuznetsov, A.; Tamarina, N.; Philipson, L.H. Candidates for nonselective cation channels and store-operated channels in insulin-secreting cells. Diabetes 2002, 51, S183–S189. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Chu, X.; Tong, Q.; Cheung, J.Y.; Conrad, K.; Masker, K.; Miller, B.A. A novel TRPM2 isoform inhibits calcium influx and susceptibility to cell death. J. Biol. Chem. 2003, 278, 16222–16229. [Google Scholar] [CrossRef] [PubMed]
- McHugh, D.; Flemming, R.; Xu, S.Z.; Perraud, A.L.; Beech, D.J. Critical intracellular Ca2+ dependence of transient receptor potential melastatin 2 (TRPM2) cation channel activation. J. Biol. Chem. 2003, 278, 11002–11006. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.H.; Roth, B.; Lu, W.; Du, J. Ligand recognition and gating mechanism through three ligand-binding sites of human TRPM2 channel. Elife 2019, 8, 18. [Google Scholar] [CrossRef]
- Yu, P.L.; Liu, Z.M.; Yu, X.F.; Ye, P.W.; Liu, H.; Xue, X.W.; Yang, L.X.; Li, Z.T.; Wu, Y.; Fang, C.; et al. Direct Gating of the TRPM2 Channel by cADPR via Specific Interactions with the ADPR Binding Pocket. Cell Rep. 2019, 27, 3684–3695. [Google Scholar] [CrossRef] [PubMed]
- Toth, B.; Iordanov, I.; Csanady, L. Ruling out pyridine dinucleotides as true TRPM2 channel activators reveals novel direct agonist ADP-ribose-2 ‘-phosphate. J. Gen. Physiol. 2015, 145, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Fliegert, R.; Bauche, A.; Wolf Perez, A.M.; Watt, J.M.; Rozewitz, M.D.; Winzer, R.; Janus, M.; Gu, F.; Rosche, A.; Harneit, A.; et al. 2’-Deoxyadenosine 5’-diphosphoribose is an endogenous TRPM2 superagonist. Nat. Chem. Biol. 2017, 13, 1036–1044. [Google Scholar] [CrossRef]
- Perraud, A.L.; Takanishi, C.L.; Shen, B.; Kang, S.; Smith, M.K.; Schmitz, C.; Knowles, H.M.; Ferraris, D.; Li, W.; Zhang, J.; et al. Accumulation of free ADP-ribose from mitochondria mediates oxidative stress-induced gating of TRPM2 cation channels. J. Biol. Chem. 2005, 280, 6138–6148. [Google Scholar] [CrossRef]
- Biden, T.J.; Schmitz-Peiffer, C.; Burchfield, J.G.; Gurisik, E.; Cantley, J.; Mitchell, C.J.; Carpenter, L. The diverse roles of protein kinase C in pancreatic beta-cell function. Biochem. Soc. Trans. 2008, 36, 916–919. [Google Scholar] [CrossRef]
- Starkus, J.G.; Poerzgen, P.; Layugan, K.; Kawabata, K.G.; Goto, J.I.; Suzuki, S.; Myers, G.; Kelly, M.; Penner, R.; Fleig, A.; et al. Scalaradial Is a Potent Inhibitor of Transient Receptor Potential Melastatin 2 (TRPM2) Ion Channels. J. Nat. Prod. 2017, 80, 2741–2750. [Google Scholar] [CrossRef]
- Fourgeaud, L.; Dvorak, C.; Faouzi, M.; Starkus, J.; Sandeo, S.; Wang, Q.; Lord, B.; Coate, H.; Taylor, N.; He, Y.B.; et al. Pharmacology of JNJ-28583113: A novel TRPM2 antagonist. Eur. J. Pharmacol. 2019, 853, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, H.; Luo, X.; Wang, Y.X.; Liu, Y.; Jin, H.W.; Liu, Z.M.; Yang, W.; Yu, P.L.; Zhang, L.R.; et al. Design, synthesis and biological activities of 2,3-dihydroquinazolin-4(1H)-one derivatives as TRPM2 inhibitors. Eur. J. Med. Chem. 2018, 152, 235–252. [Google Scholar] [CrossRef] [PubMed]
- Kheradpezhouh, E.; Barritt, G.J.; Rychkov, G.Y. Curcumin inhibits activation of TRPM2 channels in rat hepatocytes. Redox Biol. 2016, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Heiner, I.; Eisfeld, J.; Warnstedt, M.; Radukina, N.; Jungling, E.; Luckhoff, A. Endogenous ADP-ribose enables calcium-regulated cation currents through TRPM2 channels in neutrophil granulocytes. Biochem J. 2006, 398, 225–232. [Google Scholar] [CrossRef]
- Kashio, M.; Tominaga, M. Redox Signal-mediated Enhancement of the Temperature Sensitivity of Transient Receptor Potential Melastatin 2 (TRPM2) Elevates Glucose-induced Insulin Secretion from Pancreatic Islets. J. Biol. Chem. 2015, 290, 12435–12442. [Google Scholar] [CrossRef]
- Uchida, K.; Dezaki, K.; Damdindorj, B.; Inada, H.; Shiuchi, T.; Mori, Y.; Yada, T.; Minokoshi, Y.; Tominaga, M. Lack of TRPM2 impaired insulin secretion and glucose metabolisms in mice. Diabetes 2011, 60, 119–126. [Google Scholar] [CrossRef]
- Pang, B.; Kim, S.; Li, D.; Ma, Z.; Sun, B.; Zhang, X.; Wu, Z.; Chen, L. Glucagon-like peptide-1 potentiates glucose-stimulated insulin secretion via the transient receptor potential melastatin 2 channel. Exp. Ther. Med. 2017, 14, 5219–5227. [Google Scholar] [CrossRef] [PubMed]
- Yosida, M.; Dezaki, K.; Uchida, K.; Kodera, S.; Lam, N.V.; Ito, K.; Rita, R.S.; Yamada, H.; Shimomura, K.; Ishikawa, S.; et al. Involvement of cAMP/EPAC/TRPM2 Activation in Glucose- and Incretin-Induced Insulin Secretion. Diabetes 2014, 63, 3394–3403. [Google Scholar] [CrossRef]
- Kim, B.J.; Park, K.H.; Yim, C.Y.; Takasawa, S.; Okamoto, H.; Im, M.J.; Kim, U.H. Generation of nicotinic acid adenine dinucleotide phosphate and cyclic ADP-ribose by glucagon-like peptide-1 evokes Ca2+ signal that is essential for insulin secretion in mouse pancreatic islets. Diabetes 2008, 57, 868–878. [Google Scholar] [CrossRef]
- Ito, K.; Dezaki, K.; Yoshida, M.; Yamada, H.; Miura, R.; Rita, R.S.; Ookawara, S.; Tabei, K.; Kawakami, M.; Hara, K.; et al. Endogenous alpha 2A-Adrenoceptor-Operated Sympathoadrenergic Tones Attenuate Insulin Secretion via cAlViP/TRPhfi2 Signaling. Diabetes 2017, 66, 699–709. [Google Scholar] [CrossRef]
- Kurashina, T.; Dezaki, K.; Yoshida, M.; Rita, R.S.; Ito, K.; Taguchi, M.; Miura, R.; Tominaga, M.; Ishibashi, S.; Kakei, M.; et al. The beta-cell GHSR and downstream cAMP/TRPM2 signaling account for insulinostatic and glycemic effects of ghrelin. Sci. Rep. 2015, 5, 13. [Google Scholar] [CrossRef]
- Du, J.; Xie, J.; Yue, L. Modulation of TRPM2 by acidic pH and the underlying mechanisms for pH sensitivity. J. Gen. Physiol. 2009, 134, 471–488. [Google Scholar] [CrossRef] [PubMed]
- Juntti-Berggren, L.; Civelek, V.N.; Berggren, P.O.; Schultz, V.; Corkey, B.E.; Tornheim, K. Glucose-stimulated increase in cytoplasmic pH precedes increase in free Ca2+ in pancreatic beta-cells. A possible role for pyruvate. J. Biol. Chem. 1994, 269, 14391–14395. [Google Scholar] [PubMed]
- Gylfe, E.; Hellman, B. The heat production of pancreatic beta-cells stimulated by glucose. Acta Physiol. Scand. 1975, 93, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Silva-Alves, J.M.; Mares-Guia, T.R.; Oliveira, J.S.; Costa-Silva, C.; Bretz, P.; Araujo, S.; Ferreira, E.; Coimbra, C.; Sogayar, M.C.; Reis, R.; et al. Glucose-induced heat production, insulin secretion and lactate production in isolated Wistar rat pancreatic islets. Thermochim. Acta. 2008, 474, 67–71. [Google Scholar] [CrossRef]
- Ohta, M.; Nelson, D.; Nelson, J.; Meglasson, M.D.; Erecinska, M. Oxygen and temperature dependence of stimulated insulin secretion in isolated rat islets of Langerhans. J. Biol. Chem. 1990, 265, 17525–17532. [Google Scholar] [PubMed]
- Rajagopal, M.C.; Brown, J.W.; Gelda, D.; Valavala, K.V.; Wang, H.; Llano, D.A.; Gillette, R.; Sinha, S. Transient heat release during induced mitochondrial proton uncoupling. Commun. Biol. 2019, 2, 6. [Google Scholar] [CrossRef]
- Ishii, M.; Hagiwara, T.; Mori, Y.; Shimizu, S. Involvement of TRPM2 and L-type Ca2+ channels in Ca2+ entry and cell death induced by hydrogen peroxide in rat beta-cell line RIN-5F. J. Toxicol. Sci. 2014, 39, 199–209. [Google Scholar] [CrossRef]
- Herson, P.S.; Lee, K.; Pinnock, R.D.; Hughes, J.; Ashford, M.L. Hydrogen peroxide induces intracellular calcium overload by activation of a non-selective cation channel in an insulin-secreting cell line. J. Biol. Chem. 1999, 274, 833–841. [Google Scholar] [CrossRef]
- Fonfria, E.; Marshall, I.C.; Boyfield, I.; Skaper, S.D.; Hughes, J.P.; Owen, D.E.; Zhang, W.; Miller, B.A.; Benham, C.D.; McNulty, S. Amyloid beta-peptide(1-42) and hydrogen peroxide-induced toxicity are mediated by TRPM2 in rat primary striatal cultures. J. Neurochem. 2005, 95, 715–723. [Google Scholar] [CrossRef]
- Eizirik, D.L.; Pipeleers, D.G.; Ling, Z.; Welsh, N.; Hellerstrom, C.; Andersson, A. Major species differences between humans and rodents in the susceptibility to pancreatic beta-cell injury. Proc. Natl. Acad. Sci. USA 1994, 91, 9253–9256. [Google Scholar] [CrossRef] [PubMed]
- Li, F.F.; Munsey, T.S.; Sivaprasadarao, A. TRPM2-mediated rise in mitochondrial Zn2+ promotes palmitate-induced mitochondrial fission and pancreatic beta-cell death in rodents. Cell Death Differ. 2017, 24, 1999–2012. [Google Scholar] [CrossRef]
- Scharenberg, A.M. TRPM2 and pancreatic beta-cell responses to oxidative stress. Islets 2009, 1, 165–166. [Google Scholar] [CrossRef] [PubMed]
- Manna, P.T.; Munsey, T.S.; Abuarab, N.; Li, F.F.; Asipu, A.; Howell, G.; Sedo, A.; Yang, W.; Naylor, J.; Beech, D.J.; et al. TRPM2-mediated intracellular Zn2+ release triggers pancreatic beta-cell death. Biochem. J. 2015, 466, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Mirnikjoo, B.; Balasubramanian, K.; Schroit, A.J. Mobilization of lysosomal calcium regulates the externalization of phosphatidylserine during apoptosis. J. Biol. Chem. 2009, 284, 6918–6923. [Google Scholar] [CrossRef]
- Oberwinkler, J.; Lis, A.; Giehl, K.M.; Flockerzi, V.; Philipp, S.E. Alternative splicing switches the divalent cation selectivity of TRPM3 channels. J. Biol. Chem. 2005, 280, 22540–22548. [Google Scholar] [CrossRef]
- Wagner, T.F.; Loch, S.; Lambert, S.; Straub, I.; Mannebach, S.; Mathar, I.; Dufer, M.; Lis, A.; Flockerzi, V.; Philipp, S.E.; et al. Transient receptor potential M3 channels are ionotropic steroid receptors in pancreatic beta cells. Nat. Cell Biol. 2008, 10, 1421–1430. [Google Scholar] [CrossRef]
- Held, K.; Kichko, T.; De Clercq, K.; Klaassen, H.; Van Bree, R.; Vanherck, J.C.; Marchand, A.; Reeh, P.W.; Chaltin, P.; Voets, T.; et al. Activation of TRPM3 by a potent synthetic ligand reveals a role in peptide release. Proc. Natl. Acad. Sci. USA 2015, 112, E1363–E1372. [Google Scholar] [CrossRef]
- Klose, C.; Straub, I.; Riehle, M.; Ranta, F.; Krautwurst, D.; Ullrich, S.; Meyerhof, W.; Harteneck, C. Fenamates as TRP channel blockers: Mefenamic acid selectively blocks TRPM3. Br. J. Pharmacol. 2011, 162, 1757–1769. [Google Scholar] [CrossRef]
- Mayer, S.I.; Muller, I.; Mannebach, S.; Endo, T.; Thiel, G. Signal Transduction of Pregnenolone Sulfate in Insulinoma Cells ACTIVATION OF EGR-1 EXPRESSION INVOLVING TRPM3, VOLTAGE-GATED CALCIUM CHANNELS, ERK, AND TERNARY COMPLEX FACTORS. J. Biol. Chem. 2011, 286, 10084–10096. [Google Scholar] [CrossRef]
- Grimm, C.; Kraft, R.; Sauerbruch, S.; Schultz, G.; Harteneck, C. Molecular and functional characterization of the melastatin-related cation channel TRPM3. J. Biol. Chem. 2003, 278, 21493–21501. [Google Scholar] [CrossRef]
- Badheka, D.; Borbiro, I.; Rohacs, T. Transient receptor potential melastatin 3 is a phosphoinositide-dependent ion channel. J. Gen. Physiol. 2015, 146, 65–77. [Google Scholar] [CrossRef]
- Xie, B.C.; Nguyen, P.M.; Gucek, A.; Thonig, A.; Barg, S.; Idevall-Hagren, O. Plasma Membrane Phosphatidylinositol 4,5-Bisphosphate Regulates Ca2+-Influx and Insulin Secretion from Pancreatic beta Cells. Cell Chem. Biol. 2016, 23, 816–826. [Google Scholar] [CrossRef]
- Wagner, T.F.; Drews, A.; Loch, S.; Mohr, F.; Philipp, S.E.; Lambert, S.; Oberwinkler, J. TRPM3 channels provide a regulated influx pathway for zinc in pancreatic beta cells. Pflug. Arch. 2010, 460, 755–765. [Google Scholar] [CrossRef] [PubMed]
- Vriens, J.; Owsianik, G.; Hofmann, T.; Philipp, S.E.; Stab, J.; Chen, X.D.; Benoit, M.; Xue, F.Q.; Janssens, A.; Kerselaers, S.; et al. TRPM3 Is a Nociceptor Channel Involved in the Detection of Noxious Heat. Neuron 2011, 70, 482–494. [Google Scholar] [CrossRef] [PubMed]
- Krugel, U.; Straub, I.; Beckmann, H.; Schaefer, M. Primidone inhibits TRPM3 and attenuates thermal nociception in vivo. Pain 2017, 158, 856–867. [Google Scholar] [CrossRef] [PubMed]
- Guinamard, R.; Salle, L.; Simard, C. The non-selective monovalent cationic channels TRPM4 and TRPM5. Adv. Exp. Med. Biol. 2011, 704, 147–171. [Google Scholar] [PubMed]
- Sturgess, N.C.; Hales, C.N.; Ashford, M.L. Inhibition of a calcium-activated, non-selective cation channel, in a rat insulinoma cell line, by adenine derivatives. Febs Lett. 1986, 208, 397–400. [Google Scholar] [CrossRef]
- Nilius, B.; Prenen, J.; Voets, T.; Droogmans, G. Intracellular nucleotides and polyamines inhibit the Ca2+-activated cation channel TRPM4b. Pflug. Arch. 2004, 448, 70–75. [Google Scholar] [CrossRef]
- Leech, C.A.; Habener, J.F. A role for Ca2+-sensitive nonselective cation channels in regulating the membrane potential of pancreatic beta-cells. Diabetes 1998, 47, 1066–1073. [Google Scholar] [CrossRef]
- Cho, C.H.; Lee, Y.S.; Kim, E.; Hwang, E.M.; Park, J.Y. Physiological functions of the TRPM4 channels via protein interactions. Bmb Rep. 2015, 48, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Demion, M.; Bois, P.; Launay, P.; Guinamard, R. TRPM4, a Ca2+-activated nonselective cation channel in mouse sino-atrial node cells. Cardiovasc. Res. 2007, 73, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Sturgess, N.C.; Kozlowski, R.Z.; Carrington, C.A.; Hales, C.N.; Ashford, M.L. Effects of sulphonylureas and diazoxide on insulin secretion and nucleotide-sensitive channels in an insulin-secreting cell line. Br. J. Pharm. 1988, 95, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Nilius, B.; Prenen, J.; Tang, J.S.; Wang, C.B.; Owsianik, G.; Janssens, A.; Voets, T.; Zhu, M.X. Regulation of the Ca2+ sensitivity of the nonselective cation channel TRPM4. J. Biol. Chem. 2005, 280, 6423–6433. [Google Scholar] [CrossRef]
- Nilius, B.; Mahieu, F.; Prenen, J.; Janssens, A.; Owsianik, G.; Vennekens, R.; Voets, T. The Ca2+-activated cation channel TRPM4 is regulated by phosphatidylinositol 4,5-biphosphate. Embo. J. 2006, 25, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Thore, S.; Wuttke, A.; Tengholm, A. Rapid turnover of phosphatidylinositol-4,5-bisphosphate in insulin-secreting cells mediated by Ca2+ and the ATP-to-ADP ratio. Diabetes 2007, 56, 818–826. [Google Scholar] [CrossRef][Green Version]
- Marigo, V.; Courville, K.; Hsu, W.H.; Feng, J.M.; Cheng, H. TRPM4 impacts on Ca2+ signals during agonist-induced insulin secretion in pancreatic beta-cells. Mol. Cell Endocrinol. 2009, 299, 194–203. [Google Scholar] [CrossRef]
- Nelson, P.L.; Zolochevska, O.; Figueiredo, M.L.; Soliman, A.; Hsu, W.H.; Feng, J.M.; Zhang, H.; Cheng, H. Regulation of Ca2+-entry in pancreatic alpha-cell line by transient receptor potential melastatin 4 plays a vital role in glucagon release. Mol. Cell. Endocrinol. 2011, 335, 126–134. [Google Scholar] [CrossRef]
- Cheng, H.; Beck, A.; Launay, P.; Gross, S.A.; Stokes, A.J.; Kinet, J.P.; Fleig, A.; Penner, R. TRPM4 controls insulin secretion in pancreatic beta-cells. Cell Calcium 2007, 41, 51–61. [Google Scholar] [CrossRef]
- Ma, Z.; Bjorklund, A.; Islam, M.S. A TRPM4 Inhibitor 9-Phenanthrol Inhibits Glucose- and Glucagon-Like Peptide 1-Induced Insulin Secretion from Rat Islets of Langerhans. J. Diabetes Res. 2017, 2017, 5131785. [Google Scholar] [CrossRef]
- Shigeto, M.; Ramracheya, R.; Tarasov, A.I.; Cha, C.Y.; Chibalina, M.V.; Hastoy, B.; Philippaert, K.; Reinbothe, T.; Rorsman, N.; Salehi, A.; et al. GLP-1 stimulates insulin secretion by PKC-dependent TRPM4 and TRPM5 activation. J. Clin. Investig. 2015, 125, 4714–4728. [Google Scholar] [CrossRef] [PubMed]
- Vennekens, R.; Olausson, J.; Meissner, M.; Bloch, W.; Mathar, I.; Philipp, S.E.; Schmitz, F.; Weissgerber, P.; Nilius, B.; Flockerzi, V.; et al. Increased IgE-dependent mast cell activation and anaphylactic responses in mice lacking the calcium-activated nonselective cation channel TRPM4. Nat. Immunol. 2007, 8, 312–320. [Google Scholar] [CrossRef]
- Farashi, S.; Sasanpour, P.; Rafii-Tabar, H. The role of the transient receptor potential melastatin5 (TRPM5) channels in the pancreatic beta-cell electrical activity: A computational modeling study. Comput. Biol. Chem. 2018, 76, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Colsoul, B.; Schraenen, A.; Lemaire, K.; Quintens, R.; Van Lommel, L.; Segal, A.; Owsianik, G.; Talavera, K.; Voets, T.; Margolskee, R.F.; et al. Loss of high-frequency glucose-induced Ca2+ oscillations in pancreatic islets correlates with impaired glucose tolerance in Trpm5-/- mice. Proc. Natl. Acad. Sci. USA 2010, 107, 5208–5213. [Google Scholar] [CrossRef] [PubMed]
- Oike, H.; Wakamori, M.; Mori, Y.; Nakanishi, H.; Taguchi, R.; Misaka, T.; Matsumoto, I.; Abe, K. Arachidonic acid can function as a signaling modulator by activating the TRPM5 cation channel in taste receptor cells. Biochim. Biophys. Acta. 2006, 1761, 1078–1084. [Google Scholar] [CrossRef] [PubMed]
- Wolf, B.A.; Turk, J.; Sherman, W.R.; McDaniel, M.L. Intracellular Ca2+ mobilization by arachidonic acid. Comparison with myo-inositol 1,4,5-trisphosphate in isolated pancreatic islets. J. Biol Chem 1986, 261, 3501–3511. [Google Scholar]
- Liu, D.; Liman, E.R. Intracellular Ca2+ and the phospholipid PIP2 regulate the taste transduction ion channel TRPM5. Proc. Natl. Acad. Sci. USA 2003, 100, 15160–15165. [Google Scholar] [CrossRef]
- Philippaert, K.; Pironet, A.; Mesuere, M.; Sones, W.; Vermeiren, L.; Kerselaers, S.; Pinto, S.; Segal, A.; Antoine, N.; Gysemans, C.; et al. Steviol glycosides enhance pancreatic beta-cell function and taste sensation by potentiation of TRPM5 channel activity. Nat. Commun. 2017, 8, 14733. [Google Scholar] [CrossRef]
- Ivask, M.; Hugill, A.; Koks, S. RNA-sequencing of WFS1-deficient pancreatic islets. Physiol. Rep. 2016, 4, 12. [Google Scholar] [CrossRef]
- Kyriazis, G.A.; Soundarapandian, M.M.; Tyrberg, B. Sweet taste receptor signaling in beta cells mediates fructose-induced potentiation of glucose-stimulated insulin secretion. Proc. Natl. Acad. Sci. USA 2012, 109, E524–E532. [Google Scholar] [CrossRef]
- Larsson, M.H.; Hakansson, P.; Jansen, F.P.; Magnell, K.; Brodin, P. Ablation of TRPM5 in Mice Results in Reduced Body Weight Gain and Improved Glucose Tolerance and Protects from Excessive Consumption of Sweet Palatable Food when Fed High Caloric Diets. PLoS ONE 2015, 10, 19. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Glendinning, J.I.; Gillman, J.; Zamer, H.; Margolskee, R.F.; Sclafani, A. The role of T1r3 and Trpm5 in carbohydrate-induced obesity in mice. Physiol. Behav. 2012, 107, 50–58. [Google Scholar] [CrossRef]
- Colsoul, B.; Jacobs, G.; Philippaert, K.; Owsianik, G.; Segal, A.; Nilius, B.; Voets, T.; Schuit, F.; Vennekens, R. Insulin downregulates the expression of the Ca2+-activated nonselective cation channel TRPM5 in pancreatic islets from leptin-deficient mouse models. Pflug. Arch. 2014, 466, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Ketterer, C.; Mussig, K.; Heni, M.; Dudziak, K.; Randrianarisoa, E.; Wagner, R.; Machicao, F.; Stefan, N.; Holst, J.J.; Fritsche, A.; et al. Genetic variation within the TRPM5 locus associates with prediabetic phenotypes in subjects at increased risk for type 2 diabetes. Metabolism 2011, 60, 1325–1333. [Google Scholar] [CrossRef] [PubMed]
- Tabur, S.; Oztuzcu, S.; Duzen, I.V.; Eraydin, A.; Eroglu, S.; Ozkaya, M.; Demiryurek, A.T. Role of the transient receptor potential (TRP) channel gene expressions and TRP melastatin (TRPM) channel gene polymorphisms in obesity-related metabolic syndrome. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 1388–1397. [Google Scholar]
- Gommers, L.M.M.; Hill, T.G.; Ashcroft, F.M.; de Baaij, J.H.F. Low extracellular magnesium does not impair glucose-stimulated insulin secretion. PLoS ONE 2019, 14, 13. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, C.; Perraud, A.L.; Johnson, C.O.; Inabe, K.; Smith, M.K.; Penner, R.; Kurosaki, T.; Fleig, A.; Scharenberg, A.M. Regulation of vertebrate cellular Mg2+ homeostasis by TRPM7. Cell 2003, 114, 191–200. [Google Scholar] [CrossRef]
- Abiria, S.A.; Krapivinsky, G.; Sah, R.; Santa-Cruz, A.G.; Chaudhuri, D.; Zhang, J.; Adstamongkonkul, P.; DeCaen, P.G.; Clapham, D.E. TRPM7 senses oxidative stress to release Zn2+ from unique intracellular vesicles. Proc. Natl. Acad. Sci. USA 2017, 114, E6079–E6088. [Google Scholar] [CrossRef]
- Monteilh-Zoller, M.K.; Hermosura, M.C.; Nadler, M.J.; Scharenberg, A.M.; Penner, R.; Fleig, A. TRPM7 provides an ion channel mechanism for cellular entry of trace metal ions. J. Gen. Physiol. 2003, 121, 49–60. [Google Scholar] [CrossRef]
- Chen, Y.W.; Yang, C.Y.; Huang, C.F.; Hung, D.Z.; Leung, Y.M.; Liu, S.H. Heavy metals, islet function and diabetes development. Islets 2009, 1, 169–176. [Google Scholar] [CrossRef]
- Romero, J.R.; Castonguay, A.J.; Barton, N.S.; Germer, S.; Martin, M.; Zee, R.Y.L. Gene variation of the transient receptor potential cation channel, subfamily M, members 6 (TRPM6) and 7 (TRPM7), and type 2 diabetes mellitus: A case-control study. Transl. Res. 2010, 156, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Fagelskiold, A.J.; Kannisto, K.; Bostrom, A.; Hadrovic, B.; Farre, C.; Eweida, M.; Wester, K.; Islam, M.S. Insulin-secreting INS-1E cells express functional TRPV1 channels. Islets 2012, 4, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Rebellato, P.; Islam, M.S. [6]-shogaol induces Ca(2)(+) signals by activating the TRPV1 channels in the rat insulinoma INS-1E cells. JOP 2014, 15, 33–37. [Google Scholar] [PubMed]
- Diaz-Garcia, C.M.; Morales-Lazaro, S.L.; Sanchez-Soto, C.; Velasco, M.; Rosenbaum, T.; Hiriart, M. Role for the TRPV1 Channel in Insulin Secretion from Pancreatic Beta Cells. J. Membr. Biol. 2014, 247, 479–491. [Google Scholar] [CrossRef] [PubMed]
- Gram, D.X.; Ahren, B.; Nagy, I.; Olsen, U.B.; Brand, C.L.; Sundler, F.; Tabanera, R.; Svendsen, O.; Carr, R.D.; Santha, P.; et al. Capsaicin-sensitive sensory fibers in the islets of Langerhans contribute to defective insulin secretion in Zucker diabetic rat, an animal model for some aspects of human type 2 diabetes. Eur. J. Neurosci. 2007, 25, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Razavi, R.; Chan, Y.; Afifiyan, F.N.; Liu, X.J.; Wan, X.; Yantha, J.; Tsui, H.; Tang, L.; Tsai, S.; Santamaria, P.; et al. TRPV1+ sensory neurons control beta cell stress and islet inflammation in autoimmune diabetes. Cell 2006, 127, 1123–1135. [Google Scholar] [CrossRef] [PubMed]
- Akiba, Y.; Kato, S.; Katsube, K.; Nakamura, M.; Takeuchi, K.; Ishii, H.; Hibi, T. Transient receptor potential vanilloid subfamily 1 expressed in pancreatic islet beta cells modulates insulin secretion in rats. Biochem Biophys Res. Commun. 2004, 321, 219–225. [Google Scholar] [CrossRef]
- Mori, N.; Kurata, M.; Yamazaki, H.; Matsumura, S.; Hashimoto, T.; Kanazawa, K.; Nadamoto, T.; Inoue, K.; Fushiki, T. Allyl isothiocyanate increases carbohydrate oxidation through enhancing insulin secretion by TRPV1. Biosci. Biotechnol. Biochem. 2018, 82, 698–708. [Google Scholar] [CrossRef]
- Sadeh, M.; Glazer, B.; Landau, Z.; Wainstein, J.; Bezaleli, T.; Dabby, R.; Hanukoglu, A.; Boaz, M.; Leshinsky-Silver, E. Association of the M315I Variant in the Transient Receptor Potential Vanilloid Receptor-1 (TRPV1) Gene with Type 1 Diabetes in an Ashkenazi Jewish Population. Isr. Med. Assoc. J. 2013, 15, 477–480. [Google Scholar]
- Zhong, B.H.; Ma, S.T.; Wang, D.H. TRPV1 Mediates Glucose-induced Insulin Secretion Through Releasing Neuropeptides. In Vivo 2019, 33, 1431–1437. [Google Scholar] [CrossRef]
- Karam, J.B.; Cai, W.K.; Mohamed, R.; Huang, T.W.; Meng, L.Q.; Homan, E.P.; Dirice, E.; Kahn, C.R.; El Ouaamari, A. TRPV1 neurons regulate beta-cell function in a sex-dependent manner. Mol. Metab. 2018, 18, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, S.; Scheurink, A.J.; Steffens, A.B.; Ahren, B. Involvement of capsaicin-sensitive nerves in regulation of insulin secretion and glucose tolerance in conscious mice. Am. J. Physiol. 1994, 267, R1071–R1077. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.J.; Yan, Z.C.; Zhong, J.; Chen, J.; Ni, Y.X.; Li, L.; Ma, L.Q.; Zhao, Z.G.; Liu, D.Y.; Zhu, Z.M. Transient Receptor Potential Vanilloid 1 Activation Enhances Gut Glucagon-Like Peptide-1 Secretion and Improves Glucose Homeostasis. Diabetes 2012, 61, 2155–2165. [Google Scholar] [CrossRef]
- Lee, E.; Jung, D.Y.; Kim, J.H.; Patel, P.R.; Hu, X.; Lee, Y.; Azuma, Y.; Wang, H.F.; Tsitsilianos, N.; Shafiq, U.; et al. Transient receptor potential vanilloid type-1 channel regulates diet-induced obesity, insulin resistance, and leptin resistance. Faseb J. 2015, 29, 3182–3192. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Zhang, X.; Lee, N.R.; Jin, H.S. TRPV1 Gene Polymorphisms Are Associated with Type 2 Diabetes by Their Interaction with Fat Consumption in the Korean Genome Epidemiology Study. J. Nutrigenet. Nutr. 2016, 9, 47–61. [Google Scholar] [CrossRef]
- Sawatani, T.; Kaneko, Y.K.; Doutsu, I.; Ogawa, A.; Ishikawa, T. TRPV2 channels mediate insulin secretion induced by cell swelling in mouse pancreatic beta-cells. Am. J. Physiol.-Cell Physiol. 2019, 316, C434–C443. [Google Scholar] [CrossRef]
- Aoyagi, K.; Ohara-Imaizumi, M.; Nishiwaki, C.; Nakamichi, Y.; Nagamatsu, S. Insulin/phosphoinositide 3-kinase pathway accelerates the glucose-induced first-phase insulin secretion through TrpV2 recruitment in pancreatic beta-cells. Biochem. J. 2010, 432, 375–386. [Google Scholar] [CrossRef]
- Hisanaga, E.; Nagasawa, M.; Ueki, K.; Kulkarni, R.N.; Mori, M.; Kojima, I. Regulation of calcium-permeable TRPV2 channel by insulin in pancreatic beta-cells. Diabetes 2009, 58, 174–184. [Google Scholar] [CrossRef]
- Lin, Y.; Sun, Z.J. Antiaging Gene Klotho Enhances Glucose-Induced Insulin Secretion by Up-Regulating Plasma Membrane Levels of TRPV2 in MIN6 beta-Cells. Endocrinology 2012, 153, 3029–3039. [Google Scholar] [CrossRef]
- Digel, I. Primary thermosensory events in cells. Adv. Exp. Med. Biol. 2011, 704, 451–468. [Google Scholar]
- Casas, S.; Novials, A.; Reimann, F.; Gomis, R.; Gribble, F.M. Calcium elevation in mouse pancreatic beta cells evoked by extracellular human islet amyloid polypeptide involves activation of the mechanosensitive ion channel TRPV4. Diabetologia 2008, 51, 2252–2262. [Google Scholar] [CrossRef]
- Skrzypski, M.; Kakkassery, M.; Mergler, S.; Grotzinger, C.; Khajavi, N.; Sassek, M.; Szczepankiewicz, D.; Wiedenmann, B.; Nowak, K.W.; Strowski, M.Z. Activation of TRPV4 channel in pancreatic INS-1E beta cells enhances glucose-stimulated insulin secretion via calcium-dependent mechanisms. Febs Lett. 2013, 587, 3281–3287. [Google Scholar] [CrossRef] [PubMed]
- Billert, M.; Skrzypski, M.; Sassek, M.; Szczepankiewicz, D.; Wojciechowicz, T.; Mergler, S.; Strowski, M.Z.; Nowak, K.W. TRPV4 regulates insulin mRNA expression and INS-1E cell death via ERK1/2 and NO-dependent mechanisms. Cell. Signal. 2017, 35, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Skrzypski, M.; Khajavi, N.; Mergler, S.; Szczepankiewicz, D.; Kolodziejski, P.A.; Metzke, D.; Wojciechowicz, T.; Billert, M.; Nowak, K.W.; Strowski, M.Z. TRPV6 channel modulates proliferation of insulin secreting INS-1E beta cell line. Biochim. Biophys. Acta.-Mol. Cell Res. 2015, 1853, 3202–3210. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, M.; Zhang, W.K.; Benvin, N.M.; Zhou, X.; Su, D.; Li, H.; Wang, S.; Michailidis, I.E.; Tong, L.; Li, X.; et al. Structural basis of dual Ca(2+)/pH regulation of the endolysosomal TRPML1 channel. Nat. Struct. Mol. Biol. 2017, 24, 205–213. [Google Scholar] [CrossRef]
- Di Paola, S.; Scotto-Rosato, A.; Medina, D.L. TRPML1: The Ca((2+))retaker of the lysosome. Cell Calcium 2018, 69, 112–121. [Google Scholar] [CrossRef]
- Sun, M.; Goldin, E.; Stahl, S.; Falardeau, J.L.; Kennedy, J.C.; Acierno, J.S., Jr.; Bove, C.; Kaneski, C.R.; Nagle, J.; Bromley, M.C.; et al. Mucolipidosis type IV is caused by mutations in a gene encoding a novel transient receptor potential channel. Hum. Mol Genet. 2000, 9, 2471–2478. [Google Scholar] [CrossRef]
- Dong, X.P.; Wang, X.; Shen, D.; Chen, S.; Liu, M.; Wang, Y.; Mills, E.; Cheng, X.; Delling, M.; Xu, H. Activating mutations of the TRPML1 channel revealed by proline-scanning mutagenesis. J. Biol. Chem. 2009, 284, 32040–32052. [Google Scholar] [CrossRef]
- Nagata, K.; Zheng, L.; Madathany, T.; Castiglioni, A.J.; Bartles, J.R.; Garcia-Anoveros, J. The varitint-waddler (Va) deafness mutation in TRPML3 generates constitutive, inward rectifying currents and causes cell degeneration. Proc. Natl. Acad. Sci U S A 2008, 105, 353–358. [Google Scholar] [CrossRef]
- Busch, T.; Kottgen, M.; Hofherr, A. TRPP2 ion channels: Critical regulators of organ morphogenesis in health and disease. Cell Calcium 2017, 66, 25–32. [Google Scholar] [CrossRef]
- Meents, J.E.; Ciotu, C.I.; Fischer, M.J.M. TRPA1: A molecular view. J. Neurophysiol. 2019, 121, 427–443. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.S.; Zhong, L.L.; Hsieh, T.H.; Abooj, M.; Bishnoi, M.; Hughes, L.; Premkumar, L.S. Expression of Transient Receptor Potential Ankyrin 1 (TRPA1) and Its Role in Insulin Release from Rat Pancreatic Beta Cells. PLoS ONE 2012, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.Z.; Chen, X.J.; Cerne, R.; Syed, S.K.; Ficorilli, J.V.; Cabrera, O.; Obukhov, A.G.; Efanov, A.M. Catechol estrogens stimulate insulin secretion in pancreatic beta-cells via activation of the transient receptor potential A1 (TRPA1) channel. J. Biol. Chem. 2019, 294, 2935–2946. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Murali, K.Y.; Tandon, V.; Murthy, P.S.; Chandra, R. Insulinotropic effect of cinnamaldehyde on transcriptional regulation of pyruvate kinase, phosphoenolpyruvate carboxykinase, and GLUT4 translocation in experimental diabetic rats. Chem. Biol. Interact. 2010, 186, 72–81. [Google Scholar] [CrossRef]
- Babes, A.; Fischer, M.J.M.; Filipovic, M.; Engel, M.A.; Flonta, M.L.; Reeh, P.W. The anti-diabetic drug glibenclamide is an agonist of the transient receptor potential Ankyrin 1 (TRPA1) ion channel. Eur. J. Pharmacol. 2013, 704, 15–22. [Google Scholar] [CrossRef]
- Sulis, P.M.; Dambros, B.F.; Mascarello, A.; dos Santos, A.R.S.; Yunes, R.A.; Nunes, R.J.; Frederico, M.J.S.; Silva, F. Sulfonyl(thio)urea derivative induction of insulin secretion is mediated by potassium, calcium, and sodium channel signal transduction. J. Cell. Physiol. 2019, 234, 10138–10147. [Google Scholar] [CrossRef]
- Kong, X.C.; Tu, Y.F.; Li, B.F.; Zhang, L.M.; Feng, L.X.; Wang, L.X.; Zhang, L.; Zhou, H.R.; Hua, X.X.; Ma, X.S. Roux-en-Y gastric bypass enhances insulin secretion in type 2 diabetes via FXR-mediated TRPA1 expression. Mol. Metab. 2019, 29, 1–11. [Google Scholar] [CrossRef]
- Andersson, D.A.; Filipovic, M.R.; Gentry, C.; Eberhardt, M.; Vastani, N.; Leffler, A.; Reeh, P.; Bevan, S. Streptozotocin Stimulates the Ion Channel TRPA1 Directly: INVOLVEMENT OF PEROXYNITRITE. J. Biol. Chem. 2015, 290, 15185–15196. [Google Scholar] [CrossRef]
- Prawitt, D.; Monteilh-Zoller, M.K.; Brixel, L.; Spangenberg, C.; Zabel, B.; Fleig, A.; Penner, R. TRPM5 is a transient Ca2+-activated cation channel responding to rapid changes in [Ca2+]i. Proc. Natl. Acad. Sci. USA 2003, 100, 15166–15171. [Google Scholar] [CrossRef]
- Krishnan, K.; Ma, Z.; Bjorklund, A.; Islam, M.S. Role of transient receptor potential melastatin-like subtype 5 channel in insulin secretion from rat beta-cells. Pancreas 2014, 43, 597–604. [Google Scholar] [CrossRef]
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Islam, M.S. Molecular Regulations and Functions of the Transient Receptor Potential Channels of the Islets of Langerhans and Insulinoma Cells. Cells 2020, 9, 685. https://doi.org/10.3390/cells9030685
Islam MS. Molecular Regulations and Functions of the Transient Receptor Potential Channels of the Islets of Langerhans and Insulinoma Cells. Cells. 2020; 9(3):685. https://doi.org/10.3390/cells9030685
Chicago/Turabian StyleIslam, Md. Shahidul. 2020. "Molecular Regulations and Functions of the Transient Receptor Potential Channels of the Islets of Langerhans and Insulinoma Cells" Cells 9, no. 3: 685. https://doi.org/10.3390/cells9030685
APA StyleIslam, M. S. (2020). Molecular Regulations and Functions of the Transient Receptor Potential Channels of the Islets of Langerhans and Insulinoma Cells. Cells, 9(3), 685. https://doi.org/10.3390/cells9030685





