From Synthesis to Utilization: The Ins and Outs of Mitochondrial Heme
Abstract
1. Introduction
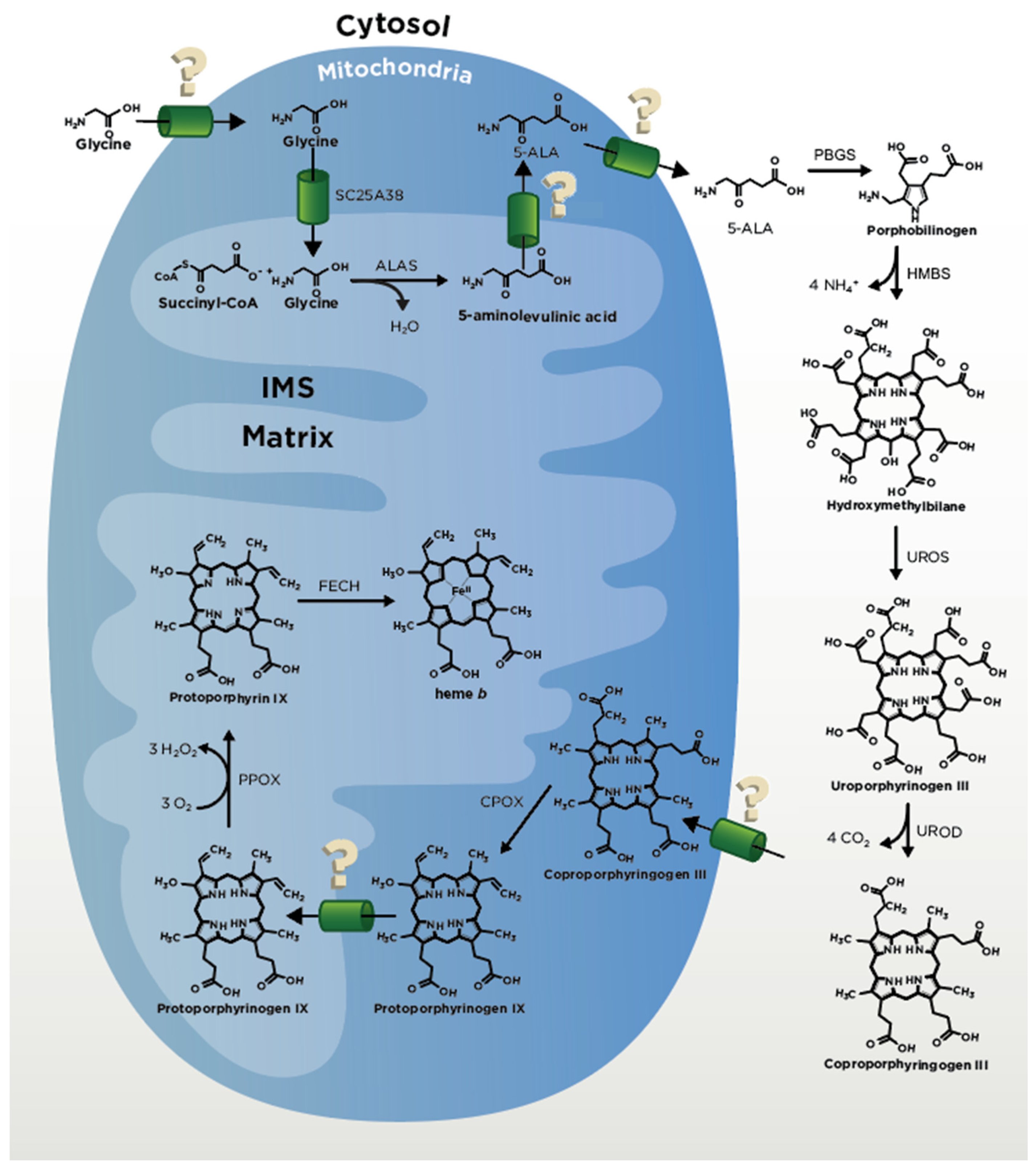
2. Heme Biosynthesis
2.1. ALA Production
2.2. CPgenIII Formation
2.3. Coproporphyrinogen Oxidase (CPOX) and Protoporphyrinogen Oxidase (PPOX)
2.4. Ferrochelatase
2.5. Anemias and Porphyrias
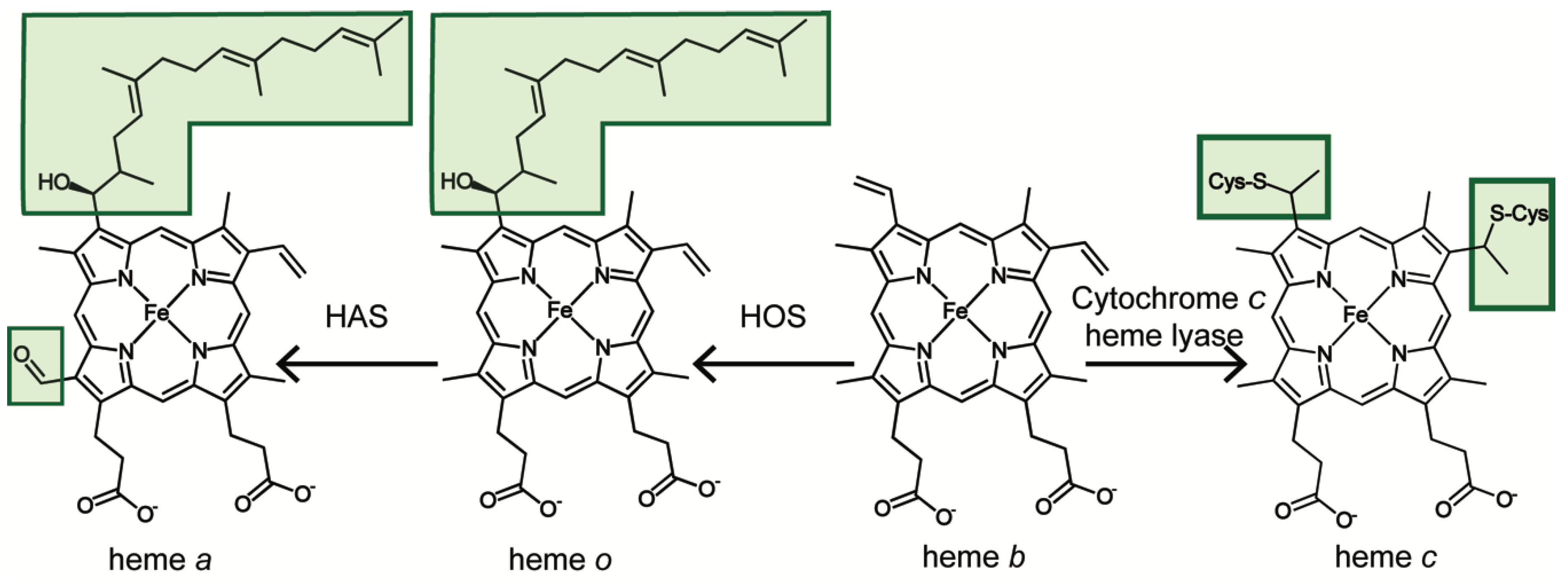
3. From Heme b to Hemes c, o and a
3.1. Mitochondrial Heme b Pathways
3.2. Heme c Pathway
3.3. Heme a Pathway
3.3.1. Heme o Synthase
3.3.2. Heme a Synthase
3.3.3. Other Proteins Related to Heme a Biogenesis
3.4. Heme c and Heme a Pathway-Related Diseases
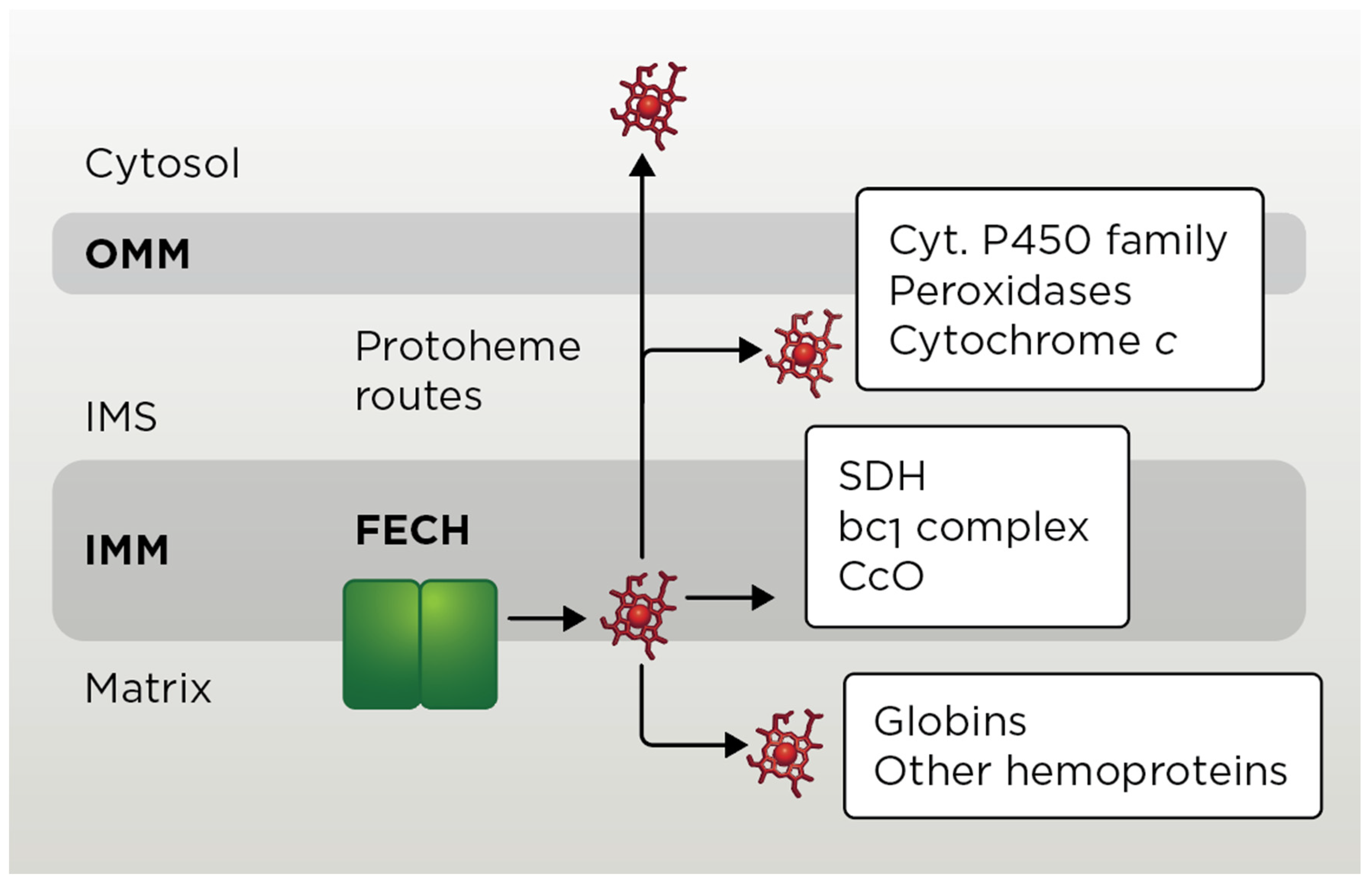
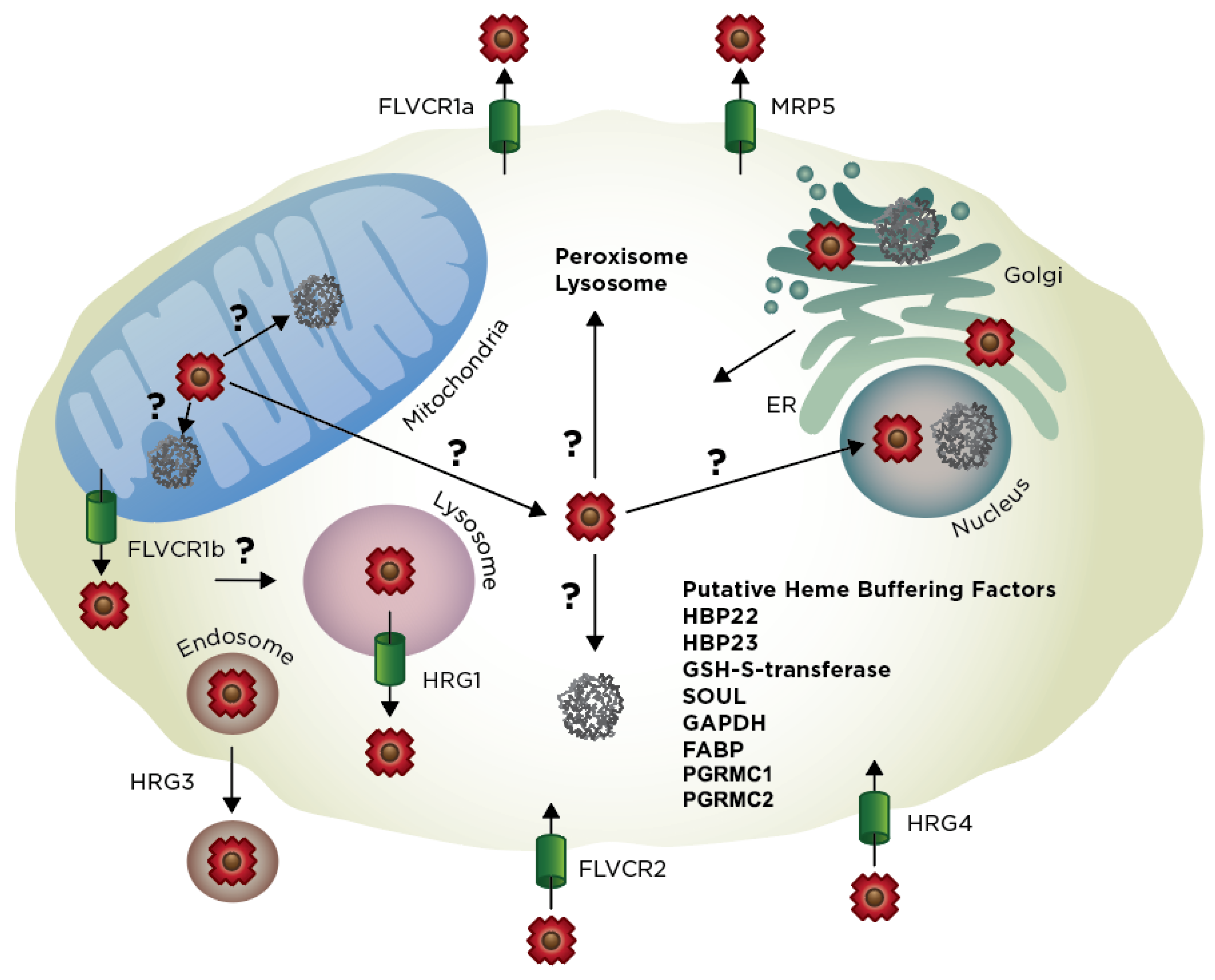
4. Extra-Mitochondrial Heme Trafficking
4.1. Exit of Mitochondrial Heme
4.2. Import of Exogenous Heme
4.3. Exogenous vs. Endogenous Heme
4.4. Heme Trafficking Factors
5. Multi-Model Comparison of Eukaryotic Heme Homeostasis
6. New Methods to Probe Heme Trafficking
7. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CO | Carbon monoxide |
| IMS | Mitochondrial intermembrane space |
| TCA | Tricarboxic acid |
| IMM | Inner mitochondrial membrane |
| 5-ALA | 5-aminolevulunic acid |
| ALAS | Aminolevulunic acid synthase |
| PBGS | Porphobilinogen synthase |
| PBG | Porphobilinogen |
| HMB | Hydroxymethylbilane |
| HMBS | Hydroxymethylbilane synthase |
| UPgen III | Uroporphyrinogen III |
| UROS | Uroporphyrinogen synthase |
| CPgen III | Coproporphyrinogen III |
| UROD | Uroporphyrinogen decarboxylase |
| PPgen IX | Protoporphyrinogen IX |
| CPOX | Coproporphyrinogen oxidase |
| Fe-PPIX | Iron-protoporphyrin IX |
| PPIX | Protoporphyrin IX |
| PPOX | Protoporphyrinogen oxidase |
| FECH | Ferrochelatase |
| PLP | Pyridoxal 5’-phosphate |
| OMM | Outer mitochondrial membrane |
| SLC | Solute carrier |
| α-KG | A-ketoglutarate |
| MTS | Mitochondria-targeting sequence |
| HRM | Heme regulatory motif |
| IRP | Iron regulatory protein |
| ATP | Adenosine triphosphate |
| ADP | Adenosine diphosphate |
| AAA+ | ATP hydrolase associate with various cellular activities |
| ANT | Adenine nucleotide translocator |
| KDH | A-ketoglutarate dehydrogenase |
| CcO | Cytochrome c oxidase |
| ETC | Electron transport chain |
| SDH | Succinate dehydrogenase |
| CCHL | Cytochrome c heme lyase |
| HCCS | Holocytochrome c synthase |
| MLS | Microphthalmia with linear skin defects |
| TMD | Transmembrane domain |
| FDX | Ferredoxin |
| MAMs | Mitochondria-associated membranes |
| ERMES | Endoplasmic reticulum-mitochondria encounter structure |
| MDV | Mitochondrial-derived vesicle |
| HRG | Heme responsive gene |
| ZnMP | Zinc mesoporphyrin |
| CHO | Chinese hamster ovary |
| LDL | Low-density lipoprotein |
| HDL | High-density lipoprotein |
| HO | Heme oxygenase |
| FeLV | Feline leukemia virus |
| ABC | ATP-binding cassette |
| BRCP | Breast cancer resistance protein |
| HBP | Heme-binding protein |
| FABP | Fatty acid-binding protein |
| GAPDH | Glyceraldehyde phosphate dehydrogenase |
| PGRMC1/2 | Progesterone receptor membrane component 1/2 |
| EGFP | Enhanced green fluorescent protein |
| ECFP | Enhanced cyan fluorescent protein |
| EYFP | Enhanced yellow fluorescent protein |
| HS | Heme sensor |
| FRET | Forster resonance energy transfer |
References
- Severance, S.; Hamza, I. Trafficking of heme and porphyrins in metazoa. Chem. Rev. 2009, 109, 4596–4616. [Google Scholar] [CrossRef] [PubMed]
- Hanna, D.A.; Martinez-Guzman, O.; Reddi, A.R. Heme gazing: Illuminating eukaryotic heme trafficking, dynamics, and signaling with fluorescent heme sensors. Biochemistry 2017, 56, 1815–1823. [Google Scholar] [CrossRef] [PubMed]
- Reddi, A.R.; Hamza, I. Heme mobilization in animals: A metallolipid’s journey. Acc. Chem. Res. 2016, 49, 1104–1110. [Google Scholar] [CrossRef]
- Ponka, P. Cell biology of heme. Am. J. Med. Sci. 1999, 318, 241–256. [Google Scholar] [CrossRef]
- Tsiftsoglou, A.S.; Tsamadou, A.I.; Papadopoulou, L.C. Heme as key regulator of major mammalian cellular functions: Molecular, cellular, and pharmacological aspects. Pharmacol. Ther. 2006, 111, 327–345. [Google Scholar] [CrossRef]
- Kim, H.J.; Khalimonchuk, O.; Smith, P.M.; Winge, D.R. Structure, function, and assembly of heme centers in mitochondrial respiratory complexes. Biochim. Biophys. Acta 2012, 1823, 1604–1616. [Google Scholar] [CrossRef]
- Mense, S.M.; Zhang, L. Heme: A versatile signaling molecule controlling the activities of diverse regulators ranging from transcription factors to map kinases. Cell Res. 2006, 16, 681–692. [Google Scholar] [CrossRef]
- Faller, M.; Matsunaga, M.; Yin, S.; Loo, J.A.; Guo, F. Heme is involved in microrna processing. Nat. Struct. Mol. Biol. 2007, 14, 23–29. [Google Scholar] [CrossRef]
- Quick-Cleveland, J.; Jacob, J.P.; Weitz, S.H.; Shoffner, G.; Senturia, R.; Guo, F. The dgcr8 rna-binding heme domain recognizes primary micrornas by clamping the hairpin. Cell Rep. 2014, 7, 1994–2005. [Google Scholar] [CrossRef]
- Igarashi, K.; Sun, J. The heme-bach1 pathway in the regulation of oxidative stress response and erythroid differentiation. Antioxid Redox Signal 2006, 8, 107–118. [Google Scholar] [CrossRef]
- Raghuram, S.; Stayrook, K.R.; Huang, P.; Rogers, P.M.; Nosie, A.K.; McClure, D.B.; Burris, L.L.; Khorasanizadeh, S.; Burris, T.P.; Rastinejad, F. Identification of heme as the ligand for the orphan nuclear receptors rev-erbalpha and rev-erbbeta. Nat. Struct. Mol. Biol. 2007, 14, 1207–1213. [Google Scholar] [CrossRef]
- Hamza, I.; Dailey, H.A. One ring to rule them all: Trafficking of heme and heme synthesis intermediates in the metazoans. Biochim. Biophys. Acta 2012, 1823, 1617–1632. [Google Scholar] [CrossRef]
- Chiabrando, D.; Vinchi, F.; Fiorito, V.; Mercurio, S.; Tolosano, E. Heme in pathophysiology: A matter of scavenging, metabolism and trafficking across cell membranes. Front. Pharmacol. 2014, 5, 61. [Google Scholar] [CrossRef]
- Keel, S.B.; Doty, R.T.; Yang, Z.; Quigley, J.G.; Chen, J.; Knoblaugh, S.; Kingsley, P.D.; De Domenico, I.; Vaughn, M.B.; Kaplan, J.; et al. A heme export protein is required for red blood cell differentiation and iron homeostasis. Science 2008, 319, 825–828. [Google Scholar] [CrossRef]
- Haldar, M.; Kohyama, M.; So, A.Y.; Kc, W.; Wu, X.; Briseno, C.G.; Satpathy, A.T.; Kretzer, N.M.; Arase, H.; Rajasekaran, N.S.; et al. Heme-mediated spi-c induction promotes monocyte differentiation into iron-recycling macrophages. Cell 2014, 156, 1223–1234. [Google Scholar] [CrossRef]
- Dutra, F.F.; Bozza, M.T. Heme on innate immunity and inflammation. Front. Pharmacol. 2014, 5, 115. [Google Scholar] [CrossRef]
- Desmard, M.; Boczkowski, J.; Poderoso, J.; Motterlini, R. Mitochondrial and cellular heme-dependent proteins as targets for the bioactive function of the heme oxygenase/carbon monoxide system. Antioxid Redox Signal 2007, 9, 2139–2155. [Google Scholar] [CrossRef]
- Kim, H.P.; Ryter, S.W.; Choi, A.M. Co as a cellular signaling molecule. Annu. Rev. Pharmacol. Toxicol. 2006, 46, 411–449. [Google Scholar] [CrossRef]
- Shen, J.; Sheng, X.; Chang, Z.; Wu, Q.; Wang, S.; Xuan, Z.; Li, D.; Wu, Y.; Shang, Y.; Kong, X.; et al. Iron metabolism regulates p53 signaling through direct heme-p53 interaction and modulation of p53 localization, stability, and function. Cell Rep. 2014, 7, 180–193. [Google Scholar] [CrossRef]
- Kumar, S.; Bandyopadhyay, U. Free heme toxicity and its detoxification systems in human. Toxicol. Lett. 2005, 157, 175–188. [Google Scholar] [CrossRef]
- Sassa, S. Why heme needs to be degraded to iron, biliverdin ixalpha, and carbon monoxide? Antioxid Redox Signal 2004, 6, 819–824. [Google Scholar]
- Wu, M.L.; Ho, Y.C.; Lin, C.Y.; Yet, S.F. Heme oxygenase-1 in inflammation and cardiovascular disease. Am. J. Cardiovasc. Dis. 2011, 1, 150–158. [Google Scholar]
- Schipper, H.M.; Song, W.; Zukor, H.; Hascalovici, J.R.; Zeligman, D. Heme oxygenase-1 and neurodegeneration: Expanding frontiers of engagement. J. Neurochem. 2009, 110, 469–485. [Google Scholar] [CrossRef]
- Atamna, H.; Killilea, D.W.; Killilea, A.N.; Ames, B.N. Heme deficiency may be a factor in the mitochondrial and neuronal decay of aging. Proc. Natl. Acad. Sci. USA 2002, 99, 14807–14812. [Google Scholar] [CrossRef]
- Atamna, H.; Frey, W.H., 2nd. A role for heme in alzheimer’s disease: Heme binds amyloid beta and has altered metabolism. Proc. Natl. Acad. Sci. USA 2004, 101, 11153–11158. [Google Scholar] [CrossRef]
- Dailey, H.A.; Dailey, T.A.; Gerdes, S.; Jahn, D.; Jahn, M.; O’Brian, M.R.; Warren, M.J. Prokaryotic heme biosynthesis: Multiple pathways to a common essential product. Microbiol. Mol. Biol. Rev. 2017, 81, e00048-16. [Google Scholar] [CrossRef]
- Labbe-Bois, R.A.L.P. Tetrapyrrole and heme biosynthesis in the yeast sacchromyces cerevisiae. In Biosynthesis of Heme and Cholorophylls; Dailey, H.A., Ed.; Green Pub. Associates and Wiley-Interscience: New York, NY, USA, 1990; pp. 235–285. [Google Scholar]
- Ajioka, R.S.; Phillips, J.D.; Kushner, J.P. Biosynthesis of heme in mammals. Biochim. Biophys. Acta 2006, 1763, 723–736. [Google Scholar] [CrossRef]
- May, B.K.; Dogra, S.C.; Sadlon, T.J.; Bhasker, C.R.; Cox, T.C.; Bottomley, S.S. Molecular regulation of heme biosynthesis in higher vertebrates. Prog. Nucleic Acid Res. Mol. Biol. 1995, 51, 1–51. [Google Scholar]
- Sun, F.; Cheng, Y.; Chen, C. Regulation of heme biosynthesis and transport in metazoa. Sci. China Life Sci. 2015, 58, 757–764. [Google Scholar] [CrossRef][Green Version]
- Hunter, G.A.; Ferreira, G.C. 5-aminolevulinate synthase: Catalysis of the first step of heme biosynthesis. Cell Mol. Biol. (Noisy-le-grand) 2009, 55, 102–110. [Google Scholar]
- Hunter, G.A.; Ferreira, G.C. Molecular enzymology of 5-aminolevulinate synthase, the gatekeeper of heme biosynthesis. Biochim. Biophys. Acta 2011, 1814, 1467–1473. [Google Scholar] [CrossRef]
- Guernsey, D.L.; Jiang, H.; Campagna, D.R.; Evans, S.C.; Ferguson, M.; Kellogg, M.D.; Lachance, M.; Matsuoka, M.; Nightingale, M.; Rideout, A.; et al. Mutations in mitochondrial carrier family gene slc25a38 cause nonsyndromic autosomal recessive congenital sideroblastic anemia. Nat. Genet. 2009, 41, 651–653. [Google Scholar] [CrossRef]
- Lunetti, P.; Damiano, F.; De Benedetto, G.; Siculella, L.; Pennetta, A.; Muto, L.; Paradies, E.; Marobbio, C.M.; Dolce, V.; Capobianco, L. Characterization of human and yeast mitochondrial glycine carriers with implications for heme biosynthesis and anemia. J. Biol. Chem. 2016, 291, 19746–19759. [Google Scholar] [CrossRef]
- Fernandez-Murray, J.P.; Prykhozhij, S.V.; Dufay, J.N.; Steele, S.L.; Gaston, D.; Nasrallah, G.K.; Coombs, A.J.; Liwski, R.S.; Fernandez, C.V.; Berman, J.N.; et al. Glycine and folate ameliorate models of congenital sideroblastic anemia. PLoS Genet. 2016, 12, e1005783. [Google Scholar] [CrossRef]
- Shemin, D.; Kumin, S. The mechanism of porphyrin formation; the formation of a succinyl intermediate from succinate. J. Biol. Chem. 1952, 198, 827–837. [Google Scholar]
- Labbe, R.F.; Kurumada, T.; Onisawa, J. The role of succinyl-coa synthetase in the control of heme biosynthesis. Biochim. Biophys. Acta 1965, 111, 403–415. [Google Scholar] [CrossRef]
- Furuyama, K.; Sassa, S. Interaction between succinyl coa synthetase and the heme-biosynthetic enzyme alas-e is disrupted in sideroblastic anemia. J. Clin. Investig. 2000, 105, 757–764. [Google Scholar] [CrossRef]
- Burch, J.S.; Marcero, J.R.; Maschek, J.A.; Cox, J.E.; Jackson, L.K.; Medlock, A.E.; Phillips, J.D.; Dailey, H.A., Jr. Glutamine via alpha-ketoglutarate dehydrogenase provides succinyl-coa for heme synthesis during erythropoiesis. Blood 2018, 132, 987–998. [Google Scholar] [CrossRef]
- Astner, I.; Schulze, J.O.; Van den Heuvel, J.; Jahn, D.; Schubert, W.D.; Heinz, D.W. Crystal structure of 5-aminolevulinate synthase, the first enzyme of heme biosynthesis, and its link to xlsa in humans. EMBO J. 2005, 24, 3166–3177. [Google Scholar] [CrossRef]
- Brown, B.L.; Kardon, J.R.; Sauer, R.T.; Baker, T.A. Structure of the mitochondrial aminolevulinic acid synthase, a key heme biosynthetic enzyme. Structure 2018, 26, 580–589 e584. [Google Scholar] [CrossRef]
- Fratz, E.J.; Clayton, J.; Hunter, G.A.; Ducamp, S.; Breydo, L.; Uversky, V.N.; Deybach, J.C.; Gouya, L.; Puy, H.; Ferreira, G.C. Human erythroid 5-aminolevulinate synthase mutations associated with x-linked protoporphyria disrupt the conformational equilibrium and enhance product release. Biochemistry 2015, 54, 5617–5631. [Google Scholar] [CrossRef]
- Duncan, R.; Faggart, M.A.; Cornell, N.W. Phylogenetic analysis of the 5-aminolevulinate synthase gene. Biol. Bull. 1997, 193, 247–248. [Google Scholar] [CrossRef]
- Lathrop, J.T.; Timko, M.P. Regulation by heme of mitochondrial protein transport through a conserved amino acid motif. Science 1993, 259, 522–525. [Google Scholar] [CrossRef]
- Dailey, T.A.; Woodruff, J.H.; Dailey, H.A. Examination of mitochondrial protein targeting of haem synthetic enzymes: In vivo identification of three functional haem-responsive motifs in 5-aminolaevulinate synthase. Biochem. J. 2005, 386, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, M.; Gora, M.; Rytka, J. Identification of rate-limiting steps in yeast heme biosynthesis. Biochem. Biophys. Res. Commun. 2003, 310, 1247–1253. [Google Scholar] [CrossRef] [PubMed]
- Furuyama, K.; Kaneko, K.; Vargas, P.D. Heme as a magnificent molecule with multiple missions: Heme determines its own fate and governs cellular homeostasis. Tohoku J. Exp. Med. 2007, 213, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Bishop, D.F.; Henderson, A.S.; Astrin, K.H. Human delta-aminolevulinate synthase: Assignment of the housekeeping gene to 3p21 and the erythroid-specific gene to the x chromosome. Genomics 1990, 7, 207–214. [Google Scholar] [CrossRef]
- Yamamoto, M.; Hayashi, N.; Kikuchi, G. Evidence for the transcriptional inhibition by heme of the synthesis of delta-aminolevulinate synthase in rat liver. Biochem. Biophys. Res. Commun. 1982, 105, 985–990. [Google Scholar] [CrossRef]
- Sassa, S.; Granick, S. Induction of -aminolevulinic acid synthetase in chick embryo liver cells in cluture. Proc. Natl. Acad. Sci. USA 1970, 67, 517–522. [Google Scholar] [CrossRef]
- Yamamoto, M.; Hayashi, N.; Kikuchi, G. Translational inhibition by heme of the synthesis of hepatic delta-aminolevulinate synthase in a cell-free system. Biochem. Biophys. Res. Commun. 1983, 115, 225–231. [Google Scholar] [CrossRef]
- Hamilton, J.W.; Bement, W.J.; Sinclair, P.R.; Sinclair, J.F.; Alcedo, J.A.; Wetterhahn, K.E. Heme regulates hepatic 5-aminolevulinate synthase mrna expression by decreasing mrna half-life and not by altering its rate of transcription. Arch. Biochem. Biophys. 1991, 289, 387–392. [Google Scholar] [CrossRef]
- Tian, Q.; Li, T.; Hou, W.; Zheng, J.; Schrum, L.W.; Bonkovsky, H.L. Lon peptidase 1 (lonp1)-dependent breakdown of mitochondrial 5-aminolevulinic acid synthase protein by heme in human liver cells. J. Biol. Chem. 2011, 286, 26424–26430. [Google Scholar] [CrossRef]
- Munakata, H.; Sun, J.Y.; Yoshida, K.; Nakatani, T.; Honda, E.; Hayakawa, S.; Furuyama, K.; Hayashi, N. Role of the heme regulatory motif in the heme-mediated inhibition of mitochondrial import of 5-aminolevulinate synthase. J. Biochem. 2004, 136, 233–238. [Google Scholar] [CrossRef]
- Aich, A.; Freundlich, M.; Vekilov, P.G. The free heme concentration in healthy human erythrocytes. Blood Cells Mol. Dis. 2015, 55, 402–409. [Google Scholar] [CrossRef]
- Fujiwara, T.; O’Geen, H.; Keles, S.; Blahnik, K.; Linnemann, A.K.; Kang, Y.A.; Choi, K.; Farnham, P.J.; Bresnick, E.H. Discovering hematopoietic mechanisms through genome-wide analysis of gata factor chromatin occupancy. Mol. Cell 2009, 36, 667–681. [Google Scholar] [CrossRef]
- Kaneko, K.; Furuyama, K.; Fujiwara, T.; Kobayashi, R.; Ishida, H.; Harigae, H.; Shibahara, S. Identification of a novel erythroid-specific enhancer for the alas2 gene and its loss-of-function mutation which is associated with congenital sideroblastic anemia. Haematologica 2014, 99, 252–261. [Google Scholar] [CrossRef]
- Doyle, F.; Tenenbaum, S.A. Trans-regulation of rna-binding protein motifs by microrna. Front. Genet. 2014, 5, 79. [Google Scholar] [CrossRef]
- Sanchez, M.; Galy, B.; Schwanhaeusser, B.; Blake, J.; Bahr-Ivacevic, T.; Benes, V.; Selbach, M.; Muckenthaler, M.U.; Hentze, M.W. Iron regulatory protein-1 and -2: Transcriptome-wide definition of binding mrnas and shaping of the cellular proteome by iron regulatory proteins. Blood 2011, 118, e168–e179. [Google Scholar] [CrossRef]
- Chung, J.; Anderson, S.A.; Gwynn, B.; Deck, K.M.; Chen, M.J.; Langer, N.B.; Shaw, G.C.; Huston, N.C.; Boyer, L.F.; Datta, S.; et al. Iron regulatory protein-1 protects against mitoferrin-1-deficient porphyria. J. Biol. Chem. 2014, 289, 7835–7843. [Google Scholar] [CrossRef]
- Ishikawa, H.; Kato, M.; Hori, H.; Ishimori, K.; Kirisako, T.; Tokunaga, F.; Iwai, K. Involvement of heme regulatory motif in heme-mediated ubiquitination and degradation of irp2. Mol. Cell 2005, 19, 171–181. [Google Scholar] [CrossRef]
- Yien, Y.Y.; Ducamp, S.; Van der Vorm, L.N.; Kardon, J.R.; Manceau, H.; Kannengiesser, C.; Bergonia, H.A.; Kafina, M.D.; Karim, Z.; Gouya, L.; et al. Mutation in human clpx elevates levels of delta-aminolevulinate synthase and protoporphyrin ix to promote erythropoietic protoporphyria. Proc. Natl. Acad. Sci. USA 2017, 114, e8045–e8052. [Google Scholar] [CrossRef]
- Abu-Farha, M.; Niles, J.; Willmore, W.G. Erythroid-specific 5-aminolevulinate synthase protein is stabilized by low oxygen and proteasomal inhibition. Biochem. Cell Biol. 2005, 83, 620–630. [Google Scholar] [CrossRef]
- Nilsson, R.; Schultz, I.J.; Pierce, E.L.; Soltis, K.A.; Naranuntarat, A.; Ward, D.M.; Baughman, J.M.; Paradkar, P.N.; Kingsley, P.D.; Culotta, V.C.; et al. Discovery of genes essential for heme biosynthesis through large-scale gene expression analysis. Cell Metab. 2009, 10, 119–130. [Google Scholar] [CrossRef]
- Schubert, H.L.; Erskine, P.T.; Cooper, J.B. 5-aminolaevulinic acid dehydratase, porphobilinogen deaminase and uroporphyrinogen iii synthase. In Tetrapyrroles: Birth, Life, and Death; Warren, M.J., Smith, A.G., Eds.; Landes Bioscience: Austin, TX, USA, 2009; pp. 43–73. [Google Scholar]
- Krishnamurthy, P.C.; Du, G.; Fukuda, Y.; Sun, D.; Sampath, J.; Mercer, K.E.; Wang, J.; Sosa-Pineda, B.; Murti, K.G.; Schuetz, J.D. Identification of a mammalian mitochondrial porphyrin transporter. Nature 2006, 443, 586–589. [Google Scholar] [CrossRef]
- Helias, V.; Saison, C.; Ballif, B.A.; Peyrard, T.; Takahashi, J.; Takahashi, H.; Tanaka, M.; Deybach, J.C.; Puy, H.; Le Gall, M.; et al. Abcb6 is dispensable for erythropoiesis and specifies the new blood group system langereis. Nat. Genet. 2012, 44, 170–173. [Google Scholar] [CrossRef]
- Ulrich, D.L.; Lynch, J.; Wang, Y.; Fukuda, Y.; Nachagari, D.; Du, G.; Sun, D.; Fan, Y.; Tsurkan, L.; Potter, P.M.; et al. Atp-dependent mitochondrial porphyrin importer abcb6 protects against phenylhydrazine toxicity. J. Biol. Chem. 2012, 287, 12679–12690. [Google Scholar] [CrossRef]
- Fukuda, Y.; Cheong, P.L.; Lynch, J.; Brighton, C.; Frase, S.; Kargas, V.; Rampersaud, E.; Wang, Y.; Sankaran, V.G.; Yu, B.; et al. The severity of hereditary porphyria is modulated by the porphyrin exporter and lan antigen abcb6. Nat. Commun. 2016, 7, 12353. [Google Scholar] [CrossRef]
- Rhee, H.W.; Zou, P.; Udeshi, N.D.; Martell, J.D.; Mootha, V.K.; Carr, S.A.; Ting, A.Y. Proteomic mapping of mitochondria in living cells via spatially restricted enzymatic tagging. Science 2013, 339, 1328–1331. [Google Scholar] [CrossRef]
- Azuma, M.; Kabe, Y.; Kuramori, C.; Kondo, M.; Yamaguchi, Y.; Handa, H. Adenine nucleotide translocator transports haem precursors into mitochondria. PLoS ONE 2008, 3, e3070. [Google Scholar] [CrossRef]
- Yien, Y.Y.; Robledo, R.F.; Schultz, I.J.; Takahashi-Makise, N.; Gwynn, B.; Bauer, D.E.; Dass, A.; Yi, G.; Li, L.; Hildick-Smith, G.J.; et al. Tmem14c is required for erythroid mitochondrial heme metabolism. J. Clin. Investig. 2014, 124, 4294–4304. [Google Scholar] [CrossRef]
- Shaw, G.C.; Cope, J.J.; Li, L.; Corson, K.; Hersey, C.; Ackermann, G.E.; Gwynn, B.; Lambert, A.J.; Wingert, R.A.; Traver, D.; et al. Mitoferrin is essential for erythroid iron assimilation. Nature 2006, 440, 96–100. [Google Scholar] [CrossRef]
- Muhlenhoff, U.; Hoffmann, B.; Richter, N.; Rietzschel, N.; Spantgar, F.; Stehling, O.; Uzarska, M.A.; Lill, R. Compartmentalization of iron between mitochondria and the cytosol and its regulation. Eur. J. Cell Biol. 2015, 94, 292–308. [Google Scholar] [CrossRef]
- Lane, D.J.; Merlot, A.M.; Huang, M.L.; Bae, D.H.; Jansson, P.J.; Sahni, S.; Kalinowski, D.S.; Richardson, D.R. Cellular iron uptake, trafficking and metabolism: Key molecules and mechanisms and their roles in disease. Biochim. Biophys. Acta 2015, 1853, 1130–1144. [Google Scholar] [CrossRef]
- Korolnek, T.; Hamza, I. Like iron in the blood of the people: The requirement for heme trafficking in iron metabolism. Front. Pharmacol. 2014, 5, 126. [Google Scholar] [CrossRef]
- Chen, C.; Paw, B.H. Cellular and mitochondrial iron homeostasis in vertebrates. Biochim. Biophys. Acta 2012, 1823, 1459–1467. [Google Scholar] [CrossRef]
- Wu, C.K.; Dailey, H.A.; Rose, J.P.; Burden, A.; Sellers, V.M.; Wang, B.C. The 2.0 a structure of human ferrochelatase, the terminal enzyme of heme biosynthesis. Nat. Struct. Biol. 2001, 8, 156–160. [Google Scholar] [CrossRef]
- Dailey, H.A.; Finnegan, M.G.; Johnson, M.K. Human ferrochelatase is an iron-sulfur protein. Biochemistry 1994, 33, 403–407. [Google Scholar] [CrossRef]
- Crouse, B.R.; Sellers, V.M.; Finnegan, M.G.; Dailey, H.A.; Johnson, M.K. Site-directed mutagenesis and spectroscopic characterization of human ferrochelatase: Identification of residues coordinating the [2fe-2s] cluster. Biochemistry 1996, 35, 16222–16229. [Google Scholar] [CrossRef]
- Shah, D.I.; Takahashi-Makise, N.; Cooney, J.D.; Li, L.; Schultz, I.J.; Pierce, E.L.; Narla, A.; Seguin, A.; Hattangadi, S.M.; Medlock, A.E.; et al. Mitochondrial atpif1 regulates haem synthesis in developing erythroblasts. Nature 2012, 491, 608–612. [Google Scholar] [CrossRef]
- Medlock, A.E.; Shiferaw, M.T.; Marcero, J.R.; Vashisht, A.A.; Wohlschlegel, J.A.; Phillips, J.D.; Dailey, H.A. Identification of the mitochondrial heme metabolism complex. PLoS ONE 2015, 10, e0135896. [Google Scholar] [CrossRef]
- Piel, R.B., 3rd; Shiferaw, M.T.; Vashisht, A.A.; Marcero, J.R.; Praissman, J.L.; Phillips, J.D.; Wohlschlegel, J.A.; Medlock, A.E. A novel role for progesterone receptor membrane component 1 (pgrmc1): A partner and regulator of ferrochelatase. Biochemistry 2016, 55, 5204–5217. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Dailey, H.A.; Paw, B.H. Ferrochelatase forms an oligomeric complex with mitoferrin-1 and abcb10 for erythroid heme biosynthesis. Blood 2010, 116, 628–630. [Google Scholar] [CrossRef] [PubMed]
- Taketani, S.; Kakimoto, K.; Ueta, H.; Masaki, R.; Furukawa, T. Involvement of abc7 in the biosynthesis of heme in erythroid cells: Interaction of abc7 with ferrochelatase. Blood 2003, 101, 3274–3280. [Google Scholar] [CrossRef]
- Maio, N.; Kim, K.S.; Holmes-Hampton, G.; Singh, A.; Rouault, T.A. Dimeric ferrochelatase bridges abcb7 and abcb10 homodimers in an architecturally defined molecular complex required for heme biosynthesis. Haematologica 2019, 104, 1756–1767. [Google Scholar] [CrossRef]
- Thompson, A.M.; Reddi, A.R.; Shi, X.; Goldbeck, R.A.; Moenne-Loccoz, P.; Gibney, B.R.; Holman, T.R. Measurement of the heme affinity for yeast dap1p, and its importance in cellular function. Biochemistry 2007, 46, 14629–14637. [Google Scholar] [CrossRef]
- Craven, R.J.; Mallory, J.C.; Hand, R.A. Regulation of iron homeostasis mediated by the heme-binding protein dap1 (damage resistance protein 1) via the p450 protein erg11/cyp51. J. Biol. Chem. 2007, 282, 36543–36551. [Google Scholar] [CrossRef]
- Hughes, A.L.; Powell, D.W.; Bard, M.; Eckstein, J.; Barbuch, R.; Link, A.J.; Espenshade, P.J. Dap1/pgrmc1 binds and regulates cytochrome p450 enzymes. Cell Metab. 2007, 5, 143–149. [Google Scholar] [CrossRef]
- Galmozzi, A.; Kok, B.P.; Kim, A.S.; Montenegro-Burke, J.R.; Lee, J.Y.; Spreafico, R.; Mosure, S.; Albert, V.; Cintron-Colon, R.; Godio, C.; et al. Pgrmc2 is an intracellular haem chaperone critical for adipocyte function. Nature 2019, 576, 138–142. [Google Scholar] [CrossRef]
- Sweeny, E.A.; Singh, A.B.; Chakravarti, R.; Martinez-Guzman, O.; Saini, A.; Haque, M.M.; Garee, G.; Dans, P.D.; Hannibal, L.; Reddi, A.R.; et al. Glyceraldehyde-3-phosphate dehydrogenase is a chaperone that allocates labile heme in cells. J. Biol. Chem. 2018, 293, 14557–14568. [Google Scholar] [CrossRef]
- Hanna, D.A.; Harvey, R.M.; Martinez-Guzman, O.; Yuan, X.; Chandrasekharan, B.; Raju, G.; Outten, F.W.; Hamza, I.; Reddi, A.R. Heme dynamics and trafficking factors revealed by genetically encoded fluorescent heme sensors. Proc. Natl. Acad. Sci. USA 2016, 113, 7539–7544. [Google Scholar] [CrossRef]
- Phillips, J.D. Heme biosynthesis and the porphyrias. Mol. Genet. Metab. 2019, 128, 164–177. [Google Scholar] [CrossRef]
- Karim, Z.; Lyoumi, S.; Nicolas, G.; Deybach, J.C.; Gouya, L.; Puy, H. Porphyrias: A 2015 update. Clin. Res. Hepatol. Gastroenterol. 2015, 39, 412–425. [Google Scholar] [CrossRef]
- Schmitt, C.; Lenglet, H.; Yu, A.; Delaby, C.; Benecke, A.; Lefebvre, T.; Letteron, P.; Paradis, V.; Wahlin, S.; Sandberg, S.; et al. Recurrent attacks of acute hepatic porphyria: Major role of the chronic inflammatory response in the liver. J. Intern. Med. 2018, 284, 78–91. [Google Scholar] [CrossRef]
- Fraser, D.J.; Podvinec, M.; Kaufmann, M.R.; Meyer, U.A. Drugs mediate the transcriptional activation of the 5-aminolevulinic acid synthase (alas1) gene via the chicken xenobiotic-sensing nuclear receptor (cxr). J. Biol. Chem. 2002, 277, 34717–34726. [Google Scholar] [CrossRef]
- Rifkind, A.B.; Gillette, P.N.; Song, C.S.; Kappas, A. Induction of hepatic delta-amino-levulinic acid synthetase by oral contraceptive steroids. J. Clin. Endocrinol. Metab. 1970, 30, 330–335. [Google Scholar] [CrossRef]
- Handschin, C.; Lin, J.; Rhee, J.; Peyer, A.K.; Chin, S.; Wu, P.H.; Meyer, U.A.; Spiegelman, B.M. Nutritional regulation of hepatic heme biosynthesis and porphyria through pgc-1alpha. Cell 2005, 122, 505–515. [Google Scholar] [CrossRef]
- Whatley, S.D.; Ducamp, S.; Gouya, L.; Grandchamp, B.; Beaumont, C.; Badminton, M.N.; Elder, G.H.; Holme, S.A.; Anstey, A.V.; Parker, M.; et al. C-terminal deletions in the alas2 gene lead to gain of function and cause x-linked dominant protoporphyria without anemia or iron overload. Am. J. Hum. Genet. 2008, 83, 408–414. [Google Scholar] [CrossRef]
- Hoggins, M.; Dailey, H.A.; Hunter, C.N.; Reid, J.D. Direct measurement of metal ion chelation in the active site of human ferrochelatase. Biochemistry 2007, 46, 8121–8127. [Google Scholar] [CrossRef]
- Jeney, V.; Balla, J.; Yachie, A.; Varga, Z.; Vercellotti, G.M.; Eaton, J.W.; Balla, G. Pro-oxidant and cytotoxic effects of circulating heme. Blood 2002, 100, 879–887. [Google Scholar] [CrossRef]
- Mogi, T. Biosynthesis and role of heme o and heme a. In The Iron and Cobalt Pigments: Biosynthesis, Structure, and Degradation; Kadish, K.M., Smith, K.M., Guilard, R., Eds.; Academic Press: Amsterdam, The Netherlands, 2003; pp. 157–181. [Google Scholar]
- Barros, M.H.; Carlson, C.G.; Glerum, D.M.; Tzagoloff, A. Involvement of mitochondrial ferredoxin and cox15p in hydroxylation of heme o. FEBS Lett. 2001, 492, 133–138. [Google Scholar] [CrossRef]
- Brown, K.R.; Allan, B.M.; Do, P.; Hegg, E.L. Identification of novel hemes generated by heme a synthase: Evidence for two successive monooxygenase reactions. Biochemistry 2002, 41, 10906–10913. [Google Scholar] [CrossRef]
- Brown, K.R.; Brown, B.M.; Hoagland, E.; Mayne, C.L.; Hegg, E.L. Heme a synthase does not incorporate molecular oxygen into the formyl group of heme a. Biochemistry 2004, 43, 8616–8624. [Google Scholar] [CrossRef]
- Hederstedt, L. Heme a biosynthesis. Biochim. Biophys. Acta 2012, 1817, 920–927. [Google Scholar] [CrossRef]
- Puustinen, A.; Wikstrom, M. The heme groups of cytochrome o from escherichia coli. Proc. Natl. Acad. Sci. USA 1991, 88, 6122–6126. [Google Scholar] [CrossRef]
- Mogi, T.; Saiki, K.; Anraku, Y. Biosynthesis and functional role of haem o and haem a. Mol. Microbiol. 1994, 14, 391–398. [Google Scholar] [CrossRef]
- Cassanova, N.; O’Brien, K.M.; Stahl, B.T.; McClure, T.; Poyton, R.O. Yeast flavohemoglobin, a nitric oxide oxidoreductase, is located in both the cytosol and the mitochondrial matrix: Effects of respiration, anoxia, and the mitochondrial genome on its intracellular level and distribution. J. Biol. Chem. 2005, 280, 7645–7653. [Google Scholar] [CrossRef]
- Yun, C.H.; Crofts, A.R.; Gennis, R.B. Assignment of the histidine axial ligands to the cytochrome bh and cytochrome bl components of the bc1 complex from rhodobacter sphaeroides by site-directed mutagenesis. Biochemistry 1991, 30, 6747–6754. [Google Scholar] [CrossRef]
- Maklashina, E.; Rajagukguk, S.; McIntire, W.S.; Cecchini, G. Mutation of the heme axial ligand of escherichia coli succinate-quinone reductase: Implications for heme ligation in mitochondrial complex ii from yeast. Biochim. Biophys. Acta 2010, 1797, 747–754. [Google Scholar] [CrossRef]
- Yankovskaya, V.; Horsefield, R.; Tornroth, S.; Luna-Chavez, C.; Miyoshi, H.; Leger, C.; Byrne, B.; Cecchini, G.; Iwata, S. Architecture of succinate dehydrogenase and reactive oxygen species generation. Science 2003, 299, 700–704. [Google Scholar] [CrossRef]
- Soto, I.C.; Fontanesi, F.; Myers, R.S.; Hamel, P.; Barrientos, A. A heme-sensing mechanism in the translational regulation of mitochondrial cytochrome c oxidase biogenesis. Cell Metab. 2012, 16, 801–813. [Google Scholar] [CrossRef]
- Djavadi-Ohaniance, L.; Rudin, Y.; Schatz, G. Identification of enzymically inactive apocytochrome c peroxidase in anaerobically grown saccharomyces cerevisiae. J. Biol. Chem. 1978, 253, 4402–4407. [Google Scholar] [PubMed]
- Guiard, B. Structure, expression and regulation of a nuclear gene encoding a mitochondrial protein: The yeast l(+)-lactate cytochrome c oxidoreductase (cytochrome b2). EMBO J. 1985, 4, 3265–3272. [Google Scholar] [CrossRef] [PubMed]
- Hildenbeutel, M.; Hegg, E.L.; Stephan, K.; Gruschke, S.; Meunier, B.; Ott, M. Assembly factors monitor sequential hemylation of cytochrome b to regulate mitochondrial translation. J. Cell Biol. 2014, 205, 511–524. [Google Scholar] [CrossRef]
- Kranz, R.G.; Richard-Fogal, C.; Taylor, J.S.; Frawley, E.R. Cytochrome c biogenesis: Mechanisms for covalent modifications and trafficking of heme and for heme-iron redox control. Microbiol. Mol. Biol. Rev. 2009, 73, 510–528. [Google Scholar] [CrossRef] [PubMed]
- Babbitt, S.E.; San Francisco, B.; Mendez, D.L.; Lukat-Rodgers, G.S.; Rodgers, K.R.; Bretsnyder, E.C.; Kranz, R.G. Mechanisms of mitochondrial holocytochrome c synthase and the key roles played by cysteines and histidine of the heme attachment site, cys-xx-cys-his. J. Biol. Chem. 2014, 289, 28795–28807. [Google Scholar] [CrossRef] [PubMed]
- Bernard, D.G.; Gabilly, S.T.; Dujardin, G.; Merchant, S.; Hamel, P.P. Overlapping specificities of the mitochondrial cytochrome c and c1 heme lyases. J. Biol. Chem. 2003, 278, 49732–49742. [Google Scholar] [CrossRef]
- Babbitt, S.E.; Sutherland, M.C.; San Francisco, B.; Mendez, D.L.; Kranz, R.G. Mitochondrial cytochrome c biogenesis: No longer an enigma. Trends Biochem. Sci. 2015, 40, 446–455. [Google Scholar] [CrossRef]
- Bernard, D.G.; Quevillon-Cheruel, S.; Merchant, S.; Guiard, B.; Hamel, P.P. Cyc2p, a membrane-bound flavoprotein involved in the maturation of mitochondrial c-type cytochromes. J. Biol. Chem. 2005, 280, 39852–39859. [Google Scholar] [CrossRef]
- Corvest, V.; Murrey, D.A.; Hirasawa, M.; Knaff, D.B.; Guiard, B.; Hamel, P.P. The flavoprotein cyc2p, a mitochondrial cytochrome c assembly factor, is a nad(p)h-dependent haem reductase. Mol. Microbiol. 2012, 83, 968–980. [Google Scholar] [CrossRef]
- Sun, Y.; Benabbas, A.; Zeng, W.; Kleingardner, J.G.; Bren, K.L.; Champion, P.M. Investigations of heme distortion, low-frequency vibrational excitations, and electron transfer in cytochrome c. Proc. Natl. Acad. Sci. USA 2014, 111, 6570–6575. [Google Scholar] [CrossRef]
- Khalimonchuk, O.; Rodel, G. Biogenesis of cytochrome c oxidase. Mitochondrion 2005, 5, 363–388. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, S.; Shinzawa-Itoh, K.; Nakashima, R.; Yaono, R.; Yamashita, E.; Inoue, N.; Yao, M.; Fei, M.J.; Libeu, C.P.; Mizushima, T.; et al. Redox-coupled crystal structural changes in bovine heart cytochrome c oxidase. Science 1998, 280, 1723–1729. [Google Scholar] [CrossRef] [PubMed]
- Khalimonchuk, O.; Bestwick, M.; Meunier, B.; Watts, T.C.; Winge, D.R. Formation of the redox cofactor centers during cox1 maturation in yeast cytochrome oxidase. Mol. Cell Biol. 2010, 30, 1004–1017. [Google Scholar] [CrossRef] [PubMed]
- Khalimonchuk, O.; Bestwick, M.; Meunier, B.; Watts, T.C.; Winge, D.R. Correction for khalimonchuk et al., “formation of the redox cofactor centers during cox1 maturation in yeast cytochrome oxidase”. Mol. Cell Biol. 2017, 37, 1004–1017. [Google Scholar] [CrossRef]
- Mick, D.U.; Fox, T.D.; Rehling, P. Inventory control: Cytochrome c oxidase assembly regulates mitochondrial translation. Nat. Rev. Mol. Cell Biol. 2011, 12, 14–20. [Google Scholar] [CrossRef]
- Soto, I.C.; Fontanesi, F.; Liu, J.; Barrientos, A. Biogenesis and assembly of eukaryotic cytochrome c oxidase catalytic core. Biochim. Biophys. Acta 2012, 1817, 883–897. [Google Scholar] [CrossRef]
- Dennerlein, S.; Rehling, P. Human mitochondrial cox1 assembly into cytochrome c oxidase at a glance. J. Cell Sci. 2015, 128, 833–837. [Google Scholar] [CrossRef]
- Timon-Gomez, A.; Nyvltova, E.; Abriata, L.A.; Vila, A.J.; Hosler, J.; Barrientos, A. Mitochondrial cytochrome c oxidase biogenesis: Recent developments. Semin. Cell Dev. Biol. 2017, 76, 163–178. [Google Scholar] [CrossRef]
- Bestwick, M.; Khalimonchuk, O.; Pierrel, F.; Winge, D.R. The role of coa2 in hemylation of yeast cox1 revealed by its genetic interaction with cox10. Mol. Cell Biol. 2010, 30, 172–185. [Google Scholar] [CrossRef][Green Version]
- Khalimonchuk, O.; Kim, H.; Watts, T.; Perez-Martinez, X.; Winge, D.R. Oligomerization of heme o synthase in cytochrome oxidase biogenesis is mediated by cytochrome oxidase assembly factor coa2. J. Biol. Chem. 2012, 287, 26715–26726. [Google Scholar] [CrossRef]
- Cheng, W.; Li, W. Structural insights into ubiquinone biosynthesis in membranes. Science 2014, 343, 878–881. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, Y.; Hegg, E.L. Regulation of the heme a biosynthetic pathway: Differential regulation of heme a synthase and heme o synthase in saccharomyces cerevisiae. J. Biol. Chem. 2009, 284, 839–847. [Google Scholar] [CrossRef] [PubMed]
- Reedy, C.J.; Gibney, B.R. Heme protein assemblies. Chem. Rev. 2004, 104, 617–649. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, J.; Reddi, A.R.; Wang, Z.; Khodaverdian, B.; Hegg, E.L.; Gibney, B.R. Evaluating the roles of the heme a side chains in cytochrome c oxidase using designed heme proteins. Biochemistry 2006, 45, 12530–12538. [Google Scholar] [CrossRef]
- Niwa, S.; Takeda, K.; Kosugi, M.; Tsutsumi, E.; Mogi, T.; Miki, K. Crystal structure of heme a synthase from bacillus subtilis. Proc. Natl. Acad. Sci. USA 2018, 115, 11953–11957. [Google Scholar] [CrossRef]
- Swenson, S.; Cannon, A.; Harris, N.J.; Taylor, N.G.; Fox, J.L.; Khalimonchuk, O. Analysis of oligomerization properties of heme a synthase provides insights into its function in eukaryotes. J. Biol. Chem. 2016, 291, 10411–10425. [Google Scholar] [CrossRef]
- Zhang, L.; Hach, A. Molecular mechanism of heme signaling in yeast: The transcriptional activator hap1 serves as the key mediator. Cell Mol. Life Sci. 1999, 56, 415–426. [Google Scholar] [CrossRef]
- Barros, M.H.; Nobrega, F.G.; Tzagoloff, A. Mitochondrial ferredoxin is required for heme a synthesis in saccharomyces cerevisiae. J. Biol. Chem. 2002, 277, 9997–10002. [Google Scholar] [CrossRef]
- Lill, R.; Muhlenhoff, U. Iron-sulfur protein biogenesis in eukaryotes: Components and mechanisms. Annu. Rev. Cell Dev. Biol. 2006, 22, 457–486. [Google Scholar] [CrossRef]
- Stehling, O.; Lill, R. The role of mitochondria in cellular iron-sulfur protein biogenesis: Mechanisms, connected processes, and diseases. Cold Spring Harb. Perspect Biol. 2013, 5, a011312. [Google Scholar] [CrossRef]
- Bureik, M.; Schiffler, B.; Hiraoka, Y.; Vogel, F.; Bernhardt, R. Functional expression of human mitochondrial cyp11b2 in fission yeast and identification of a new internal electron transfer protein, etp1. Biochemistry 2002, 41, 2311–2321. [Google Scholar] [CrossRef] [PubMed]
- Sheftel, A.D.; Stehling, O.; Pierik, A.J.; Elsasser, H.P.; Muhlenhoff, U.; Webert, H.; Hobler, A.; Hannemann, F.; Bernhardt, R.; Lill, R. Humans possess two mitochondrial ferredoxins, fdx1 and fdx2, with distinct roles in steroidogenesis, heme, and fe/s cluster biosynthesis. Proc. Natl. Acad. Sci. USA 2010, 107, 11775–11780. [Google Scholar] [CrossRef] [PubMed]
- McEwen, J.E.; Hong, K.H.; Park, S.; Preciado, G.T. Sequence and chromosomal localization of two pet genes required for cytochrome c oxidase assembly in saccharomyces cerevisiae. Curr. Genet. 1993, 23, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Barros, M.H.; Tzagoloff, A. Regulation of the heme a biosynthetic pathway in saccharomyces cerevisiae. FEBS Lett. 2002, 516, 119–123. [Google Scholar] [CrossRef]
- Taylor, N.G.; Swenson, S.; Harris, N.J.; Germany, E.M.; Fox, J.L.; Khalimonchuk, O. The assembly factor pet117 couples heme a synthase activity to cytochrome oxidase assembly. J. Biol. Chem. 2017, 292, 1815–1825. [Google Scholar] [CrossRef] [PubMed]
- Bareth, B.; Dennerlein, S.; Mick, D.U.; Nikolov, M.; Urlaub, H.; Rehling, P. The heme a synthase cox15 associates with cytochrome c oxidase assembly intermediates during cox1 maturation. Mol. Cell Biol. 2013, 33, 4128–4137. [Google Scholar] [CrossRef] [PubMed]
- Bundschuh, F.A.; Hannappel, A.; Anderka, O.; Ludwig, B. Surf1, associated with leigh syndrome in humans, is a heme-binding protein in bacterial oxidase biogenesis. J. Biol. Chem. 2009, 284, 25735–25741. [Google Scholar] [CrossRef]
- Hannappel, A.; Bundschuh, F.A.; Ludwig, B. Role of surf1 in heme recruitment for bacterial cox biogenesis. Biochim. Biophys. Acta 2012, 1817, 928–937. [Google Scholar] [CrossRef]
- Bestwick, M.; Jeong, M.Y.; Khalimonchuk, O.; Kim, H.; Winge, D.R. Analysis of leigh syndrome mutations in the yeast surf1 homolog reveals a new member of the cytochrome oxidase assembly factor family. Mol. Cell Biol. 2010, 30, 4480–4491. [Google Scholar] [CrossRef]
- Zhu, Z.; Yao, J.; Johns, T.; Fu, K.; De Bie, I.; Macmillan, C.; Cuthbert, A.P.; Newbold, R.F.; Wang, J.; Chevrette, M.; et al. Surf1, encoding a factor involved in the biogenesis of cytochrome c oxidase, is mutated in leigh syndrome. Nat. Genet. 1998, 20, 337–343. [Google Scholar] [CrossRef]
- Pierrel, F.; Khalimonchuk, O.; Cobine, P.A.; Bestwick, M.; Winge, D.R. Coa2 is an assembly factor for yeast cytochrome c oxidase biogenesis that facilitates the maturation of cox1. Mol. Cell Biol. 2008, 28, 4927–4939. [Google Scholar] [CrossRef] [PubMed]
- Khalimonchuk, O.; Jeong, M.Y.; Watts, T.; Ferris, E.; Winge, D.R. Selective oma1 protease-mediated proteolysis of cox1 subunit of cytochrome oxidase in assembly mutants. J. Biol. Chem. 2012, 287, 7289–7300. [Google Scholar] [CrossRef] [PubMed]
- Babbitt, S.E.; San Francisco, B.; Bretsnyder, E.C.; Kranz, R.G. Conserved residues of the human mitochondrial holocytochrome c synthase mediate interactions with heme. Biochemistry 2014, 53, 5261–5271. [Google Scholar] [CrossRef] [PubMed]
- Indrieri, A.; Conte, I.; Chesi, G.; Romano, A.; Quartararo, J.; Tate, R.; Ghezzi, D.; Zeviani, M.; Goffrini, P.; Ferrero, I.; et al. The impairment of hccs leads to mls syndrome by activating a non-canonical cell death pathway in the brain and eyes. EMBO Mol. Med. 2013, 5, 280–293. [Google Scholar] [CrossRef] [PubMed]
- Valnot, I.; Von Kleist-Retzow, J.C.; Barrientos, A.; Gorbatyuk, M.; Taanman, J.W.; Mehaye, B.; Rustin, P.; Tzagoloff, A.; Munnich, A.; Rotig, A. A mutation in the human heme a:Farnesyltransferase gene (cox10 ) causes cytochrome c oxidase deficiency. Hum. Mol. Genet. 2000, 9, 1245–1249. [Google Scholar] [CrossRef]
- Antonicka, H.; Leary, S.C.; Guercin, G.H.; Agar, J.N.; Horvath, R.; Kennaway, N.G.; Harding, C.O.; Jaksch, M.; Shoubridge, E.A. Mutations in cox10 result in a defect in mitochondrial heme a biosynthesis and account for multiple, early-onset clinical phenotypes associated with isolated cox deficiency. Hum. Mol. Genet. 2003, 12, 2693–2702. [Google Scholar] [CrossRef]
- Antonicka, H.; Mattman, A.; Carlson, C.G.; Glerum, D.M.; Hoffbuhr, K.C.; Leary, S.C.; Kennaway, N.G.; Shoubridge, E.A. Mutations in cox15 produce a defect in the mitochondrial heme biosynthetic pathway, causing early-onset fatal hypertrophic cardiomyopathy. Am. J. Hum. Genet. 2003, 72, 101–114. [Google Scholar] [CrossRef]
- Alfadhel, M.; Lillquist, Y.P.; Waters, P.J.; Sinclair, G.; Struys, E.; McFadden, D.; Hendson, G.; Hyams, L.; Shoffner, J.; Vallance, H.D. Infantile cardioencephalopathy due to a cox15 gene defect: Report and review. Am. J. Med. Genet. A 2011, 155A, 840–844. [Google Scholar] [CrossRef]
- Reiter, L.T.; Murakami, T.; Koeuth, T.; Gibbs, R.A.; Lupski, J.R. The human cox10 gene is disrupted during homologous recombination between the 24 kb proximal and distal cmt1a-reps. Hum. Mol. Genet. 1997, 6, 1595–1603. [Google Scholar] [CrossRef]
- Oquendo, C.E.; Antonicka, H.; Shoubridge, E.A.; Reardon, W.; Brown, G.K. Functional and genetic studies demonstrate that mutation in the cox15 gene can cause leigh syndrome. J. Med. Genet. 2004, 41, 540–544. [Google Scholar] [CrossRef]
- Bugiani, M.; Tiranti, V.; Farina, L.; Uziel, G.; Zeviani, M. Novel mutations in cox15 in a long surviving leigh syndrome patient with cytochrome c oxidase deficiency. J. Med. Genet. 2005, 42, e28. [Google Scholar] [CrossRef] [PubMed]
- Adams, P.L.; Lightowlers, R.N.; Turnbull, D.M. Molecular analysis of cytochrome c oxidase deficiency in leigh’s syndrome. Ann. Neurol. 1997, 41, 268–270. [Google Scholar] [CrossRef] [PubMed]
- Tiranti, V.; Hoertnagel, K.; Carrozzo, R.; Galimberti, C.; Munaro, M.; Granatiero, M.; Zelante, L.; Gasparini, P.; Marzella, R.; Rocchi, M.; et al. Mutations of surf-1 in leigh disease associated with cytochrome c oxidase deficiency. Am. J. Hum. Genet. 1998, 63, 1609–1621. [Google Scholar] [CrossRef] [PubMed]
- Teraoka, M.; Yokoyama, Y.; Ninomiya, S.; Inoue, C.; Yamashita, S.; Seino, Y. Two novel mutations of surf1 in leigh syndrome with cytochrome c oxidase deficiency. Hum. Genet. 1999, 105, 560–563. [Google Scholar] [CrossRef] [PubMed]
- Poyau, A.; Buchet, K.; Bouzidi, M.F.; Zabot, M.T.; Echenne, B.; Yao, J.; Shoubridge, E.A.; Godinot, C. Missense mutations in surf1 associated with deficient cytochrome c oxidase assembly in leigh syndrome patients. Hum. Genet. 2000, 106, 194–205. [Google Scholar] [PubMed]
- Piekutowska-Abramczuk, D.; Magner, M.; Popowska, E.; Pronicki, M.; Karczmarewicz, E.; Sykut-Cegielska, J.; Kmiec, T.; Jurkiewicz, E.; Szymanska-Debinska, T.; Bielecka, L.; et al. Surf1 missense mutations promote a mild leigh phenotype. Clin. Genet. 2009, 76, 195–204. [Google Scholar] [CrossRef]
- Coenen, M.J.; Van den Heuvel, L.P.; Ugalde, C.; Ten Brinke, M.; Nijtmans, L.G.; Trijbels, F.J.; Beblo, S.; Maier, E.M.; Muntau, A.C.; Smeitink, J.A. Cytochrome c oxidase biogenesis in a patient with a mutation in cox10 gene. Ann. Neurol. 2004, 56, 560–564. [Google Scholar] [CrossRef]
- Shoubridge, E.A. Cytochrome c oxidase deficiency. Am. J. Med. Genet. 2001, 106, 46–52. [Google Scholar] [CrossRef]
- Szklarczyk, R.; Wanschers, B.F.; Cuypers, T.D.; Esseling, J.J.; Riemersma, M.; Van den Brand, M.A.; Gloerich, J.; Lasonder, E.; Van den Heuvel, L.P.; Nijtmans, L.G.; et al. Iterative orthology prediction uncovers new mitochondrial proteins and identifies c12orf62 as the human ortholog of cox14, a protein involved in the assembly of cytochrome c oxidase. Genome. Biol. 2012, 13, R12. [Google Scholar] [CrossRef]
- Renkema, G.H.; Visser, G.; Baertling, F.; Wintjes, L.T.; Wolters, V.M.; Van Montfrans, J.; De Kort, G.A.P.; Nikkels, P.G.J.; Van Hasselt, P.M.; Van der Crabben, S.N.; et al. Mutated pet117 causes complex iv deficiency and is associated with neurodevelopmental regression and medulla oblongata lesions. Hum. Genet. 2017, 136, 759–769. [Google Scholar] [CrossRef]
- Yuan, X.; Rietzschel, N.; Kwon, H.; Walter Nuno, A.B.; Hanna, D.A.; Phillips, J.D.; Raven, E.L.; Reddi, A.R.; Hamza, I. Regulation of intracellular heme trafficking revealed by subcellular reporters. Proc. Natl. Acad. Sci. USA 2016, 113, e5144–e5152. [Google Scholar] [CrossRef] [PubMed]
- Chiabrando, D.; Marro, S.; Mercurio, S.; Giorgi, C.; Petrillo, S.; Vinchi, F.; Fiorito, V.; Fagoonee, S.; Camporeale, A.; Turco, E.; et al. The mitochondrial heme exporter flvcr1b mediates erythroid differentiation. J. Clin. Investig. 2012, 122, 4569–4579. [Google Scholar] [CrossRef] [PubMed]
- Quigley, J.G.; Yang, Z.; Worthington, M.T.; Phillips, J.D.; Sabo, K.M.; Sabath, D.E.; Berg, C.L.; Sassa, S.; Wood, B.L.; Abkowitz, J.L. Identification of a human heme exporter that is essential for erythropoiesis. Cell 2004, 118, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A.; Quigley, J.G. Heme and flvcr-related transporter families slc48 and slc49. Mol. Aspects Med. 2013, 34, 669–682. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, K.; Thompson, A.M.; Goldbeck, R.A.; Shi, X.; Whitman, S.; Oh, E.; Zhiwu, Z.; Vulpe, C.; Holman, T.R. Spectroscopic and biochemical characterization of heme binding to yeast dap1p and mouse pgrmc1p. Biochemistry 2005, 44, 16729–16736. [Google Scholar] [CrossRef]
- Kaluka, D.; Batabyal, D.; Chiang, B.Y.; Poulos, T.L.; Yeh, S.R. Spectroscopic and mutagenesis studies of human pgrmc1. Biochemistry 2015, 54, 1638–1647. [Google Scholar] [CrossRef]
- Min, L.; Strushkevich, N.V.; Harnastai, I.N.; Iwamoto, H.; Gilep, A.A.; Takemori, H.; Usanov, S.A.; Nonaka, Y.; Hori, H.; Vinson, G.P.; et al. Molecular identification of adrenal inner zone antigen as a heme-binding protein. FEBS J. 2005, 272, 5832–5843. [Google Scholar] [CrossRef]
- Peluso, J.J.; Liu, X.; Gawkowska, A.; Lodde, V.; Wu, C.A. Progesterone inhibits apoptosis in part by pgrmc1-regulated gene expression. Mol. Cell Endocrinol. 2010, 320, 153–161. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, S.Y.; Choi, H.S.; An, S.; Ryu, C.J. Epitope mapping of anti-pgrmc1 antibodies reveals the non-conventional membrane topology of pgrmc1 on the cell surface. Sci. Rep. 2019, 9, 653. [Google Scholar] [CrossRef]
- Vance, J.E. Mam (mitochondria-associated membranes) in mammalian cells: Lipids and beyond. Biochim. Biophys. Acta 2014, 1841, 595–609. [Google Scholar] [CrossRef]
- Poston, C.N.; Duong, E.; Cao, Y.; Bazemore-Walker, C.R. Proteomic analysis of lipid raft-enriched membranes isolated from internal organelles. Biochem. Biophys. Res. Commun. 2011, 415, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Cho, I.T.; Adelmant, G.; Lim, Y.; Marto, J.A.; Cho, G.; Golden, J.A. Ascorbate peroxidase proximity labeling coupled with biochemical fractionation identifies promoters of endoplasmic reticulum-mitochondrial contacts. J. Biol. Chem. 2017, 292, 16382–16392. [Google Scholar] [CrossRef]
- Schumann, U.; Subramani, S. Special delivery from mitochondria to peroxisomes. Trends Cell Biol. 2008, 18, 253–256. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.U.; Carta, L.K.; Lesuisse, E.; Hamza, I. Lack of heme synthesis in a free-living eukaryote. Proc. Natl. Acad. Sci. USA 2005, 102, 4270–4275. [Google Scholar] [CrossRef] [PubMed]
- Rajagopal, A.; Rao, A.U.; Amigo, J.; Tian, M.; Upadhyay, S.K.; Hall, C.; Uhm, S.; Mathew, M.K.; Fleming, M.D.; Paw, B.H.; et al. Haem homeostasis is regulated by the conserved and concerted functions of hrg-1 proteins. Nature 2008, 453, 1127–1131. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan, K.M.; Ayllon, V.; O’Keeffe, J.; Wang, Y.; Cox, O.T.; Loughran, G.; Forgac, M.; O’Connor, R. Heme-binding protein hrg-1 is induced by insulin-like growth factor i and associates with the vacuolar h+-atpase to control endosomal ph and receptor trafficking. J. Biol. Chem. 2010, 285, 381–391. [Google Scholar] [CrossRef]
- Yuan, X.; Protchenko, O.; Philpott, C.C.; Hamza, I. Topologically conserved residues direct heme transport in hrg-1-related proteins. J. Biol. Chem. 2012, 287, 4914–4924. [Google Scholar] [CrossRef]
- White, C.; Yuan, X.; Schmidt, P.J.; Bresciani, E.; Samuel, T.K.; Campagna, D.; Hall, C.; Bishop, K.; Calicchio, M.L.; Lapierre, A.; et al. Hrg1 is essential for heme transport from the phagolysosome of macrophages during erythrophagocytosis. Cell Metab. 2013, 17, 261–270. [Google Scholar] [CrossRef]
- Duffy, S.P.; Shing, J.; Saraon, P.; Berger, L.C.; Eiden, M.V.; Wilde, A.; Tailor, C.S. The fowler syndrome-associated protein flvcr2 is an importer of heme. Mol. Cell Biol. 2010, 30, 5318–5324. [Google Scholar] [CrossRef]
- Okazaki, T.; Yanagisawa, Y.; Nagai, T. Analysis of the affinity of each haptoglobin polymer for hemoglobin by two-dimensional affinity electrophoresis. Clin. Chim. Acta 1997, 258, 137–144. [Google Scholar] [CrossRef]
- Graversen, J.H.; Madsen, M.; Moestrup, S.K. Cd163: A signal receptor scavenging haptoglobin-hemoglobin complexes from plasma. Int. J. Biochem. Cell Biol. 2002, 34, 309–314. [Google Scholar] [CrossRef]
- Delaby, C.; Pilard, N.; Puy, H.; Canonne-Hergaux, F. Sequential regulation of ferroportin expression after erythrophagocytosis in murine macrophages: Early mrna induction by haem, followed by iron-dependent protein expression. Biochem. J. 2008, 411, 123–131. [Google Scholar] [CrossRef]
- Hrkal, Z.; Vodrázka, Z.; Kalousek, I. Transfer of heme from ferrihemoglobin and ferrihemoglobin isolated chains to hemopexin. Eur. J. Biochem. 1974, 43, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Vinchi, F.; Gastaldi, S.; Silengo, L.; Altruda, F.; Tolosano, E. Hemopexin prevents endothelial damage and liver congestion in a mouse model of heme overload. Am. J. Pathol. 2008, 173, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Hvidberg, V.; Maniecki, M.B.; Jacobsen, C.; Hojrup, P.; Moller, H.J.; Moestrup, S.K. Identification of the receptor scavenging hemopexin-heme complexes. Blood 2005, 106, 2572–2579. [Google Scholar] [CrossRef]
- Camus, S.M.; De Moraes, J.A.; Bonnin, P.; Abbyad, P.; Le Jeune, S.; Lionnet, F.; Loufrani, L.; Grimaud, L.; Lambry, J.C.; Charue, D.; et al. Circulating cell membrane microparticles transfer heme to endothelial cells and trigger vasoocclusions in sickle cell disease. Blood 2015, 125, 3805–3814. [Google Scholar] [CrossRef]
- Donadee, C.; Raat, N.J.; Kanias, T.; Tejero, J.; Lee, J.S.; Kelley, E.E.; Zhao, X.; Liu, C.; Reynolds, H.; Azarov, I.; et al. Nitric oxide scavenging by red blood cell microparticles and cell-free hemoglobin as a mechanism for the red cell storage lesion. Circulation 2011, 124, 465–476. [Google Scholar] [CrossRef]
- Kim-Shapiro, D.B.; Lee, J.; Gladwin, M.T. Storage lesion: Role of red blood cell breakdown. Transfusion 2011, 51, 844–851. [Google Scholar] [CrossRef]
- Gaggar, A.; Patel, R.P. There is blood in the water: Hemolysis, hemoglobin, and heme in acute lung injury. Am. J. Physiol. Lung. Cell Mol. Physiol. 2016, 311, L714–L718. [Google Scholar] [CrossRef]
- Kim, Y.; Abplanalp, W.A.; Jung, A.D.; Schuster, R.M.; Lentsch, A.B.; Gulbins, E.; Caldwell, C.C.; Pritts, T.A. Endocytosis of red blood cell microparticles by pulmonary endothelial cells is mediated by rab5. Shock 2018, 49, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Gollub, E.G.; Liu, K.P.; Dayan, J.; Adlersberg, M.; Sprinson, D.B. Yeast mutants deficient in heme biosynthesis and a heme mutant additionally blocked in cyclization of 2,3-oxidosqualene. J. Biol. Chem. 1977, 252, 2846–2854. [Google Scholar]
- Kim, H.J.; Jeong, M.Y.; Parnell, T.J.; Babst, M.; Phillips, J.D.; Winge, D.R. The plasma membrane protein nce102 implicated in eisosome formation rescues a heme defect in mitochondria. J. Biol. Chem. 2016, 291, 17417–17426. [Google Scholar] [CrossRef] [PubMed]
- Grossmann, G.; Malinsky, J.; Stahlschmidt, W.; Loibl, M.; Weig-Meckl, I.; Frommer, W.B.; Opekarova, M.; Tanner, W. Plasma membrane microdomains regulate turnover of transport proteins in yeast. J. Cell Biol. 2008, 183, 1075–1088. [Google Scholar] [CrossRef] [PubMed]
- Mourer, T.; Brault, A.; Labbe, S. Heme acquisition by shu1 requires nbr1 and proteins of the escrt complex in schizosaccharomyces pombe. Mol. Microbiol. 2019, 112, 1499–1518. [Google Scholar] [CrossRef] [PubMed]
- Mourer, T.; Jacques, J.F.; Brault, A.; Bisaillon, M.; Labbe, S. Shu1 is a cell-surface protein involved in iron acquisition from heme in schizosaccharomyces pombe. J. Biol. Chem. 2015, 290, 10176–10190. [Google Scholar] [CrossRef]
- Mourer, T.; Normant, V.; Labbe, S. Heme assimilation in schizosaccharomyces pombe requires cell-surface-anchored protein shu1 and vacuolar transporter abc3. J. Biol. Chem. 2017, 292, 4898–4912. [Google Scholar] [CrossRef]
- Normant, V.; Mourer, T.; Labbe, S. The major facilitator transporter str3 is required for low-affinity heme acquisition in schizosaccharomyces pombe. J. Biol. Chem. 2018, 293, 6349–6362. [Google Scholar] [CrossRef]
- Protchenko, O.; Shakoury-Elizeh, M.; Keane, P.; Storey, J.; Androphy, R.; Philpott, C.C. Role of pug1 in inducible porphyrin and heme transport in saccharomyces cerevisiae. Eukaryot Cell 2008, 7, 859–871. [Google Scholar] [CrossRef]
- Puy, H.; Gouya, L.; Deybach, J.C. Porphyrias. Lancet 2010, 375, 924–937. [Google Scholar] [CrossRef]
- Chen, C.; Samuel, T.K.; Sinclair, J.; Dailey, H.A.; Hamza, I. An intercellular heme-trafficking protein delivers maternal heme to the embryo during development in c. Elegans. Cell 2011, 145, 720–731. [Google Scholar] [CrossRef]
- Bonkovsky, H.L.; Healey, J.F.; Lourie, A.N.; Gerron, G.G. Intravenous heme-albumin in acute intermittent porphyria: Evidence for repletion of hepatic hemoproteins and regulatory heme pools. Am. J. Gastroenterol. 1991, 86, 1050–1056. [Google Scholar] [PubMed]
- Yang, Z.; Philips, J.D.; Doty, R.T.; Giraudi, P.; Ostrow, J.D.; Tiribelli, C.; Smith, A.; Abkowitz, J.L. Kinetics and specificity of feline leukemia virus subgroup c receptor (flvcr) export function and its dependence on hemopexin. J. Biol. Chem. 2010, 285, 28874–28882. [Google Scholar] [CrossRef] [PubMed]
- Korolnek, T.; Zhang, J.; Beardsley, S.; Scheffer, G.L.; Hamza, I. Control of metazoan heme homeostasis by a conserved multidrug resistance protein. Cell Metab. 2014, 19, 1008–1019. [Google Scholar] [CrossRef] [PubMed]
- Desuzinges-Mandon, E.; Arnaud, O.; Martinez, L.; Huche, F.; Di Pietro, A.; Falson, P. Abcg2 transports and transfers heme to albumin through its large extracellular loop. J. Biol. Chem. 2010, 285, 33123–33133. [Google Scholar] [CrossRef]
- Krishnamurthy, P.; Ross, D.D.; Nakanishi, T.; Bailey-Dell, K.; Zhou, S.; Mercer, K.E.; Sarkadi, B.; Sorrentino, B.P.; Schuetz, J.D. The stem cell marker bcrp/abcg2 enhances hypoxic cell survival through interactions with heme. J. Biol. Chem. 2004, 279, 24218–24225. [Google Scholar] [CrossRef] [PubMed]
- Saison, C.; Helias, V.; Ballif, B.A.; Peyrard, T.; Puy, H.; Miyazaki, T.; Perrot, S.; Vayssier-Taussat, M.; Waldner, M.; Le Pennec, P.Y.; et al. Null alleles of abcg2 encoding the breast cancer resistance protein define the new blood group system junior. Nat. Genet. 2012, 44, 174–177. [Google Scholar] [CrossRef] [PubMed]
- Kitajima, Y.; Ishii, T.; Kohda, T.; Ishizuka, M.; Yamazaki, K.; Nishimura, Y.; Tanaka, T.; Dan, S.; Nakajima, M. Mechanistic study of ppix accumulation using the jfcr39 cell panel revealed a role for dynamin 2-mediated exocytosis. Sci. Rep. 2019, 9, 8666. [Google Scholar] [CrossRef]
- Dutta, R.; Zhang, T.Y.; Kohnke, T.; Thomas, D.; Linde, M.; Gars, E.; Stafford, M.; Kaur, S.; Nakauchi, Y.; Yin, R.; et al. Enasidenib drives human erythroid differentiation independently of isocitrate dehydrogenase 2. J. Clin. Investig. 2020. [Google Scholar] [CrossRef]
- Robey, R.W.; To, K.K.; Polgar, O.; Dohse, M.; Fetsch, P.; Dean, M.; Bates, S.E. Abcg2: A perspective. Adv. Drug Deliv. Rev. 2009, 61, 3–13. [Google Scholar] [CrossRef]
- Donegan, R.K.; Moore, C.M.; Hanna, D.A.; Reddi, A.R. Handling heme: The mechanisms underlying the movement of heme within and between cells. Free Radic. Biol. Med. 2019, 133, 88–100. [Google Scholar] [CrossRef]
- Chakravarti, R.; Aulak, K.S.; Fox, P.L.; Stuehr, D.J. Gapdh regulates cellular heme insertion into inducible nitric oxide synthase. Proc. Natl. Acad. Sci. USA 2010, 107, 18004–18009. [Google Scholar] [CrossRef]
- Wu, B.; Novelli, J.; Jiang, D.; Dailey, H.A.; Landmann, F.; Ford, L.; Taylor, M.J.; Carlow, C.K.; Kumar, S.; Foster, J.M.; et al. Interdomain lateral gene transfer of an essential ferrochelatase gene in human parasitic nematodes. Proc. Natl. Acad. Sci. USA 2013, 110, 7748–7753. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, J.; Hamza, I. Lessons from bloodless worms: Heme homeostasis in c. Elegans. Biometals 2015, 28, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Braz, G.R.; Coelho, H.S.; Masuda, H.; Oliveira, P.L. A missing metabolic pathway in the cattle tick boophilus microplus. Curr. Biol. 1999, 9, 703–706. [Google Scholar] [CrossRef]
- Heggland, E.I.; Eichner, C.; Stove, S.I.; Martinez, A.; Nilsen, F.; Dondrup, M. A scavenger receptor b (cd36)-like protein is a potential mediator of intestinal heme absorption in the hematophagous ectoparasite lepeophtheirus salmonis. Sci. Rep. 2019, 9, 4218. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.Y.; Hamm, J.; Xu, X.; Genschmer, K.; Zhong, M.; Lebensburger, J.; Marques, M.B.; Kerby, J.D.; Pittet, J.F.; Gaggar, A.; et al. Absorbance and redox based approaches for measuring free heme and free hemoglobin in biological matrices. Redox Biol. 2016, 9, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Fronticelli, C.; Bucci, E. Acetone extraction of heme from myoglobin and hemoglobin at acid ph. Biochim. Biophys. Acta 1963, 78, 530–531. [Google Scholar] [CrossRef]
- Thomas, J.; Weinstein, J.D. Measurement of heme efflux and heme content in isolated developing chloroplasts. Plant Physiol. 1990, 94, 1414–1423. [Google Scholar] [CrossRef]
- Sinclair, P.R.; Gorman, N.; Jacobs, J.M. Measurement of heme concentration. Curr. Protoc. Toxicol. 1999, 00. [Google Scholar] [CrossRef]
- Woods, J.S.; Simmonds, P.L. Hplc methods for analysis of porphyrins in biological media. Curr. Protoc. Toxicol. 2001, 7, 8.9.1–8.9.17. [Google Scholar] [CrossRef]
- Paul, K.; Theorell, H.; Akeson, A. The molar light absorption of pyridine ferroprotoporphyrin (pyridine haemochromogen). Acta Chem. Scand 1953, 7, 1284–1287. [Google Scholar] [CrossRef]
- Barr, I.; Guo, F. Pyridine hemochromagen assay for determining the concentration of heme in purified protein solutions. Bio-protocol 2015, 5, e1594. [Google Scholar] [CrossRef] [PubMed]
- Fuhrhop, J.-H.; Smith, K.M. Laboratory Methods in Porphyrin and Metalloporphyrin Research; Elsevier Science & Technology: Amsterdam, The Netherlands, 1975. [Google Scholar]
- Marcero, J.R.; Piel Iii, R.B.; Burch, J.S.; Dailey, H.A. Rapid and sensitive quantitation of heme in hemoglobinized cells. Biotechniques 2016, 61, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Blake, R.C.I.; Griff, M.N. In situ spectroscopy on intact leptospirillum ferrooxidans reveals that reduced cytochrome 579 is an obligatory intermediate in the aerobic iron respiratory chain. Front. Microbiol. 2012, 3, 136. [Google Scholar] [CrossRef]
- Correia, M.A.; Sinclair, P.R.; De Matteis, F. Cytochrome p450 regulation: The interplay between its heme and apoprotein moieties in synthesis, assembly, repair, and disposal. Drug Metab. Rev. 2011, 43, 1–26. [Google Scholar] [CrossRef]
- Guengerich, F.P.; Martin, M.V.; Sohl, C.D.; Cheng, Q. Measurement of cytochrome p450 and nadph-cytochrome p450 reductase. Nat. Protoc. 2009, 4, 1245–1251. [Google Scholar] [CrossRef]
- Hanna, D.A.; Hu, R.; Kim, H.; Martinez-Guzman, O.; Torres, M.P.; Reddi, A.R. Heme bioavailability and signaling in response to stress in yeast cells. J. Biol. Chem. 2018, 293, 12378–12393. [Google Scholar] [CrossRef]
- Dick, R.; Murray, B.P.; Reid, M.J.; Correia, M.A. Structure--function relationships of rat hepatic tryptophan 2,3-dioxygenase: Identification of the putative heme-ligating histidine residues. Arch. Biochem. Biophys. 2001, 392, 71–78. [Google Scholar] [CrossRef]
- Nelp, M.T.; Kates, P.A.; Hunt, J.T.; Newitt, J.A.; Balog, A.; Maley, D.; Zhu, X.; Abell, L.; Allentoff, A.; Borzilleri, R.; et al. Immune-modulating enzyme indoleamine 2,3-dioxygenase is effectively inhibited by targeting its apo-form. Proc. Natl. Acad. Sci. USA 2018, 115, 3249–3254. [Google Scholar] [CrossRef]
- Atamna, H.; Brahmbhatt, M.; Atamna, W.; Shanower, G.A.; Dhahbi, J.M. Apohrp-based assay to measure intracellular regulatory heme. Metallomics 2015, 7, 309–321. [Google Scholar] [CrossRef]
- Song, Y.; Yang, M.; Wegner, S.V.; Zhao, J.; Zhu, R.; Wu, Y.; He, C.; Chen, P.R. A genetically encoded fret sensor for intracellular heme. ACS Chem. Biol. 2015, 10, 1610–1615. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Sligar, S.G.; Wand, A.J. Solution structure of apocytochrome b562. Nat. Struct. Biol. 1994, 1, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Abshire, J.R.; Rowlands, C.J.; Ganesan, S.M.; So, P.T.; Niles, J.C. Quantification of labile heme in live malaria parasites using a genetically encoded biosensor. Proc. Natl. Acad. Sci. USA 2017, 114, E2068–E2076. [Google Scholar] [CrossRef] [PubMed]
- Strommen, D.P.; Nakamoto, K. Resonance raman spectroscopy. J. Chem. Educ. 1977, 54, 474. [Google Scholar] [CrossRef]
- Keren, S.; Zavaleta, C.; Cheng, Z.; De La Zerda, A.; Gheysens, O.; Gambhir, S. Noninvasive molecular imaging of small living subjects using raman spectroscopy. Proc. Nat. Acad. Sci. USA 2008, 105, 5844–5849. [Google Scholar] [CrossRef]
- Bonifacio, A.; Finaurini, S.; Krafft, C.; Parapini, S.; Taramelli, D.; Sergo, V. Spatial distribution of heme species in erythrocytes infected with plasmodium falciparum by use of resonance raman imaging and multivariate analysis. Anal. Bioanal. Chem. 2008, 392, 1277–1282. [Google Scholar] [CrossRef]
- Boyer, D.; Tamarat, P.; Maali, A.; Lounis, B.; Orrit, M. Photothermal imaging of nanometer-sized metal particles among scatterers. Science 2002, 297, 1160–1163. [Google Scholar] [CrossRef]
- Lu, S.; Min, W.; Chong, S.; Holtom, G.R.; Xie, X.S. Label-free imaging of heme proteins with two-photon excited photothermal lens microscopy. Appl. Phys. Lett. 2010, 96, 113701. [Google Scholar] [CrossRef]
- Chen, A.J.; Yuan, X.; Li, J.; Dong, P.; Hamza, I.; Cheng, J.-X. Label-free imaging of heme dynamics in living organisms by transient absorption microscopy. Anal. Chem. 2018, 90, 3395–3401. [Google Scholar] [CrossRef]
- Wei, L.; Min, W. Pump-probe optical microscopy for imaging nonfluorescent chromophores. Anal. Bioanal. Chem. 2012, 403, 2197–2202. [Google Scholar] [CrossRef]
- Min, W.; Lu, S.; Chong, S.; Roy, R.; Holtom, G.R.; Xie, X.S. Imaging chromophores with undetectable fluorescence by stimulated emission microscopy. Nature 2009, 461, 1105–1109. [Google Scholar] [CrossRef] [PubMed]
- Domingue, S.R.; Bartels, R.A.; Chicco, A.J.; Wilson, J.W. Transient absorption imaging of hemes with 2-color, independently tunable visible-wavelength ultrafast source. Biomed. Opt. Express 2017, 8, 2807–2821. [Google Scholar] [CrossRef] [PubMed]
| Heme Homeostatic Process | Enzyme | Saccharomyces cerevisiae | Caenorhabditis elegans | Homo sapiens |
|---|---|---|---|---|
| Heme Synthesis | 5-aminolevulinic acid synthase | Hem1 | ✖ | ALAS1/ALAS2 |
| Porphobilinogen synthase | Hem2 | ✖ | PBGS | |
| Hydroxymethylbilane synthase | Hem3 | ✖ | HMBS | |
| Uroporphyrinogen synthase | Hem4 | ✖ | UROS | |
| Uroporphyrinogen decarboxylase | Hem12 | ✖ | UROD | |
| Coproporphyrinogen oxidase | Hem13 | ✖ | CPOX | |
| Protoporphyrinogen oxidase | Hem14 | ✖ | PPOX | |
| Ferrochelatase | Hem15 | fecl-1 | FECH | |
| Heme Degradation | Heme oxygenase | Hmx1 | ? | Hmox1/Hmox2 |
| Heme Import | FLVCR2 | ✖ | ✔ | ✔ |
| HRG4 | ✖ | ✔ | ✖ | |
| Heme Export | FLVCR1 | ✖ | ✖ | ✔ |
| MRP5 | ✖ | mrp-5 | ABCC5 | |
| Pug1 | ✔ | ✖ | ✖ | |
| HRG3 | ✖ | ✔ | ✖ | |
| Heme Trafficking | PGRMC1/2 | Dap1 | vem-1 | PGRMC1/2 |
| GAPDH | Tdh1/2/3 | gpd1/2/3/4 | GAPDH | |
| HRG1 | ✖ | ✔ | ✔ |
| Approaches | Methods | Advantages | Disadvantages |
|---|---|---|---|
| In Situ Label Free Imaging | Transient Absorption Microscopy Resonance Raman Imaging 2 Photon Photothermal Lens Microscopy | Subcellular resolution (<1 μm) Non-invasive Can probe heme dynamics in living cells | Signals dominated by most abundant and/or highly absorbing species Low-throughput Requires specialized equipment/expertise |
| In Situ Imaging of Labile Heme using Fluorescent Heme Sensors | HS1 CISDY-9 CHY | Subcellular resolution (<1 μm) Direct probe of labile “bioavailable” heme Can probe heme dynamics in living cells High-throughput | May perturb heme homeostasis Possible selection bias depending on the nature of the sensor Extended time resolved studies precluded by photobleaching |
| Assays for Endogenous Markers of Heme Bioavailability | Horseradish Peroxidase Tryptophan 2,3 Dioxygenase (TDO) Indoleamine-2,3-Dioxygenase (IDO) Cytochrome P450 Catalase Transcription Factors | Measurement of heme accessible to endogenous hemoproteins No genetic perturbations | Disruption of cells and tissues Time consuming Difficult to get fast time resolution |
| Assays for Total Heme | HPLC | Resolve different heme types | Time consuming Disruption of cells and tissues Low-throughput |
| Porphyrin Fluorescence | nM sensitivity High-throughput | Disruption of cells and tissues | |
| UV/vis Absorbance Spectroscopy | CLARiTY | Sensitive measurements in turbid samples Possible to measure heme and hemoproteins in intact cells | Signals dominated by most abundant and/or highly absorbing species Low-throughput Requires specialized equipment |
| Pyridine Hemochromagen | Broadly accessible Inexpensive | Disruption of cells and tissues |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Swenson, S.A.; Moore, C.M.; Marcero, J.R.; Medlock, A.E.; Reddi, A.R.; Khalimonchuk, O. From Synthesis to Utilization: The Ins and Outs of Mitochondrial Heme. Cells 2020, 9, 579. https://doi.org/10.3390/cells9030579
Swenson SA, Moore CM, Marcero JR, Medlock AE, Reddi AR, Khalimonchuk O. From Synthesis to Utilization: The Ins and Outs of Mitochondrial Heme. Cells. 2020; 9(3):579. https://doi.org/10.3390/cells9030579
Chicago/Turabian StyleSwenson, Samantha A., Courtney M. Moore, Jason R. Marcero, Amy E. Medlock, Amit R. Reddi, and Oleh Khalimonchuk. 2020. "From Synthesis to Utilization: The Ins and Outs of Mitochondrial Heme" Cells 9, no. 3: 579. https://doi.org/10.3390/cells9030579
APA StyleSwenson, S. A., Moore, C. M., Marcero, J. R., Medlock, A. E., Reddi, A. R., & Khalimonchuk, O. (2020). From Synthesis to Utilization: The Ins and Outs of Mitochondrial Heme. Cells, 9(3), 579. https://doi.org/10.3390/cells9030579







