Asbestos and Intrahepatic Cholangiocarcinoma
Abstract
1. Introduction
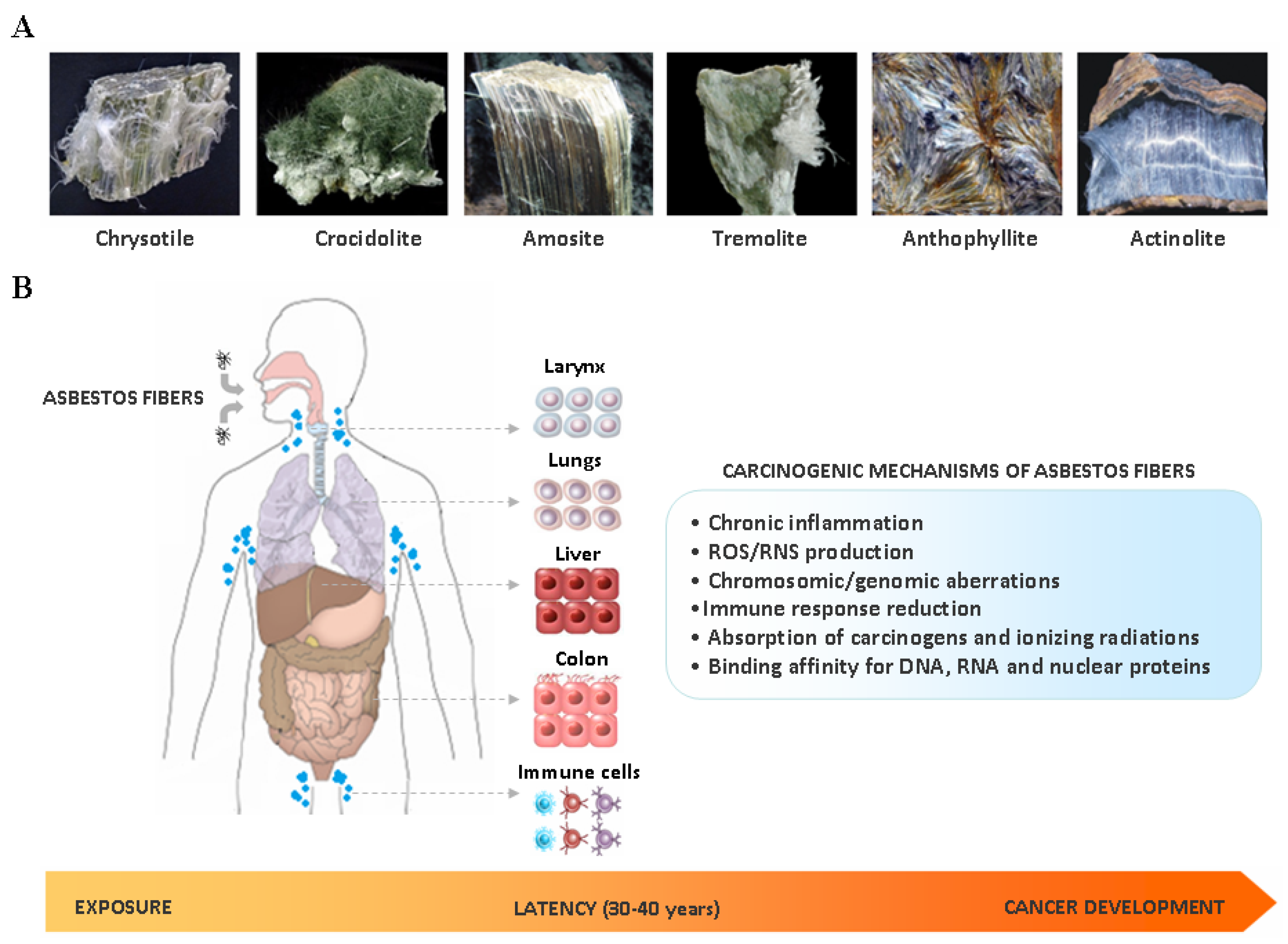
2. Asbestos Carcinogenesis
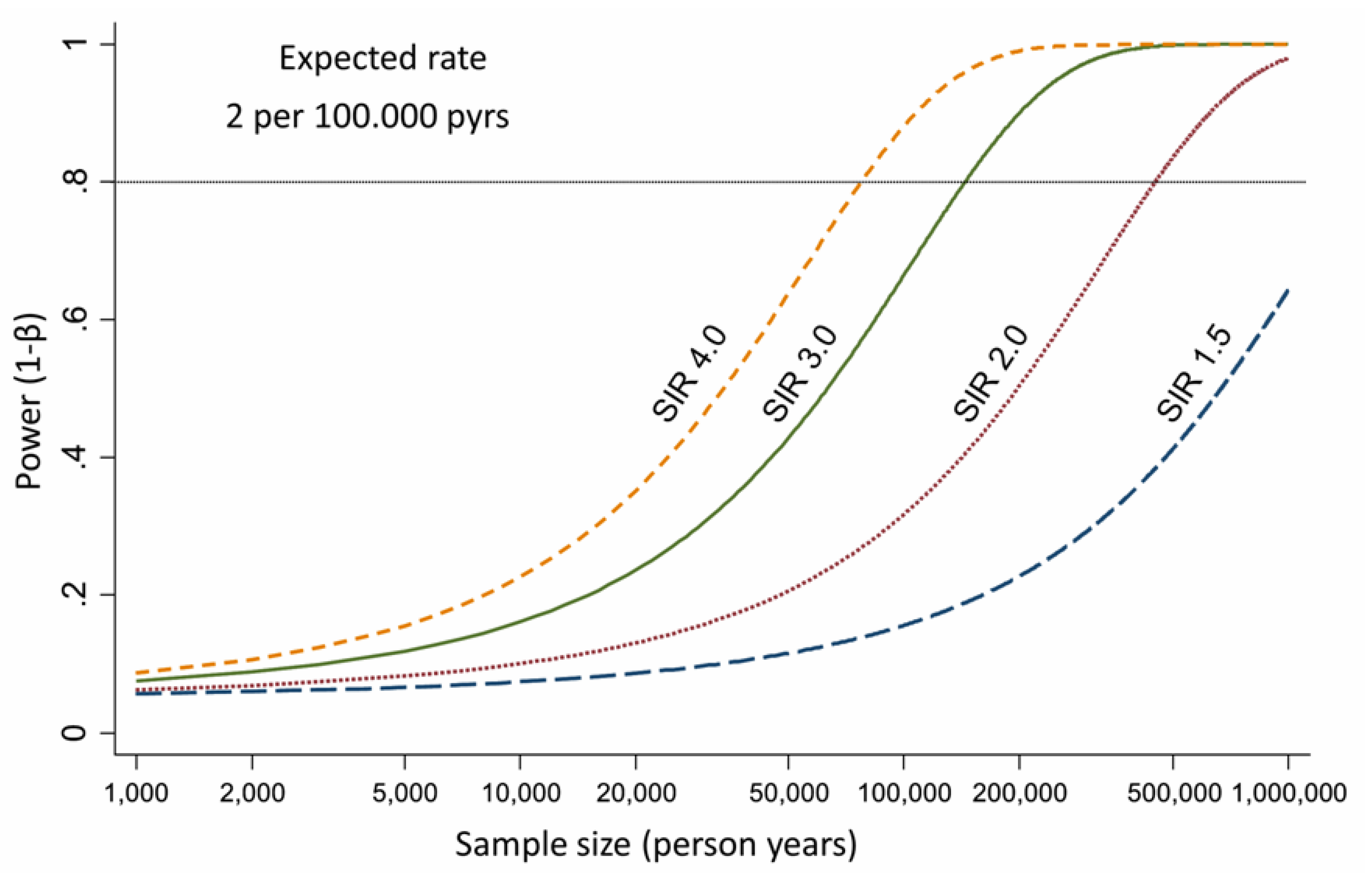
3. Epidemiological Evidences about Asbestos Exposure and ICC Development
4. Adult Liver, Biliary Tree and Stem Cell Niches
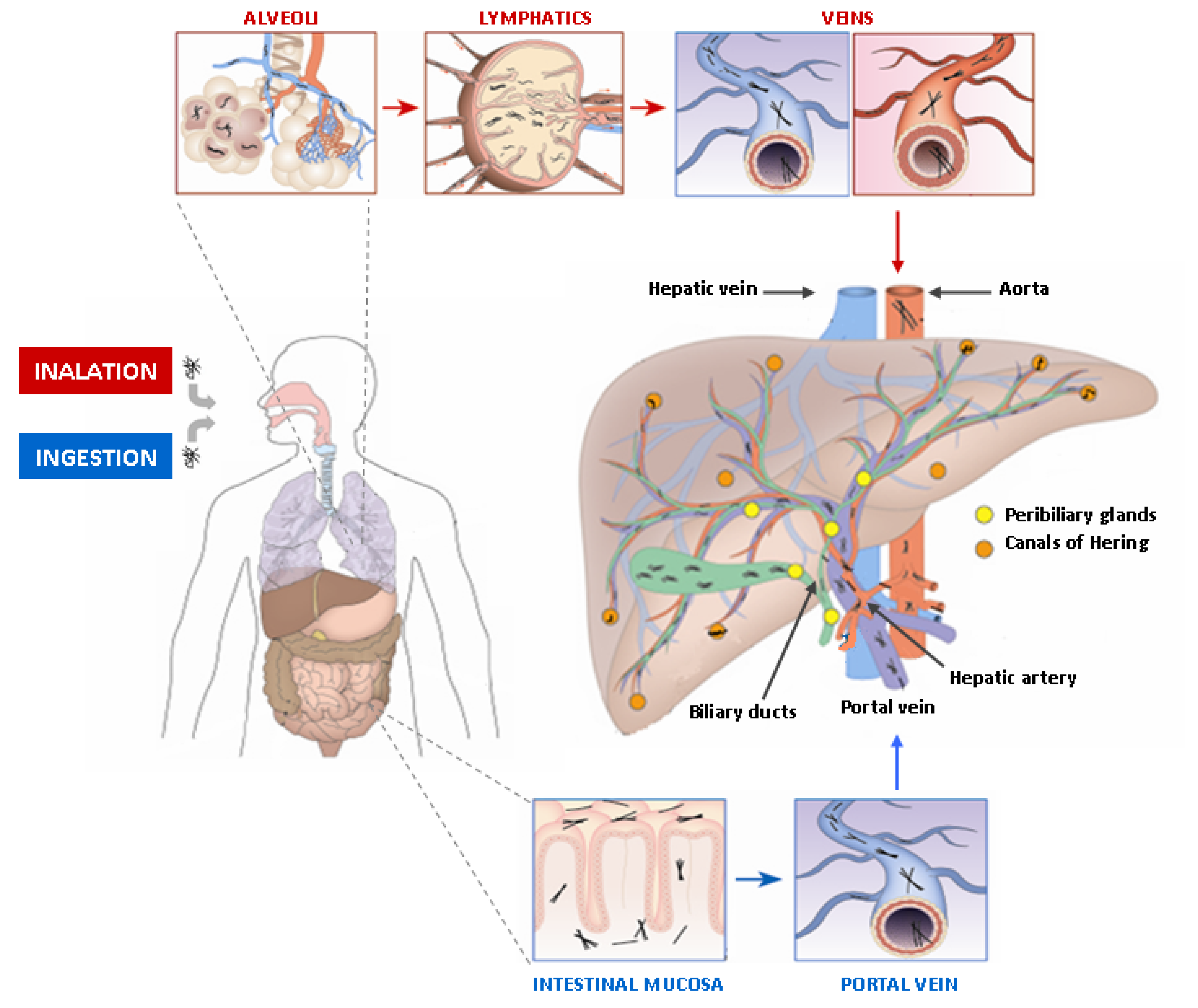
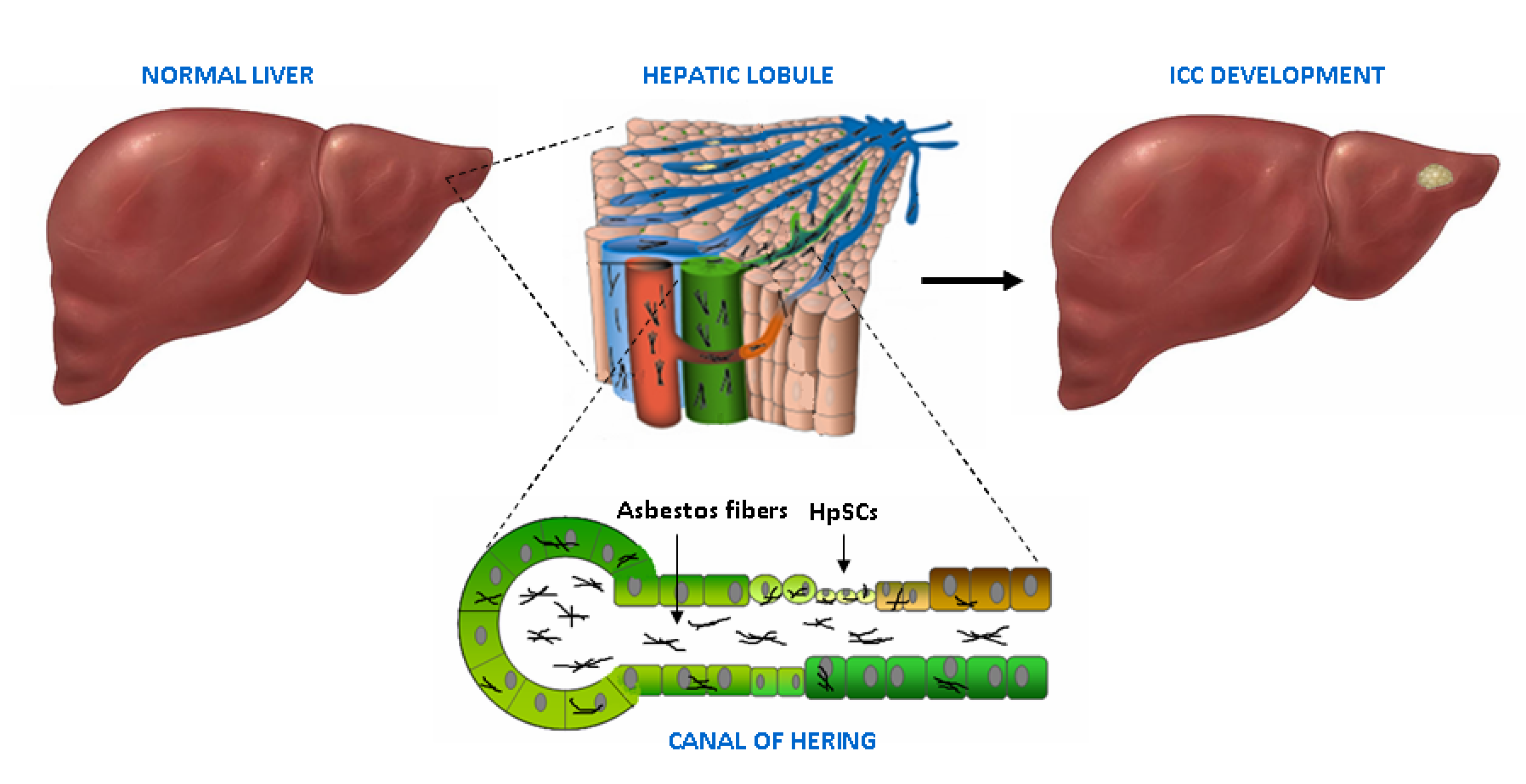
5. Asbestos Fibers in the Liver and the Biliary Tract
6. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Banales, J.M.; Cardinale, V.; Carpino, G.; Marzioni, M.; Andersen, J.B.; Invernizzi, P.; Lind, G.E.; Folseraas, T.; Forbes, S.J.; Fouassier, L.; et al. Expert consensus document: Cholangiocarcinoma: Current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA). Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 261–280. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; Tavolari, S.; Brandi, G. Cholangiocarcinoma: Epidemiology and risk factors. Liver Int. 2019, 39 (Suppl. 1), 19–31. [Google Scholar] [CrossRef] [PubMed]
- Smittenaar, C.R.; Petersen, K.A.; Stewart, K.; Moitt, N. Cancer incidence and mortality projections in the UK until 2035. Br. J. Cancer 2016, 115, 1147–1155. [Google Scholar] [CrossRef] [PubMed]
- Brandi, G.; Farioli, A.; Astolfi, A.; Biasco, G.; Tavolari, S. Genetic heterogeneity in cholangiocarcinoma: A major challenge for targeted therapies. Oncotarget 2015, 6, 14744–14753. [Google Scholar] [CrossRef] [PubMed]
- Case, B.W.; Abraham, J.L.; Meeker, G.; Pooley, F.D.; Pinkerton, K.E. Applying definitions of “asbestos” to environmental and “low-dose” exposure levels and health effects, particularly malignant mesothelioma. J. Toxicol. Environ. Health B Crit. Rev. 2011, 14, 3–39. [Google Scholar] [CrossRef] [PubMed]
- Burki, T. Health experts concerned over India’s asbestos industry. Lancet 2010, 375, 626–627. [Google Scholar] [CrossRef]
- Yao, S.; DellaVentura, G.; Petibois, C. Analytical characterization of cell-asbestos fiber interactions in lung pathogenesis. Anal. Bioanal. Chem. 2010, 397, 2079–2089. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer. Arsenic, metals, fibres, and dusts. IARC Monogr. Eval. Carcinog. Risks Hum. 2012, 100, 11–465. [Google Scholar]
- Padmore, T.; Stark, C.; Turkevich, L.A.; Champion, J.A. Quantitative analysis of the role of fiber length on phagocytosis and inflammatory response by alveolar macrophages. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 58–67. [Google Scholar] [CrossRef]
- Ospina, D.; Villegas, V.E.; Rodriguez-Leguizamon, G.; Rondon-Lagos, M. Analyzing biological and molecular characteristics and genomic damage induced by exposure to asbestos. Cancer Manag. Res. 2019, 11, 4997–5012. [Google Scholar] [CrossRef]
- Abu-Remaileh, M.; Bender, S.; Raddatz, G.; Ansari, I.; Cohen, D.; Gutekunst, J.; Musch, T.; Linhart, H.; Breiling, A.; Pikarsky, E.; et al. Chronic inflammation induces a novel epigenetic program that is conserved in intestinal adenomas and in colorectal cancer. Cancer Res 2015, 75, 2120–2130. [Google Scholar] [CrossRef] [PubMed]
- Chernova, T.; Murphy, F.A.; Galavotti, S.; Sun, X.M.; Powley, I.R.; Grosso, S.; Schinwald, A.; Zacarias-Cabeza, J.; Dudek, K.M.; Dinsdale, D.; et al. Long-Fiber Carbon Nanotubes Replicate Asbestos-Induced Mesothelioma with Disruption of the Tumor Suppressor Gene Cdkn2a (Ink4a/Arf). Curr. Biol. 2017, 27, 3302–3314.e3306. [Google Scholar] [CrossRef] [PubMed]
- Nagai, H.; Ishihara, T.; Lee, W.H.; Ohara, H.; Okazaki, Y.; Okawa, K.; Toyokuni, S. Asbestos surface provides a niche for oxidative modification. Cancer Sci. 2011, 102, 2118–2125. [Google Scholar] [CrossRef] [PubMed]
- Heintz, N.H.; Janssen-Heininger, Y.M.; Mossman, B.T. Asbestos, lung cancers, and mesotheliomas: From molecular approaches to targeting tumor survival pathways. Am. J. Respir. Cell Mol. Biol. 2010, 42, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, E.; Makishima, A.; Hagino, K.; Okabe, K. Accumulation of radium in ferruginous protein bodies formed in lung tissue: Association of resulting radiation hotspots with malignant mesothelioma and other malignancies. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2009, 85, 229–239. [Google Scholar] [CrossRef] [PubMed][Green Version]
- DiPaolo, J.A.; DeMarinis, A.J.; Doniger, J. Asbestos and benzo(a)pyrene synergism in the transformation of Syrian hamster embryo cells. Pharmacology 1983, 27, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Cortez Bde, A.; Quassollo, G.; Caceres, A.; Machado-Santelli, G.M. The fate of chrysotile-induced multipolar mitosis and aneuploid population in cultured lung cancer cells. PLoS ONE 2011, 6, e18600. [Google Scholar] [CrossRef]
- Zhang, T.; Lv, L.; Huang, Y.; Ren, X.; Shi, Q. Chromosome nondisjunction during bipolar mitoses of binucleated intermediates promote aneuploidy formation along with multipolar mitoses rather than chromosome loss in micronuclei induced by asbestos. Oncotarget 2017, 8, 11030–11041. [Google Scholar] [CrossRef][Green Version]
- Kubo, Y.; Takenaka, H.; Nagai, H.; Toyokuni, S. Distinct affinity of nuclear proteins to the surface of chrysotile and crocidolite. J. Clin. Biochem. Nutr. 2012, 51, 221–226. [Google Scholar] [CrossRef]
- Matsuzaki, H.; Kumagai-Takei, N.; Lee, S.; Maeda, M.; Sada, N.; Hatayama, T.; Yamamoto, S.; Ikeda, M.; Yoshitome, K.; Min, Y.; et al. Search for biomarkers of asbestos exposure and asbestos-induced cancers in investigations of the immunological effects of asbestos. Environ. Health Prev. Med. 2017, 22, 53. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, A.; Pellegrini, L.; Dey, A.; Larson, D.; Tanji, M.; Flores, E.G.; Kendrick, B.; Lapid, D.; Powers, A.; Kanodia, S.; et al. Minimal asbestos exposure in germline BAP1 heterozygous mice is associated with deregulated inflammatory response and increased risk of mesothelioma. Oncogene 2016, 35, 1996–2002. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Kadariya, Y.; Cheung, M.; Pei, J.; Talarchek, J.; Sementino, E.; Tan, Y.; Menges, C.W.; Cai, K.Q.; Litwin, S.; et al. Germline mutation of Bap1 accelerates development of asbestos-induced malignant mesothelioma. Cancer Res. 2014, 74, 4388–4397. [Google Scholar] [CrossRef] [PubMed]
- Brandi, G.; Deserti, M.; Palloni, A.; Turchetti, D.; Zuntini, R.; Frega, G.; De Lorenzo, S.; Abbati, F.; Rizzo, A.; Di Marco, M.; et al. Intrahepatic cholangiocarcinoma development in a patient with a novel BAP1 germline mutation and low exposure to asbestos. Oncotarget. submitted for publication.
- Takahashi, K.; Landrigan, P.J.; Ramazzini, C. The Global Health Dimensions of Asbestos and Asbestos-Related Diseases. Ann. Glob. Health 2016, 82, 209–213. [Google Scholar] [CrossRef]
- Selikoff, I.J.; Seidman, H. Cancer of the pancreas among asbestos insulation workers. Cancer 1981, 47, 1469–1473. [Google Scholar] [CrossRef]
- Sanden, A.; Naslund, P.E.; Jarvholm, B. Mortality in lung and gastrointestinal cancer among shipyard workers. Int. Arch. Occup. Environ. Health 1985, 55, 277–283. [Google Scholar] [CrossRef]
- Raffn, E.; Villadsen, E.; Lynge, E. Colorectal cancer in asbestos cement workers in Denmark. Am. J. Ind. Med. 1996, 30, 267–272. [Google Scholar] [CrossRef]
- Brandi, G.; Di Girolamo, S.; Farioli, A.; de Rosa, F.; Curti, S.; Pinna, A.D.; Ercolani, G.; Violante, F.S.; Biasco, G.; Mattioli, S. Asbestos: A hidden player behind the cholangiocarcinoma increase? Findings from a case-control analysis. Cancer Causes Control 2013, 24, 911–918. [Google Scholar] [CrossRef]
- Farioli, A.; Straif, K.; Brandi, G.; Curti, S.; Kjaerheim, K.; Martinsen, J.I.; Sparen, P.; Tryggvadottir, L.; Weiderpass, E.; Biasco, G.; et al. Occupational exposure to asbestos and risk of cholangiocarcinoma: A population-based case-control study in four Nordic countries. Occup. Environ. Med. 2018, 75, 191–198. [Google Scholar] [CrossRef]
- Welzel, T.M.; McGlynn, K.A.; Hsing, A.W.; O’Brien, T.R.; Pfeiffer, R.M. Impact of classification of hilar cholangiocarcinomas (Klatskin tumors) on the incidence of intra- and extrahepatic cholangiocarcinoma in the United States. J. Natl. Cancer Inst. 2006, 98, 873–875. [Google Scholar] [CrossRef]
- Brandi, G.; Venturi, M.; Pantaleo, M.A.; Ercolani, G.; Gico. Cholangiocarcinoma: Current opinion on clinical practice diagnostic and therapeutic algorithms: A review of the literature and a long-standing experience of a referral center. Dig. Liver Dis. 2016, 48, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, B. A simple estimator of minimum detectable relative risk, sample size, or power in cohort studies. Am. J. Epidemiol. 1987, 126, 356–358. [Google Scholar] [CrossRef] [PubMed]
- Bertuccio, P.; Bosetti, C.; Levi, F.; Decarli, A.; Negri, E.; La Vecchia, C. A comparison of trends in mortality from primary liver cancer and intrahepatic cholangiocarcinoma in Europe. Ann. Oncol. 2013, 24, 1667–1674. [Google Scholar] [CrossRef] [PubMed]
- Selikoff, I.J.; Seidman, H. Asbestos-associated deaths among insulation workers in the United States and Canada, 1967–1987. Ann. N. Y. Acad. Sci. 1991, 643, 1–14. [Google Scholar] [CrossRef]
- Battista, G.; Belli, S.; Comba, P.; Fiumalbi, C.; Grignoli, M.; Loi, F.; Orsi, D.; Paredes, I. Mortality due to asbestos-related causes among railway carriage construction and repair workers. Occup. Med. (Lond.) 1999, 49, 536–539. [Google Scholar] [CrossRef]
- Berry, G.; Newhouse, M.L.; Wagner, J.C. Mortality from all cancers of asbestos factory workers in east London 1933–1980. Occup. Environ. Med. 2000, 57, 782–785. [Google Scholar] [CrossRef]
- Wingren, G. Mortality and cancer incidence in a Swedish art glassworks—An updated cohort study. Int. Arch. Occup. Environ. Health 2004, 77, 599–603. [Google Scholar] [CrossRef]
- Hein, M.J.; Stayner, L.T.; Lehman, E.; Dement, J.M. Follow-up study of chrysotile textile workers: Cohort mortality and exposure-response. Occup. Environ. Med. 2007, 64, 616–625. [Google Scholar] [CrossRef]
- Pira, E.; Pelucchi, C.; Piolatto, P.G.; Negri, E.; Discalzi, G.; La Vecchia, C. First and subsequent asbestos exposures in relation to mesothelioma and lung cancer mortality. Br. J. Cancer 2007, 97, 1300–1304. [Google Scholar] [CrossRef][Green Version]
- Clin, B.; Morlais, F.; Dubois, B.; Guizard, A.V.; Desoubeaux, N.; Marquignon, M.F.; Raffaelli, C.; Paris, C.; Galateau-Salle, F.; Launoy, G.; et al. Occupational asbestos exposure and digestive cancers—A cohort study. Aliment. Pharmacol. Ther. 2009, 30, 364–374. [Google Scholar] [CrossRef]
- Wang, X.; Lin, S.; Yu, I.; Qiu, H.; Lan, Y.; Yano, E. Cause-specific mortality in a Chinese chrysotile textile worker cohort. Cancer Sci. 2013, 104, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Hogstedt, C.; Jansson, C.; Hugosson, M.; Tinnerberg, H.; Gustavsson, P. Cancer incidence in a cohort of Swedish chimney sweeps, 1958–2006. Am. J. Public Health 2013, 103, 1708–1714. [Google Scholar] [CrossRef] [PubMed]
- Boulanger, M.; Morlais, F.; Bouvier, V.; Galateau-Salle, F.; Guittet, L.; Marquignon, M.F.; Paris, C.; Raffaelli, C.; Launoy, G.; Clin, B. Digestive cancers and occupational asbestos exposure: Incidence study in a cohort of asbestos plant workers. Occup. Environ. Med. 2015, 72, 792–797. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.T.; Lin, Y.J.; Li, C.Y.; Tsai, P.J.; Yang, C.Y.; Liou, S.H.; Wu, T.N. Cancer Attributable to Asbestos Exposure in Shipbreaking Workers: A Matched-Cohort Study. PLoS ONE 2015, 10, e0133128. [Google Scholar] [CrossRef] [PubMed]
- Pira, E.; Romano, C.; Violante, F.S.; Farioli, A.; Spatari, G.; La Vecchia, C.; Boffetta, P. Updated mortality study of a cohort of asbestos textile workers. Cancer Med. 2016, 5, 2623–2628. [Google Scholar] [CrossRef] [PubMed]
- Pira, E.; Romano, C.; Donato, F.; Pelucchi, C.; Vecchia, C.; Boffetta, P. Mortality from cancer and other causes among Italian chrysotile asbestos miners. Occup. Environ. Med. 2017, 74, 558–563. [Google Scholar] [CrossRef]
- Luberto, F.; Ferrante, D.; Silvestri, S.; Angelini, A.; Cuccaro, F.; Nannavecchia, A.M.; Oddone, E.; Vicentini, M.; Barone-Adesi, F.; Cena, T.; et al. Cumulative asbestos exposure and mortality from asbestos related diseases in a pooled analysis of 21 asbestos cement cohorts in Italy. Environ. Health 2019, 18, 71. [Google Scholar] [CrossRef]
- Wikman, H.; Ruosaari, S.; Nymark, P.; Sarhadi, V.K.; Saharinen, J.; Vanhala, E.; Karjalainen, A.; Hollmen, J.; Knuutila, S.; Anttila, S. Gene expression and copy number profiling suggests the importance of allelic imbalance in 19p in asbestos-associated lung cancer. Oncogene 2007, 26, 4730–4737. [Google Scholar] [CrossRef][Green Version]
- Nymark, P.; Guled, M.; Borze, I.; Faisal, A.; Lahti, L.; Salmenkivi, K.; Kettunen, E.; Anttila, S.; Knuutila, S. Integrative analysis of microRNA, mRNA and aCGH data reveals asbestos- and histology-related changes in lung cancer. Genes Chromosomes Cancer 2011, 50, 585–597. [Google Scholar] [CrossRef]
- Nymark, P.; Aavikko, M.; Makila, J.; Ruosaari, S.; Hienonen-Kempas, T.; Wikman, H.; Salmenkivi, K.; Pirinen, R.; Karjalainen, A.; Vanhala, E.; et al. Accumulation of genomic alterations in 2p16, 9q33.1 and 19p13 in lung tumours of asbestos-exposed patients. Mol. Oncol. 2013, 7, 29–40. [Google Scholar] [CrossRef]
- Maki-Nevala, S.; Sarhadi, V.K.; Knuuttila, A.; Scheinin, I.; Ellonen, P.; Lagstrom, S.; Ronty, M.; Kettunen, E.; Husgafvel-Pursiainen, K.; Wolff, H.; et al. Driver Gene and Novel Mutations in Asbestos-Exposed Lung Adenocarcinoma and Malignant Mesothelioma Detected by Exome Sequencing. Lung 2016, 194, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Krieger, T.; Simons, B.D. Dynamic stem cell heterogeneity. Development 2015, 142, 1396–1406. [Google Scholar] [CrossRef] [PubMed]
- Chacon-Martinez, C.A.; Koester, J.; Wickstrom, S.A. Signaling in the stem cell niche: Regulating cell fate, function and plasticity. Development 2018, 145. [Google Scholar] [CrossRef] [PubMed]
- Overi, D.; Carpino, G.; Cardinale, V.; Franchitto, A.; Safarikia, S.; Onori, P.; Alvaro, D.; Gaudio, E. Contribution of Resident Stem Cells to Liver and Biliary Tree Regeneration in Human Diseases. Int. J. Mol. Sci. 2018, 19, 2917. [Google Scholar] [CrossRef]
- Wiemann, S.U.; Satyanarayana, A.; Tsahuridu, M.; Tillmann, H.L.; Zender, L.; Klempnauer, J.; Flemming, P.; Franco, S.; Blasco, M.A.; Manns, M.P.; et al. Hepatocyte telomere shortening and senescence are general markers of human liver cirrhosis. FASEB J. 2002, 16, 935–942. [Google Scholar] [CrossRef]
- Marshall, A.; Rushbrook, S.; Davies, S.E.; Morris, L.S.; Scott, I.S.; Vowler, S.L.; Coleman, N.; Alexander, G. Relation between hepatocyte G1 arrest, impaired hepatic regeneration, and fibrosis in chronic hepatitis C virus infection. Gastroenterology 2005, 128, 33–42. [Google Scholar] [CrossRef]
- Sato, K.; Marzioni, M.; Meng, F.; Francis, H.; Glaser, S.; Alpini, G. Ductular Reaction in Liver Diseases: Pathological Mechanisms and Translational Significances. Hepatology 2019, 69, 420–430. [Google Scholar] [CrossRef]
- Williams, M.J.; Clouston, A.D.; Forbes, S.J. Links between hepatic fibrosis, ductular reaction, and progenitor cell expansion. Gastroenterology 2014, 146, 349–356. [Google Scholar] [CrossRef]
- Lanzoni, G.; Cardinale, V.; Carpino, G. The hepatic, biliary, and pancreatic network of stem/progenitor cell niches in humans: A new reference frame for disease and regeneration. Hepatology 2016, 64, 277–286. [Google Scholar] [CrossRef]
- Alvaro, D.; Gaudio, E. Liver Capsule: Biliary Tree Stem Cell Subpopulations. Hepatology 2016, 64, 644. [Google Scholar] [CrossRef]
- Boulter, L.; Lu, W.Y.; Forbes, S.J. Differentiation of progenitors in the liver: A matter of local choice. J. Clin. Investig. 2013, 123, 1867–1873. [Google Scholar] [CrossRef]
- Carpino, G.; Cardinale, V.; Onori, P.; Franchitto, A.; Berloco, P.B.; Rossi, M.; Wang, Y.; Semeraro, R.; Anceschi, M.; Brunelli, R.; et al. Biliary tree stem/progenitor cells in glands of extrahepatic and intraheptic bile ducts: An anatomical in situ study yielding evidence of maturational lineages. J. Anat. 2012, 220, 186–199. [Google Scholar] [CrossRef]
- Cardinale, V.; Wang, Y.; Carpino, G.; Cui, C.B.; Gatto, M.; Rossi, M.; Berloco, P.B.; Cantafora, A.; Wauthier, E.; Furth, M.E.; et al. Multipotent stem/progenitor cells in human biliary tree give rise to hepatocytes, cholangiocytes, and pancreatic islets. Hepatology 2011, 54, 2159–2172. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lanzoni, G.; Carpino, G.; Cui, C.B.; Dominguez-Bendala, J.; Wauthier, E.; Cardinale, V.; Oikawa, T.; Pileggi, A.; Gerber, D.; et al. Biliary tree stem cells, precursors to pancreatic committed progenitors: Evidence for possible life-long pancreatic organogenesis. Stem Cells 2013, 31, 1966–1979. [Google Scholar] [CrossRef] [PubMed]
- Stavraka, C.; Rush, H.; Ross, P. Combined hepatocellular cholangiocarcinoma (cHCC-CC): An update of genetics, molecular biology, and therapeutic interventions. J. Hepatocell. Carcinoma 2019, 6, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Szendroi, M.; Nemeth, L.; Vajta, G. Asbestos bodies in a bile duct cancer after occupational exposure. Environ. Res. 1983, 30, 270–280. [Google Scholar] [CrossRef]
- Grosso, F.; Randi, L.; Croce, A.; Mirabelli, D.; Libener, R.; Magnani, C.; Bellis, D.; Allegrina, M.; Bertolotti, M.; Degiovanni, D.; et al. Asbestos fibers in the gallbladder of patients affected by benign biliary tract diseases. Eur. J. Gastroenterol. Hepatol. 2015, 27, 860–864. [Google Scholar] [CrossRef] [PubMed]
- Grosso, F.; Croce, A.; Trincheri, N.F.; Mariani, N.; Libener, R.; Degiovanni, D.; Rinaudo, C. Asbestos fibres detected by scanning electron microscopy in the gallbladder of patients with malignant pleural mesothelioma (MPM). J. Microsc. 2017, 266, 48–54. [Google Scholar] [CrossRef]
- Croce, A.; Capella, S.; Belluso, E.; Grosso, F.; Mariani, N.; Libener, R.; Rinaudo, C. Asbestos fibre burden in gallbladder: A case study. Micron 2018, 105, 98–104. [Google Scholar] [CrossRef]
- Grosso, F.; Croce, A.; Libener, R.; Mariani, N.; Pastormerlo, M.; Maconi, A.; Rinaudo, C. Asbestos fiber identification in liver from cholangiocarcinoma patients living in an asbestos polluted area: A preliminary study. Tumori J. 2019, 105, 404–410. [Google Scholar] [CrossRef]
- Miserocchi, G.; Sancini, G.; Mantegazza, F.; Chiappino, G. Translocation pathways for inhaled asbestos fibers. Environ. Health 2008, 7, 4. [Google Scholar] [CrossRef] [PubMed]
- Kohyama, N.; Suzuki, Y. Analysis of asbestos fibers in lung parenchyma, pleural plaques, and mesothelioma tissues of North American insulation workers. Ann. N. Y. Acad. Sci. 1991, 643, 27–52. [Google Scholar] [CrossRef] [PubMed]
- Cook, P.M. Review of published studies on gut penetration by ingested asbestos fibers. Environ. Health Perspect. 1983, 53, 121–130. [Google Scholar] [CrossRef]
- Nishimura, Y.; Maeda, M.; Kumagai-Takei, N.; Lee, S.; Matsuzaki, H.; Wada, Y.; Nishiike-Wada, T.; Iguchi, H.; Otsuki, T. Altered functions of alveolar macrophages and NK cells involved in asbestos-related diseases. Environ. Health Prev. Med. 2013, 18, 198–204. [Google Scholar] [CrossRef] [PubMed][Green Version]
| Risk Factor | Association with ICC |
|---|---|
| Bile duct cysts/Caroli’s disease | very strong |
| Primary sclerosing cholangitis/cholangitis | very strong * |
| Hepatolithiasis | strong/very strong |
| Cholelithiasis/choledocholithiasis | moderate/strong |
| Cirrhosis | strong/very strong |
| HBV/HCV infection | moderate/strong |
| Hemochromatosis | moderate |
| Inflammatory bowel disease/chronic pancreatitis | moderate |
| Duodenal/gastric ulcer | weak/modest |
| O. viverrini/C. sinensis infection | strong * |
| Diabete type II | weak/modest |
| Obesity | weak/modest * |
| NAFLD/NASH | strong |
| Alcohol | moderate |
| Cigarette smoking | weak/modest |
| Thorotrast | very strong * |
| 1,2-dichloropropane | very strong * |
| Reference | Period | Cohort | Workers’ Category | SMR or SIR * (95% CI) | Tumor Site |
|---|---|---|---|---|---|
| Selikoff I. et al., 1991 [34] | 1967–1987 | 17800 (M) | Insulator workers | 1.08 2.61 | Liver Bile ducts + Gallbladder |
| Battista G. 1999 [35] | 1945–1970 | 734 (M) | Railway workers | 241 (126–420) | Liver |
| Berry G. et al., 2000 [36] | 1933–1980 | 5000 (M/F) | Factory workers | 2.66 (1.28–4.89) | Liver + Bile ducts + Gallbladder |
| Wingren G. 2004 [37] | 1964–1997 | 1229 (M/F) | Art glassworks | * 2.00 (0.41–5.84) (M) * 4.35 (0,75–10.59) (F) | Liver + Bile ducts |
| Hein MJ. et al., 2007 [38] | 1940–2001 | 3072 (M/F) | Textile workers | 1.05 (0.51–1.94) | Liver + Biliary tract |
| Pira E. et al., 2007 [39] | 1946–1984 | 1966 (M/F) | Textile workers | 237 (118–425) | Liver |
| Clin B. et al., 2009 [40] | 1978–2004 | 2024 (M/F) | Textile workers | * 1.61 (0.86–2.75) * 1.92 (0.38–5.6) | Liver Biliary tract |
| Wang X. et al., 2013 [41] | 1972–2008 | 586 (M) 272 (F) | Textile workers | 1.34 (0.81–2.21) – | Liver + Bile duct |
| Hogstedt T. et al., 2013 [42] | 1958–2006 | 6320 (M/F) | Chimney sweeps | * 2.48 (1.47–3.91) * 1.6 (0.19–5.78) | Liver Bile ducts |
| Boulanger M. et al., 2015 [43] | 1978–2009 | 2024 (M/F) | Textile workers | * 1.85 (1.09–2.92) (M) * 2.84 (0.76–7.26) (M) | Liver Biliary tract |
| * 1.85 (1.09–2.92) (F) * 2.84 (0.76–7.26) (F) | Liver Biliary tract | ||||
| Wu W. et al., 2015 [44] | 1975–1989 | 4427 (M/F) | Shipbreaking workers | 1.6 (1.08–2.36) | Liver + Intrahepatic bile ducts |
| Pira E. et al., 2016 [45] | 1946–2013 | 1977 (M/F) | Textile workers | 1.06 (0.55–1.86) | Liver |
| Pira E. et al., 2017 [46] | 1946–2014 | 1056 (M) | Miners | 0,65 (0.21–1.51) | Liver |
| Luberto F. et al., 2019 [47] | 1934–2006 | 13076 (M/F) | Cement workers | 0.99 (0.81–1.20) (M) 0.84 (0.42–1.60) (F) | Liver + Intrahepatic bile ducts |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brandi, G.; Tavolari, S. Asbestos and Intrahepatic Cholangiocarcinoma. Cells 2020, 9, 421. https://doi.org/10.3390/cells9020421
Brandi G, Tavolari S. Asbestos and Intrahepatic Cholangiocarcinoma. Cells. 2020; 9(2):421. https://doi.org/10.3390/cells9020421
Chicago/Turabian StyleBrandi, Giovanni, and Simona Tavolari. 2020. "Asbestos and Intrahepatic Cholangiocarcinoma" Cells 9, no. 2: 421. https://doi.org/10.3390/cells9020421
APA StyleBrandi, G., & Tavolari, S. (2020). Asbestos and Intrahepatic Cholangiocarcinoma. Cells, 9(2), 421. https://doi.org/10.3390/cells9020421





