Mechanistic Roles of Matrilin-2 and Klotho in Modulating the Inflammatory Activity of Human Aortic Valve Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Isolation and Culture of Human AVICs
2.3. Immunoblotting
2.4. Quantitative qPCR
2.5. Immunofluorescence Staining
2.6. ELISA
2.7. Statistical Analysis
3. Results
3.1. Matrilin-2 Induces the Inflammatory Responses in Human AVICs
3.2. Matrilin-2 Activates PKR and NF-κB in Human AVICs
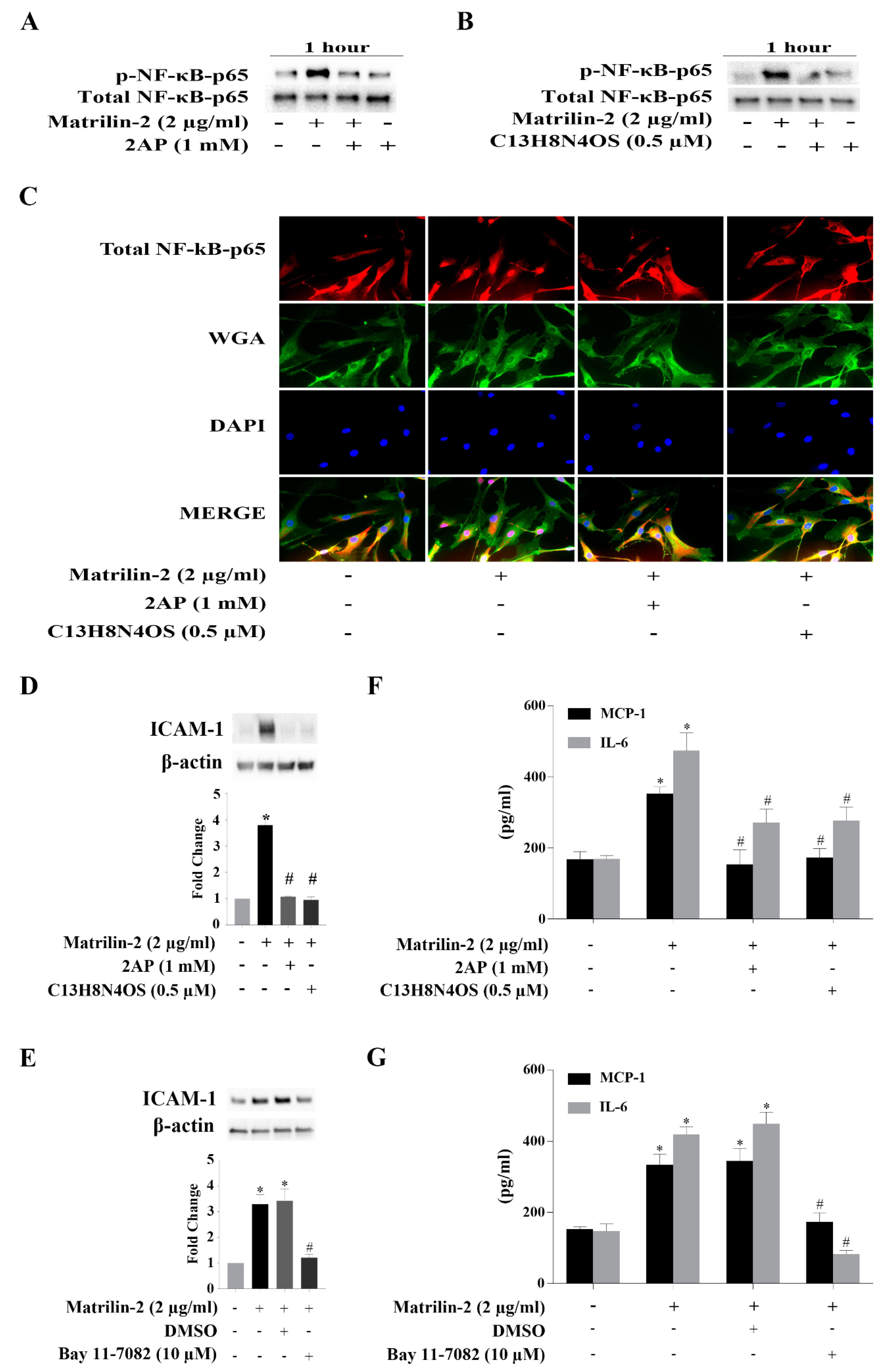
3.3. The PKR-NF-κB Pathway Mediates Matrilin-2–induced Inflammatory Responses
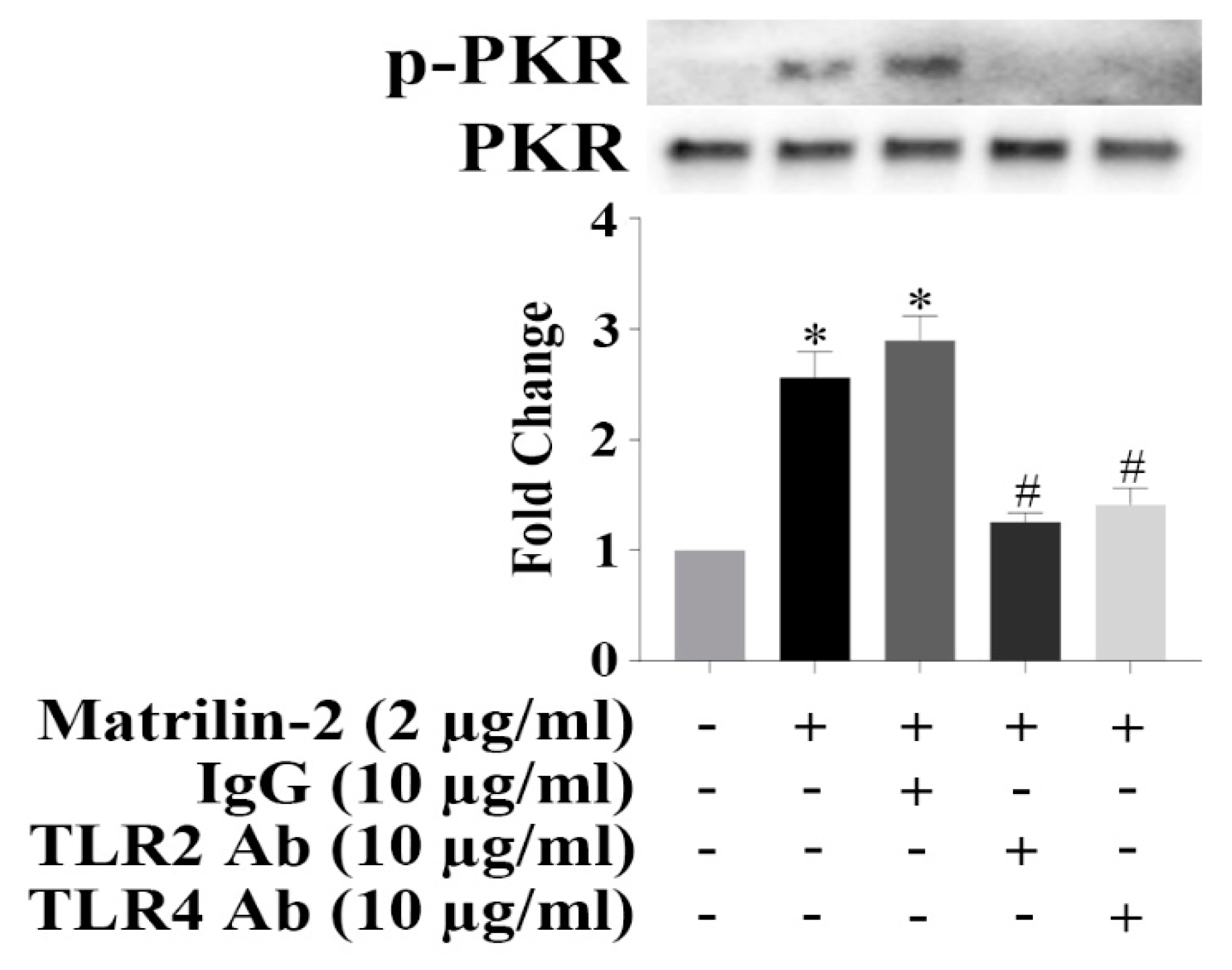
3.4. TLR2 and TLR4 Activate PKR to Induce the Inflammatory Responses in Human AVICs
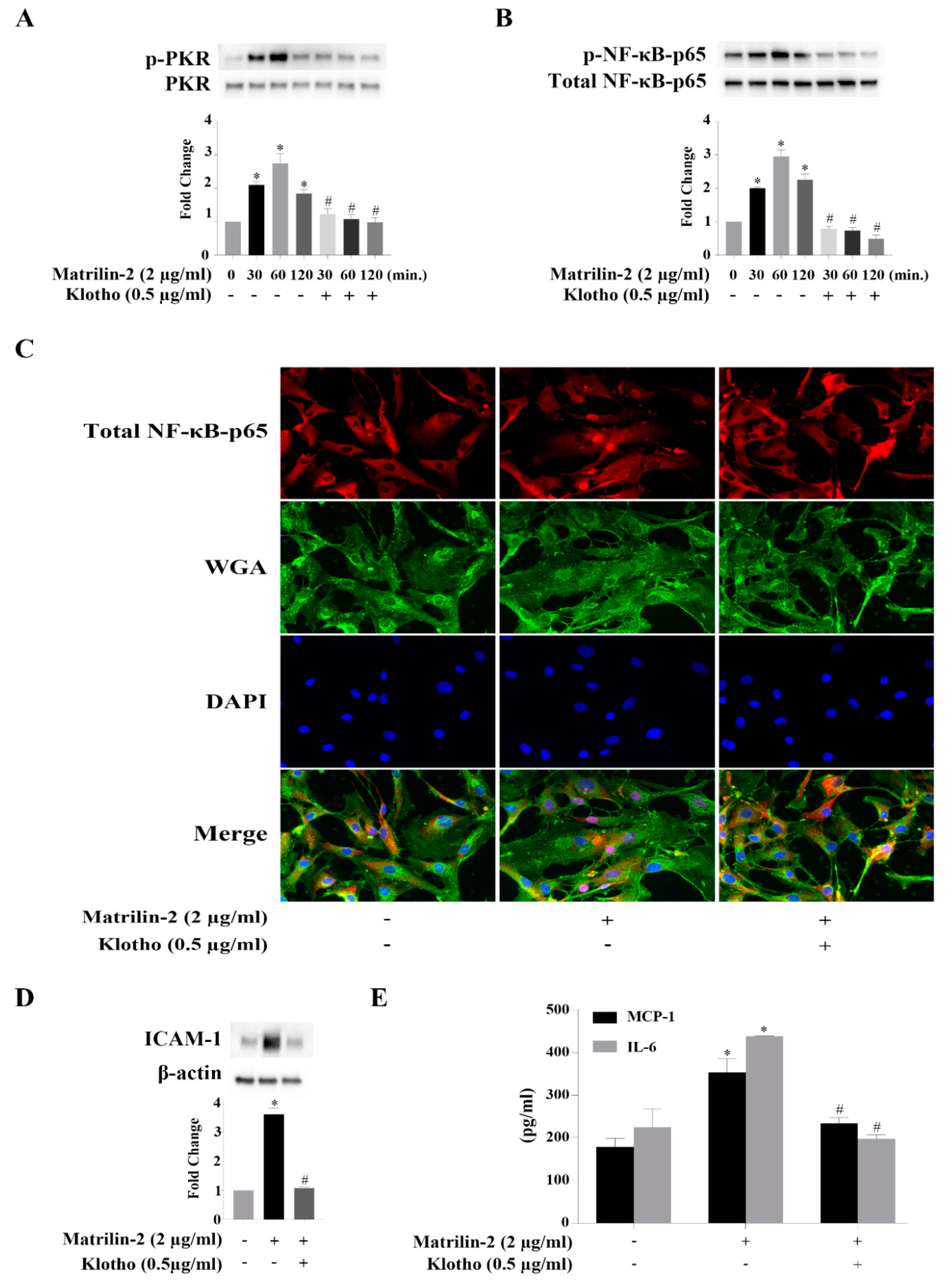
3.5. Klotho Suppresses Matrilin-2-induced Inflammatory Responses in Human AVICs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Yutzey, K.E.; Demer, L.L.; Body, S.C.; Huggins, G.S.; Towler, D.A.; Giachelli, C.M.; Hofmann-Bowman, M.A.; Mortlock, D.P.; Rogers, M.B.; Sadeghi, M.M. Calcific aortic valve disease: A consensus summary from the alliance of investigators on calcific aortic valve disease. Arter. Thromb. Vasc. Biol. 2014, 34, 2387–2393. [Google Scholar] [CrossRef]
- Lindman, B.R.; Clavel, M.A.; Mathieu, P.; Iung, B.; Lancellotti, P.; Otto, C.M.; Pibarot, P. Calcific aortic stenosis. Nat. Rev. Dis. Primers 2016, 2, 16006. [Google Scholar] [CrossRef]
- Hutcheson, J.D.; Aikawa, E.; Merryman, W.D. Potential drug targets for calcific aortic valve disease. Nat. Rev. Cardiol. 2014, 11, 218–231. [Google Scholar] [CrossRef]
- Lindman, B.R.; Bonow, R.O.; Otto, C.M. Current management of calcific aortic stenosis. Circ. Res. 2013, 113, 223–237. [Google Scholar] [CrossRef]
- Rajamannan, N.M.; Evans, F.J.; Aikawa, E.; Grande-Allen, K.J.; Demer, L.L.; Heistad, D.D.; Simmons, C.A.; Masters, K.S.; Mathieu, P.; O’Brien, K.D. Calcific aortic valve disease: Not simply a degenerative process. Circulation 2011, 124, 1783–1791. [Google Scholar] [CrossRef]
- García-Rodríguez, C.; Parra-Izquierdo, I.; Castaños-Mollor, I.; López, J.; San Román, J.A.; Sánchez Crespo, M. Toll-like receptors, inflammation, and calcific aortic valve disease. Front. Physiol. 2018, 9, 201. [Google Scholar] [CrossRef]
- Peacock, J.D.; Levay, A.K.; Gillaspie, D.B.; Tao, G.; Lincoln, J. Reduced sox9 function promotes heart valve calcification phenotypes in vivo. Circ. Res. 2010, 106, 712–719. [Google Scholar] [CrossRef]
- Mathieu, P.; Bouchareb, R.; Boulanger, M.C. Innate and adaptive immunity in calcific aortic valve disease. J. Immunol. Res. 2015, 851945. [Google Scholar] [CrossRef]
- Aikawa, E.; Otto, C.M. Look more closely at the valve: Imaging calcific aortic valve disease. Circulation 2012, 125, 9–11. [Google Scholar] [CrossRef]
- Pawade, T.A.; Newby, D.E.; Dweck, M.R. Calcification in aortic stenosis: The skeleton key. J. Am. Coll. Cardiol. 2015, 66, 561–577. [Google Scholar] [CrossRef]
- Parolari, A.; Loardi, C.; Mussoni, L.; Cavallotti, L.; Camera, M.; Biglioli, P.; Tremoli, E.; Alamanni, F. Nonrheumatic calcific aortic stenosis: An overview from basic science to pharmacological prevention. Eur. J. Cardiothorac. Surg. 2009, 35, 493–504. [Google Scholar] [CrossRef]
- Weiss, R.M.; Miller, J.D.; Heistad, D.D. Fibrocalcific aortic valve disease: Opportunity to understand disease mechanisms using mouse models. Circ. Res. 2013, 113, 209–222. [Google Scholar] [CrossRef]
- Hjortnaes, J.; Shapero, K.; Goettsch, C.; Hutcheson, J.D.; Keegan, J.; Kluin, J.; Mayer, J.E.; Bischoff, J.; Aikawa, E. Valvular interstitial cells suppress calcification of valvular endothelial cells. Atherosclerosis 2015, 242, 251–260. [Google Scholar] [CrossRef]
- Liu, A.C.; Joag, V.R.; Gotlieb, A.I. The emerging role of valve interstitial cell phenotypes in regulating heart valve pathobiology. Am. J. Pathol. 2007, 171, 1407–1418. [Google Scholar] [CrossRef]
- Zeng, Q.; Jin, C.; Ao, L.; Cleveland, J.C., Jr.; Song, R.; Xu, D.; Fullerton, D.A.; Meng, X. Cross-talk between the Toll-like receptor 4 and Notch1 pathways augments the inflammatory response in the interstitial cells of stenotic human aortic valves. Circulation 2012, 126, S222–S230. [Google Scholar] [CrossRef]
- Taylor, P.M.; Batten, P.; Brand, N.J.; Thomas, P.S.; Yacoub, M.H. The cardiac valve interstitial cell. Int. J. Biochem. Cell. Biol. 2003, 35, 113–118. [Google Scholar] [CrossRef]
- Bossé, Y.; Mathieu, P.; Pibarot, P. Genomics: The next step to elucidate the etiology of calcific aortic valve stenosis. J. Am. Coll. Cardiol. 2008, 51, 1327–1336. [Google Scholar] [CrossRef]
- Towler, D.A. Molecular and cellular aspects of calcific aortic valve disease. Circ. Res. 2013, 113, 198–208. [Google Scholar] [CrossRef]
- Babu, A.N.; Meng, X.; Zou, N.; Yang, X.; Wang, M.; Song, Y.; Cleveland, J.C.; Weyant, M.; Banerjee, A.; Fullerton, D.A. Lipopolysaccharide stimulation of human aortic valve interstitial cells activates inflammation and osteogenesis. Ann. Thorac. Surg. 2008, 86, 71–76. [Google Scholar] [CrossRef]
- Meng, X.; Ao, L.; Song, Y.; Babu, A.; Yang, X.; Wang, M.; Weyant, M.J.; Dinarello, C.A.; Cleveland Jr, J.C.; Fullerton, D.A. Expression of functional Toll-like receptors 2 and 4 in human aortic valve interstitial cells: Potential roles in aortic valve inflammation and stenosis. Am. J. Physiol. Cell Physiol. 2008, 294, C29–C35. [Google Scholar] [CrossRef]
- Sverdlov, A.L.; Ngo, D.T.; Chapman, M.J.; Ali, O.A.; Chirkov, Y.Y.; Horowitz, J.D. Pathogenesis of aortic stenosis: Not just a matter of wear and tear. Am. J. Cardiovasc. Dis. 2011, 1, 185–199. [Google Scholar]
- Schaefer, L. Complexity of danger: The diverse nature of damage-associated molecular patterns. J. Biol. Chem. 2014, 289, 35237–35245. [Google Scholar] [CrossRef]
- Babelova, A.; Moreth, K.; Tsalastra-Greul, W.; Zeng-Brouwers, J.; Eickelberg, O.; Young, M.F.; Bruckner, P.; Pfeilschifter, J.; Schaefer, R.M.; Gröne, H.-J. Biglycan, a danger signal that activates the NLRP3 inflammasome via toll-like and P2X receptors. J. Biol. Chem. 2009, 284, 24035–24048. [Google Scholar] [CrossRef]
- Merline, R.; Moreth, K.; Beckmann, J.; Nastase, M.V.; Zeng-Brouwers, J.; Tralhão, J.G.; Lemarchand, P.; Pfeilschifter, J.; Schaefer, R.M.; Iozzo, R.V. Signaling by the matrix proteoglycan decorin controls inflammation and cancer through PDCD4 and MicroRNA-21. Sci. Signal. 2011, 4, ra75. [Google Scholar] [CrossRef]
- Schaefer, L. Extracellular matrix molecules: Endogenous danger signals as new drug targets in kidney diseases. Curr. Opin. Pharmacol. 2010, 10, 185–190. [Google Scholar] [CrossRef]
- Klatt, A.R.; Becker, A.-K.A.; Neacsu, C.D.; Paulsson, M.; Wagener, R. The matrilins: Modulators of extracellular matrix assembly. Int. J. Biochem. Cell Biol. 2011, 43, 320–330. [Google Scholar] [CrossRef]
- Deák, F.; Piecha, D.; Bachrati, C.; Paulsson, M.; Kiss, I. Primary structure and expression of matrilin-2, the closest relative of cartilage matrix protein within the von Willebrand factor type A-like module superfamily. J. Biol. Chem. 1997, 272, 9268–9274. [Google Scholar] [CrossRef]
- Deák, F.; Wagener, R.; Kiss, I.; Paulsson, M. The matrilins: A novel family of oligomeric extracellular matrix proteins. Matrix Biol. 1999, 18, 55–64. [Google Scholar] [CrossRef]
- Piecha, D.; Muratoglu, S.; Mörgelin, M.; Hauser, N.; Studer, D.; Kiss, I.; Paulsson, M.; Deák, F. Matrilin-2, a large, oligomeric matrix protein, is expressed by a great variety of cells and forms fibrillar networks. J. Biol. Chem. 1999, 274, 13353–13361. [Google Scholar] [CrossRef]
- Zhang, S.; Peng, J.; Guo, Y.; Javidiparsijani, S.; Wang, G.; Wang, Y.; Liu, H.; Liu, J.; Luo, J. Matrilin-2 is a widely distributed extracellular matrix protein and a potential biomarker in the early stage of osteoarthritis in articular cartilage. Biomed. Res. Int. 2014, 2014, 986127. [Google Scholar] [CrossRef]
- Jonas, A.; Thiem, S.; Kuhlmann, T.; Wagener, R.; Aszodi, A.; Nowell, C.; Hagemeier, K.; Laverick, L.; Perreau, V.; Jokubaitis, V. Axonally derived matrilin-2 induces proinflammatory responses that exacerbate autoimmune neuroinflammation. J. Clin. Investig. 2014, 124, 5042–5056. [Google Scholar] [CrossRef]
- Szabó, E.; Korpos, É.; Batmunkh, E.; Lotz, G.; Holczbauer, Á.; Kovalszky, I.; Deák, F.; Kiss, I.; Schaff, Z.; Kiss, A. Expression of matrilin-2 in liver cirrhosis and hepatocellular carcinoma. Pathol. Oncol. Res. 2008, 14, 15–22. [Google Scholar] [CrossRef]
- Li, F.; Song, R.; Ao, L.; Reece, T.B.; Cleveland, J.C.; Dong, N.; Fullerton, D.A.; Meng, X. ADAMTS5 deficiency in calcified aortic valves is associated with elevated pro-osteogenic activity in valvular interstitial cells. Arter. Thromb. Vasc. Biol. 2017, 37, 1339–1351. [Google Scholar] [CrossRef]
- Liu, F.; Wu, S.; Ren, H.; Gu, J. Klotho suppresses RIG-I-mediated senescence-associated inflammation. Nat. Cell Biol. 2011, 13, 254–262. [Google Scholar] [CrossRef]
- Xu, Y.; Sun, Z. Molecular basis of Klotho: From gene to function in aging. Endocr. Rev. 2015, 36, 174–193. [Google Scholar] [CrossRef]
- Zhao, Y.; Banerjee, S.; Dey, N.; LeJeune, W.S.; Sarkar, P.S.; Brobey, R.; Rosenblatt, K.P.; Tilton, R.G.; Choudhary, S. Klotho depletion contributes to increased inflammation in kidney of the db/db mouse model of diabetes via RelA (serine) 536 phosphorylation. Diabetes 2011, 60, 1907–1916. [Google Scholar] [CrossRef]
- Li, L.; Wang, Y.; Gao, W.; Yuan, C.; Zhang, S.; Zhou, H.; Huang, M.; Yao, X. Klotho reduction in alveolar macrophages contributes to CSE-induced inflammation in chronic obstructive pulmonary disease. J. Biol. Chem. 2015, 290, 27890–27900. [Google Scholar] [CrossRef]
- Hui, H.; Zhai, Y.; Ao, L.; Cleveland, J.C., Jr.; Liu, H.; Fullerton, D.A.; Meng, X. Klotho suppresses the inflammatory responses and ameliorates cardiac dysfunction in aging endotoxemic mice. Oncotarget 2017, 8, 15663–15676. [Google Scholar] [CrossRef]
- Li, F.; Yao, Q.; Ao, L.; Cleveland, J.C.; Dong, N.; Fullerton, D.A.; Meng, X. Klotho suppresses high phosphate-induced osteogenic responses in human aortic valve interstitial cells through inhibition of Sox9. J. Mol. Med. 2017, 95, 739–751. [Google Scholar] [CrossRef]
- Lu, B.; Nakamura, T.; Inouye, K.; Li, J.; Tang, Y.; Lundbäck, P.; Valdes-Ferrer, S.I.; Olofsson, P.S.; Kalb, T.; Roth, J. Novel role of PKR in inflammasome activation and HMGB1 release. Nature 2012, 488, 670–674. [Google Scholar] [CrossRef]
- Ohukainen, P.; Ruskoaho, H.; Rysa, J. Cellular mechanisms of valvular thickening in early and intermediate calcific aortic valve disease. Curr. Cardiol. Rev. 2018, 14, 264–271. [Google Scholar] [CrossRef]
- Hulin, A.; Hego, A.; Lancellotti, P.; Oury, C. Advances in pathophysiology of calcific aortic valve disease propose novel molecular therapeutic targets. Front. Cardiovasc. Med. 2018, 5, 21. [Google Scholar] [CrossRef]
- Kostyunin, A.E.; Yuzhalin, A.E.; Ovcharenko, E.A.; Kutikhin, A.G. Development of calcific aortic valve disease: Do we know enough for new clinical trials? J. Mol. Cell Cardiol. 2019, 132, 189–209. [Google Scholar] [CrossRef]
- Song, R.; Ao, L.; Zhao, K.-S.; Zheng, D.; Venardos, N.; Fullerton, D.A.; Meng, X. Soluble biglycan induces the production of ICAM-1 and MCP-1 in human aortic valve interstitial cells through TLR2/4 and the ERK1/2 pathway. Inflamm. Res. 2014, 63, 703–710. [Google Scholar] [CrossRef][Green Version]
- Schober, A. Chemokines in vascular dysfunction and remodeling. Arter. Thromb. Vasc. Biol. 2008, 28, 1950–1959. [Google Scholar] [CrossRef]
- Galkina, E.; Ley, K. Vascular adhesion molecules in atherosclerosis. Arter. Thromb. Vasc. Biol. 2007, 27, 2292–2301. [Google Scholar] [CrossRef]
- Leopold, J.A. Cellular mechanisms of aortic valve calcification. Circ. Cardiovasc. Interv. 2012, 5, 605–614. [Google Scholar] [CrossRef]
- Stark, G.R.; Kerr, I.M.; Williams, B.R.; Silverman, R.H.; Schreiber, R.D. How cells respond to interferons. Annu. Rev. Biochem. 1998, 67, 227–264. [Google Scholar] [CrossRef]
- Farabaugh, K.T.; Majumder, M.; Guan, B.-J.; Jobava, R.; Wu, J.; Krokowski, D.; Gao, X.-H.; Schuster, A.; Longworth, M.; Chan, E.D. Protein Kinase R mediates the inflammatory response induced by hyperosmotic stress. Mol. Cell. Biol. 2017, 37, e00521-16. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB signaling in inflammation. Signal. Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Oeckinghaus, A.; Ghosh, S. The NF-κB family of transcription factors and its regulation. Cold Spring Harb. Perspect. Biol. 2009, 1, a000034. [Google Scholar] [CrossRef]
- Giridharan, S.; Srinivasan, M. Mechanisms of NF-κB p65 and strategies for therapeutic manipulation. J. Inflamm. Res. 2018, 11, 407–419. [Google Scholar] [CrossRef]
- Barton, G.M.; Medzhitov, R. Toll-like receptor signaling pathways. Science 2003, 300, 1524–1525. [Google Scholar] [CrossRef]
- Garcia, M.A.; Gil, J.; Ventoso, I.; Guerra, S.; Domingo, E.; Rivas, C.; Esteban, M. Impact of protein kinase PKR in cell biology: From antiviral to antiproliferative action. Microbiol. Mol. Biol. Rev. 2006, 70, 1032–1060. [Google Scholar] [CrossRef] [PubMed]
- Dalton, G.D.; Xie, J.; An, S.-W.; Huang, C.-L. New insights into the mechanism of action of soluble klotho. Front. Endocrinol. 2017, 8, 323. [Google Scholar] [CrossRef]
- Zou, D.; Wu, W.; He, Y.; Ma, S.; Gao, J. The role of klotho in chronic kidney disease. BMC Nephrol. 2018, 19, 285. [Google Scholar] [CrossRef]
- Zhu, L.; Stein, L.R.; Kim, D.; Ho, K.; Yu, G.-Q.; Zhan, L.; Larsson, T.E.; Mucke, L. Klotho controls the brain–immune system interface in the choroid plexus. Proc. Natl. Acad. Sci. USA 2018, 115, E11388–E11396. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
The, E.; Yao, Q.; Zhang, P.; Zhai, Y.; Ao, L.; Fullerton, D.A.; Meng, X. Mechanistic Roles of Matrilin-2 and Klotho in Modulating the Inflammatory Activity of Human Aortic Valve Cells. Cells 2020, 9, 385. https://doi.org/10.3390/cells9020385
The E, Yao Q, Zhang P, Zhai Y, Ao L, Fullerton DA, Meng X. Mechanistic Roles of Matrilin-2 and Klotho in Modulating the Inflammatory Activity of Human Aortic Valve Cells. Cells. 2020; 9(2):385. https://doi.org/10.3390/cells9020385
Chicago/Turabian StyleThe, Erlinda, Qingzhou Yao, Peijian Zhang, Yufeng Zhai, Lihua Ao, David A. Fullerton, and Xianzhong Meng. 2020. "Mechanistic Roles of Matrilin-2 and Klotho in Modulating the Inflammatory Activity of Human Aortic Valve Cells" Cells 9, no. 2: 385. https://doi.org/10.3390/cells9020385
APA StyleThe, E., Yao, Q., Zhang, P., Zhai, Y., Ao, L., Fullerton, D. A., & Meng, X. (2020). Mechanistic Roles of Matrilin-2 and Klotho in Modulating the Inflammatory Activity of Human Aortic Valve Cells. Cells, 9(2), 385. https://doi.org/10.3390/cells9020385



