WNT5a-ROR Signaling Is Essential for Alveologenesis
Abstract
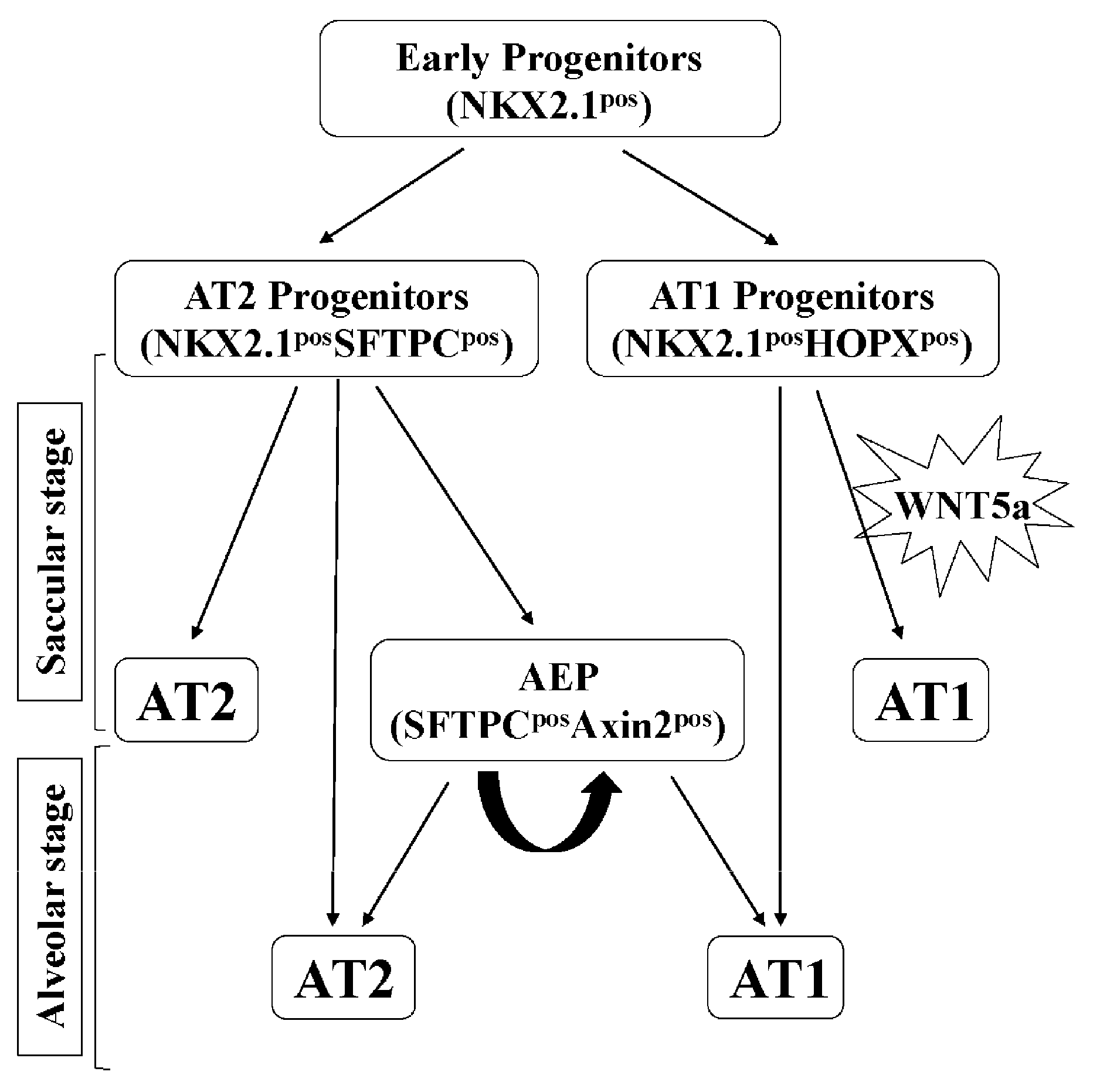
1. Introduction
2. Materials and Methods
2.1. Mouse Breeding and Genotyping
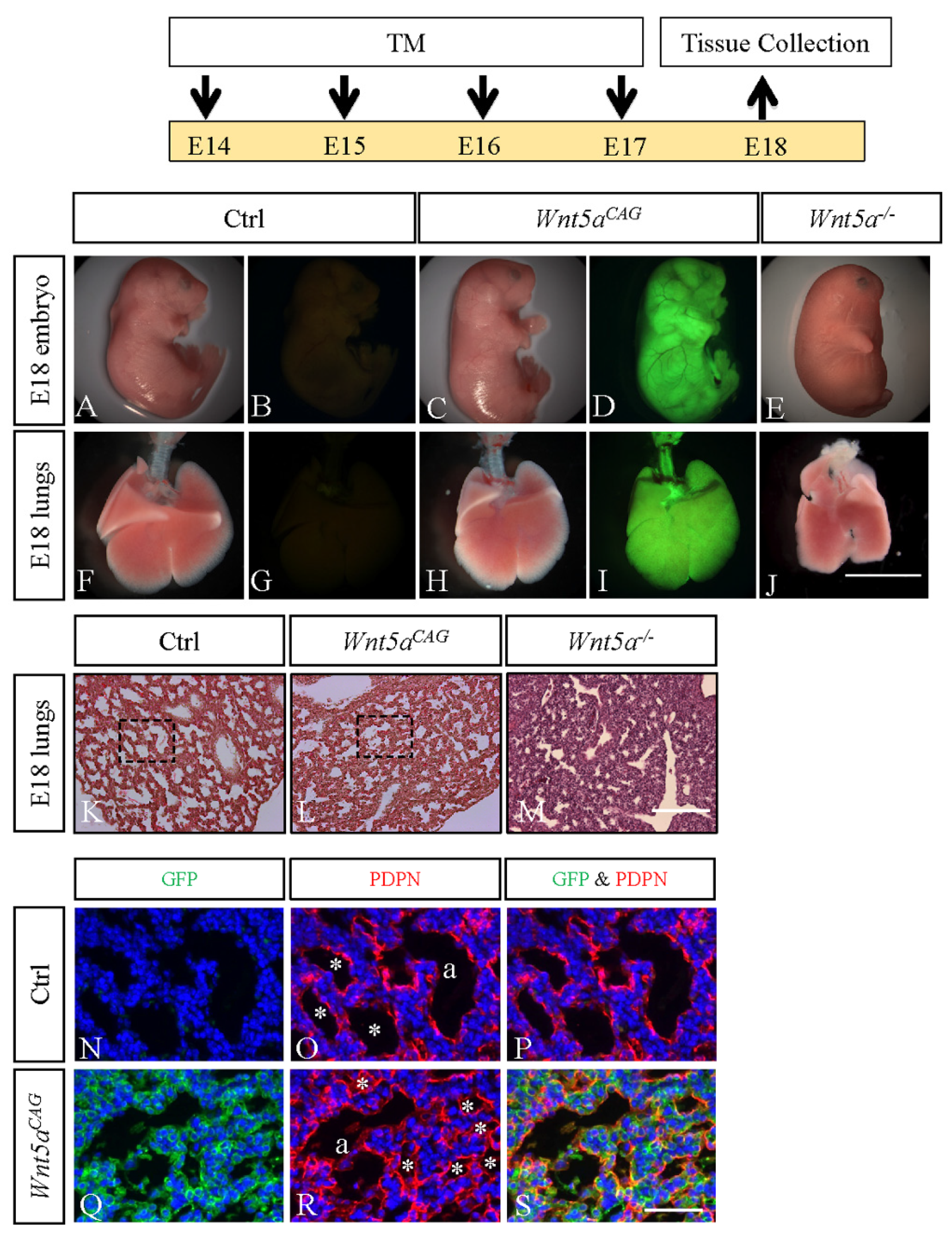
2.2. Tamoxifen Administration
2.3. Human Neonatal Lung Samples
2.4. Immunofluorescent Staining
2.5. Neonatal Lung Fibroblast Isolation, Treatment and Migration Assay
2.6. Real-time Quantitative Polymerase Chain Reaction (real-time RT-PCR)
2.7. Statistics Analysis
3. Results
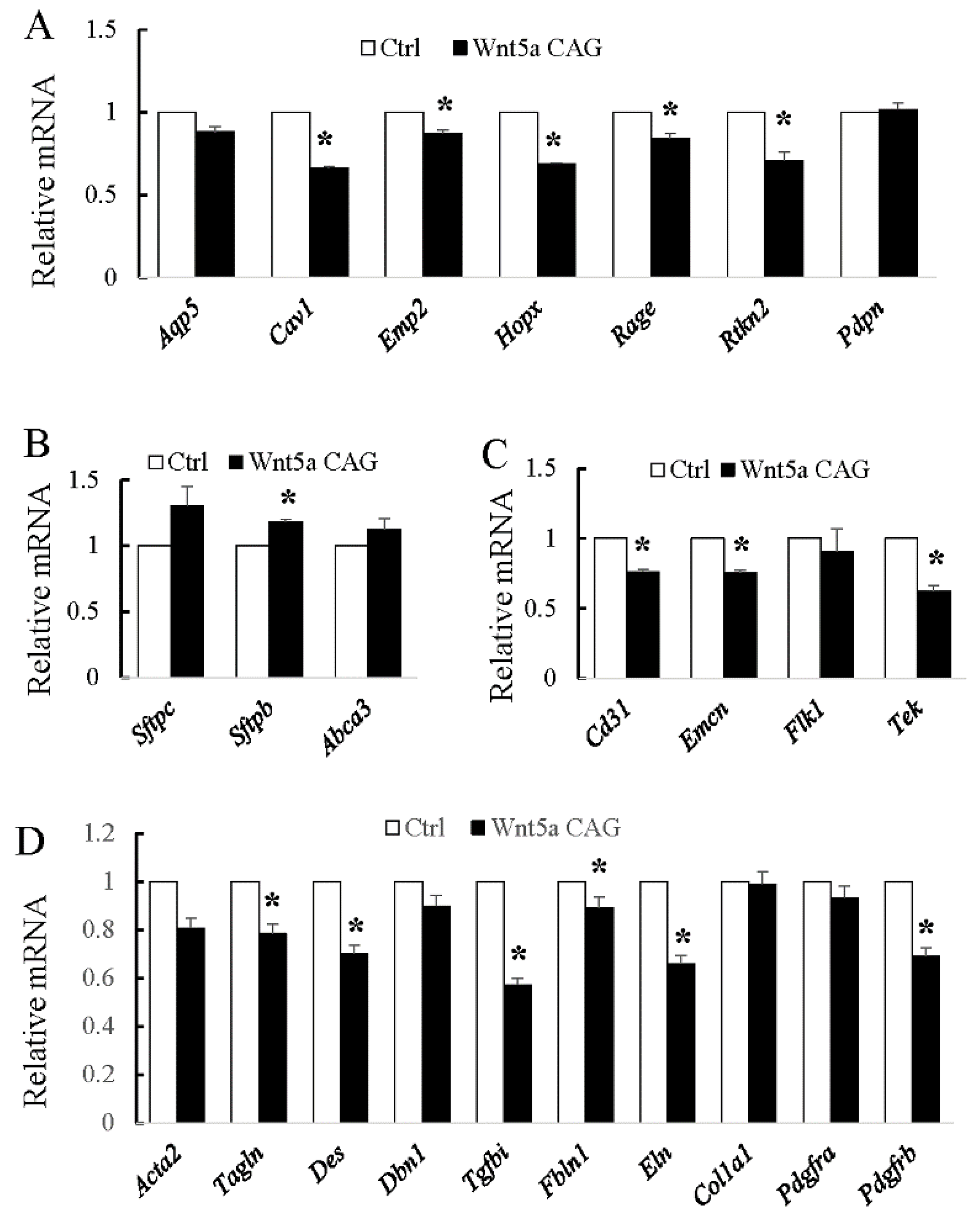
3.1. WNT5a is Required for Differentiation of Multiple Cell Types During Saccular Stage Lung Development
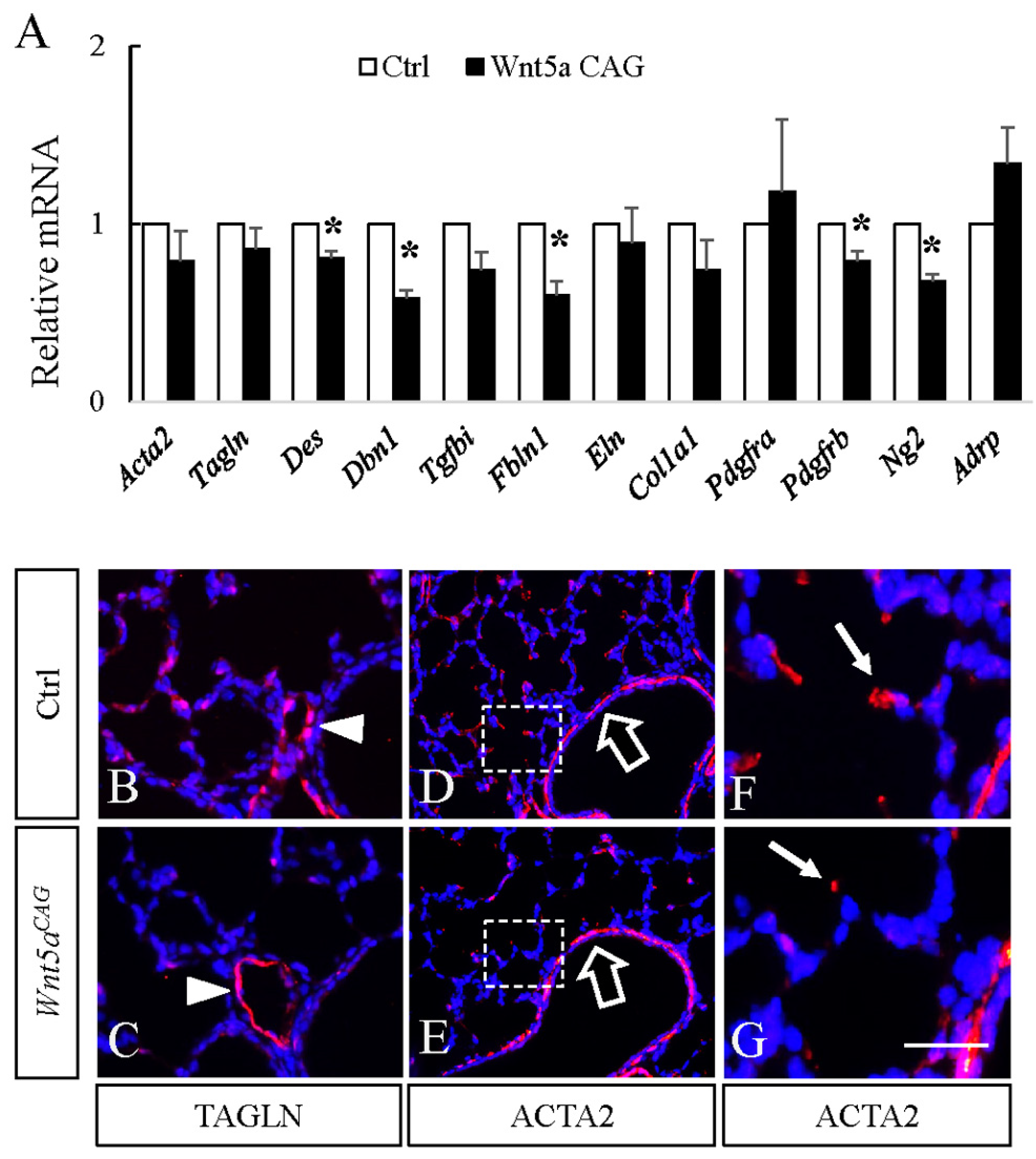
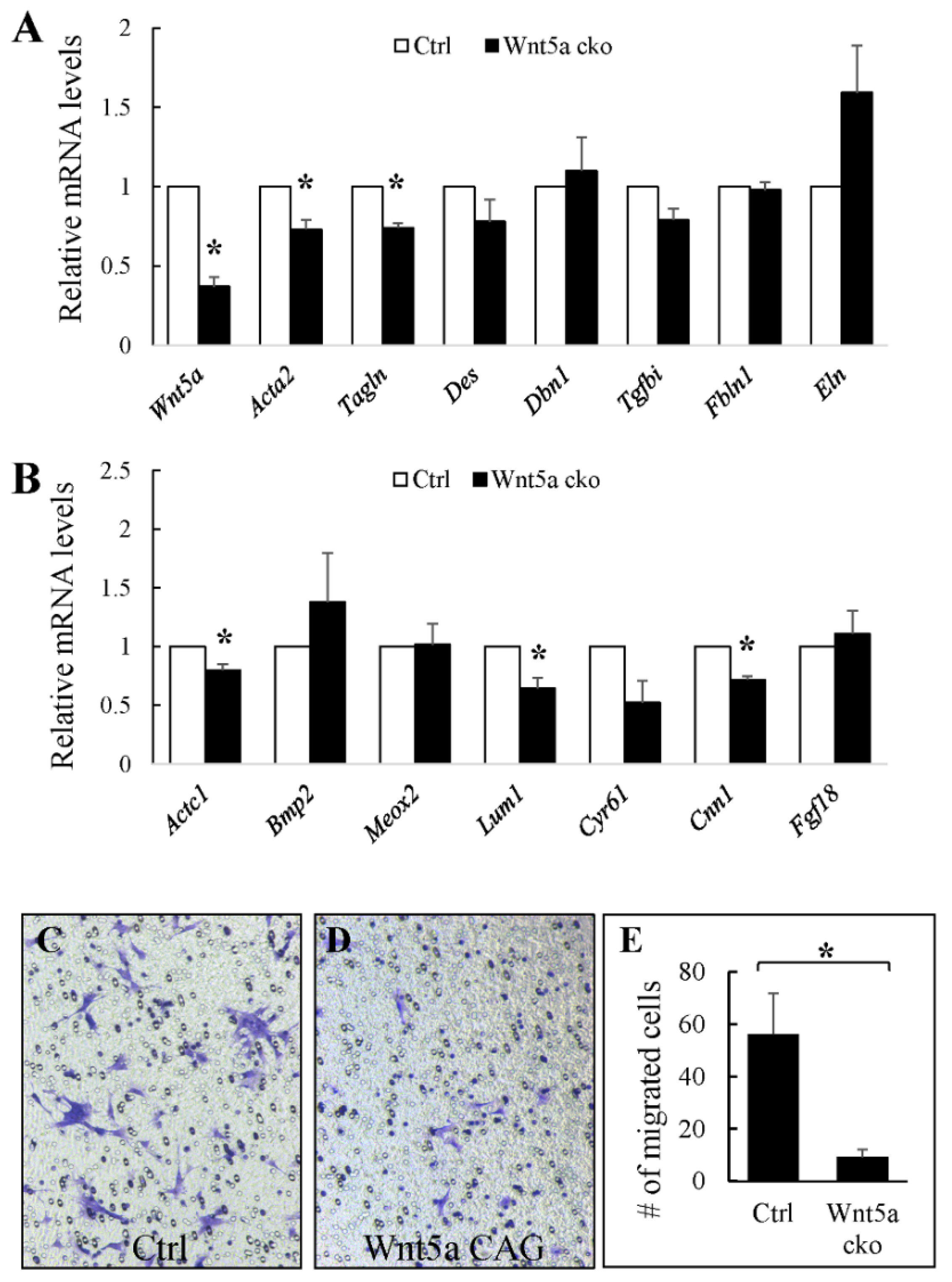
3.2. WNT5a is Required for Myofibroblast Differentiation and Migration During Alveolar Stage Lung Development
3.3. RORs are Essential for Myofibroblast Migration During Alveolar Stage Lung Development
3.4. Relevance to Human Neonatal Lung Disease
4. Discussions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shi, W.; Bellusci, S.; Warburton, D. Lung Development and Adult Lung Diseases. Chest 2007, 132, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Frank, D.B.; Penkala, I.J.; Zepp, J.A.; Sivakumar, A.; Linares-Saldana, R.; Zacharias, W.J.; Stolz, K.G.; Pankin, J.; Lu, M.; Wang, Q.; et al. Early lineage specification defines alveolar epithelial ontogeny in the murine lung. Proc. Natl. Acad. Sci. 2019, 116, 4362–4371. [Google Scholar] [CrossRef] [PubMed]
- Branchfield, K.; Li, R.; Lungova, V.; Verheyden, J.M.; McCulley, D.; Sun, X. A three-dimensional study of alveologenesis in mouse lung. Dev. Biol. 2016, 409, 429–441. [Google Scholar] [CrossRef] [PubMed]
- Boström, H.; Willetts, K.; Pekny, M.; Levéen, P.; Lindahl, P.; Hedstrand, H.; Pekna, M.; Hellström, M.; Gebre-Medhin, S.; Schalling, M.; et al. PDGF-A Signaling Is a Critical Event in Lung Alveolar Myofibroblast Development and Alveogenesis. Cell 1996, 85, 863–873. [Google Scholar] [CrossRef]
- Lindahl, P.; Karlsson, L.; Hellström, M.; Gebre-Medhin, S.; Willetts, K.; Heath, J.K.; Betsholtz, C. Alveogenesis failure in PDGF-A-deficient mice is coupled to lack of distal spreading of alveolar smooth muscle cell progenitors during lung development. Development 1997, 124, 3943–3953. [Google Scholar]
- Li, C.; Li, M.; Li, S.; Xing, Y.; Yang, C.-Y.; Li, A.; Borok, Z.; De Langhe, S.; Minoo, P. Progenitors of secondary crest myofibroblasts are developmentally committed in early lung mesoderm. Stem Cells 2015, 33, 999–1012. [Google Scholar] [CrossRef]
- Kikuchi, A.; Yamamoto, H.; Kishida, S. Multiplicity of the interactions of Wnt proteins and their receptors. Cell. Signal 2007, 19, 659–671. [Google Scholar] [CrossRef]
- Endo, M.; Nishita, M.; Fujii, M.; Minami, Y. Insight into the Role of Wnt5a-Induced Signaling in Normal and Cancer Cells. Int. Rev. Cell Mol. Biol. 2015, 314, 117–148. [Google Scholar]
- Nabhan, A.N.; Brownfield, D.G.; Harbury, P.B.; Krasnow, M.A.; Desai, T.J. Single-cell Wnt signaling niches maintain stemness of alveolar type 2 cells. Science 2018, 359, 1118–1123. [Google Scholar] [CrossRef]
- Li, C.; Xiao, J.; Hormi, K.; Borok, Z.; Minoo, P. Wnt5a participates in distal lung morphogenesis. Dev. Boil. 2002, 248, 68–81. [Google Scholar] [CrossRef]
- Yuan, K.; Shamskhou, E.A.; Orcholski, M.E.; Nathan, A.; Reddy, S.; Honda, H.; Mani, V.; Zeng, Y.; Ozen, M.O.; Wang, L.; et al. Loss of Endothelium-Derived Wnt5a Is Associated With Reduced Pericyte Recruitment and Small Vessel Loss in Pulmonary Arterial Hypertension. Circulation 2019, 139, 1710–1724. [Google Scholar] [CrossRef] [PubMed]
- Baarsma, H.A.; Skronska-Wasek, W.; Mutze, K.; Ciolek, F.; Wagner, D.E.; John-Schuster, G.; Heinzelmann, K.; Günther, A.; Bracke, K.R.; Dagouassat, M.; et al. Correction: Noncanonical WNT-5A signaling impairs endogenous lung repair in COPD. J. Exp. Med. 2017, 214, 565. [Google Scholar] [CrossRef] [PubMed]
- Vuga, L.J.; Ben-Yehudah, A.; Kovkarova-Naumovski, E.; Oriss, T.; Gibson, K.F.; Feghali-Bostwick, C.; Kaminski, N. WNT5A Is a Regulator of Fibroblast Proliferation and Resistance to Apoptosis. Am. J. Respir. Cell Mol. Boil. 2009, 41, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Kumawat, K.; Menzen, M.H.; Slegtenhorst, R.M.; Halayko, A.J.; Schmidt, M.; Gosens, R. TGF-β-Activated Kinase 1 (TAK1) Signaling Regulates TGF-β-Induced WNT-5A Expression in Airway Smooth Muscle Cells via Sp1 and β-Catenin. Plos One 2014, 9, e94801. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Loscertales, M.; Mikels, A.J.; Hu, J.K.-H.; Donahoe, P.K.; Roberts, D.J. Chick pulmonary Wnt5a directs airway and vascular tubulogenesis. Development 2008, 135, 1365–1376. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Hu, L.; Xiao, J.; Chen, H.; Li, J.T.; Bellusci, S.; Delanghe, S.; Minoo, P. Wnt5a regulates Shh and Fgf10 signaling during lung development. Dev. Boil. 2005, 287, 86–97. [Google Scholar] [CrossRef]
- Nomi, M.; Oishi, I.; Kani, S.; Suzuki, H.; Matsuda, T.; Yoda, A.; Kitamura, M.; Itoh, K.; Takeuchi, S.; Takeda, K.; et al. Loss of mRor1 Enhances the Heart and Skeletal Abnormalities in mRor2-Deficient Mice: Redundant and Pleiotropic Functions of mRor1 and mRor2 Receptor Tyrosine Kinases. Mol. Cell. Boil. 2001, 21, 8329–8335. [Google Scholar] [CrossRef]
- Ho, H.-Y.H.; Susman, M.W.; Bikoff, J.B.; Ryu, Y.K.; Jonas, A.M.; Hu, L.; Kuruvilla, R.; Greenberg, M.E. Wnt5a–Ror–Dishevelled signaling constitutes a core developmental pathway that controls tissue morphogenesis. Proc. Natl. Acad. Sci. USA 2012, 109, 4044–4051. [Google Scholar] [CrossRef]
- Mikels, A.J.; Nusse, R. Purified Wnt5a protein activates or inhibits beta-catenin-TCF signaling depending on receptor context. PLoS Boil. 2006, 4, e115. [Google Scholar]
- Fukuda, T.; Chen, L.; Endo, T.; Tang, L.; Lu, D.; Castro, J.E.; Widhopf, G.F.; Rassenti, L.Z.; Cantwell, M.J.; Prussak, C.E.; et al. Antisera induced by infusions of autologous Ad-CD154-leukemia B cells identify ROR1 as an oncofetal antigen and receptor for Wnt5a. Proc. Natl. Acad. Sci. U.S.A. 2008, 105, 3047–3052. [Google Scholar] [CrossRef]
- Li, C.; Bellusci, S.; Borok, Z.; Minoo, P. Non-canonical WNT signalling in the lung. J. Biochem. 2015, 158, 355–365. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ryu, Y.K.; Collins, S.E.; Ho, H.-Y.H.; Zhao, H.; Kuruvilla, R. An autocrine Wnt5a-Ror signaling loop mediates sympathetic target innervation. Dev. Boil. 2013, 377, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.; Joyner, A.L. Dynamic Changes in the Response of Cells to Positive Hedgehog Signaling during Mouse Limb Patterning. Cell 2004, 118, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Go, D.; Krenitsky, D.L.; Huyck, H.L.; Solleti, S.K.; Lunger, V.A.; Metlay, L.; Srisuma, S.; Wert, S.E.; Mariani, T.J.; et al. Genome-Wide Transcriptional Profiling Reveals Connective Tissue Mast Cell Accumulation in Bronchopulmonary Dysplasia. Am. J. Respir. Crit. Care Med. 2012, 186, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Lee, M.K.; Gao, F.; Webster, S.; Di, H.; Duan, J.; Yang, C.-Y.; Bhopal, N.; Peinado, N.; Pryhuber, G.; et al. Secondary crest myofibroblast PDGFRα controls the elastogenesis pathway via a secondary tier of signaling networks during alveologenesis. Development 2019, 146, dev176354. [Google Scholar] [CrossRef]
- McGowan, S.E.; Grossmann, R.E.; Kimani, P.W.; Holmes, A.J. Platelet-Derived Growth Factor Receptor-Alpha-Expressing Cells Localize to the Alveolar Entry Ring and Have Characteristics of Myofibroblasts During Pulmonary Alveolar Septal Formation. Anat. Rec. Adv. Integr. Anat. Evol. Boil. 2008, 291, 1649–1661. [Google Scholar] [CrossRef]
- Yamaguchi, T.P.; Bradley, A.; McMahon, A.P.; Jones, S. A Wnt5a pathway underlies outgrowth of multiple structures in the vertebrate embryo. Development 1999, 126, 1211–1223. [Google Scholar]
- Rieger, M.E.; Zhou, B.; Solomon, N.; Sunohara, M.; Li, C.; Nguyen, C.; Liu, Y.; Pan, J.-H.; Minoo, P.; Crandall, E.D.; et al. p300/β-Catenin Interactions Regulate Adult Progenitor Cell Differentiation Downstream of WNT5a/Protein Kinase C (PKC)*. J. Boil. Chem. 2016, 291, 6569–6582. [Google Scholar] [CrossRef]
- Thurlbeck, W.M. Measurement of pulmonary emphysema. Am. Rev. Respir. Dis. 1967, 95, 752–764. [Google Scholar]
- D’Hulst, A.I.; Vermaelen, K.Y.; Brusselle, G.G.; Joos, G.F.; Pauwels, R.A. Time course of cigarette smoke-induced pulmonary inflammation in mice. Eur. Respir. J. 2005, 26, 204–213. [Google Scholar] [CrossRef]
- Moiseenko, A.; Kheirollahi, V.; Chao, C.-M.; Ahmadvand, N.; Quantius, J.; Wilhelm, J.; Herold, S.; Ahlbrecht, K.; Morty, R.E.; Rizvanov, A.A.; et al. Origin and characterization of alpha smooth muscle actin-positive cells during murine lung development. Stem Cells 2017, 35, 1566–1578. [Google Scholar] [CrossRef] [PubMed]
- Frank, D.B.; Peng, T.; Zepp, J.A.; Snitow, M.; Vincent, T.L.; Penkala, I.J.; Cui, Z.; Herriges, M.J.; Morley, M.P.; Zhou, S.; et al. Emergence of a Wave of Wnt Signaling that Regulates Lung Alveologenesis by Controlling Epithelial Self-Renewal and Differentiation. Cell Rep. 2016, 17, 2312–2325. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Dijk, V.; Ng-Blichfeldt, J.P.; Bos, I.S.T.; Ciminieri, C.; Königshoff, M.; Kistemaker, L.E.; Gosens, R.; Wu, X.; Van Dijk, E.M.; et al. Mesenchymal WNT-5A/5B Signaling Represses Lung Alveolar Epithelial Progenitors. Cells 2019, 8, 1147. [Google Scholar] [CrossRef] [PubMed]
- Cornett, B.; Snowball, J.; Varisco, B.M.; Lang, R.; Whitsett, J.; Sinner, D. Wntless is required for peripheral lung differentiation and pulmonary vascular development. Dev. Boil. 2013, 379, 38–52. [Google Scholar] [CrossRef]
- Cheng, C.-W.; Yeh, J.-C.; Fan, T.-P.; Smith, S.K.; Charnock-Jones, D.S. Wnt5a-mediated non-canonical Wnt signalling regulates human endothelial cell proliferation and migration. Biochem. Biophys. Res. Commun. 2008, 365, 285–290. [Google Scholar] [CrossRef]
- Davidson, L.M.; Berkelhamer, S.K. Bronchopulmonary Dysplasia: Chronic Lung Disease of Infancy and Long-Term Pulmonary Outcomes. J. Clin. Med. 2017, 6, 4. [Google Scholar] [CrossRef]
- Husain, A.N.; Siddiqui, N.H.; Stocker, J. Pathology of arrested acinar development in postsurfactant bronchopulmonary dysplasia. Hum. Pathol. 1998, 29, 710–717. [Google Scholar] [CrossRef]
- Sucre, J.M.; Deutsch, G.H.; Jetter, C.S.; Ambalavanan, N.; Benjamin, J.T.; Gleaves, L.A.; Millis, B.A.; Young, L.R.; Blackwell, T.S.; Kropski, J.A.; et al. A Shared Pattern of β-Catenin Activation in Bronchopulmonary Dysplasia and Idiopathic Pulmonary Fibrosis. Am. J. Pathol. 2018, 188, 853–862. [Google Scholar] [CrossRef]
- Dasgupta, C.; Sakurai, R.; Wang, Y.; Guo, P.; Ambalavanan, N.; Torday, J.S.; Rehan, V.K. Hyperoxia-induced neonatal rat lung injury involves activation of TGF-{beta} and Wnt signaling and is protected by rosiglitazone. Am. J. Physiol. Cell. Mol. Physiol. 2009, 296, L1031–L1041. [Google Scholar] [CrossRef]
- Xu, W.; Xu, B.; Zhao, Y.; Yang, N.; Liu, C.; Wen, G.; Zhang, B. Wnt5a reverses the inhibitory effect of hyperoxia on transdifferentiation of alveolar epithelial type II cells to type I cells. J. Physiol. Biochem. 2015, 71, 823–838. [Google Scholar] [CrossRef]
- Taylor, S.K.; Sakurai, R.; Sakurai, T.; Rehan, V.K. Inhaled Vitamin D: A Novel Strategy to Enhance Neonatal Lung Maturation. Lung 2016, 194, 931–943. [Google Scholar] [CrossRef] [PubMed]
- Little, D.R.; Gerner-Mauro, K.N.; Flodby, P.; Crandall, E.D.; Borok, Z.; Akiyama, H.; Kimura, S.; Ostrin, E.J.; Chen, J. Transcriptional control of lung alveolar type 1 cell development and maintenance by NK homeobox 2-1. Proc. Natl. Acad. Sci. U.S.A. 2019, 116, 20545–20555. [Google Scholar] [CrossRef] [PubMed]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Smith, S.M.; Peinado, N.; Gao, F.; Li, W.; Lee, M.K.; Zhou, B.; Bellusci, S.; Pryhuber, G.S.; Ho, H.-Y.H.; et al. WNT5a-ROR Signaling Is Essential for Alveologenesis. Cells 2020, 9, 384. https://doi.org/10.3390/cells9020384
Li C, Smith SM, Peinado N, Gao F, Li W, Lee MK, Zhou B, Bellusci S, Pryhuber GS, Ho H-YH, et al. WNT5a-ROR Signaling Is Essential for Alveologenesis. Cells. 2020; 9(2):384. https://doi.org/10.3390/cells9020384
Chicago/Turabian StyleLi, Changgong, Susan M Smith, Neil Peinado, Feng Gao, Wei Li, Matt K Lee, Beiyun Zhou, Saverio Bellusci, Gloria S Pryhuber, Hsin-Yi Henry Ho, and et al. 2020. "WNT5a-ROR Signaling Is Essential for Alveologenesis" Cells 9, no. 2: 384. https://doi.org/10.3390/cells9020384
APA StyleLi, C., Smith, S. M., Peinado, N., Gao, F., Li, W., Lee, M. K., Zhou, B., Bellusci, S., Pryhuber, G. S., Ho, H.-Y. H., Borok, Z., & Minoo, P. (2020). WNT5a-ROR Signaling Is Essential for Alveologenesis. Cells, 9(2), 384. https://doi.org/10.3390/cells9020384




