Abstract
Alzheimer’s disease (AD) is a devastating neurodegenerative disorder and a leading cause of dementia, with accumulation of amyloid-beta (Aβ) and neurofibrillary tangles (NFTs) as defining pathological features. AD presents a serious global health concern with no cure to date, reflecting the complexity of its pathogenesis. Recent evidence indicates that neuroinflammation serves as the link between amyloid deposition, Tau pathology, and neurodegeneration. The high mobility group box 1 (HMGB1) protein, an initiator and activator of neuroinflammatory responses, has been involved in the pathogenesis of neurodegenerative diseases, including AD. HMGB1 is a typical damage-associated molecular pattern (DAMP) protein that exerts its biological activity mainly through binding to the receptor for advanced glycation end products (RAGE) and toll-like receptor 4 (TLR4). RAGE and TLR4 are key components of the innate immune system that both bind to HMGB1. Targeting of HMGB1, RAGE, and TLR4 in experimental AD models has demonstrated beneficial effects in halting AD progression by suppressing neuroinflammation, reducing Aβ load and production, improving spatial learning, and inhibiting microglial stimulation. Herein, we discuss the contribution of HMGB1 and its receptor signaling in neuroinflammation and AD pathogenesis, providing evidence of its beneficial effects upon therapeutic targeting.
1. Introduction
Alzheimer’s disease (AD) is a progressive complex neurodegenerative disorder and an emerging global health concern, afflicting around 50 million worldwide [1]. AD can be described by a steady decline in cognitive function leading to dementia in aging population. The neuropathological hallmarks of AD include acquisition of amyloid-β (Aβ) peptide into the amyloid plaques and intraneuronal neurofibrillary tangles (NFTs), comprising of accumulated Tau protein due to hyper- and/or abnormal phosphorylation [2].
AD occurs in two major forms, known as sporadic AD and familial AD. The former is most common (90% of AD cases), affecting people of any age, but mainly above the age of 65 years and it is often referred as late-onset AD (LOAD) [3]. The aetiology of sporadic AD is not well understood, but it has been associated with several genetic, environmental, and lifestyle factors [4]. On the contrary, familial AD is a less prominent form, with an earlier onset. Familial AD has been associated with mutations in three major genes: Aβ precursor protein (APP), presenilin1 (PSEN1), and presenilin 2 (PSEN2), which induce abnormal overproduction of Aβ [5].
Currently, the available AD drugs provide only symptomatic relief without altering the disease progression, thus reflecting the pressing need of effective and safe disease modifying therapies for AD. Most treatment efforts have been focused on modulation of Aβ accumulation mainly through gamma-secretase inhibition, passive vaccination, or amyloid immunotherapy [6], but with poor clinical outcomes [7].
The repeated failure of AD clinical trials has shifted drug development towards neuroinflammation, which serves as the link between amyloid deposition, Tau pathology, and neurodegeneration [8]. Among several mediators, high mobility group box 1 (HMGB1) protein has been involved in the initiation and activation of neuroinflammatory responses under pathological conditions. HMGB1 protein is the only family member that has been abundantly and most ubiquitously expressed [9] out of the four proteins (HMGB1, HMGB2, HMGB3, and HMGB4), gaining increased attention in recent time. HMGB1 protein is composed of a chain of 215 amino acids with a molecular weight of 25 kDa, comprising of two DNA binding domains (Box A and Box B) and a negatively charged C-terminal [10,11]. HMGB1 exists in three isoforms (fully reduced HMGB1, sulfonyl HMGB1, and disulfide HMGB1). However, disulfide HMGB1 is the only isoform exhibiting pro-inflammatory cytokine-like activity [12]. The functions of HMGB1 mainly depend on its location, binding partners, and redox states [13,14].
HMGB1 functions as an archetypal alarmin and a typical damage-associated molecular pattern (DAMPs) molecule [15]. Alarmins, being endogenous molecules that can be released into the extracellular settings upon cellular stress or damage, have been shown to activate the immune system [16]. The most well-known alarmins include HMGB1, S100s, heat shock proteins (HSPs), IL-1a, uric acid, cathelicidins, defensins, and thymosins [17]. HMGB1 acts as a chemotactic or pro-inflammatory mediator through direct binding to the receptor for advanced glycation end products (RAGE) and toll-like receptor-4 (TLR4) [11]. RAGE plays a significant role in neurodegeneration, whereas TLR4, being an immune cell receptor, regulates immune response [18]. Both receptors share common signaling pathways to induce inflammation [11] and they have been implicated in the pathogenesis of several diseases with therapeutic targeting potential [12].
Accumulative evidence highlights the pathogenic role of HMGB1, RAGE, and TLR4 signaling in AD onset. Upregulation of HMGB1, RAGE, and TLR4 protein levels has been detected in AD peripheral samples [19,20,21]. Moreover, elevated HMGB1 expression was detected in hippocampal neuronal cells of the Aβ25–35-induced AD-related model of neuroinflammation and has been correlated with AD progression [18]. In addition, activation of RAGE signaling in AD has been implicated in the production and aggregation of Aβ, NFTs formation, disruption of synaptic transmission, and neuronal degeneration [22]. At the same time, TLR4 activation has been implicated in the induction of neuroinflammation and Aβ deposition [23].
Herein, we discuss the pathogenic role of HMGB1 and its principal receptors in AD pathology along with their biomarker potential and the promising clinical outcome of blocking/inhibiting HMGB1, RAGE, and TLR4 in AD experimental studies.
2. The Pivotal Role of Neuroinflammation in AD Onset
Neuroinflammation has emerged as an important feature of AD, presenting a link between accumulation of Aβ and NFTs [24]. It has been associated with AD progression through the activation of astrocytes and microglia, and may present both a driving factor of the disease as well as a response to pathogenic events [25].
There is evidence that inflammation, along with sustained activation of microglia and other immune cells, takes place in AD [24,26,27]. Aβ presence has been shown to induce microglial release of pro-inflammatory cytokines and initiation of APP production, leading to increased Aβ production [28,29]. It is possible that neuroinflammation has an additive effect and serves as a risk factor that increases disease severity by exacerbating Aβ and Tau pathology [24]. Its presence has been associated with several other neurodegenerative dementias, including Parkinson’s disease dementia (PDD), frontotemporal dementia (FTD), and Lewy body dementia (LBD) [30]. Enhanced neuroinflammation due to overexpression of pro-inflammatory cytokines has been previously involved in the elevation of hyperphosphorylated Tau and in the decline of hippocampal function [31].
In this context, HMGB1 has been demonstrated to mediate neuroinflammation, and participate in the process of neurodegeneration [32,33]. These data suggest that development of novel pharmacological modulators that can modulate HMGB1 and its receptors (RAGE and TLR4) and control or reduce neuroinflammation may present additional therapeutic strategies to current AD treatment.
3. Evidence of HMGB1 Implication in AD Pathogenesis
HMGB1 is a DNA binding protein localized in the nucleus that translocates to the cytoplasm and eventually is released to the extracellular space during cell activation and apoptosis. Upon stimulation, HMGB1 undergoes post-translational modifications (PTMs) and, based on the redox status of cysteine residues (at positions 23, 45, and 106), it can initiate cytokine production via TLR4 or induce chemotaxis through its interaction with chemokine CXCL12 [34,35]. When released extracellularly, HMGB1 becomes a double-edged sword during neural development and neurodegeneration [33].
Recent studies indicate the activation of HMGB1 in AD experimental models. HMGB1 was found localized in the nucleus and the cytoplasm of hippocampal neuron cultures, in Aβ25–35-induced AD-related model of neuroinflammation. Upon Aβ25–35-treatment, a higher expression of RAGE and TLR4-NF-ĸB (at mRNA and protein level), along with inflammatory mediators (HMGB1, IL-1β, IL-6, and TNF-α), was observed in hippocampal neuronal cells. This finding strongly suggests that neuroinflammation is a crucial contributor of AD and implicates HMGB1 in mediating AD pathogenesis through activation of RAGE/TLR4 signaling, being correlated with AD progression [18].
Treatment of mouse microglial (N9) cell line with Aβ (1000 nM) was shown to upregulate HMGB1, IL-1β, and nod-like receptor protein 3 (NLRP3)-inflammasome, indicating a plausible contribution to microglial activation and subsequent inflammation in AD [36]. This is further confirmed by the fact that dipeptidyl vinyl sulfone attenuates Aβ-induced activation of inflammatory process as evident from downregulation of HMGB1, NLRP3, and IL-1β, indicating a reduction of Aβ-induced microglial activation as an emerging approach against AD [36].
In an animal model of early AD monitoring, using 5xFAD transgenic mice, HMGB1 was shown to initiate neurite degeneration with TLR4-myristoylated alanine-rich C-kinase substrate (MARCKS), triggering MARCKS phosphorylation [37]. In fact, HMGB1 initiated neurite degeneration independent of Aβ and the process of Aβ aggregation by disrupting the balance between different Aβ isoforms [37]. HMGB1 was released from necrotic neurons with intracellular Aβ, thus proposing that HMGB1 occurs downstream of the established intracellular Aβ toxicity. The association between HMGB1 and Aβ is therefore bi-directional and HMGB1 may be considered as an independent AD mediator, closely related to the amyloid cascade [37].
The impact of extracellular HMGB1 in the microglial phagocytosis of Aβ40 and Aβ42 has been explored in the perspective of AD [38,39]. A HMGB1 injection with Aβ42 impeded the Aβ42 clearance from the ipsilateral rat hippocampus. Aβ42-induced neurodegeneration was also increased by extracellular HMGB1. Moreover, by inhibiting the microglial phagocytosis, HMGB1 enhanced Aβ mediated neurotoxicity and stabilized the formation of Aβ monomers [38], suggesting its pathogenic role. Therefore, inhibition of extracellular HMGB1 might be a potential therapeutic approach against AD.
A previous study demonstrated the binding affinity between HMGB1 and Aβ40, where the combination of HMGB1 with Aβ and Aβ40 was immunoprecipitated with A-Sepharose-linked antibodies against HMGB1 or Aβ [39]. Mechanistically, extracellular HMGB1 might act as a chaperone for Aβ and reduce the microglial Aβ clearance by interfering with Aβ40 degradation and Aβ42 internalization by microglia. Therefore, extracellular HMGB1 was found to attenuate microglial Aβ clearance and possibly contribute to AD progression [39] by interacting with RAGE and TLR4 that are involved in microglial Aβ phagocytosis [40,41].
The p35−/−/Tg2576 (KO/Tg) mice model of AD with the deletion of p35 (a neuronal activator of CDK5) exhibited synaptic dysfunction and increased neuronal cell death, which is correlated with activated microglial infiltration and upregulated HMGB1 expression. Importantly, microglial infiltration in the dentate gyrus (DG) and CA1 led to increased HMGB1 secretion, which might increase neuronal apoptosis when combined with Aβ [42]. Therefore, blocking HMGB1 expression may provide protection against neuronal cell death.
Despite several studies on adult hippocampal neurogenesis (AHN) in AD [43,44], no similar conclusion or mechanisms of regulation have been identified [45]. This is evident of the controversial studies that showed AD to downregulate hippocampal neurogenesis with progression of disease [46] or to increase hippocampal neurogenesis [47]. Nevertheless, disruption of AHN at early stages might mediate the AD pathogenesis, indicating a therapeutic approach towards its prevention and treatment [48].
In this regard, HMGB1 has emerged as an inducer of differentiation of neural progenitor cells (NPCs). A study using TgCRND8 mice (an animal model of FAD) demonstrated no significant growth of new mature neurons in the hippocampi when compared to WT mice, indicating decreased survival and/or integration of newborn neurons. Of importance, HMGB1 and Aβ1–42 activated a potential reparative mechanism by promoting neuronal differentiation of adult hippocampal NPCs via the activation of the RAGE/NF-κB cascade [49], thus demonstrating the pro-neurogenic potential of HMGB1. It is therefore evident that HMGB1 activation in AD occurs through the interaction with Aβ and that it is not only a risk factor but may also exert a restorative effect in AHN.
Disruption of learning and memory represents important hallmarks of AD and intracerebroventricular (ICV) injection of HMGB1 (10 μg) in WT, TLR4−/−, and RAGE−/− mice was shown to block these functions. The HMGB1 injection was found to affect memory encoding, as demonstrated by reduced novel object preference index in novel object recognition test (NORT). The HMGB1 amnesic effect was mediated by RAGE and TLR4, as evident by the blockade of memory impairment upon injection of TLR4 antagonist in RAGE-deficient mice [50].
4. Implication of RAGE in AD
RAGE belongs to the immunoglobulin (Ig) superfamily [51] and is widely expressed on several cell types, ranging from vascular cells (endothelial and smooth muscle cells) to immune/inflammatory cells (neutrophils, monocytes/macrophages, lymphocytes, and dendritic cells) [52,53,54,55]. RAGE binds to several DAMPs (including AGEs, HMGB1, S100s, and DNA) and mediates differential cellular responses, being a crucial regulator of the innate immune response. Due to its ability to identify a range of structurally unrelated endogenous and exogenous ligands, RAGE is regarded as a pattern recognition receptor (PRR) [56]. The PRRs comprise of TLRs, nucleotide-binding oligomerization-like receptors, and several other DNA sensors.
There is evidence that RAGE signaling is implicated in an array of inflammatory [57] and neurodegenerative diseases [58], including AD [22,59]. Amyloid plaques in AD develop due to the overproduction of Aβ and/or the failure of Aβ clearance, promoting its deposition. Of importance, RAGE has been demonstrated to play a crucial role in Aβ production and at the failure of Aβ clearance [22]. RAGE, due to its molecular structure and nature, facilitates circulating plasma Aβ entry into the brain through BBB [60]. Along with its decoy receptor soluble RAGE (sRAGE), they may confer protection against AD pathogenesis by manipulating the transport of Aβ into the brain or by modulating the inflammatory mechanisms [61]. sRAGE, an isoform of RAGE lacking the transmembrane domain, competes with the cell-surface RAGE for ligand binding, and has been shown to be involved in the removal or neutralization of circulating ligands, acting as a decoy molecule [61].
RAGE activation induces Aβ production and the aberrant hyperphosphorylation of Tau. It can activate microglia and astrocytes in a reactive as well as in an inflammatory state, thus aggravating AD pathogenesis by inducing a cycle of inflammation and cellular stress [62]. In a cross-sectional clinical study of different dementia patient types, the levels of AGEs and its receptor RAGE were found upregulated in AD, whereas the levels of sRAGE were decreased, indicating that AGE, RAGE, and sRAGE might contribute to AD pathology [63].
In a clinical study of AD and non-demented (ND) patients, the upregulation of RAGE levels in the hippocampus and inferior frontal cortex in post-mortem AD patients was correlated with the severity of brain pathology. Increased immunoreactivity of RAGE has been observed mainly in neurons, microglia, and astrocytes in the affected areas of AD patients [40]. An earlier clinical study unraveled that microvascular RAGE levels were increased with AD onset and were upregulated consecutively in relation to AD severity. In concert, a significant upregulation in endothelial RAGE immunoreactivity was observed in severe Braak V-VI AD patients compared to aged-controls, as well as in patients with early AD pathology. Similarly, a notable elevation in endothelial RAGE immunoreactivity was observed in patients exhibiting early AD-like symptoms compared to aged controls with no reported AD pathology [64].
RAGE has also been demonstrated as a modulating cofactor of the Aβ impact on neuronal function. An experimental study investigating the effects of RAGE in an Aβ-rich environment employed a transgenic (Tg) mouse model with targeted neuronal overexpression of RAGE and mutant APP (mAPP). Double Tg mice (mutant APP/RAGE) exhibited early spatial learning and memory impairments, supplemented by an altered activation of synaptic plasticity markers (CREB and MAPK) and amplified neuropathologic findings, before the detection of these alterations in mAPP mice [65]. On the contrary, when Tg mice with a dominant-negative RAGE construct targeted to neurons were crossed with mAPP animals, protection of spatial learning/memory deficits and reduced neuropathological changes were observed. These findings suggest that RAGE acts as a cofactor for Aβ-mediated neuronal perturbation in experimental models, with a potential as a therapeutic target to improve cellular disruption [65].
Similarly, Tg mice expressing mAPP in neurons and RAGE in microglia showed increased production of IL-1β and TNF-α, enhanced infiltration of microglia and astrocytes, Aβ aggregation, decreased AChE activity, and rapid disruption of spatial learning/memory [66]. RAGE-facilitated generation of pro-inflammatory mediators enhanced accumulation of Aβ via a positive feedback loop, activating the RAGE receptor that will ultimately exacerbate neuroinflammation and amyloid pathology [66]. Earlier studies investigating mAPP mice with genetic deletion of RAGE (mAPP/RO) have shed more light on the impact of RAGE on accumulation of Aβ, amyloid pathology, learning and memory impairments, indicating that an additional underlying mechanism is a part of its role in the cleavage of APP to release Aβ [67]. The cytosolic domain of neuronal RAGE was implicated in the abnormal APP processing and Aβ production via the enhancement of β- and γ-secretase activity. The deletion of RAGE was found to block the initiation of GSK3β and p38 MAPK signaling axis in the Aβ milieu of mAPP mice. Furthermore, RAGE deficiency conferred a defensive effect on the learning and memory deficits in mice overexpressing mutated APP [67]. These findings further support the benefits of targeting RAGE to block the aberrant APP-Aβ metabolism and hinder AD progression.
Overall, there are several RAGE-related signaling axes including the RAGE/Ca2+/calmodulin-dependent protein kinase kinase-β (CaMKK-β)-AMPK, the RAGE/extracellular signal regulated kinase 1/2 (ERK1/2), RAGE/GSK-3β, and RAGE/NF-κB that have been implicated in AD, which are all associated with the regulation of abnormal Tau hyperphosphorylation and Aβ pathology [22].
5. TLR4 Involvement in AD Pathogenesis
TLRs belong to the family of microbe-sensing receptors and contribute to innate immune defense against infection through binding to microbial molecules [68]. To date, 10 TLRs located either at the cellular surface (TLR1, TLR2, TLR 4, TLR5, TLR6, and TLR10) or in the endosome (TLR3, TLR7, TLR8, and TLR 9) have been reported [69]. TLR4 is a transmembrane protein that belongs to the PRRs family [70], which is widely explored in the context of AD pathology. The binding of pathogen-associated molecular patterns (PAMPs) to TLR4 activates the NF-κB signaling axis, resulting in the synthesis and secretion of inflammatory cytokines [71]. Due to the known contribution of the innate immune system in AD, TLR4 has received increased attention and has been extensively studied in AD [72]. TLR4 is also instrumental in driving the binding of fibrillary amyloid and its phagocytosis by microglia in AD [73]. The presence of amyloid is detrimental in activating the TLR4-mediated NF-κB/MAPK inflammatory axis, promoting the discharge of pro-inflammatory and neurotoxic cytokines (IL-1 β, IL-6, and TNF-α) [23,74]. Importantly, microglial activation by Aβ has been shown to require a functional receptor complex of TLR4, MD-2, and CD14 [23].
Additionally, loss-of-function mutations of the TLR4 gene have been associated with inhibition of microglial and monocytic activation by accumulated amyloid peptide, leading to a decreased expression of the inflammatory markers (IL-6 and TNF-α) and nitric oxide, implicating TLR4 in the neuroinflammation of AD. Moreover, TLR4 mRNA was found elevated in APP-overexpressing mice. TLR4 was also shown to mediate Aβ-induced microglial neurotoxicity, as well as Aβ-mediated activation of murine microglia and human monocytes [23].
Despite the fact that innate immune/inflammatory responses contribute to the AD pathology, the underlying mechanism is not completely understood. In a study investigating the contribution of TLR4 in Aβ-induced upregulation of cytokines and chemokines, Aβ-induced microglial and astrocytes stimulation, and migration of leukocytes, there was an upregulation of TNF-α, IL-1β, IL-10, and IL-17 levels in the brain of TLR4 WT AD mice. However, elevation of these cytokines was not reported in TLR4-mutant AD mice as compared to the TLR4-mutant non-transgenic littermates. In addition, the expression levels of the microglia marker CD11b and the reactive astrocyte marker GFAP were upregulated in the brain of TLR4-mutant AD mice compared to TLR4-WT AD mice, without difference at the levels of the common leukocyte antigen CD45. This TLR4-dependent upregulation of cytokines in the AD mouse model indicates the involvement of TLR4 signaling in disease progression and its potential therapeutic targeting [75].
The triggering receptor expressed on myeloid cells 2 (TREM2) protein, a crucial innate immune receptor in the brain, behaves as a protective mechanism against AD where TLRs play a significant role. TREM2 overexpression was associated with upregulation of the cellular activity of Aβ1–42, and promoted its clearance by BV-2 cells, while it decreased the expression of inflammatory markers (IL-1β, IL-6, and TNF-α). It also contributed to decreased expression of other members of TLR family in BV-2 cells, such as TLR4, TLR2, and TLR6 [76]. Overall, TREM2 was shown to attenuate Aβ1–42-mediated neuroinflammation in BV-2 cells via downregulation of TLR signaling pathway. TLR4-driven inflammation was further negatively controlled by TREM2 [77]. Lipopolysaccharide (LPS) injection into the APP/PS1 transgenic AD model mimics systemic inflammation in the development of AD whereby TLR4 expression was upregulated. On the contrary, expression of TREM2 was markedly decreased in APP/PS1 mice, reflecting that the negative modulatory effect of TREM2 on inflammation could be inhibited by LPS-induced hyperactive TLR4. Thus, an imbalance of TLR4/TREM2 may present a potential link between AD and systemic inflammation [78].
In a clinical study of post-mortem human brains, an upregulation of the TLR4, IL-6, and TNF-α mRNA levels was observed at the frontal cortex of AD subjects as compared to age-matched controls [21]. Similarly, in a mouse model of hippocampal differentiation (at 7 days post-lesion) without amyloidosis (i.e., the entorhinal cortex lesioned mouse), hippocampal TLR4 and IL-1β mRNA expression levels were significantly elevated compared to sham-lesioned mice. However, during reinnervation phase (at 21 days post-lesion) there was no significant difference at the TLR4, IL-1β, IL-6, and TNF-α mRNA levels compared to sham-lesioned mice [21]. This finding suggests that the contribution of TLR4 in neuroinflammatory process during AD is not only triggered by amyloidosis, but also by an amyloid independent differentiation process that occurs in the early phases of the disease [21].
A study investigating the role of TLR4 signaling and microglial activation in early stages of AD pathology reported that a non-functional mutation in the TLR4 gene reduced Aβ-induced activation of microglia in the AD mice model at 5 months of age, when the brain deposits of Aβ usually increase. In fact, no difference was noted in the cerebral Aβ deposits and buffer-soluble Aβ amounts between TLR4 wild-type (TLR4W Tg) and TLR4 mutant AD (TLR4M Tg) mice at the early stages of β-amyloidosis [79]. This finding indicates that TLR4 signaling does not alter the production of Aβ and the onset of Aβ deposition. On the contrary, the 9-month-old TLR4M Tg mice exhibited an elevation in the quantity of cerebral Aβ deposits and soluble Aβ42, associated with special learning impairment and decreased CCL3 expression, suggesting that microglial activation via TLR4 could be neuroprotective [79].
Furthermore, the TLR signaling axis contributes to the clearance of Aβ-deposits in the AD brain. The contribution of TLR4 in amyloidogenesis has been revealed in vivo. The Mo/Hu APPswe PS1dE9 mice, which are homozygous for a destructive mutation of TLR4 (TlrLps-d/TlrLps-d), showed increased diffuse and fibrillar Aβ deposits compared to TLR4-WT mouse models [41], indicating that manipulation of the innate immune responses via the TLR4 axis may decrease Aβ load and cell injuries in AD brain.
LPS was shown to activate a greater number of microglia in the young TgAPP/PS1 mice (without Aβ deposition) compared to young WT mice, whereas its ability to activate microglia in old TgAPP/PS1 mice is less prominent (with Aβ deposition) as compared to old WT mice. TLR4 signaling is disrupted in TgAPP/PS1 mice, explaining the remarkable contrast in TLR4 signaling activation between WT and TgAPP/PS1 mice, as well as before and after Aβ deposition in the brain [80]. Hence, microglial TLR4 signaling is inhibited in the AD mouse model, indicating that dysregulated TLR4 signaling may be associated with Aβ accumulation in the brain [80].
The relationship between neuroinflammation, autophagic activity, and TLR4 stimulation has also been investigated in Tau transgenic AD mice. TLR4 stimulation through LPS injection triggers microglial/macrophage inflammatory activation, further enhancing the autophagic flux in the mouse brain. Moreover, chronic mild TLR4 stimulation improves AD-related pathology, as well as synaptic impairments, in Tau-transgenic mice [81].
Activation of TLR signaling can further aggravate AD via initiation of the inflammatory process, Aβ deposition, and oxidative stress [82]. TLR4 is not only essential for regulation of the inflammatory process, but also for the uptake as well as the phagocytic elimination of Aβ plaques [41]. TLR4 activates the phagocytosis of Aβ peptides [73,83], as well as contributes to the formation of Aβ plaque [84,85].
Taken all together, it is evident that modulation of TLR4 signaling pathways could exert a significant impact on AD pathology, mainly by changing the inflammatory state of microglia/macrophages [86].
6. HMGB1, RAGE, and TLR4 as Potential Clinical Biomarkers of AD
AD is a multifactorial disease that develops gradually with symptoms progressing with time, reflecting the need for early intervention [87]. In this regard, exploring biomarkers in AD that can predict the disease and monitor its progression while providing insight into the outcome of therapy are needed. The cerebrospinal fluid (CSF) levels of Aβ, fragments, and p-Tau or total-Tau are extensively used biomarkers for AD [88,89], but their diagnostic accuracy varies between different centers [90]. Furthermore, there is a growing interest in exploring biomarkers of AD that relate to neurodegeneration and BBB dysfunction [91]. This section focuses on novel potential AD biomarkers which are well implicated in AD pathology, such as HMGB1 and its principal receptors (RAGE and TLR4).
A clinical study validating the non-invasive clinical biomarkers of BBB dysfunction and neuroinflammation to evaluate the progression towards neurodegeneration in mild cognitive impairment (MCI) and AD patients detected upregulated expression and/or release of serum HMGB1 and sRAGE, correlated with Aβ levels in AD patients [92]. Interestingly, the elevation of serum HMGB1 levels was observed in patients with MCI as compared to controls or AD patients. Moreover, soluble thrombomodulin (sTM) antigen (a marker of BBB disruption) activity was significantly upregulated in MCI and AD patients. These findings suggest that HMGB1 and sRAGE may act as clinical biomarkers for AD progression [92]. A similar type of upregulation was also reported in the brain tissues of AD patients, suggesting that HMGB1 might accumulate in either extracellular or intracellular regions [19]. Detection of HMGB1 on Aβ40 plaques in AD brains using a specific anti-Aβ40 antibody demonstrated that HMGB1 accumulates extracellularly on Aβ plaques containing Aβ40 in AD brains [39]. A similar type of HMGB1 immunoreactivity was noted in the senile plaques, where levels of HMGB1 protein were found upregulated in AD brains [38]. Evaluation of the HMGB1 concentrations in the CSF of human AD patients showed that HMGB1 levels were unchanged in healthy controls and FTLD patients. However, in a group of AD patients, upregulation of HMGB1 levels was associated with a rapid progression of dementia, further suggesting that CSF levels of HMGB1 might represent a marker of neurodegenerative progression [37].
In a clinical study, RAGE was found co-localized near neuritic plaque deposits in the cells of Aβ-comprising blood vessels, and in endothelial, neuronal, and microglial cells in AD brain tissue at much higher concentrations compared to age-matched control-derived tissues [93]. In plasma samples of AD patients, the affinity-purified IgGs binding to fragment of RAGE were elevated by three-fold, whereas RAGE, IgG, and Aβ titers were negatively correlated with cognitive status compared to control samples. However, individuals with severe cognitive impairment tend to demonstrate higher IgG titers [94]. These data suggest that the measurement of specific Aβ and RAGE IgGs and/or their protein complex might represent a confirmatory test for AD or AD susceptibility [94].
Increased RAGE expression was observed in the capillaries of the AD brain as compared to controls. The significant negative correlations obtained between the Aβ burden of amyloid plaques and RAGE-positive capillaries in AD brains suggest that RAGE is a crucial factor that influences Aβ burden [95]. In an effort to elucidate the AD-related alterations in BBB-associated Aβ receptors, RAGE immunoreactivity was detected in neurons from control hippocampi, whereas a significant reduction in neuronal RAGE immunoreactivity was observed in AD cases. However, a higher concentration of RAGE was detected in AD hippocampi as compared to controls by Western immunoblotting. These observations suggest that AD is linked with changes in the relative distribution of RAGE in the human hippocampus [96].
Significant elevation in the expression of RAGE levels was also observed in post-mortem AD patients (hippocampus and inferior frontal cortex) where the increased RAGE expressions were positively correlated with the severity of brain pathology [40]. Decreased expression levels of sRAGE, which inhibits RAGE signaling, have been reported in the plasma of patients with AD when compared to those with vascular dementia or normal controls [97].
A clinical study conducted in Northern Han Chinese populations demonstrated an increased plasma level of TLR4 in the peripheral blood mononuclear cells (PBMCs) along with elevated TLR4 mRNA and protein levels in LOAD patients compared to healthy controls [98]. On the contrary, a downregulation of TLR4 protein expression was observed in plasma/serum of AD patients who had more Aβ plaques than in patients with other dementia-related diseases [99]. A population study in northern Italy including 626 AD patients also reported that the +896A TLR4 pro-inflammatory allele was overrepresented in AD patients, suggesting that TLR4 single nucleotide polymorphism (SNP) may be a genetic marker of AD susceptibility [100].
7. HMGB1, RAGE, and TLR4 Inhibition/Blockade as a Potential Therapy against AD
Based on the evidence that HMGB1, RAGE, and TLR4 contribute to the pathogenesis of AD, their targeting might be instrumental in elucidating the plausible underlying mechanism associated with AD pathogenesis. Up to date, several HMGB1, RAGE, and TLR4 blocking/inhibiting strategies have demonstrated promising outcomes against AD on experimental studies and are discussed below.
7.1. Effects of HMGB1 Neutralization in AD
The available therapeutic strategies to block/inhibit extracellular HMGB1 include anti-HMGB1 monoclonal antibody (mAb), specific HMGB1 inhibitors (glycyrrhizin and its derivatives), and HMGB1 interference (shRNA) in AD-like experimental settings.
The therapeutic use of anti-HMGB1 antibodies against several HMGB1-mediated pathologies was mainly focused on elucidating the involvement of HMGB1 in several pathological conditions, validation of protein targets, and the efficacy of potential therapeutic approaches [101].
The therapeutic potential of anti-HMGB1 mAb has been recently reviewed in several HMGB1-mediated diseases including PD, epilepsy, TBI, and AD [102]. The anti-HMGB1 mAb treatment in 5xFAD mice ameliorated cognitive impairment at a similar level to WT mice. Administration of the anti-HMGB1 mAb during 1-6 and 3-6 months of age significantly decreased the DNA damage in the cerebral cortex of 5xFAD mice to the normal levels (at 6 months). However, treatment with anti-HMGB1 mAb did not modulate the expression of human APP in the brains of 5xFAD mice but inhibited the HMGB1-induced elevation of Aβ monomers and oligomers [37]. In addition, anti-HMGB1 mAb treatment increased the microglia-specific marker, Iba1, mainly around Aβ aggregates, and enhanced phagocytosis of the Aβ-HMGB1 complex. Anti-HMGB1 mAb further blocked HMGB1 activity with TLR4, inhibited phosphorylation of MARCKS at Ser46, and prevented neurite degeneration, indicating its beneficial effects in modifying disease progression [37].
Glycyrrhizin is a small-molecule inhibitor of extracellular HMGB1 cytokine activity [103] with the potential to penetrate BBB [104]. The neuroprotective effect of glycyrrhizin has been demonstrated in an array of neurological disorders, including epilepsy [105], TBI [106], and PD [107]. Although the therapeutic effect of glycyrrhizin has not been demonstrated in AD mice with developed Aβ pathology, its effects have been extensively evaluated against several AD-like pathologies such as LPS-induced neuroinflammation and cognitive deficits, as well as in surgery-induced cognitive decline. Glycyrrhizin and its derivatives have exerted promising effects mainly by inhibiting HMGB1, improving memory deficits, and reducing the levels of inflammatory cytokines [108,109,110]. The experimental studies with promising outcomes upon HMGB1 inhibition in AD-like pathologies are summarized in Table 1.

Table 1.
Summaries of studies reporting HMGB1 targeted therapies in AD and related pathology.
The complexity of HMGB1 inhibition/blockade is attributed to the existence of three different isoforms of HMGB1 with distinct and different functions [111]. Among the three HMGB1 isoforms, disulphide-HMGB1 is the only isoform possessing pro-inflammatory cytokine-like activity that activates macrophages/monocytes and other cells to produce cytokines, as well as inflammatory mediators [14]. Of importance, therapeutic targeting of HMGB1 in AD might be immature at present since it is implicated at multiple levels in the regulation of immune response, and the precise underlying mechanism of its involvement in AD has not been completely understood. Despite this limiting aspect, the encouraging data of HMGB1 neutralization mAb, glycyrrhizin, and its derivatives in AD-like pathologies suggest that it may present a promising target and needs further investigation [112].
7.2. Effects of RAGE Inhibition in AD
Several pre-clinical and clinical studies have reported the beneficial effects of RAGE inhibitors in AD-like conditions. Genetic blockade of RAGE in mAPP (mAPP/RO) mice exhibited decreased cerebral amyloid pathology with suppressed abnormal APP-Aβ metabolism by downregulating activity of β- and γ-secretase. It also ameliorated learning and memory impairment as compared to mAPP mice [67]. Furthermore, mAPP mice deficient to RAGE (mAPP/DN-RAGE) demonstrated reduced production of Aβ40 and Aβ42 and lowered activity of β- and γ-secretase as compared to mAPP mice. RAGE-deleted mAPP brain exhibited inhibition of p38 MAP kinase and GSK3β activity. These findings indicate the therapeutic potential of RAGE targeting based on its ability to inhibit APP-Aβ metabolism and hinder the progression of AD [67]. Inhibition of neuronal RAGE could demonstrate cytoprotective effects by preserving neuronal function at early stages of the disease. This is further supported by studies of transgenic mice with a dominant-negative RAGE construct targeted to neurons crossed with mAPP animals which exhibited protection of spatial learning/memory deficits and reduced neuropathological alterations, reflecting that RAGE can improve cellular dysfunction [65].
Moreover, several HMGB1 inhibition approaches, including TTP488, sRAGE-mesenchymal stem cells (MSCs), FPS-ZM1, matrine, pentamidine, hesperidin, and linguizhugan, have revealed promising outcomes in experimental AD models mainly by inhibiting RAGE expression, decreasing production of Aβ, reducing Aβ deposition, oxidative stress, and inflammatory cytokines, while improving spatial learning and memory (Table 2) [113,114,115,116,117,118].

Table 2.
Summaries of studies reporting RAGE inhibition in AD and related pathology.
Although pre-clinical studies of RAGE inhibition in AD strengthen the fact that RAGE might represent a potential therapeutic target, future pre-clinical and clinical studies are warranted to evaluate the safety and therapeutic efficacy of RAGE antagonists against AD [22].
7.3. Effects of TLR4 Blockade in AD
Aβ-mediated TLR4 activation actively promotes neuroinflammation in AD, suggesting that blockade/inhibition of TLR4 activation may suppress neuroinflammatory processes [75].
In an experimental study of immunological preconditioning with TLR4 agonist LPS, Monophosphoryl lipid A (MPL) was shown to upregulate IFN-β-positive cells and reduce hippocampal TNF-α-positive cells of Aβ-treated rats. Reduction in TNF-α could be the result of increased IFN-β levels via the TLR4 signaling axis [119]. The release of IFN-β upon pre-treatment with TLR4 agonists might be a promising neuroprotective strategy against neurodegeneration in AD. Spontaneous loss-of-function mutation in the TLR4 gene suppressed activation of microglia and monocytes by aggregated Alzheimer’s amyloid peptide, leading to the downregulation of inflammatory markers IL-6, TNF-α, and NO [23]. TLR4 stimulation with detoxified ligand MPL ameliorated AD-like pathology in APPswe/PS1 mice, as evident by the reduced number and size of Aβ deposits, as well as by the quantity of soluble Aβ in the brain [120].
A wide range of therapeutic compounds (Table 3) have demonstrated their efficacy in animal models of AD-like pathologies, mainly by inhibiting TLR4 expression, suppressing microglial activation and pro-inflammatory cytokine levels, ameliorating learning and memory functions, inhibiting oxidative stress, and reducing apoptotic cell death and Aβ load (number and size of Aβ deposit) [120,121,122,123,124,125,126].

Table 3.
Summaries of pre-clinical studies investigating TLR4 inhibition in AD-like pathology.
These findings indicate that therapeutic targeting of TLR4 may present a promising therapeutic approach for the symptomatic improvement and slowing of AD progression.
8. Discussion and Future Implications
AD is the major cause of dementia worldwide, accounting for 50–70% of all cases [127]. AD is considered as a complex disorder, with multiple molecules and several contributing factors playing a significant role [128]. At the early stages of AD, the immune system contributes to the elimination of amyloid peptides. However, with disease progression, inability to clear toxic Aβ peptides along with an activation of the innate immune system might lead to the initiation of chronic inflammatory phenomena in the brain [129,130]. To date, there is lack of disease-modifying therapy against AD despite the tremendous research efforts, reflecting the increased complexity of the disease. There is an intensive need to explore novel therapeutic strategies that will prevent AD and/or retard the disease progression. However, due to the lack of precise understanding about the mechanisms underlying AD pathogenesis, the development of treatment strategies against AD is complex, which is evident by the repeated failure of drugs in the clinical trial.
Intervention at the early pathological stages of AD is considered of primary importance and HMGB1 with its receptors (RAGE and TLR4) has gained increased attention in AD pathogenesis (Figure 1). Upregulation of HMGB1, RAGE, and TLR4 levels in AD patients [92,95,98] and experimental models [18] indicate their involvement in the disease pathogenesis, presenting a possible risk factor and a therapeutic target.
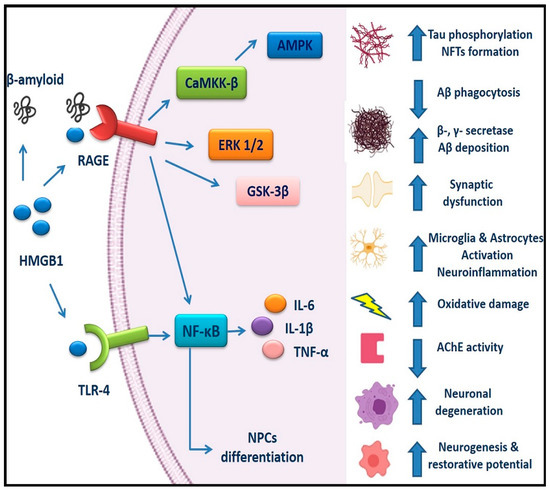
Figure 1.
HMGB1/RAGE and HMGB1/TLR4 signaling pathways in AD: HMGB1 can interact with extracellular Aβ peptides and decrease Aβ deposition by inhibiting Aβ clearance by microglia, as well as increasing β- and γ-secretase activity. RAGE enhances production of Aβ, abnormal Tau hyperphosphorylation, and NFTs formation. HMGB1/RAGE and HMGB1/TLR4 signaling induce neuroinflammation by activating the NF-κB pathway, increasing production of pro-inflammatory cytokines including TNF-α, IL-6, and IL-1β, activating microglia and astrocytes in a reactive and inflammatory state, and thus aggravating the AD pathogenesis through a vicious cycle of inflammation and oxidative damage. RAGE/CaMKK-β-AMPK, the RAGE/ERK1/2, RAGE/GSK-3β, and RAGE/NF-κB pathways have been involved in the regulation of abnormal Tau hyperphosphorylation and Aβ pathology. RAGE signaling has been also implicated in synaptic dysfunction, reduced AChE activity, and neurodegeneration. However, activation of RAGE/NF-κB pathway by HMGB1 in adult NPCs promotes neuronal differentiation and formation of new neurons, leading to increased adult neurogenesis. In addition, HMGB1 may play dual roles in AD pathogenesis, since it can also contribute to reparative mechanisms in the AD brain. AD, Alzheimer’s disease; HMGB1, High mobility group box 1; RAGE, Receptor for advanced glycation end products; TLR4, Toll-like receptor 4; Aβ, Amyloid beta; NFTs, Neurofibrillary tangles; CaMKK-β,Ca2+/calmodulin-dependent protein kinase kinase-β; ERK1/2, Extracellular signal regulated kinase ½; NPCs, Neural progenitor cells; IL, Interleukin; NF-κβ, Nuclear factor κ light chain enhancer of activated β cells; TNF-α, Tumor necrosis factor-α.
Interestingly, inhibition/blockade of HMGB1, RAGE, and TLR4 in experimental AD-like pathologies demonstrated promising outcomes by modifying AD progression through suppression of neuroinflammation, reduction of Aβ load (number and size of Aβ deposit) and Aβ production, improvement of spatial learning and inhibition of microglial activation (Figure 2) (Table 1, Table 2 and Table 3).
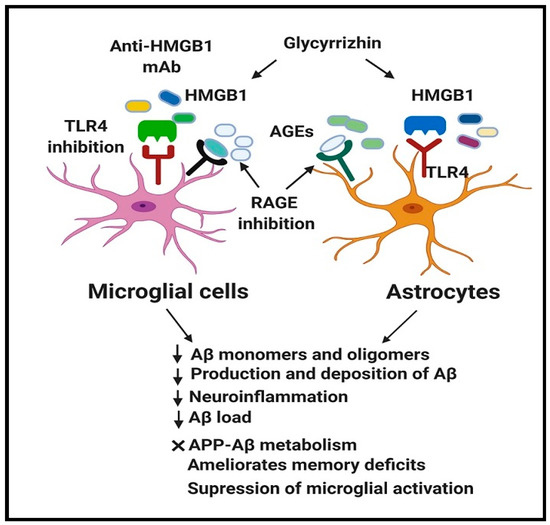
Figure 2.
Beneficial effect of HMGB1, RAGE, and TLR4 inhibition in AD.
Therapeutic blockade of HMGB1, RAGE, and TLR4 in AD using their respective inhibitors demonstrated promising outcomes in modifying AD progression through inhibition of HMGB1, RAGE, and TLR4 expression. Moreover, neutralization of HMGB1, RAGE, and TLR4 leads to the decrease in Aβ monomers and oligomers, suppression of abnormal APP-Aβ metabolism, inhibition of neuroinflammation, reduction of Aβ load (number and size of Aβ deposit) and Aβ production, amelioration of spatial learning and memory deficits, and suppression of microglial activation. These reflect that HMGB1, RAGE, and TLR4 might represent promising therapeutic targets against AD. AD, Alzheimer’s disease; HMGB1, High mobility group box 1; RAGE, Receptor for advanced glycation end products; TLR4, Toll-like receptor 4; Aβ, Amyloid beta; APP, Amyloid precursor protein; AGEs, Advanced glycation end products.
However, on a broader aspect, we should acknowledge the fact that developing treatment strategies for AD is much complex. Also, it is worth noting that due to the multifactorial, heterogeneous, progressive, and interactive pathophysiology of AD, there is a need for personalized combinatorial treatment that differs based on patients’ medical history and disease stages [131].
The encouraging outcome of HMGB1, RAGE, and TLR4 blockade/inhibition indicates that extensive research is highly demanded to elucidate their pathogenic role in AD, along with future clinical studies to validate their therapeutic potential.
Author Contributions
Y.N.P. conceived, carried out the literature review, and drafted the manuscript. E.A., I.O., K.A., and M.F.S. re-drafted the manuscript. C.P. extensively revised the manuscript, provided critical revision and contributed to the final manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work does not receive any sort of financial assistance from any funding agency.
Acknowledgments
Y.N.P. would like to acknowledge Monash University Malaysia for awarding with HDR Scholarship.
Conflicts of Interest
The authors declare that they have no competing interests.
Abbreviations
AD, Alzheimer’s disease AD; HMGB1, High mobility group box 1; RAGE, Receptor for advanced glycation end products; TLRs, Toll-like receptors; DAMP, Damage-associated molecular patterns; APP, Amyloid precursor protein; NFTs, Neurofibrillary tangles; PHFs, Paired helical filaments; AGER, Advanced glycation end product specific receptor; PRRs, Pathogen recognition receptors; AHN, Adult hippocampal neurogenesis; NPCs, Neural progenitor cells; BBB, Blood-brain barrier; CNS, Central nervous system; PTMs, Post-translational modifications; MARCKS, Myristoylated alanine-rich C-kinase substrate; TREM2, Triggering receptor expressed on myeloid cells 2; DCs, Dendritic cells; GFAP, Glial fibrillary acidic protein; IL, Interleukin; IBA1, ionized calcium-binding adapter molecule 1; iNOS, Inducible nitric oxide synthase; NF-κB, Nuclear factor κ light chain enhancer of activated B cells; TNF-α, Tumor necrosis factor-α, LPS, Lipopolysaccharide; CD, Cluster of differentiation; STAT3, Signal transducer and activator of transcription 3; NLRP3, Nod-like receptor protein 3; ICV, Intracerebroventricular; CSF, Cerebrospinal fluid; MCI, Mild cognitive impairment; LOAD, late-onset AD.
References
- Tavana, J.P.; Rosene, M.; Jensen, N.O.; Ridge, P.G.; Kauwe, J.S.; Karch, C.M. RAB10: An Alzheimer’s disease resilience locus and potential drug target. Clin. Interv. Aging 2019, 14, 73. [Google Scholar] [CrossRef] [PubMed]
- Laurent, C.; Buée, L.; Blum, D. Tau and neuroinflammation: What impact for Alzheimer’s disease and tauopathies? Biomed. J. 2018, 41, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Saido, T. Alzheimer’s disease as proteolytic disorders: Anabolism and catabolism of β-amyloid. Neurobiol. Aging 1998, 19, S69–S75. [Google Scholar] [CrossRef]
- Piaceri, I.; Nacmias, B.; Sorbi, S. Genetics of familial and sporadic Alzheimer’s disease. Front. Biosci. Elite Ed. 2013, 5, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Bertram, L.; Lill, C.M.; Tanzi, R.E. The genetics of Alzheimer disease: Back to the future. Neuron 2010, 68, 270–281. [Google Scholar] [CrossRef]
- Salomone, S.; Caraci, F.; Leggio, G.M.; Fedotova, J.; Drago, F. New pharmacological strategies for treatment of Alzheimer’s disease: Focus on disease modifying drugs. Br. J. Clin. Pharmacol. 2012, 73, 504–517. [Google Scholar] [CrossRef]
- Castellani, R.J.; Perry, G. Pathogenesis and disease-modifying therapy in Alzheimer’s disease: The flat line of progress. Arch. Med. Res. 2012, 43, 694–698. [Google Scholar] [CrossRef]
- Van Eldik, L.J.; Carrillo, M.C.; Cole, P.E.; Feuerbach, D.; Greenberg, B.D.; Hendrix, J.A.; Kennedy, M.; Kozauer, N.; Margolin, R.A.; Molinuevo, J.L. The roles of inflammation and immune mechanisms in Alzheimer’s disease. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2016, 2, 99–109. [Google Scholar] [CrossRef]
- Štros, M. HMGB proteins: Interactions with DNA and chromatin. Biochim. Biophys. Acta BBA Gene Regul. Mech. 2010, 1799, 101–113. [Google Scholar] [CrossRef]
- Andersson, U.; Yang, H.; Harris, H. High-mobility group box 1 protein (HMGB1) operates as an alarmin outside as well as inside cells. In Seminars in Immunology; Academic Press: New York, NY, USA, 2018; pp. 40–48. [Google Scholar]
- Paudel, Y.N.; Angelopoulou, E.; Piperi, C.; Balasubramaniam, V.R.; Othman, I.; Shaikh, M.F. Enlightening the role of high mobility group box 1 (HMGB1) in inflammation: Updates on receptor signalling. Eur. J. Pharmacol. 2019, 858, 172487. [Google Scholar] [CrossRef]
- Andersson, U.; Yang, H.; Harris, H. Extracellular HMGB1 as a therapeutic target in inflammatory diseases. Expert Opin. Ther. Targets 2018, 22, 263–277. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Zhao, X.; Antoine, D.; Xiao, X.; Wang, H.; Andersson, U.; Billiar, T.R.; Tracey, K.J.; Lu, B. Regulation of posttranslational modifications of HMGB1 during immune responses. Antioxid. Redox Signal. 2016, 24, 620–634. [Google Scholar] [CrossRef] [PubMed]
- Andersson, U.; Antoine, D.; Tracey, K. Expression of Concern: The functions of HMGB 1 depend on molecular localization and post-Translational modifications. J. Intern. Med. 2014, 276, 420–424. [Google Scholar] [CrossRef]
- Bianchi, M.E. HMGB1 loves company. J. Leukoc. Biol. 2009, 86, 573–576. [Google Scholar] [CrossRef] [PubMed]
- Klune, J.R.; Dhupar, R.; Cardinal, J.; Billiar, T.R.; Tsung, A. HMGB1: Endogenous danger signaling. Mol. Med. 2008, 14, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, M.E. DAMPs, PAMPs and alarmins: All we need to know about danger. J. Leukoc. Biol. 2007, 81, 1–5. [Google Scholar] [CrossRef]
- Nan, K.; Han, Y.; Fang, Q.; Huang, C.; Yu, L.; Ge, W.; Xiang, F.; Tao, Y.-X.; Cao, H.; Li, J. HMGB1 gene silencing inhibits neuroinflammation via down-regulation of NF-κB signaling in primary hippocampal neurons induced by Aβ25–35. Int. Immunopharmacol. 2019, 67, 294–301. [Google Scholar] [CrossRef]
- Takata, K.; Kitamura, Y.; Kakimura, J.-I.; Shibagaki, K.; Tsuchiya, D.; Taniguchi, T.; Smith, M.A.; Perry, G.; Shimohama, S. Role of high mobility group protein-1 (HMG1) in amyloid-β homeostasis. Biochem. Biophys. Res. Commun. 2003, 301, 699–703. [Google Scholar] [CrossRef]
- Du Yan, S.; Chen, X.; Fu, J.; Chen, M.; Zhu, H.; Roher, A.; Slattery, T.; Zhao, L.; Nagashima, M.; Morser, J. RAGE and amyloid-β peptide neurotoxicity in Alzheimer’s disease. Nature 1996, 382, 685. [Google Scholar] [CrossRef]
- Miron, J.; Picard, C.; Frappier, J.; Dea, D.; Theroux, L.; Poirier, J. TLR4 gene expression and pro-inflammatory cytokines in Alzheimer’s disease and in response to hippocampal deafferentation in rodents. J. Alzheimer’s Dis. 2018, 63, 1547–1556. [Google Scholar] [CrossRef]
- Cai, Z.; Liu, N.; Wang, C.; Qin, B.; Zhou, Y.; Xiao, M.; Chang, L.; Yan, L.-J.; Zhao, B. Role of RAGE in Alzheimer’s disease. Cell. Mol. Neurobiol. 2016, 36, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Walter, S.; Letiembre, M.; Liu, Y.; Heine, H.; Penke, B.; Hao, W.; Bode, B.; Manietta, N.; Walter, J.; Schulz-Schüffer, W. Role of the toll-like receptor 4 in neuroinflammation in Alzheimer’s disease. Cell. Physiol. Biochem. 2007, 20, 947–956. [Google Scholar] [CrossRef] [PubMed]
- Kinney, J.W.; Bemiller, S.M.; Murtishaw, A.S.; Leisgang, A.M.; Lamb, B.T. Inflammation as a central mechanism in Alzheimer’s disease. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2018, 4, 575–590. [Google Scholar] [CrossRef] [PubMed]
- Heppner, F.L.; Ransohoff, R.M.; Becher, B. Immune attack: The role of inflammation in Alzheimer disease. Nat. Rev. Neurosci. 2015, 16, 358. [Google Scholar] [CrossRef] [PubMed]
- Chaney, A.; Williams, S.R.; Boutin, H. In vivo molecular imaging of neuroinflammation in Alzheimer’s disease. J. Neurochem. 2019, 149, 438–451. [Google Scholar] [CrossRef]
- Heneka, M.T.; O’Banion, M.K.; Terwel, D.; Kummer, M.P. Neuroinflammatory processes in Alzheimer’s disease. J. Neural Transm. 2010, 117, 919–947. [Google Scholar] [CrossRef]
- Hu, J.; Akama, K.T.; Krafft, G.A.; Chromy, B.A.; Van Eldik, L.J. Amyloid-β peptide activates cultured astrocytes: Morphological alterations, cytokine induction and nitric oxide release. Brain Res. 1998, 785, 195–206. [Google Scholar] [CrossRef]
- Zhu, M.; Wang, X.; Sun, L.; Schultzberg, M.; Hjorth, E. Can inflammation be resolved in Alzheimer’s disease? Ther. Adv. Neurol. Disord. 2018, 11, 1756286418791107. [Google Scholar] [CrossRef]
- Pasqualetti, G.; Brooks, D.J.; Edison, P. The role of neuroinflammation in dementias. Curr. Neurol. Neurosci. Rep. 2015, 15, 17. [Google Scholar] [CrossRef]
- Dionisio-Santos, D.A.; Olschowka, J.A.; O’Banion, M.K. Exploiting microglial and peripheral immune cell crosstalk to treat Alzheimer’s disease. J. Neuroinflamm. 2019, 16, 74. [Google Scholar] [CrossRef]
- Chi, W.; Chen, H.; Li, F.; Zhu, Y.; Yin, W.; Zhuo, Y. HMGB1 promotes the activation of NLRP3 and caspase-8 inflammasomes via NF-κB pathway in acute glaucoma. J. Neuroinflamm. 2015, 12, 137. [Google Scholar] [CrossRef] [PubMed]
- Fang, P.; Schachner, M.; Shen, Y.-Q. HMGB1 in development and diseases of the central nervous system. Mol. Neurobiol. 2012, 45, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Harris, H.E.; Andersson, U.; Pisetsky, D.S. HMGB1: A multifunctional alarmin driving autoimmune and inflammatory disease. Nat. Rev. Rheumatol. 2012, 8, 195. [Google Scholar] [CrossRef] [PubMed]
- Magna, M.; Pisetsky, D.S. The role of HMGB1 in the pathogenesis of inflammatory and autoimmune diseases. Mol. Med. 2014, 20, 138–146. [Google Scholar] [CrossRef]
- Falcão, A.S.; Carvalho, L.A.; Lidónio, G.A.; Vaz, A.R.; Lucas, S.D.; Moreira, R.; Brites, D. Dipeptidyl vinyl sulfone as a novel chemical tool to inhibit HMGB1/NLRP3-inflammasome and inflamma-miRs in Aβ-mediated microglial inflammation. ACS Chem. Neurosci. 2016, 8, 89–99. [Google Scholar] [CrossRef]
- Fujita, K.; Motoki, K.; Tagawa, K.; Chen, X.; Hama, H.; Nakajima, K.; Homma, H.; Tamura, T.; Watanabe, H.; Katsuno, M. HMGB1, a pathogenic molecule that induces neurite degeneration via TLR4-MARCKS, is a potential therapeutic target for Alzheimer’s disease. Sci. Rep. 2016, 6, 31895. [Google Scholar] [CrossRef]
- Takata, K.; Kitamura, Y.; Tsuchiya, D.; Kawasaki, T.; Taniguchi, T.; Shimohama, S. High mobility group box protein-1 inhibits microglial Aβ clearance and enhances Aβ neurotoxicity. J. Neurosci. Res. 2004, 78, 880–891. [Google Scholar] [CrossRef]
- Takata, K.; Takada, T.; Ito, A.; Asai, M.; Tawa, M.; Saito, Y.; Ashihara, E.; Tomimoto, H.; Kitamura, Y.; Shimohama, S. Microglial Amyloid-β1-40 Phagocytosis Dysfunction Is Caused by High-Mobility Group Box Protein-1: Implications for the Pathological Progression of Alzheimer’s Disease. Int. J. Alzheimer’s Dis. 2012, 2012, 685739. [Google Scholar] [CrossRef]
- Lue, L.-F.; Walker, D.G.; Brachova, L.; Beach, T.G.; Rogers, J.; Schmidt, A.M.; Stern, D.M.; Du Yan, S. Involvement of microglial receptor for advanced glycation endproducts (RAGE) in Alzheimer’s disease: Identification of a cellular activation mechanism. Exp. Neurol. 2001, 171, 29–45. [Google Scholar] [CrossRef]
- Tahara, K.; Kim, H.-D.; Jin, J.-J.; Maxwell, J.A.; Li, L.; Fukuchi, K.-I. Role of toll-like receptor signalling in Aβ uptake and clearance. Brain 2006, 129, 3006–3019. [Google Scholar] [CrossRef]
- Jang, A.; Liew, H.; Kim, Y.-M.; Choi, H.; Kim, S.; Hyung Lee, S.; Ohshima, T.; Mikoshiba, K.; Suh, Y.-H. p35 deficiency accelerates HMGB-1-mediated neuronal death in the early stages of an Alzheimer’s disease mouse model. Curr. Alzheimer Res. 2013, 10, 829–843. [Google Scholar] [CrossRef]
- Tobin, M.K.; Musaraca, K.; Disouky, A.; Shetti, A.; Bheri, A.; Honer, W.G.; Kim, N.; Dawe, R.J.; Bennett, D.A.; Arfanakis, K. Human Hippocampal Neurogenesis Persists in Aged Adults and Alzheimer’s Disease Patients. Cell Stem Cell 2019, 24, 974–982. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Jiménez, E.P.; Flor-García, M.; Terreros-Roncal, J.; Rábano, A.; Cafini, F.; Pallas-Bazarra, N.; Ávila, J.; Llorens-Martín, M. Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer’s disease. Nat. Med. 2019, 25, 554. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Zheng, M.; Zhang, T.; He, G. Hippocampal neurogenesis in the APP/PS1/nestin-GFP triple transgenic mouse model of Alzheimer’s disease. Neuroscience 2016, 314, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Donovan, M.H.; Yazdani, U.; Norris, R.D.; Games, D.; German, D.C.; Eisch, A.J. Decreased adult hippocampal neurogenesis in the PDAPP mouse model of Alzheimer’s disease. J. Comp. Neurol. 2006, 495, 70–83. [Google Scholar] [CrossRef] [PubMed]
- Jin, K.; Galvan, V.; Xie, L.; Mao, X.O.; Gorostiza, O.F.; Bredesen, D.E.; Greenberg, D.A. Enhanced neurogenesis in Alzheimer’s disease transgenic (PDGF-APPSw, Ind) mice. Proc. Natl. Acad. Sci. USA 2004, 101, 13363–13367. [Google Scholar] [CrossRef]
- Choi, S.H.; Tanzi, R.E. Is Alzheimer’s Disease a Neurogenesis Disorder? Cell Stem Cell 2019, 25, 7–8. [Google Scholar] [CrossRef]
- Meneghini, V.; Bortolotto, V.; Francese, M.T.; Dellarole, A.; Carraro, L.; Terzieva, S.; Grilli, M. High-mobility group box-1 protein and β-amyloid oligomers promote neuronal differentiation of adult hippocampal neural progenitors via receptor for advanced glycation end products/nuclear factor-κB axis: Relevance for Alzheimer’s disease. J. Neurosci. 2013, 33, 6047–6059. [Google Scholar] [CrossRef]
- Mazarati, A.; Maroso, M.; Iori, V.; Vezzani, A.; Carli, M. High-mobility group box-1 impairs memory in mice through both toll-like receptor 4 and receptor for advanced glycation end products. Exp. Neurol. 2011, 232, 143–148. [Google Scholar] [CrossRef]
- MacLean, M.; Derk, J.; Ruiz, H.H.; Juranek, J.K.; Ramasamy, R.; Schmidt, A.M. The Receptor for Advanced Glycation End Products (RAGE) and DIAPH1: Implications for vascular and neuroinflammatory dysfunction in disorders of the central nervous system. Neurochem. Int. 2019, 126, 154–164. [Google Scholar] [CrossRef]
- Avalos, A.M.; Kiefer, K.; Tian, J.; Christensen, S.; Shlomchik, M.; Coyle, A.J.; Marshak-Rothstein, A. RAGE-independent autoreactive B cell activation in response to chromatin and HMGB1/DNA immune complexes. Autoimmunity 2010, 43, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Daffu, G.; Shen, X.; Senatus, L.; Thiagarajan, D.; Abedini, A.; del Pozo, C.H.; Rosario, R.; Song, F.; Friedman, R.A.; Ramasamy, R. RAGE suppresses ABCG1-mediated macrophage cholesterol efflux in diabetes. Diabetes 2015, 64, 4046–4060. [Google Scholar] [CrossRef] [PubMed]
- Dumitriu, I.E.; Baruah, P.; Bianchi, M.E.; Manfredi, A.A.; Rovere-Querini, P. Requirement of HMGB1 and RAGE for the maturation of human plasmacytoid dendritic cells. Eur. J. Immunol. 2005, 35, 2184–2190. [Google Scholar] [CrossRef] [PubMed]
- Moser, B.; Szabolcs, M.; Ankersmit, H.; Lu, Y.; Qu, W.; Weinberg, A.; Herold, K.; Schmidt, A. Blockade of RAGE suppresses alloimmune reactions in vitro and delays allograft rejection in murine heart transplantation. Am. J. Transplant. 2007, 7, 293–302. [Google Scholar] [CrossRef]
- Chavakis, T.; Bierhaus, A.; Al-Fakhri, N.; Schneider, D.; Witte, S.; Linn, T.; Nagashima, M.; Morser, J.; Arnold, B.; Preissner, K.T. The pattern recognition receptor (RAGE) is a counterreceptor for leukocyte integrins: A novel pathway for inflammatory cell recruitment. J. Exp. Med. 2003, 198, 1507–1515. [Google Scholar] [CrossRef]
- Hudson, B.I.; Lippman, M.E. Targeting RAGE signaling in inflammatory disease. Annu. Rev. Med. 2018, 69, 349–364. [Google Scholar] [CrossRef]
- Juranek, J.; Ray, R.; Banach, M.; Rai, V. Receptor for advanced glycation end-products in neurodegenerative diseases. Rev. Neurosci. 2015, 26, 691–698. [Google Scholar] [CrossRef]
- Yan, S.D.; Bierhaus, A.; Nawroth, P.P.; Stern, D.M. RAGE and Alzheimer’s disease: A progression factor for amyloid-β-induced cellular perturbation? J. Alzheimer’s Dis. 2009, 16, 833–843. [Google Scholar] [CrossRef]
- Deane, R.; Du Yan, S.; Submamaryan, R.K.; LaRue, B.; Jovanovic, S.; Hogg, E.; Welch, D.; Manness, L.; Lin, C.; Yu, J. RAGE mediates amyloid-β peptide transport across the blood-brain barrier and accumulation in brain. Nat. Med. 2003, 9, 907. [Google Scholar] [CrossRef]
- Srikanth, V.; Maczurek, A.; Phan, T.; Steele, M.; Westcott, B.; Juskiw, D.; Münch, G. Advanced glycation endproducts and their receptor RAGE in Alzheimer’s disease. Neurobiol. Aging 2011, 32, 763–777. [Google Scholar] [CrossRef]
- Chaney, M.O.; Stine, W.B.; Kokjohn, T.A.; Kuo, Y.-M.; Esh, C.; Rahman, A.; Luehrs, D.C.; Schmidt, A.M.; Stern, D.; Du Yan, S. RAGE and amyloid beta interactions: Atomic force microscopy and molecular modeling. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2005, 1741, 199–205. [Google Scholar] [CrossRef]
- Xu, X.-Y.; Deng, C.-Q.; Wang, J.; Deng, X.-J.; Xiao, Q.; Li, Y.; He, Q.; Fan, W.-H.; Quan, F.-Y.; Zhu, Y.-P. Plasma levels of soluble receptor for advanced glycation end products in Alzheimer’s disease. Int. J. Neurosci. 2017, 127, 454–458. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.C.; Tavares, R.; Johanson, C.E.; Hovanesian, V.; Donahue, J.E.; Gonzalez, L.; Silverberg, G.D.; Stopa, E.G. Hippocampal RAGE immunoreactivity in early and advanced Alzheimer’s disease. Brain Res. 2008, 1230, 273–280. [Google Scholar] [CrossRef]
- Arancio, O.; Zhang, H.P.; Chen, X.; Lin, C.; Trinchese, F.; Puzzo, D.; Liu, S.; Hegde, A.; Yan, S.F.; Stern, A. RAGE potentiates Aβ-induced perturbation of neuronal function in transgenic mice. EMBO J. 2004, 23, 4096–4105. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.; Lue, L.-F.; Yan, S.; Xu, H.; Luddy, J.S.; Chen, D.; Walker, D.G.; Stern, D.M.; Yan, S.; Schmidt, A.M. RAGE-dependent signaling in microglia contributes to neuroinflammation, Aβ accumulation, and impaired learning/memory in a mouse model of Alzheimer’s disease. FASEB J. 2010, 24, 1043–1055. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.; Yu, Q.; Arancio, O.; Chen, D.; Gore, S.S.; Yan, S.S.; Yan, S.F. RAGE mediates Aβ accumulation in a mouse model of Alzheimer’s disease via modulation of β-and γ-secretase activity. Hum. Mol. Genet. 2018, 27, 1002–1014. [Google Scholar] [CrossRef]
- Kang, J.Y.; Lee, J.-O. Structural biology of the Toll-like receptor family. Annu. Rev. Biochem. 2011, 80, 917–941. [Google Scholar] [CrossRef]
- Bode, J.G.; Ehlting, C.; Häussinger, D. The macrophage response towards LPS and its control through the p38MAPK–STAT3 axis. Cell. Signal. 2012, 24, 1185–1194. [Google Scholar] [CrossRef]
- Kurt-Jones, E.A.; Popova, L.; Kwinn, L.; Haynes, L.M.; Jones, L.P.; Tripp, R.A.; Walsh, E.E.; Freeman, M.W.; Golenbock, D.T.; Anderson, L.J. Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nat. Immunol. 2000, 1, 398. [Google Scholar] [CrossRef]
- Vaure, C.; Liu, Y. A comparative review of toll-like receptor 4 expression and functionality in different animal species. Front. Immunol. 2014, 5, 316. [Google Scholar] [CrossRef]
- Le Page, A.; Dupuis, G.; Frost, E.H.; Larbi, A.; Pawelec, G.; Witkowski, J.M.; Fulop, T. Role of the peripheral innate immune system in the development of Alzheimer’s disease. Exp. Gerontol. 2018, 107, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Reed-Geaghan, E.G.; Savage, J.C.; Hise, A.G.; Landreth, G.E. CD14 and toll-like receptors 2 and 4 are required for fibrillar Aβ-stimulated microglial activation. J. Neurosci. 2009, 29, 11982–11992. [Google Scholar] [CrossRef] [PubMed]
- Ajit, D. Toll-like receptors 2 and 4 mediate Abeta (1-42) activation of the innate immune response in a human monocytic cell line. J. Neurochem. 2008, 104, 524–533. [Google Scholar]
- Jin, J.-J.; Kim, H.-D.; Maxwell, J.A.; Li, L.; Fukuchi, K.-I. Toll-like receptor 4-dependent upregulation of cytokines in a transgenic mouse model of Alzheimer’s disease. J. Neuroinflamm. 2008, 5, 23. [Google Scholar] [CrossRef] [PubMed]
- Long, H.; Zhong, G.; Wang, C.; Zhang, J.; Zhang, Y.; Luo, J.; Shi, S. TREM2 Attenuates Aβ1-42-Mediated Neuroinflammation in BV-2 Cells by Downregulating TLR Signaling. Neurochem. Res. 2019, 44, 1830–1839. [Google Scholar] [CrossRef] [PubMed]
- Ito, H.; Hamerman, J.A. TREM-2, triggering receptor expressed on myeloid cell-2, negatively regulates TLR responses in dendritic cells. Eur. J. Immunol. 2012, 42, 176–185. [Google Scholar] [CrossRef]
- Zhou, J.; Yu, W.; Zhang, M.; Tian, X.; Li, Y.; Lü, Y. Imbalance of Microglial TLR4/TREM2 in LPS-Treated APP/PS1 Transgenic Mice: A Potential Link Between Alzheimer’s Disease and Systemic Inflammation. Neurochem. Res. 2019, 44, 1138–1151. [Google Scholar] [CrossRef]
- Song, M.; Jin, J.; Lim, J.-E.; Kou, J.; Pattanayak, A.; Rehman, J.A.; Kim, H.-D.; Tahara, K.; Lalonde, R.; Fukuchi, K.-I. TLR4 mutation reduces microglial activation, increases Aβ deposits and exacerbates cognitive deficits in a mouse model of Alzheimer’s disease. J. Neuroinflamm. 2011, 8, 92. [Google Scholar] [CrossRef]
- Go, M.; Kou, J.; Lim, J.-E.; Yang, J.; Fukuchi, K.-I. Microglial response to LPS increases in wild-type mice during aging but diminishes in an Alzheimer’s mouse model: Implication of TLR4 signaling in disease progression. Biochem. Biophys. Res. Commun. 2016, 479, 331–337. [Google Scholar] [CrossRef]
- Qin, Y.; Liu, Y.; Hao, W.; Decker, Y.; Tomic, I.; Menger, M.D.; Liu, C.; Fassbender, K. Stimulation of TLR4 Attenuates Alzheimer’s Disease–Related Symptoms and Pathology in Tau-Transgenic Mice. J. Immunol. 2016, 197, 3281–3292. [Google Scholar] [CrossRef]
- Hickman, S.E.; El Khoury, J. TREM2 and the neuroimmunology of Alzheimer’s disease. Biochem. Pharmacol. 2014, 88, 495–498. [Google Scholar] [CrossRef] [PubMed]
- Tricker, E.; Cheng, G. With a little help from my friends: Modulation of phagocytosis through TLR activation. Cell Res. 2008, 18, 711. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, I.R.; Hao, C.; Munoz, D.G. Role of microglia in senile plaque formation. Neurobiol. Aging 1995, 16, 797–804. [Google Scholar] [CrossRef]
- Sheng, J.G.; Bora, S.H.; Xu, G.; Borchelt, D.R.; Price, D.L.; Koliatsos, V.E. Lipopolysaccharide-induced-neuroinflammation increases intracellular accumulation of amyloid precursor protein and amyloid β peptide in APPswe transgenic mice. Neurobiol. Dis. 2003, 14, 133–145. [Google Scholar] [CrossRef]
- Boutajangout, A.; Wisniewski, T. The innate immune system in Alzheimer’s disease. Int. J. Cell Biol. 2013, 2013, 576383. [Google Scholar] [CrossRef]
- Morgan, A.R.; Touchard, S.; Leckey, C.; O’Hagan, C.; Nevado-Holgado, A.J.; Barkhof, F.; Bertram, L.; Blin, O.; Bos, I.; Dobricic, V. Inflammatory biomarkers in Alzheimer’s disease plasma. Alzheimer’s Dement. 2019, 15, 776–787. [Google Scholar] [CrossRef]
- Bayer, A.J. The role of biomarkers and imaging in the clinical diagnosis of dementia. Age Ageing 2018, 47, 641–643. [Google Scholar] [CrossRef]
- Molinuevo, J.L.; Gispert, J.D.; Dubois, B.; Heneka, M.T.; Lleo, A.; Engelborghs, S.; Pujol, J.; de Souza, L.C.; Alcolea, D.; Jessen, F. The AD-CSF-index discriminates Alzheimer’s disease patients from healthy controls: A validation study. J. Alzheimer’s Dis. 2013, 36, 67–77. [Google Scholar] [CrossRef]
- Ritchie, C.; Smailagic, N.; Noel-Storr, A.H.; Ukoumunne, O.; Ladds, E.C.; Martin, S. CSF tau and the CSF tau/ABeta ratio for the diagnosis of Alzheimer’s disease dementia and other dementias in people with mild cognitive impairment (MCI). Cochrane Database Syst. Rev. 2017. [Google Scholar] [CrossRef]
- Zetterberg, H.; Schott, J.M. Biomarkers for Alzheimer’s disease beyond amyloid and tau. Nat. Med. 2019, 25, 201. [Google Scholar] [CrossRef]
- Festoff, B.W.; Sajja, R.K.; van Dreden, P.; Cucullo, L. HMGB1 and thrombin mediate the blood-brain barrier dysfunction acting as biomarkers of neuroinflammation and progression to neurodegeneration in Alzheimer’s disease. J. Neuroinflamm. 2016, 13, 194. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, N.; Toki, S.; Chowei, H.; Saito, T.; Nakano, N.; Hayashi, Y.; Takeuchi, M.; Makita, Z. Immunohistochemical distribution of the receptor for advanced glycation end products in neurons and astrocytes in Alzheimer’s disease. Brain Res. 2001, 888, 256–262. [Google Scholar] [CrossRef]
- Mruthinti, S.; Buccafusco, J.J.; Hill, W.D.; Waller, J.L.; Jackson, T.W.; Zamrini, E.Y.; Schade, R.F. Autoimmunity in Alzheimer’s disease: Increased levels of circulating IgGs binding Aβ and RAGE peptides. Neurobiol. Aging 2004, 25, 1023–1032. [Google Scholar] [CrossRef] [PubMed]
- Jeynes, B.; Provias, J. Evidence for altered LRP/RAGE expression in Alzheimer lesion pathogenesis. Curr. Alzheimer Res. 2008, 5, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Donahue, J.E.; Flaherty, S.L.; Johanson, C.E.; Duncan, J.A.; Silverberg, G.D.; Miller, M.C.; Tavares, R.; Yang, W.; Wu, Q.; Sabo, E. RAGE, LRP-1, and amyloid-beta protein in Alzheimer’s disease. Acta Neuropathol. 2006, 112, 405–415. [Google Scholar] [CrossRef]
- Emanuele, E.; D’Angelo, A.; Tomaino, C.; Binetti, G.; Ghidoni, R.; Politi, P.; Bernardi, L.; Maletta, R.; Bruni, A.C.; Geroldi, D. Circulating levels of soluble receptor for advanced glycation end products in Alzheimer disease and vascular dementia. Arch. Neurol. 2005, 62, 1734–1736. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, L.-Z.; Yu, J.-T.; Chi, Z.-F.; Tan, L. Increased expressions of TLR2 and TLR4 on peripheral blood mononuclear cells from patients with Alzheimer’s disease. J. Neurol. Sci. 2012, 315, 67–71. [Google Scholar] [CrossRef]
- Kilic, U.; Elibol, B.; Uysal, O.; Kilic, E.; Yulug, B.; Sakul, A.S.; Yildiz, G.B. Specific alterations in the circulating levels of the SIRT1, TLR4, and IL7 proteins in patients with dementia. Exp. Gerontol. 2018, 111, 203–209. [Google Scholar] [CrossRef]
- Balistreri, C.; Grimaldi, M.; Chiappelli, M.; Licastro, F.; Castiglia, L.; Listì, F.; Vasto, S.; Lio, D.; Caruso, C.; Candore, G. Association between the polymorphisms of TLR4 and CD14 genes and Alzheimer’s disease. Curr. Pharm. Des. 2008, 14, 2672–2677. [Google Scholar] [CrossRef]
- Musumeci, D.; Roviello, G.N.; Montesarchio, D. An overview on HMGB1 inhibitors as potential therapeutic agents in HMGB1-related pathologies. Pharmacol. Ther. 2014, 141, 347–357. [Google Scholar] [CrossRef]
- Nishibori, M.; Mori, S.; Takahashi, H.K. Anti-HMGB1 monoclonal antibody therapy for a wide range of CNS and PNS diseases. J. Pharmacol. Sci. 2019, 140, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Paudel, Y.N.; Angelopoulou, E.; Semple, B.; Piperi, C.; Othman, I.; Shaikh, M.F. Potential neuroprotective effect of the HMGB1 inhibitor Glycyrrhizin in neurological disorders. ACS Chem. Neurosci. 2020. [Google Scholar] [CrossRef] [PubMed]
- Okuma, Y.; Liu, K.; Wake, H.; Liu, R.; Nishimura, Y.; Hui, Z.; Teshigawara, K.; Haruma, J.; Yamamoto, Y.; Yamamoto, H. Glycyrrhizin inhibits traumatic brain injury by reducing HMGB1–RAGE interaction. Neuropharmacology 2014, 85, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.J.; Wang, L.; Zhang, B.; Gao, F.; Yang, C.M. Glycyrrhizin, an HMGB1 inhibitor, exhibits neuroprotective effects in rats after lithium-pilocarpine-induced status epilepticus. J. Pharm. Pharmacol. 2019, 71, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Webster, K.M.; Shultz, S.R.; Ozturk, E.; Dill, L.K.; Sun, M.; Casillas-Espinosa, P.; Jones, N.C.; Crack, P.J.; O’Brien, T.J.; Semple, B.D. Targeting high-mobility group box protein 1 (HMGB1) in pediatric traumatic brain injury: Chronic neuroinflammatory, behavioral, and epileptogenic consequences. Exp. Neurol. 2019, 320, 112979. [Google Scholar] [CrossRef]
- Santoro, M.; Maetzler, W.; Stathakos, P.; Martin, H.L.; Hobert, M.A.; Rattay, T.W.; Gasser, T.; Forrester, J.V.; Berg, D.; Tracey, K.J. In-vivo evidence that high mobility group box 1 exerts deleterious effects in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model and Parkinson’s disease which can be attenuated by glycyrrhizin. Neurobiol. Dis. 2016, 91, 59–68. [Google Scholar] [CrossRef]
- Song, J.-H.; Lee, J.-W.; Shim, B.; Lee, C.-Y.; Choi, S.; Kang, C.; Sohn, N.-W.; Shin, J.-W. Glycyrrhizin alleviates neuroinflammation and memory deficit induced by systemic lipopolysaccharide treatment in mice. Molecules 2013, 18, 15788–15803. [Google Scholar] [CrossRef]
- Liu, W.; Huang, S.; Li, Y.; Zhang, K.; Zheng, X. Suppressive effect of glycyrrhizic acid against lipopolysaccharide-induced neuroinflammation and cognitive impairment in C57 mice via toll-like receptor 4 signaling pathway. Food Nutr. Res. 2019, 63. [Google Scholar] [CrossRef]
- Chen, X.; Hua, H.-P.; Liang, L.; Liu, L.-J. The oral pretreatment of glycyrrhizin prevents surgery-induced cognitive impairment in aged mice by reducing neuroinflammation and Alzheimer’s-related pathology via HMGB1 inhibition. J. Mol. Neurosci. 2017, 63, 385–395. [Google Scholar]
- Yang, H.; Wang, H.; Chavan, S.S.; Andersson, U. High mobility group box protein 1 (HMGB1): The prototypical endogenous danger molecule. Mol. Med. 2015, 21, S6–S12. [Google Scholar] [CrossRef]
- Balducci, C.; Forloni, G. Novel targets in Alzheimer’s disease: A special focus on microglia. Pharmacol. Res. 2018, 130, 402–413. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, C.; Capoccia, E.; Iuvone, T.; Cuomo, R.; Sarnelli, G.; Steardo, L.; Esposito, G. S100B inhibitor pentamidine attenuates reactive gliosis and reduces neuronal loss in a mouse model of Alzheimer’s disease. Biomed. Res. Int. 2015, 2015, 508342. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Cai, Y.; Cheng, W.; Liu, G.; Zhao, J.; Cao, H.; Tao, H.; Wang, Y.; Yin, M.; Liu, T. A novel, multi-target natural drug candidate, matrine, improves cognitive deficits in Alzheimer’s disease transgenic mice by inhibiting Aβ aggregation and blocking the RAGE/Aβ axis. Mol. Neurobiol. 2017, 54, 1939–1952. [Google Scholar] [CrossRef] [PubMed]
- Deane, R.; Singh, I.; Sagare, A.P.; Bell, R.D.; Ross, N.T.; LaRue, B.; Love, R.; Perry, S.; Paquette, N.; Deane, R.J. A multimodal RAGE-specific inhibitor reduces amyloid β–mediated brain disorder in a mouse model of Alzheimer disease. J. Clin. Investig. 2012, 122, 1377–1392. [Google Scholar] [CrossRef]
- Kostura, M.J.; Kindy, M.S.; Burstein, A.; Valcarce, C.; Polisetti, D.; Andrews, R.; Mjalli, A.M. Efficacy of Rage Antagonists in Murine Model of Alzheimer’s Disease. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2014, 10, P638–P639. [Google Scholar] [CrossRef]
- Hu, Q.; Yu, B.; Chen, Q.; Wang, Y.; Ling, Y.; Sun, S.; Shi, Y.; Zhou, C. Effect of Linguizhugan decoction on neuroinflammation and expression disorder of the amyloid β-related transporters RAGE and LRP-1 in a rat model of Alzheimer’s disease. Mol. Med. Rep. 2018, 17, 827–834. [Google Scholar] [CrossRef]
- Hong, Y.; An, Z. Hesperidin attenuates learning and memory deficits in APP/PS1 mice through activation of Akt/Nrf2 signaling and inhibition of RAGE/NF-κB signaling. Arch. Pharm. Res. 2018, 41, 655–663. [Google Scholar] [CrossRef]
- Yousefi, N.; Sotoodehnejadnematalahi, F.; Heshmati-Fakhr, N.; Sayyah, M.; Hoseini, M.; Ghassemi, S.; Aliakbari, S.; Pourbadie, H.G. Prestimulation of Microglia Through TLR4 Pathway Promotes Interferon Beta Expression in a Rat Model of Alzheimer’s Disease. J. Mol. Neurosci. 2019, 67, 495–503. [Google Scholar] [CrossRef]
- Michaud, J.-P.; Hallé, M.; Lampron, A.; Thériault, P.; Préfontaine, P.; Filali, M.; Tribout-Jover, P.; Lanteigne, A.-M.; Jodoin, R.; Cluff, C. Toll-like receptor 4 stimulation with the detoxified ligand monophosphoryl lipid A improves Alzheimer’s disease-related pathology. Proc. Natl. Acad. Sci. USA 2013, 110, 1941–1946. [Google Scholar] [CrossRef]
- Shi, S.; Liang, D.; Chen, Y.; Xie, Y.; Wang, Y.; Wang, L.; Wang, Z.; Qiao, Z. Gx-50 reduces β-amyloid-induced TNF-α, IL-1β, NO, and PGE2 expression and inhibits NF-κB signaling in a mouse model of Alzheimer’s disease. Eur. J. Immunol. 2016, 46, 665–676. [Google Scholar] [CrossRef]
- Jin, X.; Liu, M.Y.; Zhang, D.F.; Zhong, X.; Du, K.; Qian, P.; Yao, W.F.; Gao, H.; Wei, M.J. Baicalin mitigates cognitive impairment and protects neurons from microglia-mediated neuroinflammation via suppressing NLRP 3 inflammasomes and TLR 4/NF-κB signaling pathway. CNS Neurosci. Ther. 2019, 25, 575–590. [Google Scholar] [CrossRef] [PubMed]
- Ikram, M.; Muhammad, T.; Rehman, S.U.; Khan, A.; Jo, M.G.; Ali, T.; Kim, M.O. Hesperetin confers neuroprotection by regulating Nrf2/TLR4/NF-κB signaling in an Aβ mouse model. Mol. Neurobiol. 2019, 6, 6293–6309. [Google Scholar] [CrossRef] [PubMed]
- Guan, F.; Zhou, X.; Li, P.; Wang, Y.; Liu, M.; Li, F.; Cui, Y.; Huang, T.; Yao, M.; Zhang, Y. MG53 attenuates lipopolysaccharide-induced neurotoxicity and neuroinflammation via inhibiting TLR4/NF-κB pathway in vitro and in vivo. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2019, 95, 109684. [Google Scholar] [CrossRef] [PubMed]
- Capiralla, H.; Vingtdeux, V.; Zhao, H.; Sankowski, R.; Al-Abed, Y.; Davies, P.; Marambaud, P. Resveratrol mitigates lipopolysaccharide-and Aβ-mediated microglial inflammation by inhibiting the TLR4/NF-κB/STAT signaling cascade. J. Neurochem. 2012, 120, 461–472. [Google Scholar] [CrossRef]
- Pourbadie, H.G.; Sayyah, M.; Khoshkholgh-Sima, B.; Choopani, S.; Nategh, M.; Motamedi, F.; Shokrgozar, M.A. Early minor stimulation of microglial TLR2 and TLR4 receptors attenuates Alzheimer’s disease–related cognitive deficit in rats: Behavioral, molecular, and electrophysiological evidence. Neurobiol. Aging 2018, 70, 203–216. [Google Scholar] [CrossRef]
- Querfurth, H.W.; LaFerla, F.M. Mechanisms of disease. N. Engl. J. Med. 2010, 362, 329–344. [Google Scholar] [CrossRef]
- Lahiri, D.K. Lessons from Alzheimer’s Disease (AD) Clinical Trials: Instead of “A-Drug”, AD-D prevention to Avert AD. Curr. Alzheimer Res. 2019, 16, 279–280. [Google Scholar] [CrossRef]
- McGeer, P.L.; McGeer, E.G. Inflammation, autotoxicity and Alzheimer disease. Neurobiol. Aging 2001, 22, 799–809. [Google Scholar] [CrossRef]
- McGeer, P.L.; McGeer, E.G. Local neuroinflammation and the progression of Alzheimer’s disease. J. Neurovirol. 2002, 8, 529–538. [Google Scholar] [CrossRef]
- Hampel, H.; Mesulam, M.-M.; Cuello, A.C.; Farlow, M.R.; Giacobini, E.; Grossberg, G.T.; Khachaturian, A.S.; Vergallo, A.; Cavedo, E.; Snyder, P.J. The cholinergic system in the pathophysiology and treatment of Alzheimer’s disease. Brain 2018, 141, 1917–1933. [Google Scholar] [CrossRef]
- Papaevgeniou, N.; Sakellari, M.; Jha, S.; Tavernarakis, N.; Holmberg, C.I.; Gonos, E.S.; Chondrogianni, N. 18α-Glycyrrhetinic acid proteasome activator decelerates aging and Alzheimer’s disease progression in caenorhabditis elegans and neuronal cultures. Antioxid. Redox Signal. 2016, 25, 855–869. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Son, M.; Choi, J.; Lee, S.; Byun, K. sRAGE prolonged stem cell survival and suppressed RAGE-related inflammatory cell and T lymphocyte accumulations in an Alzheimer’s disease model. Biochem. Biophys. Res. Commun. 2018, 495, 807–813. [Google Scholar] [CrossRef] [PubMed]
- Criscuolo, C.; Fontebasso, V.; Middei, S.; Stazi, M.; Ammassari-Teule, M.; Yan, S.S.; Origlia, N. Entorhinal Cortex dysfunction can be rescued by inhibition of microglial RAGE in an Alzheimer’s disease mouse model. Sci. Rep. 2017, 7, 42370. [Google Scholar] [CrossRef] [PubMed]
- Sabbagh, M.N.; Agro, A.; Bell, J.; Aisen, P.S.; Schweizer, E.; Galasko, D. PF-04494700, an oral inhibitor of receptor for advanced glycation end products (RAGE), in Alzheimer’s disease. Alzheimer Dis. Assoc. Disord. 2011, 25, 206. [Google Scholar] [CrossRef]
- Galasko, D.; Bell, J.; Mancuso, J.Y.; Kupiec, J.W.; Sabbagh, M.N.; van Dyck, C.; Thomas, R.G.; Aisen, P.S. Clinical trial of an inhibitor of RAGE-Aβ interactions in Alzheimer disease. Neurology 2014, 82, 1536–1542. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).