Regulation of Synaptic Development by Astrocyte Signaling Factors and Their Emerging Roles in Substance Abuse
Abstract
1. Introduction
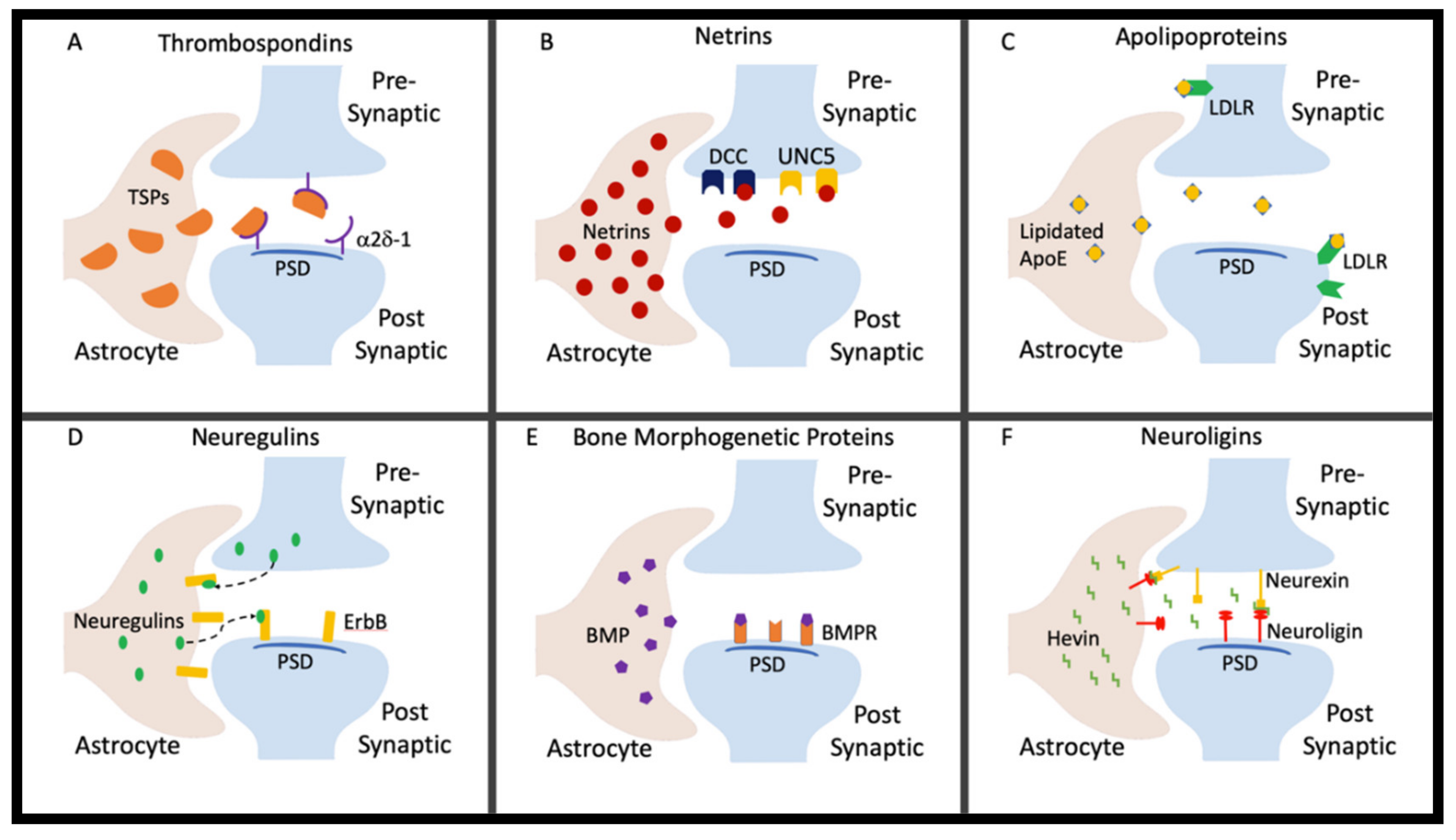
2. Thrombospondins
3. Netrins
4. Apolipoproteins
5. Neuregulins
6. Bone Morphogenetic Proteins
7. Neuroligins
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kubotera, H.; Ikeshima-Kataoka, H.; Hatashita, Y.; Allegra Mascaro, A.L.; Pavone, F.S.; Inoue, T. Astrocytic endfeet re-cover blood vessels after removal by laser ablation. Sci. Rep. 2019, 9, 1263. [Google Scholar] [CrossRef] [PubMed]
- Ventura, R.; Harris, K.M. Three-dimensional relationships between hippocampal synapses and astrocytes. J. Neurosci. 1999, 19, 6897–6906. [Google Scholar] [CrossRef] [PubMed]
- Bushong, E.A.; Martone, M.E.; Jones, Y.Z.; Ellisman, M.H. Protoplasmic astrocytes in CA1 stratum radiatum occupy separate anatomical domains. J. Neurosci. 2002, 22, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Oberheim, N.A.; Takano, T.; Han, X.; He, W.; Lin, J.H.; Wang, F.; Xu, Q.; Wyatt, J.D.; Pilcher, W.; Ojemann, J.G.; et al. Uniquely hominid features of adult human astrocytes. J. Neurosci. 2009, 29, 3276–3287. [Google Scholar] [CrossRef]
- Ullian, E.M.; Sapperstein, S.K.; Christopherson, K.S.; Barres, B.A. Control of synapse number by glia. Science 2001, 291, 657–661. [Google Scholar] [CrossRef]
- Christopherson, K.S.; Ullian, E.M.; Stokes, C.C.; Mullowney, C.E.; Hell, J.W.; Agah, A.; Lawler, J.; Mosher, D.F.; Bornstein, P.; Barres, B.A. Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell 2005, 120, 421–433. [Google Scholar] [CrossRef]
- Farhy-Tselnicker, I.; Allen, N.J. Astrocytes, neurons, synapses: A tripartite view on cortical circuit development. Neural Dev. 2018, 13, 7. [Google Scholar] [CrossRef]
- Wiese, S.; Karus, M.; Faissner, A. Astrocytes as a source for extracellular matrix molecules and cytokines. Front. Pharmacol. 2012, 3, 120. [Google Scholar] [CrossRef]
- Zamanian, J.L.; Xu, L.; Foo, L.C.; Nouri, N.; Zhou, L.; Giffard, R.G.; Barres, B.A. Genomic analysis of reactive astrogliosis. J. Neurosci. 2012, 32, 6391–6410. [Google Scholar] [CrossRef]
- Wong, D.; Dorovini-Zis, K.; Vincent, S.R. Cytokines, nitric oxide, and cGMP modulate the permeability of an in vitro model of the human blood-brain barrier. Exp. Neurol. 2004, 190, 446–455. [Google Scholar] [CrossRef]
- Fan, Y.; Shen, F.; Chen, Y.; Hao, Q.; Liu, W.; Su, H.; Young, W.L.; Yang, G.Y. Overexpression of netrin-1 induces neovascularization in the adult mouse brain. J. Cereb. Blood Flow Metab. 2008, 28, 1543–1551. [Google Scholar] [CrossRef]
- Song, H.; Stevens, C.F.; Gage, F.H. Astroglia induce neurogenesis from adult neural stem cells. Nature 2002, 417, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Dickson, B.J. Molecular mechanisms of axon guidance. Science 2002, 298, 1959–1964. [Google Scholar] [CrossRef]
- Khakh, B.S.; Sofroniew, M.V. Diversity of astrocyte functions and phenotypes in neural circuits. Nat. Neurosci. 2015, 18, 942–952. [Google Scholar] [CrossRef]
- Matias, I.; Morgado, J.; Gomes, F.C.A. Astrocyte Heterogeneity: Impact to Brain Aging and Disease. Front. Aging Neurosci. 2019, 11, 59. [Google Scholar] [CrossRef] [PubMed]
- Clarke, L.E.; Liddelow, S.A.; Chakraborty, C.; Munch, A.E.; Heiman, M.; Barres, B.A. Normal aging induces A1-like astrocyte reactivity. Proc. Natl. Acad. Sci. USA 2018, 115, E1896–E1905. [Google Scholar] [CrossRef] [PubMed]
- Karve, I.P.; Taylor, J.M.; Crack, P.J. The contribution of astrocytes and microglia to traumatic brain injury. Br. J. Pharmacol 2016, 173, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Munch, A.E.; Chung, W.S.; Peterson, T.C.; et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef]
- Pekny, M.; Wilhelmsson, U.; Pekna, M. The dual role of astrocyte activation and reactive gliosis. Neurosci. Lett. 2014, 565, 30–38. [Google Scholar] [CrossRef]
- Zakharova, M.; Ziegler, H.K. Paradoxical anti-inflammatory actions of TNF-alpha: Inhibition of IL-12 and IL-23 via TNF receptor 1 in macrophages and dendritic cells. J. Immunol. 2005, 175, 5024–5033. [Google Scholar] [CrossRef]
- Lee, C.T.; Boeshore, K.L.; Wu, C.; Becker, K.G.; Errico, S.L.; Mash, D.C.; Freed, W.J. Cocaine promotes primary human astrocyte proliferation via JNK-dependent up-regulation of cyclin A2. Restor. Neurol. Neurosci. 2016, 34, 965–976. [Google Scholar] [CrossRef] [PubMed]
- Lasic, E.; Lisjak, M.; Horvat, A.; Bozic, M.; Sakanovic, A.; Anderluh, G.; Verkhratsky, A.; Vardjan, N.; Jorgacevski, J.; Stenovec, M.; et al. Astrocyte Specific Remodeling of Plasmalemmal Cholesterol Composition by Ketamine Indicates a New Mechanism of Antidepressant Action. Sci. Rep. 2019, 9, 10957. [Google Scholar] [CrossRef] [PubMed]
- Stansfield, W.E.; Ranek, M.; Pendse, A.; Schisler, J.C.; Wang, S.; Pulinilkunnil, T.; Willis, M.S. The Pathophysiology of Cardiac Hypertrophy and Heart Failure. In Cellular and Molecular Pathobiology of Cardiovascular Disease; Willis, M.S., Homeister, J.W., Stone, J.R., Eds.; Elsevier Academic Press: Cambridge, MA, USA, 2014; pp. 51–78. [Google Scholar]
- Risher, W.C.; Eroglu, C. Thrombospondins as key regulators of synaptogenesis in the central nervous system. Matrix Biol. 2012, 31, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Eroglu, C.; Allen, N.J.; Susman, M.W.; O’Rourke, N.A.; Park, C.Y.; Ozkan, E.; Chakraborty, C.; Mulinyawe, S.B.; Annis, D.S.; Huberman, A.D.; et al. Gabapentin receptor alpha2delta-1 is a neuronal thrombospondin receptor responsible for excitatory CNS synaptogenesis. Cell 2009, 139, 380–392. [Google Scholar] [CrossRef] [PubMed]
- Risher, W.C.; Kim, N.; Koh, S.; Choi, J.E.; Mitev, P.; Spence, E.F.; Pilaz, L.J.; Wang, D.; Feng, G.; Silver, D.L.; et al. Thrombospondin receptor alpha2delta-1 promotes synaptogenesis and spinogenesis via postsynaptic Rac1. J. Cell Biol. 2018, 217, 3747–3765. [Google Scholar] [CrossRef]
- Lu, Z.; Kipnis, J. Thrombospondin 1--a key astrocyte-derived neurogenic factor. FASEB J. 2010, 24, 1925–1934. [Google Scholar] [CrossRef]
- Spencer, S.; Brown, R.M.; Quintero, G.C.; Kupchik, Y.M.; Thomas, C.A.; Reissner, K.J.; Kalivas, P.W. alpha2delta-1 signaling in nucleus accumbens is necessary for cocaine-induced relapse. J. Neurosci. 2014, 34, 8605–8611. [Google Scholar] [CrossRef]
- Risher, M.L.; Sexton, H.G.; Risher, W.C.; Wilson, W.A.; Fleming, R.L.; Madison, R.D.; Moore, S.D.; Eroglu, C.; Swartzwelder, H.S. Adolescent Intermittent Alcohol Exposure: Dysregulation of Thrombospondins and Synapse Formation are Associated with Decreased Neuronal Density in the Adult Hippocampus. Alcohol Clin. Exp. Res. 2015, 39, 2403–2413. [Google Scholar] [CrossRef]
- Hakanen, J.; Duprat, S.; Salminen, M. Netrin1 is required for neural and glial precursor migrations into the olfactory bulb. Dev. Biol. 2011, 355, 101–114. [Google Scholar] [CrossRef]
- Yung, A.R.; Nishitani, A.M.; Goodrich, L.V. Phenotypic analysis of mice completely lacking netrin 1. Development 2015, 142, 3686–3691. [Google Scholar] [CrossRef]
- Lai Wing Sun, K.; Correia, J.P.; Kennedy, T.E. Netrins: Versatile extracellular cues with diverse functions. Development 2011, 138, 2153–2169. [Google Scholar] [PubMed]
- Harter, P.N.; Bunz, B.; Dietz, K.; Hoffmann, K.; Meyermann, R.; Mittelbronn, M. Spatio-temporal deleted in colorectal cancer (DCC) and netrin-1 expression in human foetal brain development. Neuropathol. Appl. Neurobiol. 2010, 36, 623–635. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Chen, R.; Chen, H.; Zhang, Y.; Chen, J.; Lin, P.; Lan, Q.; Yuan, Q.; Lai, Y.; Jiang, X.; et al. Netrin-1 Promotes Synaptic Formation and Axonal Regeneration via JNK1/c-Jun Pathway after the Middle Cerebral Artery Occlusion. Front. Cell Neurosci. 2018, 12, 13. [Google Scholar] [CrossRef] [PubMed]
- Goldman, J.S.; Ashour, M.A.; Magdesian, M.H.; Tritsch, N.X.; Harris, S.N.; Christofi, N.; Chemali, R.; Stern, Y.E.; Thompson-Steckel, G.; Gris, P.; et al. Netrin-1 promotes excitatory synaptogenesis between cortical neurons by initiating synapse assembly. J. Neurosci. 2013, 33, 17278–17289. [Google Scholar] [CrossRef]
- Matsukawa, H.; Akiyoshi-Nishimura, S.; Zhang, Q.; Lujan, R.; Yamaguchi, K.; Goto, H.; Yaguchi, K.; Hashikawa, T.; Sano, C.; Shigemoto, R.; et al. Netrin-G/NGL complexes encode functional synaptic diversification. J. Neurosci. 2014, 34, 15779–15792. [Google Scholar] [CrossRef] [PubMed]
- Kelai, S.; Ramoz, N.; Moalic, J.M.; Noble, F.; Mechawar, N.; Imbeaud, S.; Turecki, G.; Simonneau, M.; Gorwood, P.; Maussion, G. Netrin G1: Its downregulation in the nucleus accumbens of cocaine-conditioned mice and genetic association in human cocaine dependence. Addict. Biol. 2018, 23, 448–460. [Google Scholar] [CrossRef] [PubMed]
- Serafini, T.; Colamarino, S.A.; Leonardo, E.D.; Wang, H.; Beddington, R.; Skarnes, W.C.; Tessier-Lavigne, M. Netrin-1 is required for commissural axon guidance in the developing vertebrate nervous system. Cell 1996, 87, 1001–1014. [Google Scholar] [CrossRef]
- Elliott, D.A.; Weickert, C.S.; Garner, B. Apolipoproteins in the brain: Implications for neurological and psychiatric disorders. Clin. Lipidol. 2010, 51, 555–573. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Q. Cholesterol metabolism and homeostasis in the brain. Protein Cell 2015, 6, 254–264. [Google Scholar] [CrossRef]
- Liu, C.C.; Liu, C.C.; Kanekiyo, T.; Xu, H.; Bu, G. Apolipoprotein E and Alzheimer disease: Risk, mechanisms and therapy. Nat. Rev. Neurol. 2013, 9, 106–118. [Google Scholar] [CrossRef]
- Harris, F.M.; Tesseur, I.; Brecht, W.J.; Xu, Q.; Mullendorff, K.; Chang, S.; Wyss-Coray, T.; Mahley, R.W.; Huang, Y. Astroglial regulation of apolipoprotein E expression in neuronal cells. Implications for Alzheimer’s disease. J. Biol. Chem. 2004, 279, 3862–3868. [Google Scholar] [CrossRef] [PubMed]
- Spagnuolo, M.S.; Donizetti, A.; Iannotta, L.; Aliperti, V.; Cupidi, C.; Bruni, A.C.; Cigliano, L. Brain-derived neurotrophic factor modulates cholesterol homeostasis and Apolipoprotein E synthesis in human cell models of astrocytes and neurons. J. Cell Physiol. 2018, 233, 6925–6943. [Google Scholar] [CrossRef] [PubMed]
- Koistinaho, M.; Lin, S.; Wu, X.; Esterman, M.; Koger, D.; Hanson, J.; Higgs, R.; Liu, F.; Malkani, S.; Bales, K.R.; et al. Apolipoprotein E promotes astrocyte colocalization and degradation of deposited amyloid-beta peptides. Nat. Med. 2004, 10, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Cartocci, V.; Servadio, M.; Trezza, V.; Pallottini, V. Can Cholesterol Metabolism Modulation Affect Brain Function and Behavior? J. Cell Physiol. 2017, 232, 281–286. [Google Scholar] [CrossRef]
- Fernandez, C.G.; Hamby, M.E.; McReynolds, M.L.; Ray, W.J. The Role of APOE4 in Disrupting the Homeostatic Functions of Astrocytes and Microglia in Aging and Alzheimer’s Disease. Front. Aging Neurosci. 2019, 11, 14. [Google Scholar] [CrossRef]
- Farrer, L.A.; Cupples, L.A.; Haines, J.L.; Hyman, B.; Kukull, W.A.; Mayeux, R.; Myers, R.H.; Pericak-Vance, M.A.; Risch, N.; van Duijn, C.M. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA 1997, 278, 1349–1356. [Google Scholar] [CrossRef]
- Li, L.; Liu, M.S.; Li, G.Q.; Tang, J.; Liao, Y.; Zheng, Y.; Guo, T.L.; Kang, X.; Yuan, M.T. Relationship between Apolipoprotein Superfamily and Parkinson’s Disease. Chin. Med. J. (Engl) 2017, 130, 2616–2623. [Google Scholar] [CrossRef]
- Lewohl, J.M.; Wang, L.; Miles, M.F.; Zhang, L.; Dodd, P.R.; Harris, R.A. Gene expression in human alcoholism: Microarray analysis of frontal cortex. Alcohol Clin. Exp. Res. 2000, 24, 1873–1882. [Google Scholar] [CrossRef]
- Bechtholt, A.J.; Smith, R.; Raber, J.; Cunningham, C.L. Enhanced ethanol-, but not cocaine-induced, conditioned place preference in Apoe(-/-) mice. Pharmacol. Biochem. Behav. 2004, 77, 783–792. [Google Scholar] [CrossRef]
- Djousse, L.; Himali, J.J.; Beiser, A.; Kelly-Hayes, M.; Wolf, P.A. Apolipoprotein e, alcohol consumption, and risk of ischemic stroke: The Framingham Heart Study revisited. J. Stroke Cerebrovasc. Dis. 2009, 18, 384–388. [Google Scholar] [CrossRef][Green Version]
- Harwood, D.G.; Kalechstein, A.; Barker, W.W.; Strauman, S.; St George-Hyslop, P.; Iglesias, C.; Loewenstein, D.; Duara, R. The effect of alcohol and tobacco consumption, and apolipoprotein E genotype, on the age of onset in Alzheimer’s disease. Int. J. Geriatr. Psychiatry 2010, 25, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Falls, D.L. Neuregulins and the neuromuscular system: 10 years of answers and questions. J. Neurocytol. 2003, 32, 619–647. [Google Scholar] [CrossRef]
- Yang, X.; Kuo, Y.; Devay, P.; Yu, C.; Role, L. A cysteine-rich isoform of neuregulin controls the level of expression of neuronal nicotinic receptor channels during synaptogenesis. Neuron 1998, 20, 255–270. [Google Scholar] [CrossRef]
- Talmage, D.A. Mechanisms of neuregulin action. Novartis Found. Symp. 2008, 289, 74–84. [Google Scholar] [PubMed]
- Deakin, I.H.; Godlewska, B.R.; Walker, M.A.; Huang, G.J.; Schwab, M.H.; Nave, K.A.; Law, A.J.; Harrison, P.J. Altered hippocampal gene expression and structure in transgenic mice overexpressing neuregulin 1 (Nrg1) type I. Transl. Psychiatry 2018, 8, 229. [Google Scholar] [CrossRef] [PubMed]
- Deakin, I.H.; Nissen, W.; Law, A.J.; Lane, T.; Kanso, R.; Schwab, M.H.; Nave, K.A.; Lamsa, K.P.; Paulsen, O.; Bannerman, D.M.; et al. Transgenic overexpression of the type I isoform of neuregulin 1 affects working memory and hippocampal oscillations but not long-term potentiation. Cereb. Cortex 2012, 22, 1520–1529. [Google Scholar] [CrossRef]
- Vaht, M.; Laas, K.; Kiive, E.; Parik, J.; Veidebaum, T.; Harro, J. A functional neuregulin-1 gene variant and stressful life events: Effect on drug use in a longitudinal population-representative cohort study. J. Psychopharmacol 2017, 31, 54–61. [Google Scholar] [CrossRef]
- Chen, D.; Zhao, M.; Mundy, G.R. Bone morphogenetic proteins. Growth Factors 2004, 22, 233–241. [Google Scholar] [CrossRef]
- Jha, M.K.; Kim, J.H.; Song, G.J.; Lee, W.H.; Lee, I.K.; Lee, H.W.; An, S.S.A.; Kim, S.; Suk, K. Functional dissection of astrocyte-secreted proteins: Implications in brain health and diseases. Prog. Neurobiol. 2018, 162, 37–69. [Google Scholar] [CrossRef]
- Mabie, P.C.; Mehler, M.F.; Marmur, R.; Papavasiliou, A.; Song, Q.; Kessler, J.A. Bone morphogenetic proteins induce astroglial differentiation of oligodendroglial-astroglial progenitor cells. J. Neurosci. 1997, 17, 4112–4120. [Google Scholar] [CrossRef]
- Cole, A.E.; Murray, S.S.; Xiao, J. Bone Morphogenetic Protein 4 Signalling in Neural Stem and Progenitor Cells during Development and after Injury. Stem Cells Int. 2016, 2016, 9260592. [Google Scholar] [CrossRef] [PubMed]
- Sykaras, N.; Opperman, L.A. Bone morphogenetic proteins (BMPs): How do they function and what can they offer the clinician? J. Oral Sci. 2003, 45, 57–73. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, J.S.; Yetz-Aldape, J.; Wang, E.A. Bone morphogenetic proteins induce differentiation in astrocyte lineage cells. Growth Factors 1994, 11, 53–69. [Google Scholar] [CrossRef] [PubMed]
- Wilkemeyer, M.F.; Pajerski, M.; Charness, M.E. Alcohol inhibition of cell adhesion in BMP-treated NG108-15 cells. Alcohol Clin. Exp. Res. 1999, 23, 1711–1720. [Google Scholar] [CrossRef]
- Skowronska, K.; Obara-Michlewska, M.; Zielinska, M.; Albrecht, J. NMDA Receptors in Astrocytes: In Search for Roles in Neurotransmission and Astrocytic Homeostasis. Int. J. Mol. Sci. 2019, 20, 309. [Google Scholar] [CrossRef]
- Dorit, R.; Jun, W. The NMDA Receptor and Alcohol Addiction. In Biology of the NMDA Receptor; Van Dongen, A.M., Ed.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2009. [Google Scholar]
- Newcomer, J.W.; Farber, N.B.; Olney, J.W. NMDA receptor function, memory, and brain aging. Dialogues Clin. Neurosci. 2000, 2, 219–232. [Google Scholar]
- Dalvi, P.; O’Brien-Ladner, A.; Dhillon, N.K. Downregulation of bone morphogenetic protein receptor axis during HIV-1 and cocaine-mediated pulmonary smooth muscle hyperplasia: Implications for HIV-related pulmonary arterial hypertension. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 2585–2595. [Google Scholar] [CrossRef]
- Varoqueaux, F.; Jamain, S.; Brose, N. Neuroligin 2 is exclusively localized to inhibitory synapses. Eur. J. Cell Biol. 2004, 83, 449–456. [Google Scholar] [CrossRef]
- Stogsdill, J.A.; Ramirez, J.; Liu, D.; Kim, Y.H.; Baldwin, K.T.; Enustun, E.; Ejikeme, T.; Ji, R.-R.; Eroglu, C. Astrocytic neuroligins control astrocyte morphogenesis and synaptogenesis. Nature 2017, 551, 192–197. [Google Scholar] [CrossRef]
- Song, J.Y.; Ichtchenko, K.; Sudhof, T.C.; Brose, N. Neuroligin 1 is a postsynaptic cell-adhesion molecule of excitatory synapses. Proc. Natl. Acad. Sci. USA 1999, 96, 1100–1105. [Google Scholar] [CrossRef]
- Budreck, E.C.; Scheiffele, P. Neuroligin-3 is a neuronal adhesion protein at GABAergic and glutamatergic synapses. Eur. J. Neurosci. 2007, 26, 1738–1748. [Google Scholar] [CrossRef] [PubMed]
- Octeau, J.C.; Chai, H.; Jiang, R.; Bonanno, S.L.; Martin, K.C.; Khakh, B.S. An Optical Neuron-Astrocyte Proximity Assay at Synaptic Distance Scales. Neuron 2018, 98, 49–66.e49. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Stogsdill, J.A.; Pulimood, N.S.; Dingsdale, H.; Kim, Y.H.; Pilaz, L.J.; Kim, I.H.; Manhaes, A.C.; Rodrigues, W.S.; Pamukcu, A.; et al. Astrocytes Assemble Thalamocortical Synapses by Bridging NRX1alpha and NL1 via Hevin. Cell 2016, 164, 183–196. [Google Scholar] [CrossRef] [PubMed]
- Sudhof, T.C. Neuroligins and neurexins link synaptic function to cognitive disease. Nature 2008, 455, 903–911. [Google Scholar] [CrossRef]
- Hishimoto, A.; Liu, Q.R.; Drgon, T.; Pletnikova, O.; Walther, D.; Zhu, X.G.; Troncoso, J.C.; Uhl, G.R. Neurexin 3 polymorphisms are associated with alcohol dependence and altered expression of specific isoforms. Hum. Mol. Genet. 2007, 16, 2880–2891. [Google Scholar] [CrossRef]
- Herman, M.A.; Jahr, C.E. Extracellular glutamate concentration in hippocampal slice. J. Neurosci. 2007, 27, 9736–9741. [Google Scholar] [CrossRef]
- Jackson, J.G.; O’Donnell, J.C.; Takano, H.; Coulter, D.A.; Robinson, M.B. Neuronal activity and glutamate uptake decrease mitochondrial mobility in astrocytes and position mitochondria near glutamate transporters. J. Neurosci. 2014, 34, 1613–1624. [Google Scholar] [CrossRef]
- Bolewska, P.; Martin, B.I.; Orlando, K.A.; Rhoads, D.E. Sequential Changes in Brain Glutamate and Adenosine A1 Receptors May Explain Severity of Adolescent Alcohol Withdrawal after Consumption of High Levels of Alcohol. Neurosci. J. 2019, 2019, 5950818. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Walker, C.D.; Risher, W.C.; Risher, M.-L. Regulation of Synaptic Development by Astrocyte Signaling Factors and Their Emerging Roles in Substance Abuse. Cells 2020, 9, 297. https://doi.org/10.3390/cells9020297
Walker CD, Risher WC, Risher M-L. Regulation of Synaptic Development by Astrocyte Signaling Factors and Their Emerging Roles in Substance Abuse. Cells. 2020; 9(2):297. https://doi.org/10.3390/cells9020297
Chicago/Turabian StyleWalker, Christopher D., W. Christopher Risher, and Mary-Louise Risher. 2020. "Regulation of Synaptic Development by Astrocyte Signaling Factors and Their Emerging Roles in Substance Abuse" Cells 9, no. 2: 297. https://doi.org/10.3390/cells9020297
APA StyleWalker, C. D., Risher, W. C., & Risher, M.-L. (2020). Regulation of Synaptic Development by Astrocyte Signaling Factors and Their Emerging Roles in Substance Abuse. Cells, 9(2), 297. https://doi.org/10.3390/cells9020297



