Capillary Rarefaction in Obesity and Metabolic Diseases—Organ-Specificity and Possible Mechanisms
Abstract
1. Introduction
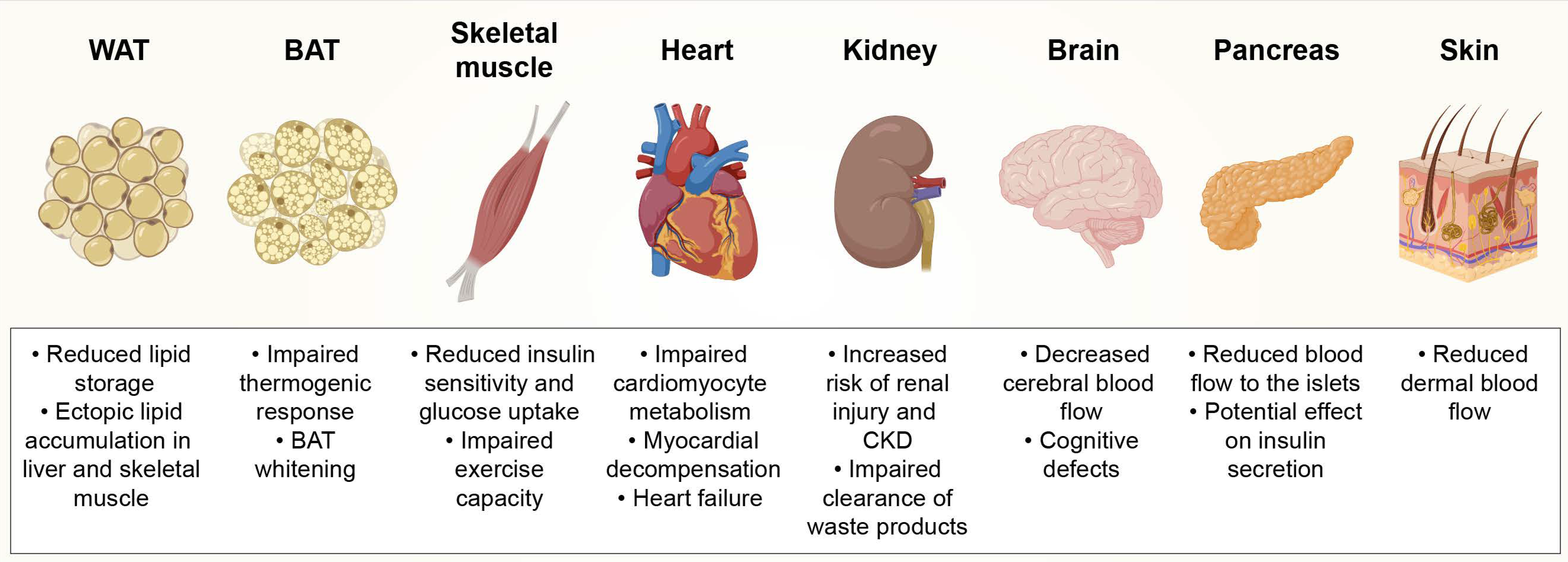
2. Obesity-Induced Capillary Rarefaction in Different Tissues
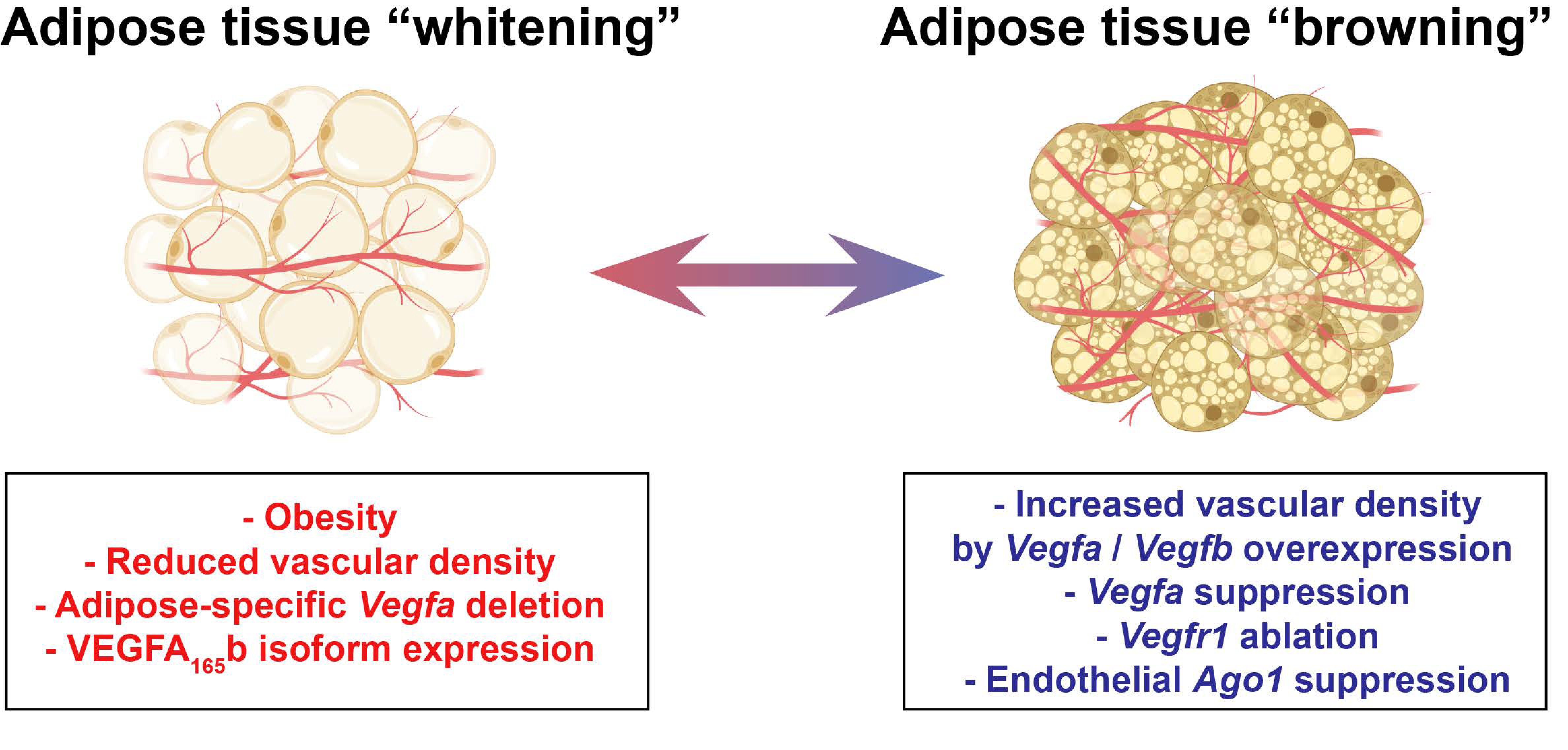
2.1. Adipose Tissue (AT)
2.2. Skeletal Muscle
2.3. Heart
2.4. Kidneys
2.5. Brain
2.6. Pancreas
2.7. Skin
3. Factors Regulating Capillary Density in Obesity
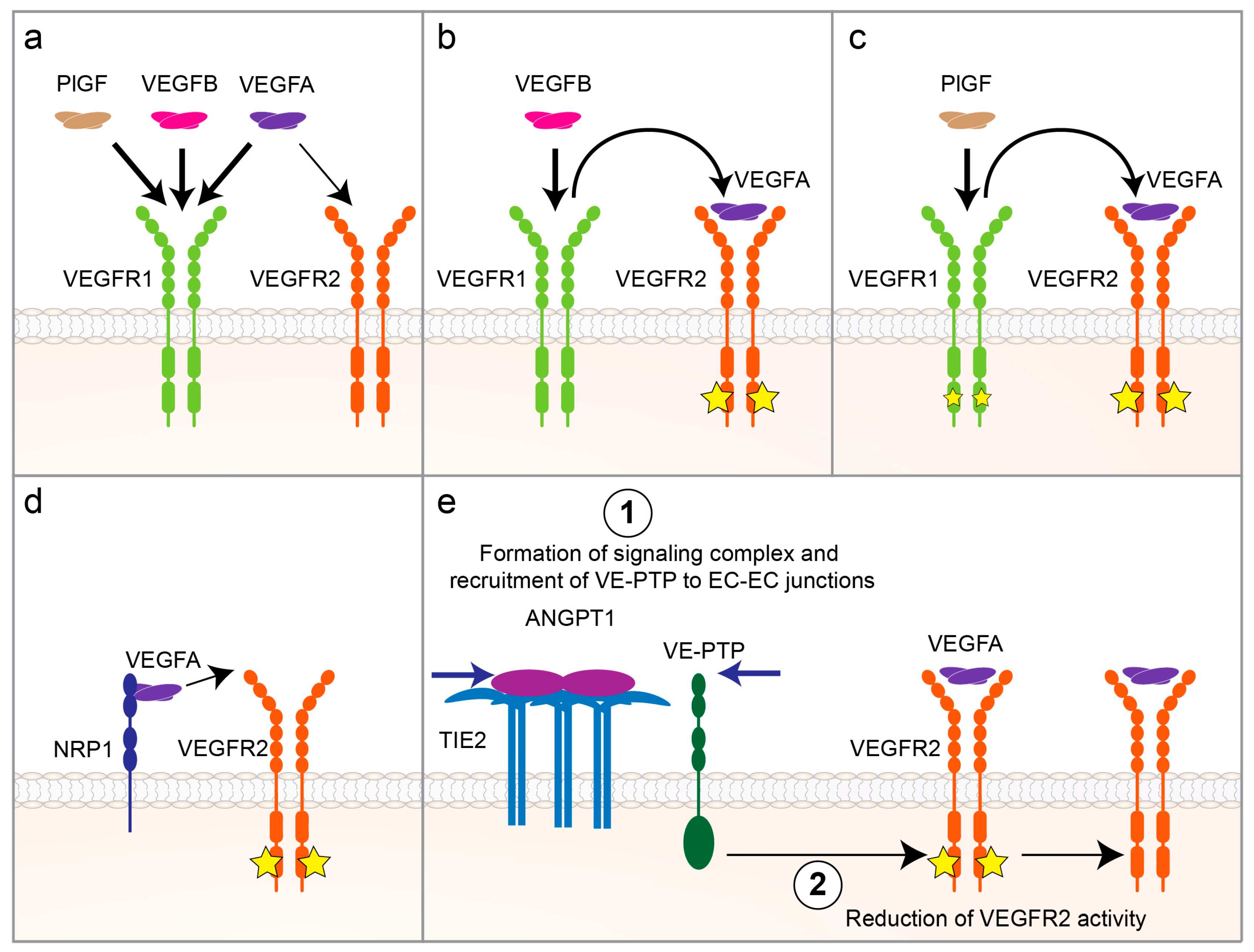
3.1. VEGF/VEGFR Signaling
3.2. Inflammatory Mediators
3.2.1. Soluble Factors
3.2.2. Adhesion Molecules
3.2.3. WNT5A
3.2.4. PlGF
3.2.5. Nitric Oxide (NO)
3.2.6. Apelin
3.3. MicroRNA (miRNA) Pathways
3.4. Other Factors
3.4.1. FOXO1
3.4.2. MAP4K4
3.4.3. SIRT3
3.4.4. Thymosin-β4 (Tβ4)
3.4.5. Extracellular Matrix (ECM)
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AAV | adeno-associated virus |
| AGO1 | argonaute 1 |
| AMPK | adenosine monophosphate-activated protein kinase |
| ANGPT | angiopoietin |
| APJ | apelin receptor |
| AT | adipose tissue |
| ATPase | adenosine triphosphatase |
| BAT | brown adipose tissue |
| BMI | body mass index |
| CCL5 | chemokine C-C motif ligand 5 |
| CKD | chronic kidney disease |
| COX2 | cyclooxygenase-2 |
| DM | diabetes mellitus |
| EC | endothelial cell |
| ECM | extracellular matrix |
| eNO | endothelial nitric oxide |
| ET | exercise training |
| eWAT | epididymal white adipose tissue |
| FFA | free fatty acid |
| FOXO1 | forkhead box O transcription factor 1 |
| gWAT | gonadal white adipose tissue |
| HFD | high-fat diet |
| HFHS | high-fat, high-sucrose |
| HIF-1α | hypoxia-inducible factor 1-alpha |
| HUVEC | human umbilical vein endothelial cell |
| ICAM-1 | intercellular cell adhesion molecule-1 |
| IF | immunofluorescence |
| IHC | immunohistochemical |
| IL | interleukin |
| JNK | c-Jun N-terminal kinase |
| KO | knockout |
| MAP4K4 | mitogen-activated protein kinase kinase kinase kinase 4 |
| MAPK8 | mitogen-activated protein kinase 8 |
| MCP-1 | monocyte chemoattractant protein-1 |
| Micro-CT | micro-computed tomography |
| miRNA | microRNA |
| MPO | myeloperoxidase |
| NAD | nicotinamide adenine dinucleotide |
| NO | nitric oxide |
| NRP | neuropilin |
| Pi3rR2 | phosphoinositide-3-kinase regulatory subunit 2 |
| PlGF | placental growth factor |
| PORH | post-occlusive reactive hyperemia |
| rAAV | recombinant adeno-associated virus |
| rAAV.Tβ4 | recombinant adeno-associated virus encoding thymosin-β4 |
| RAS | renal artery stenosis |
| SIRT3 | sirtuin 3 |
| sWAT | subcutaneous white adipose tissue |
| T2DM | type 2 diabetes mellitus |
| TEM | transmission electron microscopy |
| Th1 | type 1 T helper cell |
| Th17 | type 17 T helper cell |
| TNF-α | tumor necrosis factor alpha |
| Tβ4 | thymosin-beta 4 |
| VCAM-1 | vascular cell adhesion molecule-1 |
| VE-PTP | vascular endothelial protein tyrosine phosphatase |
| VEGF | vascular endothelial growth factor |
| VEGFR | vascular endothelial growth factor receptor |
| vWAT | visceral white adipose tissue |
| WAT | white adipose tissue |
| WNT5A | Wingless-related integration site family member 5A |
References
- Cinti, S. Obesity, Type 2 Diabetes and the Adipose Organ: A Pictorial Atlas from Research to Clinical Applications; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Eroschenko, V.P.; Di Fiore, M.S. DiFiore’s Atlas of Histology with Functional Correlations; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013. [Google Scholar]
- Sun, K.; Kusminski, C.M.; Scherer, P.E. Adipose tissue remodeling and obesity. J. Clin. Investig. 2011, 121, 2094–2101. [Google Scholar] [CrossRef]
- Spencer, M.; Unal, R.; Zhu, B.; Rasouli, N.; McGehee, R.E., Jr.; Peterson, C.A.; Kern, P.A. Adipose tissue extracellular matrix and vascular abnormalities in obesity and insulin resistance. J. Clin. Endocrinol. Metab. 2011, 96, E1990–E1998. [Google Scholar] [CrossRef]
- Pasarica, M.; Sereda, O.R.; Redman, L.M.; Albarado, D.C.; Hymel, D.T.; Roan, L.E.; Rood, J.C.; Burk, D.H.; Smith, S.R. Reduced adipose tissue oxygenation in human obesity: Evidence for rarefaction, macrophage chemotaxis, and inflammation without an angiogenic response. Diabetes 2009, 58, 718–725. [Google Scholar] [CrossRef] [PubMed]
- Belligoli, A.; Compagnin, C.; Sanna, M.; Favaretto, F.; Fabris, R.; Busetto, L.; Foletto, M.; Dal Pra, C.; Serra, R.; Prevedello, L.; et al. Characterization of subcutaneous and omental adipose tissue in patients with obesity and with different degrees of glucose impairment. Sci. Rep. 2019, 9, 11333. [Google Scholar] [CrossRef] [PubMed]
- Gealekman, O.; Guseva, N.; Hartigan, C.; Apotheker, S.; Gorgoglione, M.; Gurav, K.; Tran, K.V.; Straubhaar, J.; Nicoloro, S.; Czech, M.P.; et al. Depot-specific differences and insufficient subcutaneous adipose tissue angiogenesis in human obesity. Circulation 2011, 123, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, I.; Aprahamian, T.; Kikuchi, R.; Shimizu, A.; Papanicolaou, K.N.; MacLauchlan, S.; Maruyama, S.; Walsh, K. Vascular rarefaction mediates whitening of brown fat in obesity. J. Clin. Invest. 2014, 124, 2099–2112. [Google Scholar] [CrossRef]
- Sung, H.K.; Doh, K.O.; Son, J.E.; Park, J.G.; Bae, Y.; Choi, S.; Nelson, S.M.; Cowling, R.; Nagy, K.; Michael, I.P.; et al. Adipose vascular endothelial growth factor regulates metabolic homeostasis through angiogenesis. Cell Metab. 2013, 17, 61–72. [Google Scholar] [CrossRef]
- Goossens, G.H.; Bizzarri, A.; Venteclef, N.; Essers, Y.; Cleutjens, J.P.; Konings, E.; Jocken, J.W.; Cajlakovic, M.; Ribitsch, V.; Clement, K.; et al. Increased adipose tissue oxygen tension in obese compared with lean men is accompanied by insulin resistance, impaired adipose tissue capillarization, and inflammation. Circulation 2011, 124, 67–76. [Google Scholar] [CrossRef]
- Danforth, E., Jr. Failure of adipocyte differentiation causes type II diabetes mellitus? Nat. Genet. 2000, 26, 13. [Google Scholar] [CrossRef]
- Virtue, S.; Vidal-Puig, A. Adipose tissue expandability, lipotoxicity and the Metabolic Syndrome--an allostatic perspective. Biochim. Biophys. Acta 2010, 1801, 338–349. [Google Scholar] [CrossRef]
- Hardy, O.T.; Czech, M.P.; Corvera, S. What causes the insulin resistance underlying obesity? Curr. Opin. Endocrinol. Diabetes Obes. 2012, 19, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Cannon, B.; Nedergaard, J. Brown adipose tissue: Function and physiological significance. Physiol. Rev. 2004, 84, 277–359. [Google Scholar] [CrossRef] [PubMed]
- Scheele, C.; Wolfrum, C. Brown Adipose Crosstalk in Tissue Plasticity and Human Metabolism. Endocr. Rev. 2020, 41. [Google Scholar] [CrossRef] [PubMed]
- Baron, A.D.; Brechtel, G.; Wallace, P.; Edelman, S.V. Rates and tissue sites of non-insulin- and insulin-mediated glucose uptake in humans. Am. J. Physiol. 1988, 255, E769–E774. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.A.; Ferrannini, E.; Sato, Y.; Felig, P.; Wahren, J. Synergistic interaction between exercise and insulin on peripheral glucose uptake. J. Clin. Investig. 1981, 68, 1468–1474. [Google Scholar] [CrossRef]
- Agapitov, A.V.; Correia, M.L.; Sinkey, C.A.; Dopp, J.M.; Haynes, W.G. Impaired skeletal muscle and skin microcirculatory function in human obesity. J. Hypertens. 2002, 20, 1401–1405. [Google Scholar] [CrossRef]
- Gavin, T.P.; Stallings, H.W., 3rd; Zwetsloot, K.A.; Westerkamp, L.M.; Ryan, N.A.; Moore, R.A.; Pofahl, W.E.; Hickner, R.C. Lower capillary density but no difference in VEGF expression in obese vs. lean young skeletal muscle in humans. J. Appl. Physiol. 2005, 98, 315–321. [Google Scholar] [CrossRef]
- Vinet, A.; Karpoff, L.; Walther, G.; Startun, A.; Obert, P.; Goret, L.; Dauzat, M.; Perez-Martin, A. Vascular reactivity at rest and during exercise in middle-aged obese men: Effects of short-term, low-intensity, exercise training. Int. J. Obes. 2011, 35, 820–828. [Google Scholar] [CrossRef]
- van Haare, J.; Kooi, M.E.; Vink, H.; Post, M.J.; van Teeffelen, J.W.; Slenter, J.; Munts, C.; Cobelens, H.; Strijkers, G.J.; Koehn, D.; et al. Early impairment of coronary microvascular perfusion capacity in rats on a high fat diet. Cardiovasc. Diabetol. 2015, 14, 150. [Google Scholar] [CrossRef]
- Frisbee, J.C.; Goodwill, A.G.; Frisbee, S.J.; Butcher, J.T.; Brock, R.W.; Olfert, I.M.; DeVallance, E.R.; Chantler, P.D. Distinct temporal phases of microvascular rarefaction in skeletal muscle of obese Zucker rats. Am. J. Physiol. Heart Circ. Physiol. 2014, 307, H1714–H1728. [Google Scholar] [CrossRef]
- Toblli, J.E.; Cao, G.; DeRosa, G.; Di Gennaro, F.; Forcada, P. Angiotensin-converting enzyme inhibition and angiogenesis in myocardium of obese Zucker rats. Am. J. Hypertens. 2004, 17, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Gruber, C.; Kohlstedt, K.; Loot, A.E.; Fleming, I.; Kummer, W.; Muhlfeld, C. Stereological characterization of left ventricular cardiomyocytes, capillaries, and innervation in the nondiabetic, obese mouse. Cardiovasc. Pathol. 2012, 21, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Leopoldo, A.S.; Sugizaki, M.M.; Lima-Leopoldo, A.P.; do Nascimento, A.F.; Luvizotto Rde, A.; de Campos, D.H.; Okoshi, K.; Dal Pai-Silva, M.; Padovani, C.R.; Cicogna, A.C. Cardiac remodeling in a rat model of diet-induced obesity. Can. J. Cardiol. 2010, 26, 423–429. [Google Scholar] [CrossRef]
- Campbell, D.J.; Somaratne, J.B.; Prior, D.L.; Yii, M.; Kenny, J.F.; Newcomb, A.E.; Kelly, D.J.; Black, M.J. Obesity is associated with lower coronary microvascular density. PLoS ONE 2013, 8, e81798. [Google Scholar] [CrossRef] [PubMed]
- Machado, M.V.; Vieira, A.B.; da Conceicao, F.G.; Nascimento, A.R.; da Nobrega, A.C.L.; Tibirica, E. Exercise training dose differentially alters muscle and heart capillary density and metabolic functions in an obese rat with metabolic syndrome. Exp. Physiol. 2017, 102, 1716–1728. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Quesada, C.; Cavalera, M.; Biernacka, A.; Kong, P.; Lee, D.W.; Saxena, A.; Frunza, O.; Dobaczewski, M.; Shinde, A.; Frangogiannis, N.G. Thrombospondin-1 induction in the diabetic myocardium stabilizes the cardiac matrix in addition to promoting vascular rarefaction through angiopoietin-2 upregulation. Circ. Res. 2013, 113, 1331–1344. [Google Scholar] [CrossRef]
- Sugawara, T.; Fujii, S.; Zaman, A.K.; Goto, D.; Furumoto, T.; Imagawa, S.; Dong, J.; Sakuma, I.; Jesmin, S.; Togashi, H.; et al. Coronary capillary network remodeling and hypofibrinolysis in aged obese diabetic rats: Implications for increased myocardial vulnerability to ischemia. Mol. Cell. Biochem. 2003, 248, 165–170. [Google Scholar] [CrossRef]
- Hinkel, R.; Howe, A.; Renner, S.; Ng, J.; Lee, S.; Klett, K.; Kaczmarek, V.; Moretti, A.; Laugwitz, K.L.; Skroblin, P.; et al. Diabetes Mellitus-Induced Microvascular Destabilization in the Myocardium. J. Am. Coll. Cardiol. 2017, 69, 131–143. [Google Scholar] [CrossRef]
- Messer, J.V.; Wagman, R.J.; Levine, H.J.; Neill, W.A.; Krasnow, N.; Gorlin, R. Patterns of human myocardial oxygen extraction during rest and exercise. J. Clin. Invest. 1962, 41, 725–742. [Google Scholar] [CrossRef]
- Oka, T.; Komuro, I. Molecular mechanisms underlying the transition of cardiac hypertrophy to heart failure. Circ. J. 2008, 72 (Suppl. A), A13–A16. [Google Scholar] [CrossRef]
- Walsh, K.; Shiojima, I. Cardiac growth and angiogenesis coordinated by intertissue interactions. J. Clin. Investig. 2007, 117, 3176–3179. [Google Scholar] [CrossRef] [PubMed]
- Motivala, A.A.; Rose, P.A.; Kim, H.M.; Smith, Y.R.; Bartnik, C.; Brook, R.D.; Muzik, O.; Duvernoy, C.S. Cardiovascular risk, obesity, and myocardial blood flow in postmenopausal women. J. Nucl. Cardiol. 2008, 15, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Kondo, I.; Mizushige, K.; Hirao, K.; Nozaki, S.; Tsuji, T.; Masugata, H.; Kohno, M.; Matsuo, H. Ultrasonographic assessment of coronary flow reserve and abdominal fat in obesity. Ultrasound Med. Biol. 2001, 27, 1199–1205. [Google Scholar] [CrossRef]
- Tona, F.; Serra, R.; Di Ascenzo, L.; Osto, E.; Scarda, A.; Fabris, R.; Montisci, R.; Famoso, G.; Tellatin, S.; Foletto, M.; et al. Systemic inflammation is related to coronary microvascular dysfunction in obese patients without obstructive coronary disease. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Skorecki, K.; Chertow, G.M.; Marsden, P.A.; Taal, M.W.; Alan, S.; Luyckx, V. Brenner & Rector’s the kidney; Elsevier: Philadelphia, PA, USA, 2016. [Google Scholar]
- Sorop, O.; Olver, T.D.; van de Wouw, J.; Heinonen, I.; van Duin, R.W.; Duncker, D.J.; Merkus, D. The microcirculation: A key player in obesity-associated cardiovascular disease. Cardiovasc. Res. 2017, 113, 1035–1045. [Google Scholar] [CrossRef]
- de Vries, A.P.; Ruggenenti, P.; Ruan, X.Z.; Praga, M.; Cruzado, J.M.; Bajema, I.M.; D’Agati, V.D.; Lamb, H.J.; Pongrac Barlovic, D.; Hojs, R.; et al. Fatty kidney: Emerging role of ectopic lipid in obesity-related renal disease. Lancet Diabetes Endocrinol. 2014, 2, 417–426. [Google Scholar] [CrossRef]
- Kramer, H. Obesity and chronic kidney disease. Contrib Nephrol 2006, 151, 1–18. [Google Scholar] [CrossRef]
- Li, Z.; Woollard, J.R.; Wang, S.; Korsmo, M.J.; Ebrahimi, B.; Grande, J.P.; Textor, S.C.; Lerman, A.; Lerman, L.O. Increased glomerular filtration rate in early metabolic syndrome is associated with renal adiposity and microvascular proliferation. Am. J. Physiol. Renal. Physiol. 2011, 301, F1078–F1087. [Google Scholar] [CrossRef]
- Iliescu, R.; Chade, A.R. Progressive renal vascular proliferation and injury in obese Zucker rats. Microcirculation 2010, 17, 250–258. [Google Scholar] [CrossRef]
- Martens, R.J.; Henry, R.M.; Houben, A.J.; van der Kallen, C.J.; Kroon, A.A.; Schalkwijk, C.G.; Schram, M.T.; Sep, S.J.; Schaper, N.C.; Dagnelie, P.C.; et al. Capillary Rarefaction Associates with Albuminuria: The Maastricht Study. J. Am. Soc. Nephrol. 2016, 27, 3748–3757. [Google Scholar] [CrossRef]
- Hall, J.E.; do Carmo, J.M.; da Silva, A.A.; Wang, Z.; Hall, M.E. Obesity-induced hypertension: Interaction of neurohumoral and renal mechanisms. Circ. Res. 2015, 116, 991–1006. [Google Scholar] [CrossRef] [PubMed]
- Futatsugi, K.; Tokuyama, H.; Shibata, S.; Naitoh, M.; Kanda, T.; Minakuchi, H.; Yamaguchi, S.; Hayashi, K.; Minamishima, Y.A.; Yanagita, M.; et al. Obesity-induced kidney injury is attenuated by amelioration of aberrant PHD2 activation in proximal tubules. Sci. Rep. 2016, 6, 36533. [Google Scholar] [CrossRef] [PubMed]
- Kuwahara, S.; Hosojima, M.; Kaneko, R.; Aoki, H.; Nakano, D.; Sasagawa, T.; Kabasawa, H.; Kaseda, R.; Yasukawa, R.; Ishikawa, T.; et al. Megalin-Mediated Tubuloglomerular Alterations in High-Fat Diet-Induced Kidney Disease. J. Am. Soc. Nephrol. 2016, 27, 1996–2008. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, Z.L.; Woollard, J.R.; Eirin, A.; Ebrahimi, B.; Crane, J.A.; Zhu, X.Y.; Pawar, A.S.; Krier, J.D.; Jordan, K.L.; et al. Obesity-metabolic derangement preserves hemodynamics but promotes intrarenal adiposity and macrophage infiltration in swine renovascular disease. Am. J. Physiol. Renal Physiol. 2013, 305, F265–F276. [Google Scholar] [CrossRef] [PubMed]
- Tsuboi, N.; Utsunomiya, Y.; Hosoya, T. Obesity-related glomerulopathy and the nephron complement. Nephrol. Dial. Transplant. 2013, 28 (Suppl. 4), iv108–iv113. [Google Scholar] [CrossRef] [PubMed]
- Griffin, K.A.; Kramer, H.; Bidani, A.K. Adverse renal consequences of obesity. Am. J. Physiol. Renal Physiol. 2008, 294, F685–F696. [Google Scholar] [CrossRef] [PubMed]
- Joles, J.A.; Koomans, H.A. Causes and consequences of increased sympathetic activity in renal disease. Hypertension 2004, 43, 699–706. [Google Scholar] [CrossRef]
- Anderson, S.; Halter, J.B.; Hazzard, W.R.; Himmelfarb, J.; Horne, F.M.; Kaysen, G.A.; Kusek, J.W.; Nayfield, S.G.; Schmader, K.; Tian, Y.; et al. Prediction, progression, and outcomes of chronic kidney disease in older adults. J. Am. Soc. Nephrol. 2009, 20, 1199–1209. [Google Scholar] [CrossRef]
- Ter Maaten, J.M.; Damman, K.; Verhaar, M.C.; Paulus, W.J.; Duncker, D.J.; Cheng, C.; van Heerebeek, L.; Hillege, H.L.; Lam, C.S.; Navis, G.; et al. Connecting heart failure with preserved ejection fraction and renal dysfunction: The role of endothelial dysfunction and inflammation. Eur J. Heart Fail. 2016, 18, 588–598. [Google Scholar] [CrossRef]
- Dorrance, A.M.; Matin, N.; Pires, P.W. The effects of obesity on the cerebral vasculature. Curr. Vasc. Pharmacol. 2014, 12, 462–472. [Google Scholar] [CrossRef]
- Alosco, M.L.; Spitznagel, M.B.; Raz, N.; Cohen, R.; Sweet, L.H.; Colbert, L.H.; Josephson, R.; van Dulmen, M.; Hughes, J.; Rosneck, J.; et al. Obesity interacts with cerebral hypoperfusion to exacerbate cognitive impairment in older adults with heart failure. Cerebrovasc. Dis. Extra. 2012, 2, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Willeumier, K.C.; Taylor, D.V.; Amen, D.G. Elevated BMI is associated with decreased blood flow in the prefrontal cortex using SPECT imaging in healthy adults. Obesity (Silver Spring) 2011, 19, 1095–1097. [Google Scholar] [CrossRef] [PubMed]
- Selim, M.; Jones, R.; Novak, P.; Zhao, P.; Novak, V. The effects of body mass index on cerebral blood flow velocity. Clin. Auton. Res. 2008, 18, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Chantler, P.D.; Shrader, C.D.; Tabone, L.E.; d’Audiffret, A.C.; Huseynova, K.; Brooks, S.D.; Branyan, K.W.; Grogg, K.A.; Frisbee, J.C. Cerebral Cortical Microvascular Rarefaction in Metabolic Syndrome is Dependent on Insulin Resistance and Loss of Nitric Oxide Bioavailability. Microcirculation 2015, 22, 435–445. [Google Scholar] [CrossRef]
- Fu, Z.; Wu, J.; Nesil, T.; Li, M.D.; Aylor, K.W.; Liu, Z. Long-term high-fat diet induces hippocampal microvascular insulin resistance and cognitive dysfunction. Am. J. Physiol. Endocrinol. Metab. 2017, 312, E89–E97. [Google Scholar] [CrossRef]
- Estato, V.; Nascimento, A.; Antunes, B.; Gomes, F.; Coelho, L.; Rangel, R.; Garzoni, L.; Daliry, A.; Bousquet, P.; Tibirica, E. Cerebral Microvascular Dysfunction and Inflammation Are Improved by Centrally Acting Antihypertensive Drugs in Metabolic Syndrome. Metab. Syndr. Relat. Disord. 2017, 15, 26–35. [Google Scholar] [CrossRef]
- Tucsek, Z.; Toth, P.; Tarantini, S.; Sosnowska, D.; Gautam, T.; Warrington, J.P.; Giles, C.B.; Wren, J.D.; Koller, A.; Ballabh, P.; et al. Aging exacerbates obesity-induced cerebromicrovascular rarefaction, neurovascular uncoupling, and cognitive decline in mice. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 1339–1352. [Google Scholar] [CrossRef]
- Strazzullo, P.; D’Elia, L.; Cairella, G.; Garbagnati, F.; Cappuccio, F.P.; Scalfi, L. Excess body weight and incidence of stroke: Meta-analysis of prospective studies with 2 million participants. Stroke 2010, 41, e418–e426. [Google Scholar] [CrossRef]
- Smith, E.; Hay, P.; Campbell, L.; Trollor, J.N. A review of the association between obesity and cognitive function across the lifespan: Implications for novel approaches to prevention and treatment. Obes. Rev. 2011, 12, 740–755. [Google Scholar] [CrossRef]
- Fowler, J.L.; Lee, S.S.; Wesner, Z.C.; Olehnik, S.K.; Kron, S.J.; Hara, M. Three-Dimensional Analysis of the Human Pancreas. Endocrinology 2018, 159, 1393–1400. [Google Scholar] [CrossRef]
- Hayden, M.R.; Karuparthi, P.R.; Habibi, J.; Lastra, G.; Patel, K.; Wasekar, C.; Manrique, C.M.; Ozerdem, U.; Stas, S.; Sowers, J.R. Ultrastructure of islet microcirculation, pericytes and the islet exocrine interface in the HIP rat model of diabetes. Exp. Biol. Med. (Maywood) 2008, 233, 1109–1123. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Brissova, M.; Reinert, R.B.; Nyman, L.; Liu, E.H.; Thompson, C.; Shostak, A.; Shiota, M.; Takahashi, T.; Powers, A.C. Pancreatic islet vasculature adapts to insulin resistance through dilation and not angiogenesis. Diabetes 2013, 62, 4144–4153. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, P.O.; Andersson, A.; Jansson, L. Pancreatic islet blood flow in normal and obese-hyperglycemic (ob/ob) mice. Am. J. Physiol. 1996, 271, E990–E995. [Google Scholar] [CrossRef] [PubMed]
- Charkoudian, N. Skin blood flow in adult human thermoregulation: How it works, when it does not, and why. Mayo Clin. proc. 2003, 78, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Altintas, A.A.; Aust, M.C.; Kramer, R.; Vogt, P.M.; Altintas, M.A. In vivo reflectance-mode confocal microscopy assessments: Impact of overweight on human skin microcirculation and histomorphology. J. Biomed. Opt. 2016, 21, 36009. [Google Scholar] [CrossRef] [PubMed]
- De Ciuceis, C.; Rossini, C.; Porteri, E.; La Boria, E.; Corbellini, C.; Mittempergher, F.; Di Betta, E.; Petroboni, B.; Sarkar, A.; Agabiti-Rosei, C.; et al. Circulating endothelial progenitor cells, microvascular density and fibrosis in obesity before and after bariatric surgery. Blood Press. 2013, 22, 165–172. [Google Scholar] [CrossRef]
- Francischetti, E.A.; Tibirica, E.; da Silva, E.G.; Rodrigues, E.; Celoria, B.M.; de Abreu, V.G. Skin capillary density and microvascular reactivity in obese subjects with and without metabolic syndrome. Microvasc. Res. 2011, 81, 325–330. [Google Scholar] [CrossRef]
- Karaman, S.; Leppanen, V.M.; Alitalo, K. Vascular endothelial growth factor signaling in development and disease. Development 2018, 145. [Google Scholar] [CrossRef]
- Simons, M.; Gordon, E.; Claesson-Welsh, L. Mechanisms and regulation of endothelial VEGF receptor signalling. Nat. Rev. Mol. Cell Biol. 2016, 17, 611–625. [Google Scholar] [CrossRef]
- Bagchi, M.; Kim, L.A.; Boucher, J.; Walshe, T.E.; Kahn, C.R.; D’Amore, P.A. Vascular endothelial growth factor is important for brown adipose tissue development and maintenance. FASEB J. 2013, 27, 3257–3271. [Google Scholar] [CrossRef]
- Elias, I.; Franckhauser, S.; Ferre, T.; Vila, L.; Tafuro, S.; Munoz, S.; Roca, C.; Ramos, D.; Pujol, A.; Riu, E.; et al. Adipose tissue overexpression of vascular endothelial growth factor protects against diet-induced obesity and insulin resistance. Diabetes 2012, 61, 1801–1813. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Wernstedt Asterholm, I.; Kusminski, C.M.; Bueno, A.C.; Wang, Z.V.; Pollard, J.W.; Brekken, R.A.; Scherer, P.E. Dichotomous effects of VEGF-A on adipose tissue dysfunction. Proc. Natl. Acad. Sci. USA 2012, 109, 5874–5879. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Ji, Y.; Zhang, L.; Zhang, Y.; Zhang, S.; An, Y.; Liu, P.; Zheng, Y. Resistance to obesity by repression of VEGF gene expression through induction of brown-like adipocyte differentiation. Endocrinology 2012, 153, 3123–3132. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhang, Y.; Yang, Y.; Lim, S.; Cao, Z.; Rak, J.; Cao, Y. Vascular endothelial growth factor-dependent spatiotemporal dual roles of placental growth factor in modulation of angiogenesis and tumor growth. Proc. Natl. Acad. Sci. USA 2013, 110, 13932–13937. [Google Scholar] [CrossRef] [PubMed]
- Plein, A.; Fantin, A.; Ruhrberg, C. Neuropilin regulation of angiogenesis, arteriogenesis, and vascular permeability. Microcirculation 2014, 21, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Eklund, L.; Kangas, J.; Saharinen, P. Angiopoietin-Tie signalling in the cardiovascular and lymphatic systems. Clin. Sci. (Lond) 2017, 131, 87–103. [Google Scholar] [CrossRef]
- Ngo, D.T.; Farb, M.G.; Kikuchi, R.; Karki, S.; Tiwari, S.; Bigornia, S.J.; Bates, D.O.; LaValley, M.P.; Hamburg, N.M.; Vita, J.A.; et al. Antiangiogenic actions of vascular endothelial growth factor-A165b, an inhibitory isoform of vascular endothelial growth factor-A, in human obesity. Circulation 2014, 130, 1072–1080. [Google Scholar] [CrossRef]
- Robciuc, M.R.; Kivela, R.; Williams, I.M.; de Boer, J.F.; van Dijk, T.H.; Elamaa, H.; Tigistu-Sahle, F.; Molotkov, D.; Leppanen, V.M.; Kakela, R.; et al. VEGFB/VEGFR1-Induced Expansion of Adipose Vasculature Counteracts Obesity and Related Metabolic Complications. Cell Metab. 2016, 23, 712–724. [Google Scholar] [CrossRef]
- Seki, T.; Hosaka, K.; Fischer, C.; Lim, S.; Andersson, P.; Abe, M.; Iwamoto, H.; Gao, Y.; Wang, X.; Fong, G.H.; et al. Ablation of endothelial VEGFR1 improves metabolic dysfunction by inducing adipose tissue browning. J. Exp. Med. 2018, 215, 611–626. [Google Scholar] [CrossRef]
- Elias, I.; Franckhauser, S.; Bosch, F. New insights into adipose tissue VEGF-A actions in the control of obesity and insulin resistance. Adipocyte 2013, 2, 109–112. [Google Scholar] [CrossRef]
- Park, J.; Kim, M.; Sun, K.; An, Y.A.; Gu, X.; Scherer, P.E. VEGF-A-Expressing Adipose Tissue Shows Rapid Beiging and Enhanced Survival After Transplantation and Confers IL-4-Independent Metabolic Improvements. Diabetes 2017, 66, 1479–1490. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Miao, Y.; Luo, Y.; Sriram, K.; Qi, Z.; Lin, F.M.; Gu, Y.; Lai, C.H.; Hsu, C.Y.; Peterson, K.L.; et al. Suppression of Endothelial AGO1 Promotes Adipose Tissue Browning and Improves Metabolic Dysfunction. Circulation 2020, 142, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, M.; Hotamisligil, G.S. Damned if you do, damned if you don’t: The conundrum of adipose tissue vascularization. Cell Metab. 2013, 17, 7–9. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bonner, J.S.; Lantier, L.; Hasenour, C.M.; James, F.D.; Bracy, D.P.; Wasserman, D.H. Muscle-specific vascular endothelial growth factor deletion induces muscle capillary rarefaction creating muscle insulin resistance. Diabetes 2013, 62, 572–580. [Google Scholar] [CrossRef]
- Arsic, N.; Zacchigna, S.; Zentilin, L.; Ramirez-Correa, G.; Pattarini, L.; Salvi, A.; Sinagra, G.; Giacca, M. Vascular endothelial growth factor stimulates skeletal muscle regeneration in vivo. Mol. Ther. 2004, 10, 844–854. [Google Scholar] [CrossRef]
- Karvinen, H.; Pasanen, E.; Rissanen, T.T.; Korpisalo, P.; Vahakangas, E.; Jazwa, A.; Giacca, M.; Yla-Herttuala, S. Long-term VEGF-A expression promotes aberrant angiogenesis and fibrosis in skeletal muscle. Gene Ther. 2011, 18, 1166–1172. [Google Scholar] [CrossRef]
- Koller, G.M.; Schafer, C.; Kemp, S.S.; Aguera, K.N.; Lin, P.K.; Forgy, J.C.; Griffin, C.T.; Davis, G.E. Proinflammatory Mediators, IL (Interleukin)-1beta, TNF (Tumor Necrosis Factor) alpha, and Thrombin Directly Induce Capillary Tube Regression. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 365–377. [Google Scholar] [CrossRef]
- Wernstedt Asterholm, I.; Tao, C.; Morley, T.S.; Wang, Q.A.; Delgado-Lopez, F.; Wang, Z.V.; Scherer, P.E. Adipocyte inflammation is essential for healthy adipose tissue expansion and remodeling. Cell Metab. 2014, 20, 103–118. [Google Scholar] [CrossRef]
- Eringa, E.C.; Bakker, W.; van Hinsbergh, V.W. Paracrine regulation of vascular tone, inflammation and insulin sensitivity by perivascular adipose tissue. Vascul. Pharmacol. 2012, 56, 204–209. [Google Scholar] [CrossRef]
- Schinzari, F.; Tesauro, M.; Cardillo, C. Endothelial and Perivascular Adipose Tissue Abnormalities in Obesity-Related Vascular Dysfunction: Novel Targets for Treatment. J. Cardiovasc. Pharmacol. 2017, 69, 360–368. [Google Scholar] [CrossRef]
- Ziccardi, P.; Nappo, F.; Giugliano, G.; Esposito, K.; Marfella, R.; Cioffi, M.; D’Andrea, F.; Molinari, A.M.; Giugliano, D. Reduction of inflammatory cytokine concentrations and improvement of endothelial functions in obese women after weight loss over one year. Circulation 2002, 105, 804–809. [Google Scholar] [CrossRef] [PubMed]
- Ijzerman, R.G.; Voordouw, J.J.; Van Weissenbruch, M.M.; Yudkin, J.S.; Serne, E.H.; Delemarre-van de Waal, H.A.; Stehouwer, C.D. TNF-alpha levels are associated with skin capillary recruitment in humans: A potential explanation for the relationship between TNF-alpha and insulin resistance. Clin. Sci. 2006, 110, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Scalia, R. The microcirculation in adipose tissue inflammation. Rev. Endocr. Metab. Disord. 2013, 14, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Fuster, J.J.; Zuriaga, M.A.; Ngo, D.T.; Farb, M.G.; Aprahamian, T.; Yamaguchi, T.P.; Gokce, N.; Walsh, K. Noncanonical Wnt signaling promotes obesity-induced adipose tissue inflammation and metabolic dysfunction independent of adipose tissue expansion. Diabetes 2015, 64, 1235–1248. [Google Scholar] [CrossRef]
- Kikuchi, R.; Nakamura, K.; MacLauchlan, S.; Ngo, D.T.; Shimizu, I.; Fuster, J.J.; Katanasaka, Y.; Yoshida, S.; Qiu, Y.; Yamaguchi, T.P.; et al. An antiangiogenic isoform of VEGF-A contributes to impaired vascularization in peripheral artery disease. Nat. Med. 2014, 20, 1464–1471. [Google Scholar] [CrossRef]
- Karki, S.; Ngo, D.T.M.; Farb, M.G.; Park, S.Y.; Saggese, S.M.; Hamburg, N.M.; Carmine, B.; Hess, D.T.; Walsh, K.; Gokce, N. WNT5A regulates adipose tissue angiogenesis via antiangiogenic VEGF-A165b in obese humans. Am. J. Physiol. Heart Circ. Physiol. 2017, 313, H200–H206. [Google Scholar] [CrossRef]
- Lijnen, H.R.; Christiaens, V.; Scroyen, I.; Voros, G.; Tjwa, M.; Carmeliet, P.; Collen, D. Impaired adipose tissue development in mice with inactivation of placental growth factor function. Diabetes 2006, 55, 2698–2704. [Google Scholar] [CrossRef]
- Kang, M.; Jeong, J.; Lee, J.; Park, S.; Sung, Y.; Choi, M.; Kwon, W.; Jang, S.; Choi, K.S.; Choo, Y.S.; et al. Placental growth factor (PlGF) is linked to inflammation and metabolic disorders in mice with diet-induced obesity. Endocr. J. 2018, 65, 437–447. [Google Scholar] [CrossRef]
- Goodwill, A.G.; Frisbee, S.J.; Stapleton, P.A.; James, M.E.; Frisbee, J.C. Impact of chronic anticholesterol therapy on development of microvascular rarefaction in the metabolic syndrome. Microcirculation 2009, 16, 667–684. [Google Scholar] [CrossRef]
- Lv, X.; Kong, J.; Chen, W.D.; Wang, Y.D. The Role of the Apelin/APJ System in the Regulation of Liver Disease. Front. Pharmacol. 2017, 8, 221. [Google Scholar] [CrossRef]
- Kunduzova, O.; Alet, N.; Delesque-Touchard, N.; Millet, L.; Castan-Laurell, I.; Muller, C.; Dray, C.; Schaeffer, P.; Herault, J.P.; Savi, P.; et al. Apelin/APJ signaling system: A potential link between adipose tissue and endothelial angiogenic processes. FASEB J. 2008, 22, 4146–4153. [Google Scholar] [CrossRef] [PubMed]
- Castan-Laurell, I.; Dray, C.; Attane, C.; Duparc, T.; Knauf, C.; Valet, P. Apelin, diabetes, and obesity. Endocrine 2011, 40, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yue, P.; Jin, H.; Aillaud, M.; Deng, A.C.; Azuma, J.; Asagami, T.; Kundu, R.K.; Reaven, G.M.; Quertermous, T.; Tsao, P.S. Apelin is necessary for the maintenance of insulin sensitivity. Am. J. Physiol. Endocrinol. Metab. 2010, 298, E59–E67. [Google Scholar] [CrossRef]
- Yamamoto, T.; Habata, Y.; Matsumoto, Y.; Yasuhara, Y.; Hashimoto, T.; Hamajyo, H.; Anayama, H.; Fujii, R.; Fuse, H.; Shintani, Y.; et al. Apelin-transgenic mice exhibit a resistance against diet-induced obesity by increasing vascular mass and mitochondrial biogenesis in skeletal muscle. Biochim. Biophys. Acta 2011, 1810, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Sawane, M.; Kajiya, K.; Kidoya, H.; Takagi, M.; Muramatsu, F.; Takakura, N. Apelin inhibits diet-induced obesity by enhancing lymphatic and blood vessel integrity. Diabetes 2013, 62, 1970–1980. [Google Scholar] [CrossRef] [PubMed]
- Gomes, J.L.; Fernandes, T.; Soci, U.P.; Silveira, A.C.; Barretti, D.L.; Negrao, C.E.; Oliveira, E.M. Obesity Downregulates MicroRNA-126 Inducing Capillary Rarefaction in Skeletal Muscle: Effects of Aerobic Exercise Training. Oxid. Med. Cell. Longev. 2017, 2017, 2415246. [Google Scholar] [CrossRef]
- Fernandes, T.; Casaes, L.; Soci, U.; Silveira, A.; Gomes, J.; Barretti, D.; Roque, F.; Oliveira, E. Exercise Training Restores the Cardiac Microrna-16 Levels Preventing Microvascular Rarefaction in Obese Zucker Rats. Obes. Facts 2018, 11, 15–24. [Google Scholar] [CrossRef]
- Fernandes, T.; Magalhaes, F.C.; Roque, F.R.; Phillips, M.I.; Oliveira, E.M. Exercise training prevents the microvascular rarefaction in hypertension balancing angiogenic and apoptotic factors: Role of microRNAs-16, -21, and -126. Hypertension 2012, 59, 513–520. [Google Scholar] [CrossRef]
- Wilhelm, K.; Happel, K.; Eelen, G.; Schoors, S.; Oellerich, M.F.; Lim, R.; Zimmermann, B.; Aspalter, I.M.; Franco, C.A.; Boettger, T.; et al. FOXO1 couples metabolic activity and growth state in the vascular endothelium. Nature 2016, 529, 216–220. [Google Scholar] [CrossRef]
- Rudnicki, M.; Abdifarkosh, G.; Nwadozi, E.; Ramos, S.V.; Makki, A.; Sepa-Kishi, D.M.; Ceddia, R.B.; Perry, C.G.; Roudier, E.; Haas, T.L. Endothelial-specific FoxO1 depletion prevents obesity-related disorders by increasing vascular metabolism and growth. Elife 2018, 7. [Google Scholar] [CrossRef]
- Roth Flach, R.J.; DiStefano, M.T.; Danai, L.V.; Senol-Cosar, O.; Yawe, J.C.; Kelly, M.; Garcia Menendez, L.; Czech, M.P. Map4k4 impairs energy metabolism in endothelial cells and promotes insulin resistance in obesity. Am. J. Physiol. Endocrinol. Metab. 2017, 313, E303–E313. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Vaka, V.R.; He, X.; Booz, G.W.; Chen, J.X. High-fat diet induces cardiac remodelling and dysfunction: Assessment of the role played by SIRT3 loss. J. Cell. Mol. Med. 2015, 19, 1847–1856. [Google Scholar] [CrossRef] [PubMed]
- Huynh, K. Diabetes: Diabetes-induced microvascular destabilization. Nat. Rev. Cardiol. 2017, 14, 128–129. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Ojeda, F.J.; Mendez-Gutierrez, A.; Aguilera, C.M.; Plaza-Diaz, J. Extracellular Matrix Remodeling of Adipose Tissue in Obesity and Metabolic Diseases. Int J. Mol. Sci. 2019, 20. [Google Scholar] [CrossRef]
- Ucuzian, A.A.; Gassman, A.A.; East, A.T.; Greisler, H.P. Molecular mediators of angiogenesis. J. Burn Care Res. 2010, 31, 158–175. [Google Scholar] [CrossRef]
- Sun, K.; Tordjman, J.; Clement, K.; Scherer, P.E. Fibrosis and adipose tissue dysfunction. Cell Metab. 2013, 18, 470–477. [Google Scholar] [CrossRef]
- Nakadate, K.; Motojima, K.; Tanaka-Nakadate, S. Dilatation of sinusoidal capillary and swelling of sinusoidal fenestration in obesity: An ultrastructural study. Ultrastruct. Pathol. 2015, 39, 30–37. [Google Scholar] [CrossRef]
- Brock, R.W.; Dorman, R.B. Obesity, insulin resistance and hepatic perfusion. Microcirculation 2007, 14, 339–347. [Google Scholar] [CrossRef]
- Zhi, Z.; Chao, J.R.; Wietecha, T.; Hudkins, K.L.; Alpers, C.E.; Wang, R.K. Noninvasive imaging of retinal morphology and microvasculature in obese mice using optical coherence tomography and optical microangiography. Invest. Ophthalmol. Vis. Sci. 2014, 55, 1024–1030. [Google Scholar] [CrossRef]
- Liang, J.; Li, Y.; Chen, L.; Xia, W.; Wu, G.; Tong, X.; Su, C.; He, J.; Lin, X.; Tao, J. Systemic microvascular rarefaction is correlated with dysfunction of late endothelial progenitor cells in mild hypertension: A substudy of EXCAVATION-CHN1. J. Transl. Med. 2019, 17, 368. [Google Scholar] [CrossRef]
- Pontes-Quero, S.; Fernandez-Chacon, M.; Luo, W.; Lunella, F.F.; Casquero-Garcia, V.; Garcia-Gonzalez, I.; Hermoso, A.; Rocha, S.F.; Bansal, M.; Benedito, R. High mitogenic stimulation arrests angiogenesis. Nat. Commun. 2019, 10, 2016. [Google Scholar] [CrossRef] [PubMed]
- Jonk, A.M.; Houben, A.J.; de Jongh, R.T.; Serne, E.H.; Schaper, N.C.; Stehouwer, C.D. Microvascular dysfunction in obesity: A potential mechanism in the pathogenesis of obesity-associated insulin resistance and hypertension. Physiology (Bethesda) 2007, 22, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Vogt, C.J.; Schmid-Schonbein, G.W. Microvascular endothelial cell death and rarefaction in the glucocorticoid-induced hypertensive rat. Microcirculation 2001, 8, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Almaca, J.; Weitz, J.; Rodriguez-Diaz, R.; Pereira, E.; Caicedo, A. The Pericyte of the Pancreatic Islet Regulates Capillary Diameter and Local Blood Flow. Cell Metab. 2018, 27, 630–644. [Google Scholar] [CrossRef]
- Schrimpf, C.; Teebken, O.E.; Wilhelmi, M.; Duffield, J.S. The role of pericyte detachment in vascular rarefaction. J. Vasc. Res. 2014, 51, 247–258. [Google Scholar] [CrossRef]
- Onogi, Y.; Wada, T.; Kamiya, C.; Inata, K.; Matsuzawa, T.; Inaba, Y.; Kimura, K.; Inoue, H.; Yamamoto, S.; Ishii, Y.; et al. PDGFRbeta Regulates Adipose Tissue Expansion and Glucose Metabolism via Vascular Remodeling in Diet-Induced Obesity. Diabetes 2017, 66, 1008–1021. [Google Scholar] [CrossRef]
- Dunford, E.C.; Leclair, E.; Aiken, J.; Mandel, E.R.; Haas, T.L.; Birot, O.; Riddell, M.C. The effects of voluntary exercise and prazosin on capillary rarefaction and metabolism in streptozotocin-induced diabetic male rats. J. Appl. Physiol. (1985) 2017, 122, 492–502. [Google Scholar] [CrossRef]
- Frisbee, J.C.; Samora, J.B.; Peterson, J.; Bryner, R. Exercise training blunts microvascular rarefaction in the metabolic syndrome. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H2483–H2492. [Google Scholar] [CrossRef]
- Honkala, S.M.; Motiani, P.; Kivela, R.; Hemanthakumar, K.A.; Tolvanen, E.; Motiani, K.K.; Eskelinen, J.J.; Virtanen, K.A.; Kemppainen, J.; Heiskanen, M.A.; et al. Exercise training improves adipose tissue metabolism and vasculature regardless of baseline glucose tolerance and sex. BMJ Open Diabetes Res. Care 2020, 8. [Google Scholar] [CrossRef]
- Xian, Y.; Chen, Z.; Deng, H.; Cai, M.; Liang, H.; Xu, W.; Weng, J.; Xu, F. Exenatide mitigates inflammation and hypoxia along with improved angiogenesis in obese fat tissue. J. Endocrinol. 2019, 242, 79–89. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paavonsalo, S.; Hariharan, S.; Lackman, M.H.; Karaman, S. Capillary Rarefaction in Obesity and Metabolic Diseases—Organ-Specificity and Possible Mechanisms. Cells 2020, 9, 2683. https://doi.org/10.3390/cells9122683
Paavonsalo S, Hariharan S, Lackman MH, Karaman S. Capillary Rarefaction in Obesity and Metabolic Diseases—Organ-Specificity and Possible Mechanisms. Cells. 2020; 9(12):2683. https://doi.org/10.3390/cells9122683
Chicago/Turabian StylePaavonsalo, Satu, Sangeetha Hariharan, Madeleine H. Lackman, and Sinem Karaman. 2020. "Capillary Rarefaction in Obesity and Metabolic Diseases—Organ-Specificity and Possible Mechanisms" Cells 9, no. 12: 2683. https://doi.org/10.3390/cells9122683
APA StylePaavonsalo, S., Hariharan, S., Lackman, M. H., & Karaman, S. (2020). Capillary Rarefaction in Obesity and Metabolic Diseases—Organ-Specificity and Possible Mechanisms. Cells, 9(12), 2683. https://doi.org/10.3390/cells9122683






