Abstract
The majority of cancer patients will be treated with radiotherapy, either alone or together with chemotherapy and/or surgery. Optimising the balance between tumour control and the probability of normal tissue side effects is the primary goal of radiation treatment. Therefore, it is imperative to understand the effects that irradiation will have on both normal and cancer tissue. The more classical lab models of immortal cell lines and in vivo animal models have been fundamental to radiobiological studies to date. However, each of these comes with their own limitations and new complementary models are required to fill the gaps left by these traditional models. In this review, we discuss how organoids, three-dimensional tissue-resembling structures derived from tissue-resident, embryonic or induced pluripotent stem cells, overcome the limitations of these models and thus have a growing importance in the field of radiation biology research. The roles of organoids in understanding radiation-induced tissue responses and in moving towards precision medicine are examined. Finally, the limitations of organoids in radiobiology and the steps being made to overcome these limitations are considered.
1. Introduction—Optimising the Therapeutic Window of Radiation Treatment
With an ever-aging population the number of people diagnosed with cancer is constantly growing [1]. Therefore, there is an even greater onus on the need to develop both current and new methods to enhance the efficacy of cancer treatments. Traditional cancer treatments, such as radiotherapy, chemotherapy and surgery, are still the most common modalities, but newer treatments such as immunotherapy are becoming more and more prevalent. Radiotherapy (either alone or in combination with surgery and/or chemotherapy) is used to treat over half of all cancer patients, with a curative intent in the majority of these cases [2,3]. Furthermore, the number of patients undergoing radiotherapy is predicted to increase even further due to an aging and growing population, as well as rapid technological advances in radiotherapy delivery practices [4]. The primary goal of radiotherapy, as with all other forms of cancer treatment, is to maximise the therapeutic window. The therapeutic window describes the balance between the probability of increasing tumour cell kill while minimising the probability of normal tissue complications. This can be achieved by using drugs which target the intrinsic vulnerabilities of a tumour to make it more susceptible than healthy tissue, or alternatively by physically targeting the tumour with greater accuracy and minimising the co-irradiated normal healthy tissue (Figure 1).
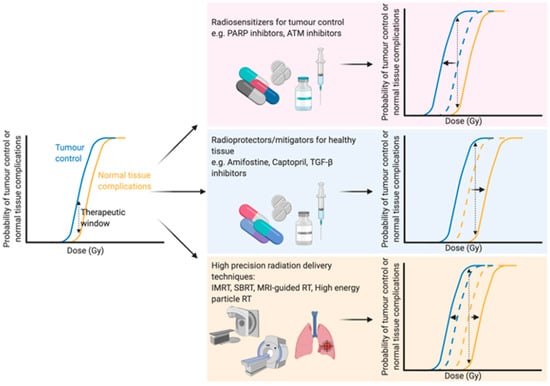
Figure 1.
Optimising the therapeutic window of radiotherapy. The therapeutic window describes the balance between the probability of tumour control (blue line) and normal tissue complications (yellow line). There are three main rationales behind broadening the therapeutic window in radiation treatment: (1) Increasing tumour sensitivity using radiosensitisers, reducing the dose required for tumour kill (blue line shifts to the left), (2) protecting normal tissue using radioprotectors or mitigators, thus increasing the tolerable dose of normal tissue (shifting the yellow line to the right) or (3) high precision dose delivery which can reduce the volume of co-irradiated normal tissue (effectively shifting the yellow line to the right) while in the case of charged particles an increased relative biological effectiveness reduces the dose required for tumour control. Abbreviations: PARP; poly-ADP ribose polymerase, ATM; ataxia telangiectasia mutated, TGF-β; transforming growth factor beta, IMRT; intensity-modulated radiation therapy, SBRT; stereotactic body radiation therapy, MRI; magnetic resonance imaging. Created with BioRender.com.
The development of high precision means of dose delivery, such as intensity modulated radiation therapy [5], stereotactic radiation therapy [6] and charged particle radiotherapy [7], have allowed for substantial reductions in co-irradiated normal tissue during therapy. These strategies enable better sparing of crucial organs [8] or sub-regions [9] within organs during treatment or dose escalation to the tumour. Furthermore, real-time advanced imaging, such as magnetic resonance imaging (MRI), during radiation therapy has been suggested as a means to further optimise the delivery of radiation to the target tumour with an increased sparing of the surrounding healthy tissue. Initial in vitro studies showed no changes in survival in response to X-rays when a magnetic field of 1.5 T was applied [10]. Indeed, combining X-ray therapy with MRI-guidance has been successfully applied in clinical practice to increase the accuracy of dose delivery and thus spare a greater proportion of healthy tissue [11]. Particle therapies, such as proton therapies, can modulate the dose to encompass the whole tumour in a so-called “spread-out Bragg peak” with a minimised entrance dose and negligible exit dose, sparing healthy tissue [12,13]. Furthermore, MRI-guided proton therapy has also been proposed [14,15] and early in vitro findings suggest that a magnetic field perpendicular to the radiation beam has no effect on the radiobiological effectiveness of the dose [16], while a magnetic field longitudinal to the beam slightly changes the effectiveness [17], emphasising the potential of such advances in a clinical setting. Further advances in radiation delivery include FLASH radiotherapy, which delivers ultra-high dose rates of ionising radiation which are believed to reduce normal tissue complications compared to conventional dose rates [18], although the therapeutic window of FLASH therapy still needs to be addressed [19].
All of these technological advances in the field of radiation beam delivery have significantly reduced the amount of co-irradiated healthy tissue during radiation treatment; however, none of these developments can completely eliminate dose to the surrounding tissue. Therefore, it is still necessary to develop in vivo and in vitro models to improve understanding of the mechanisms involved to better protect and/or regenerate normal tissue or to target intrinsic vulnerabilities of a tumour to enhance radiotherapy efficacy. These models should also take the therapeutic window into account as there is often an overlap between these mechanisms in both normal tissue and tumours, a feature which is regretfully often overlooked. Here we discuss one such in vitro model which could potentially allow the comparison of normal tissue and tumour responses at a patient-specific level, organoids, and the ever-growing role it has in radiobiological studies. We examine the strength of organoids in mechanistic studies in both normal and diseased tissue, but also examine the prospects of organoids in a more personalised medicine approach for patients. Finally, we discuss (potential) developments within the field of organoid research that could further benefit the radiobiology world.
2. The Need for New Models in Radiobiology
Since the beginning of the use of radiation treatment for cancer, radiobiology has made use of many different models to understand the molecular pathways triggered by radiation and to determine the consequences of radiation at a cellular, organ and system level in order to maximise the therapeutic window (Figure 2). Traditional cell cultures have been fundamental to radiobiology research, with many different techniques and findings crucial to other fields of biology, such as the development of the clonogenic survival assay [20,21]. Moreover, cell lines are highly amenable to high throughput drug screens, which in the field of radiobiology facilitates the efficient screening of large panels of potential radiosensitising agents over a radiation dose range [22]. However, cell lines cultured in two dimensions lack many features that are crucial to the overall response and survival of organisms following irradiation, such as cellular heterogeneity, cell–matrix interactions, “real” cell–cell interactions, a correct morphology and polarity, and functional relevance such as cytokine secretion [23,24]. Therefore, while they are invaluable, findings in cell lines often overstate findings, such as survival, compared to in vivo [25,26] and must therefore be treated with caution when translating to a more clinical patient setting.
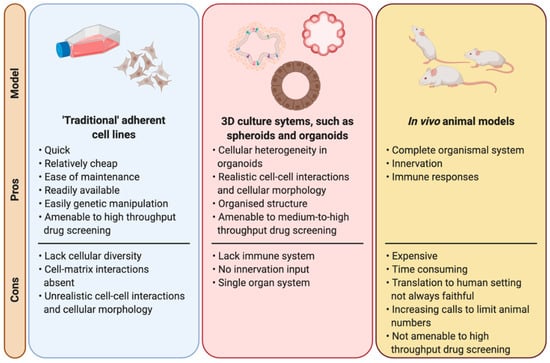
Figure 2.
Laboratory models used in radiation biology. A comparison between the main laboratory-based models used in radiobiological studies highlighting the pros and cons of each model. Created with BioRender.com.
Another model which has always been considered as a cornerstone for radiobiological research are in vivo animal models. Obviously animal models overcome many of the limitations of cell lines mentioned earlier, but they come with their own drawbacks, such as translatability to human settings, they are time consuming and expensive. Animal models are the most complete model available to researchers with the complete diversity of cell types and molecular interactions on an organismal level, as opposed to being constricted simply to a single cell type of a particular tissue when working with cell lines. Animal models are amenable to genetic manipulation and genetically modified animals offer the opportunity to study the impact of disease specific mutations, which in radiobiology allows researchers to study the effects on radio-resistance or -sensitivity of particular cancer associated mutations, such as p53 [27,28] or Atm [29,30]. Furthermore, in vitro and in silico findings should always be confirmed in vivo as the final step prior to human translation, and therefore animal models will remain crucial to biomedical and radiobiological research. However, with an increasing growing pressure on researchers to limit (or even eradicate) the use of animals in research [31], it is necessary to find and implement alternative models in the search for treatments to a wide variety of diseases, not just cancer.
Organoids, three-dimensional in vitro structures derived from induced pluripotent stem cells, embryonic stem cells or tissue specific resident stem/progenitor cells [32,33], offer a “steppingstone” between more traditional in vitro cell lines and in vivo animal models. Organoids are self-assembling structures which resemble the tissue of origin [32,33,34]. They contain multiple cell types [32], overcoming the lack of cellular diversity of cell lines, although vasculature and endothelial cells are generally absent from these cultures. Distinct nomenclature has been proposed in some fields to distinguish between different 3D in vitro cultures, such as the suggested nomenclature differences between “enteroids”, “colonoids” and “organoids” in the gastrointestinal field [35]. Furthermore, the term “tumouroids” is frequently used for tumour-derived organoids (or tumour-like organoids). Therefore, it should be noted that here we use the term “organoids” to encompass all self-organising 3D cellular structures derived from embryonic stem cells, induced pluripotent stem cells or tissue-resident stem/progenitor cells which contain multiple different cell types found within the tissue of origin. This is based on the definitions proposed by Lancaster and Knoblich (2014) [32] and Clevers (2016) [33].
As they are cultured in three dimensions, the cellular interactions and morphology become more “realistic” allowing for endpoint readouts which more closely resemble clinical observations. Furthermore, many organoid cultures have been shown to secrete functional enzymes under the right conditions [36], while transplantation of cultured organoids into murine models has been shown to rescue injured phenotypes [37]. Following radiation treatment, normal tissue stem cells are crucial to tissue regeneration. Conversely, cancer stem cells have increased radioresistance, repopulate tumours and are more prone to metastasize [38]. Therefore, it is important to be able to assess stem cell responses and the dynamics of those responses within the cellular heterogeneity (consisting of stem cells, progenitors and differentiated cells) of the tissue of origin. As they are derived from stem/progenitor cells, organoids can be used as a readout for such cells in an environment encompassing such heterogeneity [26]. Organoids are crucial to the studies of the mechanistic sequalae to irradiation, but also have an increasing role and potential in a more personalised approach to determining individual patient treatments. However, when designing experiments using organoids, researchers should always consider the question on hand when deciding which model (tissue-derived organoids, embryonic stem cell-derived or induced pluripotent stem cell-derived) to be used. For example, in cancer studies using organoids, pluripotent stem cell-derived and CRISPR-edited normal tissue-derived organoids can mimic germline mutations and thus allow accurate assessment of specific mutations in oncogenesis [39]. However, for treatment response studies, patient-derived organoids may represent a more suitable model, as they can encompass the true complexity of the disease, such microsatellite and chromosomal instabilities [40,41].
3. Organoids and Regeneration of Radiation-Induced Damaged Tissue
Since the identification of Lgr5 as a marker for intestinal stem cells [42], one of the most studied and established organoid models are the gastrointestinal “mini-gut” organoids. Originally established from mouse small intestinal stem cells [43], organoid “mini-gut” models have subsequently been established from human stem cells [44], as well as from various different locations along the gastrointestinal tract, including stomach [45], colon [44] and oesophagus [46]. Furthermore, pluripotent stem cells have been utilised to successfully generate intestinal [47] and oesophageal [48] organoid cultures. These models have opened novel avenues of study for intestinal development, cancer progression [49] and other diseases, such cystic fibrosis [50].
While there have been only a limited number of studies using organoids to investigate radiation-induced gastrointestinal injury, some recent studies have used organoids to complement and reinforce important insights from in vivo mouse studies [51,52,53]. Wang et al. [51] demonstrated using intestinal crypt organoids that selective inhibition of radiation-induced p53-mediated apoptosis using CHIR99021, an inhibitor of glycogen synthase kinase-3 (GSK-3), can protect intestinal stem cells against radiation due to an increased survival of Lgr5+ cells. This was recapitulated in vivo, indicating a pivotal role for p53 post-translational modifications in intestinal stem cell responses to irradiation [51]. More recently, using intestinal organoids from mouse jejunum and human colon, Bhanja et al. [52] revealed the potential of BCN057, an anti-neoplastic small molecular agent, to mitigate radiation-induced gastrointestinal syndrome in normal tissue. Interestingly, BCN057 did not have a radiomitigative effect in tumour-derived organoids with these findings again mimicking in vivo findings. The same group also investigated the potential of repurposing auranofin, an anti-rheumatoid drug containing gold, as a radioprotective agent against intestinal injury [53]. In both in vivo mice and ex vivo human colon organoids treatment with auranofin significantly reduced the toxicity of radiation [53]. Furthermore, Martin et al. [54] recently demonstrated that the profile of the Lgr5+ stem cell population of the large and small intestines following irradiation of organoids could act as a marker for predicting the sensitivity of these organs to radiation. The authors validated their approach using organoids with a well-established in vivo microcolony assay which quantifies the number of regenerating crypts per small intestinal circumference [54,55]. This assay is regarded as a benchmark assay for establishing the radiosensitivity of intestinal stem cell survival and highlights the potential of intestinal organoids to predict radiation responses [54]. These studies demonstrate the strength of “mini-gut” organoids as a model for radiation studies of the gastrointestinal tract and also the opportunities for radiobiological studies in other organoid systems, particularly in tissues which lack accurate in vitro models for radiobiological studies.
Radiotherapy is used to treat the majority of head and neck cancer patients, either alone or in combination with surgery and/or chemotherapy [56]. Frequently, irradiation of head and neck tumours leads to the unavoidable co-irradiation of salivary glands, with almost half of head and neck cancer patients subsequently suffering from radiation-induced xerostomia due to hyposalivation. This drastically impacts on the quality of life of patients due to impaired chewing, swallowing, speaking and an increased risk of oral infections [57]. In vivo studies using rats have shown that sparing a region of the salivary gland which contains a high density of tissue specific stem/progenitor cells has been shown to reduce the effects of salivary gland irradiation [9]. Therapeutic options are available to stimulate salivary gland flow post-irradiation but are limited in their effectiveness [57]. Therefore, a need for a more long-term strategy for salivary gland regeneration following radiotherapy remains [58]. While in vivo animal models have provided a wealth of knowledge as to the mechanisms behind salivary gland regeneration following injury, including radiation-induced damage [59,60,61,62,63,64], there is a limited number of in vitro systems to accurately study salivary glands following irradiation. Thus there is a growing niche for new models such as organotypic slice cultures [65] and organoids in the area of salivary gland radiation research.
Recently, our group has established protocols for the isolation and expansion of both murine [66] and human [67] submandibular salivary gland stem/progenitor cells. Using these protocols, we have shown that transplantation of enriched murine or human stem/progenitor cell populations improved functional readouts of irradiated mice salivary glands [37,67,68]. However, this effect may not only be directly from the expansion of the stem/progenitor cells in the transplanted tissue, but also due to paracrine effects of the transplanted cells acting on the recipient tissue [67]. Another recent study by Tanaka et al. has demonstrated the ability to derive salivary gland stem cells from embryonic stem cells [69]. Upon transplantation into parotid gland-defective mice, the induced salivary gland cells (transplanted either alone or together with mesenchymal cells) were capable of generating mature salivary gland tissue. The newly generated tissue was also shown to be functional as demonstrated by an increased saliva secretion in transplanted mice [69]. Combined, these studies hold significant preclinical promise for studying the mechanisms behind salivary gland regeneration and amelioration of salivary gland damage, both irradiation and non-irradiation induced damage [67,69]. However, the translation of any embryonic stem cell derived treatment [69] to a clinical application is always likely to be hindered by ethical concerns [70] and safety concerns regarding tumorigenicity [71].
Our models have been successfully utilised to study the survival responses of salivary gland stem/progenitor cells [26]. The salivary gland stem/progenitor organoids demonstrated a disproportionate sensitivity to low dose of radiation which was recapitulated in a functional low dose sensitivity in vivo [72]. While low dose hypersensitivity is not a new phenomenon [73,74], this was the first study to show the relevance of this phenomenon in stem/progenitor cells, with a potential clinical relevance. Furthermore, we have recently developed a protocol for the culturing of parotid salivary gland organoids and demonstrated that parotid gland stem cells display a similar radiosensitivity as those of submandibular salivary glands [75]. Importantly, as organoids are derived from stem/progenitor cell populations, they allow for the study of a more stem/progenitor specific response. As stem/progenitor cells play a prominent role in tissue regeneration following irradiation, models which allow for the understanding of these cells are crucial to protecting these tissues.
Another tissue in which the use of radiation is highly limited due to radiation-induced toxicity is the liver. Along with lung, breast, colorectal and pancreatic cancers, liver cancer deaths are one of the highest of all cancer-related deaths each year [76], while the prognosis is extremely poor due to limited treatment options [77]. The use of radiation treatment for liver cancer is severely hindered by the development of radiation-induced liver disease [78], a consequence which can also impede the utilisation of radiotherapy for other abdominal tumours in proximity to the liver, such as gastrointestinal cancers [79]. Much of what is known regarding radiation-induced liver disease is from retrospective clinical studies [79], as current lab models for studying it are limited with in vitro studies generally limited to cell lines lacking cellular heterogeneity and functionality. The recently developed models of both mouse [80] and human [81] derived liver organoid cultures from tissue resident stem cells, as well as pluripotent stem cell-derived liver cultures [82,83,84,85], may represent an ideal model for studying radiation-induced liver disease in the future. These models display cellular, functional activity and have structural organisation, while they have been successfully utilised to study genetic liver disorders mimicking the clinical pathology [81] and drug-induced liver injury [86]. Understanding the mechanisms of radiation-induced liver disease may eventually allow for increased treatment options for liver cancers.
4. A Platform for Treatment Response Studies; Moving towards Personalised Treatment?
The concept of precision treatments has been of growing interest in many fields of research in recent years, particularly oncology, as there is a wide variability of patient responses to standard “one size fits all” treatment regimens. In some cases, genetic factors which can be specifically targeted in a “personalised” manner are already known, for example non-small cell lung cancer patients with an activating mutation in tyrosine kinase are particularly sensitive to treatment with tyrosine kinases inhibitors such as gefitinib [87]. However, for other cancers, such as oesophageal cancers and locally advanced rectal cancers, there are currently no accurate predictors of patient responses to treatment. The standard of care for oesophageal cancer consists of neo-adjuvant chemoradiotherapy followed by surgery, with a complete pathological response observed in approximately a quarter at the time of surgery but no response in approximately one fifth of patients [88,89]. Similarly, for neoadjuvant chemoradiotherapy treatment of colorectal cancer while approximately one fifth of patients show a complete pathological response, almost 40% of patients show no benefit to the treatment [90]. In both cancers, patients would clearly benefit from more robust pre-treatment predictive models.
Therefore, there has been a concentrated effort in the field of organoids to establish reliable predictors of colorectal cancer treatment response to both chemotherapy alone [91,92] and neoadjuvant chemoradiotherapy [93,94]. Van de Wetering et al. [91] established colorectal cancer organoids, alongside paired healthy tissue, and demonstrated that the organoids recapitulated the genetic profiles and mutational spectra of the tumours of origin. Furthermore, by performing screening of 83 compounds, including both clinically used drugs and experimental compounds, the authors showed that the organoids facilitated the high-content drug screening [91], which could facilitate precision treatments in the future. Interestingly, a later study by Ooft et al. [92] investigating treatment response of metastatic colorectal cancer using organoids, was able to predict accuracy of irinotecan monotherapy and 5-flurouracil/irinotecan dual therapy, with 80% and 83.3% respectively. While greater accuracy is required to implement predictive models in a clinical setting, these studies show the developing potential of organoids in precision medicine. Furthermore, in recent years, there has been an increasing number of studies aimed at identifying and repurposing already available drugs as radiosensitisers [95,96,97,98]. Drugs which can be repurposed offer cheaper and quicker alternatives to developing new drugs from scratch, while many of the adverse side effects are already known [99]. The possibilities to quickly and accurately screen drugs, as shown in the studies of van de Wetering et al. [91] and Ooft et al. [92], in cancer organoids will greatly increase the possibilities in precision medicine and further benefit the search for potentiators of radiation therapy.
Indeed, recent studies by Ganesh et al. [93] and Yao et al. [94] have focussed on rectal cancer organoids for predicting patient responses to neoadjuvant chemoradiotherapy (Table 1 summarises the different cancer organoids that have been used in studies of radiation responses). Both studies further consolidated other evidence that rectal cancer organoids faithfully recapitulate the tumours of origin, performing histopathological and mutational comparisons between the two [93,94]. Moreover, Ganesh et al. showed that upon xenotransplantation of the organoids into mice they were found to metastasise to the same locations as the original tumours. Importantly, upon treating the organoids with chemotherapeutic drugs (such as 5-Flurouracil and oxaliplatin) heterogeneous treatment responses correlated with the clinical progression-free survival of patients. Interestingly, organoids which displayed resistance to radiation were derived from patients who either were resistant to therapy or showed disease recurrence following treatment [93]. Yao et al. [94] also correlated the therapeutic clinical outcomes to the standard neoadjuvant chemoradiotherapy with the organoid outcomes following treatment 5-Flurouracil, irinotecan or radiation. In sixty-eight out of the 80 patient-derived organoid lines generated, at least one of the three treatment courses was found to be predictive of the patient’s tumour regression score after surgery [94]. Furthermore, in a recent study, Pasch et al. established patient-derived cancer organoids and were prospectively able to predict the treatment response of a patient with metastatic colon cancer [100]. These studies combined with the works of van de Wetering et al. and Ooft et al. provide a significant step towards a model for patient-specific response prediction.

Table 1.
Radiation response studies using different cancer organoid models.
A recent study also established an organoid model for metastatic gastrointestinal cancers which were histologically, genetically and molecularly similar to the tumour of origin [104]. Following drug treatment of the organoids, the outcomes were compared with the clinical outcomes of the patients enrolled in Phase I/II clinical trials and were found to closely mimic the clinical outcomes of the patients [104]. Moreover, the study successfully identified differential inter- and intra-patient responses to common chemotherapeutic agents for gastrointestinal cancer treatment [104]. This study represents an important advance for organoids in the field of personalised precision medicine.
As mentioned above, currently the ability to predict patient responses to chemoradiotherapy for oesophageal cancer is also extremely limited. Great strides are being made towards the optimisation of imaging techniques for predicting treatment outcomes for oesophageal cancer treatment [89,105,106,107]; however, there is still no means to accurately predict patient outcomes. Recent advances in the culturing of oesophageal adenocarcinoma organoids have established new models to study the development and heterogeneity of the disease [108]. The established patient-derived oesophageal adenocarcinoma organoids shared histopathological features with patient-matched tumour samples and genetic mutations were conserved at a patient-specific level [108]. They further showed a loss of cellular polarity, which is often considered a hallmark of cancer. Drug screening in the organoids revealed a highly diverse range of responses, which tallies with the difficulties in predicting patient responses. However, the diversity of the responses remained throughout passaging, indicating the stability of the model through time [108]. Unfortunately, the findings of this study were somewhat limited due to a low success rate of establishing organoids (organoids were established from only 10 out of 32 patients). Reasons for a low success yield included failure to initiate culture, infection, fibroblast overgrowth, and arrested growth [108], while others also working on developing oesophageal adenocarcinoma organoids have recently identified the presence of Barrett’s epithelium as another potential contamination source in culture [109]. These new models will be essential to opening new avenues for testing new drugs and treatment regimens for oesophageal adenocarcinomas. Furthermore, as mentioned above, radiotherapy is an also important arm of treatment for other cancers in the head and neck region. Recently established protocols for generating organoids from oral mucosa and head and neck squamous cell carcinomas may facilitate a more personalised treatment planning for more tumours in this region [101]. Comparisons of the responses of tumour organoids with matched normal tissue organoids may even allow for studies of the therapeutic window on a personalised scale.
Glioblastoma is a highly aggressive brain tumour with an extremely poor prognosis for patients for whom radiotherapy is an integral arm of treatment [110]. This remains the case even with significant advances in the understanding of glioblastoma development, cellular heterogeneity within the tumour, and the role of cancer stem cells play in this [111,112,113]. Many of the models used for studying glioblastoma utilise adherent monolayers which, although they have been highly revealing of the mechanisms of glioma stem cell resistance [114], have thus far not been representative of the tumour microenvironment or levels of therapeutic resistance of glioblastoma seen in vivo. However, recently new organoid models have been established that could shed light on the initiation, development, tumour invasion, and treatment of glioblastoma. In two independent studies, Bian et al. [115] and Ogawa et al. [116] utilised CRISPR/Cas9 genome editing technology to manipulate cerebral organoids towards tumorigenesis. In both studies, cells derived from the generated tumour organoids exhibited epithelial-mesenchymal properties, indicative that they are representative of the invasive mesenchymal subtype of glioblastoma. Indeed, the cells were invasive when seeded with normal cerebral organoids [115,116] and were capable of forming tumours when xeno-transplanted into mouse recipients [116]. While neither group determined radiation responses of the glioblastoma organoids, Bian et al. demonstrated that CRISPR/Cas9 generated glioblastoma organoid models are appropriate for preclinical in vitro drug screening [115].
Indeed, studies which have investigated the radiosensitivity of glioblastoma organoids have demonstrated that they more closely resemble in vivo tumour sensitivity than monolayer cultures [102,103]. Furthermore, importantly, particularly from a radiobiology point-of-view, Hubert et al. showed that although the non-stem cells of the organoids were radiosensitive, the tumour-initiating cancer stem cells were indeed resistant [102], recapitulating important in vivo findings from previous studies [114]. Although these glioblastoma models offer excellent platforms to study glioblastoma development and biology, and to test new treatments, the duration of culturing generally does not facilitate rapid screening for a more personalised approach to treatment. However, recently a robust and rapid (within 1-2 weeks) protocol for establishing glioblastoma organoids capable of facilitating moderate to high throughput screening for a potentially more personalised response prediction [117].
5. The Future Directions of Organoid Models in Radiation Biology
Despite a growing role for organoids in radiobiology (as well as other fields of biology) and continuous advances of the models to faithfully simulate the tissue of origin, organoids still have limitations. However, these drawbacks may represent opportunities. Opportunities for researchers to optimise and improve current organoid systems, and opportunities to complement their research with other techniques, such as clinical imaging techniques for enhancing patient treatment response predictions.
While organoids consist of heterogeneous cell types and are cultured in three dimensions, they still lack important microenvironmental cues, such as sympathetic and parasympathetic innervation and immune cells (such as macrophages and cytokines). These are crucial factors in both development and regeneration of tissue. There is growing evidence for the role of parasympathetic innervation in salivary gland development [118] and regeneration [119], including following radiation-induced damage [120]. Finding means to accurately mimic autonomic innervation in organoids may be important to fully utilising them as models for regeneration in tissues with a similar architecture to the salivary glands. Similarly, the lack of stroma and immune cells in organoids in response to both injury and treatment are important factors which still need addressing especially considering the rising number of applications of immunotherapy. In the aforementioned study of Ooft et al. [92], while the patient-derived organoids were predictive of patient response to irinotecan-based treatments, they were not predictive of 5-FU–oxaliplatin combination therapy, which the authors suggest may, at least in part, be done to the lack of crucial stroma and immune system interactions. Recent advances have been made to overcome these issues, with Neal et al. [121] successfully developing patient-derived organoids with the T-cell spectra of the original tumours capable of modelling the immune checkpoint blockade. Alternatively, co-culturing of organoids with immune cells will offer a theoretically more realistic tissue response. Indeed, in co-culture experiments with macrophages and mammary organoids, macrophages were shown to migrate to organoids with an increased migration rate towards irradiated organoids [122].
Furthermore, stroma also plays an important role in radiation responses, of both normal and tumour tissue. In organoid cultures derived from whole tissue biopsies (without stem cell selection) stromal cells and effects can be found within the culture system [123]. However, in organoid cultures from selected stem cells stroma is absent, and therefore stromal co-culturing is necessary to recapitulate the effects of the tissue’s stroma. In prostate organoids, an increased viability and maintained branching was induced upon co-culture with prostate stroma [124]. Furthermore, the generation of organoids derived from prostate cancer was also improved upon stromal co-culture. These effects were suggested to be primarily due to direct contact with stromal cells and the expression of factors, such as TGF-β, by the stromal cells [124]. Besides the advance that this model represents in development and disease studies, the co-culture of organoids with tissue-specific stromal cells could have important implications for treatment responses, due to the important role of stromal cells [125] and the effects of signalling factors, such as TGF-β [126], in tissue responses.
Radiation-induced bystander effects have been suggested to act both proximally [127,128] and distally [129,130] to the site of irradiation; however, organoids derived from a single tissue currently do not recapitulate such interactions. Various anti-cancer therapies, including radiation, are known to induce senescence and an induction of a senescent associated secretory phenotype [131,132] which, it has been suggested, can in turn contribute to therapy-induced normal tissue side effects [133]. Studies using cultured media from irradiated cells has long been shown to induce paracrine bystander effects in non-irradiated cells [134] and such techniques may be insightful into the effects of secreted SASP proteins on untreated cells or organoids. Indeed, our group recently demonstrated that cultured media from irradiation-induced senescent organoids inhibits organoid forming efficiency in freshly passaged salivary gland-derived organoids [135]; however, these models still lack a true interaction between treated and non-treated organoids and the potential paracrine effects of other tissues in their vicinity in vivo.
Furthermore, both organ–organ, tumour–organ and vasculature interactions are generally absent in organoid cultures. Some of the glioblastoma organoid studies mentioned above elegantly show that cancer cells and healthy cells can be cultured together as organoids allowing for the study of tumour invasion [103,115,116]. Moreover, these models may be useful in revealing new therapeutic targets for tumour radiosensitisation or normal tissue radioprotection. Implementing organoid models alongside newly-established microfluidic devices which allow for the study of metabolic gradients [136] in radiation studies has the potential to reveal valuable insights of how such as signalling gradients can influence both irradiated and non-irradiated cells in perhaps a physiological relevant setting than organoids alone. Indeed, gut-on-a-chip models have recently been utilised in studies of radiation-induced intestinal injury and faithfully mimicked epithelial cell loss due to reactive oxygen production as seen in vivo [137] and may represent an excellent model for complementary studies to the abovementioned “mini-gut” organoid models in radiation studies. Organ-on-a-chip devices have been established for various other tissues, including lung [138], kidney [139] and liver [140]. The capacity of these platforms to mimic functional mechanics, such as breathing movements in lung-on-a-chip [138], could potentially offer more physiologically relevant models to complement and add a translational element to findings from organoid radiation studies. Furthermore, multiple chamber “on-a-chip” devices [141] could overcome the limitation of organoids of studying organs in isolation, in which each chamber potentially could contain cells from different tissues, vessels, stroma or nerves.
Radiation-induced endothelial cell loss and vascular damage are known to be major contributors to the response of both normal tissue and tumours [142,143]. Vasculopathy significantly increases the chances of ischemic stroke following radiation treatment [144], while preclinical models have been used to demonstrate that vascular remodelling is a major contributor to radiation-induced lung toxicity [145]. The vasculature of a tissue is essential for nutrient availability and regeneration following damage, as well as effective engraftment after tissue transplantation [146]. Furthermore, the response of tumour vasculature, particularly vasculogenesis, has also been shown to play a key role in tumour recurrence following radiation treatment [147]. While radiation can initially control the tumour, a reduced flow through tumour blood vessels and increased hypoxia can induce the hypoxia inducible factor-1 pathway. This in turn can activate pathways to re-promote vasculature and can subsequently cause tumour regrowth [143]. Therefore, it is important that in vitro models, particularly tumour models, can recapitulate such vasculature features. Recently, many techniques have been established to engineer vascularisation of organoids, including bioprinting, implantation into highly vascularised tissue and growing organoids in the vicinity of endothelial cell monolayers [148]. Vascularised organoids, such as recently-established vascularised cortical organoids [149,150] and tumour organoids [151], offer new opportunities to study disease pathology but also to study the impact vascularisation can have on treatment (including radiation) responses.
Finally, although there are many different protocols and technical considerations for the isolation and propagation of organoids, they are often arduous and time consuming. In order to have enough cells or organoids to test still often requires weeks to months of culturing. This is of particular importance for the development of organoids as a model for predicting patient responses in proposed precision therapies, where it is frequently necessary to treat patients as soon as possible. Protocols are being established to reduce culture time of organoids while maintaining fidelity of the systems of various different tissue origins (such as the aforementioned glioblastoma organoid model [117]); however, it is important that organoid models are further optimised for rapid and accurate screening of responses before implementation in a personalised medicine.
Despite their limitations, the future of organoid models in the field of radiobiology remains bright. As highlighted, many valuable studies are already overcoming the shortcomings of organoids, and as our knowledge and availability of organoid models grow, so too will their place in radiobiology. New organoid models can potentially shed some much-needed light on tissues which are perhaps less studied or highly limiting to the clinical application of radiation treatment, such as the liver. Moreover, while it could be questioned if a response prediction accuracy of approximately 80–85% is good enough, this will surely only improve as the models themselves are further optimised. Combining clinical patient imaging techniques currently used to predict patient responses, such as PET/CT, with the in vitro predictions from organoids may in the future bring around more accurate means to forecast treatment outcomes. Organoids could also potentially be used in discovery and validation of radiation biomarker and in radiomics. Understanding the mechanisms behind tissue regeneration are key to mitigating radiation-induced side effects, whether it is by stem cell therapy or through druggable targets to protect against damage, and organoids have already proven themselves as excellent models for such studies.
Author Contributions
Writing—original draft preparation, P.W.N.; writing—editing and reviewing, P.W.N. and R.P.C.; All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Dutch Cancer Society (KWF-grant number 10417).
Acknowledgments
While we have tried to give a broad overview of the state of organoids in radiation biology, there are many other important works in this area of the field that unfortunately we could not include due to manuscript constraints.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fitzmaurice, C.; Allen, C.; Barber, R.M.; Barregard, L.; Bhutta, Z.A.; Brenner, H.; Dicker, D.J.; Chimed-Orchir, O.; Dandona, R.; Dandona, L.; et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study Global Burden. JAMA Oncol. 2017, 3, 524–548. [Google Scholar] [CrossRef] [PubMed]
- Moding, E.J.; Kastan, M.B.; Kirsch, D.G. Strategies for optimizing the response of cancer and normal tissues to radiation. Nat. Rev. Drug Discov. 2013, 12, 526–542. [Google Scholar] [CrossRef] [PubMed]
- Barker, H.E.; Paget, J.T.E.; Khan, A.A.; Harrington, K.J. The tumour microenvironment after radiotherapy: Mechanisms of resistance and recurrence. Nat. Rev. Cancer 2015, 15, 409–425. [Google Scholar] [CrossRef] [PubMed]
- Borras, J.M.; Lievens, Y.; Barton, M.; Corral, J.; Ferlay, J.; Bray, F.; Grau, C. How many new cancer patients in Europe will require radiotherapy by 2025? An ESTRO-HERO analysis. Radiother. Oncol. 2016, 119, 5–11. [Google Scholar] [CrossRef]
- Wang, X.; Eisbruch, A. IMRT for head and neck cancer: Reducing xerostomia and dysphagia. J. Radiat. Res. 2016, 57, i69–i75. [Google Scholar] [CrossRef] [PubMed]
- Folkert, M.R.; Timmerman, R.D. Stereotactic ablative body radiosurgery (SABR) or Stereotactic body radiation therapy (SBRT). Adv. Drug Deliv. Rev. 2017, 109, 3–14. [Google Scholar] [CrossRef]
- Durante, M.; Loeffler, J.S. Charged particles in radiation oncology. Nat. Rev. Clin. Oncol. 2010, 7, 37–43. [Google Scholar] [CrossRef]
- Nutting, C.M.; Morden, J.P.; Harrington, K.J.; Urbano, T.G.; Bhide, S.A.; Clark, C.; Miles, E.A.; Miah, A.B.; Newbold, K.; Tanay, M.A.; et al. Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): A phase 3 multicentre randomised controlled trial. Lancet Oncol. 2011, 12, 127–136. [Google Scholar] [CrossRef]
- Van Luijk, P.; Pringle, S.; Deasy, J.O.; Moiseenko, V.V.; Faber, H.; Hovan, A.; Baanstra, M.; Van Der Laan, H.P.; Kierkels, R.G.J.; Van Der Schaaf, A.; et al. Sparing the region of the salivary gland containing stem cells preserves saliva production after radiotherapy for head and neck cancer. Sci. Transl. Med. 2015, 7, 305ra147. [Google Scholar] [CrossRef]
- Wang, L.; Hoogcarspel, S.J.; Wen, Z.; van Vulpen, M.; Molkentine, D.P.; Kok, J.; Lin, S.H.; Broekhuizen, R.; Ang, K.K.; Bovenschen, N.; et al. Biological responses of human solid tumor cells to X-ray irradiation within a 1.5-Tesla magnetic field generated by a magnetic resonance imaging–linear accelerator. Bioelectromagnetics 2016, 37, 471–480. [Google Scholar] [CrossRef]
- Raaymakers, B.W.; Jürgenliemk-Schulz, I.M.; Bol, G.H.; Glitzner, M.; Kotte, A.N.T.J.; Van Asselen, B.; De Boer, J.C.J.; Bluemink, J.J.; Hackett, S.L.; Moerland, M.A.; et al. First patients treated with a 1.5 T MRI-Linac: Clinical proof of concept of a high-precision, high-field MRI guided radiotherapy treatment. Phys. Med. Biol. 2017, 62, L41–L50. [Google Scholar] [CrossRef] [PubMed]
- Lomax, A.J. Charged particle therapy: The physics of interaction. Cancer J. 2009, 15, 285–291. [Google Scholar] [CrossRef]
- Newhauser, W.D.; Zhang, R. The physics of proton therapy. Phys. Med. Biol. 2015, 60, R155–R209. [Google Scholar] [CrossRef] [PubMed]
- Oborn, B.M.; Dowdell, S.; Metcalfe, P.E.; Crozier, S.; Mohan, R.; Keall, P.J. Proton beam deflection in MRI fields: Implications for MRI-guided proton therapy. Med. Phys. 2015, 42, 2113–2124. [Google Scholar] [CrossRef]
- Oborn, B.M.; Dowdell, S.; Metcalfe, P.E.; Crozier, S.; Mohan, R.; Keall, P.J. Future of medical physics: Real-time MRI-guided proton therapy: Real-time. Med. Phys. 2017, 44, e77–e90. [Google Scholar] [CrossRef]
- Nagle, P.W.; van Goethem, M.J.; Kempers, M.; Kiewit, H.; Knopf, A.; Langendijk, J.A.; Brandenburg, S.; van Luijk, P.; Coppes, R.P. In vitro biological response of cancer and normal tissue cells to proton irradiation not affected by an added magnetic field. Radiother. Oncol. 2019, 137, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Inaniwa, T.; Suzuki, M.; Sato, S.; Muramatsu, M.; Noda, A.; Iwata, Y.; Kanematsu, N.; Shirai, T.; Noda, K. Effect of External Magnetic Fields on Biological Effectiveness of Proton Beams. Int. J. Radiat. Oncol. Biol. Phys. 2020, 106, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.D.; Hammond, E.M.; Higgins, G.S.; Petersson, K. Ultra-High Dose Rate (FLASH) Radiotherapy: Silver Bullet or Fool’s Gold? Front. Oncol. 2020, 9, 1563. [Google Scholar] [CrossRef]
- Vozenin, M.-C.; Baumann, M.; Coppes, R.P.; Bourhis, J. FLASH radiotherapy International Workshop. Radiother. Oncol. 2019, 139, 1–3. [Google Scholar] [CrossRef]
- Barendsen, G.W. Dose-survival curves of human cells in tissue culture irradiated with alpha-, beta-, 20-kV. X- and 200-kV. X-radiation. Nature 1962, 193, 1153–1155. [Google Scholar] [CrossRef]
- Franken, N.A.P.; Rodermond, H.M.; Stap, J.; Haveman, J.; van Bree, C. Clonogenic assay of cells in vitro. Nat. Protoc. 2006, 1, 2315–2319. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wang, M.; Kern, A.M.; Khaled, S.; Han, J.; Yeap, B.Y.; Hong, T.S.; Settleman, J.; Benes, C.H.; Held, K.D.; et al. Adapting a drug screening platform to discover associations of molecular targeted radiosensitizers with genomic biomarkers. Mol. Cancer Res. 2015, 13, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Pampaloni, F.; Reynaud, E.G.; Stelzer, E.H.K. The third dimension bridges the gap between cell culture and live tissue. Nat. Rev. Mol. Cell Biol. 2007, 8, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Langhans, S.A. Three-dimensional in vitro cell culture models in drug discovery and drug repositioning. Front. Pharmacol. 2018, 9, 6. [Google Scholar] [CrossRef] [PubMed]
- Eke, I.; Cordes, N. Radiobiology goes 3D: How ECM and cell morphology impact on cell survival after irradiation. Radiother. Oncol. 2011, 99, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Nagle, P.W.; Hosper, N.A.; Ploeg, E.M.; Van Goethem, M.J.; Brandenburg, S.; Langendijk, J.A.; Chiu, R.K.; Coppes, R.P. The in Vitro Response of Tissue Stem Cells to Irradiation with Different Linear Energy Transfers. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 103–111. [Google Scholar] [CrossRef]
- Lee, J.M.; Abrahamson, J.L.A.; Kandel, R.; Donehower, L.A.; Bernstein, A. Susceptibility to radiation-carcinogenesis and accumulation of chromosomal breakage in p53 deficient mice. Oncogene 1994, 9, 3731–3736. [Google Scholar]
- Kemp, C.J.; Wheldon, T.; Balmain, A. P53-deficient mice are extremely susceptible to radiation-induced tumorigenesis. Nat. Genet. 1994, 8, 66–69. [Google Scholar] [CrossRef]
- Hammond, E.M.; Muschel, R.J. Radiation and ATM inhibition: The heart of the matter. J. Clin. Investig. 2014, 124, 3289–3291. [Google Scholar] [CrossRef]
- Moding, E.J.; Lee, C.L.; Castle, K.D.; Oh, P.; Mao, L.; Zha, S.; Min, H.D.; Ma, Y.; Das, S.; Kirsch, D.G. ATM deletion with dual recombinase technology preferentially radiosensitizes tumor endothelium. J. Clin. Investig. 2014, 124, 3325–3338. [Google Scholar] [CrossRef]
- De Boo, J.; Hendriksen, C. Reduction strategies in animal research: A review of scientific approaches at the intra-experimental, supra-experimental and extra-experimental levels. ATLA Altern. Lab. Anim. 2005, 33, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, M.A.; Knoblich, J.A. Organogenesisin a dish: Modeling development and disease using organoid technologies. Science 2014, 345, 1247125. [Google Scholar] [CrossRef]
- Clevers, H. Modeling Development and Disease with Organoids. Cell 2016, 165, 1586–1597. [Google Scholar] [CrossRef] [PubMed]
- Dutta, D.; Heo, I.; Clevers, H. Disease Modeling in Stem Cell-Derived 3D Organoid Systems. Trends Mol. Med. 2017, 23, 393–410. [Google Scholar] [CrossRef] [PubMed]
- Stelzner, M.; Helmrath, M.; Dunn, J.C.Y.; Henning, S.J.; Houchen, C.W.; Kuo, C.; Lynch, J.; Li, L.; Magness, S.T.; Martin, M.G.; et al. A nomenclature for intestinal in vitro cultures. Am. J. Physiol.-Gastrointest. Liver Physiol. 2012, 302, G1359–G1363. [Google Scholar] [CrossRef] [PubMed]
- Shankar, A.S.; Du, Z.; Mora, H.T.; van den Bosch, T.P.P.; Korevaar, S.S.; Van den Berg-Garrelds, I.M.; Bindels, E.; Lopez-Iglesias, C.; Groningen, M.C.; Gribnau, J.; et al. Human kidney organoids produce functional renin. Kidney Int. 2020, in press. [Google Scholar] [CrossRef]
- Maimets, M.; Rocchi, C.; Bron, R.; Pringle, S.; Kuipers, J.; Giepmans, B.N.G.; Vries, R.G.J.; Clevers, H.; De Haan, G.; Van Os, R.; et al. Long-Term in Vitro Expansion of Salivary Gland Stem Cells Driven by Wnt Signals. Stem Cell Rep. 2016, 6, 150–162. [Google Scholar] [CrossRef]
- Wang, D.; Plukker, J.T.M.; Coppes, R.P. Cancer stem cells with increased metastatic potential as a therapeutic target for esophageal cancer. Semin. Cancer Biol. 2017, 44, 60–66. [Google Scholar] [CrossRef]
- Matano, M.; Date, S.; Shimokawa, M.; Takano, A.; Fujii, M.; Ohta, Y.; Watanabe, T.; Kanai, T.; Sato, T. Modeling colorectal cancer using CRISPR-Cas9-mediated engineering of human intestinal organoids. Nat. Med. 2015, 21, 256–262. [Google Scholar] [CrossRef]
- Seidlitz, T.; Merker, S.R.; Rothe, A.; Zakrzewski, F.; Von Neubeck, C.; Grützmann, K.; Sommer, U.; Schweitzer, C.; Schölch, S.; Uhlemann, H.; et al. Human gastric cancer modelling using organoids. Gut 2019, 68, 207–217. [Google Scholar] [CrossRef]
- Li, X.; Larsson, P.; Ljuslinder, I.; Öhlund, D.; Myte, R.; Löfgren-Burström, A.; Zingmark, C.; Ling, A.; Edin, S.; Palmqvist, R. Ex vivo organoid cultures reveal the importance of the tumor microenvironment for maintenance of colorectal cancer stem cells. Cancers 2020, 12, 923. [Google Scholar] [CrossRef] [PubMed]
- Barker, N.; Van Es, J.H.; Kuipers, J.; Kujala, P.; Van Den Born, M.; Cozijnsen, M.; Haegebarth, A.; Korving, J.; Begthel, H.; Peters, P.J.; et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 2007, 449, 1003–1007. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Vries, R.G.; Snippert, H.J.; Van De Wetering, M.; Barker, N.; Stange, D.E.; Van Es, J.H.; Abo, A.; Kujala, P.; Peters, P.J.; et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 2009, 459, 262–265. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Stange, D.E.; Ferrante, M.; Vries, R.G.J.; Van Es, J.H.; Van Den Brink, S.; Van Houdt, W.J.; Pronk, A.; Van Gorp, J.; Siersema, P.D.; et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology 2011, 141, 1762–1772. [Google Scholar] [CrossRef] [PubMed]
- Barker, N.; Huch, M.; Kujala, P.; van de Wetering, M.; Snippert, H.J.; van Es, J.H.; Sato, T.; Stange, D.E.; Begthel, H.; van den Born, M.; et al. Lgr5+ve Stem Cells Drive Self-Renewal in the Stomach and Build Long-Lived Gastric Units In Vitro. Cell Stem Cell 2010, 6, 25–36. [Google Scholar] [CrossRef]
- DeWard, A.D.; Cramer, J.; Lagasse, E. Cellular heterogeneity in the mouse esophagus implicates the presence of a nonquiescent epithelial stem cell population. Cell Rep. 2014, 9, 701–711. [Google Scholar] [CrossRef]
- Spence, J.R.; Mayhew, C.N.; Rankin, S.A.; Kuhar, M.F.; Vallance, J.E.; Tolle, K.; Hoskins, E.E.; Kalinichenko, V.V.; Wells, S.I.; Zorn, A.M.; et al. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature 2011, 470, 105–110. [Google Scholar] [CrossRef]
- Trisno, S.L.; Philo, K.E.D.; McCracken, K.W.; Catá, E.M.; Ruiz-Torres, S.; Rankin, S.A.; Han, L.; Nasr, T.; Chaturvedi, P.; Rothenberg, M.E.; et al. Esophageal Organoids from Human Pluripotent Stem Cells Delineate Sox2 Functions during Esophageal Specification. Cell Stem Cell 2018, 23, 501–515. [Google Scholar] [CrossRef]
- Drost, J.; Van Boxtel, R.; Blokzijl, F.; Mizutani, T.; Sasaki, N.; Sasselli, V.; De Ligt, J.; Behjati, S.; Grolleman, J.E.; Van Wezel, T.; et al. Use of CRISPR-modified human stem cell organoids to study the origin of mutational signatures in cancer. Science 2017, 358, 234–238. [Google Scholar] [CrossRef]
- Dekkers, J.F.; Wiegerinck, C.L.; De Jonge, H.R.; Bronsveld, I.; Janssens, H.M.; De Winter-De Groot, K.M.; Brandsma, A.M.; De Jong, N.W.M.; Bijvelds, M.J.C.; Scholte, B.J.; et al. A functional CFTR assay using primary cystic fibrosis intestinal organoids. Nat. Med. 2013, 19, 939–945. [Google Scholar] [CrossRef]
- Wang, X.; Wei, L.; Cramer, J.M.; Leibowitz, B.J.; Judge, C.; Epperly, M.; Greenberger, J.; Wang, F.; Li, L.; Stelzner, M.G.; et al. Pharmacologically blocking p53-dependent apoptosis protects intestinal stem cells and mice from radiation. Sci. Rep. 2015, 5, 8566. [Google Scholar] [CrossRef] [PubMed]
- Bhanja, P.; Norris, A.; Gupta-Saraf, P.; Hoover, A.; Saha, S. BCN057 induces intestinal stem cell repair and mitigates radiation-induced intestinal injury. Stem Cell Res. Ther. 2018, 9, 26. [Google Scholar] [CrossRef] [PubMed]
- Nag, D.; Bhanja, P.; Riha, R.; Sanchez-Guerrero, G.; Kimler, B.F.; Tsue, T.T.; Lominska, C.; Saha, S. Auranofin protects intestine against radiation injury by modulating p53/p21 pathway and radiosensitizes human colon tumor. Clin. Cancer Res. 2019, 25, 4791–4807. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.L.; Adileh, M.; Hsu, K.S.; Hua, G.; Lee, S.G.; Li, C.; Fuller, J.D.; Rotolo, J.A.; Bodo, S.; Klingler, S.; et al. Organoids reveal that inherent radiosensitivity of small and large intestinal stem cells determines organ sensitivity. Cancer Res. 2020, 256, 1219–1227. [Google Scholar] [CrossRef] [PubMed]
- Withers, H.R.; Elkind, M.M. Microcolony survival assay for cells of mouse intestinal mucosa exposed to radiation. Int. J. Radiat. Biol. 1970, 17, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Nutting, C. Radiotherapy in head and neck cancer management: United Kingdom National Multidisciplinary Guidelines. J. Laryngol. Otol. 2016, 130, S66–S67. [Google Scholar] [CrossRef][Green Version]
- Vissink, A.; Mitchell, J.B.; Baum, B.J.; Limesand, K.H.; Jensen, S.B.; Fox, P.C.; Elting, L.S.; Langendijk, J.A.; Coppes, R.P.; Reyland, M.E. Clinical management of salivary gland hypofunction and xerostomia in head-and-neck cancer patients: Successes and barriers. Int. J. Radiat. Oncol. Biol. Phys. 2010, 78, 983–991. [Google Scholar] [CrossRef]
- Rocchi, C.; Emmerson, E. Mouth-Watering Results: Clinical Need, Current Approaches, and Future Directions for Salivary Gland Regeneration. Trends Mol. Med. 2020, 26, 649–669. [Google Scholar] [CrossRef]
- Zeilstra, L.J.W.; Vissink, A.; Konings, A.W.T.; Coppes, R.P. Radiation induced cell loss in rat submandibular gland and its relation to gland function. Int. J. Radiat. Biol. 2000, 76, 419–429. [Google Scholar] [CrossRef]
- Coppes, R.P.; Vissink, A.; Konings, A.W.T. Comparison of radiosensitivity of rat parotid and submandibular glands after different radiation schedules. Radiother. Oncol. 2002, 63, 321–328. [Google Scholar] [CrossRef]
- Marmary, Y.; Adar, R.; Gaska, S.; Wygoda, A.; Maly, A.; Cohen, J.; Eliashar, R.; Mizrachi, L.; Orfaig-Geva, C.; Baum, B.J.; et al. Radiation-induced loss of salivary gland function is driven by cellular senescence and prevented by IL6 modulation. Cancer Res. 2016, 76, 1170–1180. [Google Scholar] [CrossRef] [PubMed]
- Emmerson, E.; May, A.J.; Nathan, S.; Cruz-Pacheco, N.; Lizama, C.O.; Maliskova, L.; Zovein, A.C.; Shen, Y.; Muench, M.O.; Knox, S.M. SOX2 regulates acinar cell development in the salivary gland. eLife 2017, 6, e26620. [Google Scholar] [CrossRef] [PubMed]
- Ninche, N.; Kwak, M.; Ghazizadeh, S. Diverse epithelial cell populations contribute to the regeneration of secretory units in injured salivary glands. Development 2020, 147, dev192807. [Google Scholar] [CrossRef] [PubMed]
- Lombaert, I.M.A.; Patel, V.N.; Jones, C.E.; Villier, D.C.; Canada, A.E.; Moore, M.R.; Berenstein, E.; Zheng, C.; Goldsmith, C.M.; Chorini, J.A.; et al. CERE-120 Prevents Irradiation-Induced Hypofunction and Restores Immune Homeostasis in Porcine Salivary Glands. Mol. Ther.-Methods Clin. Dev. 2020, 18, 839–855. [Google Scholar] [CrossRef] [PubMed]
- Meyer, R.; Wong, W.Y.; Guzman, R.; Burd, R.; Limesand, K. Radiation treatment of organotypic cultures from submandibular and parotid salivary glands models key in vivo characteristics. J. Vis. Exp. 2019, 2019, 59484. [Google Scholar] [CrossRef]
- Nanduri, L.S.Y.; Baanstra, M.; Faber, H.; Rocchi, C.; Zwart, E.; De Haan, G.; Van Os, R.; Coppes, R.P. Purification and Ex vivo expansion of fully functional salivary gland stem cells. Stem Cell Rep. 2014, 3, 957–964. [Google Scholar] [CrossRef]
- Pringle, S.; Maimets, M.; Van Der Zwaag, M.; Stokman, M.A.; Van Gosliga, D.; Zwart, E.; Witjes, M.J.H.; De Haan, G.; Van Os, R.; Coppes, R.P. Human salivary gland stem cells functionally restore radiation damaged salivary glands. Stem Cells 2016, 34, 640–652. [Google Scholar] [CrossRef]
- Nanduri, L.S.Y.; Maimets, M.; Pringle, S.A.; Van Der Zwaag, M.; Van Os, R.P.; Coppes, R.P. Regeneration of irradiated salivary glands with stem cell marker expressing cells. Radiother. Oncol. 2011, 99, 367–372. [Google Scholar] [CrossRef]
- Tanaka, J.; Ogawa, M.; Hojo, H.; Kawashima, Y.; Mabuchi, Y.; Hata, K.; Nakamura, S.; Yasuhara, R.; Takamatsu, K.; Irié, T.; et al. Generation of orthotopically functional salivary gland from embryonic stem cells. Nat. Commun. 2018, 9, 4216. [Google Scholar] [CrossRef]
- Lo, B.; Parham, L. Ethical issues in stem cell research. Endocr. Rev. 2009, 30, 204–213. [Google Scholar] [CrossRef]
- Nori, S.; Okada, Y.; Nishimura, S.; Sasaki, T.; Itakura, G.; Kobayashi, Y.; Renault-Mihara, F.; Shimizu, A.; Koya, I.; Yoshida, R.; et al. Long-term safety issues of iPSC-based cell therapy in a spinal cord injury model: Oncogenic transformation with epithelial-mesenchymal transition. Stem Cell Rep. 2015, 4, 360–373. [Google Scholar] [CrossRef] [PubMed]
- Nagle, P.W.; Hosper, N.A.; Barazzuol, L.; Jellema, A.L.; Baanstra, M.; Van Goethem, M.J.; Brandenburg, S.; Giesen, U.; Langendijk, J.A.; Van Luijk, P.; et al. Lack of DNA damage response at low radiation doses in adult stem cells contributes to organ dysfunction. Clin. Cancer Res. 2018, 24, 6583–6593. [Google Scholar] [CrossRef] [PubMed]
- Marples, B.; Joiner, M.C. The response of Chinese hamster V79 cells to low radiation doses: Evidence of enhanced sensitivity of the whole cell population. Radiat. Res. 1993, 133, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.M.; Marples, B.; Lynch, T.H.; Hollywood, D.; Marignol, L. Exposure to low dose ionising radiation: Molecular and clinical consequences. Cancer Lett. 2014, 349, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Serrano Martinez, P.; Cinat, D.; van Luijk, P.; Baanstra, M.; de Haan, G.; Pringle, S.; Coppes, R.P. Mouse parotid salivary gland organoids for the in vitro study of stem cell radiation response. Oral Dis. 2020. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Llovet, J.M.; Villanueva, A.; Lachenmayer, A.; Finn, R.S. Advances in targeted therapies for hepatocellular carcinoma in the genomic era. Nat. Rev. Clin. Oncol. 2015, 12, 408–424. [Google Scholar] [CrossRef]
- Guha, C.; Kavanagh, B.D. Hepatic Radiation Toxicity: Avoidance and Amelioration. Semin. Radiat. Oncol. 2011, 21, 256–263. [Google Scholar] [CrossRef]
- Kim, J.; Jung, Y. Radiation-induced liver disease: Current understanding and future perspectives. Exp. Mol. Med. 2017, 49, e359. [Google Scholar] [CrossRef]
- Huch, M.; Dorrell, C.; Boj, S.F.; Van Es, J.H.; Li, V.S.W.; Van De Wetering, M.; Sato, T.; Hamer, K.; Sasaki, N.; Finegold, M.J.; et al. In vitro expansion of single Lgr5 + liver stem cells induced by Wnt-driven regeneration. Nature 2013, 494, 247–250. [Google Scholar] [CrossRef]
- Huch, M.; Gehart, H.; Van Boxtel, R.; Hamer, K.; Blokzijl, F.; Verstegen, M.M.A.; Ellis, E.; Van Wenum, M.; Fuchs, S.A.; De Ligt, J.; et al. Long-term culture of genome-stable bipotent stem cells from adult human liver. Cell 2015, 160, 299–312. [Google Scholar] [CrossRef]
- Hay, D.C.; Zhao, D.; Fletcher, J.; Hewitt, Z.A.; McLean, D.; Urruticoechea-Uriguen, A.; Black, J.R.; Elcombe, C.; Ross, J.A.; Wolf, R.; et al. Efficient Differentiation of Hepatocytes from Human Embryonic Stem Cells Exhibiting Markers Recapitulating Liver Development In Vivo. Stem Cells 2008, 26, 894–902. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, G.J.; Hay, D.C.; Park, I.H.; Fletcher, J.; Hannoun, Z.; Payne, C.M.; Dalgetty, D.; Black, J.R.; Ross, J.A.; Samuel, K.; et al. Generation of functional human hepatic endoderm from human induced pluripotent stem cells. Hepatology 2010, 51, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Takebe, T.; Sekine, K.; Enomura, M.; Koike, H.; Kimura, M.; Ogaeri, T.; Zhang, R.R.; Ueno, Y.; Zheng, Y.W.; Koike, N.; et al. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature 2013, 499, 481–484. [Google Scholar] [CrossRef] [PubMed]
- Takebe, T.; Sekine, K.; Kimura, M.; Yoshizawa, E.; Ayano, S.; Koido, M.; Funayama, S.; Nakanishi, N.; Hisai, T.; Kobayashi, T.; et al. Massive and Reproducible Production of Liver Buds Entirely from Human Pluripotent Stem Cells. Cell Rep. 2017, 21, 2661–2670. [Google Scholar] [CrossRef] [PubMed]
- Shinozawa, T.; Kimura, M.; Cai, Y.; Saiki, N.; Yoneyama, Y.; Ouchi, R.; Koike, H.; Maezawa, M.; Zhang, R.-R.; Dunn, A.; et al. High-Fidelity Drug Induced Liver Injury Screen Using Human PSC-derived Organoids. Gastroenterology 2020, in press. [Google Scholar] [CrossRef]
- Lynch, T.J.; Bell, D.W.; Sordella, R.; Gurubhagavatula, S.; Okimoto, R.A.; Brannigan, B.W.; Harris, P.L.; Haserlat, S.M.; Supko, J.G.; Haluska, F.G.; et al. Activating Mutations in the Epidermal Growth Factor Receptor Underlying Responsiveness of Non–Small-Cell Lung Cancer to Gefitinib. N. Engl. J. Med. 2004, 350, 2129–2139. [Google Scholar] [CrossRef]
- van Hagen, P.; Hulshof, M.C.C.M.; van Lanschot, J.J.B.; Steyerberg, E.W.; Henegouwen, M.V.; Wijnhoven, B.P.L.; Richel, D.J.; Nieuwenhuijzen, G.A.P.; Hospers, G.A.P.; Bonenkamp, J.J.; et al. Preoperative Chemoradiotherapy for Esophageal or Junctional Cancer. N. Engl. J. Med. 2012, 366, 2074–2084. [Google Scholar] [CrossRef]
- Sjoquist, K.M.; Burmeister, B.H.; Smithers, B.M.; Zalcberg, J.R.; Simes, R.J.; Barbour, A.; Gebski, V. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: An updated meta-analysis. Lancet Oncol. 2011, 12, 681–692. [Google Scholar] [CrossRef]
- Janakiraman, H.; Zhu, Y.; Becker, S.A.; Wang, C.; Cross, A.; Curl, E.; Lewin, D.; Hoffman, B.J.; Warren, G.W.; Hill, E.G.; et al. Modeling rectal cancer to advance neoadjuvant precision therapy. Int. J. Cancer 2020, 147, 1405–1418. [Google Scholar] [CrossRef]
- Van De Wetering, M.; Francies, H.E.; Francis, J.M.; Bounova, G.; Iorio, F.; Pronk, A.; Van Houdt, W.; Van Gorp, J.; Taylor-Weiner, A.; Kester, L.; et al. Prospective derivation of a living organoid biobank of colorectal cancer patients. Cell 2015, 161, 933–945. [Google Scholar] [CrossRef] [PubMed]
- Ooft, S.N.; Weeber, F.; Dijkstra, K.K.; McLean, C.M.; Kaing, S.; van Werkhoven, E.; Schipper, L.; Hoes, L.; Vis, D.J.; van de Haar, J.; et al. Patient-derived organoids can predict response to chemotherapy in metastatic colorectal cancer patients. Sci. Transl. Med. 2019, 11, eaay2574. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, K.; Wu, C.; O’Rourke, K.P.; Szeglin, B.C.; Zheng, Y.; Sauvé, C.E.G.; Adileh, M.; Wasserman, I.; Marco, M.R.; Kim, A.S.; et al. A rectal cancer organoid platform to study individual responses to chemoradiation. Nat. Med. 2019, 25, 1607–1614. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Xu, X.; Yang, L.; Zhu, J.; Wan, J.; Shen, L.; Xia, F.; Fu, G.; Deng, Y.; Pan, M.; et al. Patient-Derived Organoids Predict Chemoradiation Responses of Locally Advanced Rectal Cancer. Cell Stem Cell 2020, 26, 17–26. [Google Scholar] [CrossRef]
- Brown, S.L.; Kolozsvary, A.; Isrow, D.M.; Al Feghali, K.; Lapanowski, K.; Jenrow, K.A.; Kim, J.H. A novel mechanism of high dose radiation sensitization by metformin. Front. Oncol. 2019, 9, 247. [Google Scholar] [CrossRef] [PubMed]
- Spiegelberg, L.; van Hoof, S.J.; Biemans, R.; Lieuwes, N.G.; Marcus, D.; Niemans, R.; Theys, J.; Yaromina, A.; Lambin, P.; Verhaegen, F.; et al. Evofosfamide sensitizes esophageal carcinomas to radiation without increasing normal tissue toxicity. Radiother. Oncol. 2019, 141, 247–255. [Google Scholar] [CrossRef]
- Zhang, L.; Bochkur Dratver, M.; Yazal, T.; Dong, K.; Nguyen, A.; Yu, G.; Dao, A.; Bochkur Dratver, M.; Duhachek-Muggy, S.; Bhat, K.; et al. Mebendazole Potentiates Radiation Therapy in Triple-Negative Breast Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2019, 103, 195–207. [Google Scholar] [CrossRef]
- Broadfield, L.A.; Marcinko, K.; Tsakiridis, E.; Zacharidis, P.G.; Villani, L.; Lally, J.S.V.; Menjolian, G.; Maharaj, D.; Mathurin, T.; Smoke, M.; et al. Salicylate enhances the response of prostate cancer to radiotherapy. Prostate 2019, 79, 489–497. [Google Scholar] [CrossRef]
- Pushpakom, S.; Iorio, F.; Eyers, P.A.; Escott, K.J.; Hopper, S.; Wells, A.; Doig, A.; Guilliams, T.; Latimer, J.; McNamee, C.; et al. Drug repurposing: Progress, challenges and recommendations. Nat. Rev. Drug Discov. 2018, 18, 41–58. [Google Scholar] [CrossRef]
- Pasch, C.A.; Favreau, P.F.; Yueh, A.E.; Babiarz, C.P.; Gillette, A.A.; Sharick, J.T.; Karim, M.R.; Nickel, K.P.; DeZeeuw, A.K.; Sprackling, C.M.; et al. Patient-derived cancer organoid cultures to predict sensitivity to chemotherapy and radiation. Clin. Cancer Res. 2019, 25, 5376–5387. [Google Scholar] [CrossRef]
- Driehuis, E.; Kolders, S.; Spelier, S.; Lõhmussaar, K.; Willems, S.M.; Devriese, L.A.; de Bree, R.; de Ruiter, E.J.; Korving, J.; Begthel, H.; et al. Oral mucosal organoids as a potential platform for personalized cancer therapy. Cancer Discov. 2019, 9, 852–871. [Google Scholar] [CrossRef] [PubMed]
- Hubert, C.G.; Rivera, M.; Spangler, L.C.; Wu, Q.; Mack, S.C.; Prager, B.C.; Couce, M.; McLendon, R.E.; Sloan, A.E.; Rich, J.N. A three-dimensional organoid culture system derived from human glioblastomas recapitulates the hypoxic gradients and cancer stem cell heterogeneity of tumors found in vivo. Cancer Res. 2016, 76, 2465–2477. [Google Scholar] [CrossRef] [PubMed]
- Linkous, A.; Balamatsias, D.; Snuderl, M.; Edwards, L.; Miyaguchi, K.; Milner, T.; Reich, B.; Cohen-Gould, L.; Storaska, A.; Nakayama, Y.; et al. Modeling Patient-Derived Glioblastoma with Cerebral Organoids. Cell Rep. 2019, 26, 3203–3211. [Google Scholar] [CrossRef] [PubMed]
- Vlachogiannis, G.; Hedayat, S.; Vatsiou, A.; Jamin, Y.; Fernández-Mateos, J.; Khan, K.; Lampis, A.; Eason, K.; Huntingford, I.; Burke, R.; et al. Patient-derived organoids model treatment response of metastatic gastrointestinal cancers. Science 2018, 359, 920–926. [Google Scholar] [CrossRef]
- Roedl, J.B.; Halpern, E.F.; Colen, R.R.; Sahani, D.V.; Fischman, A.J.; Blake, M.A. Metabolic tumor width parameters as determined on PET/CT predict disease-free survival and treatment response in squamous cell carcinoma of the esophagus. Mol. Imaging Biol. 2009, 11, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Monjazeb, A.M.; Riedlinger, G.; Aklilu, M.; Geisinger, K.R.; Mishra, G.; Isom, S.; Clark, P.; Levine, E.A.; Blackstock, A.W. Outcomes of patients with esophageal cancer staged with [18F] fluorodeoxyglucose positron emission tomography (FDG-PET): Can postchemoradiotherapy FDG-PET predict the utility of resection? J. Clin. Oncol. 2010, 28, 4714–4721. [Google Scholar] [CrossRef]
- Omloo, J.M.T.; Van Heijl, M.; Hoekstra, O.S.; Van Berge Henegouwen, M.I.; Van Lanschot, J.J.B.; Sloof, G.W. FDG-PET parameters as prognostic factor in esophageal cancer patients: A review. Ann. Surg. Oncol. 2011, 18, 3338–3352. [Google Scholar] [CrossRef]
- Li, X.; Francies, H.E.; Secrier, M.; Perner, J.; Miremadi, A.; Galeano-Dalmau, N.; Barendt, W.J.; Letchford, L.; Leyden, G.M.; Goffin, E.K.; et al. Organoid cultures recapitulate esophageal adenocarcinoma heterogeneity providing a model for clonality studies and precision therapeutics. Nat. Commun. 2018, 9, 2983. [Google Scholar] [CrossRef]
- Derouet, M.F.; Allen, J.; Wilson, G.W.; Ng, C.; Radulovich, N.; Kalimuthu, S.; Tsao, M.S.; Darling, G.E.; Yeung, J.C. Towards personalized induction therapy for esophageal adenocarcinoma: Organoids derived from endoscopic biopsy recapitulate the pre-treatment tumor. Sci. Rep. 2020, 10, 14514. [Google Scholar] [CrossRef]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef]
- Pollard, S.M.; Yoshikawa, K.; Clarke, I.D.; Danovi, D.; Stricker, S.; Russell, R.; Bayani, J.; Head, R.; Lee, M.; Bernstein, M.; et al. Glioma Stem Cell Lines Expanded in Adherent Culture Have Tumor-Specific Phenotypes and Are Suitable for Chemical and Genetic Screens. Cell Stem Cell 2009, 4, 568–580. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.P.; Tirosh, I.; Trombetta, J.J.; Shalek, A.K.; Gillespie, S.M.; Wakimoto, H.; Cahill, D.P.; Nahed, B.V.; Curry, W.T.; Martuza, R.L.; et al. Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science 2014, 344, 1396–1401. [Google Scholar] [CrossRef] [PubMed]
- Lan, X.; Jörg, D.J.; Cavalli, F.M.G.; Richards, L.M.; Nguyen, L.V.; Vanner, R.J.; Guilhamon, P.; Lee, L.; Kushida, M.M.; Pellacani, D.; et al. Fate mapping of human glioblastoma reveals an invariant stem cell hierarchy. Nature 2017, 549, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Bao, S.; Wu, Q.; McLendon, R.E.; Hao, Y.; Shi, Q.; Hjelmeland, A.B.; Dewhirst, M.W.; Bigner, D.D.; Rich, J.N. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 2006, 444, 756–760. [Google Scholar] [CrossRef] [PubMed]
- Bian, S.; Repic, M.; Guo, Z.; Kavirayani, A.; Burkard, T.; Bagley, J.A.; Krauditsch, C.; Knoblich, J.A. Genetically engineered cerebral organoids model brain tumor formation. Nat. Methods 2018, 15, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, J.; Pao, G.M.; Shokhirev, M.N.; Verma, I.M. Glioblastoma Model Using Human Cerebral Organoids. Cell Rep. 2018, 23, 1220–1229. [Google Scholar] [CrossRef] [PubMed]
- Jacob, F.; Salinas, R.D.; Zhang, D.Y.; Nguyen, P.T.T.; Schnoll, J.G.; Wong, S.Z.H.; Thokala, R.; Sheikh, S.; Saxena, D.; Prokop, S.; et al. A Patient-Derived Glioblastoma Organoid Model and Biobank Recapitulates Inter- and Intra-tumoral Heterogeneity. Cell 2020, 180, 188–204. [Google Scholar] [CrossRef]
- Ferreira, J.N.; Hoffman, M.P. Interactions between developing nerves and salivary glands. Organogenesis 2013, 9, 152–158. [Google Scholar] [CrossRef]
- Knox, S.M.; Lombaert, I.M.A.; Haddox, C.L.; Abrams, S.R.; Cotrim, A.; Wilson, A.J.; Hoffman, M.P. Parasympathetic stimulation improves epithelial organ regeneration. Nat. Commun. 2013, 4, 1494. [Google Scholar] [CrossRef]
- Emmerson, E.; May, A.J.; Berthoin, L.; Cruz-Pacheco, N.; Nathan, S.; Mattingly, A.J.; Chang, J.L.; Ryan, W.R.; Tward, A.D.; Knox, S.M. Salivary glands regenerate after radiation injury through SOX2-mediated secretory cell replacement. EMBO Mol. Med. 2018, 10, e8051. [Google Scholar] [CrossRef]
- Neal, J.T.; Li, X.; Zhu, J.; Giangarra, V.; Grzeskowiak, C.L.; Ju, J.; Liu, I.H.; Chiou, S.H.; Salahudeen, A.A.; Smith, A.R.; et al. Organoid Modeling of the Tumor Immune Microenvironment. Cell 2018, 175, 1972–1988. [Google Scholar] [CrossRef] [PubMed]
- Hacker, B.C.; Gomez, J.D.; Silvera Batista, C.A.; Rafat, M. Growth and characterization of irradiated organoids from mammary glands. J. Vis. Exp. 2019, 2019, 59293. [Google Scholar] [CrossRef] [PubMed]
- Kretzschmar, K.; Clevers, H. Organoids: Modeling Development and the Stem Cell Niche in a Dish. Dev. Cell 2016, 38, 590–600. [Google Scholar] [CrossRef] [PubMed]
- Richards, Z.; McCray, T.; Marsili, J.; Zenner, M.L.; Manlucu, J.T.; Garcia, J.; Kajdacsy-Balla, A.; Murray, M.; Voisine, C.; Murphy, A.B.; et al. Prostate Stroma Increases the Viability and Maintains the Branching Phenotype of Human Prostate Organoids. iScience 2019, 12, 304–317. [Google Scholar] [CrossRef]
- Hellevik, T.; Martinez-Zubiaurre, I. Radiotherapy and the tumor stroma: The importance of dose and fractionation. Front. Oncol. 2014, 4, 1. [Google Scholar] [CrossRef]
- Farhood, B.; Khodamoradi, E.; Hoseini-Ghahfarokhi, M.; Motevaseli, E.; Mirtavoos-Mahyari, H.; Eleojo Musa, A.; Najafi, M. TGF-β in radiotherapy: Mechanisms of tumor resistance and normal tissues injury. Pharmacol. Res. 2020, 155, 104745. [Google Scholar] [CrossRef]
- Khan, M.A.; Hill, R.P.; Van Dyk, J. Partial volume rat lung irradiation: An evaluation of early DNA damage. Int. J. Radiat. Oncol. Biol. Phys. 1998, 40, 467–476. [Google Scholar] [CrossRef]
- Khan, M.A.; Van Dyk, J.; Yeung, I.W.T.; Hill, R.P. Partial volume rat lung irradiation; Assessment of early DNA damage in different lung regions and effect of radical scavengers. Radiother. Oncol. 2003, 66, 95–102. [Google Scholar] [CrossRef]
- Koturbash, I.; Loree, J.; Kutanzi, K.; Koganow, C.; Pogribny, I.; Kovalchuk, O. In Vivo Bystander Effect: Cranial X-Irradiation Leads to Elevated DNA Damage, Altered Cellular Proliferation and Apoptosis, and Increased p53 Levels in Shielded Spleen. Int. J. Radiat. Oncol. Biol. Phys. 2008, 70, 554–562. [Google Scholar] [CrossRef]
- Mancuso, M.; Pasquali, E.; Leonardi, S.; Tanori, M.; Rebessi, S.; Di Majo, V.; Pazzaglia, S.; Toni, M.P.; Pimpinella, M.; Covelli, V.; et al. Oncogenic bystander radiation effects in Patched heterozygous mouse cerebellum. Proc. Natl. Acad. Sci. USA 2008, 105, 12445–12450. [Google Scholar] [CrossRef]
- Igarashi, K.; Sakimoto, I.; Kataoka, K.; Ohta, K.; Miura, M. Radiation-induced senescence-like phenotype in proliferating and plateau-phase vascular endothelial cells. Exp. Cell Res. 2007, 313, 3326–3336. [Google Scholar] [CrossRef] [PubMed]
- Chiche, A.; Le Roux, I.; von Joest, M.; Sakai, H.; Aguín, S.B.; Cazin, C.; Salam, R.; Fiette, L.; Alegria, O.; Flamant, P.; et al. Injury-Induced Senescence Enables In Vivo Reprogramming in Skeletal Muscle. Cell Stem Cell 2017, 20, 407–414. [Google Scholar] [CrossRef]
- Demaria, M.; O’Leary, M.N.; Chang, J.; Shao, L.; Liu, S.; Alimirah, F.; Koenig, K.; Le, C.; Mitin, N.; Deal, A.M.; et al. Cellular senescence promotes adverse effects of chemotherapy and cancer relapse. Cancer Discov. 2017, 7, 165–176. [Google Scholar] [CrossRef]
- Ryan, L.A.; Smith, R.W.; Seymour, C.B.; Mothersill, C.E. Dilution of irradiated cell conditioned medium and the bystander effect. Radiat. Res. 2008, 169, 188–196. [Google Scholar] [CrossRef]
- Peng, X.; Wu, Y.; Brouwer, U.; van Vliet, T.; Wang, B.; Demaria, M.; Barazzuol, L.; Coppes, R.P. Cellular senescence contributes to radiation-induced hyposalivation by affecting the stem/progenitor cell niche. Cell Death Dis. 2020, 11, 854. [Google Scholar] [CrossRef]
- Manfrin, A.; Tabata, Y.; Paquet, E.R.; Vuaridel, A.R.; Rivest, F.R.; Naef, F.; Lutolf, M.P. Engineered signaling centers for the spatially controlled patterning of human pluripotent stem cells. Nat. Methods 2019, 16, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Jalili-Firoozinezhad, S.; Prantil-Baun, R.; Jiang, A.; Potla, R.; Mammoto, T.; Weaver, J.C.; Ferrante, T.C.; Kim, H.J.; Cabral, J.M.S.; Levy, O.; et al. Modeling radiation injury-induced cell death and countermeasure drug responses in a human Gut-on-a-Chip article. Cell Death Dis. 2018, 9, 223. [Google Scholar] [CrossRef] [PubMed]
- Huh, D.; Matthews, B.D.; Mammoto, A.; Montoya-Zavala, M.; Yuan Hsin, H.; Ingber, D.E. Reconstituting organ-level lung functions on a chip. Science 2010, 328, 1662–1668. [Google Scholar] [CrossRef]
- Jang, K.J.; Suh, K.Y. A multi-layer microfluidic device for efficient culture and analysis of renal tubular cells. Lab Chip 2010, 10, 36–42. [Google Scholar] [CrossRef]
- Carraro, A.; Hsu, W.M.; Kulig, K.M.; Cheung, W.S.; Miller, M.L.; Weinberg, E.J.; Swart, E.F.; Kaazempur-Mofrad, M.; Borenstein, J.T.; Vacanti, J.P.; et al. In vitro analysis of a hepatic device with intrinsic microvascular-based channels. Biomed. Microdevices 2008, 10, 795–805. [Google Scholar] [CrossRef]
- Sung, J.H.; Kam, C.; Shuler, M.L. A microfluidic device for a pharmacokinetic-pharmacodynamic (PK-PD) model on a chip. Lab Chip 2010, 10, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Milliat, F.; François, A.; Isoir, M.; Deutsch, E.; Tamarat, R.; Tarlet, G.; Atfi, A.; Validire, P.; Bourhis, J.; Sabourin, J.C.; et al. Influence of endothelial cells on vascular smooth muscle cells phenotype after irradiation: Implication in radiation-induced vascular damages. Am. J. Pathol. 2006, 169, 1484–1495. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.M. Radiation Damage to Tumor Vasculature Initiates a Program That Promotes Tumor Recurrences. Int. J. Radiat. Oncol. Biol. Phys. 2020, 108, 734–744. [Google Scholar] [CrossRef] [PubMed]
- Plummer, C.; Henderson, R.D.; O’Sullivan, J.D.; Read, S.J. Ischemic stroke and transient ischemic attack after head and neck radiotherapy: A review. Stroke 2011, 42, 2410–2418. [Google Scholar] [CrossRef]
- Ghobadi, G.; Bartelds, B.; Van Der Veen, S.J.; Dickinson, M.G.; Brandenburg, S.; Berger, R.M.F.; Langendijk, J.A.; Coppes, R.P.; Van Luijk, P. Lung irradiation induces pulmonary vascular remodelling resembling pulmonary arterial hypertension. Thorax 2012, 67, 334–341. [Google Scholar] [CrossRef]
- Pellegata, A.F.; Tedeschi, A.M.; De Coppi, P. Whole organ tissue vascularization: Engineering the tree to develop the fruits. Front. Bioeng. Biotechnol. 2018, 6, 56. [Google Scholar] [CrossRef]
- Kioi, M.; Vogel, H.; Schultz, G.; Hoffman, R.M.; Harsh, G.R.; Brown, J.M. Inhibition of vasculogenesis, but not angiogenesis, prevents the recurrence of glioblastoma after irradiation in mice. J. Clin. Investig. 2010, 120, 694–705. [Google Scholar] [CrossRef]
- Grebenyuk, S.; Ranga, A. Engineering organoid vascularization. Front. Bioeng. Biotechnol. 2019, 7, 39. [Google Scholar] [CrossRef]
- Cakir, B.; Xiang, Y.; Tanaka, Y.; Kural, M.H.; Parent, M.; Kang, Y.J.; Chapeton, K.; Patterson, B.; Yuan, Y.; He, C.S.; et al. Engineering of human brain organoids with a functional vascular-like system. Nat. Methods 2019, 16, 1169–1175. [Google Scholar] [CrossRef]
- Shi, Y.; Sun, L.; Wang, M.; Liu, J.; Zhong, S.; Li, R.; Li, P.; Guo, L.; Fang, A.; Chen, R.; et al. Vascularized human cortical organoids (vOrganoids) model cortical development in vivo. PLoS Biol. 2020, 18, e3000705. [Google Scholar] [CrossRef]
- Wörsdörfer, P.; Dalda, N.; Kern, A.; Krüger, S.; Wagner, N.; Kwok, C.K.; Henke, E.; Ergün, S. Generation of complex human organoid models including vascular networks by incorporation of mesodermal progenitor cells. Sci. Rep. 2019, 9, 15663. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).