The Relevance of Aquaporins for the Physiology, Pathology, and Aging of the Female Reproductive System in Mammals
Abstract
1. Introduction
2. Aquaporins in the Female Mammalian Reproductive System
2.1. The Expression of Aquaporins in the Vagina
2.2. Aquaporins and the Functioning of the Ovary
2.3. Aquaporins and the Functioning of the Uterus
2.4. Aquaporins and the Functioning of the Placenta
3. Aquaporins and Reproductive Aging
4. Aquaporins in Female Reproductive Tract Disorders
4.1. Polycystic Ovary Syndrome (PCOS)
4.2. Ovarian Cancer
4.3. Cervical Cancer
4.4. Endometrial Diseases
5. Aquaporins and Related Proteins
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Preston, G.M.; Carroll, T.P.; Guggino, W.B.; Agre, P. Appearance of water channels in Xenopus oocytes expressing red cell CHIP28 protein. Science 1992, 256, 385–387. [Google Scholar] [CrossRef]
- Mathai, J.C.; Agre, P. Hourglass pore-forming domains restrict aquaporin-1 tetramer assembly. Biochemistry 1999, 38, 923–928. [Google Scholar] [CrossRef]
- Sha, X.Y.; Xiong, Z.F.; Liu, H.S.; Di, X.D.; Ma, T.H. Maternal-fetal fluid balance and aquaporins: From molecule to physiology. Acta Pharmacol. Sin. 2011, 32, 716–720. [Google Scholar] [CrossRef]
- Ducza, E.; Csányi, A.; Gáspár, R. Aquaporins during pregnancy: Their function and significance. Int. J. Mol. Sci. 2017, 18, 2593. [Google Scholar] [CrossRef] [PubMed]
- Törnroth-Horsefield, S.; Hedfalk, K.; Fischer, G.; Lindkvist-Petersson, K.; Neutze, R. Structural insights into eukaryotic aquaporin regulation. FEBS Lett. 2010, 584, 2580–2588. [Google Scholar] [CrossRef] [PubMed]
- Agre, P.; King, L.S.; Yasui, M.; Guggino, W.B.; Ottersen, O.P.; Fujiyoshi, Y.; Engel, A.; Nielsen, S. Aquaporin water channels—From atomic structure to clinical medicine. J. Physiol. 2002, 542 Pt 1, 3–16. [Google Scholar] [CrossRef]
- Huang, H.F.; He, R.H.; Sun, C.C.; Zhang, Y.; Meng, Q.X.; Ma, Y.Y. Function of aquaporins in female and male reproductive systems. Hum. Reprod. Update 2006, 12, 785–795. [Google Scholar] [CrossRef]
- Yasui, M.; Kwon, T.H.; Knepper, M.A.; Nielsen, S.; Agre, P. Aquaporin-6: An intracellular vesicle water channel protein in renal epithelia. Proc. Natl. Acad. Sci. USA 1999, 10, 5808–5813. [Google Scholar] [CrossRef]
- Saparov, S.M.; Liu, K.; Agre, P.; Pohl, P. Fast and selective ammonia transport by aquaporin-8. J. Biol. Chem. 2007, 282, 5296–5301. [Google Scholar] [CrossRef]
- Tsukaguchi, H.; Shayakul, C.; Berger, U.V.; Mackenzie, B.; Devidas, S.; Guggino, W.B.; Van Hoek, A.N.; Hediger, M.A. Molecular characterization of a broad selectivity neutral solute channel. J. Biol. Chem. 1998, 273, 24737–24743. [Google Scholar] [CrossRef]
- Ozu, M.; Galizia, L.; Acuña, C.; Amodeo, G. Aquaporins: More Than Functional Monomers in a Tetrameric Arrangement. Cells 2018, 7, 209. [Google Scholar] [CrossRef] [PubMed]
- Benga, G.; Popescu, O.; Borza, V.; Pop, V.I.; Muresan, A.; Mocsy, I.; Brain, A.; Wrigglesworth, J.M. Water permeability in human erythrocytes: Identification of membrane proteins involved in water transport. Eur. J. Cell Biol. 1986, 41, 252–262. [Google Scholar] [PubMed]
- Agre, P.; Preston, G.M.; Smith, B.L.; Jung, J.S.; Raina, S.; Moon, C.; Guggino, W.B.; Nielsen, S. Aquaporin CHIP: The archetypal molecular water channel. Am. J. Physiol. Ren. Fluid Electrolyte Physiol. 1993, 265 Pt 2, F463–F476. [Google Scholar] [CrossRef]
- Day, R.E.; Kitchen, P.; Owen, D.S.; Bland, C.; Marshall, L.; Conner, A.C.; Bill, R.M.; Conner, M.T. Human aquaporins: Regulators of transcellular water flow. Biochim. Biophys. Acta Gen. Subj. 2014, 1840, 1492–1506. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yu, H.; Koide, S.S. The water channel gene in human uterus. Biochem. Mol. Biol. Int. 1994, 32, 371–377. [Google Scholar] [PubMed]
- Li, X.J.; Yu, H.M.; Koide, S.S. Regulation of water channel gene (AQP-CHIP) expression by estradiol and anordiol in rat uterus. Yaoxue Xuebao 1997, 32, 586–592. [Google Scholar]
- Ferré-Dolcet, L.; Yeste, M.; Vendrell, M.; Rigau, T.; Rodríguez-Gil, J.E.; del Alamo, M.M.R. Uterine and placental specific localization of AQP2 and AQP8 is related with changes of serum progesterone levels in pregnant queens. Theriogenology 2020, 142, 149–157. [Google Scholar] [CrossRef]
- Jablonski, E.M.; McConnell, N.A.; Hughes, F.M.; Huet-Hudson, Y.M. Estrogen Regulation of Aquaporins in the Mouse Uterus: Potential Roles in Uterine Water Movement. Biol. Reprod. 2003, 69, 1481–1487. [Google Scholar] [CrossRef]
- Richard, C.; Gao, J.; Brown, N.; Reese, J. Aquaporin water channel genes are differentially expressed and regulated by ovarian steroids during the periimplantation period in the mouse. Endocrinology 2003, 144, 1533–1541. [Google Scholar] [CrossRef]
- Lindsay, L.A.; Murphy, C.R. Redistribution of aquaporins 1 and 5 in the rat uterus is dependent on progesterone: A study with light and electron microscopy. Reproduction 2006, 131, 369–378. [Google Scholar] [CrossRef]
- Kim, S.O.; Oh, K.J.; Lee, H.S.; Ahn, K.; Kim, S.W.; Park, K. Expression of aquaporin water channels in the vagina in premenopausal women. J. Sex. Med. 2011, 8, 1925–1930. [Google Scholar] [CrossRef] [PubMed]
- Gannon, B.J.; Warnest, G.M.; Carati, C.J.; Verco, C.J. Aquaporin-1 expression in visceral smooth muscle cells of female rat reproductive tract. J. Smooth Muscle Res. 2000, 36, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.O.; Lee, H.S.; Ahn, K.; Park, K. Effect of estrogen deprivation on the expression of aquaporins and nitric oxide synthases in rat vagina. J. Sex. Med. 2009, 6, 1579–1586. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.H.; Chen, R.; Talafu, T.; Nijiati, R.; Lalai, S. Significance and expression of aquaporin 1, 3, 8 in cervical carcinoma in xinjiang uygur women of China. Asian Pac. J. Cancer Prev. 2012, 13, 1971–1975. [Google Scholar] [CrossRef]
- Feng, C.; Sun, C.C.; Wang, T.T.; He, R.H.; Sheng, J.Z.; Huang, H.F. Decreased expression of endometrial vessel AQP1 and endometrial epithelium AQP2 related to anovulatory uterine bleeding in premenopausal women. Menopause 2008, 15, 648–654. [Google Scholar] [CrossRef]
- Skowronski, M.T.; Kwon, T.H.; Nielsen, S. Immunolocalization of aquaporin 1, 5, and 9 in the female pig reproductive system. J. Histochem. Cytochem. 2009, 57, 61–67. [Google Scholar] [CrossRef]
- Skowronski, M.T. Distribution and quantitative changes in amounts of aquaporin 1, 5 and 9 in the pig uterus during the estrous cycle and early pregnancy. Reprod. Biol. Endocrinol. 2010, 8, 109. [Google Scholar] [CrossRef]
- Skowronski, M.T.; Skowronska, A.; Nielsen, S. Fluctuation of aquaporin 1, 5, and 9 expression in the pig oviduct during the estrous cycle and early pregnancy. J. Histochem. Cytochem. 2011, 59, 419–427. [Google Scholar] [CrossRef]
- Skowronski, M.T.; Frackowiak, L.; Skowronska, A. Expression of aquaporin 1 in the pig peri-ovarian vascular complex during the estrous cycle and early pregnancy. Reprod. Biol. 2011, 11, 210–223. [Google Scholar] [CrossRef]
- Aralla, M.; Borromeo, V.; Groppetti, D.; Secchi, C.; Cremonesi, F.; Arrighi, S. A collaboration of aquaporins handles water transport in relation to the estrous cycle in the bitch uterus. Theriogenology 2009, 72, 310–321. [Google Scholar] [CrossRef]
- Ji, Y.F.; Chen, L.Y.; Xu, K.H.; Yao, J.F.; Shi, Y.F.; Shanguan, X.J. Reduced expression of aquaporin 9 in tubal ectopic pregnancy. J. Mol. Histol. 2013, 44, 167–173. [Google Scholar] [CrossRef]
- Yang, J.H.; Shi, Y.F.; Cheng, Q.; Qian, Y.L. Protein and mRNA expression of aquaporin-1 in epithelial ovarian tumors and its clinic significance. Zhonghua Fu Chan Ke Za Zhi 2005, 40, 623–626. [Google Scholar]
- Yang, J.H.; Shi, Y.F.; Chen, X.D.; Qi, W.J. The influence of aquaporin-1 and microvessel density on ovarian carcinogenesis and ascites formation. Int. J. Gynecol. Cancer 2006, 16 (Suppl. S1), 400–405. [Google Scholar] [CrossRef]
- Thoroddsen, A.; Dahm-Kähler, P.; Lind, A.K.; Weijdegard, B.; Lindenthal, B.; Müller, J.; Brännström, M. The water permeability channels aquaporins 1-4 are differentially expressed in granulosa and theca cells of the preovulatory follicle during precise stages of human ovulation. J. Clin. Endocrinol. Metab. 2011, 96, 1021–1028. [Google Scholar] [CrossRef]
- Escobar, J.; Gormaz, M.; Arduini, A.; Gosens, K.; Martinez, A.; Perales, A.; Escrig, R.; Tormos, E.; Roselló, M.; Orellana, C.; et al. Expression of aquaporins early in human pregnancy. Early Hum. Dev. 2012, 88, 589–594. [Google Scholar] [CrossRef]
- Prat, C.; Blanchon, L.; Borel, V.; Gallot, D.; Herbet, A.; Bouvier, D.; Marceau, G.; Sapin, V. Ontogeny of aquaporins in human fetal membranes. Biol. Reprod. 2012, 86, 48. [Google Scholar] [CrossRef]
- Zhu, X.Q.; Jiang, S.S.; Zhu, X.J.; Zou, S.W.; Wang, Y.H.; Hu, Y.C. Expression of Aquaporin 1 and Aquaporin 3 in Fetal Membranes and Placenta in Human Term Pregnancies with Oligohydramnios. Placenta 2009, 30, 670–676. [Google Scholar] [CrossRef]
- Štulc, J. Placental transfer of inorganic ions and water. Physiol. Rev. 1997, 77, 805–836. [Google Scholar] [CrossRef]
- Xiong, Y.; Tan, Y.J.; Xiong, Y.M.; Huang, Y.T.; Hu, X.L.; Lu, Y.C.; Ye, Y.H.; Wang, T.T.; Zhang, D.; Jin, F.; et al. Expression of aquaporins in human embryos and potential role of AQP3 and AQP7 in preimplantation mouse embryo development. Cell. Physiol. Biochem. 2013, 31, 649–658. [Google Scholar] [CrossRef]
- Beall, M.H.; Wang, S.; Yang, B.; Chaudhri, N.; Amidi, F.; Ross, M.G. Placental and Membrane Aquaporin Water Channels: Correlation with Amniotic Fluid Volume and Composition. Placenta 2007, 28, 421–428. [Google Scholar] [CrossRef]
- Offenberg, H.; Barcroft, L.C.; Caveney, A.; Viuff, D.; Thomsen, P.D.; Watson, A.J. mRNAs encoding aquaporins are present during murine preimplantation development. Mol. Reprod. Dev. 2000, 57, 323–330. [Google Scholar] [CrossRef][Green Version]
- Liu, H.; Koukoulas, I.; Ross, M.C.; Wang, S.; Wintour, E.M. Quantitative comparison of placental expression of three aquaporin genes. Placenta 2004, 25, 475–478. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Han, H.J.; Kim, S.W.; Jung, S.I.; Kim, S.O.; Lee, H.S.; Lee, M.N.; Ahn, K. Expression of aquaporin water channels in rat vagina: Potential role in vaginal lubrication. J. Sex. Med. 2008, 5, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Hildenbrand, A.; Stavreus-Evers, A.; Lalitkumar, P.G.L.; Nielsen, S.; Mints, M.; Gemzell-Danielsson, K. Aquaporin 1 is expressed in the human endometrium during normal cycle and increases after mifepristone treatment. Int. J. Mol. Med. 2008, 22, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Hildenbrand, A.; Lalitkumar, L.; Nielsen, S.; Gemzell-Danielsson, K.; Stavreus-Evers, A. Expression of aquaporin 2 in human endometrium. Fertil. Steril. 2006, 86, 1452–1458. [Google Scholar] [CrossRef] [PubMed]
- He, R.H.; Sheng, J.Z.; Luo, Q.; Jin, F.; Wang, B.; Qian, Y.L.; Zhou, C.Y.; Sheng, X.; Huang, H.F. Aquaporin-2 expression in human endometrium correlates with serum ovarian steroid hormones. Life Sci. 2006, 79, 423–429. [Google Scholar] [CrossRef]
- Mobasheri, A.; Wray, S.; Marples, D. Distribution of AQP2 and AQP3 water channels in human tissue microarrays. J. Mol. Histol. 2005, 36, 1–14. [Google Scholar] [CrossRef]
- Zhu, J.; Xia, J.; Jiang, J.; Jiang, R.; He, Y.; Lin, H. Effects of estrogen deprivation on expression of aquaporins in rat vagina. Menopause 2015, 22, 893–898. [Google Scholar] [CrossRef]
- Yin, Y.; Lin, C.; Ma, L. Msx2 promotes vaginal epithelial differentiation and Wolffian duct regression and dampens the vaginal response to diethylstilbestrol. Mol. Endocrinol. 2006, 20, 1535–1546. [Google Scholar] [CrossRef][Green Version]
- Anderson, J.; Brown, N.; Mahendroo, M.S.; Reese, J. Utilization of different aquaporin water channels in the mouse cervix during pregnancy and parturition and in models of preterm and delayed cervical ripening. Endocrinology 2006, 147, 130–140. [Google Scholar] [CrossRef]
- Soh, Y.M.; Tiwari, A.; Mahendroo, M.; Conrad, K.P.; Parry, L.J. Relaxin regulates hyaluronan synthesis and aquaporins in the cervix of late pregnant mice. Endocrinology 2012, 12, 6054–6064. [Google Scholar] [CrossRef] [PubMed]
- Edashige, K.; Sakamoto, M.; Kasai, M. Expression of mRNAs of the aquaporin family in mouse oocytes and embryos. Cryobiology 2000, 40, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Edashige, K.; Ohta, S.; Tanaka, M.; Kuwano, T.; Valdez, D.M.; Hara, T.; Jin, B.; Takahashi, S.I.; Seki, S.; Koshimoto, C.; et al. The role of aquaporin 3 in the movement of water and cryoprotectants in mouse morulae. Biol. Reprod. 2007, 77, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Offenberg, H.; Thomsen, P.D. Functional challenge affects aquaporin mRNA abundance in mouse blastocysts. Mol. Reprod. Dev. 2005, 71, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, L.A.; Murphy, C.R. Aquaporins are upregulated in glandular epithelium at the time of implantation in the rat. J. Mol. Histol. 2007, 38, 87–95. [Google Scholar] [CrossRef]
- Brañes, M.C.; Morales, B.; Ríos, M.; Villalón, M.J. Regulation of the immunoexpression of aquaporin 9 by ovarian hormones in the rat oviductal epithelium. Am. J. Physiol. Cell Physiol. 2005, 288, C1048–C1057. [Google Scholar] [CrossRef]
- McConnell, N.A.; Yunus, R.S.; Gross, S.A.; Bost, K.L.; Clemens, M.G.; Hughes, F.M. Water permeability of an ovarian antral follicle is predominantly transcellular and mediated by aquaporins. Endocrinology 2002, 143, 2905–2912. [Google Scholar] [CrossRef]
- Su, W.; Guan, X.; Zhang, D.; Sun, M.; Yang, L.; Yi, F.; Hao, F.; Feng, X.; Ma, T. Occurrence of multi-oocyte follicles in aquaporin 8-deficient mice. Reprod. Biol. Endocrinol. 2013, 11, 88. [Google Scholar] [CrossRef]
- West-Farrell, E.R.; Xu, M.; Gomberg, M.A.; Chow, Y.H.; Woodruff, T.K.; Shea, L.D. The mouse follicle microenvironment regulates antrum formation and steroid production: Alterations in gene expression profiles. Biol. Reprod. 2009, 80, 432–439. [Google Scholar] [CrossRef][Green Version]
- Liu, H.S.; Hao, R.Z.; Song, X.F.; Xiong, Z.F. Aquaporin 8 expression in human placenta and fetal membrane. J. Clin. Rehabil. Tissue Eng. Res. 2009, 28, 333–336. [Google Scholar] [CrossRef]
- Wang, S.; Chen, J.; Au, K.T.; Ross, M.G. Expression of aquaporin 8 and its up-regulation by cyclic adenosine monophosphate in human WISH cells. Am. J. Obstet. Gynecol. 2003, 188, 997–1001. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Kallichanda, N.; Song, W.; Ramirez, B.A.; Ross, M.G. Expression of aquaporin-8 in human placenta and chorioamniotic membranes: Evidence of molecular mechanism for intramembranous amniotic fluid resorption. Am. J. Obstet. Gynecol. 2001, 185, 1226–1231. [Google Scholar] [CrossRef] [PubMed]
- Barcroft, L.C.; Offenberg, H.; Thomsen, P.; Watson, A.J. Aquaporin proteins in murine trophectoderm mediate transepithelial water movements during cavitation. Dev. Biol. 2003, 256, 342–354. [Google Scholar] [CrossRef]
- Bell, C.E.; Larivière, N.M.K.; Watson, P.H.; Watson, A.J. Mitogen-activated protein kinase (MAPK) pathways mediate embryonic responses to culture medium osmolarity by regulating Aquaporin 3 and 9 expression and localization, as well as embryonic apoptosis. Hum. Reprod. 2009, 24, 1373–1386. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chen, J.; Beall, M.; Zhou, W.; Ross, M.G. Expression of aquaporin 9 in human chorioamniotic membranes and placenta. Am. J. Obstet. Gynecol. 2004, 191, 2160–2167. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Jiang, S.; Hu, Y.; Zheng, X.; Zou, S.; Wang, Y.; Zhu, X. The expression of aquaporin 8 and aquaporin 9 in fetal membranes and placenta in term pregnancies complicated by idiopathic polyhydramnios. Early Hum. Dev. 2010, 86, 657–663. [Google Scholar] [CrossRef]
- Wang, S.; Chen, J.; Huang, B.; Ross, M.G. Cloning and cellular expression of aquaporin 9 in ovine fetal membranes. Am. J. Obstet. Gynecol. 2005, 193, 841–848. [Google Scholar] [CrossRef]
- De Wilde, J.; Wilting, S.M.; Meijer, C.J.L.M.; Van De Wiel, M.A.; Ylstra, B.; Snijders, P.J.F.; Steenbergen, R.D.M. Gene expression profiling to identify markers associated with deregulated hTERT in HPV-transformed keratinocytes and cervical cancer. Int. J. Cancer 2008, 122, 877–888. [Google Scholar] [CrossRef]
- Enders, A.C.; Schlafke, S. A morphological analysis of the early implantation stages in the rat. Am. J. Anat. 1967, 120, 185–225. [Google Scholar] [CrossRef]
- Wang, S.; Amidi, F.; Beall, M.; Gui, L.; Ross, M.G. Aquaporin 3 Expression in Human Fetal Membranes and its Up-regulation by Cyclic Adenosine Monophosphate in Amnion Epithelial Cell Culture. J. Soc. Gynecol. Investig. 2006, 13, 181–185. [Google Scholar] [CrossRef]
- De Falco, M.; Cobellis, L.; Torella, M.; Acone, G.; Varano, L.; Sellitti, A.; Ragucci, A.; Coppola, G.; Cassandro, R.; Laforgia, V.; et al. Down-regulation of aquaporin 4 in human placenta throughout pregnancy. In Vivo 2007, 21, 813–817. [Google Scholar] [PubMed]
- te Velde, E.R.; Pearson, P.L. The variability of female reproductive ageing. Hum. Reprod. Update 2002, 8, 141–154. [Google Scholar] [CrossRef]
- Selesniemi, K.; Lee, H.J.; Tilly, J.L. Moderate caloric restriction initiated in rodents during adulthood sustains function of the female reproductive axis into advanced chronological age. Aging Cell 2008, 7, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Garg, N.; Sinclair, D.A. Oogonial stem cells as a model to study age-associated infertility in women. Reprod. Fertil. Dev. 2015, 27, 969–974. [Google Scholar] [CrossRef] [PubMed]
- Harrison, B.J.; Hilton, T.N.; Rivière, R.N.; Ferraro, Z.M.; Deonandan, R.; Walker, M.C. Advanced maternal age: Ethical and medical considerations for assisted reproductive technology. Int. J. Women’s Health 2017, 9, 561–570. [Google Scholar] [CrossRef]
- Kemkes-Grottenthaler, A. Postponing or rejecting parenthood? Results of a survey among female academic professionals. J. Biosoc. Sci. 2003, 35, 213–226. [Google Scholar] [CrossRef]
- Marshall, A.L.; Arora, V.M.; Salles, A. Physician Fertility: A Call to Action. Acad. Med. 2020, 95, 679–681. [Google Scholar] [CrossRef]
- Patel, A.; Sharma, P.S.V.N.; Kumar, P. “In cycles of dreams, despair, and desperation:” Research perspectives on infertility specific distress in patients undergoing fertility treatments. J. Hum. Reprod. Sci. 2018, 11, 320–328. [Google Scholar] [CrossRef]
- Katz, P.; Showstack, J.; Smith, J.F.; Nachtigall, R.D.; Millstein, S.G.; Wing, H.; Eisenberg, M.L.; Pasch, L.A.; Croughan, M.S.; Adler, N. Costs of infertility treatment: Results from an 18-month prospective cohort study. Fertil. Steril. 2011, 95, 915–921. [Google Scholar] [CrossRef]
- Bittles, A.H.; Bower, C.; Hussain, R.; Glasson, E.J. The four ages of Down syndrome. Eur. J. Public Health 2007, 17, 221–225. [Google Scholar] [CrossRef]
- Cotterill, M.; Harris, S.; Collado, F.; Lu, J.; Huntriss, J.; Campbell, B.; Picton, H. The activity and copy number of mitochondrial DNA in ovine oocytes throughout oogenesis in vivo and during oocyte maturation in vitro. Mol. Hum. Reprod. 2013, 19, 444. [Google Scholar] [CrossRef] [PubMed]
- Dumollard, R.; Duchen, M.; Carroll, J. The Role of Mitochondrial Function in the Oocyte and Embryo. Curr. Top. Dev. Biol. 2007, 77, 21–49. [Google Scholar] [CrossRef] [PubMed]
- Leese, H.J.; Barton, A.M. Pyruvate and glucose uptake by mouse ova and preimplantation embryos. J. Reprod. Fertil. 1984, 72, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Wilding, M.; Dale, B.; Marino, M.; Di Matteo, L.; Alviggi, C.; Pisaturo, M.L.; Lombardi, L.; De Placido, G. Mitochondrial aggregation patterns and activity in human oocytes and preimplantation embryos. Hum. Reprod. 2001, 16, 909–917. [Google Scholar] [CrossRef]
- Takeuchi, T.; Neri, Q.V.; Katagiri, Y.; Rosenwaks, Z.; Palermo, G.D. Effect of treating induced mitochondrial damage on embryonic development and epigenesis. Biol. Reprod. 2005, 72, 584–592. [Google Scholar] [CrossRef] [PubMed]
- Thouas, G.A.; Trounson, A.O.; Jones, G.M. Developmental effects of sublethal mitochondrial injury in mouse oocytes. Biol. Reprod. 2006, 74, 969–977. [Google Scholar] [CrossRef] [PubMed]
- Wyman, A.; Pinto, A.B.; Sheridan, R.; Moley, K.H. One-cell zygote transfer from diabetic to nondiabetic mouse results in congenital malformations and growth retardation in offspring. Endocrinology 2008, 149, 466–469. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.W.; Dickinson, B.C.; Chang, C.J. Aquaporin-3 mediates hydrogen peroxide uptake to regulate downstream intracellular signaling. Proc. Natl. Acad. Sci. USA 2010, 107, 15681–15686. [Google Scholar] [CrossRef]
- Bertolotti, M.; Bestetti, S.; García-Manteiga, J.M.; Medraño-Fernandez, I.; Dal Mas, A.; Malosio, M.L.; Sitia, R. Tyrosine Kinase signal modulation: A matter of H2O2 membrane permeability? Antioxid. Redox Signal. 2013, 19, 1447–1451. [Google Scholar] [CrossRef] [PubMed]
- Bertolotti, M.; Farinelli, G.; Galli, M.; Aiuti, A.; Sitia, R. AQP8 transports NOX2-generated H2O2 across the plasma membrane to promote signaling in B cells. J. Leukoc. Biol. 2016, 100, 1071–1079. [Google Scholar] [CrossRef] [PubMed]
- Sega, F.V.D.; Prata, C.; Zambonin, L.; Angeloni, C.; Rizzo, B.; Hrelia, S.; Fiorentini, D. Intracellular cysteine oxidation is modulated by aquaporin-8-mediated hydrogen peroxide channeling in leukaemia cells. BioFactors 2017, 43, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Calamita, G.; Ferri, D.; Gena, P.; Liquori, G.E.; Cavalier, A.; Thomas, D.; Svelto, M. The inner mitochondrial membrane has aquaporin-8 water channels and is highly permeable to water. J. Biol. Chem. 2005, 280, 17149–17153. [Google Scholar] [CrossRef] [PubMed]
- Laforenza, U.; Pellavio, G.; Marchetti, A.L.; Omes, C.; Todaro, F.; Gastaldi, G. Aquaporin-mediated water and hydrogen peroxide transport is involved in normal human spermatozoa functioning. Int. J. Mol. Sci. 2017, 18, 66. [Google Scholar] [CrossRef] [PubMed]
- Marchissio, M.J.; Francés, D.E.A.; Carnovale, C.E.; Marinelli, R.A. Mitochondrial aquaporin-8 knockdown in human hepatoma HepG2 cells causes ROS-induced mitochondrial depolarization and loss of viability. Toxicol. Appl. Pharmacol. 2012, 264, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Chauvigné, F.; Boj, M.; Finn, R.N.; Cerdà, J. Mitochondrial aquaporin-8-mediated hydrogen peroxide transport is essential for teleost spermatozoon motility. Sci. Rep. 2015, 5, 7789. [Google Scholar] [CrossRef] [PubMed]
- Siekevitz, P.; Watson, M.L. Some cytochemical characteristics of a phosphorylating digitonin preparation of mitochondria. BBA Biochim. Biophys. Acta 1957, 25, 274–279. [Google Scholar] [CrossRef]
- Edashige, K.; Yamaji, Y.; Kleinhans, F.W.; Kasai, M. Artificial expression of aquaporin-3 improves the survival of mouse oocytes after cryopreservation. Biol. Reprod. 2003, 68, 87–94. [Google Scholar] [CrossRef]
- Tamma, G.; Valenti, G.; Grossini, E.; Donnini, S.; Marino, A.; Marinelli, R.A.; Calamita, G. Aquaporin membrane channels in oxidative stress, cell signaling, and aging: Recent advances and research trends. Oxid. Med. Cell. Longev. 2018, 2018. [Google Scholar] [CrossRef]
- Sega, F.V.D.; Zambonin, L.; Fiorentini, D.; Rizzo, B.; Caliceti, C.; Landi, L.; Hrelia, S.; Prata, C. Specific aquaporins facilitate Nox-produced hydrogen peroxide transport through plasma membrane in leukaemia cells. Biochim. Biophys. Acta Mol. Cell Res. 2014, 1843, 806–814. [Google Scholar] [CrossRef]
- Dajani, S.; Saripalli, A.; Sharma-Walia, N. Water transport proteins–aquaporins (AQPs) in cancer biology. Oncotarget 2018, 9, 36392–36405. [Google Scholar] [CrossRef]
- Mobasheri, A.; Marples, D. Expression of the AQP-1 water channel in normal human tissues: A semiquantitative study using tissue microarray technology. Am. J. Physiol. Cell Physiol. 2004, 286. [Google Scholar] [CrossRef] [PubMed]
- Otterbach, F.; Callies, R.; Adamzik, M.; Kimmig, R.; Siffert, W.; Schmid, K.W.; Bankfalvi, A. Aquaporin 1 (AQP1) expression is a novel characteristic feature of a particularly aggressive subgroup of basal-like breast carcinomas. Breast Cancer Res. Treat. 2010, 120, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Sun, C.C.; Zhou, C.Y.; Huang, H.F. Expression of aquaporin-1 in normal, hyperplasic, and carcinomatous endometria. Int. J. Gynecol. Obstet. 2008, 101, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Yusupov, M.; Razzokov, J.; Cordeiro, R.M.; Bogaerts, A. Transport of Reactive Oxygen and Nitrogen Species across Aquaporin: A Molecular Level Picture. Oxidative Med. Cell. Longev. 2019, 17, 2930504. [Google Scholar] [CrossRef]
- Qu, F.; Wang, F.F.; Lu, X.E.; Dong, M.Y.; Sheng, J.Z.; Lv, P.P.; Ding, G.L.; Shi, B.W.; Zhang, D.; Huang, H.F. Altered aquaporin expression in women with polycystic ovary syndrome: Hyperandrogenism in follicular fluid inhibits aquaporin-9 in granulosa cells through the phosphatidylinositol 3-kinase pathway. Hum. Reprod. 2010, 25, 1441–1450. [Google Scholar] [CrossRef]
- Verkman, A.S. Role of aquaporin water channels in eye function. Exp. Eye Res. 2003, 76, 137–143. [Google Scholar] [CrossRef]
- Goetsch, A.L.; Kimelman, D.; Woodruff, T.K. Polycystic Ovary Syndrome. In Fertility Preservation and Restoration for Patients with Complex Medical Conditions; Springer International Publishing: Cham, Switzerland, 2017; pp. 231–248. [Google Scholar]
- Lai, D.; Wang, F.; Yao, X.; Zhang, Q.; Wu, X.; Xiang, C. Human endometrial mesenchymal stem cells restore ovarian function through improving the renewal of germline stem cells in a mouse model of premature ovarian failure. J. Transl. Med. 2015, 13, 155. [Google Scholar] [CrossRef]
- Wawrzkiewicz-Jałowiecka, A.; Kowalczyk, K.; Pluta, D.; Blukacz, Ł.; Madej, P. The role of aquaporins in polycystic ovary syndrome—A way towards a novel drug target in PCOS. Med. Hypotheses 2017, 102, 23–27. [Google Scholar] [CrossRef]
- Verkman, A.S.; Anderson, M.O.; Papadopoulos, M.C. Aquaporins: Important but elusive drug targets. Nat. Rev. Drug Discov. 2014, 13, 259–277. [Google Scholar] [CrossRef]
- Xiong, Z.; Li, B.; Wang, L.; Zeng, X.; Li, B.; Sha, X.; Liu, H. AQP8 and AQP9 expression in patients with polycystic ovary syndrome and its association with in vitro fertilization-embryo transfer outcomes. Exp. Ther. Med. 2019, 18, 755–760. [Google Scholar] [CrossRef]
- Sales, A.D.; Lobo, C.H.; Carvalho, A.A.; Moura, A.A.; Rodrigues, A.P.R. Structure, function, and localization of aquaporins: Their possible implications on gamete cryopreservation. Genet. Mol. Res. 2013, 12, 6718–6732. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Jee, B.C.; Kim, S.K.; Kim, H.; Lee, J.R.; Suh, C.S.; Kim, S.H. Expressions of aquaporin family in human luteinized granulosa cells and their correlations with IVF outcomes. Hum. Reprod. 2016, 31, 822–831. [Google Scholar] [CrossRef] [PubMed]
- Saadoun, S.; Papadopoulos, M.C.; Hara-Chikuma, M.; Verkman, A.S. Impairment of angiogenesis and cell migration by targeted aquaporin-1 gene disruption. Nature 2005, 434, 786–792. [Google Scholar] [CrossRef] [PubMed]
- Verkman, A.S. Aquaporins in clinical medicine. Annu. Rev. Med. 2012, 63, 303–316. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Verkman, A.S.; Hu, J.; Verkman, A.S. Increased migration and metastatic potential of tumor cells expressing aquaporin water channels. FASEB J. 2006, 20, 1892–1894. [Google Scholar] [CrossRef] [PubMed]
- Mobasheri, A.; Airley, R.; Hewitt, S.M.; Marples, D. Heterogeneous expression of the aquaporin 1 (AQP1) water channel in tumors of the prostate, breast, ovary, colon and lung: A study using high density multiple human tumor tissue microarrays. Int. J. Oncol. 2005, 26, 1149–1158. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Feng, L.; Zhu, Z.; Zheng, M.; Wang, D.; Chen, Z.; Sun, H. Aquaporins as diagnostic and therapeutic targets in cancer: How far we are? J. Transl. Med. 2015, 13, 96. [Google Scholar] [CrossRef]
- Torre, L.A.; Trabert, B.; DeSantis, C.E.; Miller, K.D.; Samimi, G.; Runowicz, C.D.; Gaudet, M.M.; Jemal, A.; Siegel, R.L. Ovarian cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 284–296. [Google Scholar] [CrossRef]
- Yang, J.H.; Yu, Y.Q.; Yan, C. xiao Localisation and expression of aquaporin subtypes in epithelial ovarian tumours. Histol. Histopathol. 2011, 26, 1197–1205. [Google Scholar] [CrossRef]
- Jin, P.Y.; Lu, Y.C.; Li, L.; Han, Q.F. Co action of CFTR and AQP1 increases permeability of peritoneal epithelial cells on estrogen-induced ovarian hyper stimulation syndrome. BMC Cell Biol. 2012, 13, 23. [Google Scholar] [CrossRef]
- Yang, J.; Yan, C.; Zheng, W.; Chen, X. Proliferation inhibition of cisplatin and aquaporin 5 expression in human ovarian cancer cell CAOV3. Arch. Gynecol. Obstet. 2012, 285, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Yang, J.; Shen, L.; Chen, X. Inhibitory effect of Epigallocatechin gallate on ovarian cancer cell proliferation associated with aquaporin 5 expression. Arch. Gynecol. Obstet. 2012, 285, 459–467. [Google Scholar] [CrossRef]
- Frede, J.; Fraser, S.P.; Oskay-Özcelik, G.; Hong, Y.; Braicu, E.I.; Sehouli, J.; Gabra, H.; Djamgoz, M.B.A. Ovarian cancer: Ion channel and aquaporin expression as novel targets of clinical potential. Eur. J. Cancer 2013, 49, 2331–2344. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Miyamoto, M.; Takano, M.; Furuya, K.; Tsuda, H. Different Prognostic Implications of Aquaporin-1 and Aquaporin-5 Expression among Different Histological Types of Ovarian Carcinoma. Pathol. Oncol. Res. 2020, 26, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Yu, H.; Zhang, Y.; Zeng, J.; Cai, C.; Shi, R. Decreased expression of aquaporin 1 correlates with clinicopathological features of patients with cervical cancer. Onco Targets Ther. 2019, 12, 2843–2851. [Google Scholar] [CrossRef]
- Chen, R.; Shi, Y.; Amiduo, R.; Tuokan, T.; Suzuk, L. Expression and Prognostic Value of Aquaporin 1, 3 in Cervical Carcinoma in Women of Uygur Ethnicity from Xinjiang, China. PLoS ONE 2014, 9, e98576. [Google Scholar] [CrossRef]
- Ji, C.; Cao, C.; Lu, S.; Kivlin, R.; Amaral, A.; Kouttab, N.; Yang, H.; Chu, W.; Bi, Z.; Di, W.; et al. Curcumin attenuates EGF-induced AQP3 up-regulation and cell migration in human ovarian cancer cells. Cancer Chemother. Pharmacol. 2008, 62, 857–865. [Google Scholar] [CrossRef]
- Zhang, T.; Zhao, C.; Chen, D.; Zhou, Z. Overexpression of AQP5 in cervical cancer: Correlation with clinicopathological features and prognosis. Med. Oncol. 2012, 29, 1998–2004. [Google Scholar] [CrossRef]
- Shen, Q.; Lin, W.; Luo, H.; Zhao, C.; Cheng, H.; Jiang, W.; Zhu, X. Differential Expression of Aquaporins in Cervical Precursor Lesions and Invasive Cervical Cancer. Reprod. Sci. 2016, 23, 1551–1558. [Google Scholar] [CrossRef]
- Zou, L.B.; Zhang, R.J.; Tan, Y.J.; Ding, G.L.; Shi, S.; Zhang, D.; He, R.H.; Liu, A.X.; Wang, T.T.; Leung, P.C.K.; et al. Identification of estrogen response element in the aquaporin-2 gene that mediates estrogen-induced cell migration and invasion in human endometrial carcinoma. J. Clin. Endocrinol. Metab. 2011, 96. [Google Scholar] [CrossRef]
- Jiang, X.X.; Fei, X.W.; Zhao, L.; Ye, X.L.; Xin, L.B.; Qu, Y.; Xu, K.H.; Wu, R.J.; Lin, J. Aquaporin 5 plays a role in estrogen-induced ectopic implantation of endometrial stromal cells in endometriosis. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.X.; Xu, K.H.; Ma, J.Y.; Tian, Y.H.; Guo, X.Y.; Lin, J.; Wu, R.J. Reduced migration of Ishikawa cells associated with downregulation of aquaporin-5. Oncol. Lett. 2012, 4, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Huebert, R.C.; Vasdev, M.M.; Shergill, U.; Das, A.; Huang, B.Q.; Charlton, M.R.; LaRusso, N.F.; Shah, V.H. Aquaporin-1 facilitates angiogenic invasion in the pathological neovasculature that accompanies cirrhosis. Hepatology 2010, 52, 238–248. [Google Scholar] [CrossRef]
- Kirchebner, P.; Marth, C.; Mayer, I.; Daxenbichler, G. Metabolism of E1 and E2 in Ishikawa endometrium carcinoma cells: Influence of TNFα. J. Steroid Biochem. Mol. Biol. 1991, 39, 221–222. [Google Scholar] [CrossRef]
- Jiang, X.X.; Wu, R.J.; Xu, K.H.; Zhou, C.Y.; Guo, X.Y.; Sun, Y.L.; Lin, J. Immunohistochemical detection of aquaporin expression in eutopic and ectopic endometria from women with endometriomas. Fertil. Steril. 2010, 94, 1229–1234. [Google Scholar] [CrossRef] [PubMed]
- Cassará, M.C.; Menzel, V.A.; Hinsch, K.D.; Wrenzycki, C.; Hinsch, E. Voltage-dependent anion channels 1 and 2 are expressed in porcine oocytes. Biosci. Rep. 2010, 30, 193–200. [Google Scholar] [CrossRef][Green Version]
- Shad, K.F.; Salman, S.; Afridi, S.; Tariq, M.; Asghar, S. Introductory Chapter. Ion Channels. In Ion Channels in Health and Sickness; IntechOpen Limited: London, UK, 2018. [Google Scholar] [CrossRef]
- Ratan, N.M. Types of Ion Channels in the Body. 2018. Available online: https://www.news-medical.net/health/Types-of-Ion-Channels-in-the-Body.aspx (accessed on 26 October 2018).
- Brown, S.G.; Publicover, S.J.; Barratt, C.L.R.; Martins da Silva, S.J. Human sperm ion channel (dys)function: Implications for fertilization. Hum. Reprod. Update 2019, 25, 758–776. [Google Scholar] [CrossRef]
- Singh, A.P.; Rajender, S. CatSper channel, sperm function and male fertility. Reprod. Biomed. Online 2015, 30, 28–38. [Google Scholar] [CrossRef]
- Avidan, N.; Tamary, H.; Dgany, O.; Cattan, D.; Pariente, A.; Thulliez, M.; Borot, N.; Moati, L.; Barthelme, A.; Shalmon, L.; et al. CATSPER2, a human autosomal nonsyndromic male infertility gene. Eur. J. Hum. Genet. 2003, 11, 497–502. [Google Scholar] [CrossRef]
- Lishko, P.V.; Mannowetz, N. CatSper: A unique calcium channel of the sperm flagellum. Curr. Opin. Physiol. 2018, 2, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.-J.; Clapham, D.E.; Garbers, D.L.; Ren, D. CatSper and Two-Pore channels (version 2019.4) in the IUPHAR/BPS Guide to Pharmacology Database. IUPHAR/BPS Guid. Pharmacol. CITE 2019, 2019. [Google Scholar] [CrossRef]
- Ruas, M.; Chuang, K.-T.; Davis, L.C.; Al-Douri, A.; Tynan, P.W.; Tunn, R.; Teboul, L.; Galione, A.; Parrington, J. TPC1 Has Two Variant Isoforms, and Their Removal Has Different Effects on Endo-Lysosomal Functions Compared to Loss of TPC2. Mol. Cell. Biol. 2014, 34, 3981–3992. [Google Scholar] [CrossRef] [PubMed]
- Grøndahl, M.L.; Borup, R.; Lee, Y.B.; Myrhøj, V.; Meinertz, H.; Sørensen, S. Differences in gene expression of granulosa cells from women undergoing controlled ovarian hyperstimulation with either recombinant follicle-stimulating hormone or highly purified human menopausal gonadotropin. Fertil. Steril. 2009, 91, 1820–1830. [Google Scholar] [CrossRef]
- Lee, B.; Yoon, S.Y.; Malcuit, C.; Parys, J.B.; Fissore, R.A. Inositol 1,4,5-trisphosphate receptor 1 degradation in mouse eggs and impact on [Ca2+]i oscillations. J. Cell. Physiol. 2010, 222, 238–247. [Google Scholar] [CrossRef]
- Malcuit, C.; Knott, J.G.; He, C.; Wainwright, T.; Parys, J.B.; Robl, J.M.; Fissore, R.A. Fertilization and Inositol 1,4,5-Trisphosphate (IP3)-Induced Calcium Release in Type-1 Inositol 1,4,5-Trisphosphate Receptor Down-Regulated Bovine Eggs1. Biol. Reprod. 2005, 73, 2–13. [Google Scholar] [CrossRef]
- Quinton, P.M. Cystic fibrosis: A disease in electrolyte transport. FASEB J. 1990, 4, 2709–2710. [Google Scholar] [CrossRef]
- Quinton, P.M. Physiological basis of cystic fibrosis: A historical perspective. Physiol. Rev. 1999, 79. [Google Scholar] [CrossRef]
- Rowe, S.M.; Miller, S.; Sorscher, E.J. Cystic fibrosis. N. Engl. J. Med. 2005, 352, 1992–2001. [Google Scholar] [CrossRef]
- Chan, H.C.; Ruan, Y.C.; He, Q.; Chen, M.H.; Chen, H.; Xu, W.M.; Chen, W.Y.; Xie, C.; Zhang, X.H.; Zhou, Z. The cystic fibrosis transmembrane conductance regulator in reproductive health and disease. J. Physiol. 2009, 587, 2187–2195. [Google Scholar] [CrossRef]
- Chan, L.N.; Tsang, L.L.; Rowlands, D.K.; Rochelle, L.G.; Boucher, R.C.; Liu, C.Q.; Chan, H.C. Distribution and regulation of ENaC subunit and CFTR mRNA expression in murine female reproductive tract. J. Membr. Biol. 2002, 185, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Decker, W.K.; Craigen, W.J. The tissue-specific, alternatively spliced single ATG exon of the type 3 voltage-dependent anion channel gene does not create a truncated protein isoform in vivo. Mol. Genet. Metab. 2000, 70, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Decker, W.K.; Bowles, K.R.; Schatte, E.C.; Towbin, J.A.; Craigen, W.J. Revised fine mapping of the human voltage-dependent anion channel loci by radiation hybrid analysis. Mamm. Genome 1999, 10, 1041–1042. [Google Scholar] [CrossRef] [PubMed]
- Sampson, M.J.; Lovell, R.S.; Craigen, W.J. The murine voltage-dependent anion channel gene family. Conserved structure and function. J. Biol. Chem. 1997, 272, 18966–18973. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Wang, Z.; Zhang, W.; Wang, X. Expression and localization of voltage-dependent anion channels (VDAC) in human spermatozoa. Biochem. Biophys. Res. Commun. 2009, 378, 366–370. [Google Scholar] [CrossRef]
- Messina, A.; Reina, S.; Guarino, F.; De Pinto, V. VDAC isoforms in mammals. Biochim. Biophys. Acta Biomembr. 2012, 1818, 1466–1476. [Google Scholar] [CrossRef]
- Shoshan-Barmatz, V.; Krelin, Y.; Shteinfer-Kuzmine, A.; Arif, T. Voltage-dependent anion channel 1 as an emerging drug target for novel anti-cancer therapeutics. Front. Oncol. 2017, 7. [Google Scholar] [CrossRef]
- Kwon, W.S.; Park, Y.J.; Mohamed, E.S.A.; Pang, M.G. Voltage-dependent anion channels are a key factor of male fertility. Fertil. Steril. 2013, 99, 354–361. [Google Scholar] [CrossRef]
- Hinsch, K.D.; De Pinto, V.; Aires, V.A.; Schneider, X.; Messina, A.; Hinsch, E. Voltage-dependent Anion-selective Channels VDAC2 and VDAC3 Are Abundant Proteins in Bovine Outer Dense Fibers, a Cytoskeletal Component of the Sperm Flagellum. J. Biol. Chem. 2004, 279, 15281–15288. [Google Scholar] [CrossRef]
- Triphan, X.; Menzel, V.A.; Petrunkina, A.M.; Cassará, M.C.; Wemheuer, W.; Hinsch, K.D.; Hinsch, E. Localisation and function of voltage-dependent anion channels (VDAC) in bovine spermatozoa. Pflug. Arch. Eur. J. Physiol. 2008, 455, 677–686. [Google Scholar] [CrossRef]
- Gandini, L.; Lenzi, A.; Lombarde, F.; Pacifici, R.; Dondero, F. Immature germ cell separation using a modified discontinuous Percoll gradient technique in human semen. Hum. Reprod. 1999, 14, 1022–1027. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Larsen, W.J. Biological implications of gap junction structure, distribution and composition: A review. Tissue Cell 1983, 15, 645–671. [Google Scholar] [CrossRef]
- Munari-Silem, Y.; Rousset, B. Gap junction-mediated cell-to-cell communication in endocrine glands Molecular and functional aspects: A review. Eur. J. Endocrinol. 1996, 135, 251–264. [Google Scholar] [CrossRef]
- Sánchez, A.; Castro, C.; Flores, D.L.; Gutiérrez, E.; Baldi, P. Gap junction channels of innexins and connexins: Relations and computational perspectives. Int. J. Mol. Sci. 2019, 20, 2476. [Google Scholar] [CrossRef] [PubMed]
- Assef, Y.A.; Damiano, A.E.; Zotta, E.; Ibarra, C.; Kotsias, B.A. CFTR in K562 human leukemic cells. Am. J. Physiol. Cell Physiol. 2003, 285. [Google Scholar] [CrossRef] [PubMed]
- Abraham, E.H.; Sterling, K.M.; Kim, R.J.; Salikhova, A.Y.; Huffman, H.B.; Crockett, M.A.; Johnston, N.; Parker, H.W.; Boyle, W.E.; Hartov, A.; et al. Erythrocyte membrane ATP binding cassette (ABC) proteins: MRP1 and CFTR as well as CD39 (ecto-apyrase) involved in RBC ATP transport and elevated blood plasma ATP of cystic fibrosis. Blood Cells Mol. Dis. 2001, 27, 165–180. [Google Scholar] [CrossRef]
- Kamal, D.A.M.; Ibrahim, S.F.; Mokhtar, M.H. Androgen effect on connexin expression in the mammalian female reproductive system: A systematic review. Bosn. J. Basic Med. Sci. 2020, 20, 293–302. [Google Scholar] [CrossRef]
- Winterhager, E.; Kidder, G.M. Gap junction connexins in female reproductive organs: Implications for women’s reproductive health. Hum. Reprod. Update 2015, 21, 340–352. [Google Scholar] [CrossRef]
- Kempisty, B.; Ziółkowska, A.; Piotrowska, H.; Ciesiółka, S.; Antosik, P.; Bukowska, D.; Zawierucha, P.; Woźna, M.; Jaśkowski, J.M.; Brüssow, K.P.; et al. Short-term cultivation of porcine cumulus cells influences the cyclin-dependent kinase 4 (Cdk4) and connexin 43 (Cx43) protein expression—A real-time cell proliferation approach. J. Reprod. Dev. 2013, 59, 339–345. [Google Scholar] [CrossRef]
- Zabner, J.; Couture, L.A.; Smith, A.E.; Welsh, M.J. Correction of cAMP-stimulated fluid secretion in cystic fibrosis airway epithelia: Efficiency of adenovirus-mediated gene transfer in vitro. Hum. Gene Ther. 1994, 5, 585–593. [Google Scholar] [CrossRef]
- Johnson, L.G.; Olsen, J.C.; Sarkadi, B.; Moore, K.L.; Swanstrom, R.; Boucher, R.C. Efficiency of gene transfer for restoration of normal airway epithelial function in cystic fibrosis. Nat. Genet. 1992, 2, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Wong, P.Y.D. CFTR gene and male fertility. Mol. Hum. Reprod. 1998, 4, 107–110. [Google Scholar] [CrossRef] [PubMed]
- Kotsias, B.A.; Salim, M.; Peracchia, L.L.; Peracchia, C. Interplay between cystic fibrosis transmembrane regulator and gap junction channels made of connexins 45, 40, 32 and 50 expressed in oocytes. J. Membr. Biol. 2006, 214, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghoul, K.J.; Kirk, T.; Kuszak, A.J.; Zoltoski, R.K.; Shiels, A.; Kuszak, J.R. Lens structure in MIP-deficient mice. Anat. Rec. Part A Discov. Mol. Cell. Evol. Biol. 2003, 273, 714–730. [Google Scholar] [CrossRef]
- Yu, X.S.; Yin, X.; Lafer, E.M.; Jiang, J.X. Developmental regulation of the direct interaction between the intracellular loop of connexin 45.6 and the C terminus of Major intrinsic protein (aquaporin-0). J. Biol. Chem. 2005, 280, 22081–22090. [Google Scholar] [CrossRef]
- Yu, X.S.; Jiang, J.X. Interaction of major intrinsic protein (aquaporin-0) with fiber connexins in lens development. J. Cell Sci. 2004, 117, 871–880. [Google Scholar] [CrossRef]
- Rash, J.E.; Yasumura, T. Direct immunogold labeling of connexins and aquaporin-4 in freeze-fracture replicas of liver, brain, and spinal cord: Factors limiting quantitative analysis. Cell Tissue Res. 1999, 296, 307–321. [Google Scholar] [CrossRef]
- Nicchia, G.P.; Srinivas, M.; Li, W.; Brosnan, C.F.; Frigeri, A.; Spray, D.C. New possible roles for aquaporin-4 in astrocytes: Cell cytoskeleton and functional relationship with connexin43. FASEB J. 2005, 19, 1674–1676. [Google Scholar] [CrossRef]
- Wicki-Stordeur, L.E.; Swayne, L.A. Large pore ion and metabolite-permeable channel regulation of postnatal ventricular zone neural stem and progenitor cells: Interplay between aquaporins, connexins, and pannexins? Stem Cells Int. 2012, 2012. [Google Scholar] [CrossRef]
- Corsini, N.S.; Knoblich, J.A. Tracing Stem Cell Division in Adult Neurogenesis. Cell Stem Cell 2018, 22, 143–145. [Google Scholar] [CrossRef]
- Katoozi, S.; Skauli, N.; Zahl, S.; Deshpande, T.; Ezan, P.; Palazzo, C.; Steinhäuser, C.; Frigeri, A.; Cohen-Salmon, M.; Ottersen, O.P.; et al. Uncoupling of the Astrocyte Syncytium Differentially Affects AQP4 Isoforms. Cells 2020, 9, 382. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Liu, X.; Liu, Z.; Su, Z. Interactions of connexin 43 and aquaporin-4 in the formation of glioma-induced brain edema. Mol. Med. Rep. 2015, 11, 1188–1194. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Huang, J.; Ding, F.; Mei, J.; Zhu, J.; Liu, H.; Sun, K. Aquaporin 1 plays an important role in myocardial edema caused by cardiopulmonary bypass surgery in goat. Int. J. Mol. Med. 2013, 31, 637–643. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kurtenbach, S.; Kurtenbach, S.; Zoidl, G. Gap junction modulation and its implications for heart function. Front. Physiol. 2014, 82. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.M.; Gilula, N.B. The gap junction communication channel. Cell 1996, 84, 381–388. [Google Scholar] [CrossRef]
- Ciesiółka, S.; Budna, J.; Jopek, K.; Bryja, A.; Kranc, W.; Borys, S.; Jeseta, M.; Chachuła, A.; Ziółkowska, A.; Antosik, P.; et al. Time- and Dose-Dependent Effects of 17 Beta-Estradiol on Short-Term, Real-Time Proliferation and Gene Expression in Porcine Granulosa Cells. BioMed Res. Int. 2017, 2017, 1–9. [Google Scholar] [CrossRef]
- Kempisty, B.; Ziółkowska, A.; Piotrowska, H.; Antosik, P.; Bukowska, D.; Zawierucha, P.; Jaśkowski, J.; Brüssow, K.-P.; Nowicki, M.; Zabel, M. Expression and cellular distribution of cyclin-dependent kinase 4 (Cdk4) and connexin 43 (Cx43) in porcine oocytes before and after in vitro maturation. Acta Vet. Hung. 2014, 62, 84–95. [Google Scholar] [CrossRef][Green Version]
- Kibschull, M.; Gellhaus, A.; Carette, D.; Segretain, D.; Pointis, G.; Gilleron, J. Physiological roles of connexins and pannexins in reproductive organs. Cell. Mol. Life Sci. 2015, 72, 2879–2898. [Google Scholar] [CrossRef]
- Sosinsky, G.E.; Boassa, D.; Dermietzel, R.; Duffy, H.S.; Laird, D.W.; MacVicar, B.A.; Naus, C.C.; Penuela, S.; Scemes, E.; Spray, D.C.; et al. Pannexin channels are not gap junction hemichannels. Channels 2011, 5, 193–197. [Google Scholar] [CrossRef]
- Dahl, G. The Pannexin1 membrane channel: Distinct conformations and functions. FEBS Lett. 2018, 592, 3201–3209. [Google Scholar] [CrossRef]
- Barbe, M.T.; Monyer, H.; Bruzzone, R. Cell-cell communication beyond connexins: The pannexin channels. Physiology 2006, 21, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Dye, Z.T.; Rutledge, L.V.; Penuela, S.; Dyce, P.W. Pannexin 1 inhibition delays maturation and improves development of Bos taurus oocytes. J. Ovarian Res. 2020, 13, 1–11. [Google Scholar] [CrossRef]
- Sang, Q.; Zhang, Z.; Shi, J.; Sun, X.; Li, B.; Yan, Z.; Xue, S.; Ai, A.; Lyu, Q.; Li, W.; et al. A pannexin 1 channelopathy causes human oocyte death. Sci. Transl. Med. 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Wojtanowicz-Markiewicz, K.; Kulus, M.; Knap, S.; Kocherova, I.; Jankowski, M.; Stefańska, K.; Jeseta, M.; Piotrowska-Kempisty, H.; Bukowska, D.; Zabel, M.; et al. Expression of Selected Connexin and Aquaporin Genes and Real-Time Proliferation of Porcine Endometrial Luminal Epithelial Cells in Primary Culture Model. BioMed Res. Int. 2020, 2020. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Zhang, Y.; Sheng, Y.; Fu, X.; Cheng, H.; Zhou, R. MYBL2 guides autophagy suppressor VDAC2 in the developing ovary to inhibit autophagy through a complex of VDAC2-BECN1-BCL2L1 in mammals. Autophagy 2015, 11, 1081–1098. [Google Scholar] [CrossRef] [PubMed]
- Sampson, M.J.; Decker, W.K.; Beaudet, A.L.; Ruitenbeek, W.; Armstrong, D.; Hicks, M.J.; Craigen, W.J. Immotile Sperm and Infertility in Mice Lacking Mitochondrial Voltage-dependent Anion Channel Type 3. J. Biol. Chem. 2001, 276, 39206–39212. [Google Scholar] [CrossRef] [PubMed]
- Li, H.G.; Ding, X.F.; Liao, A.H.; Kong, X.B.; Xiong, C.L. Expression of CatSper family transcripts in the mouse testis during post-natal development and human ejaculated spermatozoa: Relationship to sperm motility. Mol. Hum. Reprod. 2007, 13, 299–306. [Google Scholar] [CrossRef]
- Jin, J.; Jin, N.; Zheng, H.; Ro, S.; Tafolla, D.; Sanders, K.M.; Yan, W. Catsper3 and Catsper4 are essential for sperm hyperactivated motility and male fertility in the mouse. Biol. Reprod. 2007, 77, 37–44. [Google Scholar] [CrossRef]
- Smyth, J.T.; Abbott, A.L.; Lee, B.; Sienaert, I.; Kasri, N.N.; De Smedt, H.; Ducibella, T.; Missiaen, L.; Parys, J.B.; Fissore, R.A. Inhibition of the inositol trisphosphate receptor of mouse eggs and A7r5 cells by KN-93 via a mechanism unrelated to Ca2+/calmodulin-dependent protein kinase II antagonism. J. Biol. Chem. 2002, 277, 35061–35070. [Google Scholar] [CrossRef]
- Carlin, R.W.; Sedlacek, R.L.; Quesnell, R.R.; Pierucci-Alves, F.; Grieger, D.M.; Schultz, B.D. PVD9902, a porcine vas deferens epithelial cell line that exhibits neurotransmitter-stimulated anion secretion and expresses numerous HCO 3- transporters. Am. J. Physiol. Cell Physiol. 2006, 290. [Google Scholar] [CrossRef]
- Tizzano, E.F.; Silver, M.M.; Chitayat, D.; Benichou, J.C.; Buchwald, M. Differential cellular expression of cystic fibrosis transmembrane regulator in human reproductive tissues: Clues for the infertility in patients with cystic fibrosis. Am. J. Pathol. 1994, 144, 906–914. [Google Scholar] [PubMed]
- Chan, H.C.; He, Q.; Ajonuma, L.C.; Wang, X.F. Epithelial ion channels in the regulation of female reproductive tract fluid microenvironment: Implications in fertility and infertility. Sheng Li Xue Bao 2007, 59, 495–504. [Google Scholar] [PubMed]
- Chan, H.C.; Shi, Q.X.; Zhou, C.X.; Wang, X.F.; Xu, W.M.; Chen, W.Y.; Chen, A.J.; Ni, Y.; Yuan, Y.Y. Critical role of CFTR in uterine bicarbonate secretion and the fertilizing capacity of sperm. Mol. Cell. Endocrinol. 2006, 250, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.F.; Zhou, C.X.; Shi, Q.X.; Yuan, Y.Y.; Yu, M.K.; Ajonuma, L.C.; Ho, L.S.; Lo, P.S.; Tsang, L.L.; Liu, Y.; et al. Involvement of CFTR in uterine bicarbonate secretion and the fertilizing capacity of sperm. Nat. Cell Biol. 2003, 5, 902–906. [Google Scholar] [CrossRef]
- Beyer, E.C.; Berthoud, V.M. Gap junction gene and protein families: Connexins, innexins, and pannexins. Biochim. Biophys. Acta Biomembr. 2018, 1860, 5–8. [Google Scholar] [CrossRef]
- Borowczyk, E.; Johnson, M.L.; Bilski, J.J.; Borowicz, P.; Redmer, D.A.; Reynolds, L.P.; Grazul-Bilska, A.T. Gap junctional connexin 37 is expressed in sheep ovaries. Endocrine 2006, 30, 223–230. [Google Scholar] [CrossRef]
- Gershon, E.; Plaks, V.; Dekel, N. Gap junctions in the ovary: Expression, localization and function. Mol. Cell. Endocrinol. 2008, 282, 18–25. [Google Scholar] [CrossRef]
- Nuttinck, F.; Peynot, N.; Humblot, P.; Massip, A.; Dessy, F.; Fléchon, J.E. Comparative immunohistochemical distribution of Connexin 37 and Connexin 43 throughout folliculogenesis in the bovine ovary. Mol. Reprod. Dev. 2000, 57, 60–66. [Google Scholar] [CrossRef]
- Pandey, A.; Gupta, N.; Gupta, S.C. Improvement of in vitro oocyte maturation with lectin supplementation and expression analysis of Cx43, GDF-9, FGF-4 and Fibronectin mRNA transcripts in Buffalo (Bubalus bubalis). J. Assist. Reprod. Genet. 2009, 26, 365–371. [Google Scholar] [CrossRef]
- Grummer, R.; Chwalisz, K.; Mulholland, J.; Traub, O.; Winterhager, E. Regulation of connexin26 and connexin43 expression in rat endometrium by ovarian steroid hormones. Biol. Reprod. 1994, 51, 1109–1116. [Google Scholar] [CrossRef]
- Ackert, C.L.; Gittens, J.E.I.; O’Brien, M.J.; Eppig, J.J.; Kidder, G.M. Intercellular communication via connexin43 gap junctions is required for ovarian folliculogenesis in the mouse. Dev. Biol. 2001, 233, 258–270. [Google Scholar] [CrossRef] [PubMed]
- Gittens, J.E.I.; Kidder, G.M. Differential contributions of connexin37 and connexin43 to oogenesis revealed in chimeric reaggregated mouse ovaries. J. Cell Sci. 2005, 118, 5071–5078. [Google Scholar] [CrossRef] [PubMed]
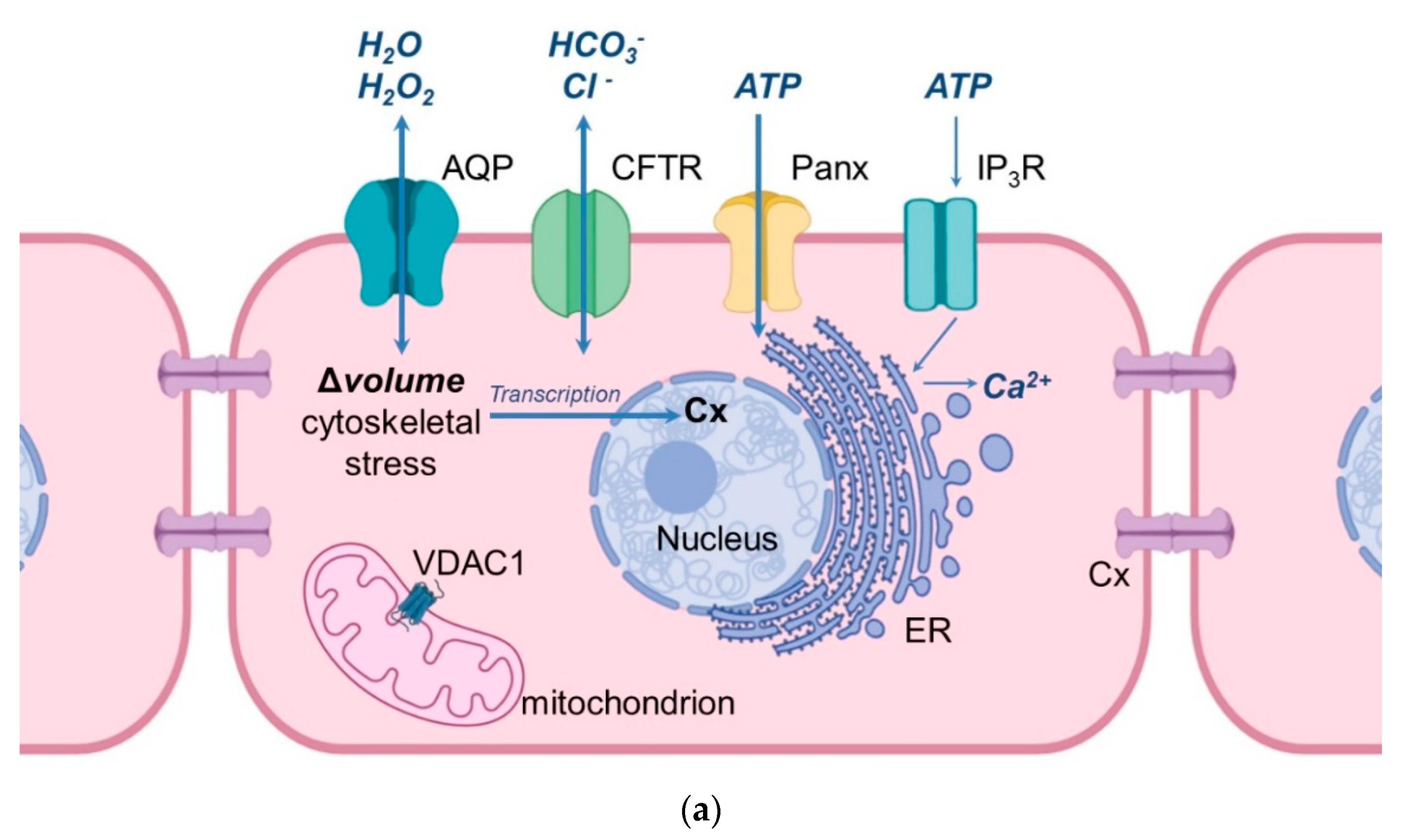

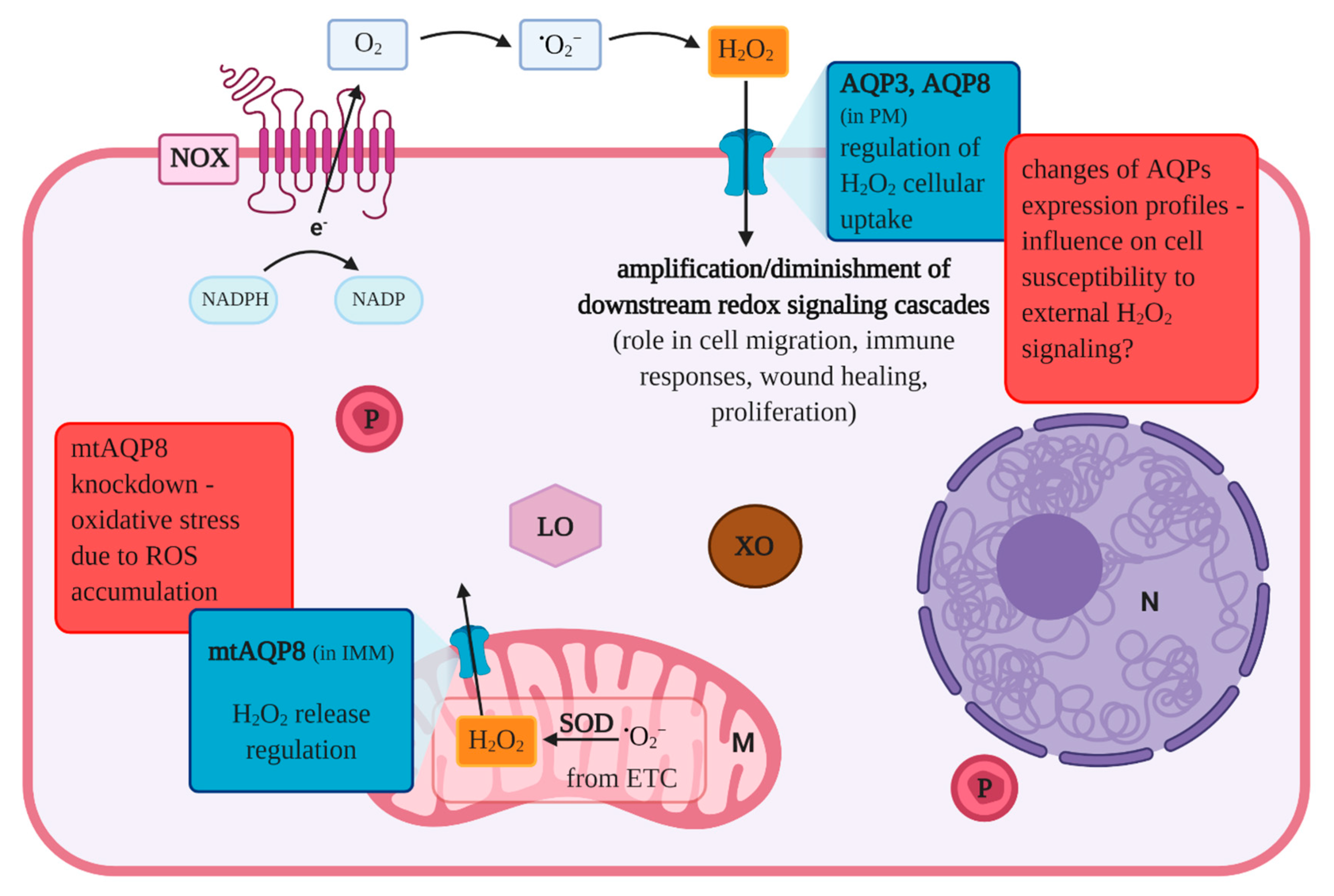
| AQP | Vagina | Cervix/Cervical Carcinoma | Uterus | Oviduct | Ovary | Follicle/Oocyte | Embryo/Amnion/Chorion |
|---|---|---|---|---|---|---|---|
| AQP1 | Human [21] Rodent [22,23] | Human [24] | Human [25] Rodent [19] Porcine [26,27,28,29] Canine [30] | Human [31] Rodent [22] Porcine [26,27,28,29] | Human [32,33,34] Porcine [26,27,28,29] | Porcine [26,27,28,29] | Human [35,36,37,38,39] Rodent [40,41] Ovine [42] |
| AQP2 | Human [21] Rodent [43] | Human [25,44,45,46] Canine [30] | Human [47] | Human [34] | Human [39] | ||
| AQP3 | Human [21] | Human [24] | Human [47] | Human [34] | Human [35,36,37,38,39] | ||
| Rodent [43,48,49] | Rodent [50,51] | Rodent [52] | Rodent [40,41,53,54] | ||||
| Ovine [42] | |||||||
| AQP4 | Rodent [49] | Rodent [50] | Rodent [19] | Human [34] | Human [35,36,37,38,39] | ||
| AQP5 | Human [21] | Rodent [19,55] | Rodent [56] | Porcine [26,27,28,29] | Human [35,39] Rodent [41] | ||
| Rodent [48] | Porcine [26,27,28,29] | Porcine [26,27,28,29] | |||||
| Canine [30] | |||||||
| AQP6 | Human [21] | Rodent [41] | |||||
| Rodent [48] | |||||||
| AQP7 | Rodent [55] | Rodent [57,58,59] | Human [39] Rodent [41,54] | ||||
| AQP8 | Humans [24] | Rodents [19,55] | Rodent [56] | Rodent [57,58,59] | Human [35,36,60,61,62] | ||
| Rodent [50] | Rodent [40,41,52,63,64] | ||||||
| Ovine [42] | |||||||
| AQP9 | Rodent [19,55] | Human [31] | Porcine [26,27,28,29] | Rodent [57] | Human [35,36,65,66] | ||
| Porcine [26,27,28,29] | Ovine [67] | ||||||
| AQP10 | Rodent [48] | ||||||
| AQP11 | Rodent [48] | Human [35,39] | |||||
| Rodent [54] | |||||||
| AQP12 | Rodent [48] | Rodent [58] | Human [39] |
| Type of Ion Channel | Type of Protein | Location | Function |
|---|---|---|---|
| Porins | VDAC1, 2, 3 | Sertoli cells [162]; GV (germinal vesicle) and MII (meiosis II) stage porcine oocytes [137] outer dense fibers of the bovine sperm flagellum; head of bovine sperm, late spermatocytes, spermatids and spermatozoa of the bovine testis [161]; GV (germinal vesicle) and MII (meiosis II) stage porcine oocytes [137]; mouse granulosa cells [197] outer dense fibers of the bovine sperm flagellum in porcine [161]; | participation in follicular development and autophagy suppression to folliculogenesis in mammals [197]; deficient males are infertile because of structural abnormalities in the sperm tail, leading to sperm immotility [198] |
| Cation channels sperm associated | CATSPER1, 2, 3, 4 | Plasma membrane of the sperm tail [144] testis [199] | key role in the motility, hyperactivation and fertilization function of sperm [141,200] |
| Inositol trisphosphate receptor | InsP3R1, 2,3 | human GCS [146] mouse oocyte [201] | proper fertilization [148] |
| CFTR | rat epididymal epithelial cells [174]; porcine vas deferens epithelial cells [202]; vagina, cervix, uterus and fallopian tubes, in rodents and humans [153,203,204]; mouse endometrial cells [205] | CFTR plays a key role in regulating Cl− secretion, and thus fluid volume in male and female reproductive tract [152,202]; sperm capacitation [206] | |
| Gap junction protein | Cxs | mouse, human, rat, pig, dog seminiferous tubules [190]; mouse, human, swine, bovine, canine ovary [207,208,209,210]; oocyte and granulosa cells (GCs) [171,211]; human, mouse and baboon endometrium [170,212] | function as nurturing the germ cell lineage; developmental competence by oocyte, communication with cumulus oophorus cells; connection between GCs population, mural—mural GCs communication; folliculogenesis [213,214] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kordowitzki, P.; Kranc, W.; Bryl, R.; Kempisty, B.; Skowronska, A.; Skowronski, M.T. The Relevance of Aquaporins for the Physiology, Pathology, and Aging of the Female Reproductive System in Mammals. Cells 2020, 9, 2570. https://doi.org/10.3390/cells9122570
Kordowitzki P, Kranc W, Bryl R, Kempisty B, Skowronska A, Skowronski MT. The Relevance of Aquaporins for the Physiology, Pathology, and Aging of the Female Reproductive System in Mammals. Cells. 2020; 9(12):2570. https://doi.org/10.3390/cells9122570
Chicago/Turabian StyleKordowitzki, Paweł, Wiesława Kranc, Rut Bryl, Bartosz Kempisty, Agnieszka Skowronska, and Mariusz T. Skowronski. 2020. "The Relevance of Aquaporins for the Physiology, Pathology, and Aging of the Female Reproductive System in Mammals" Cells 9, no. 12: 2570. https://doi.org/10.3390/cells9122570
APA StyleKordowitzki, P., Kranc, W., Bryl, R., Kempisty, B., Skowronska, A., & Skowronski, M. T. (2020). The Relevance of Aquaporins for the Physiology, Pathology, and Aging of the Female Reproductive System in Mammals. Cells, 9(12), 2570. https://doi.org/10.3390/cells9122570







