Exosomes and Extracellular Vesicles as Emerging Theranostic Platforms in Cancer Research
Abstract
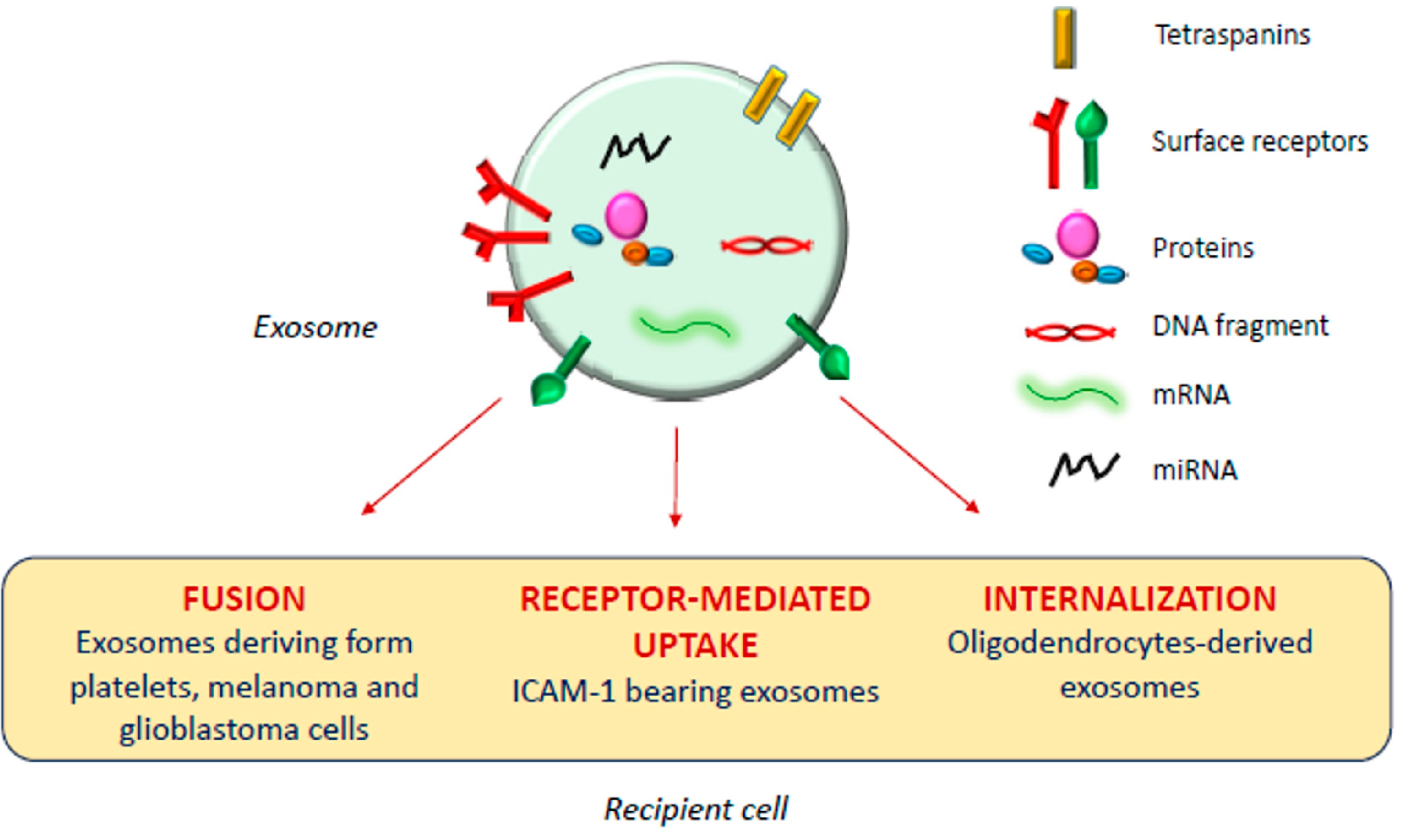
1. Introduction
1.1. Classification and Characteristics
1.2. Biological Function
1.3. Applications in Therapy
1.4. Applications in Diagnosis
2. Nanoparticle-Loaded Exosomes in Oncology
2.1. Superparamagnetic Iron Oxide and Ultrasmall Superparamagnetic Iron Oxide Nanoparticles
2.2. Quantum Dots
2.3. Gold Nanoparticles
2.4. Polymeric Nanoparticles
3. Transition Metal-Labeled Exosomes
4. Other Systems as Potential Cancer Diagnostic Tools
4.1. Bioluminiscent Agent-Loaded Evs
4.2. Nanocluster-Loaded Exosomes
4.3. Metabolic Labeled Exosomes
5. Exosomes Beyond Oncology
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Colombo, M.; Raposo, G.; Thery, C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef] [PubMed]
- Cocucci, E.; Racchetti, G.; Meldolesi, J. Shedding microvesicles: Artefacts no more. Trends Cell Biol. 2009, 19, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Hristov, M.; Erl, W.; Linder, S.; Weber, P.C. Apoptotic bodies from endothelial cells enhance the number and initiate the differentiation of human endothelial progenitor cells in vitro. Blood 2004, 104, 2761–2766. [Google Scholar] [CrossRef] [PubMed]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef]
- Vlassov, A.V.; Magdaleno, S.; Setterquist, R.; Conrad, R. Exosomes: Current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim. Biophys. Acta Gen. Subj. 2012, 1820, 940–948. [Google Scholar] [CrossRef]
- Denzer, K.; Kleijmeer, M.J.; Heijnen, H.F.G.; Stoorvogel, W.; Geuze, H.J. Exosome: From internal vesicle of the multivesicular body to intercellular signaling device. J. Cell Sci. 2000, 113, 3365–3374. [Google Scholar]
- Johnstone, R.M.; Adam, M.; Hammond, J.R.; Orr, L.; Turbide, C. Vesicle formation during reticulocyte maturation—Association of plasma-membrane activities with released vesicles (exosomes). J. Biol. Chem. 1987, 262, 9412–9420. [Google Scholar]
- Raposo, G.; Nijman, H.W.; Stoorvogel, W.; Leijendekker, R.; Harding, C.V.; Melief, C.J.M.; Geuze, H.J. B lymphocytes secrete antigen-presenting vesicles. J. Exp. Med. 1996, 183, 1161–1172. [Google Scholar] [CrossRef]
- Thery, C.; Regnault, A.; Garin, J.; Wolfers, J.; Zitvogel, L.; Ricciardi-Castagnoli, P.; Raposo, G.; Amigorena, S. Molecular characterization of dendritic cell-derived exosomes: Selective accumulation of the heat shock protein hsc73. J. Cell Biol. 1999, 147, 599–610. [Google Scholar] [CrossRef]
- Simpson, R.J.; Jensen, S.S.; Lim, J.W.E. Proteomic profiling of exosomes: Current perspectives. Proteomics 2008, 8, 4083–4099. [Google Scholar] [CrossRef]
- Hugel, B.; Carmen, M.; Martinez, M.C.; Kunzelmann, C.; Freyssinet, J.M. Membrane microparticles: Two sides of the coin. Physiology 2005, 20, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Thery, C.; Zitvogel, L.; Amigorena, S. Exosomes: Composition, biogenesis and function. Nat. Rev. Immunol. 2002, 2, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Pontecorvi, G.; Bellenghi, M.; Puglisi, R.; Care, A.; Mattia, G. Tumor-derived extracellular vesicles and microRNAs: Functional roles, diagnostic, prognostic and therapeutic options. Cytokine Growth Factor Rev. 2020, 51, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Bang, C.; Thum, T. Exosomes: New players in cell-cell communication. Int. J. Biochem. Cell Biol. 2012, 44, 2060–2064. [Google Scholar] [CrossRef] [PubMed]
- Bobrie, A.; Colombo, M.; Raposo, G.; Thery, C. Exosome secretion: Molecular mechanisms and roles in immune responses. Traffic 2011, 12, 1659–1668. [Google Scholar] [CrossRef] [PubMed]
- Chaput, N.; Thery, C. Exosomes: Immune properties and potential clinical implementations. Semin. Immunopathol. 2011, 33, 419–440. [Google Scholar] [CrossRef] [PubMed]
- Meldolesi, J. Exosomes and ectosomes in intercellular communication. Curr. Biol. 2018, 28, R435–R444. [Google Scholar] [CrossRef] [PubMed]
- Bebelman, M.P.; Bun, P.; Huveneers, S.; van Niel, G.; Pegtel, D.M.; Verweij, F.J. Real-time imaging of multivesicular body-plasma membrane fusion to quantify exosome release from single cells. Nat. Protoc. 2020, 15, 102–121. [Google Scholar] [CrossRef]
- Parolini, I.; Federici, C.; Raggi, C.; Lugini, L.; Palleschi, S.; De Milito, A.; Coscia, C.; Iessi, E.; Logozzi, M.; Molinari, A.; et al. Microenvironmental pH is a key factor for exosome traffic in tumor cells. J. Biol. Chem. 2009, 284, 34211–34222. [Google Scholar] [CrossRef]
- Fleming, A.; Sampey, G.; Chung, M.C.; Bailey, C.; van Hoek, M.L.; Kashanchi, F.; Hakami, R.M. The carrying pigeons of the cell: Exosomes and their role in infectious diseases caused by human pathogens. Pathog. Dis. 2014, 71, 107–118. [Google Scholar] [CrossRef]
- Kimura, K.; Hohjoh, H.; Fukuoka, M.; Sato, W.; Oki, S.; Tomi, C.; Yamaguchi, H.; Kondo, T.; Takahashi, R.; Yamamura, T. Circulating exosomes suppress the induction of regulatory T cells via let-7i in multiple sclerosis. Nat. Commun. 2018, 9. [Google Scholar] [CrossRef]
- Gon, Y.; Shimizu, T.; Mizumura, K.; Maruoka, S.; Hikichi, M. Molecular techniques for respiratory diseases: MicroRNA and extracellular vesicles. Respirology 2020, 25, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, R.; Kanda, T.; Yokosuka, O.; Kato, N.; Matsuoka, S.; Moriyama, M. Exosomes and hepatocellular carcinoma: From bench to bedside. Int. J. Mol. Sci. 2019, 20, 1406. [Google Scholar] [CrossRef] [PubMed]
- Abak, A.; Abhari, A.; Rahimzadeh, S. Exosomes in cancer: Small vesicular transporters for cancer progression and metastasis, biomarkers in cancer therapeutics. PEERJ 2018, 6, e4763. [Google Scholar] [CrossRef] [PubMed]
- Al-Nedawi, K.; Meehan, B.; Micallef, J.; Lhotak, V.; May, L.; Guha, A.; Rak, J. Intercellular transfer of the oncogenic receptor EGFrvIII by microvesicles derived from tumour cells. Nat. Cell Biol. 2008, 10, 619–624. [Google Scholar] [CrossRef]
- Ristorcelli, E.; Beraud, E.; Verrando, P.; Villard, C.; Lafitte, D.; Sbarra, V.; Lombardo, D.; Verine, A. Human tumor nanoparticles induce apoptosis of pancreatic cancer cells. FASEB J. 2008, 22, 3358–3369. [Google Scholar] [CrossRef]
- Zitvogel, L.; Regnault, A.; Lozier, A.; Wolfers, J.; Flament, C.; Tenza, D.; Ricciardi-Castagnoli, P.; Raposo, G.; Amigorena, S. Eradication of established murine tumors using a novel cell-free vaccine: Dendritic cell-derived exosomes. Nat. Med. 1998, 4, 594–600. [Google Scholar] [CrossRef]
- Umezu, T.; Ohyashiki, K.; Kuroda, M.; Ohyashiki, J.H. Leukemia cell to endothelial cell communication via exosomal miRNAs. Oncogene 2013, 32, 2747–2755. [Google Scholar] [CrossRef]
- Gajos-Michniewicz, A.; Duechler, M.; Czyz, M. MiRNA in melanoma-derived exosomes. Cancer Lett. 2014, 347, 29–37. [Google Scholar] [CrossRef]
- Gerloff, D.; Lutzkendorf, J.; Moritz, R.K.C.; Wersig, T.; Mader, K.; Muller, L.P.; Sunderkotter, C. Melanoma-derived exosomal miR-125b-5p educates tumor associated macrophages (TAMs) by targeting lysosomal acid lipase A (LIPA). Cancers 2020, 12, 464. [Google Scholar] [CrossRef]
- Kurahashi, R.; Kadomatsu, T.; Baba, M.; Hara, C.; Itoh, H.; Miyata, K.; Endo, M.; Morinaga, J.; Terada, K.; Araki, K.; et al. MicroRNA-204-5p: A novel candidate urinary biomarker of Xp11.2 translocation renal cell carcinoma. Cancer Sci. 2019, 110, 1897–1908. [Google Scholar] [CrossRef]
- Chalmin, F.; Ladoire, S.; Mignot, G.; Vincent, J.; Bruchard, M.; Remy-Martin, J.P.; Boireau, W.; Rouleau, A.; Simon, B.; Lanneau, D.; et al. Membrane-associated Hsp72 from tumor-derived exosomes mediates STAT3-dependent immunosuppressive function of mouse and human myeloid-derived suppressor cells. J. Clin. Investig. 2010, 120, 457–471. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak, J.; Wysoczynski, M.; Hayek, F.; Janowska-Wieczorek, A.; Ratajczak, M.Z. Membrane-derived microvesicles: Important and underappreciated mediators of cell-to-cell communication. Leukemia 2006, 20, 1487–1495. [Google Scholar] [CrossRef]
- Jung, T.; Castellana, D.; Klingbeil, P.; Hernandez, I.C.; Vitacolonna, M.; Orlicky, D.J.; Roffler, S.; Brodt, P.; Zoller, M. CD44v6 dependence of premetastatic niche preparation by exosomes. Neoplasia 2009, 11, 1093–1150. [Google Scholar] [CrossRef]
- Lima, L.G.; Chammas, R.; Monteiro, R.Q.; Moreira, M.E.C.; Barcinski, M.A. Tumor-derived microvesicles modulate the establishment of metastatic melanoma in a phosphatidylserine-dependent manner. Cancer Lett. 2009, 283, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Hood, J.L.; Roman, S.S.; Wickline, S.A. Exosomes released by melanoma cells prepare sentinel lymph nodes for tumor metastasis. Cancer Res. 2011, 71, 3792–3801. [Google Scholar] [CrossRef] [PubMed]
- Peinado, H.; Kovic, M.A.; Lavotshkin, S.; Matei, I.; Costa-Silva, B.; Moreno-Bueno, G.; Hergueta-Redondo, M.; Williams, C.; Garcia-Santos, G.; Ghajar, C.M.; et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET (vol 18, pg 883, 2012). Nat. Med. 2012, 18, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Afshari, A.; Sengupta, R.; Sebastiano, V.; Gupta, A.; Kim, Y.H.; Biol, R.P.C. Replication study: Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Elife 2018, 7, e39944. [Google Scholar] [CrossRef] [PubMed]
- Ramteke, A.; Ting, H.; Agarwal, C.; Mateen, S.; Somasagara, R.; Hussain, A.; Graner, M.; Frederick, B.; Agarwal, R.; Deep, G. Exosomes secreted under hypoxia enhance invasiveness and stemness of prostate cancer cells by targeting adherens junction molecules. Mol. Carcinog. 2015, 54, 554–565. [Google Scholar] [CrossRef]
- Chen, C.H.; Luo, Y.M.; He, W.; Zhao, Y.; Kong, Y.; Liu, H.W.; Zhong, G.Z.; Li, Y.T.; Li, J.; Huang, J.; et al. Exosomal long noncoding RNA LNMAT2 promotes lymphatic metastasis in bladder cancer. J. Clin. Investig. 2020, 130, 404–421. [Google Scholar] [CrossRef]
- Zhou, W.Y.; Fong, M.Y.; Min, Y.F.; Somlo, G.; Liu, L.; Palomares, M.R.; Yu, Y.; Chow, A.; O’Connor, S.T.F.; Chin, A.R.; et al. Cancer-secreted miR-105 destroys vascular endothelial barriers to promote metastasis. Cancer Cell 2014, 25, 501–515. [Google Scholar] [CrossRef] [PubMed]
- Fong, M.Y.; Zhou, W.Y.; Liu, L.; Alontaga, A.Y.; Chandra, M.; Ashby, J.; Chow, A.; O’Connor, S.T.F.; Li, S.S.; Chin, A.R.; et al. Breast-cancer-secreted miR-122 reprograms glucose metabolism in premetastatic niche to promote metastasis. Nat. Cell Biol. 2015, 17, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Le, M.T.N.; Hamar, P.; Guo, C.Y.; Basar, E.; Perdigao-Henriques, R.; Balaj, L.; Lieberman, J. miR-200-containing extracellular vesicles promote breast cancer cell metastasis. J. Clin. Investig. 2014, 124, 5109–5128. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhao, C.; Shi, H.; Zhang, B.; Zhang, L.; Zhang, X.; Wang, S.; Wu, X.; Yang, T.; Huang, F.; et al. Deregulated microRNAs in gastric cancer tissue-derived mesenchymal stem cells: Novel biomarkers and a mechanism for gastric cancer. Br. J. Cancer 2014, 110, 1199–1210. [Google Scholar] [CrossRef]
- Lim, W.; Kim, H.S. Exosomes as Therapeutic Vehicles for Cancer. Tissue Eng. Regen. Med. 2019, 16, 213–223. [Google Scholar] [CrossRef]
- Antimisiaris, S.G.; Mourtas, S.; Marazioti, A. Exosomes and exosome-inspired vesicles for targeted drug delivery. Pharmaceutics 2018, 10, 218. [Google Scholar] [CrossRef]
- Qiao, L.; Hu, S.Q.; Huang, K.; Su, T.; Li, Z.H.; Vandergriff, A.; Cores, J.; Dinh, P.U.; Allen, T.; Shen, D.L.; et al. Tumor cell-derived exosomes home to their cells of origin and can be used as Trojan horses to deliver cancer drugs. Theranostics 2020, 10, 3474–3487. [Google Scholar] [CrossRef]
- Lu, M.; Huang, Y.Y. Bioinspired exosome-like therapeutics and delivery nanoplatforms. Biomaterials 2020, 242, 119925. [Google Scholar] [CrossRef]
- Sato, Y.T.; Umezaki, K.; Sawada, S.; Mukai, S.; Sasaki, Y.; Harada, N.; Shiku, H.; Akiyoshi, K. Engineering hybrid exosomes by membrane fusion with liposomes. Sci. Rep. 2016, 6, 21933. [Google Scholar] [CrossRef]
- Jang, S.C.; Kim, O.Y.; Yoon, C.M.; Choi, D.S.; Roh, T.Y.; Park, J.; Nilsson, J.; Lotvall, J.; Kim, Y.K.; Gho, Y.S. Bioinspired exosome-mimetic nanovesicles for targeted delivery of chemotherapeutics to malignant tumors. ACS Nano 2013, 7, 7698–7710. [Google Scholar] [CrossRef]
- Johnsen, K.B.; Gudbergsson, J.M.; Skov, M.N.; Pilgaard, L.; Moos, T.; Duroux, M. A comprehensive overview of exosomes as drug delivery vehicles—Endogenous nanocarriers for targeted cancer therapy. Biochim. Biophys. Acta Rev. Cancer 2014, 1846, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.F.; Shi, J.B.; Li, C. Small extracellular vesicle loading systems in cancer therapy: Current status and the way forward. Cytotherapy 2019, 21, 1122–1136. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; Amreddy, N.; Babu, A.; Panneerselvam, J.; Mehta, M.; Muralidharan, R.; Chen, A.; Zhao, Y.D.; Razaq, M.; Riedinger, N.; et al. Nanosomes carrying doxorubicin exhibit potent anticancer activity against human lung cancer cells. Sci. Rep. 2016, 6, 38541. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Wang, K.Y.; Lu, Q.; Zhao, J.; Wang, M.L.; Kan, Q.M.; Zhang, H.T.; Wang, Y.J.; He, Z.G.; Sun, J. Nanosponges of circulating tumor-derived exosomes for breast cancer metastasis inhibition. Biomaterials 2020, 242, 119932. [Google Scholar] [CrossRef]
- Li, Y.J.; Wu, J.Y.; Wang, J.M.; Hu, X.B.; Cai, J.X.; Xiang, D.X. Gemcitabine loaded autologous exosomes for effective and safe chemotherapy of pancreatic cancer. Acta Biomater. 2020, 101, 519–530. [Google Scholar] [CrossRef]
- Haney, M.J.; Zhao, Y.L.; Jin, Y.S.; Li, S.M.; Bago, J.R.; Klyachko, N.L.; Kabanov, A.V.; Batrakova, E.V. Macrophage-derived extracellular vesicles as drug delivery systems for triple negative breast cancer (TNBC) therapy. J. Neuroimmune Pharmacol. 2020, 15, 487–500. [Google Scholar] [CrossRef]
- Tran, P.H.L.; Wang, T.; Yin, W.; Tran, T.T.D.; Nguyen, T.N.G.; Lee, B.J.; Duan, W. Aspirin-loaded nanoexosomes as cancer therapeutics. Int. J. Pharm. 2019, 572, 118786. [Google Scholar] [CrossRef]
- Lin, Q.; Qu, M.K.; Zhou, B.J.; Patra, H.K.; Sun, Z.H.; Luo, Q.; Yang, W.Y.; Wu, Y.C.; Zhang, Y.; Li, L.; et al. Exosome-like nanoplatform modified with targeting ligand improves anti-cancer and anti-inflammation effects of imperialine. J. Control Release 2019, 311, 104–116. [Google Scholar] [CrossRef]
- Baldari, S.; Di Rocco, G.; Magenta, A.; Picozza, M.; Toietta, G. Extracellular vesicles-encapsulated MicroRNA-125b produced in genetically modified mesenchymal stromal cells inhibits hepatocellular carcinoma cell proliferation. Cells 2019, 8, 1560. [Google Scholar] [CrossRef]
- Nie, H.F.; Xie, X.D.; Zhang, D.D.; Zhou, Y.; Li, B.F.; Li, F.Q.; Li, F.Y.; Cheng, Y.L.; Mei, H.; Meng, H.; et al. Use of lung-specific exosomes for miRNA-126 delivery in non-small cell lung cancer. Nanoscale 2020, 12, 877–887. [Google Scholar] [CrossRef]
- Wang, H.B.; Wei, H.; Wang, J.S.; Li, L.; Chen, A.Y.; Li, Z.G. MicroRNA-181d-5p-containing exosomes derived from CAFs promote EMT by regulating CDX2/HOXA5 in breast cancer. Mol. Ther. Nucleic Acids 2020, 19, 654–667. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.F.; Zhu, Y.L.; Ali, D.J.; Tian, T.; Xu, H.T.; Si, K.; Sun, B.; Chen, B.A.; Xiao, Z.D. Engineered exosomes for targeted co-delivery of miR-21 inhibitor and chemotherapeutics to reverse drug resistance in colon cancer. J. Nanobiotechnol. 2020, 18, 10. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.J.; Liu, J.Q.; Zhang, Q.L.; Liu, B.X.; Cheng, Y.; Zhang, Y.L.; Sun, Y.N.; Ge, H.; Liu, Y.Q. Exosome-mediated transfer of miR-93-5p from cancer-associated fibroblasts confer radioresistance in colorectal cancer cells by downregulating FOXA1 and upregulating TGFB3. J. Exp. Clin. Cancer Res. 2020, 39, 65. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Sawada, K.; Miyamoto, M.; Shimizu, A.; Yamamoto, M.; Kinose, Y.; Nakamura, K.; Kawano, M.; Kodama, M.; Hashimoto, K.; et al. Exploring the potential of engineered exosomes as delivery systems for tumor-suppressor microRNA replacement therapy in ovarian cancer. Biochem. Biophys. Res. Commun. 2020, 527, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Forterre, A.V.; Wang, J.H.; Delcayre, A.; Kim, K.; Green, C.; Pegram, M.D.; Jeffrey, S.S.; Matin, A.C. Extracellular vesicle-mediated in vitro transcribed mRNA delivery for treatment of HER2(+) breast cancer xenografts in mice by prodrug CB1954 without general toxicity. Mol. Cancer Ther. 2020, 19, 858–867. [Google Scholar] [CrossRef]
- Zhuang, M.J.; Chen, X.L.; Du, D.; Shi, J.M.; Deng, M.; Long, Q.; Yin, X.F.; Wang, Y.Y.; Rao, L. SPION decorated exosome delivery of TNF-alpha to cancer cell membranes through magnetism. Nanoscale 2020, 12, 173–188. [Google Scholar] [CrossRef]
- Xin, L.; Yuan, Y.W.; Liu, C.; Zhou, L.Q.; Liu, L.; Zhou, Q.; Li, S.H. Preparation of internalizing RGD-modified recombinant methioninase exosome active targeting vector and antitumor effect evaluation. Dig. Dis. Sci. 2020. [Google Scholar] [CrossRef]
- Huang, T.; Deng, C.X. Current progresses of exosomes as cancer diagnostic and prognostic biomarkers. Int. J. Biol. Sci. 2019, 15, 1–11. [Google Scholar] [CrossRef]
- van der Meel, R.; Krawczyk-Durka, M.; van Solinge, W.W.; Schiffelers, R.M. Toward routine detection of extracellular vesicles in clinical samples. Int. J. Lab. Hematol. 2014, 36, 244–253. [Google Scholar] [CrossRef]
- Shao, H.L.; Chung, J.; Lee, K.; Balaj, L.; Min, C.; Carter, B.S.; Hochberg, F.H.; Breakefield, X.O.; Lee, H.; Weissleder, R. Chip-based analysis of exosomal mRNA mediating drug resistance in glioblastoma. Nat. Commun. 2015, 6, 6999. [Google Scholar] [CrossRef]
- Kosaka, N.; Iguchi, H.; Ochiya, T. Circulating microRNA in body fluid: A new potential biomarker for cancer diagnosis and prognosis. Cancer Sci. 2010, 101, 2087–2092. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zheng, Q.P.; Bao, C.Y.; Li, S.Y.; Guo, W.J.; Zhao, J.; Chen, D.; Gu, J.R.; He, X.H.; Huang, S.L. Circular RNA is enriched and stable in exosomes: A promising biomarker for cancer diagnosis. Cell Res. 2015, 25, 981–984. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.S.; Kim, D.K.; Kim, Y.K.; Gho, Y.S. Proteomics of extracellular vesicles: Exosomes and ectosomes. Mass Spectrom. Rev. 2015, 34, 474–490. [Google Scholar] [CrossRef] [PubMed]
- Verma, M.; Lam, T.K.; Hebert, E.; Divi, R.L. Extracellular vesicles: Potential applications in cancer diagnosis, prognosis, and epidemiology. BMC Clin. Pathol. 2015, 15, 6. [Google Scholar] [CrossRef] [PubMed]
- Zong, S.F.; Zong, J.Z.; Chen, C.; Jiang, X.Y.; Zhang, Y.Z.; Wang, Z.Y.; Cui, Y.P. Single molecule localization imaging of exosomes using blinking silicon quantum dots. Nanotechnology 2018, 29, 065705. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zong, S.F.; Wang, Z.Y.; Lu, J.; Zhu, D.; Zhang, Y.Z.; Cui, Y.P. Imaging and intracellular tracking of cancer-derived exosomes using single-molecule localization-based super-resolution microscope. ACS Appl. Mater. Interfaces 2016, 8, 25825–25833. [Google Scholar] [CrossRef]
- Shang, M.Y.; Ji, J.S.; Song, C.; Gao, B.J.; Jin, J.G.; Kuo, W.P.; Kang, H.J. Extracellular vesicles: A brief overview and its role in precision medicine. In Extracellular Vesicles: Methods and Protocols; Kuo, W.P., Jia, S., Eds.; Humana Press: New York, NY, USA, 2017; Volume 1660, pp. 1–14. [Google Scholar]
- Jia, G.; Han, Y.; An, Y.L.; Ding, Y.A.; He, C.; Wang, X.H.; Tang, Q.S. NRP-1 targeted and cargo-loaded exosomes facilitate simultaneous imaging and therapy of glioma in vitro and in vivo. Biomaterials 2018, 178, 302–316. [Google Scholar] [CrossRef]
- Jiang, X.Y.; Zong, S.F.; Chen, C.; Zhang, Y.Z.; Wang, Z.Y.; Cui, Y.P. Gold-carbon dots for the intracellular imaging of cancer-derived exosomes. Nanotechnology 2018, 29, 175701. [Google Scholar] [CrossRef]
- Cao, Y.; Wu, T.T.; Zhang, K.; Meng, X.D.; Dai, W.H.; Wang, D.D.; Dong, H.F.; Zhang, X.J. Engineered exosome-mediated near-infrared-II region V2C quantum dot delivery for nucleus-target low-temperature photothermal therapy. ACS Nano 2019, 13, 1499–1510. [Google Scholar] [CrossRef]
- Pan, S.J.; Pei, L.J.; Zhang, A.M.; Zhang, Y.H.; Zhang, C.L.; Huang, M.; Huang, Z.C.; Liu, B.; Wang, L.R.; Ma, L.J.; et al. Passion fruit-like exosome-PMA/Au-BSA@Ce6 nanovehicles for real-time fluorescence imaging and enhanced targeted photodynamic therapy with deep penetration and superior retention behavior in tumor. Biomaterials 2020, 230, 119606. [Google Scholar] [CrossRef]
- Busato, A.; Bonafede, R.; Bontempi, P.; Scambi, I.; Schiaffino, L.; Benati, D.; Malatesta, M.; Sbarbati, A.; Marzola, P.; Mariotti, R. Magnetic resonance imaging of ultrasmall superparamagnetic iron oxide-labeled exosomes from stem cells: A new method to obtain labeled exosomes. Int. J. Nanomed. 2016, 11, 2481–2490. [Google Scholar]
- Busato, A.; Bonafede, R.; Bontempi, P.; Scambi, I.; Schiaffino, L.; Benati, D.; Malatesta, M.; Sbarbati, A.; Marzola, P.; Mariotti, R. Labeling and magnetic resonance imaging of exosomes isolated from adipose stem cells. Curr. Protoc. Cell Biol. 2017, 75. [Google Scholar] [CrossRef] [PubMed]
- Betzer, O.; Perets, N.; Ange, A.; Motiei, M.; Sadan, T.; Yadid, G.; Offen, D.; Popovtzer, R. In Vivo Neuroimaging of Exosomes Using Gold Nanoparticles. ACS Nano 2017, 11, 10883–10893. [Google Scholar] [CrossRef] [PubMed]
- Abello, J.; Nguyen, T.D.T.; Marasini, R.; Aryal, S.; Weiss, M.L. Biodistribution of gadolinium- and near infrared-labeled human umbilical cord mesenchymal stromal cell-derived exosomes in tumor bearing mice. Theranostics 2019, 9, 2325–2345. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Alves, V.; Rondao, T.; Sereno, J.; Neves, A.; Lino, M.; Ribeiro, A.; Abrunhosa, A.J.; Ferreira, L.S. A positron-emission tomography (PET)/magnetic resonance imaging (MRI) platform to track in vivo small extracellular vesicles. Nanoscale 2019, 11, 13243–13248. [Google Scholar] [CrossRef]
- Shi, S.X.; Li, T.T.; Wen, X.F.; Wu, S.Y.; Xiong, C.Y.; Zhao, J.; Lincha, V.R.; Chow, D.S.; Liu, Y.Y.; Sood, A.K.; et al. Copper-64 labeled PEGylated exosomes for in vivo positron emission tomography and enhanced tumor retention. Bioconjugate Chem. 2019, 30, 2675–2683. [Google Scholar] [CrossRef] [PubMed]
- Molavipordanjani, S.; Khodashenas, S.; Abedi, S.M.; Moghadam, M.F.; Mardanshahi, A.; Hosseinimehr, S.J. Tc-99m-radiolabeled HER2 targeted exosome for tumor imaging. Eur. J. Pharm. Sci. 2020, 148, 105312. [Google Scholar] [CrossRef]
- Rashid, M.H.; Borin, T.F.; Ara, R.; Alptekin, A.; Liu, Y.T.; Arbab, A.S. Generation of novel diagnostic and therapeutic exosomes to detect and deplete protumorigenic M2 macrophages. Adv. Ther. 2020, 3, 1900209. [Google Scholar] [CrossRef]
- Lai, C.P.; Tannous, B.A.; Breakefield, X.O. Noninvasive in vivo monitoring of extracellular vesicles. Biolumin. Imaging Methods Protoc. 2014, 1098, 249–258. [Google Scholar]
- Lai, C.P.; Kim, E.Y.; Badr, C.E.; Weissleder, R.; Mempel, T.R.; Tannous, B.A.; Breakefield, X.O. Visualization and tracking of tumour extracellular vesicle delivery and RNA translation using multiplexed reporters. Nat. Commun. 2015, 6, 7029. [Google Scholar] [CrossRef]
- Tayyaba; Rehman, F.U.; Shaikh, S.; Semcheddine, F.; Du, T.Y.; Jiang, H.; Wang, X.M. In situ self-assembled Ag-Fe3O4 nanoclusters in exosomes for cancer diagnosis. J. Mater. Chem. B 2020, 8, 2845–2855. [Google Scholar]
- Horgan, C.C.; Nagelkerke, A.; Whittaker, T.E.; Nele, V.; Massi, L.; Kauscher, U.; Penders, J.; Bergholt, M.S.; Hood, S.R.; Stevens, M.M. Molecular imaging of extracellular vesicles in vitro via Raman metabolic labelling. J. Mater. Chem. B 2020, 8, 4447–4459. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.F.; Zhang, F.; Wang, K.; Luo, P.C.; Wei, Y.Q.; Liu, S.Q. Activatable fluorescence imaging and targeted drug delivery via extracellular vesicle-like porous coordination polymer nanoparticles. Anal. Chem. 2019, 91, 14036–14042. [Google Scholar] [CrossRef] [PubMed]
- Rayamajhi, S.; Marasini, R.; Nguyen, T.D.T.; Plattner, B.L.; Biller, D.; Aryal, S. Strategic reconstruction of macrophage-derived extracellular vesicles as a magnetic resonance imaging contrast agent. Biomater. Sci. 2020, 8, 2887–2904. [Google Scholar] [CrossRef] [PubMed]
- Hwang, D.W.; Choi, H.; Jang, S.C.; Yoo, M.Y.; Park, J.Y.; Choi, N.E.; Oh, H.J.; Ha, S.; Lee, Y.S.; Jeong, J.M.; et al. Noninvasive imaging of radiolabeled exosome-mimetic nanovesicle using Tc-99m-HMPAO. Sci. Rep. 2015, 5, 15636. [Google Scholar] [CrossRef]
- Gupta, A.K.; Gupta, M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials 2005, 26, 3995–4021. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Kumar, A.; Bharti, A.C. Anticancer potential of curcumin: Preclinical and clinical studies. Anticancer Res. 2003, 23, 363–398. [Google Scholar]
- Luthra, P.M.; Lal, N. Prospective of curcumin, a pleiotropic signalling molecule from Curcuma longa in the treatment of Glioblastoma. Eur. J. Med. Chem. 2016, 109, 23–35. [Google Scholar] [CrossRef]
- Hamerlik, P.; Lathia, J.D.; Rasmussen, R.; Wu, Q.L.; Bartkova, J.; Lee, M.; Moudry, P.; Bartek, J.; Fischer, W.; Lukas, J.; et al. Autocrine VEGF-VEGFR2-Neuropilin-1 signaling promotes glioma stem-like cell viability and tumor growth. J. Exp. Med. 2012, 209, 507–520. [Google Scholar] [CrossRef]
- Laurent, S.; Dutz, S.; Hafeli, U.O.; Mahmoudi, M. Magnetic fluid hyperthermia: Focus on superparamagnetic iron oxide nanoparticles. Adv. Colloid Interface Sci. 2011, 166, 8–23. [Google Scholar] [CrossRef]
- Ciravolo, V.; Huber, V.; Ghedini, G.C.; Venturelli, E.; Bianchi, F.; Campiglio, M.; Morelli, D.; Villa, A.; Della Mina, P.; Menard, S.; et al. Potential role of HER2-overexpressing exosomes in countering trastuzumab-based therapy. J. Cell. Physiol. 2012, 227, 658–667. [Google Scholar] [CrossRef] [PubMed]
- Huhn, J.; Carrillo-Carrion, C.; Soliman, M.G.; Pfeiffer, C.; Valdeperez, D.; Masood, A.; Chakraborty, I.; Zhu, L.; Gallego, M.; Yue, Z.; et al. Selected standard protocols for the synthesis, phase transfer, and characterization of inorganic colloidal nanoparticles. Chem. Mater. 2017, 29, 399–461. [Google Scholar] [CrossRef]
- Rogosnitzky, M.; Branch, S. Gadolinium-based contrast agent toxicity: A review of known and proposed mechanisms. Biometals 2016, 29, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Gray, W.D.; French, K.M.; Ghosh-Choudhary, S.; Maxwell, J.T.; Brown, M.E.; Platt, M.O.; Searles, C.D.; Davis, M.E. Identification of therapeutic covariant MicroRNA clusters in hypoxia-treated cardiac progenitor cell exosomes using systems biology. Circ. Res. 2015, 116, 255–263. [Google Scholar] [CrossRef]
- Limoni, S.K.; Moghadam, M.F.; Moazzeni, S.M.; Gomari, H.; Salimi, F. Engineered exosomes for targeted transfer of siRNA to HER2 positive breast cancer cells. Appl. Biochem. Biotechnol. 2019, 187, 352–364. [Google Scholar] [CrossRef]
- Arbab, A.S.; Thiffault, C.; Navia, B.; Victor, S.J.; Hong, K.; Zhang, L.; Jiang, Q.; Varma, N.R.S.; Iskander, A.S.M.; Chopp, M. Tracking of In-111-labeled human umbilical tissue-derived cells (hUTC) in a rat model of cerebral ischemia using SPECT imaging. BMC Med. Imaging 2012, 12, 33. [Google Scholar] [CrossRef]
- Tannous, B.A.; Kim, D.E.; Fernandez, J.L.; Weissleder, R.; Breakefield, X.O. Codon-optimized Gaussia luciferase cDNA for mammalian gene expression in culture and in vivo. Mol. Ther. 2005, 11, 435–443. [Google Scholar] [CrossRef]
- Morris, L.M.; Klanke, C.A.; Lang, S.A.; Lim, F.Y.; Crombleholme, T.M. TdTomato and EGFP identification in histological sections: Insight and alternatives. Biotech. Histochem. 2010, 85, 379–387. [Google Scholar] [CrossRef]
- Shaikh, S.; Rehman, F.U.; Du, T.Y.; Jiang, H.; Yin, L.H.; Wang, X.M.; Chai, R.J. Real-time multimodal bioimaging of cancer cells and exosomes through biosynthesized iridium and iron nanoclusters. ACS Appl. Mater. Interfaces 2018, 10, 26056–26063. [Google Scholar] [CrossRef]
- Kallepitis, C.; Bergholt, M.S.; Mazo, M.M.; Leonardo, V.; Skaalure, S.C.; Maynard, S.A.; Stevens, M.M. Quantitative volumetric Raman imaging of three dimensional cell cultures. Nat. Commun. 2017, 8, 14843. [Google Scholar] [CrossRef]
- Rad, A.M.; Arbab, A.S.; Iskander, A.S.M.; Jiang, Q.; Soltanian-Zadeh, H. Quantification of superparamagenetic iron oxide (SPIO)-labeled cells using MRI. J. Magn. Reson. Imaging 2007, 26, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Thery, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7. [Google Scholar] [CrossRef] [PubMed]




| Ref. | Labeling Strategy | Parent Cells | Exosome Isolation Method | Labeling Compound | Therapeutic Compound | Loading/Labeling Procedure | Surface Engineering | Detection Technique | Tests |
|---|---|---|---|---|---|---|---|---|---|
| [78] | Nanoparticle-loaded exosomes | Raw264.7 mouse macrophages | Sequential centrifugation | SPION | Curcumin | Exogenous (electroporation) | NRP-1 binding peptide by click chemistry | MRI | In vitro: U251 cells In vivo: BALB/c nude mice transplanted with U251 cells |
| [79] | SKBR3 breast cancer cells | Exosome isolation kit | Gold-carbon QD | Exogenous (incubation exploiting targeted loading through anti-HER2 antibodies) | Fluorescence imaging | In vitro: HeLa cells | |||
| [80] | MCF-7 breast cancer cells | Exosome isolation kit | Vanadium carbide QD | Exogenous (electroporation) | RGD peptide introduced by incubating exosomes with DSPE-PEG-RGD | Photoacoustic imaging | In vitro: MCF-7, A549, NHDF cells In vivo: tumor-bearing BALB/c nude mice | ||
| [81] | Urine of gastric cancer patients | Sequential centrifugation | Chlorine-6 labeled gold NP | Exogenous (electroporation) | Fluorescence imaging | In vitro: MGC-803, Raw264.7 cells In vivo: MGC-803 tumor-bearing BALB/c-nude mice | |||
| [82,83] | Murine adipose stem cells | Exosome isolation kit | USPION | Endogenous (cell incubation) | MRI | In vitro: exosomes immobilized in an agarose matrix In vivo: C57BL/6 mice | |||
| [84] | Mesenchymal stem cells | Sequential centrifugation | Gold NP | Exogenous (incubation) | CT | In vivo: C57bl/6 mice | |||
| [85] | Transition metal-labeled exosomes | Human umbilical cord mesenchymal stem cells | Sequential centrifugation | 68Gd (complexed by DOTA) | Exogenous (lipid insertion technique with Gd-DOTA-DSPE) | MRI | In vitro: K7M2 mouse and 14B human osteosarcoma cells In vivo: immunodeficient NU/NU nude mice implanted with K7M2 cells | ||
| [86] | Human umbilical cord blood mononuclear cells | Sequential centrifugation | 64Cu (complexed by DOTA) | Exogenous (reaction between the maleimide group of DOTA and thiol groups on exosome surface) | PET/MRI | In vitro: HUVEC In vivo: C57BL/6J mice | |||
| [87] | 4T1 breast cancer cells | Sequential centrifugation | 64Cu (complexed by NOTA) | Exogenous (reaction of NOTA with exosome surface proteins) | PEG decoration using PEG5k/NHS | PET | In vivo: 4T1 tumor-bearing BALB/c mice | ||
| [88] | Mouse macrophage HEK293T cells | Sequential centrifugation | 99mTc | Exogenous (incubation with fac-[99mTc(CO)3(H2O)3]+) | DARPin G3 functionalization by transfection of the parent cells | Radioactive signal by gamma-counter | In vitro: SKOV-3, MCF-7, U87-MG, HT-29, A549 cells In vivo: BALB/c mice, SKOV-3 xenografted C57 nude mice | ||
| [89] | Human embryonic kidney HEK293 cells | Sequential centrifugation | 111In | Exogenous (incubation with 111In -oxine) | CSPGAKVRC peptide, functionalized by transfection of the parent cells | CT/SPECT | In vitro: Raw264.7 cells In vivo: 4T1 tumor-bearing Balb/c mice | ||
| [90] | Bioluminescently labeled exosomes | Human embryonic kidney 293T cells | Sequential centrifugation | Gaussia princeps luciferase (Gluc) | Endogenous (transfection of the parent cells with a gene encoding for Gluc bound to a membrane protein) | IVIS imaging | In vivo: immunodeficient athymic nude mice | ||
| [91] | Human embryonic kidney 293T cells | Sequential centrifugation | GFP, tandem dimer Tomato | Endogenous (transfection of the parent cells with a gene encoding for palmGFP/palmtdTomato) | Multiphoton intravital microscopy | In vitro: 293T cells In vivo: C57BL6 (B6) mice implanted with mouse thymoma EL-4 cells | |||
| [92] | Nanocluster loaded exosomes | HepG2 human hepatocellular carcinoma | Sequential centrifugation | Ag-nanoclusters and Fe3O4 NP | Endogenous (parent cells cultured in the presence of AgNO3 and FeCl2 forming the nanoclusters) | Flurescence bioimaging, CT, MRI | In vitro: HepG2, U87 cells | ||
| [93] | Metabolic labeled exosomes | MDA-MB-231 breast cancer cells | Ultracentrifugation and size exclusion chromatography | Deuterium | Endogenous (parent cells cultured in presence of D2O/d-Gluc/d-Chol) | Raman spectroscopic imaging | In vitro: MDA-MB-231, MCF10A cells |
| Ref. | Cell Line | Labeling Compound | Therapeutic Compound | Vesicle Preparation Method | Loading/Labeling Procedure | Detection Technique | Tests |
|---|---|---|---|---|---|---|---|
| [94] | Bel-7402 human hepatoma cancer cells | NP-encapsulated doxorubicin | NP-encapsulated doxorubicin | Coating of the NP with cell membranes through extrusion | Incubation | Fluorescence imaging | In vitro: Bel-7402, MCF-7, L-O2 cells |
| [95] | J774A.1 mouse macrophages | Gd-conjugated liposomes | Sonication and extrusion of the exosome/liposome mixture | Obtained during vesicle preparation procedure | MRI | In vitro: K7M2, NIH/3T3 cells In vivo: osteosarcoma—bearing NU/NU immunodeficient mice | |
| [96] | Raw264.7 mouse macrophages, HB1.F3 human neural stem cells | 99mTc-HMPAO | Sequential extrusion of parent cells and density gradient centrifugation | Incubation | SPECT/CT | In vivo: BALB/c mice |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ailuno, G.; Baldassari, S.; Lai, F.; Florio, T.; Caviglioli, G. Exosomes and Extracellular Vesicles as Emerging Theranostic Platforms in Cancer Research. Cells 2020, 9, 2569. https://doi.org/10.3390/cells9122569
Ailuno G, Baldassari S, Lai F, Florio T, Caviglioli G. Exosomes and Extracellular Vesicles as Emerging Theranostic Platforms in Cancer Research. Cells. 2020; 9(12):2569. https://doi.org/10.3390/cells9122569
Chicago/Turabian StyleAiluno, Giorgia, Sara Baldassari, Francesco Lai, Tullio Florio, and Gabriele Caviglioli. 2020. "Exosomes and Extracellular Vesicles as Emerging Theranostic Platforms in Cancer Research" Cells 9, no. 12: 2569. https://doi.org/10.3390/cells9122569
APA StyleAiluno, G., Baldassari, S., Lai, F., Florio, T., & Caviglioli, G. (2020). Exosomes and Extracellular Vesicles as Emerging Theranostic Platforms in Cancer Research. Cells, 9(12), 2569. https://doi.org/10.3390/cells9122569







