Pro-Inflammatory Role of AQP4 in Mice Subjected to Intrastriatal Injections of the Parkinsonogenic Toxin MPP+
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Stereotaxic Surgery and Injection of MPP+
2.3. Post-Operative Animal Care
2.4. Tissue Preparation for Light and Electron Microscopic Cytochemistry
2.5. Immunocytochemistry
2.6. Stereological Quantifications of Dopaminergic Density in the Midbrain
2.7. Electron Microscopy
2.8. Electron Microscopic Analysis
2.9. RNA Isolation and Quantitative Real Time qPCR Analysis
3. Results
3.1. Survival Rate and Clinical Appearance Post-Surgery
3.2. Unilateral Intrastriatal Injections of MPP+ Lead to Astrogliosis and Microgliosis in the Ipsilateral SN
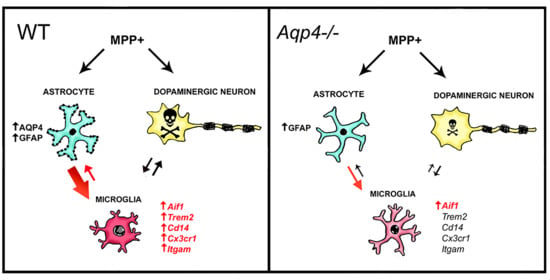
3.3. Unilateral Intrastriatal MPP+ Injections Lead to a Strong Increase in the Transcript Levels of Microglial Activating Genes in Ipsilateral Midbrain of Wild Type but Not Aqp4−/− Mice
3.4. Expression of AQP4 in SN after MPP+ Injections
3.5. Electron Microscopic Analysis of Endfoot Width
3.6. Stereological Quantification of Dopaminergic Cell Density
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nagelhus, E.A.; Ottersen, O.P. Physiological roles of Aquaporin-4 in brain. Physiol. Rev. 2013, 93, 1543–1562. [Google Scholar] [CrossRef] [PubMed]
- Mylonakou, M.N.; Petersen, P.H.; Rinvik, E.; Rojek, A.; Valdimarsdottir, E.; Zelenin, S.; Zeuthen, T.; Nielsen, S.; Ottersen, O.P.; Amiry-Moghaddam, M. Analysis of mice with targeted deletion of AQP9 gene provides conclusive evidence for expression of AQP9 in neurons. J. Neurosci. Res. 2009, 87, 1310–1322. [Google Scholar] [CrossRef] [PubMed]
- Badaut, J.; Petit, J.-M.; Brunet, J.-F.; Magistretti, P.J.; Charriaut-Marlangue, C.; Regli, L. Distribution of Aquaporin 9 in the adult rat brain: Preferential expression in catecholaminergic neurons and in glial cells. Neuroscience 2004, 128, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Badaut, J.; Lasbennes, F.; Magistretti, P.J.; Regli, L. Aquaporins in brain: Distribution, physiology, and pathophysiology. Br. J. Pharmacol. 2002, 22, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.; Nagelhus, E.A.; Amiry-Moghaddam, M.; Bourque, C.; Agre, P.; Ottersen, O.P. Specialized membrane domains for water transport in glial cells: High-resolution immunogold cytochemistry of aquaporin-4 in rat brain. J. Neurosci. 1997, 17, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Amiry-Moghaddam, M.; Frydenlund, D.; Ottersen, O. Anchoring of aquaporin-4 in brain: Molecular mechanisms and implications for the physiology and pathophysiology of water transport. Neuroscience 2004, 129, 997–1008. [Google Scholar] [CrossRef] [PubMed]
- Amiry-Moghaddam, M.; Ottersen, O.P. The molecular basis of water transport in the brain. Nat. Rev. Neurosci. 2003, 4, 991–1001. [Google Scholar] [CrossRef]
- Stahl, K.; Rahmani, S.; Prydz, A.; Skauli, N.; Macaulay, N.; Mylonakou, M.N.; Torp, R.; Skare, O.; Berg, T.; Leergaard, T.B.; et al. Targeted deletion of the aquaglyceroporin AQP9 is protective in a mouse model of Parkinson’s disease. PLoS ONE 2018, 13, e0194896. [Google Scholar] [CrossRef]
- Carbrey, J.M.; Gorelick-Feldman, D.A.; Kozono, D.; Praetorius, J.; Nielsen, S.; Agre, P. Aquaglyceroporin AQP9: Solute permeation and metabolic control of expression in liver. Proc. Natl. Acad. Sci. USA 2003, 100, 2945–2950. [Google Scholar] [CrossRef]
- Manley, G.T.; Fujimura, M.; Ma, T.; Noshita, N.; Filiz, F.; Bollen, A.W.; Chan, P.; Verkman, A. Aquaporin-4 deletion in mice reduces brain edema after acute water intoxication and ischemic stroke. Nat. Med. 2000, 6, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Vajda, Z.; Pedersen, M.; Füchtbauer, E.-M.; Wertz, K.; Stødkilde-Jørgensen, H.; Sulyok, E.; Dóczi, T.; Neely, J.D.; Agre, P.; Frokiaer, J.; et al. Delayed onset of brain edema and mislocalization of aquaporin-4 in dystrophin-null transgenic mice. Proc. Natl. Acad. Sci. USA 2002, 99, 13131–13136. [Google Scholar] [CrossRef] [PubMed]
- Frydenlund, D.S.; Bhardwaj, A.; Otsuka, T.; Mylonakou, M.N.; Yasumura, T.; Davidson, K.G.V.; Zeynalov, E.; Skare, O.; Laake, P.; Haug, F.-M.; et al. Temporary loss of perivascular aquaporin-4 in neocortex after transient middle cerebral artery occlusion in mice. Proc. Natl. Acad. Sci. USA 2006, 103, 13532–13536. [Google Scholar] [CrossRef]
- Hirt, L.; Ternon, A.; Price, M.; Mastour, N. Protective role of early aquaporin 4 induction against postischemic edema formation. Br. J. Pharmacol. 2008, 29, 423–433. [Google Scholar] [CrossRef]
- Papadopoulos, M.C.; Verkman, A.S. Aquaporin water channels in the nervous system. Nat. Rev. Neurosci. 2013, 14, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Alvestad, S.; Hammer, J.; Hoddevik, E.H.; Skare, O.; Sonnewald, U.; Amiry-Moghaddam, M.; Ottersen, O.P. Mislocalization of AQP4 precedes chronic seizures in the kainate model of temporal lobe epilepsy. Epilepsy Res. 2013, 105, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Eid, T.; Lee, T.-S.W.; Thomas, M.J.; Amiry-Moghaddam, M.; Bjørnsen, L.P.; Spencer, D.D.; Agre, P.; Ottersen, O.P.; De Lanerolle, N.C. Loss of perivascular aquaporin 4 may underlie deficient water and K + homeostasis in the human epileptogenic hippocampus. Proc. Natl. Acad. Sci. USA 2005, 102, 1193–1198. [Google Scholar] [CrossRef]
- Yang, J.; Lunde, L.L.; Paworn, N.; Tomohiro, O.; Camassa, L.M.A.; Nilsson, N.G.; Lannfelt, L.; Xu, Y.; Amiry-Moghaddam, M.; Ottersen, O.P.; et al. Loss of astrocyte polarization in the tg-ArcSwe mouse model of Alzheimer’s disease. J. Alzheimers Dis. 2011, 27, 711–722. [Google Scholar] [CrossRef]
- Pérez, E.; Barrachina, M.; Rodríguez, A.; Torrejón-Escribano, B.; Boada, M.; Hernández, I.; Sánchez, M.; Ferrer, I. Aquaporin expression in the cerebral cortex is increased at early stages of Alzheimer disease. Brain Res. 2007, 1128, 164–174. [Google Scholar] [CrossRef]
- Moftakhar, P.; Lynch, M.D.; Pomakian, J.L.; Vinters, H.V. Aquaporin expression in the brains of patients with or without cerebral amyloid angiopathy. J. Neuropathol. Exp. Neurol. 2010, 69, 1201–1209. [Google Scholar] [CrossRef]
- Hoshi, A.; Yamamoto, T.; Shimizu, K.; Ugawa, Y.; Nishizawa, M.; Takahashi, H.; Kakita, A. Characteristics of aquaporin expression surrounding senile plaques and cerebral amyloid angiopathy in Alzheimer disease. J. Neuropathol. Exp. Neurol. 2012, 71, 750–759. [Google Scholar] [CrossRef]
- Kaiser, M.; Maletzki, I.; Hülsmann, S.; Holtmann, B.; Schulz-Schaeffer, W.; Kirchhoff, F.; Bähr, M.; Neusch, C. Progressive loss of a glial potassium channel (KCNJ10) in the spinal cord of the SOD1 (G93A) transgenic mouse model of amyotrophic lateral sclerosis. J. Neurochem. 2006, 99, 900–912. [Google Scholar] [CrossRef]
- Nicaise, C.; Soyfoo, M.S.; Authelet, M.; De Decker, R.; Bataveljić, D.; Delporte, C.; Roland, P. Aquaporin-4 overexpression in rat ALS model. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 2009, 292, 207–213. [Google Scholar] [CrossRef]
- Wu, T.-T.; Su, F.-J.; Feng, Y.-Q.; Liu, B.; Li, M.-Y.; Liang, F.-Y.; Li, G.; Li, X.-J.; Zhang, Y.; Cai, Z.-Q.; et al. Mesenchymal stem cells alleviate AQP-4-dependent glymphatic dysfunction and improve brain distribution of antisense oligonucleotides in BACHD mice. STEM CELLS 2019. [Google Scholar] [CrossRef]
- Costa, C.; Tortosa, R.; Rodriguez, A.; Ferrer, I.; Torres, J.M.; Bassols, A.; Pumarola, M.B. Aquaporin 1 and aquaporin 4 overexpression in bovine spongiform encephalopathy in a transgenic murine model and in cattle field cases. Brain Res. 2007, 1175, 96–106. [Google Scholar] [CrossRef]
- Papadopoulos, M.C.; Verkman, A.S. Aquaporin-4 gene disruption in mice reduces brain swelling and mortality in pneumococcal meningitis. J. Biol. Chem. 2005, 280, 13906–13912. [Google Scholar] [CrossRef] [PubMed]
- Promeneur, D.; Lunde, L.K.; Amiry-Moghaddam, M.; Agre, P. Protective role of brain water channel AQP4 in murine cerebral malaria. Proc. Natl. Acad. Sci. USA 2012, 110, 1035–1040. [Google Scholar] [CrossRef]
- Lennon, V.A.; Kryzer, T.J.; Pittock, S.J.; Verkman, A.S.; Hinson, S.R. IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. J. Exp. Med. 2005, 202, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Gershen, L.D.; Zanotti-Fregonara, P.; Dustin, I.H.; Liow, J.-S.; Hirvonen, J.; Kreisl, W.C.; Jenko, K.J.; Inati, S.K.; Fujita, M.; Morse, C.L.; et al. Neuroinflammation in temporal lobe epilepsy measured using positron emission tomographic imaging of translocator protein. JAMA Neurol. 2015, 72, 882–888. [Google Scholar] [CrossRef] [PubMed]
- Amor, S.; Puentes, F.; Baker, D.; Van Der Valk, P. Inflammation in neurodegenerative diseases. Immunology 2010, 129, 154–169. [Google Scholar] [CrossRef]
- Troncoso-Escudero, P.; Parra, A.; Nassif, M.; Vidal, R.L. Outside in: Unraveling the role of neuroinflammation in the progression of Parkinson’s disease. Front. Neurol. 2018, 9, 860. [Google Scholar] [CrossRef]
- Li, L.; Zhang, H.; Varrin-Doyer, M.; Zamvil, S.S.; Verkman, A.S. Proinflammatory role of aquaporin-4 in autoimmune neuroinflammation. FASEB J. 2011, 25, 1556–1566. [Google Scholar] [CrossRef]
- Martinez, F.O.; Gordon, S. The M1 and M2 paradigm of macrophage activation: Time for reassessment. F1000Prime Rep. 2014, 6, 13. [Google Scholar] [CrossRef]
- Nau, G.J.; Richmond, J.F.L.; Schlesinger, A.; Jennings, E.G.; Lander, E.S.; Young, R.A. Human macrophage activation programs induced by bacterial pathogens. Proc. Natl. Acad. Sci. USA 2002, 99, 1503–1508. [Google Scholar] [CrossRef]
- Prydz, A.; Stahl, K.; Puchades, M.; Davarpaneh, N.; Nadeem, M.; Ottersen, O.P.; Gundersen, V.; Amiry-Moghaddam, M. Subcellular expression of aquaporin-4 in substantia nigra of normal and MPTP-treated mice. Neuroscience 2017, 359, 258–266. [Google Scholar] [CrossRef]
- Dauer, W.; Przedborski, S. Parkinson’s disease: Mechanisms and models. Neuron 2003, 39, 889–909. [Google Scholar] [CrossRef]
- Ofori, E.; Pasternak, O.; Planetta, P.J.; Burciu, R.; Snyder, A.; Febo, M.; Golde, T.E.; Okun, M.S.; Vaillancourt, D.E. Increased free water in the substantia nigra of Parkinson’s disease: A single-site and multi-site study. Neurobiol. Aging 2015, 36, 1097–1104. [Google Scholar] [CrossRef]
- Thrane, A.S.; Rappold, P.M.; Fujita, T.; Torres, A.; Bekar, L.K.; Takano, T.; Peng, W.; Wang, F.; Thrane, V.R.; Enger, R.; et al. Critical role of aquaporin-4 (AQP4) in astrocytic Ca2+ signaling events elicited by cerebral edema. Proc. Natl. Acad. Sci. USA 2011, 108, 846–851. [Google Scholar] [CrossRef]
- Paxinos, G.A.F.K. The Mouse Brain in Sterotaxic Coordinates, 3rd ed.; Elsevier Inc.: San Diego, CA, USA, 2007. [Google Scholar]
- Van Lookeren Campagne, M.; Oestreicher, A.B.; Buma, P.; Verkleij, A.J.; Gispen, W.H. Ultrastructural localization of adrenocorticotrophic hormone and the phosphoprotein B-50/growth-associated protein 43 in freeze-substituted, Lowicryl HM20-embedded mesencephalic central gray substance of the rat. Neuroscience 1991, 42, 517–529. [Google Scholar] [CrossRef]
- Amiry-Moghaddam, M.; Lindland, H.; Zelenin, S.; Roberg, B.Å.; Gundersen, B.B.; Petersen, P.; Rinvik, E.; Torgner, I.A.; Ottersen, O.P. Brain mitochondria contain aquaporin water channels: Evidence for the expression of a short AQP9 isoform in the inner mitochondrial membrane. FASEB J. 2005, 19, 1459–1467. [Google Scholar] [CrossRef]
- Wu, D.C.; Jackson-Lewis, V.; Vila, M.; Tieu, K.; Teismann, P.; Vadseth, C.; Choi, D.-K.; Ischiropoulos, H.; Przedborski, S. Blockade of microglial activation is neuroprotective in the 1-Methyl-4-Phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson disease. J. Neurosci. 2002, 22, 1763–1771. [Google Scholar] [CrossRef]
- Davis, E.; Foster, T.; Thomas, W. Cellular forms and functions of brain microglia. Brain Res. Bull. 1994, 34, 73–78. [Google Scholar] [CrossRef]
- Janova, H.; Böttcher, C.; Holtman, I.R.; Regen, T.; Van Rossum, D.; Götz, A.; Ernst, A.-S.; Fritsche, C.; Gertig, U.; Saiepour, N.; et al. CD14 is a key organizer of microglial responses to CNS infection and injury. Glia 2015, 64, 635–649. [Google Scholar] [CrossRef]
- Cignarella, F.; Filipello, F.; Bollman, B.; Cantoni, C.; Locca, A.; Mikesell, R.; Manis, M.; Ibrahim, A.; Deng, L.; Benitez, B.A.; et al. TREM2 activation on microglia promotes myelin debris clearance and remyelination in a model of multiple sclerosis. Acta Neuropathol. 2020, 140, 513–534. [Google Scholar] [CrossRef]
- Chen, G.; Zhou, Z.; Sha, W.; Wang, L.; Yan, F.; Yang, X.; Qin, X.; Wu, M.; Li, D.; Tian, S.; et al. A novel CX3CR1 inhibitor AZD8797 facilitates early recovery of rat acute spinal cord injury by inhibiting inflammation and apoptosis. Int. J. Mol. Med. 2020, 45, 1373–1384. [Google Scholar] [CrossRef]
- Ewolf, Y.; Eyona, S.; Ekim, K.-W.; Jung, S. Microglia, seen from the CX3CR1 angle. Front. Cell. Neurosci. 2013, 7, 26. [Google Scholar] [CrossRef]
- Jeetle, J.K.; Hagger, G.N.; Topps, S.S.; Male, D.; Rezaie, P. Microglial colonization of the developing mouse brain: The effect of CD11b deletion. Neuropathol. Appl. Neurobiol. 2002, 28, 164. [Google Scholar] [CrossRef]
- Kalkonde, Y.V.; Morgan, W.W.; Sigala, J.; Maffi, S.K.; Condello, C.; Kuziel, W.; Ahuja, S.S.; Ahuja, S.K. Chemokines in the MPTP model of Parkinson’s disease: Absence of CCL2 and its receptor CCR2 does not protect against striatal neurodegeneration. Brain Res. 2007, 1128, 1–11. [Google Scholar] [CrossRef]
- Xue, X.; Zhang, W.; Zhu, J.; Chen, X.; Zhou, S.; Xu, Z.; Hu, G.; Su, C. Aquaporin-4 deficiency reduces TGF-β1 in mouse midbrains and exacerbates pathology in experimental Parkinson’s disease. J. Cell. Mol. Med. 2019, 23, 2568–2582. [Google Scholar] [CrossRef] [PubMed]
- Lehrmann, E.; Kiefer, R.; Finsen, B.; Diemer, N.H.; Zimmer, J.; Hartung, H.P. Cytokines in cerebral ischemia: Expression of transforming growth factor beta-1 (TGF-β1) mRNA in the postischemic adult rat hippocampus. Exp. Neurol. 1995, 131, 114–123. [Google Scholar] [CrossRef]
- Spittau, B. Transforming growth factor β1-mediated anti-inflammation slows progression of midbrain dopaminergic neurodegeneration in Parkinson’s disease? Neural Regen. Res. 2015, 10, 1578–1580. [Google Scholar] [CrossRef]
- Chen, S.; Luo, D.; Streit, W.J.; Harrison, J.K. TGF-beta1 upregulates CX3CR1 expression and inhibits fractalkine-stimulated signaling in rat microglia. J. Neuroimmunol. 2002, 133, 46–55. [Google Scholar] [CrossRef]
- Farina, C.; Aloisi, F.; Meinl, E. Astrocytes are active players in cerebral innate immunity. Trends Immunol. 2007, 28, 138–145. [Google Scholar] [CrossRef]
- Guedes, J.R.; Lao, T.; Cardoso, A.L.; El Khoury, J. Roles of microglial and monocyte chemokines and their receptors in regulating Alzheimer’s disease-associated amyloid-β and Tau pathologies. Front. Neurol. 2018, 9, 549. [Google Scholar] [CrossRef]
- Clarner, T.G.; Janssen, K.; Nellessen, L.; Stangel, M.; Skripuletz, T.; Krauspe, B.; Hess, F.-M.; Denecke, B.; Beutner, C.; Linnartz-Gerlach, B.; et al. CXCL10 triggers early microglial activation in the cuprizone model. J. Immunol. 2015, 194, 3400–3413. [Google Scholar] [CrossRef]
- Dong, Y.; Yuan, Y.; Fang, Y.; Zheng, T.; Du, D.; Gao, D.; Du, J.; Liu, L.; He, Q. Effect of aquaporin 4 protein overexpression in nigrostriatal system on development of Parkinson’s disease. Int. J. Neurosci. 2020, 1–8. [Google Scholar] [CrossRef]
- Tourdias, T.; Mori, N.; Dragonu, I.; Cassagno, N.; Boiziau, C.; Aussudre, J.; Brochet, B.; Moonen, C.T.; Klaus, P.; Dousset, V. Differential aquaporin 4 expression during edema build-up and resolution phases of brain inflammation. J. Neuroinflamm. 2011, 8, 143. [Google Scholar] [CrossRef]
- Edwards, J.P.; Zhang, X.; Frauwirth, K.A.; Mosser, D.M. Biochemical and functional characterization of three activated macrophage populations. J. Leukoc. Biol. 2006, 80, 1298–1307. [Google Scholar] [CrossRef]
- Vivekanantham, S.; Shah, S.; Dewji, R.; Dewji, A.; Khatri, C.; Ologunde, R. Neuroinflammation in Parkinson’s disease: Role in neurodegeneration and tissue repair. Int. J. Neurosci. 2015, 125, 717–725. [Google Scholar] [CrossRef]
- Lee, H.-J.; Suk, J.-E.; Patrick, C.; Bae, E.-J.; Cho, J.-H.; Rho, S.; Hwang, D.; Masliah, E.; Lee, S.-J. Direct transfer of α-synuclein from neuron to astroglia causes inflammatory responses in synucleinopathies. J. Biol. Chem. 2010, 285, 9262–9272. [Google Scholar] [CrossRef]
- Hammond, S.L.; Bantle, C.M.; Popichak, K.A.; Wright, K.A.; Thompson, D.; Forero, C.; Kirkley, K.S.; Damale, P.U.; Chong, E.K.P.; Tjalkens, R.B. NF-κB signaling in astrocytes modulates brain inflammation and neuronal injury following sequential exposure to manganese and MPTP during development and Aging. Toxicol. Sci. 2020, 177, 506–520. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, B.; Sun, H.; Zhou, Y.; Liu, M.; Ding, J.; Fang, F.; Fan, Y.; Hu, G. Aquaporin-4 deficiency diminishes the differential degeneration of midbrain dopaminergic neurons in experimental Parkinson’s disease. Neurosci. Lett. 2016, 614, 7–15. [Google Scholar] [CrossRef]
- Liang, R.; Yong, S.; Huang, X.; Kong, H.; Hu, G.; Fan, Y. Aquaporin-4 mediates the suppressive effect of Lipopolysaccharide on hippocampal neurogenesis. Neuroimmunomodulation 2016, 23, 309–317. [Google Scholar] [CrossRef]
- Zhou, J.; Kong, H.; Hua, X.; Xiao, M.; Ding, J.; Hu, G. Altered blood–brain barrier integrity in adult aquaporin-4 knockout mice. NeuroReport 2008, 19, 1–5. [Google Scholar] [CrossRef]
- Eilert-Olsen, M.; Haj-Yasein, N.N.; Vindedal, G.F.; Enger, R.; Gundersen, G.A.; Hoddevik, E.H.; Petersen, P.H.; Haug, F.-M.S.; Skare, O.; Adams, M.E.; et al. Deletion of aquaporin-4 changes the perivascular glial protein scaffold without disrupting the brain endothelial barrier. Glia 2011, 60, 432–440. [Google Scholar] [CrossRef]
- García-Domínguez, I.; Veselá, K.; García-Revilla, J.; Carrillo-Jiménez, A.; Roca-Ceballos, M.A.; Santiago, M.; De Pablos, R.M.; Venero, J.L. Peripheral inflammation enhances microglia response and nigral dopaminergic cell death in an in vivo MPTP model of Parkinson’s disease. Front. Cell. Neurosci. 2018, 12, 398. [Google Scholar] [CrossRef]
- Koizumi, S.; Shigemoto-Mogami, Y.; Nasu-Tada, K.; Shinozaki, Y.; Ohsawa, K.; Tsuda, M.; Joshi, B.V.; Jacobson, K.A.; Kohsaka, S.; Inoue, K. UDP acting at P2Y6 receptors is a mediator of microglial phagocytosis. Nat. Cell Biol. 2007, 446, 1091–1095. [Google Scholar] [CrossRef]
- Pizzoni, A.; Bazzi, Z.; Di Giusto, G.; Alvarez, C.L.; Rivarola, V.; Capurro, C.; Schwarzbaum, P.J.; Ford, P. Release of ATP by TRPV4 activation is dependent upon the expression of AQP2 in renal cells. J. Cell. Physiol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Benfenati, V.; Caprini, M.; Dovizio, M.; Mylonakou, M.N.; Ferroni, S.; Ottersen, O.P.; Amiry-Moghaddam, M. An aquaporin-4/transient receptor potential vanilloid 4 (AQP4/TRPV4) complex is essential for cell-volume control in astrocytes. Proc. Natl. Acad. Sci. USA 2011, 108, 2563–2568. [Google Scholar] [CrossRef]
- Meshorer, E.; Biton, I.E.; Ben-Shaul, Y.; Ben-Ari, S.; Assaf, Y.; Soreq, H.; Cohen, Y. Chronic cholinergic imbalances promote brain diffusion and transport abnormalities. FASEB J. 2005, 19, 910–922. [Google Scholar] [CrossRef]
- Gupta, M.; Gupta, B.; Thomas, R.; Bruemmer, V.; Sladek, J.; Felten, D. Aged mice are more sensitive to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine treatment than young adults. Neurosci. Lett. 1986, 70, 326–331. [Google Scholar] [CrossRef]
- Gupta, R.; Kanungo, M. Glial molecular alterations with mouse brain development and aging: Up-regulation of the Kir4.1 and aquaporin-4. AGE 2011, 35, 59–67. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Noelker, C.; Morel, L.; Lescot, T.; Osterloh, A.; Alvarez-Fischer, D.; Breloer, M.; Henze, C.; Depboylu, C.; Skrzydelski, D.; Michel, P.P.; et al. Toll like receptor 4 mediates cell death in a mouse MPTP model of Parkinson disease. Sci. Rep. 2013, 3, 1393. [Google Scholar] [CrossRef]
- Fiebich, B.L.; Batista, C.R.A.; Saliba, S.W.; Yousif, N.M.; De Oliveira, A.C.P. Role of microglia TLRs in neurodegeneration. Front. Cell. Neurosci. 2018, 12, 329. [Google Scholar] [CrossRef]
- Mogi, M.; Harada, M.; Kondo, T.; Riederer, P.; Inagaki, H.; Minami, M.; Nagatsu, T. Interleukin-1 beta, interleukin-6, epidermal growth factor and transforming growth factor-alpha are elevated in the brain from parkinsonian patients. Neurosci. Lett. 1994, 180, 147–150. [Google Scholar] [CrossRef]
- McGeer, P.L.; Kawamata, T.; Walker, D.G.; Akiyama, H.; Tooyama, I.; McGeer, E.G. Microglia in degenerative neurological disease. Glia 1993, 7, 84–92. [Google Scholar] [CrossRef]





| Methods | Primary Antibody | Secondary Antibody | Tertiary Antibody |
|---|---|---|---|
| Immunocytochemistry | Rabbit anti-onized calcium-binding adapter molecule 1, Iba1, 1:200, Wako | Biotinylated anti-rabbit IgG (H + L) produced in goat, 1:100, Vector, Burlingame | Streptavidin-Biotinylated horse radish peroxidase complex, 1:100, GE Healthcare |
| Mouse anti-glial fibrillary acidic protein, GFAP, 1:1000, Nordic BioSite AB | Biotinylated anti-mouse IgG (H + L) produced in goat, 1:100, Vector, Burlingame | Streptavidin-Biotinylated horse radish peroxidase complex, 1:100, GE Healthcare | |
| Mouse anti-tyrosine hydroxylase, 1:1000, Chemicon | Biotinylated anti-mouse IgG (H + L) produced in goat, 1:100, Vector, Burlingame | Streptavidin-Biotinylated horse radish peroxidase complex, 1:100, GE Healthcare | |
| Immunofluorescence | Mouse anti-tyrosine hydroxylase, 1:1000, Chemicon | Alexa 488, 1:500, Thermo Fisher Scientific | |
| Rabbit anti-AQP4, 1:400, Sigma-Aldrich | Cy3 anti-rabbit, 1:500, Jackson ImmunoResearch Laboratories | ||
| Chicken anti-glial fibrillary acidic protein, GFAP, 1:1000, Nordic BioSite AB | Cy5 anti-chicken, 1:500, Jackson ImmunoResearch Laboratories |
| Gene Name | Forward Primer | Reverse Primer | Product Size | PCR Efficiency |
|---|---|---|---|---|
| Aqp4 | AGCAATTGGATTTTCCGTTG | TGAGCTCCACATCAGGACAG | 203 bp | 96% |
| Aif1 | CTGCCAGCCTAAGACAACCA | GGAATTGCTTGTTGATCCCCT | 128 bp | 99% |
| Cx3cr1 | TGCTCAGGACCTCACCATGTC | CTCAAGGCCAGGTTCAGGAG | 246 bp | 98% |
| Itgam | TGCGCGAAGGAGATATCCAG | GCCTGCGTGTGTTGTTCTTT | 108 bp | 94% |
| Cd14 | CAGAGAACACCACCGCTGTA | CACGCTCCATGGTCGGTAGA | 97 bp | 96% |
| Cxcl10 | ATGACGGGCCAGTGAGAATG | TCGTGGCAATGATCTCAACAC | 80 bp | 97% |
| Tgfb1 | AATTCCTGGCGTTACCTTGG | AGTGAGCGCTGAATCGAAAG | 139 bp | 100% |
| Trem2 | CTGGAGGACCCTCTAGATGAC | CCACAGGATGAAACCTGCCT | 116 bp | 98% |
| Gfap | GCACTCAATACGAGGCAGTG | GCTCTAGGGACTCGTTCGTG | 207 bp | 97% |
| Ubc | CGTCGAGCCCAGTGTTACCACCAAGAAGG | CCCCCATCACACCCAAGAACAAGCACAAG | 112 bp | 92% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prydz, A.; Stahl, K.; Zahl, S.; Skauli, N.; Skare, Ø.; Ottersen, O.P.; Amiry-Moghaddam, M. Pro-Inflammatory Role of AQP4 in Mice Subjected to Intrastriatal Injections of the Parkinsonogenic Toxin MPP+. Cells 2020, 9, 2418. https://doi.org/10.3390/cells9112418
Prydz A, Stahl K, Zahl S, Skauli N, Skare Ø, Ottersen OP, Amiry-Moghaddam M. Pro-Inflammatory Role of AQP4 in Mice Subjected to Intrastriatal Injections of the Parkinsonogenic Toxin MPP+. Cells. 2020; 9(11):2418. https://doi.org/10.3390/cells9112418
Chicago/Turabian StylePrydz, Agnete, Katja Stahl, Soulmaz Zahl, Nadia Skauli, Øivind Skare, Ole Petter Ottersen, and Mahmood Amiry-Moghaddam. 2020. "Pro-Inflammatory Role of AQP4 in Mice Subjected to Intrastriatal Injections of the Parkinsonogenic Toxin MPP+" Cells 9, no. 11: 2418. https://doi.org/10.3390/cells9112418
APA StylePrydz, A., Stahl, K., Zahl, S., Skauli, N., Skare, Ø., Ottersen, O. P., & Amiry-Moghaddam, M. (2020). Pro-Inflammatory Role of AQP4 in Mice Subjected to Intrastriatal Injections of the Parkinsonogenic Toxin MPP+. Cells, 9(11), 2418. https://doi.org/10.3390/cells9112418