The Tubulin Code in Mitosis and Cancer
Abstract
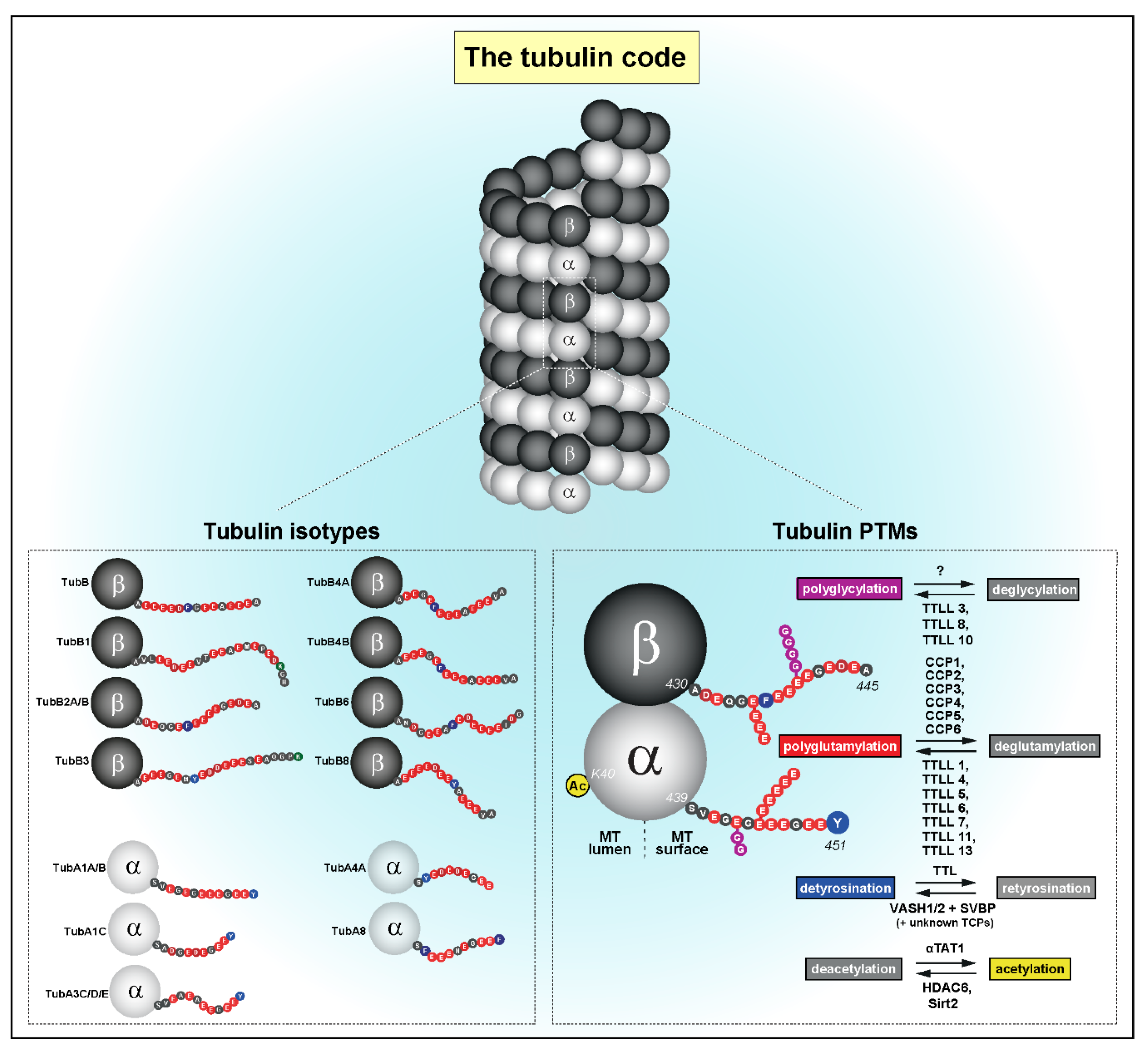
1. The Tubulin Code
2. The Tubulin Code in Mitosis
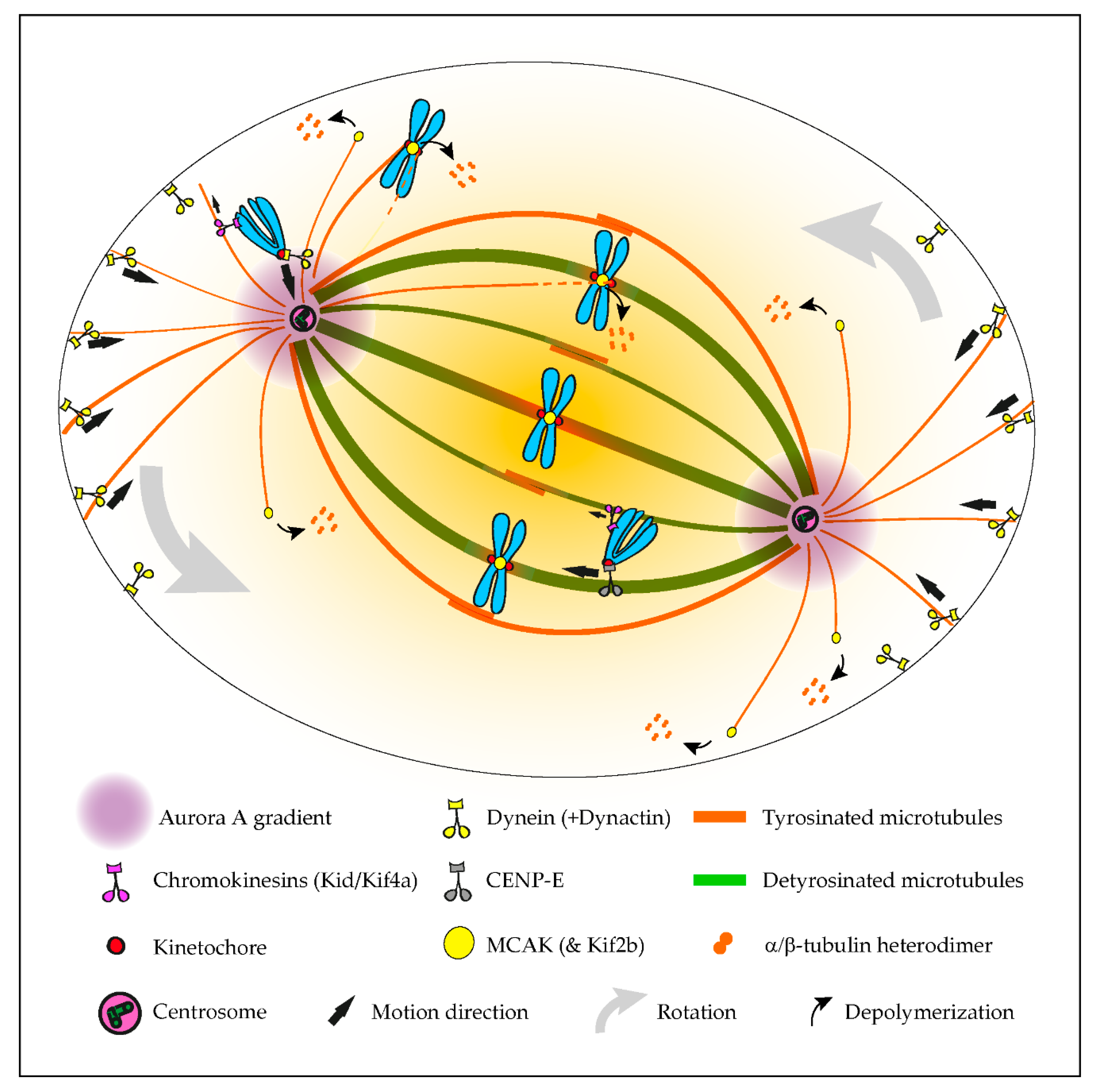
2.1. A Navigation System Guides Chromosomes to the Spindle Equator
2.2. A Mitotic Error Code
2.3. Role in Mitotic Spindle Orientation and Positioning
2.4. Roles in Centrosome Structure and Cytokinesis
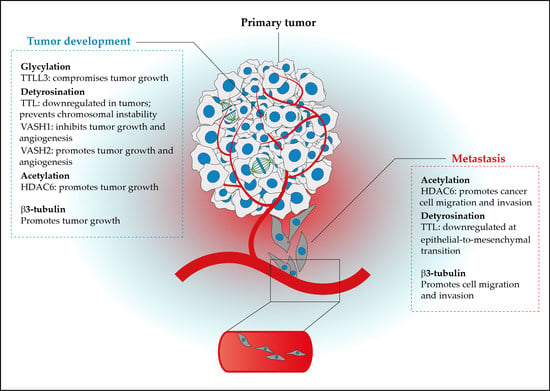
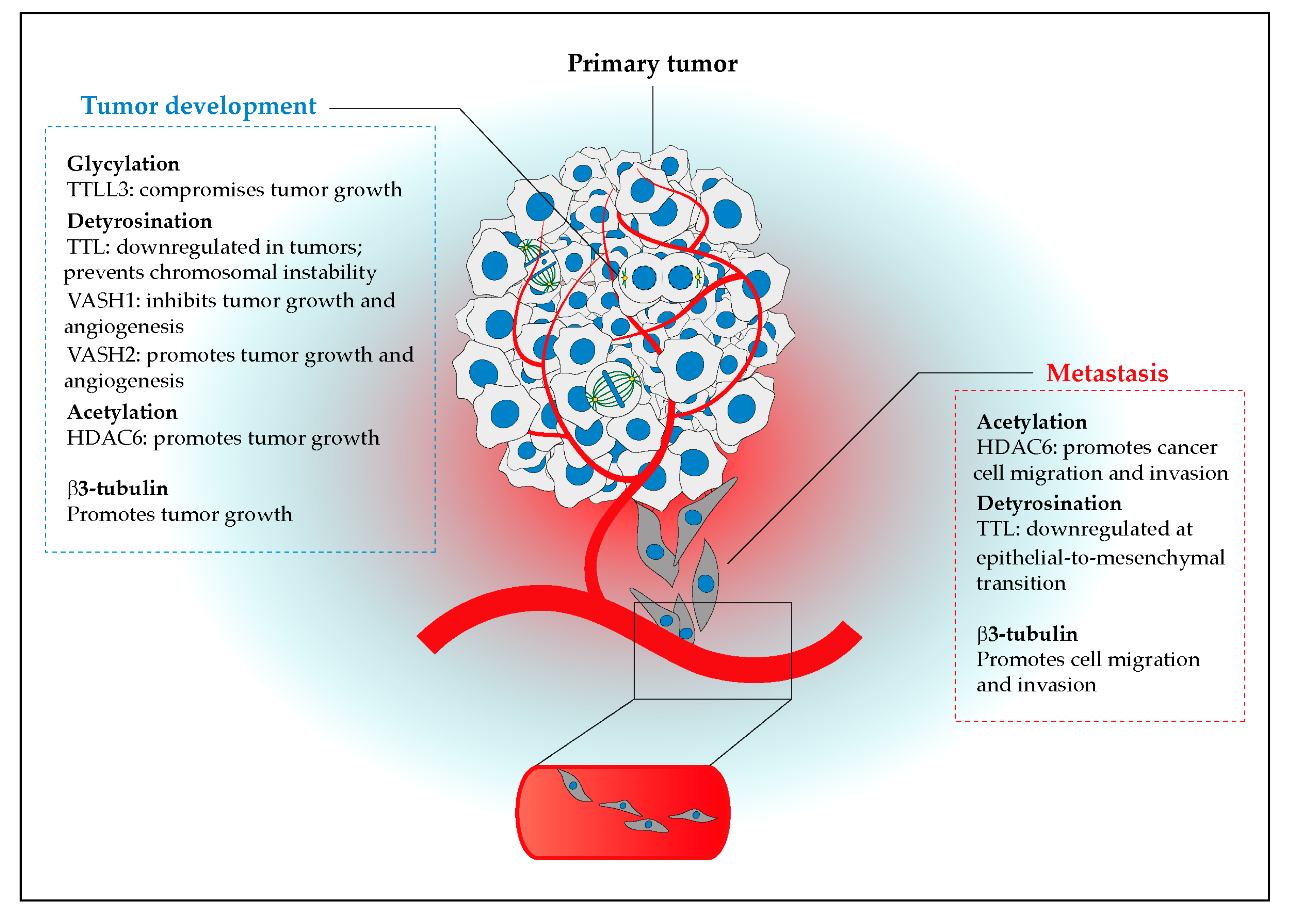
3. The Cancer Tubulin Code
3.1. (De)Regulation of Tubulin Isotypes and PTMs in Cancer
3.2. Functional Implications of the Cancer Tubulin Code
3.3. The Cancer Tubulin Code in Cell Migration and Invasion
4. How Alterations of the Tubulin Code in Mitosis Might Be Implicated in Cancer
5. Conclusions and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Desai, A.; Mitchison, T.J. Microtubule polymerization dynamics. Annu. Rev. Cell Dev. Biol. 1997, 13, 83–117. [Google Scholar] [CrossRef] [PubMed]
- Ludueña, R.F.; Banerjee, A. The isotypes of tubulin. In The Role of Microtubules in Cell Biology, Neurobiology, and Oncology; Springer: Berlin/Heidelberg, Germany, 2008; pp. 123–175. [Google Scholar]
- Janke, C. The tubulin code: Molecular components, readout mechanisms, and functions. J. Cell Biol. 2014, 206, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Verhey, K.J.; Gaertig, J. The tubulin code. Cell Cycle 2007, 6, 2152–2160. [Google Scholar] [CrossRef] [PubMed]
- Janke, C.; Magiera, M.M. The tubulin code and its role in controlling microtubule properties and functions. Nat. Rev. Mol. Cell Biol. 2020, 21, 307–326. [Google Scholar] [CrossRef]
- L’Hernault, S.W.; Rosenbaum, J.L. Chlamydomonas alpha-tubulin is posttranslationally modified by acetylation on the epsilon-amino group of a lysine. Biochemistry 1985, 24, 473–478. [Google Scholar] [CrossRef]
- Soppina, V.; Herbstman, J.F.; Skiniotis, G.; Verhey, K.J. Luminal localization of α-tubulin k40 acetylation by cryo-em analysis of fab-labeled microtubules. PLoS ONE 2012, 7, e48204. [Google Scholar] [CrossRef]
- Chu, C.W.; Hou, F.; Zhang, J.; Phu, L.; Loktev, A.V.; Kirkpatrick, D.S.; Jackson, P.K.; Zhao, Y.; Zou, H. A novel acetylation of beta-tubulin by san modulates microtubule polymerization via down-regulating tubulin incorporation. Mol. Biol. Cell 2011, 22, 448–456. [Google Scholar] [CrossRef]
- Akella, J.S.; Wloga, D.; Kim, J.; Starostina, N.G.; Lyons-Abbott, S.; Morrissette, N.S.; Dougan, S.T.; Kipreos, E.T.; Gaertig, J. Mec-17 is an alpha-tubulin acetyltransferase. Nature 2010, 467, 218–222. [Google Scholar] [CrossRef]
- Shida, T.; Cueva, J.G.; Xu, Z.; Goodman, M.B.; Nachury, M.V. The major alpha-tubulin k40 acetyltransferase alphatat1 promotes rapid ciliogenesis and efficient mechanosensation. Proc. Natl. Acad. Sci. USA 2010, 107, 21517–21522. [Google Scholar] [CrossRef]
- North, B.J.; Marshall, B.L.; Borra, M.T.; Denu, J.M.; Verdin, E. The human sir2 ortholog, sirt2, is an nad+-dependent tubulin deacetylase. Mol. Cell 2003, 11, 437–444. [Google Scholar] [CrossRef]
- Hubbert, C.; Guardiola, A.; Shao, R.; Kawaguchi, Y.; Ito, A.; Nixon, A.; Yoshida, M.; Wang, X.F.; Yao, T.P. Hdac6 is a microtubule-associated deacetylase. Nature 2002, 417, 455–458. [Google Scholar] [CrossRef]
- Aillaud, C.; Bosc, C.; Peris, L.; Bosson, A.; Heemeryck, P.; Van Dijk, J.; Le Friec, J.; Boulan, B.; Vossier, F.; Sanman, L.E.; et al. Vasohibins/svbp are tubulin carboxypeptidases (tcps) that regulate neuron differentiation. Science 2017, 358, 1448–1453. [Google Scholar] [CrossRef] [PubMed]
- Nieuwenhuis, J.; Adamopoulos, A.; Bleijerveld, O.B.; Mazouzi, A.; Stickel, E.; Celie, P.; Altelaar, M.; Knipscheer, P.; Perrakis, A.; Blomen, V.A.; et al. Vasohibins encode tubulin detyrosinating activity. Science 2017, 358, 1453–1456. [Google Scholar] [CrossRef]
- Li, F.; Li, Y.; Ye, X.; Gao, H.; Shi, Z.; Luo, X.; Rice, L.M.; Yu, H. Cryo-em structure of vash1-svbp bound to microtubules. eLife 2020, 9, e58157. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, H.; Zhu, J.; Xie, Y.; Liang, X.; Chen, Z.; Feng, Y.; Zhang, Y. Structural insights into tubulin detyrosination by vasohibins-svbp complex. Cell Discov. 2019, 5, 65. [Google Scholar] [CrossRef]
- Wang, N.; Bosc, C.; Ryul Choi, S.; Boulan, B.; Peris, L.; Olieric, N.; Bao, H.; Krichen, F.; Chen, L.; Andrieux, A.; et al. Structural basis of tubulin detyrosination by the vasohibin-svbp enzyme complex. Nat. Struct. Mol. Biol. 2019, 26, 571–582. [Google Scholar] [CrossRef]
- Li, F.; Hu, Y.; Qi, S.; Luo, X.; Yu, H. Structural basis of tubulin detyrosination by vasohibins. Nat. Struct. Mol. Biol. 2019, 26, 583–591. [Google Scholar] [CrossRef]
- Liao, S.; Rajendraprasad, G.; Wang, N.; Eibes, S.; Gao, J.; Yu, H.; Wu, G.; Tu, X.; Huang, H.; Barisic, M.; et al. Molecular basis of vasohibins-mediated detyrosination and its impact on spindle function and mitosis. Cell Res. 2019, 29, 533–547. [Google Scholar] [CrossRef]
- Ersfeld, K.; Wehland, J.; Plessmann, U.; Dodemont, H.; Gerke, V.; Weber, K. Characterization of the tubulin-tyrosine ligase. J. Cell Biol. 1993, 120, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Schroder, H.C.; Wehland, J.; Weber, K. Purification of brain tubulin-tyrosine ligase by biochemical and immunological methods. J. Cell Biol. 1985, 100, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Rogowski, K.; van Dijk, J.; Magiera, M.M.; Bosc, C.; Deloulme, J.C.; Bosson, A.; Peris, L.; Gold, N.D.; Lacroix, B.; Bosch Grau, M.; et al. A family of protein-deglutamylating enzymes associated with neurodegeneration. Cell 2010, 143, 564–578. [Google Scholar] [CrossRef]
- Tort, O.; Tanco, S.; Rocha, C.; Bieche, I.; Seixas, C.; Bosc, C.; Andrieux, A.; Moutin, M.J.; Aviles, F.X.; Lorenzo, J.; et al. The cytosolic carboxypeptidases ccp2 and ccp3 catalyze posttranslational removal of acidic amino acids. Mol. Biol. Cell 2014, 25, 3017–3027. [Google Scholar] [CrossRef] [PubMed]
- Paturle-Lafanechere, L.; Edde, B.; Denoulet, P.; Van Dorsselaer, A.; Mazarguil, H.; Le Caer, J.P.; Wehland, J.; Job, D. Characterization of a major brain tubulin variant which cannot be tyrosinated. Biochemistry 1991, 30, 10523–10528. [Google Scholar] [CrossRef] [PubMed]
- Aillaud, C.; Bosc, C.; Saoudi, Y.; Denarier, E.; Peris, L.; Sago, L.; Taulet, N.; Cieren, A.; Tort, O.; Magiera, M.M.; et al. Evidence for new c-terminally truncated variants of alpha- and beta-tubulins. Mol. Biol. Cell 2016, 27, 640–653. [Google Scholar] [CrossRef]
- Edde, B.; Rossier, J.; Le Caer, J.P.; Desbruyeres, E.; Gros, F.; Denoulet, P. Posttranslational glutamylation of alpha-tubulin. Science 1990, 247, 83–85. [Google Scholar] [CrossRef]
- Redeker, V.; Levilliers, N.; Schmitter, J.M.; Le Caer, J.P.; Rossier, J.; Adoutte, A.; Bre, M.H. Polyglycylation of tubulin: A posttranslational modification in axonemal microtubules. Science 1994, 266, 1688–1691. [Google Scholar] [CrossRef] [PubMed]
- Janke, C.; Rogowski, K.; Wloga, D.; Regnard, C.; Kajava, A.V.; Strub, J.M.; Temurak, N.; van Dijk, J.; Boucher, D.; van Dorsselaer, A.; et al. Tubulin polyglutamylase enzymes are members of the ttl domain protein family. Science 2005, 308, 1758–1762. [Google Scholar] [CrossRef]
- Van Dijk, J.; Rogowski, K.; Miro, J.; Lacroix, B.; Edde, B.; Janke, C. A targeted multienzyme mechanism for selective microtubule polyglutamylation. Mol. Cell 2007, 26, 437–448. [Google Scholar] [CrossRef]
- Kimura, Y.; Kurabe, N.; Ikegami, K.; Tsutsumi, K.; Konishi, Y.; Kaplan, O.I.; Kunitomo, H.; Iino, Y.; Blacque, O.E.; Setou, M. Identification of tubulin deglutamylase among caenorhabditis elegans and mammalian cytosolic carboxypeptidases (ccps). J. Biol. Chem. 2010, 285, 22936–22941. [Google Scholar] [CrossRef]
- Rogowski, K.; Juge, F.; van Dijk, J.; Wloga, D.; Strub, J.M.; Levilliers, N.; Thomas, D.; Bre, M.H.; Van Dorsselaer, A.; Gaertig, J.; et al. Evolutionary divergence of enzymatic mechanisms for posttranslational polyglycylation. Cell 2009, 137, 1076–1087. [Google Scholar] [CrossRef]
- Wloga, D.; Webster, D.M.; Rogowski, K.; Bre, M.H.; Levilliers, N.; Jerka-Dziadosz, M.; Janke, C.; Dougan, S.T.; Gaertig, J. Ttll3 is a tubulin glycine ligase that regulates the assembly of cilia. Dev. Cell 2009, 16, 867–876. [Google Scholar] [CrossRef] [PubMed]
- Walgren, J.L.; Vincent, T.S.; Schey, K.L.; Buse, M.G. High glucose and insulin promote o-glcnac modification of proteins, including alpha-tubulin. Am. J. Physiol. Endocrinol. Metab. 2003, 284, E424–E434. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.L.; Qin, H. O-glcnacylation regulates primary ciliary length by promoting microtubule disassembly. iScience 2019, 12, 379–391. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; Kang, J.G.; Park, S.Y.; Lee, J.; Oh, Y.J.; Cho, J.W. O-glcnacylation of tubulin inhibits its polymerization. Amino Acids 2011, 40, 809–818. [Google Scholar] [CrossRef]
- Barisic, M.; Silva e Sousa, R.; Tripathy, S.K.; Magiera, M.M.; Zaytsev, A.V.; Pereira, A.L.; Janke, C.; Grishchuk, E.L.; Maiato, H. Mitosis. Microtubule detyrosination guides chromosomes during mitosis. Science 2015, 348, 799–803. [Google Scholar] [CrossRef]
- Ferreira, L.T.; Orr, B.; Rajendraprasad, G.; Pereira, A.J.; Lemos, C.; Lima, J.T.; Guasch Boldu, C.; Ferreira, J.G.; Barisic, M.; Maiato, H. Alpha-tubulin detyrosination impairs mitotic error correction by suppressing mcak centromeric activity. J. Cell Biol. 2020, 219, e201910064. [Google Scholar] [CrossRef]
- Gundersen, G.G.; Bulinski, J.C. Distribution of tyrosinated and nontyrosinated alpha-tubulin during mitosis. J. Cell Biol. 1986, 102, 1118–1126. [Google Scholar] [CrossRef]
- Gundersen, G.G.; Kalnoski, M.H.; Bulinski, J.C. Distinct populations of microtubules: Tyrosinated and nontyrosinated alpha tubulin are distributed differently in vivo. Cell 1984, 38, 779–789. [Google Scholar] [CrossRef]
- Peris, L.; Thery, M.; Faure, J.; Saoudi, Y.; Lafanechere, L.; Chilton, J.K.; Gordon-Weeks, P.; Galjart, N.; Bornens, M.; Wordeman, L.; et al. Tubulin tyrosination is a major factor affecting the recruitment of cap-gly proteins at microtubule plus ends. J. Cell Biol. 2006, 174, 839–849. [Google Scholar] [CrossRef]
- Wilson, P.J.; Forer, A. Acetylated alpha-tubulin in spermatogenic cells of the crane fly nephrotoma-suturalis-kinetochore microtubules are selectively acetylated. Cell Motil. Cytoskelet. 1989, 14, 237–250. [Google Scholar] [CrossRef]
- Bobinnec, Y.; Moudjou, M.; Fouquet, J.P.; Desbruyeres, E.; Edde, B.; Bornens, M. Glutamylation of centriole and cytoplasmic tubulin in proliferating non-neuronal cells. Cell Motil. Cytoskelet. 1998, 39, 223–232. [Google Scholar] [CrossRef]
- Ferreira, L.T.; Figueiredo, A.C.; Orr, B.; Lopes, D.; Maiato, H. Dissecting the role of the tubulin code in mitosis. In Methods in Cell Biology; Elsevier: Amsterdam, The Netherlands, 2018; Volume 144, pp. 33–74. [Google Scholar]
- Maiato, H.; Sampaio, P.; Sunkel, C.E. Microtubule-associated proteins and their essential roles during mitosis. Int. Rev. Cytol. 2004, 241, 53–153. [Google Scholar]
- Cross, R.A.; McAinsh, A. Prime movers: The mechanochemistry of mitotic kinesins. Nat. Rev. 2014, 15, 257–271. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Tulu, U.S.; Wadsworth, P.; Rieder, C.L. Kinetochore dynein is required for chromosome motion and congression independent of the spindle checkpoint. Curr. Biol. 2007, 17, 973–980. [Google Scholar] [CrossRef]
- Hayden, J.H.; Bowser, S.S.; Rieder, C.L. Kinetochores capture astral microtubules during chromosome attachment to the mitotic spindle: Direct visualization in live newt lung cells. J. Cell Biol. 1990, 111, 1039–1045. [Google Scholar] [CrossRef]
- Li, Y.; Yu, W.; Liang, Y.; Zhu, X. Kinetochore dynein generates a poleward pulling force to facilitate congression and full chromosome alignment. Cell Res. 2007, 17, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Vorozhko, V.V.; Emanuele, M.J.; Kallio, M.J.; Stukenberg, P.T.; Gorbsky, G.J. Multiple mechanisms of chromosome movement in vertebrate cells mediated through the ndc80 complex and dynein/dynactin. Chromosoma 2008, 117, 169–179. [Google Scholar] [CrossRef]
- Pfarr, C.M.; Coue, M.; Grissom, P.M.; Hays, T.S.; Porter, M.E.; McIntosh, J.R. Cytoplasmic dynein is localized to kinetochores during mitosis. Nature 1990, 345, 263–265. [Google Scholar] [CrossRef]
- Steuer, E.R.; Wordeman, L.; Schroer, T.A.; Sheetz, M.P. Localization of cytoplasmic dynein to mitotic spindles and kinetochores. Nature 1990, 345, 266–268. [Google Scholar] [CrossRef]
- Kapoor, T.M.; Lampson, M.A.; Hergert, P.; Cameron, L.; Cimini, D.; Salmon, E.D.; McEwen, B.F.; Khodjakov, A. Chromosomes can congress to the metaphase plate before biorientation. Science 2006, 311, 388–391. [Google Scholar] [CrossRef] [PubMed]
- Wood, K.W.; Sakowicz, R.; Goldstein, L.S.; Cleveland, D.W. Cenp-e is a plus end-directed kinetochore motor required for metaphase chromosome alignment. Cell 1997, 91, 357–366. [Google Scholar] [CrossRef]
- Mann, B.J.; Wadsworth, P. Kinesin-5 regulation and function in mitosis. Trends Cell Biol. 2019, 29, 66–79. [Google Scholar] [CrossRef]
- Bakhoum, S.F.; Thompson, S.L.; Manning, A.L.; Compton, D.A. Genome stability is ensured by temporal control of kinetochore-microtubule dynamics. Nat. Cell Biol. 2009, 11, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Kline-Smith, S.L.; Khodjakov, A.; Hergert, P.; Walczak, C.E. Depletion of centromeric mcak leads to chromosome congression and segregation defects due to improper kinetochore attachments. Mol. Biol. Cell 2004, 15, 1146–1159. [Google Scholar] [CrossRef]
- Lan, W.; Zhang, X.; Kline-Smith, S.L.; Rosasco, S.E.; Barrett-Wilt, G.A.; Shabanowitz, J.; Hunt, D.F.; Walczak, C.E.; Stukenberg, P.T. Aurora b phosphorylates centromeric mcak and regulates its localization and microtubule depolymerization activity. Curr. Biol. 2004, 14, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Andrews, P.D.; Ovechkina, Y.; Morrice, N.; Wagenbach, M.; Duncan, K.; Wordeman, L.; Swedlow, J.R. Aurora b regulates mcak at the mitotic centromere. Dev. Cell 2004, 6, 253–268. [Google Scholar] [CrossRef]
- Domnitz, S.B.; Wagenbach, M.; Decarreau, J.; Wordeman, L. Mcak activity at microtubule tips regulates spindle microtubule length to promote robust kinetochore attachment. J. Cell Biol. 2012, 197, 231–237. [Google Scholar] [CrossRef]
- Reed, N.A.; Cai, D.; Blasius, T.L.; Jih, G.T.; Meyhofer, E.; Gaertig, J.; Verhey, K.J. Microtubule acetylation promotes kinesin-1 binding and transport. Curr. Biol. 2006, 16, 2166–2172. [Google Scholar] [CrossRef]
- Konishi, Y.; Setou, M. Tubulin tyrosination navigates the kinesin-1 motor domain to axons. Nat. Neurosci. 2009, 12, 559–567. [Google Scholar] [CrossRef]
- McKenney, R.J.; Huynh, W.; Tanenbaum, M.E.; Bhabha, G.; Vale, R.D. Activation of cytoplasmic dynein motility by dynactin-cargo adapter complexes. Science 2014, 345, 337–341. [Google Scholar] [CrossRef]
- McKenney, R.J.; Huynh, W.; Vale, R.D.; Sirajuddin, M. Tyrosination of alpha-tubulin controls the initiation of processive dynein-dynactin motility. EMBO J. 2016, 35, 1175–1185. [Google Scholar] [CrossRef]
- Nirschl, J.J.; Magiera, M.M.; Lazarus, J.E.; Janke, C.; Holzbaur, E.L. Alpha-tubulin tyrosination and clip-170 phosphorylation regulate the initiation of dynein-driven transport in neurons. Cell Rep. 2016, 14, 2637–2652. [Google Scholar] [CrossRef] [PubMed]
- Barisic, M.; Maiato, H. The tubulin code: A navigation system for chromosomes during mitosis. Trends Cell Biol. 2016, 26, 766–775. [Google Scholar] [CrossRef]
- Barisic, M.; Aguiar, P.; Geley, S.; Maiato, H. Kinetochore motors drive congression of peripheral polar chromosomes by overcoming random arm-ejection forces. Nat. Cell Biol. 2014, 16, 1249–1256. [Google Scholar] [CrossRef]
- Chmatal, L.; Yang, K.; Schultz, R.M.; Lampson, M.A. Spatial regulation of kinetochore microtubule attachments by destabilization at spindle poles in meiosis i. Curr. Biol. 2015, 25, 1835–1841. [Google Scholar] [CrossRef] [PubMed]
- Ye, A.A.; Deretic, J.; Hole, C.M.; Hinman, A.W.; Cimini, D.; Welburn, J.P.; Maresca, T.J. Aurora a kinase contributes to a pole-based error correction pathway. Curr. Biol. 2015, 25, 1842–1851. [Google Scholar] [CrossRef]
- Kim, Y.; Holland, A.J.; Lan, W.; Cleveland, D.W. Aurora kinases and protein phosphatase 1 mediate chromosome congression through regulation of cenp-e. Cell 2010, 142, 444–455. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.; Sousa, M.; Almeida, A.C.; Ferreira, L.T.; Costa, A.R.; Novais-Cruz, M.; Ferras, C.; Sousa, M.M.; Sampaio, P.; Belsley, M.; et al. Coherent-hybrid sted: High contrast sub-diffraction imaging using a bi-vortex depletion beam. Opt. Express 2019, 27, 8092–8111. [Google Scholar] [CrossRef]
- Steblyanko, Y.; Rajendraprasad, G.; Osswald, M.; Eibes, S.; Jacome, A.; Geley, S.; Pereira, A.J.; Maiato, H.; Barisic, M. Microtubule poleward flux in human cells is driven by the coordinated action of four kinesins. EMBO J. 2020, e105432. [Google Scholar] [CrossRef]
- Bakhoum, S.F.; Genovese, G.; Compton, D.A. Deviant kinetochore microtubule dynamics underlie chromosomal instability. Curr. Biol. 2009, 19, 1937–1942. [Google Scholar] [CrossRef]
- Bakhoum, S.F.; Ngo, B.; Laughney, A.M.; Cavallo, J.A.; Murphy, C.J.; Ly, P.; Shah, P.; Sriram, R.K.; Watkins, T.B.K.; Taunk, N.K.; et al. Chromosomal instability drives metastasis through a cytosolic DNA response. Nature 2018, 553, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Peris, L.; Wagenbach, M.; Lafanechere, L.; Brocard, J.; Moore, A.T.; Kozielski, F.; Job, D.; Wordeman, L.; Andrieux, A. Motor-dependent microtubule disassembly driven by tubulin tyrosination. J. Cell Biol. 2009, 185, 1159–1166. [Google Scholar] [CrossRef] [PubMed]
- Sirajuddin, M.; Rice, L.M.; Vale, R.D. Regulation of microtubule motors by tubulin isotypes and post-translational modifications. Nat. Cell Biol. 2014, 16, 335–344. [Google Scholar] [CrossRef]
- Silkworth, W.T.; Nardi, I.K.; Paul, R.; Mogilner, A.; Cimini, D. Timing of centrosome separation is important for accurate chromosome segregation. Mol. Biol. Cell 2012, 23, 401–411. [Google Scholar] [CrossRef]
- Nunes, V.; Dantas, M.; Castro, D.; Vitiello, E.; Wang, I.; Carpi, N.; Balland, M.; Piel, M.; Aguiar, P.; Maiato, H.; et al. Centrosome-nuclear axis repositioning drives the assembly of a bipolar spindle scaffold to ensure mitotic fidelity. Mol. Biol. Cell 2020, 31, 1675–1690. [Google Scholar] [CrossRef]
- Raaijmakers, J.A.; van Heesbeen, R.G.H.P.; Meaders, J.L.; Geers, E.F.; Fernandez-Garcia, B.; Medema, R.H.; Tanenbaum, M.E. Nuclear envelope-associated dynein drives prophase centrosome separation and enables eg5-independent bipolar spindle formation. EMBO J. 2012, 31, 4179–4190. [Google Scholar] [CrossRef]
- Kahn, O.I.; Sharma, V.; Gonzalez-Billault, C.; Baas, P.W. Effects of kinesin-5 inhibition on dendritic architecture and microtubule organization. Mol. Biol. Cell 2015, 26, 66–77. [Google Scholar] [CrossRef]
- Siller, K.H.; Doe, C.Q. Spindle orientation during asymmetric cell division. Nat. Cell Biol. 2009, 11, 365–374. [Google Scholar] [CrossRef]
- Kotak, S.; Busso, C.; Gonczy, P. Cortical dynein is critical for proper spindle positioning in human cells. J. Cell Biol. 2012, 199, 97–110. [Google Scholar] [CrossRef]
- Nguyen-Ngoc, T.; Afshar, K.; Gonczy, P. Coupling of cortical dynein and g alpha proteins mediates spindle positioning in caenorhabditis elegans. Nat. Cell Biol. 2007, 9, 1294–1302. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, D.J.; Duro, J.; Prevo, B.; Cheerambathur, D.K.; Carvalho, A.X.; Gassmann, R. Dynactin binding to tyrosinated microtubules promotes centrosome centration in c. Elegans by enhancing dynein-mediated organelle transport. PLoS Genet. 2017, 13, e1006941. [Google Scholar] [CrossRef]
- Bobinnec, Y.; Khodjakov, A.; Mir, L.M.; Rieder, C.L.; Edde, B.; Bornens, M. Centriole disassembly in vivo and its effect on centrosome structure and function in vertebrate cells. J. Cell Biol. 1998, 143, 1575–1589. [Google Scholar] [CrossRef] [PubMed]
- Abal, M.; Keryer, G.; Bornens, M. Centrioles resist forces applied on centrosomes during g2/m transition. Biol. Cell 2005, 97, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Mahecic, D.; Gambarotto, D.; Douglass, K.M.; Fortun, D.; Banterle, N.; Ibrahim, K.A.; Le Guennec, M.; Gonczy, P.; Hamel, V.; Guichard, P.; et al. Homogeneous multifocal excitation for high-throughput super-resolution imaging. Nat. Methods 2020, 17, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, B.; van Dijk, J.; Gold, N.D.; Guizetti, J.; Aldrian-Herrada, G.; Rogowski, K.; Gerlich, D.W.; Janke, C. Tubulin polyglutamylation stimulates spastin-mediated microtubule severing. J. Cell Biol. 2010, 189, 945–954. [Google Scholar] [CrossRef]
- Sharma, N.; Bryant, J.; Wloga, D.; Donaldson, R.; Davis, R.C.; Jerka-Dziadosz, M.; Gaertig, J. Katanin regulates dynamics of microtubules and biogenesis of motile cilia. J. Cell Biol. 2007, 178, 1065–1079. [Google Scholar] [CrossRef]
- Shin, S.C.; Im, S.K.; Jang, E.H.; Jin, K.S.; Hur, E.M.; Kim, E.E. Structural and molecular basis for katanin-mediated severing of glutamylated microtubules. Cell Rep. 2019, 26, 1357–1367. [Google Scholar] [CrossRef]
- Valenstein, M.L.; Roll-Mecak, A. Graded control of microtubule severing by tubulin glutamylation. Cell 2016, 164, 911–921. [Google Scholar] [CrossRef]
- Jiang, K.; Rezabkova, L.; Hua, S.S.; Liu, Q.Y.; Capitani, G.; Altelaar, A.F.M.; Heck, A.J.R.; Kammerer, R.A.; Steinmetz, M.O.; Akhmanova, A. Microtubule minus-end regulation at spindle poles by an aspm-katanin complex. Nat. Cell Biol. 2017, 19, 873. [Google Scholar] [CrossRef]
- McNally, K.; Audhya, A.; Oegema, K.; McNally, F.J. Katanin controls mitotic and meiotic spindle length. J. Cell Biol. 2006, 175, 881–891. [Google Scholar] [CrossRef]
- Zhang, D.; Rogers, G.C.; Buster, D.W.; Sharp, D.J. Three microtubule severing enzymes contribute to the "pacman-flux" machinery that moves chromosomes. J. Cell Biol. 2007, 177, 231–242. [Google Scholar] [CrossRef]
- Connell, J.W.; Lindon, C.; Luzio, J.P.; Reid, E. Spastin couples microtubule severing to membrane traffic in completion of cytokinesis and secretion. Traffic 2009, 10, 42–56. [Google Scholar] [CrossRef]
- Guizetti, J.; Schermelleh, L.; Mantler, J.; Maar, S.; Poser, I.; Leonhardt, H.; Muller-Reichert, T.; Gerlich, D.W. Cortical constriction during abscission involves helices of escrt-iii-dependent filaments. Science 2011, 331, 1616–1620. [Google Scholar] [CrossRef]
- Matsuo, M.; Shimodaira, T.; Kasama, T.; Hata, Y.; Echigo, A.; Okabe, M.; Arai, K.; Makino, Y.; Niwa, S.I.; Saya, H.; et al. Katanin p60 contributes to microtubule instability around the midbody and facilitates cytokinesis in rat cells. PLoS ONE 2013, 8, e80392. [Google Scholar] [CrossRef][Green Version]
- Thazhath, R.; Liu, C.B.; Gaertig, J. Polyglycylation domain of beta-tubulin maintains axonemal architecture and affects cytokinesis in tetrahymena. Nat. Cell Biol. 2002, 4, 256–259. [Google Scholar] [CrossRef] [PubMed]
- Boggs, A.E.; Vitolo, M.I.; Whipple, R.A.; Charpentier, M.S.; Goloubeva, O.G.; Ioffe, O.B.; Tuttle, K.C.; Slovic, J.; Lu, Y.; Mills, G.B.; et al. Alpha-tubulin acetylation elevated in metastatic and basal-like breast cancer cells promotes microtentacle formation, adhesion, and invasive migration. Cancer Res. 2015, 75, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Mialhe, A.; Lafanechere, L.; Treilleux, I.; Peloux, N.; Dumontet, C.; Bremond, A.; Panh, M.H.; Payan, R.; Wehland, J.; Margolis, R.L.; et al. Tubulin detyrosination is a frequent occurrence in breast cancers of poor prognosis. Cancer Res. 2001, 61, 5024–5027. [Google Scholar]
- Kato, C.; Miyazaki, K.; Nakagawa, A.; Ohira, M.; Nakamura, Y.; Ozaki, T.; Imai, T.; Nakagawara, A. Low expression of human tubulin tyrosine ligase and suppressed tubulin tyrosination/detyrosination cycle are associated with impaired neuronal differentiation in neuroblastomas with poor prognosis. Int. J. Cancer 2004, 112, 365–375. [Google Scholar] [CrossRef]
- Soucek, K.; Kamaid, A.; Phung, A.D.; Kubala, L.; Bulinski, J.C.; Harper, R.W.; Eiserich, J.P. Normal and prostate cancer cells display distinct molecular profiles of alpha-tubulin posttranslational modifications. Prostate 2006, 66, 954–965. [Google Scholar] [CrossRef] [PubMed]
- Whipple, R.A.; Matrone, M.A.; Cho, E.H.; Balzer, E.M.; Vitolo, M.I.; Yoon, J.R.; Ioffe, O.B.; Tuttle, K.C.; Yang, J.; Martin, S.S. Epithelial-to-mesenchymal transition promotes tubulin detyrosination and microtentacles that enhance endothelial engagement. Cancer Res. 2010, 70, 8127–8137. [Google Scholar] [CrossRef]
- Xue, X.; Gao, W.; Sun, B.; Xu, Y.; Han, B.; Wang, F.; Zhang, Y.; Sun, J.; Wei, J.; Lu, Z.; et al. Vasohibin 2 is transcriptionally activated and promotes angiogenesis in hepatocellular carcinoma. Oncogene 2013, 32, 1724–1734. [Google Scholar] [CrossRef] [PubMed]
- Li, D.W.; Sun, X.D.; Zhang, L.L.; Yan, B.; Xie, S.B.; Liu, R.M.; Liu, M.; Zhou, J. Histone deacetylase 6 and cytoplasmic linker protein 170 function together to regulate the motility of pancreatic cancer cells. Protein Cell 2014, 5, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Hu, P.; Tang, F.; Lian, H.; Chen, X.; Zhang, Y.; He, X.; Liu, W.; Xie, C. Hdac6 promotes cell proliferation and confers resistance to temozolomide in glioblastoma. Cancer Lett. 2016, 379, 134–142. [Google Scholar] [CrossRef]
- Gradilone, S.A.; Radtke, B.N.; Bogert, P.S.; Huang, B.Q.; Gajdos, G.B.; LaRusso, N.F. Hdac6 inhibition restores ciliary expression and decreases tumor growth. Cancer Res. 2013, 73, 2259–2270. [Google Scholar] [CrossRef] [PubMed]
- Kashiwaya, K.; Nakagawa, H.; Hosokawa, M.; Mochizuki, Y.; Ueda, K.; Piao, L.; Chung, S.; Hamamoto, R.; Eguchi, H.; Ohigashi, H.; et al. Involvement of the tubulin tyrosine ligase-like family member 4 polyglutamylase in pelp1 polyglutamylation and chromatin remodeling in pancreatic cancer cells. Cancer Res. 2010, 70, 4024–4033. [Google Scholar] [CrossRef] [PubMed]
- Rocha, C.; Papon, L.; Cacheux, W.; Marques Sousa, P.; Lascano, V.; Tort, O.; Giordano, T.; Vacher, S.; Lemmers, B.; Mariani, P.; et al. Tubulin glycylases are required for primary cilia, control of cell proliferation and tumor development in colon. EMBO J. 2014, 33, 2247–2260. [Google Scholar] [CrossRef]
- McCarroll, J.A.; Sharbeen, G.; Liu, J.; Youkhana, J.; Goldstein, D.; McCarthy, N.; Limbri, L.F.; Dischl, D.; Ceyhan, G.O.; Erkan, M.; et al. Betaiii-tubulin: A novel mediator of chemoresistance and metastases in pancreatic cancer. Oncotarget 2015, 6, 2235–2249. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.M.; Cao, D.; Itami, A.; Pour, P.M.; Hruban, R.H.; Maitra, A.; Ouellette, M.M. Class iii beta-tubulin, a marker of resistance to paclitaxel, is overexpressed in pancreatic ductal adenocarcinoma and intraepithelial neoplasia. Histopathology 2007, 51, 539–546. [Google Scholar] [CrossRef]
- Kanojia, D.; Morshed, R.A.; Zhang, L.; Miska, J.M.; Qiao, J.; Kim, J.W.; Pytel, P.; Balyasnikova, I.V.; Lesniak, M.S.; Ahmed, A.U. Betaiii-tubulin regulates breast cancer metastases to the brain. Mol. Cancer Ther. 2015, 14, 1152–1161. [Google Scholar] [CrossRef]
- McCarroll, J.A.; Gan, P.P.; Liu, M.; Kavallaris, M. Betaiii-tubulin is a multifunctional protein involved in drug sensitivity and tumorigenesis in non-small cell lung cancer. Cancer Res. 2010, 70, 4995–5003. [Google Scholar] [CrossRef]
- Ferrandina, G.; Zannoni, G.F.; Martinelli, E.; Paglia, A.; Gallotta, V.; Mozzetti, S.; Scambia, G.; Ferlini, C. Class iii beta-tubulin overexpression is a marker of poor clinical outcome in advanced ovarian cancer patients. Clin. Cancer Res. 2006, 12, 2774–2779. [Google Scholar] [CrossRef] [PubMed]
- Ruksha, K.; Mezheyeuski, A.; Nerovnya, A.; Bich, T.; Tur, G.; Gorgun, J.; Luduena, R.; Portyanko, A. Over-expression of betaii-tubulin and especially its localization in cell nuclei correlates with poorer outcomes in colorectal cancer. Cells 2019, 8, 25. [Google Scholar] [CrossRef] [PubMed]
- Parker, A.L.; Teo, W.S.; McCarroll, J.A.; Kavallaris, M. An emerging role for tubulin isotypes in modulating cancer biology and chemotherapy resistance. Int. J. Mol. Sci. 2017, 18, 1434. [Google Scholar] [CrossRef] [PubMed]
- Panda, D.; Miller, H.P.; Banerjee, A.; Luduena, R.F.; Wilson, L. Microtubule dynamics in vitro are regulated by the tubulin isotype composition. Proc. Natl. Acad. Sci. USA 1994, 91, 11358–11362. [Google Scholar] [CrossRef]
- Pamula, M.C.; Ti, S.C.; Kapoor, T.M. The structured core of human beta tubulin confers isotype-specific polymerization properties. J. Cell Biol. 2016, 213, 425–433. [Google Scholar] [CrossRef]
- Ti, S.C.; Alushin, G.M.; Kapoor, T.M. Human beta-tubulin isotypes can regulate microtubule protofilament number and stability. Dev. Cell 2018, 47, 175–190 e175. [Google Scholar] [CrossRef]
- Van Dijk, J.; Miro, J.; Strub, J.M.; Lacroix, B.; van Dorsselaer, A.; Edde, B.; Janke, C. Polyglutamylation is a post-translational modification with a broad range of substrates. J. Biol. Chem. 2008, 283, 3915–3922. [Google Scholar] [CrossRef]
- Regnard, C.; Desbruyeres, E.; Huet, J.C.; Beauvallet, C.; Pernollet, J.C.; Edde, B. Polyglutamylation of nucleosome assembly proteins. J. Biol. Chem. 2000, 275, 15969–15976. [Google Scholar] [CrossRef]
- Aguilar, A.; Becker, L.; Tedeschi, T.; Heller, S.; Iomini, C.; Nachury, M.V. Alpha-tubulin k40 acetylation is required for contact inhibition of proliferation and cell-substrate adhesion. Mol. Biol. Cell 2014, 25, 1854–1866. [Google Scholar] [CrossRef]
- Lee, Y.S.; Lim, K.H.; Guo, X.; Kawaguchi, Y.; Gao, Y.; Barrientos, T.; Ordentlich, P.; Wang, X.F.; Counter, C.M.; Yao, T.P. The cytoplasmic deacetylase hdac6 is required for efficient oncogenic tumorigenesis. Cancer Res. 2008, 68, 7561–7569. [Google Scholar] [CrossRef]
- Putcha, P.; Yu, J.; Rodriguez-Barrueco, R.; Saucedo-Cuevas, L.; Villagrasa, P.; Murga-Penas, E.; Quayle, S.N.; Yang, M.; Castro, V.; Llobet-Navas, D.; et al. Hdac6 activity is a non-oncogene addiction hub for inflammatory breast cancers. Breast Cancer Res. 2015, 17, 149. [Google Scholar] [CrossRef] [PubMed]
- Woan, K.V.; Lienlaf, M.; Perez-Villaroel, P.; Lee, C.; Cheng, F.; Knox, T.; Woods, D.M.; Barrios, K.; Powers, J.; Sahakian, E.; et al. Targeting histone deacetylase 6 mediates a dual anti-melanoma effect: Enhanced antitumor immunity and impaired cell proliferation. Mol. Oncol. 2015, 9, 1447–1457. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yuan, Z.; Zhang, Y.; Yong, S.; Salas-Burgos, A.; Koomen, J.; Olashaw, N.; Parsons, J.T.; Yang, X.J.; Dent, S.R.; et al. Hdac6 modulates cell motility by altering the acetylation level of cortactin. Mol. Cell 2007, 27, 197–213. [Google Scholar] [CrossRef]
- Lafanechere, L.; Courtay-Cahen, C.; Kawakami, T.; Jacrot, M.; Rudiger, M.; Wehland, J.; Job, D.; Margolis, R.L. Suppression of tubulin tyrosine ligase during tumor growth. J. Cell Sci. 1998, 111 Pt 2, 171–181. [Google Scholar]
- Sato, Y. The vasohibin family: A novel family for angiogenesis regulation. J. Biochem. 2013, 153, 5–11. [Google Scholar] [CrossRef]
- Kimura, H.; Miyashita, H.; Suzuki, Y.; Kobayashi, M.; Watanabe, K.; Sonoda, H.; Ohta, H.; Fujiwara, T.; Shimosegawa, T.; Sato, Y. Distinctive localization and opposed roles of vasohibin-1 and vasohibin-2 in the regulation of angiogenesis. Blood 2009, 113, 4810–4818. [Google Scholar] [CrossRef] [PubMed]
- Heishi, T.; Hosaka, T.; Suzuki, Y.; Miyashita, H.; Oike, Y.; Takahashi, T.; Nakamura, T.; Arioka, S.; Mitsuda, Y.; Takakura, T.; et al. Endogenous angiogenesis inhibitor vasohibin1 exhibits broad-spectrum antilymphangiogenic activity and suppresses lymph node metastasis. Am. J. Pathol. 2010, 176, 1950–1958. [Google Scholar] [CrossRef]
- Hosaka, T.; Kimura, H.; Heishi, T.; Suzuki, Y.; Miyashita, H.; Ohta, H.; Sonoda, H.; Moriya, T.; Suzuki, S.; Kondo, T.; et al. Vasohibin-1 expression in endothelium of tumor blood vessels regulates angiogenesis. Am. J. Pathol. 2009, 175, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Kitahara, S.; Suzuki, Y.; Morishima, M.; Yoshii, A.; Kikuta, S.; Shimizu, K.; Morikawa, S.; Sato, Y.; Ezaki, T. Vasohibin-2 modulates tumor onset in the gastrointestinal tract by normalizing tumor angiogenesis. Mol. Cancer 2014, 13, 99. [Google Scholar] [CrossRef]
- Koyanagi, T.; Suzuki, Y.; Saga, Y.; Machida, S.; Takei, Y.; Fujiwara, H.; Suzuki, M.; Sato, Y. In vivo delivery of sirna targeting vasohibin-2 decreases tumor angiogenesis and suppresses tumor growth in ovarian cancer. Cancer Sci. 2013, 104, 1705–1710. [Google Scholar] [CrossRef]
- Takahashi, Y.; Koyanagi, T.; Suzuki, Y.; Saga, Y.; Kanomata, N.; Moriya, T.; Suzuki, M.; Sato, Y. Vasohibin-2 expressed in human serous ovarian adenocarcinoma accelerates tumor growth by promoting angiogenesis. Mol. Cancer Res. 2012, 10, 1135–1146. [Google Scholar] [CrossRef]
- Kobayashi, M.; Wakabayashi, I.; Suzuki, Y.; Fujiwara, K.; Nakayama, M.; Watabe, T.; Sato, Y. Tubulin carboxypeptidase activity of vasohibin-1 inhibits angiogenesis by interfering with endocytosis and trafficking of pro-angiogenic factor receptors. Angiogenesis 2020. [Google Scholar] [CrossRef] [PubMed]
- Pagnamenta, A.T.; Heemeryck, P.; Martin, H.C.; Bosc, C.; Peris, L.; Uszynski, I.; Gory-Faure, S.; Couly, S.; Deshpande, C.; Siddiqui, A.; et al. Defective tubulin detyrosination causes structural brain abnormalities with cognitive deficiency in humans and mice. Hum. Mol. Genet. 2019, 28, 3391–3405. [Google Scholar] [CrossRef]
- Gu, S.; Liu, Y.; Zhu, B.; Ding, K.; Yao, T.P.; Chen, F.; Zhan, L.; Xu, P.; Ehrlich, M.; Liang, T.; et al. Loss of alpha-tubulin acetylation is associated with tgf-beta-induced epithelial-mesenchymal transition. J. Biol. Chem. 2016, 291, 5396–5405. [Google Scholar] [CrossRef]
- Bance, B.; Seetharaman, S.; Leduc, C.; Boeda, B.; Etienne-Manneville, S. Microtubule acetylation but not detyrosination promotes focal adhesion dynamics and astrocyte migration. J. Cell Sci. 2019, 132, jcs225805. [Google Scholar] [CrossRef] [PubMed]
- Haggarty, S.J.; Koeller, K.M.; Wong, J.C.; Grozinger, C.M.; Schreiber, S.L. Domain-selective small-molecule inhibitor of histone deacetylase 6 (hdac6)-mediated tubulin deacetylation. Proc. Natl. Acad. Sci. USA 2003, 100, 4389–4394. [Google Scholar] [CrossRef] [PubMed]
- Castro-Castro, A.; Janke, C.; Montagnac, G.; Paul-Gilloteaux, P.; Chavrier, P. Atat1/mec-17 acetyltransferase and hdac6 deacetylase control a balance of acetylation of alpha-tubulin and cortactin and regulate mt1-mmp trafficking and breast tumor cell invasion. Eur. J. Cell Biol. 2012, 91, 950–960. [Google Scholar] [CrossRef]
- Montagnac, G.; Meas-Yedid, V.; Irondelle, M.; Castro-Castro, A.; Franco, M.; Shida, T.; Nachury, M.V.; Benmerah, A.; Olivo-Marin, J.C.; Chavrier, P. Alphatat1 catalyses microtubule acetylation at clathrin-coated pits. Nature 2013, 502, 567–570. [Google Scholar] [CrossRef]
- Tran, A.D.; Marmo, T.P.; Salam, A.A.; Che, S.; Finkelstein, E.; Kabarriti, R.; Xenias, H.S.; Mazitschek, R.; Hubbert, C.; Kawaguchi, Y.; et al. Hdac6 deacetylation of tubulin modulates dynamics of cellular adhesions. J. Cell Sci. 2007, 120, 1469–1479. [Google Scholar] [CrossRef]
- Rey, M.; Irondelle, M.; Waharte, F.; Lizarraga, F.; Chavrier, P. Hdac6 is required for invadopodia activity and invasion by breast tumor cells. Eur. J. Cell Biol. 2011, 90, 128–135. [Google Scholar] [CrossRef]
- Whipple, R.A.; Vitolo, M.I.; Boggs, A.E.; Charpentier, M.S.; Thompson, K.; Martin, S.S. Parthenolide and costunolide reduce microtentacles and tumor cell attachment by selectively targeting detyrosinated tubulin independent from nf-kappab inhibition. Breast Cancer Res. 2013, 15, R83. [Google Scholar] [CrossRef]
- Fonrose, X.; Ausseil, F.; Soleilhac, E.; Masson, V.; David, B.; Pouny, I.; Cintrat, J.C.; Rousseau, B.; Barette, C.; Massiot, G.; et al. Parthenolide inhibits tubulin carboxypeptidase activity. Cancer Res. 2007, 67, 3371–3378. [Google Scholar] [CrossRef]
- Clemente-Ruiz, M.; Muzzopappa, M.; Milan, M. Tumor suppressor roles of cenp-e and nsl1 in drosophila epithelial tissues. Cell Cycle 2014, 13, 1450–1455. [Google Scholar] [CrossRef]
- Silk, A.D.; Zasadil, L.M.; Holland, A.J.; Vitre, B.; Cleveland, D.W.; Weaver, B.A. Chromosome missegregation rate predicts whether aneuploidy will promote or suppress tumors. Proc. Natl. Acad. Sci. USA 2013, 110, E4134–E4141. [Google Scholar] [CrossRef] [PubMed]
- Weaver, B.A.; Silk, A.D.; Montagna, C.; Verdier-Pinard, P.; Cleveland, D.W. Aneuploidy acts both oncogenically and as a tumor suppressor. Cancer Cell 2007, 11, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Zasadil, L.M.; Britigan, E.M.; Ryan, S.D.; Kaur, C.; Guckenberger, D.J.; Beebe, D.J.; Moser, A.R.; Weaver, B.A. High rates of chromosome missegregation suppress tumor progression but do not inhibit tumor initiation. Mol. Biol. Cell 2016, 27, 1981–1989. [Google Scholar] [CrossRef] [PubMed]
| Tubulin PTM (and/or Enzymes)/Isotype | Cancer | Regulation | References |
|---|---|---|---|
| Detyrosination | Prostate Cancer Cells | Upregulated | [122] |
| Poor Prognosis Breast Tumors | Upregulated | [99] | |
| Invasive Ductal Carcinoma (Breast) | Upregulated | [123] | |
| TTL | Prostate Cancer Cells | Downregulated | [122] |
| Poor Prognosis Neuroblastomas | Downregulated | [100] | |
| VASH2 | Hepatocellular carcinoma Tissues and Cell Lines | Upregulated | [124] |
| Δ2-Tubulin | Prostate Cancer Cells | Downregulated | [122] |
| Acetylation | Metastatic Breast Tumors and Cell Lines | Upregulated | [98] |
| HDAC6 | Pancreatic Tumors | Upregulated | [125] |
| Glioblastoma Tissues and Cell Lines | Upregulated | [118] | |
| Cholangiocarcinoma Cell Lines | Upregulated | [115] | |
| Glutamylation/ Polyglutamylation | Prostate Cancer Cells | Upregulated | [122] |
| TTLL4 | Pancreatic Ductal Adenocarcinoma Cells | Upregulated | [111] |
| Glycylation TTLL3 | Colon Tumors and Cell Lines | Downregulated | [110] |
| β3-tubulin | Pancreatic Tumors and Cell Lines | Upregulated | [102] |
| Pancreatic Ductal Adenocarcinoma Tissues | Upregulated | [126] | |
| Breast Cancer Brain Metastases | Upregulated | [103] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopes, D.; Maiato, H. The Tubulin Code in Mitosis and Cancer. Cells 2020, 9, 2356. https://doi.org/10.3390/cells9112356
Lopes D, Maiato H. The Tubulin Code in Mitosis and Cancer. Cells. 2020; 9(11):2356. https://doi.org/10.3390/cells9112356
Chicago/Turabian StyleLopes, Danilo, and Helder Maiato. 2020. "The Tubulin Code in Mitosis and Cancer" Cells 9, no. 11: 2356. https://doi.org/10.3390/cells9112356
APA StyleLopes, D., & Maiato, H. (2020). The Tubulin Code in Mitosis and Cancer. Cells, 9(11), 2356. https://doi.org/10.3390/cells9112356