Deletion of Acid-Sensing Ion Channel 3 Relieves the Late Phase of Neuropathic Pain by Preventing Neuron Degeneration and Promoting Neuron Repair
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Surgery
2.3. Histological Staining and Immunostaining
2.4. Tests
2.5. Statistical Analysis
3. Results
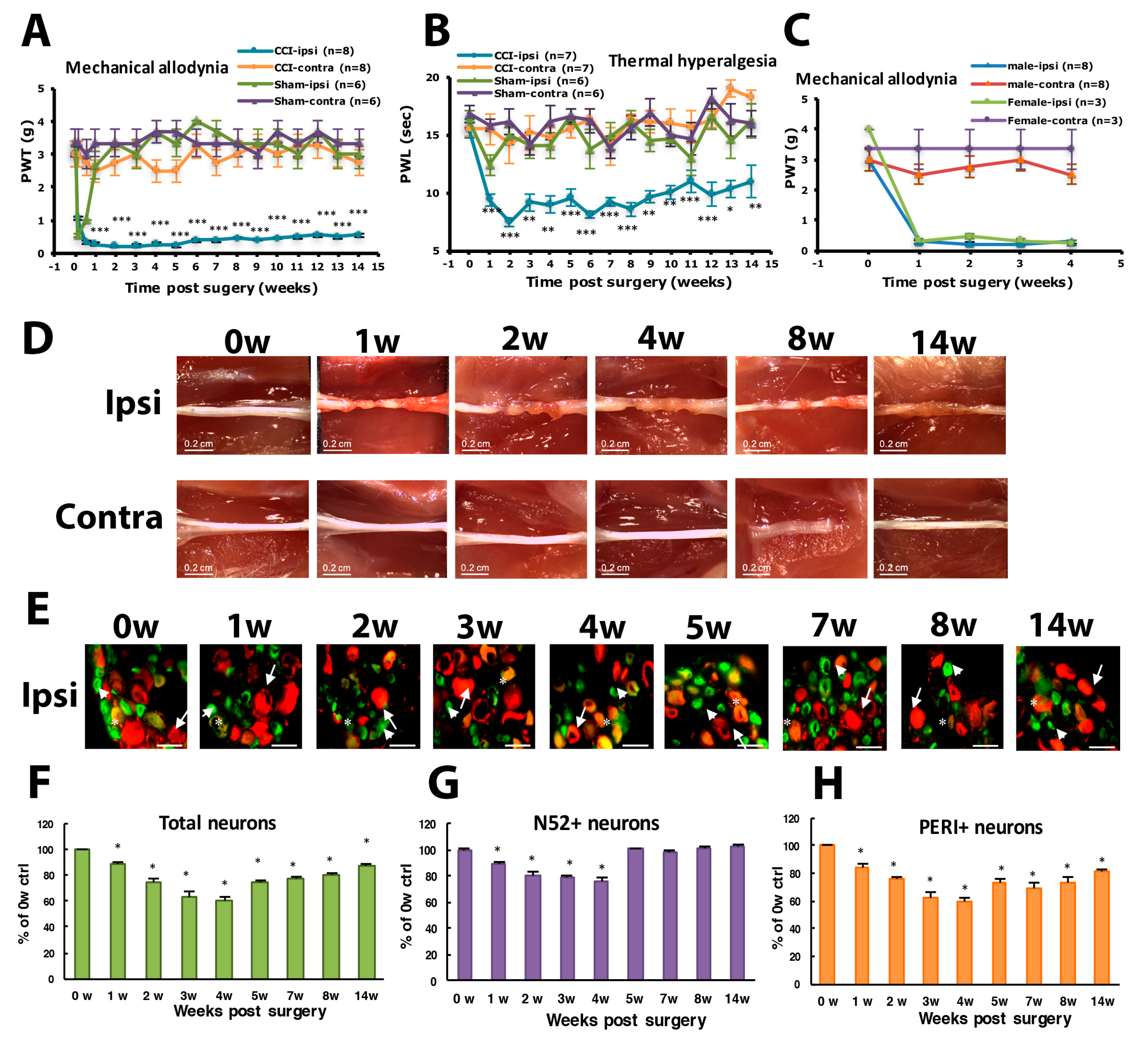
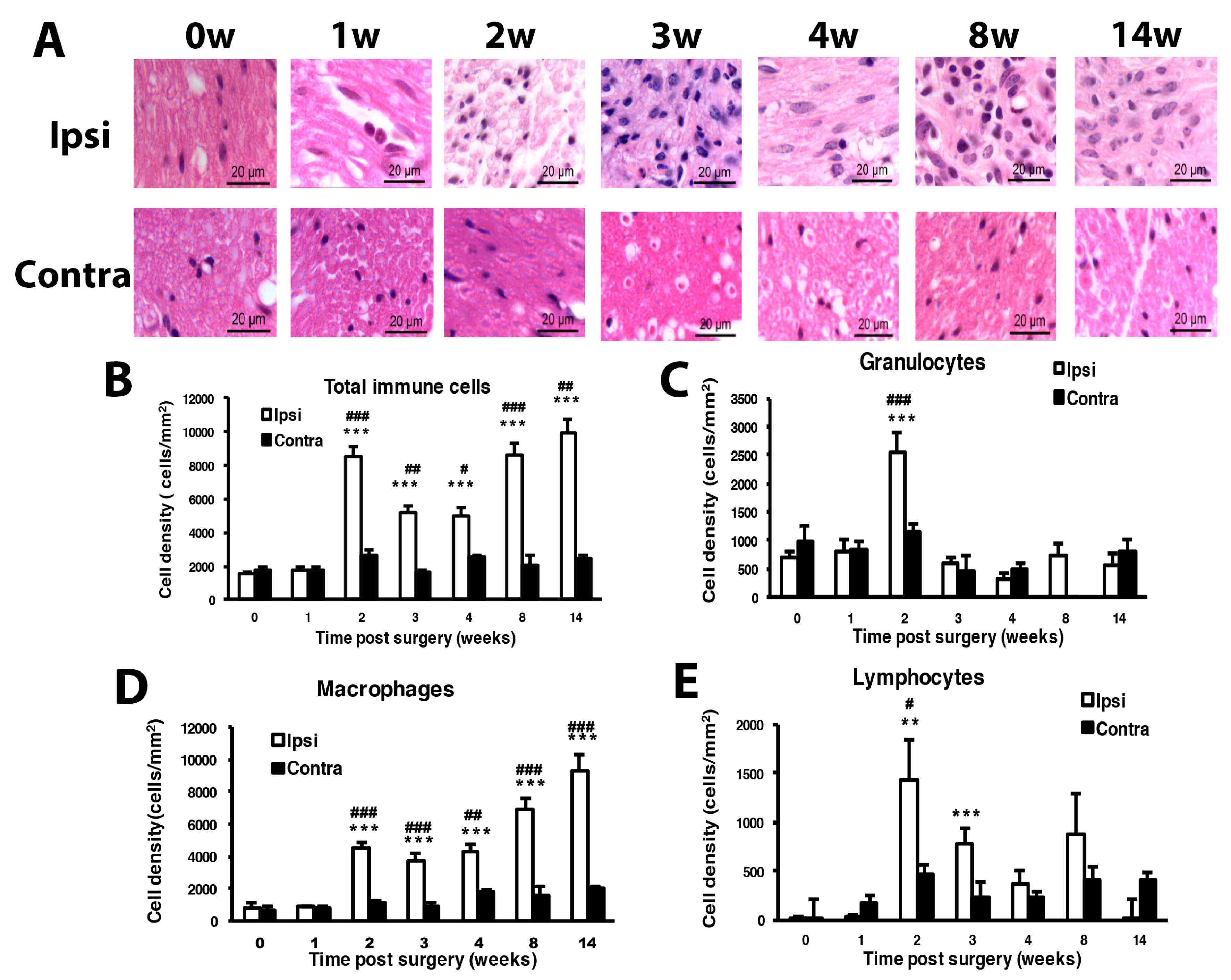
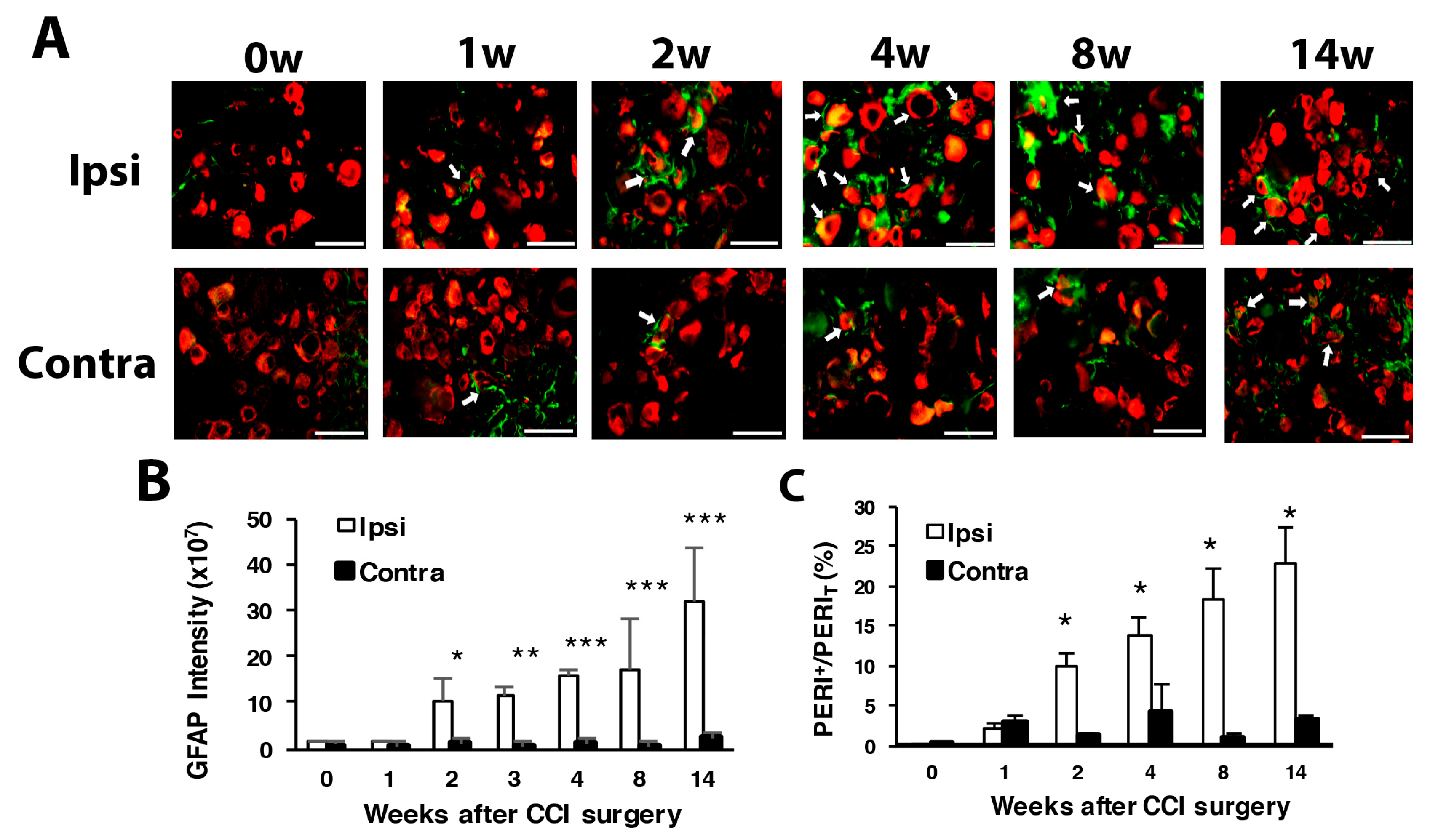
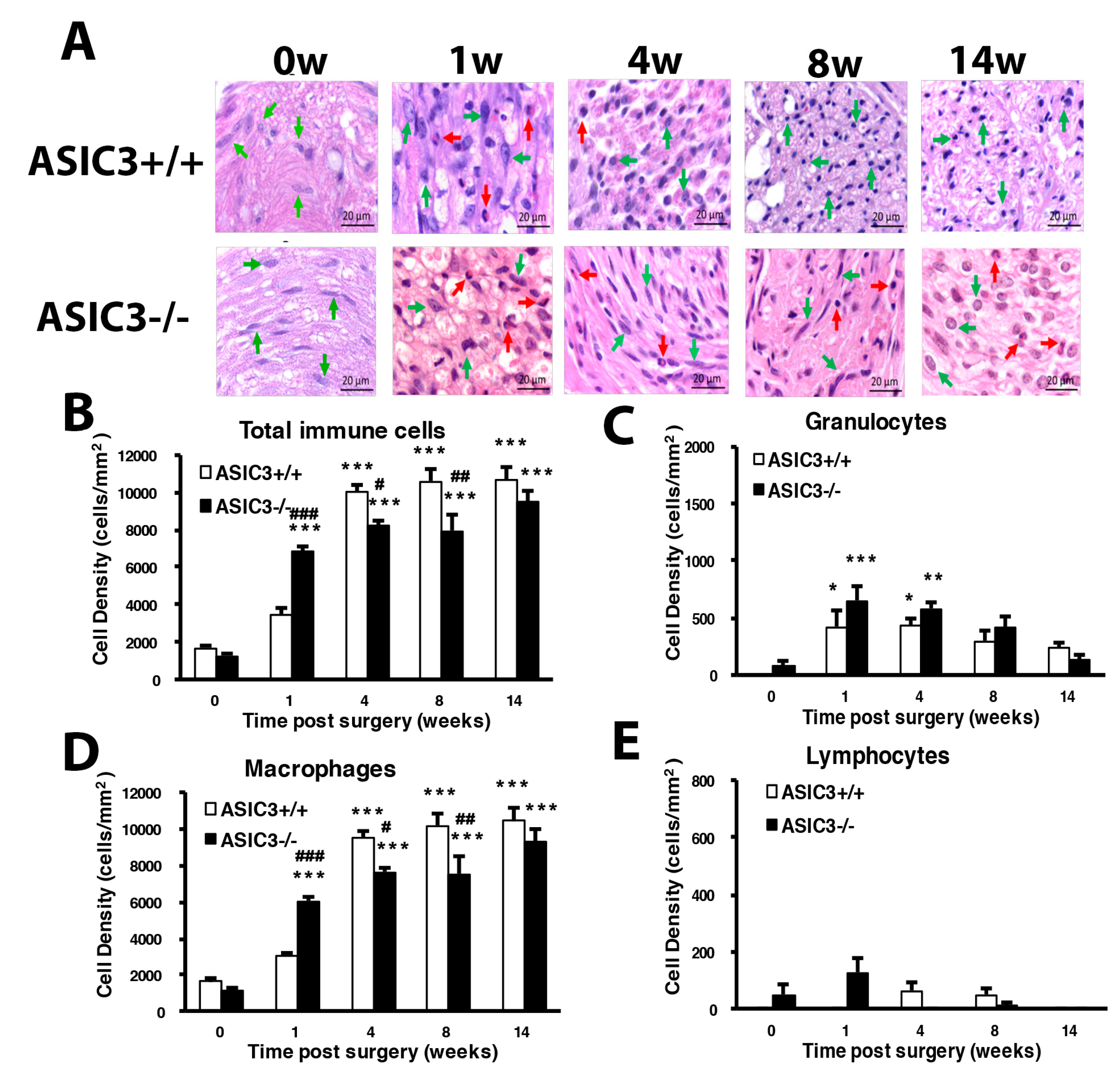
3.1. CCI of Sciatic Nerve Causes Long-Term Hyperalgesia, Inflammation, and Neuron Degeneration
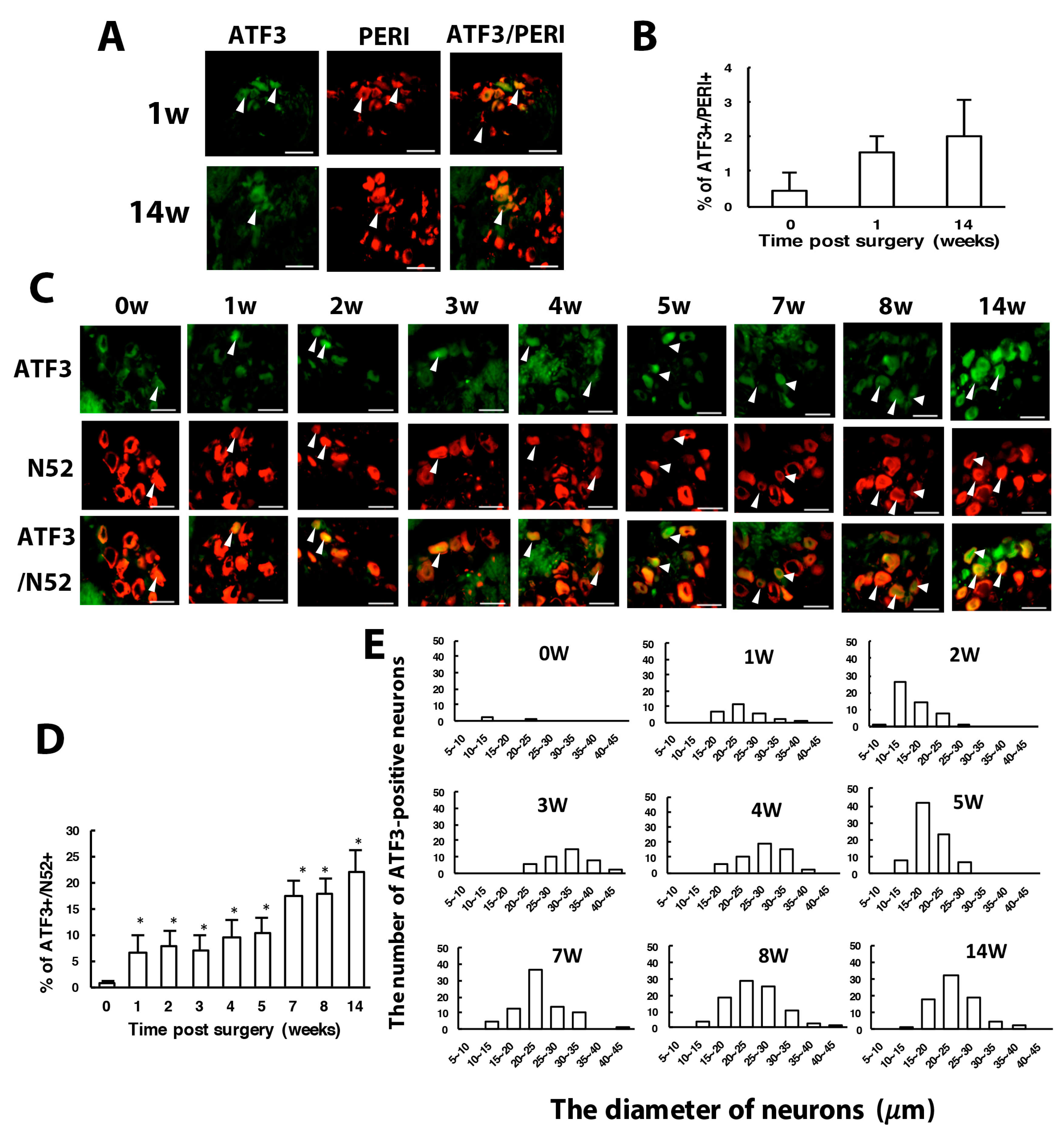
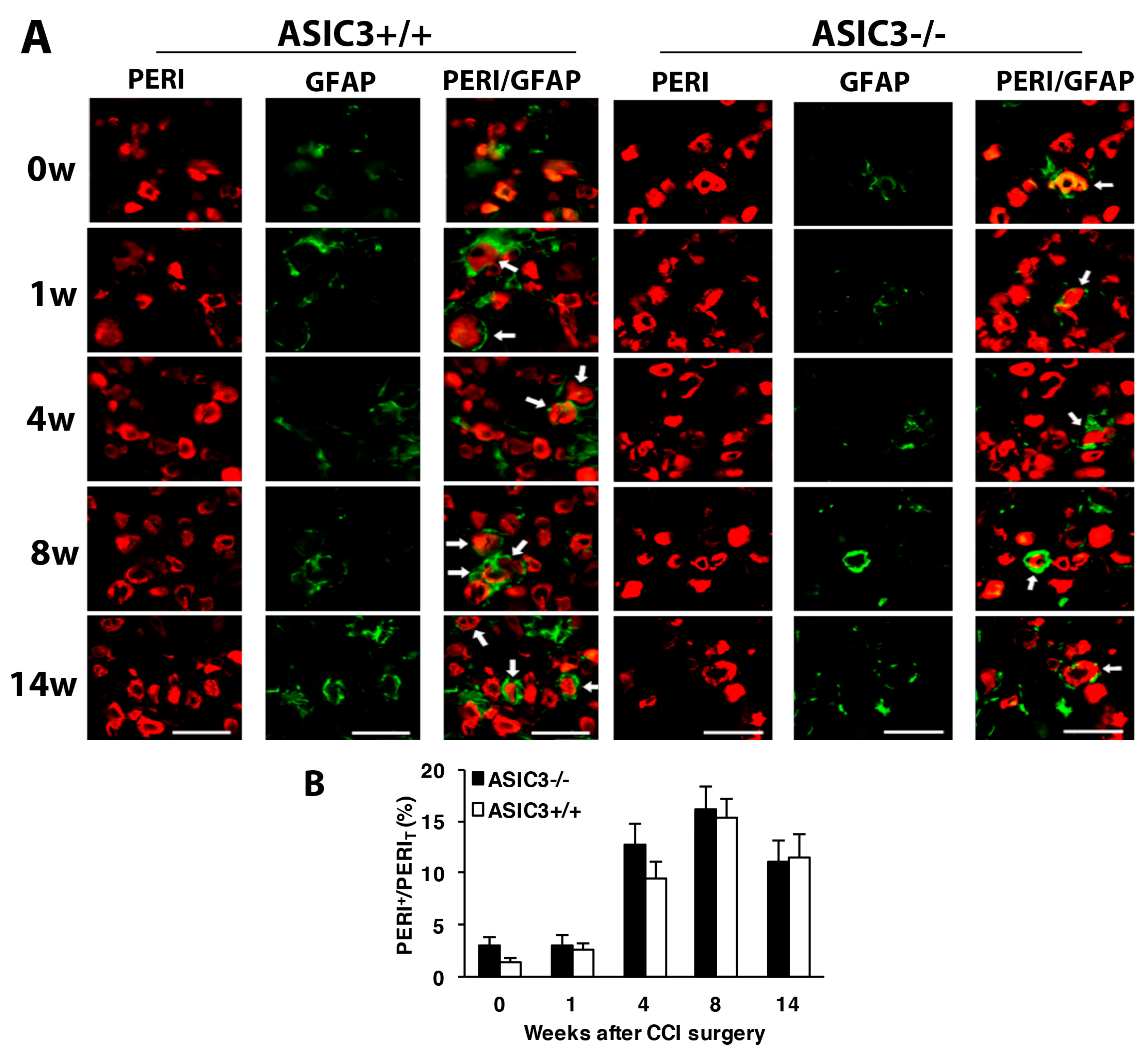
3.2. Number of SGCs and ATF3-Positive Neurons Was Increased after CCI Surgery
3.3. ASIC3 Deficiency Reverses CCI-Induced Hyperalgesia and Delays Neuron Loss in the Beginning of CCI
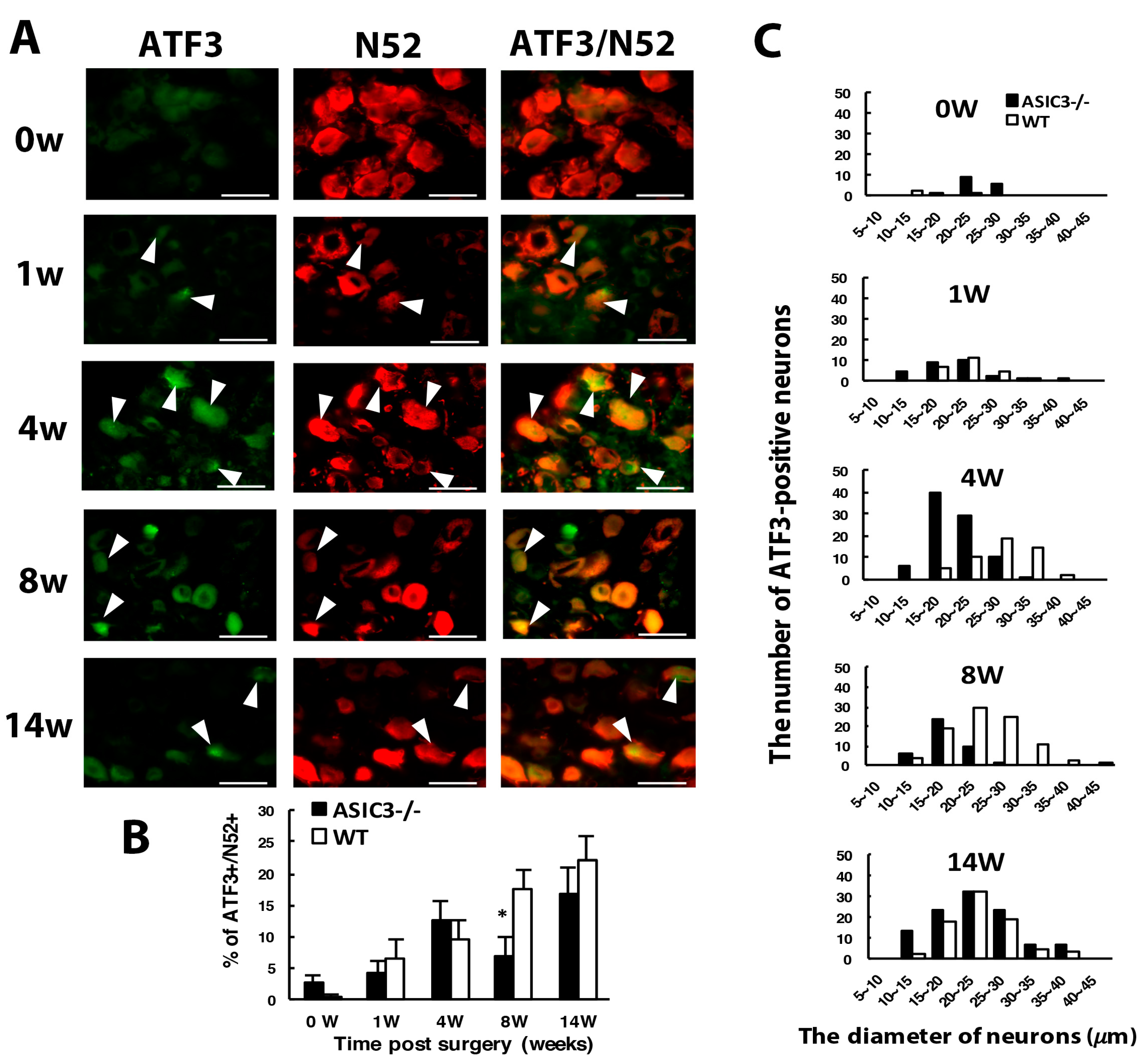
3.4. ASIC3 Deficiency Reverses the Shift from Large to Small Cells in ATF3+ Neurons, with No Alteration in Gliosis
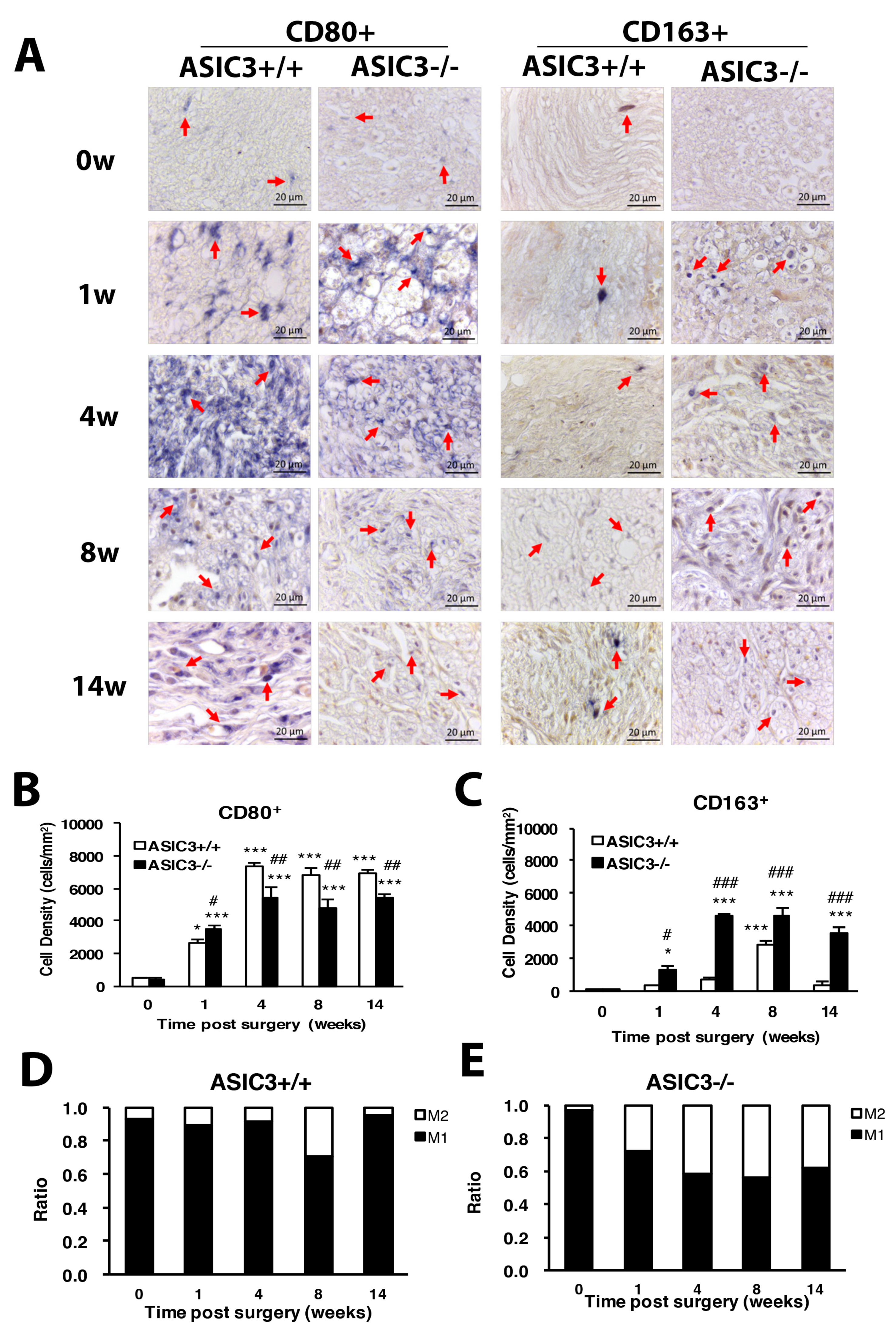
3.5. ASIC3 Deletion Alters M1/ M2 Macrophage Ratio after CCI
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Reichling, D.B.; Levine, J.D. Critical role of nociceptor plasticity in chronic pain. Trends Neurosci. 2009, 32, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Scholz, J.; Woolf, C.J. The neuropathic pain triad: Neurons, immune cells and glia. Nat. Neurosci. 2007, 10, 1361–1368. [Google Scholar] [CrossRef] [PubMed]
- MacInnis, B.L.; Campenot, R.B. Regulation of Wallerian degeneration and nerve growth factor withdrawal-induced pruning of axons of sympathetic neurons by the proteasome and the MEK/Erk pathway. Mol. Cell. Neurosci. 2005, 28, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Sacerdote, P.; Franchi, S.; Trovato, A.E.; Valsecchi, A.E.; Panerai, A.E.; Colleoni, M. Transient early expression of TNF-alpha in sciatic nerve and dorsal root ganglia in a mouse model of painful peripheral neuropathy. Neurosci. Lett. 2008, 436, 210–213. [Google Scholar] [CrossRef]
- Shamash, S.; Reichert, F.; Rotshenker, S. The cytokine network of Wallerian degeneration: Tumor necrosis factor-alpha, interleukin-1alpha, and interleukin-1beta. J. Neurosci. 2002, 22, 3052–3060. [Google Scholar] [CrossRef]
- Kim, C.F.; Moalem-Taylor, G. Detailed characterization of neuro-immune responses following neuropathic injury in mice. Brain Res. 2011, 1405, 95–108. [Google Scholar] [CrossRef]
- Mueller, M.; Wacker, K.; Ringelstein, E.B.; Hickey, W.F.; Imai, Y.; Kiefer, R. Rapid response of identified resident endoneurial macrophages to nerve injury. Am. J. Pathol. 2001, 159, 2187–2197. [Google Scholar] [CrossRef]
- Vega-Avelaira, D.; Géranton, S.M.; Fitzgerald, M. Differential regulation of immune responses and macrophage/neuron interactions in the dorsal root ganglion in young and adult rats following nerve injury. Mol. Pain 2009, 5, 70. [Google Scholar] [CrossRef]
- Finnerup, N.; Attal, N.; Haroutounian, S.; McNicol, E.; Baron, R.; Dworkin, R.H.; Gilron, I.; Haanpää, M.; Hansson, P.; Jensen, T.S.; et al. Pharmacotherapy for neuropathic pain in adults: A systematic review and meta-analysis. Lancet Neurol. 2015, 14, 162–173. [Google Scholar] [CrossRef]
- Austin, P.J.; Moalem-Taylor, G. The neuro-immune balance in neuropathic pain: Involvement of inflammatory immune cells, immune-like glial cells and cytokines. J. Neuroimmunol. 2010, 229, 26–50. [Google Scholar] [CrossRef]
- Calvo, M.; Dawes, J.M.; Bennett, D.L. The role of the immune system in the generation of neuropathic pain. Lancet Neurol. 2012, 11, 629–642. [Google Scholar] [CrossRef]
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef] [PubMed]
- Kigerl, K.A.; Gensel, J.C.; Ankeny, D.P.; Alexander, J.K.; Donnelly, D.J.; Popovich, P.G. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J. Neurosci. 2009, 29, 13435–13444. [Google Scholar] [CrossRef] [PubMed]
- Kiguchi, N.; Kobayashi, Y.; Saika, F.; Sakaguchi, H.; Maeda, T.; Kishioka, S. Peripheral interleukin-4 ameliorates inflammatory macrophage-dependent neuropathic pain. Pain 2015, 156, 684–693. [Google Scholar] [CrossRef] [PubMed]
- Pannell, M.; Labuz, D.; Celik, M.Ö.; Keye, J.; Batra, A.; Siegmund, B.; Machelska, H. Adoptive transfer of M2 macrophages reduces neuropathic pain via opioid peptides. J. Neuroinflamm. 2016, 13, 262. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.Y.; Gu, Y.; Chen, Y. Communication between neuronal somata and satellite glial cells in sensory ganglia. Glia 2013, 61, 1157–1181. [Google Scholar] [CrossRef] [PubMed]
- Ellis, A.; Bennett, D.L. Neuroinflammation and the generation of neuropathic pain. Br. J. Anaesth. 2013, 111, 26–37. [Google Scholar] [CrossRef]
- Richner, M.; Ulrichsen, M.; Elmegaard, S.L.; Dieu, R.; Pallesen, L.T.; Vaegter, C.B. Peripheral nerve injury modulates neurotrophin signaling in the peripheral and central nervous system. Mol. Neurobiol. 2014, 50, 945–970. [Google Scholar] [CrossRef]
- Tsujino, H.; Kondo, E.; Fukuoka, T.; Dai, Y.; Tokunaga, A.; Miki, K.; Yonenobu, K.; Ochi, T.; Noguchi, K. Activating transcription factor 3 (ATF3) induction by axotomy in sensory and motoneurons: A novel neuronal marker of nerve injury. Mol. Cell. Neurosci. 2000, 15, 170–182. [Google Scholar] [CrossRef]
- Averill, S.; Michael, G.J.; Shortland, P.J.; Leavesley, R.C.; King, V.R.; Bradbury, E.J.; McMahon, S.B.; Priestley, J.V. NGF and GDNF ameliorate the increase in ATF3 expression which occurs in dorsal root ganglion cells in response to peripheral nerve injury. Eur. J. Neurosci. 2004, 19, 1437–1445. [Google Scholar] [CrossRef]
- Mason, M.R.; Lieberman, A.R.; Anderson, P.N. Corticospinal neurons up-regulate a range of growth-associated genes following intracortical, but not spinal, axotomy. Eur. J. Neurosci. 2003, 18, 789–802. [Google Scholar] [CrossRef] [PubMed]
- Nakagomi, S.; Suzuki, Y.; Namikawa, K.; Kiryu-Seo, S.; Kiyama, H. Expression of the activating transcription factor 3 prevents c-Jun N-terminal kinase-induced neuronal death by promoting heat shock protein 27 expression and Akt activation. J. Neurosci. 2003, 23, 5187–5196. [Google Scholar] [CrossRef] [PubMed]
- Deval, E.; Gasull, X.; Noël, J.; Salinas, M.; Baron, A.; Diochot, S.; Lingueglia, E. Acid-sensing ion channels (ASICs): Pharmacology and implication in pain. Pharm. Ther. 2010, 128, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.H.; Sun, W.H.; Chen, C.C. Genetic exploration of the role of acid-sensing ion channels. Neuropharmocology 2015, 94, 99–118. [Google Scholar] [CrossRef]
- Jeong, S.; Lee, S.H.; Kim, Y.O.; Yoon, M.H. Antinociceptive effects of amiloride and benzamil in neuropathic pain model rats. J. Korean Med. Sci. 2013, 28, 1238–1243. [Google Scholar] [CrossRef][Green Version]
- Kong, X.; Tang, X.; Du, W.; Tong, J.; Yan, Y.; Zheng, F.; Fang, M.; Gong, F.; Tan, Z. Extracellular acidosis modulates the endocytosis and maturation of macrophages. Cell Immunol. 2013, 281, 44–50. [Google Scholar] [CrossRef]
- Voilley, N.; de Weille, J.; Mamet, J.; Lazdunski, M. Nonsteroid Anti-Inflammatory Drugs inhibit both the activity and the inflammation-induced expression of acid-sensing ion channels in nociceptors. J. Neurosci. 2001, 21, 8026–8033. [Google Scholar] [CrossRef]
- Ikeuchi, M.; Kolker, S.J.; Burnes, L.A.; Walder, R.Y.; Sluka, K.A. Role of ASIC3 in the primary and secondary hyperalgesia produced by joint inflammation in mice. Pain 2008, 137, 662–669. [Google Scholar] [CrossRef]
- Chen, W.N.; Lee, C.H.; Lin, S.H.; Wong, C.W.; Sun, W.H.; Wood, J.N.; Chen, C.C. Roles of ASIC3, TRPV1, and NaV1.8 in the transition from acute to chronic pain in a mouse model of fibromyalgia. Mol. Pain. 2014, 10, 40. [Google Scholar] [CrossRef]
- Hsieh, W.S.; Kung, C.C.; Huang, S.L.; Lin, S.C.; Sun, W.H. TDAG8, TRPV1, and ASIC3 involved in establishing hyperalgesic priming in experimental rheumatoid arthritis. Sci. Rep. 2017, 7, 8870. [Google Scholar] [CrossRef]
- Huang, Y.H.; Chang, C.Y.; Chen, C.C.; Yang, C.D.; Sun, W.H. Distinct expression of Mas1-related G-protein-coupled receptor B4 in dorsal root and trigeminal ganglia-implications for altered behaviors in acid-sensing ion channel 3-deficient mice. J. Mol. Neurosci. 2013, 51, 820–834. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.J.; Huang, C.W.; Lin, C.S.; Chang, W.H.; Sun, W.H. Expression and function of proton-sensing G protein-coupled receptors in inflammatory pain. Mol. Pain 2009, 5, 39. [Google Scholar] [CrossRef] [PubMed]
- Pearson, E.S.; Hartley, H.O. Biometrika Tables for Statisticians; Biometrika Trust: Oxford, UK, 1976; p. 289. [Google Scholar]
- Bennett, G.J.; Xie, Y.K. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain 1988, 22, 87–107. [Google Scholar] [CrossRef]
- Hargreaves, K.; Dubner, R.; Brown, F.; Flores, C.; Joris, J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 1988, 32, 77–88. [Google Scholar] [CrossRef]
- Gehrmann, J.; Monaco, S.; Kreutzberg, G.W. Spinal Cord microglial cells and DRG satellite cells rapidly respond to transection of the rat sciatic nerve. Restor. Neurol. Neurosci. 1991, 2, 181–198. [Google Scholar] [CrossRef]
- Liu, F.Y.; Sun, Y.N.; Wang, F.T.; Li, Q.; Su, L.; Zhao, Z.F.; Meng, X.L.; Zhao, H.; Wu, X.; Sun, Q.; et al. Activation of satellite glial cells in lumbar dorsal root ganglia contributes to neuropathic pain after spinal nerve ligation. Brain Res. 2012, 1427, 65–77. [Google Scholar] [CrossRef]
- Hai, T.; Hartman, M.G. The molecular biology and nomenclature of the activating transcription factor/cAMP responsive element binding family of transcription factors: Activating transcription factor proteins and homeostasis. Gene 2001, 273, 1–11. [Google Scholar] [CrossRef]
- Seijffers, R.; Allchorne, A.J.; Woolf, C.J. The transcription factor ATF-3 promotes neurite outgrowth. Mol. Cell. Neurosci. 2006, 32, 143–154. [Google Scholar] [CrossRef]
- Kiryu-Seo, S.; Ohno, N.; Kidd, G.J.; Komuro, H.; Trapp, B.D. Demyelination increases axonal stationary mitochondrial size and the speed of axonal mitochrondrial transport. J. Neurosci. 2010, 306, 6586–6666. [Google Scholar]
- Hunt, D.; Hossain-Ibrahim, K.; Mason, M.R.; Coffin, R.S.; Lieberman, A.R.; Winterbottom, J.; Anderson, P.N. ATF3 upregulation in glia during Wallerian degeneration: Differential expression in peripheral nerves and CNS white matter. BMC Neurosci. 2004, 5, 9. [Google Scholar] [CrossRef]
- Mokarram, N.; Merchant, A.; Mukhatyar, V.; Patel, G.; Bellamkonda, R.V. Effect of Modulating Macrophage Phenotype on Peripheral Nerve Repair. Biomaterials 2012, 33, 8793–8801. [Google Scholar] [CrossRef] [PubMed]
- Sha, H.; Zhang, D.; Zhang, Y.; Wen, Y.; Wang, Y. ATF3 promotes migration and M1/M2 polarization of macrophages by activating tenascin-C via Wnt/β-catenin pathway. Mol. Med. Rep. 2017, 16, 3641–3647. [Google Scholar] [CrossRef] [PubMed]
- Basbaum, A.I.; Gautron, M.; Jazat, F.; Mayes, M.; Guilbaud, G. The spectrum of fiber loss in a model of neuropathic pain in the rat: An electron microscopic study. Pain 1991, 47, 359–367. [Google Scholar] [CrossRef]
- Ramer, M.S.; French, G.D.; Bisby, M.A. Wallerian degeneration is required for both neuropathic pain and sympathetic sprouting into the DRG. Pain 1997, 72, 71–78. [Google Scholar] [CrossRef]
- Schmid, A.B.; Coppieters, M.W.; Ruitenberg, M.J.; McLachlan, E.M. Local and Remote Immune-Mediated Inflammation After Mild Peripheral Nerve Compression in Rats. J. Neuropathol. Exp. Neurol. 2013, 72, 662–680. [Google Scholar] [CrossRef]
- Shi, T.J.; Tandrup, T.; Bergman, E.; Xu, Z.Q.; Ulfhake, B.; Hökfelt, T. Effect of peripheral nerve injury on dorsal root ganglion neurons in the C57 BL/6J mouse: Marked changes both in cell numbers and neuropeptide expression. Neuroscience 2001, 105, 249–263. [Google Scholar] [CrossRef]
- Myers, R.R.; Heckman, H.M.; Rodriguez, M. Reduced Hyperalgesia in Nerve-Injured WLD Mice: Relationship to Nerve Fiber Phagocytosis, Axonal Degeneration, and Regeneration in Normal Mice. Exp. Neurol. 1996, 141, 94–101. [Google Scholar] [CrossRef]
- Sommer, C.; Schäfers, M. Painful Mononeuropathy in C57BL/Wld Mice with Delayed Wallerian Degeneration: Differential Effects of Cytokine Production and Nerve Regeneration on Thermal and Mechanical Hypersensitivity. Brain Res. 1998, 784, 154–162. [Google Scholar] [CrossRef]
- Coggeshall, R.E.; Dougherty, P.M.; Pover, C.M.; Carlton, S.M. Is large myelinated fiber loss associated with hyperalgesia in a model of experimental peripheral neuropathy in the rat? Pain 1993, 52, 233–242. [Google Scholar] [CrossRef]
- Brück, W. The role of macrophages in Wallerian degeneration. Brain Pathol. 1997, 7, 741–752. [Google Scholar] [CrossRef]
- Raggi, F.; Pelassa, S.; Pierobon, D.; Penco, F.; Gattorno, M.; Novelli, F.; Eva, A.; Varesio, L.; Giovarelli, M.; Bosco, M.C. Regulation of Human Macrophage M1-M2 Polarization Balance by Hypoxia and the Triggering Receptor Expressed on Myeloid Cells-1. Front. Immunol. 2017, 8, 1097. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Bae, D.J.; Kim, M.J.; Piao, M.L.; Kim, I.S. Extracellular low pH modulates phosphatidylserine-depedent phagocytosis in macrophages by increasing stabilin-1 expression. J. Biol. Chem. 2012, 287, 11261–11271. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kung, C.-C.; Huang, Y.-C.; Hung, T.-Y.; Teng, C.-Y.; Lee, T.-Y.; Sun, W.-H. Deletion of Acid-Sensing Ion Channel 3 Relieves the Late Phase of Neuropathic Pain by Preventing Neuron Degeneration and Promoting Neuron Repair. Cells 2020, 9, 2355. https://doi.org/10.3390/cells9112355
Kung C-C, Huang Y-C, Hung T-Y, Teng C-Y, Lee T-Y, Sun W-H. Deletion of Acid-Sensing Ion Channel 3 Relieves the Late Phase of Neuropathic Pain by Preventing Neuron Degeneration and Promoting Neuron Repair. Cells. 2020; 9(11):2355. https://doi.org/10.3390/cells9112355
Chicago/Turabian StyleKung, Chia-Chi, Yi-Chu Huang, Ting-Yun Hung, Chih-Yu Teng, Tai-Ying Lee, and Wei-Hsin Sun. 2020. "Deletion of Acid-Sensing Ion Channel 3 Relieves the Late Phase of Neuropathic Pain by Preventing Neuron Degeneration and Promoting Neuron Repair" Cells 9, no. 11: 2355. https://doi.org/10.3390/cells9112355
APA StyleKung, C.-C., Huang, Y.-C., Hung, T.-Y., Teng, C.-Y., Lee, T.-Y., & Sun, W.-H. (2020). Deletion of Acid-Sensing Ion Channel 3 Relieves the Late Phase of Neuropathic Pain by Preventing Neuron Degeneration and Promoting Neuron Repair. Cells, 9(11), 2355. https://doi.org/10.3390/cells9112355




