1. Introduction
The maintenance of cytoplasmic calcium (Ca
2+) homeostasis is important for the preservation of a normal structure and function of skeletal muscle fibers. Skeletal muscle inactivity can trigger Ca
2+ homeostasis disturbance, often characterized by cytoplasmic Ca
2+ overload [
1]. A direct consequence of this overload is the activation of calpain system-mediated protein degradation [
2]. In addition, an increased cytoplasmic Ca
2+ concentration can promote cell apoptosis [
3]. Increased protein degradation and cell apoptosis are both involved in skeletal muscle loss.
Hibernation is a unique survival strategy exhibited by various mammals in order to cope with adverse environments in winter, during which hibernators not only face the challenge of prolonged skeletal muscle inactivity, but also deal with other stresses, including hypoxia, fasting, and repeated ischemia-reperfusion during the torpor-arousal cycle. However, various studies have reported that skeletal muscle is well-maintained in hibernators during hibernation [
4,
5]. Therefore, hibernators can be considered typical anti-atrophy models, with their unique skeletal muscle preservation mechanism undoubtedly an attractive and valuable research topic.
Previous findings from our laboratory showed that, under adverse conditions over several months of hibernation, the cytoplasmic Ca
2+ concentration in skeletal muscle fibers of Daurian ground squirrels increased transiently during inter-bout arousal, partially recovered after re-entering torpor, and almost recovered to pre-hibernation levels in the post-hibernation stage, thus exhibiting good Ca
2+ homeostasis during the entire hibernation cycle [
6]. During long-term hibernation, the torpor-arousal cycle likely plays an important role in protecting skeletal muscle from atrophy by avoiding or alleviating persistent and excessive cytoplasmic Ca
2+ overload-induced protein degradation. Therefore, exploring the potential mechanisms involved in Ca
2+ homeostasis during hibernation could help reveal the mechanisms against disuse-induced skeletal muscle atrophy of hibernators. To date, however, only one study (from our lab) has reported on sarcoplasmic reticulum Ca
2+ pump (SERCA) expression in skeletal muscles during hibernation [
7]. As such, the regulatory mechanisms involved in intracellular Ca
2+ homeostasis in skeletal muscle fibers are far from having been clarified.
The level of intracellular Ca
2+ is closely related to the expression level and activity of Ca
2+ transport proteins or channels located in the plasma membrane and intracellular Ca
2+ storage membrane (mainly sarcoplasmic reticulum (SR) and mitochondria), as well as intracellular Ca
2+ binding proteins. Increased extracellular Ca
2+ influx and intracellular Ca
2+ storage/release (especially in the SR) both contribute to an increase in the intracellular Ca
2+ concentration. Store-operated Ca
2+ entry (SOCE) is the most important channel transporting extracellular Ca
2+ into the cytosol. Stromal interaction molecule-1 (STIM1) located in the endoplasmic reticulum (ER) and Orai1 (also known as calcium-release-activated calcium-modulator, CRACM1) located in the cell membrane are two essential components required for SOCE [
8,
9,
10]. With external stimulation, Ca
2+ is released from the STIM1 EF-hand domain, which triggers the aggregation and movement of STIM1 to ER/plasma membrane (PM) binding sites, as well as the Orai1 aggregation of STIM1, and leads to the activation of SOCE and Ca
2+ influx [
11,
12,
13,
14]. The ryanodine receptor (RyR) is a major SR Ca
2+ release channel. Specifically, when sensing cell membrane depolarization, exterior membrane L-type calcium channels (surface membrane and T tubules) and dihydropyridine receptors (DHPR) combine to activate RyR, resulting in substantial SR Ca
2+ release [
15,
16]. The RyR family is comprised of three isoforms (i.e., RyR1–3), with RyR1 exclusively expressed and particularly enriched in skeletal muscle [
17]. Leucine zipper-EF-hand-containing transmembrane protein 1 (LETM1) is a Ca
2+-H
+ exchanger located in the mitochondrial membrane. When the mitochondrial Ca
2+ concentration is high, LETM1 will extrude excess Ca
2+ from the mitochondria into the cytoplasm [
18]. Therefore, LETM1 is another possible contributor to elevated cytoplasmic Ca
2+ levels.
In contrast to the above mechanisms, however, the increase in Ca
2+ efflux, intracellular Ca
2+ uptake of the Ca
2+ pool, and binding capacity of free Ca
2+ binding protein in the cytoplasm all effectively decrease cytoplasmic Ca
2+. Plasma membrane Ca
2+ ATPase (PMCA) can eject Ca
2+ from the cytosol into the external medium, thereby attenuating the cytoplasmic Ca
2+ concentration. PMCA3 is the major isoform expressed in skeletal muscle [
19]. As a primary active transporter located in the SR membrane, SR/ER Ca
2+ ATPase (SERCA) can decrease cytoplasmic Ca
2+ levels by pumping Ca
2+ from the cytosol into the SR, which is one of the key factors attenuating cytoplasmic Ca
2+ overload in skeletal muscle fibers [
20]. The mitochondrial calcium uniporter (MCU) complex is considered a major channel for the transportation of Ca
2+ into mitochondria [
21]. Mitochondrial calcium uptake 1 and 2 (MICU1 and 2) are two regulatory subunits of MCU [
22]. When the Ca
2+ concentration in the intermembrane space is low, the heterodimers of MICU1 and MICU2 block the MCU channel and inhibit the entry of Ca
2+ into the mitochondria. In contrast, when the Ca
2+ level is high upon stimulation, the binding of Ca
2+ to the MICU protein elicits a conformational change, resulting in the opening of the channel and the transportation of Ca
2+ into the mitochondria [
21,
23]. Calmodulin (CALM), a Ca
2+ binding protein located in the cytoplasm, can directly reduce the concentration of cytoplasmic free Ca
2+ by combining with four Ca
2+ ions [
24]. Overall, Ca
2+ uptake channels, extrusion mechanisms, and free Ca
2+ binding proteins all contribute to intracellular Ca
2+ homeostasis.
What, then, is the role of Ca2+ channels in Ca2+ fluctuations during the torpor-arousal cycle? To answer this question, we investigated the cytoplasmic, SR, and mitochondrial Ca2+ levels in the plantaris (PL, calf muscle) and adductor magnus (AM, thigh muscle) muscles of Daurian ground squirrels during different hibernation states (i.e., summer active, pre-hibernation, late torpor (entering a new bout after more than 5 d), inter-bout arousal (arousing spontaneously for less than 12 h), early torpor (entering a new bout for less than 48 h), and post-hibernation). Furthermore, a comprehensive and time-course investigation was carried out to explore the roles of the above major Ca2+ transport proteins/channels, including SOCE, RyR1, LETM1, PMCA3, SERCA1, and MCU, as well as the major Ca2+ binding protein CALM, in the fluctuations of Ca2+ concentration throughout hibernation.
2. Materials and Methods
2.1. Animals and Groups
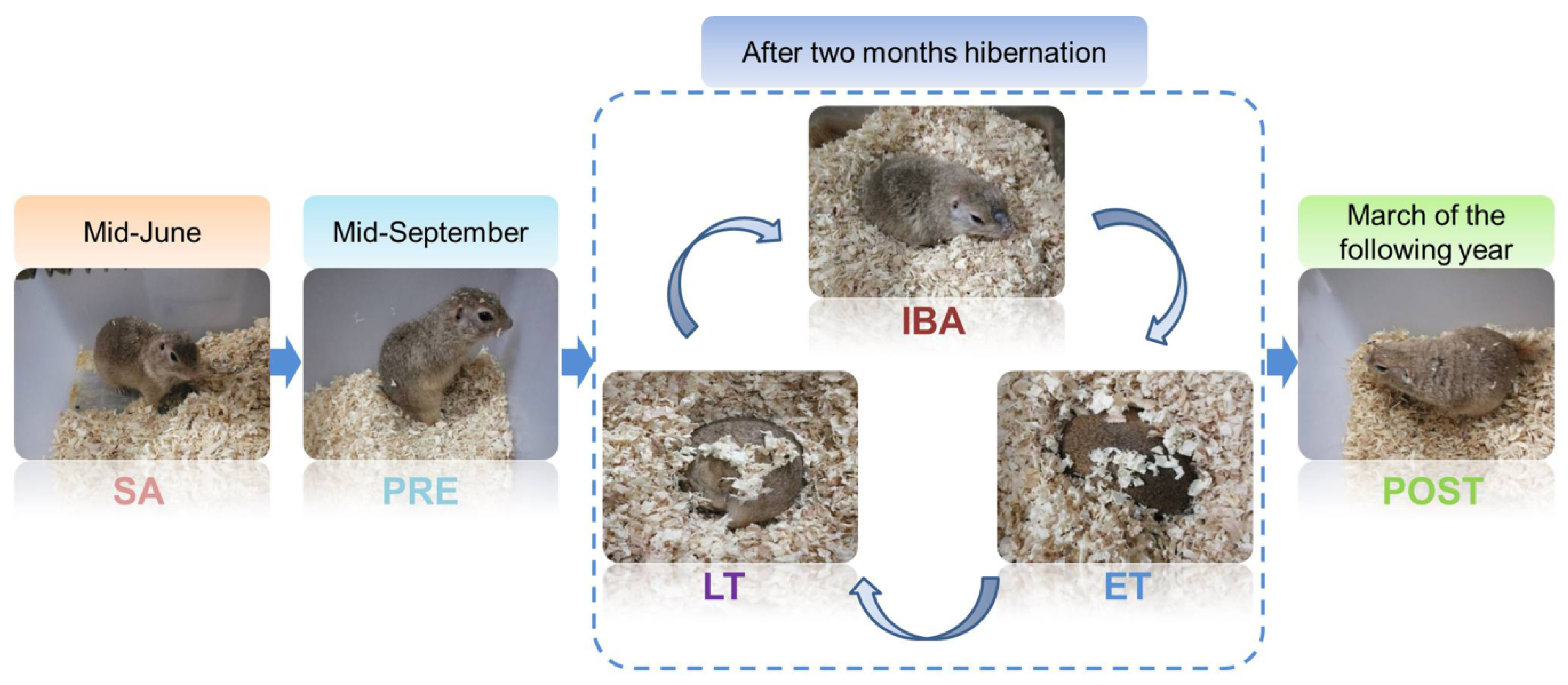
All animal procedures and care and handling protocols were in accordance with the approval granted by the Laboratory Animal Care Committee of the China Ministry of Health (approval No. MH-55). The Daurian ground squirrels used in the experimental procedures were captured from the Weinan region, Shaanxi Province, China. Upon return to the laboratory, all squirrels were maintained in an animal room under a temperature range of 18–25 °C and modified daily light conditions (coincident with local sunrise and sunset). After one month of adaptation, the adult individuals were weight-matched and divided into six groups (n = 6–8): (i) Summer active group (SA): samples were collected in mid-June; (ii) pre-hibernation group (PRE): samples were collected in mid-September; (iii) late torpor group (LT): after two months hibernation, animals entered into a new hibernation bout and were in continuous torpor for at least 5 d, with a stable body temperature (Tb) of 5–8 °C; (iv) inter-bout arousal group (IBA): after two months hibernation, animals entered into a new hibernation bout and were fully aroused, with the Tb returned to 34–37 °C for less than 12 h; (v) early torpor group (ET): after two months hibernation, animals entered into a new hibernation bout, with Tb maintained at 5–8 °C for less than 24 h; (vi) post-hibernation group in spring (POST): animals awaking from hibernation and maintaining a Tb of 36–38 °C for more than 3 d in March of the following year. Animals in the SA and PRE groups were maintained in an environment with a natural light:dark photoperiod until sacrifice. When the ground squirrels gradually entered torpor in early November, the animals were transferred to a 4–6 °C dark hibernaculum. Due to observations occurring twice a day under weak light, these animals were housed under a 2:22 light-dark cycle. The Tb of animals was measured using a visual thermometer with thermal imaging (Fluke, VT04, Everett, Washington, DC, USA). The different states of the animals used here are shown in
Figure 1.
2.2. Muscle Sample Collection and Preparation
Muscle sample collection was carried out at 9:00 am for the SA, PRE, LT, and POST groups. Due to the specific sampling procedures in the IBA and ET groups, it could not be guaranteed that sample collection in these two groups always occurred at 9:00 am. Animals were anesthetized with sodium pentobarbital (90 mg/kg). The two distinct skeletal muscles (PL and AM) were carefully isolated and surgically removed. Subsequently, left leg muscles were treated with embedding medium for frozen section cutting and staining and right leg muscles were used for all other experiments. Following surgical intrusion, the squirrels were euthanized via sodium pentobarbital overdose injection.
2.3. Skeletal Muscle Fiber Cross-Sectional Area (CSA) Determination
As described previously [
25], immunofluorescence and confocal analyses were used to measure the muscle fiber cross-sectional area (CSA) in frozen sections. Briefly, 10-μm thick frozen cross-sections were cut from the muscle mid-belly at −20 °C with a CM1850 cryostat (Leica, Wetzlar, Germany) and stored at −80 °C until further staining. After fixing in 4% paraformaldehyde for 30 min, slices were permeabilized in 0.1% Triton X-100 for 30 min, blocked with 1% bovine serum albumin (BSA) in phosphate-buffered saline (PBS) at room temperature for 60 min, and then incubated at 4 °C overnight with an anti-laminin antibody (1:500, Boster, BA1761-1, Wuhan, China) to visualize muscle fiber CSA. Subsequently, after washing them three times with PBS (10 min/time), the sections were incubated at 37 °C for 2 h with a 647-labeled IgG secondary antibody (1:400, Thermo Fisher Scientific, A-21235, Eugene, OR, USA). Finally, the slices were treated with anti-fade mounting medium (Life Technologies, 1427588, Eugene, OR, USA). Images were visualized via confocal laser scanning microscopy (Olympus, FV1000, Tokyo, Japan) at a 40× objective magnification. Image-Pro Plus 6.0 was used to measure muscle fiber CSA. In detail, eight images were captured from each sample, and the CSA of all complete muscle fibers (about 30) within each picture was then analyzed. Therefore, the CSA of ~250 muscle fibers per skeletal muscle sample was determined.
2.4. Single Muscle Fiber Isolation
We anaesthetized the squirrels with 90 mg/kg sodium pentobarbital, after which muscle samples (including the tendons) were carefully removed from the neighboring tissues and sarcolemma, ensuring that the blood and nerve supply remained intact. The muscle samples were subsequently separated into two full-length strips along the longitudinal axis using a pair of tweezers. The muscle strips, which were obtained from the same middle region, were then washed with 20 mL of PBS (137 mM sodium chloride, 2.7 mM potassium chloride, 4.3 mM disodium chloride, 1.4 mM monopotassium phosphate, pH 7.4) and digested with 3 mL of enzymatic digestion solution containing 0.35% collagenase I (Sigma-Aldrich, C0130-1G, Saint Quentin Fallavier, France), followed by orbital shaker incubation at 37 °C for 2 h and saturation with 95% O2 and 5% CO2 to ensure complete digestion of the muscle fiber samples. Finally, the digestion solution was removed with a PBS rinse and the muscle samples were added to Dulbecco’s modified Eagle’s medium (DMEM) (Hyclone, AC10221937, Pittsburgh, PA, USA) containing 10% fetal bovine serum (FBS) (Everygreen, 11011-8611, Hangzhou, China), 25 µM of N-benzyl-p-toluenesulfonamide (BTS) (TCI, B3082, Shanghai, China), and 0.1 M HEPES (Guoan, H0082, Xi’an, China), and were carefully stirred with a pipette. The digested single muscle fiber samples were finally plated on culture chamber slides and viewed via inverted microscopy (Olympus, IX2-ILL100, Tokyo, Japan).
2.5. Measurement of Cytoplasmic Ca2+
We used fluo-3-acetoxymethylester (Fluo-3/AM) (Invitrogen, Carlsbad, CA, USA), which demonstrates increased fluorescence upon Ca2+ binding, to determine cytoplasmic free Ca2+. Briefly, after washing the samples three times with fresh PBS, dye (5 mM Fluo-3/AM) was slowly added along the sides of the single muscle fibers, followed by incubation in the dark at 37 °C for 30 min. After incubation, the glass slide-mounted Fluo-3/AM-loaded fibers were washed with fresh PBS three times (20 s/time, 1-min process). The slide was quickly placed on the microscope stage, with the fibers focused in the bright field (20-s process) and scanned via laser confocal microscopy in combination with an Olympus FV10-ASW system (Tokyo, Japan) under 488-nm krypton/argon laser illumination, with fluorescence detected at 526 nm. According to their length, three to five pictures were captured at 10× objective magnification for each muscle fiber (10-s capture process for each picture). In consideration of the influence of muscle fiber size on the fluorescence intensity, the average fluorescence intensity (total fluorescence intensity/total area of selected region) was used to measure the Ca2+ levels. Specifically, the average fluorescence intensity of 10 different regions in each picture was measured using Olympus Fluoview v4.2 software. All pictures (3–5 pictures, depending on the muscle fiber length) of each fiber, with 10 muscle fibers per sample, were used for statistical analysis.
2.6. Measurement of Sarcoplasmic Reticulum Ca2+
We used magnesium-Fluo-4-acetoxymethylester (mag-Fluo-4/AM) (M14206, Thermo Fisher Scientific, Eugene, OR, USA), which demonstrates increased fluorescence upon Ca
2+ binding, to indicate SR free Ca
2+, as per Park et al. (2000) [
26]. Briefly, after washing samples twice with fresh PBS, dye (5 mM mag-Fluo-4/AM) was slowly added along the sides of the single muscle fibers, followed by incubation in the dark at 37 °C for 30 min. After incubation, the glass slide-mounted mag-Fluo-4/AM-loaded fibers were washed with fresh PBS three times (20 s/time, 1-min process). The slide was then quickly placed on the microscope stage, with the fibers focused in the bright field (20-s process) and scanned via laser confocal microscopy in combination with an Olympus FV10-ASW system (Japan) under 488-nm krypton/argon laser illumination, with fluorescence detected at 526 nm. Analysis and statistical methods were similar to those used for the measurement of cytoplasmic Ca
2+ mentioned above.
2.7. Measurement of Mitochondrial Ca2+
We used Rhod-2/AM (R1244, Thermo Fisher Scientific, USA), which demonstrates increased fluorescence upon Ca
2+ binding in the mitochondria, to determine mitochondrial free Ca
2+ [
27]. Briefly, after washing samples twice with fresh PBS, dye (5 µM Rhod-2/AM) was slowly added along the sides of the single muscle fibers, followed by incubation in the dark at 37 °C for 30 min. After incubation, the glass slide-mounted Rhod-2/AM-loaded fibers were washed with fresh PBS three times (20 s/time, 1-min process). The slide was then quickly placed on the microscope stage, and the fibers were focused in the bright field (20-s process) and scanned via laser confocal microscopy in combination with an Olympus FV10-ASW system (Japan) under 594-nm krypton/argon laser illumination, with fluorescence detected at 618 nm. Analysis and statistical methods were similar to those used for the measurement of cytoplasmic Ca
2+ mentioned above.
2.8. Total RNA Extraction and Quantitative Real-Time Polymerase Chain Reaction (RT-PCR)
As per Fu et al. (2016) [
6] and in accordance with the manufacturer’s protocols, we extracted total RNA from the muscle samples using an RNAiso Plus kit (TaKaRa Biotechnology, 9109, Dalian, China). RNA quality was characterized using the OD260/OD280 ratio, after which selected samples (those exhibiting OD260/OD280 > 1.8) were reverse transcribed into cDNA using an appropriate reagent (TaKaRa Biotechnology, RR036A China) and stored (−20 °C) for the following analyses. Here, qRT-PCR was undertaken using a SYBR Premix Ex Taq II kit (TaKaRa Biotechnology, RR820A, China), following the protocols stated by the manufacturer. The resultant dissolution and amplification curves were observed and selected, with the α-tubulin reference gene and 2
−ΔΔct method then being applied to analyze the relative mRNA concentrations of STIM1, ORAI1, RyR1, LETM1, PMCA3, SERCA1, MCU, MICU1, MICU2, CALM, and α-tubulin. The primers used for the above genes (Sangon, Nanjing, China) are listed in
Table 1.
2.9. Protein Extraction and Western Blotting Analysis
Muscle samples (~0.1 g) were weighed and fully homogenized with 1 mM RIPA Lysis Buffer (Heart, WB053A, Xi’an, China), 1% protease inhibitor cocktail (Heart, WB053B, Xi’an, China), and 1% phenylmethylsulfonyl fluoride (PMSF, Heart, WB053C, Xi’an, China). After 15 min of centrifugation at 4 °C and 15,000 rpm, the supernatants were removed and placed into new tubes, with soluble protein concentrations then detected using a PierceTM BCA Protein Quantitation kit (Thermo Fisher Scientific, 23227, USA). The supernatants were mixed with 1 × SDS loading buffer (100 mM Tris, 5% glycerol, 5% 2-β-mercaptoethanol, 4% SDS, and bromophenol blue, pH 6.8) at a 1:4 v/v ratio, followed by boiling and then storage at −20 °C for further analysis.
Western blotting procedures were as described by Zhang et al. (2017) [
28]. In brief, we first separated the muscle protein extracts using SDS-PAGE on 10% Laemmli gels (acrylamide/bisacrylamide ratio of 37.5:1 for STIM1, ORAI1, LETM1, PMCA3, SERCA1, MCU, MICU1, CALM) and on 6% Laemmli gels (acrylamide/bisacrylamide ratio of 37.5:1 for RyR1), respectively. Following electrophoresis (for 60 min at 120 V), the proteins were electrically transferred to 0.45-μm pore polyvinylidene difluoride (PVDF) membranes (Millipore, IPVH00010, Merck kGaA, Darmstadt, Germany) using the Bio-Rad (1703930) semidry transfer apparatus (Hercules, CA, USA) at 15 V for 30–40 min. We then blocked the membranes at room temperature for 2 h using 5% skim milk in TBST (containing 10 mM Tris-HCl, 150 mM NaCl, 0.05% Tween-20, pH 7.6), followed by overnight incubation at 4 °C with primary STIM1 (1:1000, CST, 5668S, Danvers, MA, USA), ORAI1 (1:1000, Thermo, MA5-15776, Eugene, OR, USA), RyR1 (1:1000, CST, 8153S, USA), LETM1 (1:1000, CST, 14997S, USA), PMCA3 (1:1000, Abcam, ab3530, Cambridge, UK), SERCA1 (1:1000, CST, 4219S, USA), MCU (1:1000, CST, 14997S, USA), MICU1 (1:750, CST, 12524S, USA), and CALM (1:1000, CST, 4830S, USA) antibodies in TBST containing 0.1% BSA. The membranes where then washed three times with TBST (10 min/time), followed by 1.5-h incubation at room temperature with horseradish peroxidase (HRP)-conjugated anti-rabbit or anti-mouse secondary antibodies (Thermo Fisher Scientific, A27014, USA). The membranes were again washed with TBST (four times × 10 min), with the resulting immunoblots being visualized using enhanced chemiluminescence reagents (Thermo Fisher Scientific, NCI5079, USA), in accordance with the manufacturer’s instructions. Blot quantification was conducted using Image-Pro Plus 6.0 software. Total protein staining of the gel was used as the normalization control for all blots. In detail, as described previously, 0.5% 2,2,2-trichloroethanol (TCE) was first added to the gel [
29,
30,
31]. After electrophoresis, the gel was irradiated on the UV platform of the electrophoresis gel imaging analysis system (G: box, GBOX Cambridge, UK) for 5 min, with the signal then being collected. As described previously [
32,
33], the original images captured with no gain were stored. After that, the fluorescence intensity of each lane (after removal of the background fluorescence intensity) was determined with Image-Pro Plus 6.0, with the internal reference being used to correct the fluorescence intensity of the target protein. Specificity detection of the complete SDS-PAGE lane for each antibody used in the present study is shown in
Figure S1.
2.10. Co-Localization Analysis of ORAI1/STIM1
Briefly, 10-μm thick frozen cross-sections were cut from the muscle mid-belly at −20 °C with a CM1850 cryostat (Leica, Wetzlar, Germany). After fixing samples in 4% paraformaldehyde for 30 min, slices were permeabilized in 0.1% Triton X-100 for 30 min, blocked with 1% BSA in PBS at room temperature for 60 min, and then incubated at 4 °C overnight with an anti-ORAI1 antibody (1:50, Thermo, MA5-15776, USA). On the second day, after washing samples three times with PBS (10 min/time), the sections were incubated at 37 °C for 2 h with an Alexa Fluor FITC-conjugated secondary antibody (1:300, Thermo Fisher Scientific, Rockford, IL, USA). After again washing samples three times with PBS (10 min/time), the slices were incubated at 4 °C overnight with an anti-STIM1 antibody (1:300, CST, 5668S, USA). On the third day, after washing samples three times with PBS (10 min/time), the sections were incubated at 37 °C for 2 h with a 647-labeled IgG secondary antibody (1:200, Thermo Fisher Scientific, A-21235, USA). The slices were then washed three times with PBS (10 min/time) and dried and treated with anti-fade mounting medium (Life Technologies, 1427588, USA). Images were visualized and captured via confocal laser scanning microscopy (Olympus, FV1000, Japan) at a 40× objective magnification with krypton/argon laser illumination at 488 and 647 nm and captured at 526 and 665 nm. As previously described [
34,
35], the co-localization of ORAI1/STIM1 was calculated by Pearson’s correlation coefficients using Image-Pro Plus 6.0.
2.11. Statistical Analysis
Data are presented as means ± SEM. SPSS Statistics 17.0 was used for all statistical tests. Group differences were determined via one-way analysis of variance (ANOVA) with Fisher’s least significant difference (LSD) post hoc test. When no homogeneity was detected, ANOVA-Dunnett’s T3 method was applied. A value of p < 0.05 was considered statistically significant.
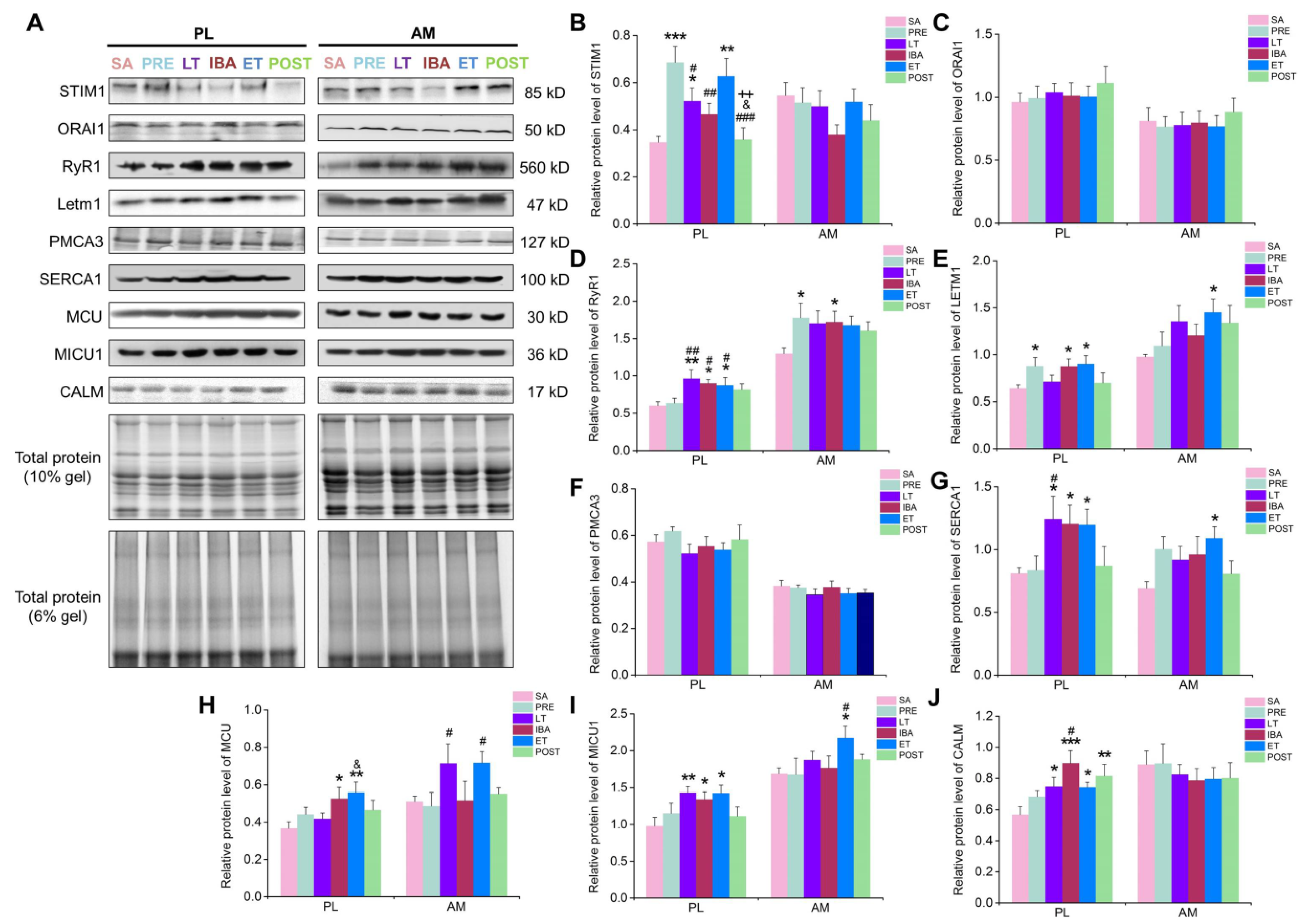
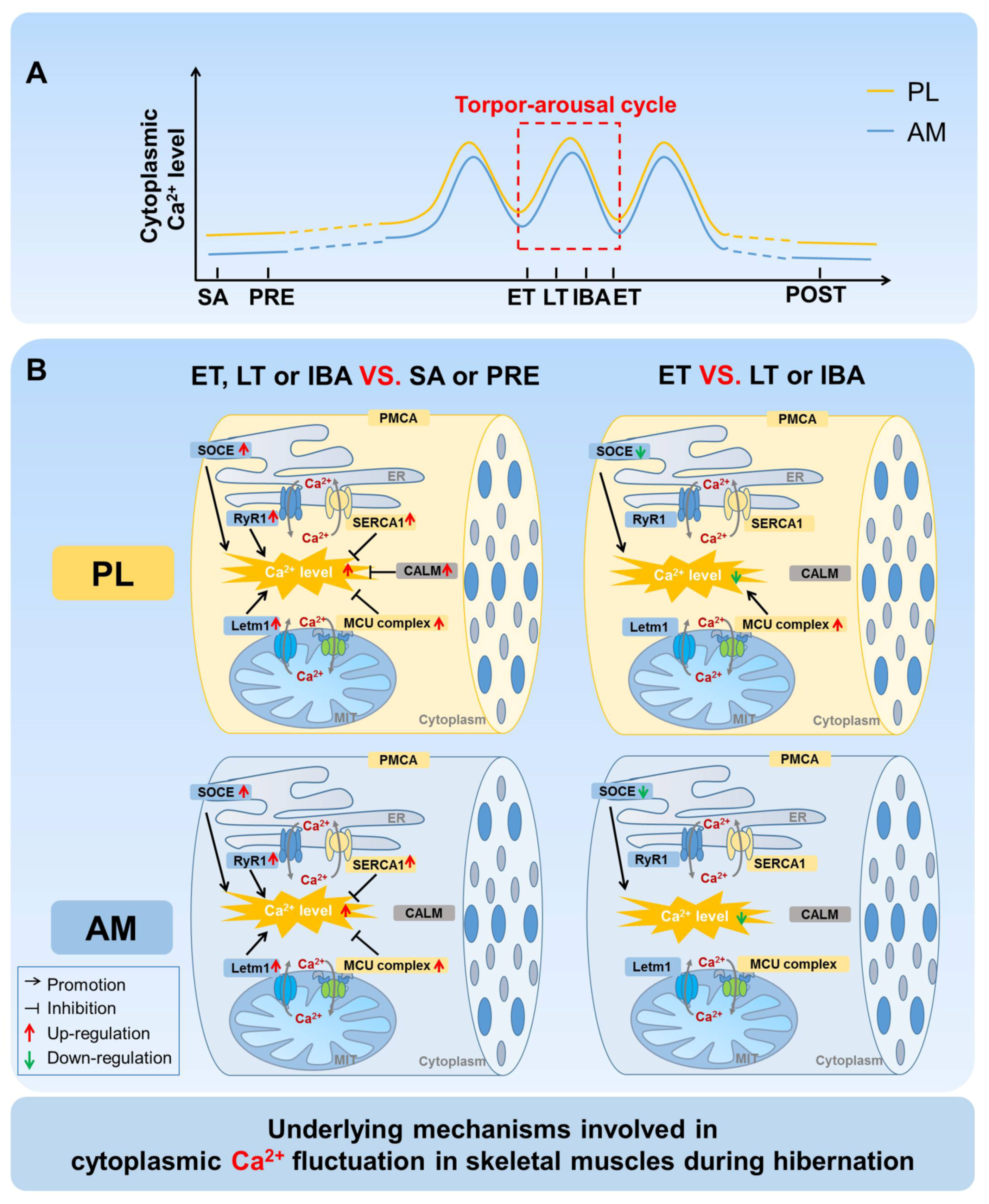
4. Discussion
A comprehensive time-course investigation was firstly carried out to measure the cytoplasmic, SR, and mitochondrial Ca
2+ levels, as well as clarify the possible Ca
2+ regulatory mechanisms, in the skeletal muscles of Daurian ground squirrels during different hibernation states. Fluctuations in intracellular Ca
2+ levels were observed during the torpor-arousal cycle, with Ca
2+ levels partially recovered when the animals aroused and then re-entered torpor (
Figure 9A). Further investigation suggested that the Ca
2+ proteins/channels and free Ca
2+ binding protein located in the cytoplasm, SR, and mitochondria all participated in the fluctuation of intracellular Ca
2+ (
Figure 9B).
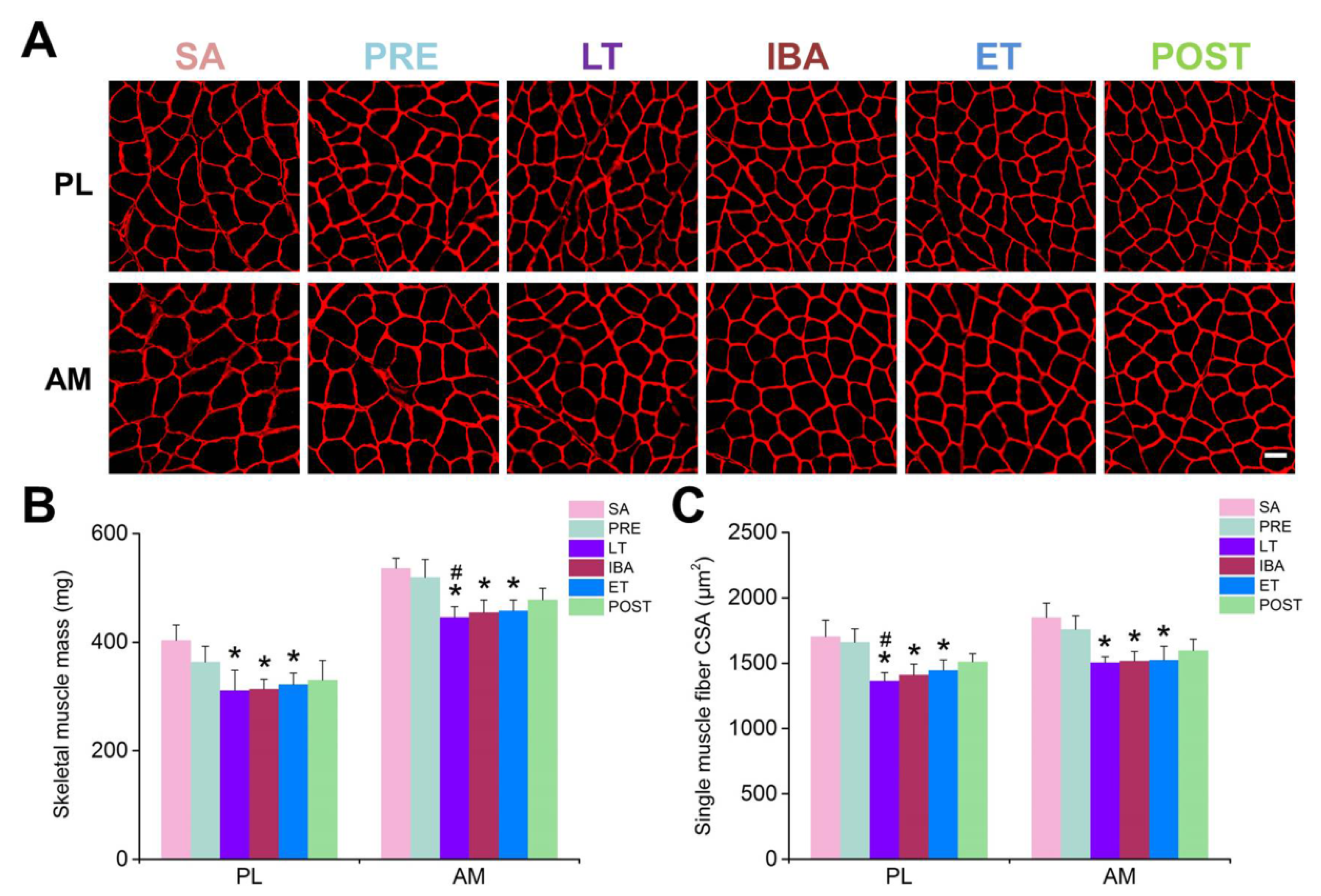
During prolonged hibernation, hibernators experience various stressful conditions, including prolonged inactivity, hypoxia, fasting, and repeated ischemia-reperfusion from the torpor-arousal cycle. Here, after several months of hindlimb inactivity during hibernation, slight decreases in muscle mass and single muscle fiber CSA (15–20%) were observed, suggesting that only limited skeletal muscle loss occurs in the PL and AM muscles, consistent with our previous report on PL and gastrocnemius muscles [
36]. Obviously, hibernators can be considered good anti-atrophy models, and their unique skeletal muscle preservation mechanisms deserve further exploration. In view of the critical role of intracellular Ca
2+ homeostasis in skeletal muscle maintenance, the present study focused on changes in Ca
2+ levels in the different compartments (including cytoplasm, SR, and mitochondria) of skeletal muscle fibers during different hibernation stages. The results showed that the cytoplasmic Ca
2+ levels in the PL and AM muscles were elevated to varying degrees during hibernation, suggesting that, similar to the phenomenon found in non-hibernators, prolonged skeletal muscle disuse during hibernation was accompanied by elevated cytoplasmic Ca
2+ levels [
37,
38,
39,
40]. However, fluctuations in cytoplasmic Ca
2+ levels were observed during the torpor-arousal cycle, with an increase during LT and partial recovery when the squirrels re-entered torpor after transient arousal. Therefore, periodic arousal from torpor may be a strategy employed by hibernators to adjust and stabilize cytoplasmic Ca
2+ levels to effectively avoid excessive Ca
2+-induced skeletal muscle loss or damage. Our previous study found that, compared with pre-hibernation levels, serum Ca
2+ concentrations in ground squirrels increased significantly during hibernation and recovered after post-hibernation [
41]. This raises the question of whether increased serum Ca
2+ concentrations during hibernation influence cytoplasmic Ca
2+ levels. SOCE is the main channel of extracellular Ca
2+ influx. Higher co-localization coefficients of its two major components, i.e., STIM1 and ORAI1, represent a higher activation probability of SOCE [
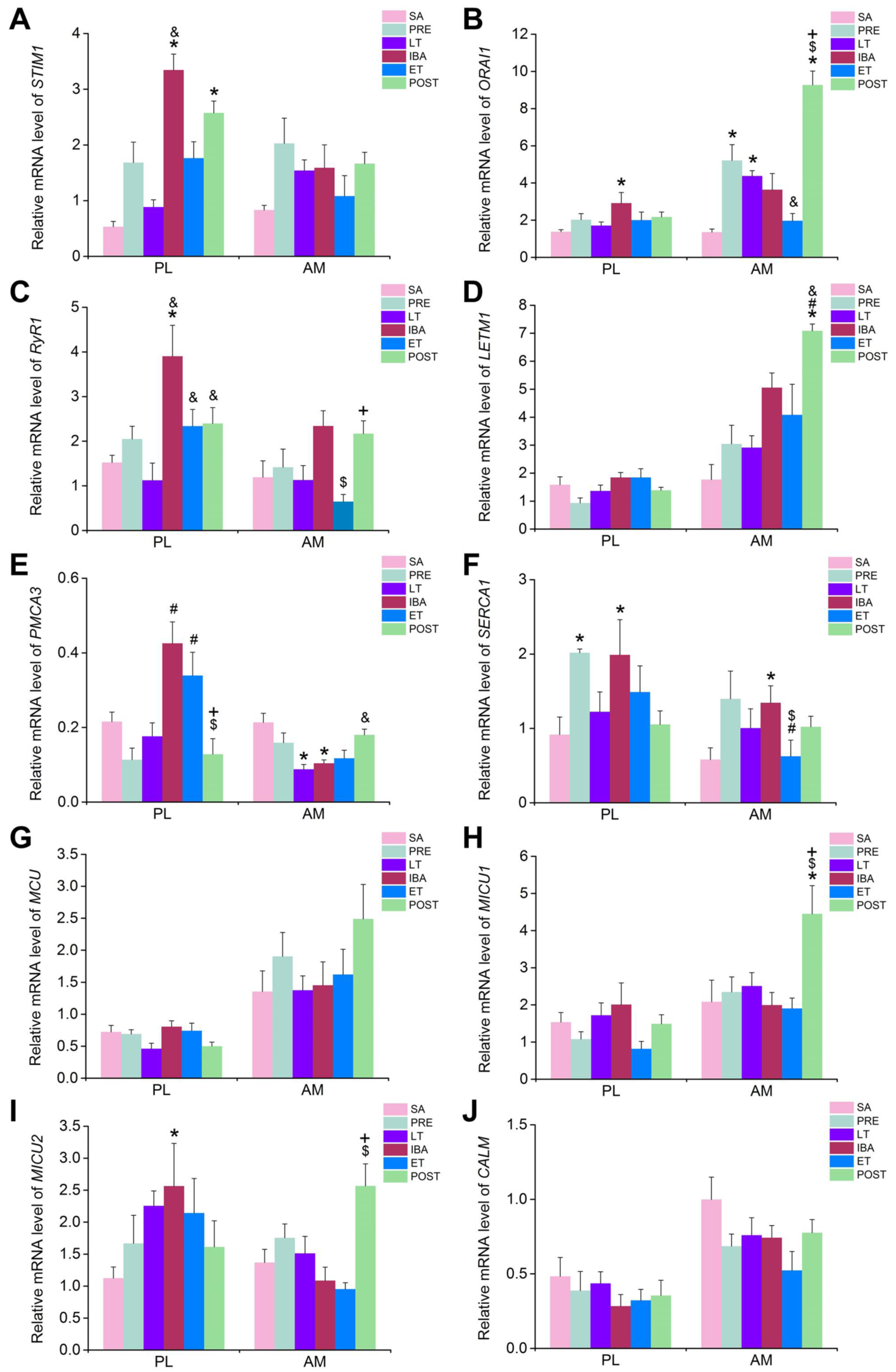
35]. Our results showed that the co-localization coefficients of STIM1 and ORAI1 increased significantly during LT and IBA, suggesting that the activation probability of SOCE increased during these two stages. Furthermore, the extracellular Ca
2+ influx mediated by SOCE may be one reason for the increased cytoplasmic Ca
2+ level during LT and IBA. In addition to extracellular Ca
2+ influx, Ca
2+ release from intracellular Ca
2+ storage can also cause elevated cytoplasmic Ca
2+ levels. RyR1 is the main Ca
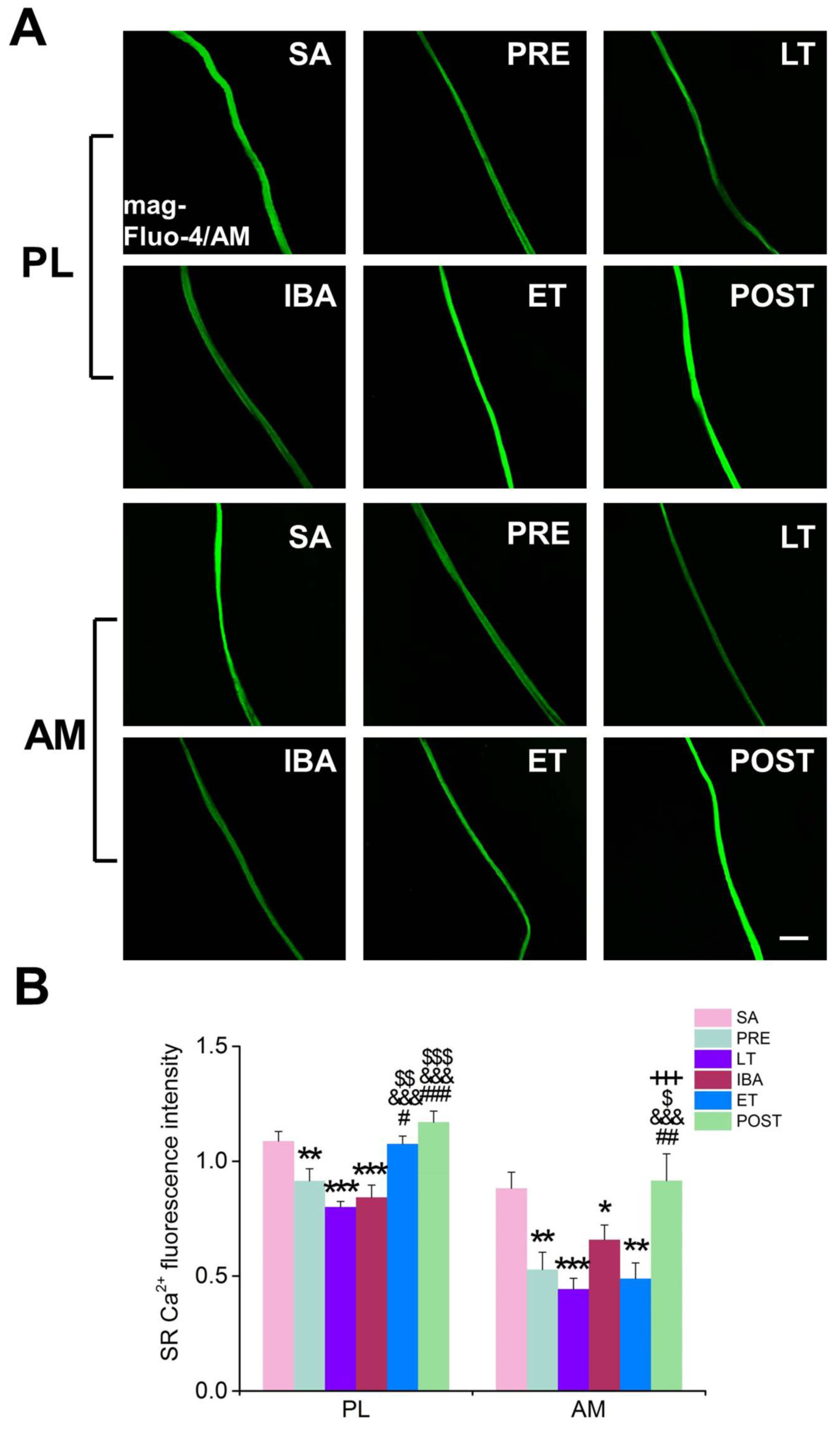
2+ release channel located in the SR. The results showed that its protein expression level was up-regulated to varying degrees during different periods of hibernation. Combined with the phenomenon that the Ca
2+ concentration in the SR showed the opposite change to that of cytoplasmic Ca
2+ during hibernation, the increased release of SR Ca
2+ was likely a major reason for the increased Ca
2+ level in skeletal muscles of ground squirrels during hibernation. In addition to SR Ca
2+ release, several proteins also mediated Ca
2+ efflux from the mitochondria to the cytoplasm. The present study showed that the protein expression of LETM1, a major channel that mediates Ca
2+ efflux in the mitochondrial membrane, was increased to varying degrees during different periods of hibernation, intimating that Ca
2+ release mediated by LETM1 in the mitochondria may also be involved in the increase in cytoplasmic Ca
2+ levels during hibernation.
As a Ca
2+ pump located in the cell membrane, PMCA3 can pump intracellular Ca
2+ out of the cell. In the current study, however, the PMCA3 protein expression levels showed no significant differences during hibernation. Therefore, PMCA3 did not appear to play a substantial role in the fluctuation of cytoplasmic Ca
2+ in hibernation. In contrast, the protein expression levels of SERCA1, a major Ca
2+ uptake channel in the SR, were significantly up-regulated during different stages of hibernation, contrary to the decrease in SERCA activity found in non-hibernating animals under disuse conditions [
42]. Our previous study showed that the protein content of SERCA2 in the soleus and extensor digitorum longus muscles of Daurian ground squirrels increased significantly during hibernation and IBA [
7]. Other studies have also reported that SERCA activity is more resistant to temperature reduction in hibernating cardiac muscle than that in non-hibernating rats [
43,
44]. Such evidence indicates that, although faced with various stresses during hibernation, including a low temperature, low metabolism, and prolonged skeletal muscle disuse, SERCA1 still maintains high activity and can pump more cytoplasmic Ca
2+ into the SR, thus avoiding an excessive increase in cytoplasmic Ca
2+ and related skeletal muscle injury. In addition, the protein expression of MCU, a major Ca
2+ absorption channel located in the mitochondrial membrane, was elevated during hibernation, and may therefore be another important mechanism for the absorption of Ca
2+ into the mitochondria and alleviation of the Ca
2+ level in the cytoplasm. However, we know that when the concentration of mitochondrial Ca
2+ reaches a certain threshold, mitochondrial depolarization is triggered and, as a consequence, the pro-apoptotic protein Bax is activated, translocated, and inserted into the outer membrane via Bax/Bax-homo-oligomerization [
45]. This is followed by the formation and opening of a mitochondrial permeability transition pore (mPTP), through which cytochrome C (a mitochondria-residing apoptogenic factor) is released into the cytosol, leading to the cleavage of nuclear DNA and cell apoptosis [
3,
46]. In the present study, the level of mitochondrial Ca
2+ only increased slightly during hibernation. Therefore, the simultaneously higher expression of MCU and LETM1 may be a strategy employed by hibernating ground squirrels to control the balance between cytoplasmic and mitochondrial Ca
2+, and thereby avoid mitochondrial Ca
2+ concentration-induced skeletal muscle damage, such as cell apoptosis. As a free Ca
2+ binding protein, CALM can relieve the elevation of cytoplasmic Ca
2+ by binding to free Ca
2+ in the cytoplasm. Our results showed that the protein expression of CALM in the PL muscle increased significantly, and the enhanced free Ca
2+ binding capacity mediated by the elevated protein expression of CALM may thus be another important mechanism in hibernating ground squirrels to alleviate cytoplasmic Ca
2+ levels during hibernation.
During the torpor-arousal cycle, the intracellular Ca2+ level showed increased-decreased fluctuation. Compared with that during LT and IBA, the cytoplasmic Ca2+ level in the skeletal muscles of the Daurian ground squirrels was down-regulated to varying degrees after re-entry into torpor (ET). Compared with levels in LT, the co-localization coefficients of STIM1 and ORAI1 decreased to varying degrees, representing decreased Ca2+ influx mediated by SOCE, which may be another critical mechanism used to avoid the intracellular Ca2+ increase caused by persistent Ca2+ influx during hibernation. In addition, we found that the higher protein expression of Ca2+ uptake channel MCU in ET may be another vital mechanism through which PL muscle can relieve cytoplasmic Ca2+ overload. It is worth noting that, after arousal from hibernation, the intracellular Ca2+ levels (i.e., cytoplasmic, SR, and mitochondrial) returned to the levels found in the SA and PRE groups, thus reflecting a strong and rapid Ca2+ recovery ability of these ground squirrels. We further found that the expression of Ca2+ transport proteins/channels or free Ca2+ binding protein also returned to the levels observed in the SA and PRE groups. This may be due to the sampling time of the POST group, which occurred 3 d after the squirrels aroused from torpor, and all physiological states had returned to normal.
By comparing and analyzing the mRNA and protein expression levels of each Ca
2+ transporter protein, we found that the levels were not always consistent in the same stage. In particular, during LT, the mRNA levels of most Ca
2+ transport proteins/channels were decreased or showed no significant change, whereas the corresponding protein expression levels were significantly increased. In view of these results, we checked the transcription and proteomic analysis results of the skeletal muscles during different hibernation stages (unpublished data) and found that the predicted results were consistent with our experimental findings. Previous studies have demonstrated that transcriptional elongation and initiation are essentially arrested during hibernation [
47,
48], which may be one of the main reasons for the lower mRNA expression of Ca
2+ transport proteins/channels in skeletal muscles of Daurian ground squirrels during LT. In other words, the inconsistent changes in mRNA and protein expression levels may be the result of total inhibition at the transcriptional level during LT, although the corresponding protein expression was not affected during hibernation.
In summary, despite experiencing stresses such as a low temperature, low metabolism, and prolonged hindlimb inactivity during hibernation, hibernating ground squirrels still possess a strong Ca2+ operation ability. Here, by regulating the activity and protein expression of Ca2+ pumps, Ca2+ channels, and Ca2+ binding proteins in the cytoplasm, SR, and mitochondrial membrane, the dynamic balance of intracellular Ca2+ homeostasis was well-maintained during hibernation. Therefore, maintaining intracellular Ca2+ homeostasis and avoiding skeletal muscle injury caused by its disturbance appear to be priority strategies employed by hibernating squirrels to cope with the various stresses induced during the torpor-arousal cycle.