Sterculic Acid: The Mechanisms of Action beyond Stearoyl-CoA Desaturase Inhibition and Therapeutic Opportunities in Human Diseases
Abstract
1. Introduction
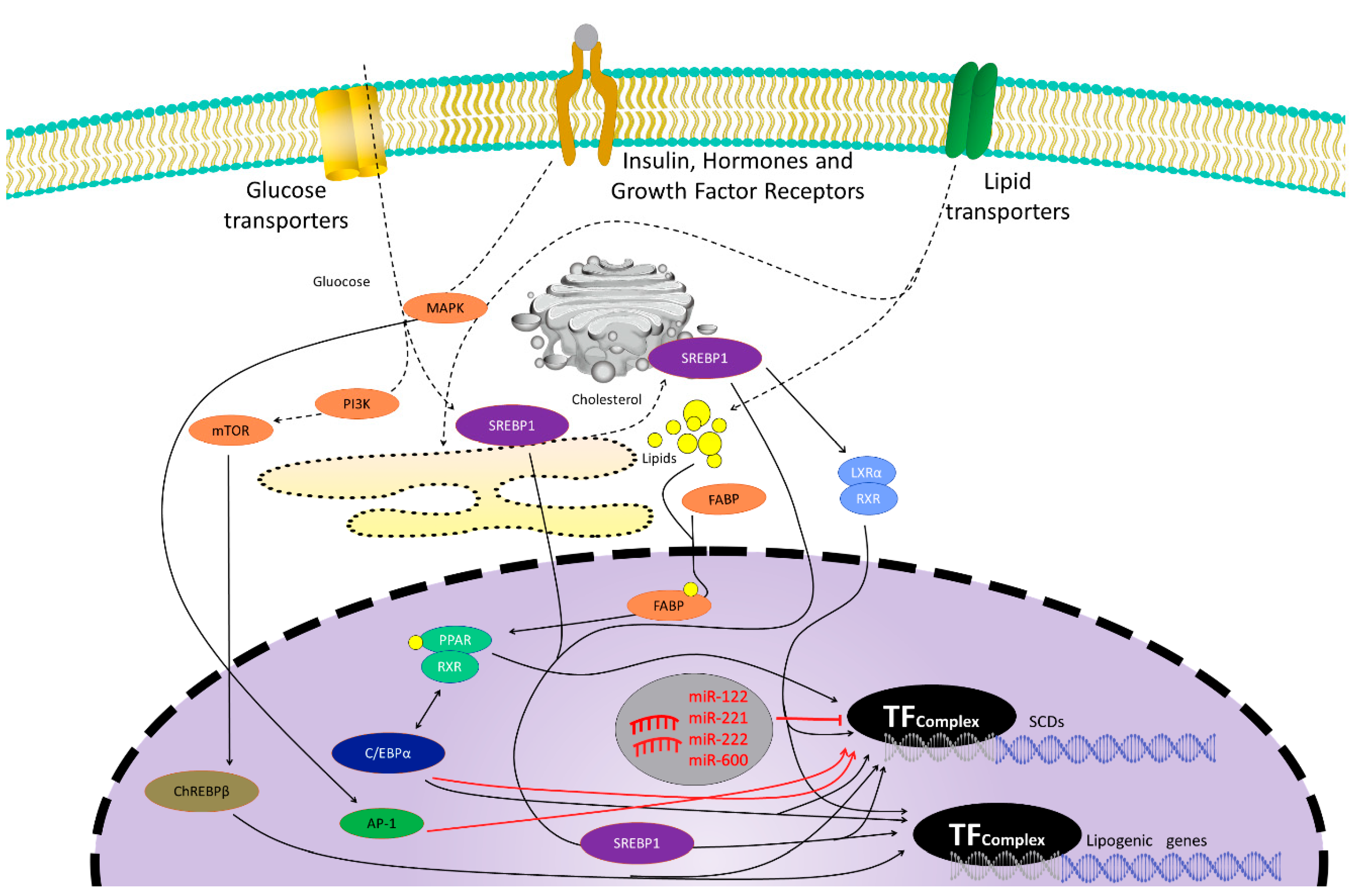
2. Stearoyl-CoA Desaturase (SCD)
2.1. Cancer
2.2. Dermatology
2.3. Alzheimer’s Disease
2.4. Liver
2.5. Atherosclerosis
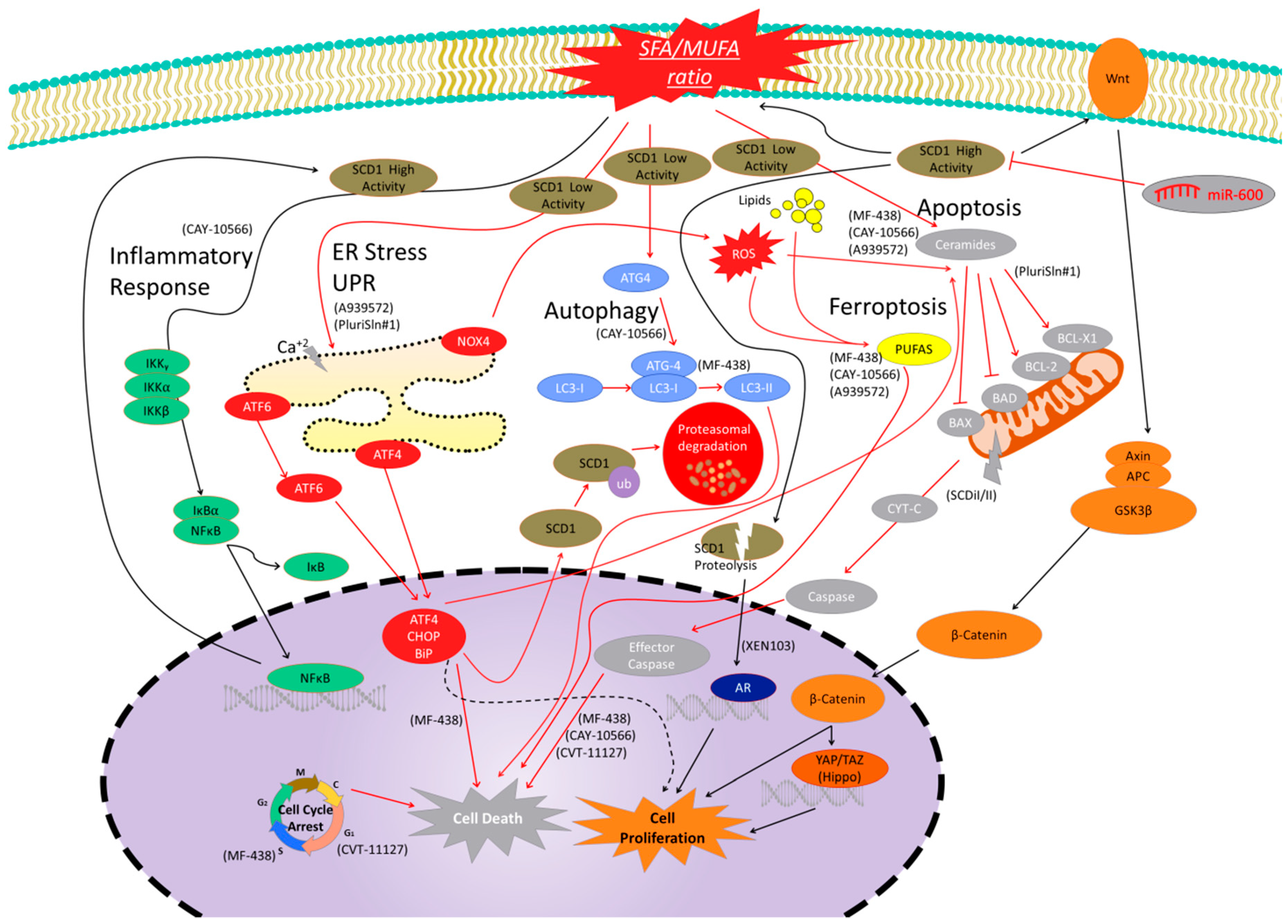
2.6. Adverse Effects of SCD1 Treatments
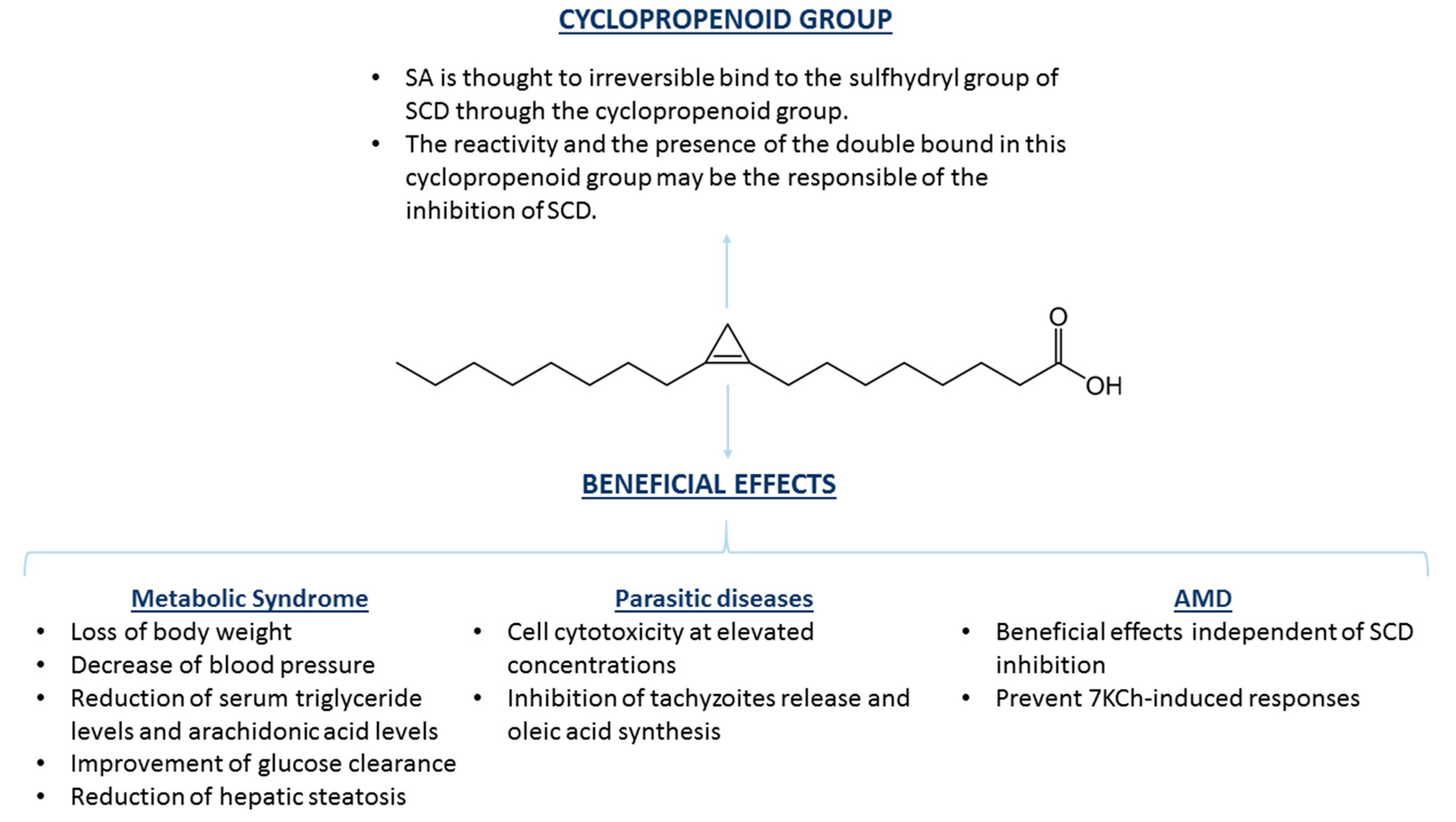
3. Sterculic Acid
3.1. Effects of SA on mRNA and Protein Expression
3.2. SA and Therapeutic Opportunities
4. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Smith, U.; Kahn, B.B. Adipose tissue regulates insulin sensitivity: Role of adipogenesis, de novo lipogenesis and novel lipids. J. Intern. Med. 2016, 280, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Xiaoli, A.M.; Yang, F. Regulation and Metabolic Significance of De Novo Lipogenesis in Adipose Tissues. Nutrients 2018, 10, 1383. [Google Scholar] [CrossRef] [PubMed]
- Aarsland, A.; Chinkes, D.; Wolfe, R.R. Hepatic and whole-body fat synthesis in humans during carbohydrate overfeeding. Am. J. Clin. Nutr. 1997, 65, 1774–1782. [Google Scholar] [CrossRef] [PubMed]
- Poudyal, H.; Brown, L. Stearoyl-CoA desaturase: A vital checkpoint in the development and progression of obesity. Endocr. Metab. Immune Disord Drug Targets 2011, 11, 217–231. [Google Scholar] [CrossRef] [PubMed]
- Paton, C.M.; Ntambi, J.M. Biochemical and physiological function of stearoyl-CoA desaturase. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E28–E37. [Google Scholar] [CrossRef]
- Enoch, H.G.; Catala, A.; Strittmatter, P. Mechanism of rat liver microsomal stearyl-CoA desaturase. Studies of the substrate specificity, enzyme-substrate interactions, and the function of lipid. J. Biol. Chem. 1976, 251, 5095–5103. [Google Scholar]
- Mosley, E.E.; McGuire, M.A. Methodology for the in vivo measurement of the delta9-desaturation of myristic, palmitic, and stearic acids in lactating dairy cattle. Lipids 2007, 42, 939–945. [Google Scholar] [CrossRef]
- Tracz-Gaszewska, Z.; Dobrzyn, P. Stearoyl-CoA Desaturase 1 as a Therapeutic Target for the Treatment of Cancer. Cancers 2019, 11, 948. [Google Scholar] [CrossRef]
- Chu, K.; Miyazaki, M.; Man, W.C.; Ntambi, J.M. Stearoyl-coenzyme A desaturase 1 deficiency protects against hypertriglyceridemia and increases plasma high-density lipoprotein cholesterol induced by liver X receptor activation. Mol. Cell Biol. 2006, 26, 6786–6798. [Google Scholar] [CrossRef]
- Kim, H.J.; Miyazaki, M.; Ntambi, J.M. Dietary cholesterol opposes PUFA-mediated repression of the stearoyl-CoA desaturase-1 gene by SREBP-1 independent mechanism. J. Lipid Res. 2002, 43, 1750–1757. [Google Scholar] [CrossRef]
- Miyazaki, M.; Dobrzyn, A.; Man, W.C.; Chu, K.; Sampath, H.; Kim, H.J.; Ntambi, J.M. Stearoyl-CoA desaturase 1 gene expression is necessary for fructose-mediated induction of lipogenic gene expression by sterol regulatory element-binding protein-1c-dependent and -independent mechanisms. J. Biol. Chem. 2004, 279, 25164–25171. [Google Scholar] [CrossRef] [PubMed]
- Piccinin, E.; Cariello, M.; De Santis, S.; Ducheix, S.; Sabba, C.; Ntambi, J.M.; Moschetta, A. Role of Oleic Acid in the Gut-Liver Axis: From Diet to the Regulation of Its Synthesis via Stearoyl-CoA Desaturase 1 (SCD1). Nutrients 2019, 11, 2283. [Google Scholar] [CrossRef] [PubMed]
- Puglisi, R.; Bellenghi, M.; Pontecorvi, G.; Gulino, A.; Petrini, M.; Felicetti, F.; Bottero, L.; Mattia, G.; Care, A. SCD5 restored expression favors differentiation and epithelial-mesenchymal reversion in advanced melanoma. Oncotarget 2018, 9, 7567–7581. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Uto, Y. Recent progress in the discovery and development of stearoyl CoA desaturase inhibitors. Chem. Phys. Lipids 2016, 197, 3–12. [Google Scholar] [CrossRef]
- Zhang, L.; Ge, L.; Parimoo, S.; Stenn, K.; Prouty, S.M. Human stearoyl-CoA desaturase: Alternative transcripts generated from a single gene by usage of tandem polyadenylation sites. Biochem. J. 1999, 340, 255–264. [Google Scholar] [CrossRef]
- Wang, J.; Yu, L.; Schmidt, R.E.; Su, C.; Huang, X.; Gould, K.; Cao, G. Characterization of HSCD5, a novel human stearoyl-CoA desaturase unique to primates. Biochem. Biophys. Res. Commun. 2005, 332, 735–742. [Google Scholar] [CrossRef]
- Tesfay, L.; Paul, B.T.; Konstorum, A.; Deng, Z.; Cox, A.O.; Lee, J.; Furdui, C.M.; Hegde, P.; Torti, F.M.; Torti, S.V. Stearoyl-CoA Desaturase 1 Protects Ovarian Cancer Cells from Ferroptotic Cell Death. Cancer Res. 2019, 79, 5355–5366. [Google Scholar] [CrossRef]
- Sinner, D.I.; Kim, G.J.; Henderson, G.C.; Igal, R.A. StearoylCoA desaturase-5: A novel regulator of neuronal cell proliferation and differentiation. PLoS ONE 2012, 7, e39787. [Google Scholar] [CrossRef]
- Bellenghi, M.; Puglisi, R.; Pedini, F.; De Feo, A.; Felicetti, F.; Bottero, L.; Sangaletti, S.; Errico, M.C.; Petrini, M.; Gesumundo, C.; et al. SCD5-induced oleic acid production reduces melanoma malignancy by intracellular retention of SPARC and cathepsin B. J. Pathol. 2015, 236, 315–325. [Google Scholar] [CrossRef]
- Wang, H.; Klein, M.G.; Zou, H.; Lane, W.; Snell, G.; Levin, I.; Li, K.; Sang, B.C. Crystal structure of human stearoyl-coenzyme A desaturase in complex with substrate. Nat. Struct. Mol. Biol. 2015, 22, 581–585. [Google Scholar] [CrossRef]
- Galbraith, L.; Leung, H.Y.; Ahmad, I. Lipid pathway deregulation in advanced prostate cancer. Pharm. Res. 2018, 131, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, M.; Man, W.C.; Ntambi, J.M. Targeted disruption of stearoyl-CoA desaturase1 gene in mice causes atrophy of sebaceous and meibomian glands and depletion of wax esters in the eyelid. J. Nutr. 2001, 131, 2260–2268. [Google Scholar] [CrossRef] [PubMed]
- Dalla Valle, A.; Vertongen, P.; Spruyt, D.; Lechanteur, J.; Suain, V.; Gaspard, N.; Brion, J.P.; Gangji, V.; Rasschaert, J. Induction of Stearoyl-CoA 9-Desaturase 1 Protects Human Mesenchymal Stromal Cells Against Palmitic Acid-Induced Lipotoxicity and Inflammation. Front. Endocrinol. 2019, 10, 726. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Strable, M.S.; Ntambi, J.M. Stearoyl CoA desaturase 1: Role in cellular inflammation and stress. Adv. Nutr. 2011, 2, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Shen, X.; Seyfert, H.M. Stearoyl-CoA desaturase 1 expression is downregulated in liver and udder during E. coli mastitis through enhanced expression of repressive C/EBP factors and reduced expression of the inducer SREBP1A. BMC Mol. Biol. 2016, 17, 16. [Google Scholar] [CrossRef]
- Shao, W.; Espenshade, P.J. Expanding roles for SREBP in metabolism. Cell Metab. 2012, 16, 414–419. [Google Scholar] [CrossRef]
- Noureddin, M.; Sanyal, A.J. Pathogenesis of NASH: The Impact of Multiple Pathways. Curr. Hepatol. Rep. 2018, 17, 350–360. [Google Scholar] [CrossRef]
- Jalil, A.; Bourgeois, T.; Menegaut, L.; Lagrost, L.; Thomas, C.; Masson, D. Revisiting the Role of LXRs in PUFA Metabolism and Phospholipid Homeostasis. Int. J. Mol. Sci. 2019, 20, 3787. [Google Scholar] [CrossRef]
- Joseph, S.B.; Laffitte, B.A.; Patel, P.H.; Watson, M.A.; Matsukuma, K.E.; Walczak, R.; Collins, J.L.; Osborne, T.F.; Tontonoz, P. Direct and indirect mechanisms for regulation of fatty acid synthase gene expression by liver X receptors. J. Biol. Chem. 2002, 277, 11019–11025. [Google Scholar] [CrossRef]
- Kovalic, A.J.; Banerjee, P.; Tran, Q.T.; Singal, A.K.; Satapathy, S.K. Genetic and Epigenetic Culprits in the Pathogenesis of Nonalcoholic Fatty Liver Disease. J. Clin. Exp. Hepatol. 2018, 8, 390–402. [Google Scholar] [CrossRef]
- Mancini, R.; Noto, A.; Pisanu, M.E.; De Vitis, C.; Maugeri-Sacca, M.; Ciliberto, G. Metabolic features of cancer stem cells: The emerging role of lipid metabolism. Oncogene 2018, 37, 2367–2378. [Google Scholar] [CrossRef] [PubMed]
- Samuel, W.; Kutty, R.K.; Duncan, T.; Vijayasarathy, C.; Kuo, B.C.; Chapa, K.M.; Redmond, T.M. Fenretinide induces ubiquitin-dependent proteasomal degradation of stearoyl-CoA desaturase in human retinal pigment epithelial cells. J. Cell Physiol. 2014, 229, 1028–1038. [Google Scholar] [CrossRef] [PubMed]
- Nagao, K.; Murakami, A.; Umeda, M. Structure and Function of Delta9-Fatty Acid Desaturase. Chem. Pharm. Bull. 2019, 67, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Mauvoisin, D.; Rocque, G.; Arfa, O.; Radenne, A.; Boissier, P.; Mounier, C. Role of the PI3-kinase/mTor pathway in the regulation of the stearoyl CoA desaturase (SCD1) gene expression by insulin in liver. J. Cell Commun. Signal. 2007, 1, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Byagowi, S.; Naserpour Farivar, T.; Najafipour, R.; Sahmani, M.; Darabi, M.; Fayezi, S.; Mirshahvaladi, S. Effect of PPARdelta agonist on stearoyl-CoA desaturase 1 in human pancreatic cancer cells: Role of MEK/ERK1/2 pathway. Can. J. Diabetes 2015, 39, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Mauvoisin, D.; Prevost, M.; Ducheix, S.; Arnaud, M.P.; Mounier, C. Key role of the ERK1/2 MAPK pathway in the transcriptional regulation of the Stearoyl-CoA Desaturase (SCD1) gene expression in response to leptin. Mol. Cell Endocrinol. 2010, 319, 116–128. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Yan, H.; Xia, M.; Chang, X.; Xu, X.; Wang, L.; Sun, X.; Lu, Y.; Bian, H.; Li, X.; et al. Metformin attenuates triglyceride accumulation in HepG2 cells through decreasing stearyl-coenzyme A desaturase 1 expression. Lipids Health Dis. 2018, 17, 114. [Google Scholar] [CrossRef]
- Rini, B.I. Temsirolimus, an inhibitor of mammalian target of rapamycin. Clin. Cancer Res. 2008, 14, 1286–1290. [Google Scholar] [CrossRef]
- Roongta, U.V.; Pabalan, J.G.; Wang, X.; Ryseck, R.P.; Fargnoli, J.; Henley, B.J.; Yang, W.P.; Zhu, J.; Madireddi, M.T.; Lawrence, R.M.; et al. Cancer cell dependence on unsaturated fatty acids implicates stearoyl-CoA desaturase as a target for cancer therapy. Mol. Cancer Res. 2011, 9, 1551–1561. [Google Scholar] [CrossRef]
- von Roemeling, C.A.; Marlow, L.A.; Pinkerton, A.B.; Crist, A.; Miller, J.; Tun, H.W.; Smallridge, R.C.; Copland, J.A. Aberrant lipid metabolism in anaplastic thyroid carcinoma reveals stearoyl CoA desaturase 1 as a novel therapeutic target. J. Clin. Endocrinol. Metab. 2015, 100, E697–E709. [Google Scholar] [CrossRef]
- Ben-David, U.; Gan, Q.F.; Golan-Lev, T.; Arora, P.; Yanuka, O.; Oren, Y.S.; Leikin-Frenkel, A.; Graf, M.; Garippa, R.; Boehringer, M.; et al. Selective elimination of human pluripotent stem cells by an oleate synthesis inhibitor discovered in a high-throughput screen. Cell Stem Cell 2013, 12, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Condello, S.; Thomes-Pepin, J.; Ma, X.; Xia, Y.; Hurley, T.D.; Matei, D.; Cheng, J.X. Lipid Desaturation Is a Metabolic Marker and Therapeutic Target of Ovarian Cancer Stem Cells. Cell Stem Cell 2017, 20, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Lynch, J.K.; Freeman, J.; Liu, B.; Xin, Z.; Zhao, H.; Serby, M.D.; Kym, P.R.; Suhar, T.S.; Smith, H.T.; et al. Discovery of potent, selective, orally bioavailable stearoyl-CoA desaturase 1 inhibitors. J. Med. Chem. 2007, 50, 3086–3100. [Google Scholar] [CrossRef]
- Mason, P.; Liang, B.; Li, L.; Fremgen, T.; Murphy, E.; Quinn, A.; Madden, S.L.; Biemann, H.P.; Wang, B.; Cohen, A.; et al. SCD1 inhibition causes cancer cell death by depleting mono-unsaturated fatty acids. PLoS ONE 2012, 7, e33823. [Google Scholar] [CrossRef]
- Pisanu, M.E.; Noto, A.; De Vitis, C.; Morrone, S.; Scognamiglio, G.; Botti, G.; Venuta, F.; Diso, D.; Jakopin, Z.; Padula, F.; et al. Blockade of Stearoyl-CoA-desaturase 1 activity reverts resistance to cisplatin in lung cancer stem cells. Cancer Lett. 2017, 406, 93–104. [Google Scholar] [CrossRef]
- Oballa, R.M.; Belair, L.; Black, W.C.; Bleasby, K.; Chan, C.C.; Desroches, C.; Du, X.; Gordon, R.; Guay, J.; Guiral, S.; et al. Development of a liver-targeted stearoyl-CoA desaturase (SCD) inhibitor (MK-8245) to establish a therapeutic window for the treatment of diabetes and dyslipidemia. J. Med. Chem. 2011, 54, 5082–5096. [Google Scholar] [CrossRef]
- Peck, B.; Schug, Z.T.; Zhang, Q.; Dankworth, B.; Jones, D.T.; Smethurst, E.; Patel, R.; Mason, S.; Jiang, M.; Saunders, R.; et al. Inhibition of fatty acid desaturation is detrimental to cancer cell survival in metabolically compromised environments. Cancer Metab. 2016, 4, 6. [Google Scholar] [CrossRef]
- Hess, D.; Chisholm, J.W.; Igal, R.A. Inhibition of stearoylCoA desaturase activity blocks cell cycle progression and induces programmed cell death in lung cancer cells. PLoS ONE 2010, 5, e11394. [Google Scholar] [CrossRef]
- Meingassner, J.G.; Aschauer, H.; Winiski, A.P.; Dales, N.; Yowe, D.; Winther, M.D.; Zhang, Z.; Stutz, A.; Billich, A. Pharmacological inhibition of stearoyl CoA desaturase in the skin induces atrophy of the sebaceous glands. J. Investig. Derm. 2013, 133, 2091–2094. [Google Scholar] [CrossRef]
- Herrera-Meza, M.S.; Mendoza-Lopez, M.R.; Garcia-Barradas, O.; Sanchez-Otero, M.G.; Silva-Hernandez, E.R.; Angulo, J.O.; Oliart-Ros, R.M. Dietary anhydrous milk fat naturally enriched with conjugated linoleic acid and vaccenic acid modify cardiovascular risk biomarkers in spontaneously hypertensive rats. Int. J. Food Sci. Nutr. 2013, 64, 575–586. [Google Scholar] [CrossRef]
- Herrera-Meza, S.; Rodriguez-Landa, J.F.; Martinez, A.J.; Herrera-Meza, G.; Fernandez-Demeneghi, R.; Reyes-Saldana, K.; Oliart-Ros, R.M. Behavioral Effect of Sterculia apetala Seed Oil Consumption in Male Zucker Rats. J. Med. Food 2017, 20, 1133–1139. [Google Scholar] [CrossRef] [PubMed]
- Ortinau, L.C.; Nickelson, K.J.; Stromsdorfer, K.L.; Naik, C.Y.; Pickering, R.T.; Haynes, R.A.; Fritsche, K.L.; Perfield, J.W., 2nd. Sterculic oil, a natural inhibitor of SCD1, improves the metabolic state of obese OLETF rats. Obesity. 2013, 21, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Ortinau, L.C.; Pickering, R.T.; Nickelson, K.J.; Stromsdorfer, K.L.; Naik, C.Y.; Haynes, R.A.; Bauman, D.E.; Rector, R.S.; Fritsche, K.L.; Perfield, J.W., 2nd. Sterculic Oil, a Natural SCD1 Inhibitor, Improves Glucose Tolerance in Obese ob/ob Mice. ISRN Endocrinol. 2012, 2012, 947323. [Google Scholar] [CrossRef] [PubMed]
- Gratraud, P.; Huws, E.; Falkard, B.; Adjalley, S.; Fidock, D.A.; Berry, L.; Jacobs, W.R., Jr.; Baird, M.S.; Vial, H.; Kremer, L. Oleic acid biosynthesis in Plasmodium falciparum: Characterization of the stearoyl-CoA desaturase and investigation as a potential therapeutic target. PLoS ONE 2009, 4, e6889. [Google Scholar] [CrossRef]
- Hao, P.; Alaraj, I.Q.; Dulayymi, J.R.; Baird, M.S.; Liu, J.; Liu, Q. Sterculic Acid and Its Analogues Are Potent Inhibitors of Toxoplasma gondii. Korean J. Parasitol. 2016, 54, 139–145. [Google Scholar] [CrossRef]
- Phetsuksiri, B.; Jackson, M.; Scherman, H.; McNeil, M.; Besra, G.S.; Baulard, A.R.; Slayden, R.A.; DeBarber, A.E.; Barry, C.E., 3rd; Baird, M.S. Unique mechanism of action of the thiourea drug isoxyl on Mycobacterium tuberculosis. J. Biol. Chem. 2003, 278, 53123–53130. [Google Scholar] [CrossRef]
- Huang, J.D.; Amaral, J.; Lee, J.W.; Larrayoz, I.M.; Rodriguez, I.R. Sterculic acid antagonizes 7-ketocholesterol-mediated inflammation and inhibits choroidal neovascularization. Biochim. Biophys. Acta 2012, 1821, 637–646. [Google Scholar] [CrossRef][Green Version]
- Huang, J.D.; Amaral, J.; Lee, J.W.; Rodriguez, I.R. 7-Ketocholesterol-induced inflammation signals mostly through the TLR4 receptor both in vitro and in vivo. PLoS ONE 2014, 9, e100985. [Google Scholar] [CrossRef]
- Ray, U.; Roy, S.S. Aberrant lipid metabolism in cancer cells—The role of oncolipid-activated signaling. FEBS J. 2018, 285, 432–443. [Google Scholar] [CrossRef]
- Igal, R.A. Stearoyl CoA desaturase-1: New insights into a central regulator of cancer metabolism. Biochim. Biophys. Acta 2016, 1861, 1865–1880. [Google Scholar] [CrossRef]
- Holder, A.M.; Gonzalez-Angulo, A.M.; Chen, H.; Akcakanat, A.; Do, K.A.; Fraser Symmans, W.; Pusztai, L.; Hortobagyi, G.N.; Mills, G.B.; Meric-Bernstam, F. High stearoyl-CoA desaturase 1 expression is associated with shorter survival in breast cancer patients. Breast Cancer Res. Treat. 2013, 137, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.M.; Jiang, Q.H.; Cai, C.; Qu, M.; Shen, W. SCD1 negatively regulates autophagy-induced cell death in human hepatocellular carcinoma through inactivation of the AMPK signaling pathway. Cancer Lett. 2015, 358, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Choi, H.; Park, S.S.; Chang, C.; Kim, E. Stearoyl CoA desaturase (SCD) facilitates proliferation of prostate cancer cells through enhancement of androgen receptor transactivation. Mol. Cells 2011, 31, 371–377. [Google Scholar] [CrossRef]
- Flowers, M.T.; Paton, C.M.; O’Byrne, S.M.; Schiesser, K.; Dawson, J.A.; Blaner, W.S.; Kendziorski, C.; Ntambi, J.M. Metabolic changes in skin caused by Scd1 deficiency: A focus on retinol metabolism. PLoS ONE 2011, 6, e19734. [Google Scholar] [CrossRef]
- Fritz, V.; Benfodda, Z.; Rodier, G.; Henriquet, C.; Iborra, F.; Avances, C.; Allory, Y.; de la Taille, A.; Culine, S.; Blancou, H.; et al. Abrogation of de novo lipogenesis by stearoyl-CoA desaturase 1 inhibition interferes with oncogenic signaling and blocks prostate cancer progression in mice. Mol. Cancer 2010, 9, 1740–1754. [Google Scholar] [CrossRef]
- Scaglia, N.; Igal, R.A. Inhibition of Stearoyl-CoA Desaturase 1 expression in human lung adenocarcinoma cells impairs tumorigenesis. Int. J. Oncol. 2008, 33, 839–850. [Google Scholar]
- Noto, A.; Raffa, S.; De Vitis, C.; Roscilli, G.; Malpicci, D.; Coluccia, P.; Di Napoli, A.; Ricci, A.; Giovagnoli, M.R.; Aurisicchio, L.; et al. Stearoyl-CoA desaturase-1 is a key factor for lung cancer-initiating cells. Cell Death Dis. 2013, 4, e947. [Google Scholar] [CrossRef]
- Noto, A.; De Vitis, C.; Pisanu, M.E.; Roscilli, G.; Ricci, G.; Catizone, A.; Sorrentino, G.; Chianese, G.; Taglialatela-Scafati, O.; Trisciuoglio, D.; et al. Stearoyl-CoA-desaturase 1 regulates lung cancer stemness via stabilization and nuclear localization of YAP/TAZ. Oncogene 2017, 36, 4573–4584. [Google Scholar] [CrossRef]
- Bruschini, S.; di Martino, S.; Pisanu, M.E.; Fattore, L.; De Vitis, C.; Laquintana, V.; Buglioni, S.; Tabbi, E.; Cerri, A.; Visca, P.; et al. CytoMatrix for a reliable and simple characterization of lung cancer stem cells from malignant pleural effusions. J. Cell. Physiol. 2019. [Google Scholar] [CrossRef]
- Piccolo, S.; Dupont, S.; Cordenonsi, M. The biology of YAP/TAZ: Hippo signaling and beyond. Physiol. Rev. 2014, 94, 1287–1312. [Google Scholar] [CrossRef]
- Zhao, G.; Cardenas, H.; Matei, D. Ovarian Cancer-Why Lipids Matter. Cancers 2019, 11, 1870. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, M.L.; van Eck, M.; Hildebrand, R.B.; Wong, B.W.; Bissada, N.; Ruddle, P.; Kontush, A.; Hussein, H.; Pouladi, M.A.; Chapman, M.J.; et al. Despite antiatherogenic metabolic characteristics, SCD1-deficient mice have increased inflammation and atherosclerosis. Arter. Thromb. Vasc. Biol. 2009, 29, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Larrayoz, I.M.; Ferrero, H.; Martisova, E.; Gil-Bea, F.J.; Ramirez, M.J.; Martinez, A. Adrenomedullin Contributes to Age-Related Memory Loss in Mice and Is Elevated in Aging Human Brains. Front. Mol. Neurosci. 2017, 10, 384. [Google Scholar] [CrossRef] [PubMed]
- Ferrero, H.; Larrayoz, I.M.; Martisova, E.; Solas, M.; Howlett, D.R.; Francis, P.T.; Gil-Bea, F.J.; Martinez, A.; Ramirez, M.J. Increased Levels of Brain Adrenomedullin in the Neuropathology of Alzheimer’s Disease. Mol. Neurobiol. 2018, 55, 5177–5183. [Google Scholar] [CrossRef] [PubMed]
- Astarita, G.; Jung, K.M.; Vasilevko, V.; Dipatrizio, N.V.; Martin, S.K.; Cribbs, D.H.; Head, E.; Cotman, C.W.; Piomelli, D. Elevated stearoyl-CoA desaturase in brains of patients with Alzheimer’s disease. PLoS ONE 2011, 6, e24777. [Google Scholar] [CrossRef] [PubMed]
- Angulo, P. Nonalcoholic fatty liver disease. N. Engl. J. Med. 2002, 346, 1221–1231. [Google Scholar] [CrossRef]
- Lyn, R.K.; Singaravelu, R.; Kargman, S.; O’Hara, S.; Chan, H.; Oballa, R.; Huang, Z.; Jones, D.M.; Ridsdale, A.; Russell, R.S.; et al. Stearoyl-CoA desaturase inhibition blocks formation of hepatitis C virus-induced specialized membranes. Sci. Rep. 2014, 4, 4549. [Google Scholar] [CrossRef]
- Pi, H.; Wang, Z.; Liu, M.; Deng, P.; Yu, Z.; Zhou, Z.; Gao, F. SCD1 activation impedes foam cell formation by inducing lipophagy in oxLDL-treated human vascular smooth muscle cells. J. Cell. Mol. Med. 2019, 23, 5259–5269. [Google Scholar] [CrossRef]
- Savransky, V.; Jun, J.; Li, J.; Nanayakkara, A.; Fonti, S.; Moser, A.B.; Steele, K.E.; Schweitzer, M.A.; Patil, S.P.; Bhanot, S.; et al. Dyslipidemia and atherosclerosis induced by chronic intermittent hypoxia are attenuated by deficiency of stearoyl coenzyme A desaturase. Circ. Res. 2008, 103, 1173–1180. [Google Scholar] [CrossRef]
- Nakaya, K.; Ayaori, M.; Uto-Kondo, H.; Sotherden, G.M.; Nishida, T.; Katamoto, H.; Miura, Y.; Takiguchi, S.; Yakushiji, E.; Iizuka, M.; et al. Overexpression of stearoyl-coenzyme A desaturase 1 in macrophages promotes reverse cholesterol transport. Biochim. Biophys. Acta 2013, 1831, 1402–1411. [Google Scholar] [CrossRef]
- Le Gros, J.E.; Jenkins, D.R.; Prestidge, R.L.; Watson, J.D. Expression of genes in cloned murine cell lines that can be maintained in both interleukin 2- and interleukin 3-dependent growth states. Immunol. Cell Biol. 1987, 65, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.M.; Rudel, L.L. Stearoyl-coenzyme A desaturase 1 inhibition and the metabolic syndrome: Considerations for future drug discovery. Curr. Opin. Lipidol. 2010, 21, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.M.; Chung, S.; Sawyer, J.K.; Degirolamo, C.; Alger, H.M.; Nguyen, T.; Zhu, X.; Duong, M.N.; Wibley, A.L.; Shah, R.; et al. Inhibition of stearoyl-coenzyme A desaturase 1 dissociates insulin resistance and obesity from atherosclerosis. Circulation 2008, 118, 1467–1475. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Dales, N.A.; Winther, M.D. Opportunities and challenges in developing stearoyl-coenzyme A desaturase-1 inhibitors as novel therapeutics for human disease. J. Med. Chem. 2014, 57, 5039–5056. [Google Scholar] [CrossRef] [PubMed]
- Busch, A.K.; Gurisik, E.; Cordery, D.V.; Sudlow, M.; Denyer, G.S.; Laybutt, D.R.; Hughes, W.E.; Biden, T.J. Increased fatty acid desaturation and enhanced expression of stearoyl coenzyme A desaturase protects pancreatic beta-cells from lipoapoptosis. Diabetes 2005, 54, 2917–2924. [Google Scholar] [CrossRef] [PubMed]
- Vriens, K.; Christen, S.; Parik, S.; Broekaert, D.; Yoshinaga, K.; Talebi, A.; Dehairs, J.; Escalona-Noguero, C.; Schmieder, R.; Cornfield, T.; et al. Evidence for an alternative fatty acid desaturation pathway increasing cancer plasticity. Immunol. Cell Biol. 2019, 566, 403–406. [Google Scholar] [CrossRef] [PubMed]
- Kadegowda, A.K.; Burns, T.A.; Pratt, S.L.; Duckett, S.K. Inhibition of stearoyl-CoA desaturase 1 reduces lipogenesis in primary bovine adipocytes. Lipids 2013, 48, 967–976. [Google Scholar] [CrossRef]
- Gomez, F.E.; Bauman, D.E.; Ntambi, J.M.; Fox, B.G. Effects of sterculic acid on stearoyl-CoA desaturase in differentiating 3T3-L1 adipocytes. Biochem. Biophys. Res. Commun. 2003, 300, 316–326. [Google Scholar] [CrossRef]
- Bichi, E.; Toral, P.G.; Hervas, G.; Frutos, P.; Gomez-Cortes, P.; Juarez, M.; de la Fuente, M.A. Inhibition of 9-desaturase activity with sterculic acid: Effect on the endogenous synthesis of cis-9 18:1 and cis-9, trans-11 18:2 in dairy sheep. J. Dairy Sci. 2012, 95, 5242–5252. [Google Scholar] [CrossRef]
- Lock, A.L.; Corl, B.A.; Barbano, D.M.; Bauman, D.E.; Ip, C. The anticarcinogenic effect of trans-11 18:1 is dependent on its conversion to cis-9, trans-11 CLA by delta9-desaturase in rats. J. Nutr. 2004, 134, 2698–2704. [Google Scholar] [CrossRef]
- Raju, P.K.; Reiser, R. Inhibition of fatty acyl desaturase by cyclopropene fatty acids. J. Biol. Chem. 1967, 242, 379–384. [Google Scholar] [PubMed]
- Jeffcoat, R.; Pollard, M.R. Studies on the inhibition of the desaturases by cyclopropenoid fatty acids. Lipids 1977, 12, 480–485. [Google Scholar] [CrossRef] [PubMed]
- Dallaire, M.P.; Taga, H.; Ma, L.; Corl, B.A.; Gervais, R.; Lebeuf, Y.; Richard, F.J.; Chouinard, P.Y. Effects of abomasal infusion of conjugated linoleic acids, Sterculia foetida oil, and fish oil on production performance and the extent of fatty acid Delta(9)-desaturation in dairy cows. J. Dairy Sci. 2014, 97, 6411–6425. [Google Scholar] [CrossRef] [PubMed]
- Major, C.A.; Ryan, K.; Bennett, A.J.; Lock, A.L.; Bauman, D.E.; Salter, A.M. Inhibition of stearoyl CoA desaturase activity induces hypercholesterolemia in the cholesterol-fed hamster. J. Lipid Res. 2008, 49, 1456–1465. [Google Scholar] [CrossRef]
- Seibert, J.T.; Abuajamieh, M.; Sanz Fernandez, M.V.; Johnson, J.S.; Kvidera, S.K.; Horst, E.A.; Mayorga, E.J.; Lei, S.; Patience, J.F.; Ross, J.W.; et al. Effects of heat stress and insulin sensitizers on pig adipose tissue. J. Anim. Sci. 2018, 96, 510–520. [Google Scholar] [CrossRef]
- Bao, X.; Thelen, J.J.; Bonaventure, G.; Ohlrogge, J.B. Characterization of cyclopropane fatty-acid synthase from Sterculia foetida. J. Biol. Chem. 2003, 278, 12846–12853. [Google Scholar] [CrossRef]
- Cao, J.; Blond, J.P.; Bezard, J. Inhibition of fatty acid delta 6- and delta 5-desaturation by cyclopropene fatty acids in rat liver microsomes. Biochim. Biophys. Acta 1993, 1210, 27–34. [Google Scholar] [CrossRef]
- Macfarlane, J.J.; Shenstone, F.S.; Vickery, J.R. Malvalic acid and its structure. Nature 1957, 179, 830–831. [Google Scholar] [CrossRef]
- Zoeller, R.A.; Wood, R. Effects of cyclopropene fatty acids on the lipid composition of the Morris hepatoma 7288C. Lipids 1984, 19, 529–538. [Google Scholar] [CrossRef]
- Allen, E.; Johnson, A.R.; Fogerty, A.C.; Pearson, J.A.; Shenstone, F.S. Inhibition by cyclopropene fatty acids of the desaturation of stearic acid in hen liver. Lipids 1967, 2, 419–423. [Google Scholar] [CrossRef]
- Lee, D.J.; Wales, J.H.; Sinnhuber, R.O. Promotion of aflatoxin-induced hepatoma growth in trout by methyl malvalate and sterculate. Cancer Res. 1971, 31, 960–963. [Google Scholar] [PubMed]
- Slayden, O.; Stormshak, F. In vivo and in vitro effects of a cyclopropenoid fatty acid on ovine corpus luteum function. Endocrinology 1990, 127, 3166–3171. [Google Scholar] [CrossRef] [PubMed]
- Corl, B.A.; Baumgard, L.H.; Dwyer, D.A.; Griinari, J.M.; Phillips, B.S.; Bauman, D.E. The role of Delta(9)-desaturase in the production of cis-9, trans-11 CLA. J. Nutr. Biochem. 2001, 12, 622–630. [Google Scholar] [CrossRef]
- Griinari, J.M.; Corl, B.A.; Lacy, S.H.; Chouinard, P.Y.; Nurmela, K.V.; Bauman, D.E. Conjugated linoleic acid is synthesized endogenously in lactating dairy cows by Delta(9)-desaturase. J. Nutr. 2000, 130, 2285–2291. [Google Scholar] [CrossRef]
- Kay, J.K.; Mackle, T.R.; Auldist, M.J.; Thomson, N.A.; Bauman, D.E. Endogenous synthesis of cis-9, trans-11 conjugated linoleic acid in dairy cows fed fresh pasture. J. Dairy Sci. 2004, 87, 369–378. [Google Scholar] [CrossRef]
- Miyazaki, M.; Kim, Y.C.; Ntambi, J.M. A lipogenic diet in mice with a disruption of the stearoyl-CoA desaturase 1 gene reveals a stringent requirement of endogenous monounsaturated fatty acids for triglyceride synthesis. J. Lipid Res. 2001, 42, 1018–1024. [Google Scholar]
- Sherling, D.H.; Perumareddi, P.; Hennekens, C.H. Metabolic Syndrome. J. Cardiovasc. Pharm. 2017, 22, 365–367. [Google Scholar] [CrossRef]
- Rodriguez, I.R.; Larrayoz, I.M. Cholesterol oxidation in the retina: Implications of 7KCh formation in chronic inflammation and age-related macular degeneration. J. lipid Res. 2010, 51, 2847–2862. [Google Scholar] [CrossRef]
- Buttari, B.; Segoni, L.; Profumo, E.; D’Arcangelo, D.; Rossi, S.; Facchiano, F.; Businaro, R.; Iuliano, L.; Rigano, R. 7-Oxo-cholesterol potentiates pro-inflammatory signaling in human M1 and M2 macrophages. Biochem. Pharm. 2013, 86, 130–137. [Google Scholar] [CrossRef]
- Hayden, J.M.; Brachova, L.; Higgins, K.; Obermiller, L.; Sevanian, A.; Khandrika, S.; Reaven, P.D. Induction of monocyte differentiation and foam cell formation in vitro by 7-ketocholesterol. J. Lipid Res. 2002, 43, 26–35. [Google Scholar]
- Pedruzzi, E.; Guichard, C.; Ollivier, V.; Driss, F.; Fay, M.; Prunet, C.; Marie, J.C.; Pouzet, C.; Samadi, M.; Elbim, C.; et al. NAD(P)H oxidase Nox-4 mediates 7-ketocholesterol-induced endoplasmic reticulum stress and apoptosis in human aortic smooth muscle cells. Mol. Cell. Biol. 2004, 24, 10703–10717. [Google Scholar] [CrossRef] [PubMed]
- Vejux, A.; Lizard, G. Cytotoxic effects of oxysterols associated with human diseases: Induction of cell death (apoptosis and/or oncosis), oxidative and inflammatory activities, and phospholipidosis. Mol. Asp. Med. 2009, 30, 153–170. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Jenner, A.M.; Shui, G.; Cheong, W.F.; Mitchell, T.W.; Nealon, J.R.; Kim, W.S.; McCann, H.; Wenk, M.R.; Halliday, G.M.; et al. Lipid pathway alterations in Parkinson’s disease primary visual cortex. PLoS ONE 2011, 6, e17299. [Google Scholar] [CrossRef] [PubMed]
- Olivier, E.; Dutot, M.; Regazzetti, A.; Leguillier, T.; Dargere, D.; Auzeil, N.; Laprevote, O.; Rat, P. P2X7-pannexin-1 and amyloid beta-induced oxysterol input in human retinal cell: Role in age-related macular degeneration? Biochimie 2016, 127, 70–78. [Google Scholar] [CrossRef]
- Phan, H.T.; Hata, T.; Morita, M.; Yoda, T.; Hamada, T.; Vestergaard, M.C.; Takagi, M. The effect of oxysterols on the interaction of Alzheimer’s amyloid beta with model membranes. Biochim. Biophys. Acta 2013, 1828, 2487–2495. [Google Scholar] [CrossRef]
- Testa, G.; Staurenghi, E.; Zerbinati, C.; Gargiulo, S.; Iuliano, L.; Giaccone, G.; Fanto, F.; Poli, G.; Leonarduzzi, G.; Gamba, P. Changes in brain oxysterols at different stages of Alzheimer’s disease: Their involvement in neuroinflammation. Redox Biol. 2016, 10, 24–33. [Google Scholar] [CrossRef]
- Moreira, E.F.; Larrayoz, I.M.; Lee, J.W.; Rodriguez, I.R. 7-Ketocholesterol is present in lipid deposits in the primate retina: Potential implication in the induction of VEGF and CNV formation. Invest. Ophthalmol. Vis. Sci. 2009, 50, 523–532. [Google Scholar] [CrossRef]
- Larrayoz, I.M.; Huang, J.D.; Lee, J.W.; Pascual, I.; Rodriguez, I.R. 7-ketocholesterol-induced inflammation: Involvement of multiple kinase signaling pathways via NFkappaB but independently of reactive oxygen species formation. Invest. Ophthalmol. Vis. Sci. 2010, 51, 4942–4955. [Google Scholar] [CrossRef]
- Pariente, A.; Pelaez, R.; Perez-Sala, A.; Larrayoz, I.M. Inflammatory and cell death mechanisms induced by 7-ketocholesterol in the retina. Implications for age-related macular degeneration. Exp. Eye. Res. 2019, 187, 107746. [Google Scholar] [CrossRef]
- Rey-Funes, M.; Larrayoz, I.M.; Fernandez, J.C.; Contartese, D.S.; Rolon, F.; Inserra, P.I.; Martinez-Murillo, R.; Lopez-Costa, J.J.; Dorfman, V.B.; Martinez, A.; et al. Methylene blue prevents retinal damage in an experimental model of ischemic proliferative retinopathy. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 310, R1011–R1019. [Google Scholar] [CrossRef]
- Rey-Funes, M.; Larrayoz, I.M.; Contartese, D.S.; Solino, M.; Sarotto, A.; Bustelo, M.; Bruno, M.; Dorfman, V.B.; Loidl, C.F.; Martinez, A. Hypothermia Prevents Retinal Damage Generated by Optic Nerve Trauma in the Rat. Sci. Rep. 2017, 7, 6966. [Google Scholar] [CrossRef] [PubMed]
| Inhibitor | Pathology | Tissue/Organ | Dose | Effect | References |
|---|---|---|---|---|---|
| A939572 (Bristol-Meyer Squibb) | Alopecia, Hypoplasia of meibomiam and sebaceous glands | Skin | 3–60 mg/Kg | Sebaceous gland atrophy, reduction of lipid content | [14] |
| Cancer | Pharynx Stomach Kidney Thyroid | 19 nM 100 mg/Kg 6–65 nM 5–100 nM | Cell growth inhibition, cell death | [38,39,40] | |
| Cancer stem cells, mouse embryos Ovarian cancer Stem cells | 75–100 nM 5 μM | Induce cell death thought ER stress, UPR Induce ferroptosis and apoptotic ceramides | [17,41] | ||
| CAY10566 (Cayman Chemical) | Cancer | Liver Ovary Colon | 7–8 nM, 5 μM 1 μM | Cell growth inhibition, decrease in the oleic content, alterations in autophagy | [14,42,43,44] |
| Cancer stem cells | 75 nM 1–5 μM | Reduce cell viability Reduce NFκB signaling Induce ferroptosis and apoptotic ceramides | [17,41,42] | ||
| PluriSIn#1 | Cancer | Cancer stem cells Cancer cell lines, Fibroblast, mouse embryos | 20 μM | Induce cell death thought ER stress, UPR, and ROS/NOS | [41] |
| MF-438 | Cancer | Thyroid Lung | 2–5 nM | Cell growth inhibition, cell death, decrease of ALDH1A levels | [14] |
| Lung cancer stem Cells Ovarian cancer Stem cells | 0.007–50 μM 1 μM | Induce cycle arrest, apoptosis, RE stress, and autophagy Induce ferroptosis and apoptotic ceramides | [17,45] | ||
| MK-8245 (Merk Frosst) | Type II diabetes, dyslipidemia, obesity | Liver | 20–60 mg/Kg | Antidyslipidemic Antidiabetic | [46] |
| SCDi I/II | Cancer | Breast and prostate cancer cell lines | 0.001 nM–100 μM | Cytochrome C dependent apoptosis and tumor growth inhibition | [47] |
| CVT-11127 | Cancer | Lung cancer cells | 1 μM | Inhibit cell cycle and induce cell apoptosis | [48] |
| XEN103 (Novartis) | Acne | Skin | 2–14 nM | Sebaceous gland atrophy Blockage of SCD1 transcription induced by androgens Phosphatidylcholine decrease of monounsaturated acyl chains | [14,49] |
| Sterculic Acid | Metabolic syndrome | Adipose tissue Liver | Diets supplemented with 0.4%–1.3% of SO | Blood pressure decrease, loss of body weight, decrease of serum triglyceride levels, decrease of arachidonic acid levels, improvement of glucose clearance, reduction of hepatic steatosis | [50,51,52,53] |
| Parasitic diseases (toxoplasmosis, malaria, and tuberculosis) | Small intestine Erythrocytes Lungs | 870 μM–1 mM, 10–100 μg/mL | Cell cytotoxicity at high concentrations, inhibition of tachyzoites release, inhibition of oleic acid synthesis | [54,55,56] | |
| Age-related macular degeneration | Retina | 1–10 μM | Counteracts the inflammatory and cytotoxic effects of 7-ketocholesterol, but seems to be independent from its capacity to inhibit SCD1 | [57,58] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peláez, R.; Pariente, A.; Pérez-Sala, Á.; Larráyoz, I.M. Sterculic Acid: The Mechanisms of Action beyond Stearoyl-CoA Desaturase Inhibition and Therapeutic Opportunities in Human Diseases. Cells 2020, 9, 140. https://doi.org/10.3390/cells9010140
Peláez R, Pariente A, Pérez-Sala Á, Larráyoz IM. Sterculic Acid: The Mechanisms of Action beyond Stearoyl-CoA Desaturase Inhibition and Therapeutic Opportunities in Human Diseases. Cells. 2020; 9(1):140. https://doi.org/10.3390/cells9010140
Chicago/Turabian StylePeláez, Rafael, Ana Pariente, Álvaro Pérez-Sala, and Ignacio M. Larráyoz. 2020. "Sterculic Acid: The Mechanisms of Action beyond Stearoyl-CoA Desaturase Inhibition and Therapeutic Opportunities in Human Diseases" Cells 9, no. 1: 140. https://doi.org/10.3390/cells9010140
APA StylePeláez, R., Pariente, A., Pérez-Sala, Á., & Larráyoz, I. M. (2020). Sterculic Acid: The Mechanisms of Action beyond Stearoyl-CoA Desaturase Inhibition and Therapeutic Opportunities in Human Diseases. Cells, 9(1), 140. https://doi.org/10.3390/cells9010140





