DNA Damage Changes Distribution Pattern and Levels of HP1 Protein Isoforms in the Nucleolus and Increases Phosphorylation of HP1β-Ser88
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Cultivation and Treatment
2.2. Immunofluorescence
2.3. Confocal Microscopy, Image Analysis and STED Microscopy
2.4. Immunoprecipitation
2.5. Western Blotting
2.6. Fluorescence Lifetime Image–Förster Resonance Energy Transfer (FLIM-FRET) Technique
2.7. Immunoaffinity Enrichment of HP1β for mass Spectrometric Analysis
2.8. Mass Spectrometric Analysis
2.9. Database Searches and Quantification of HP1β Peptide Forms
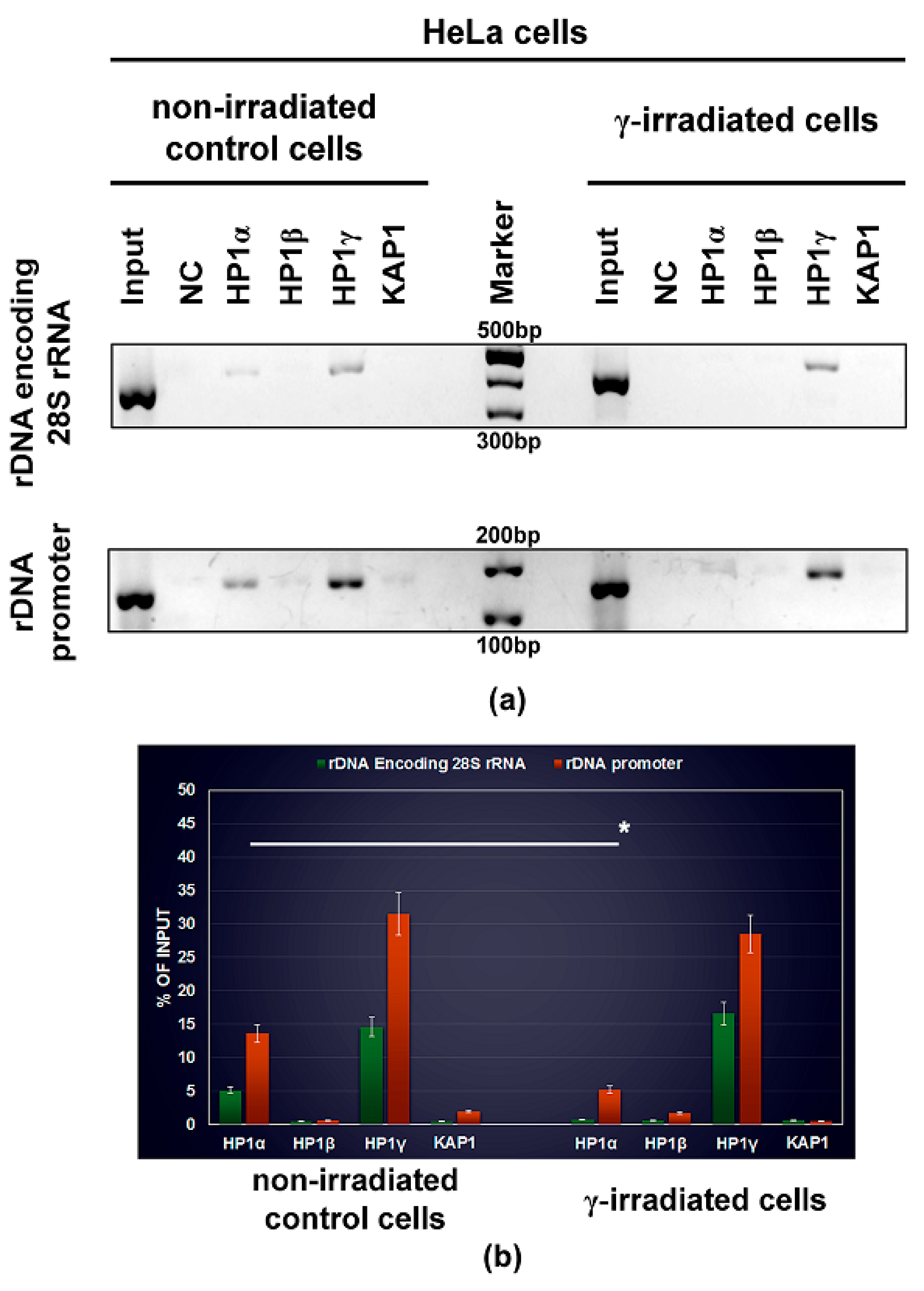
2.10. ChIP-Polymerase Chain Reaction (ChIP-PCR) Analysis of HP1 Isoforms in Ribosomal Genes
3. Results
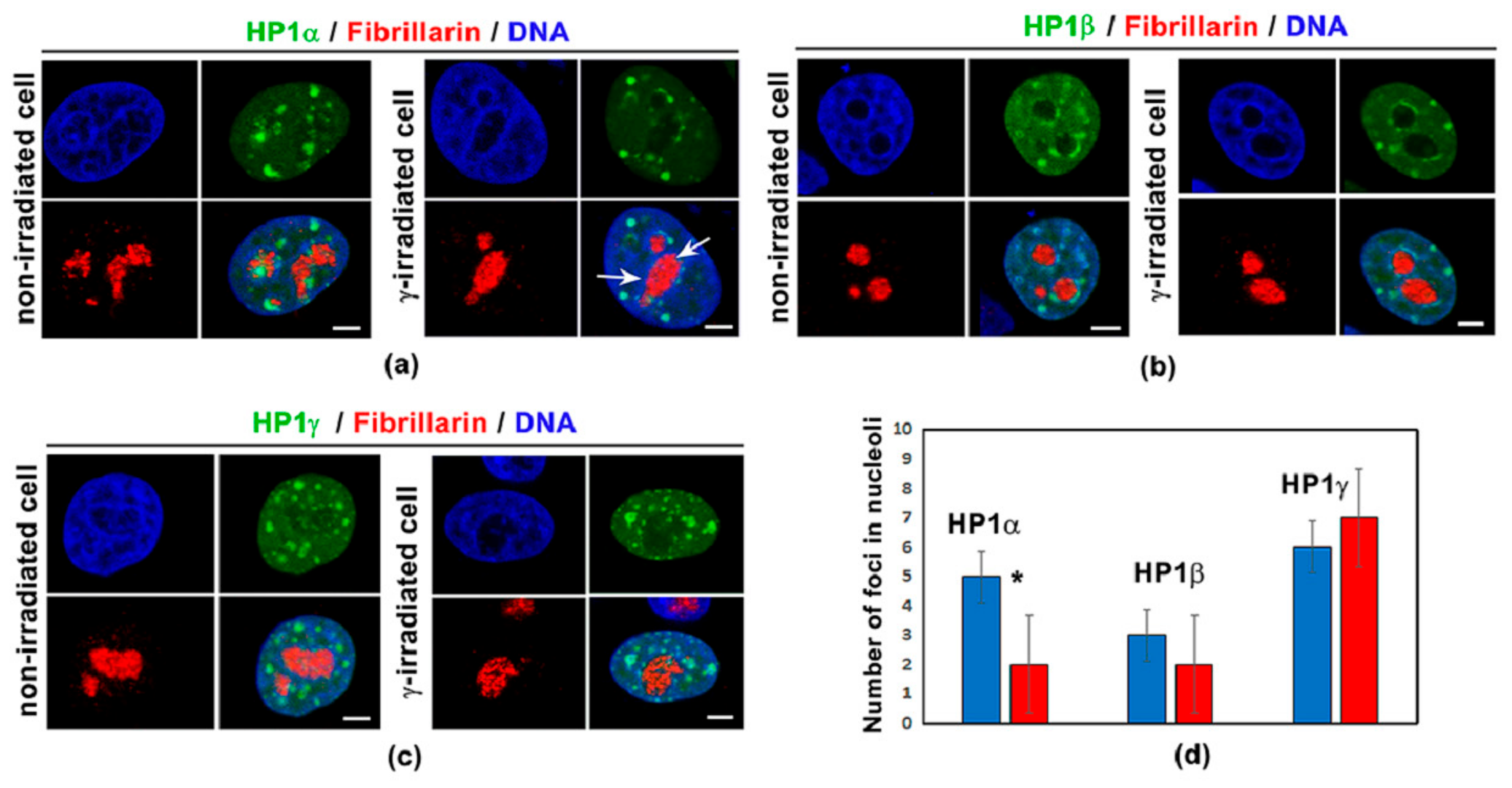
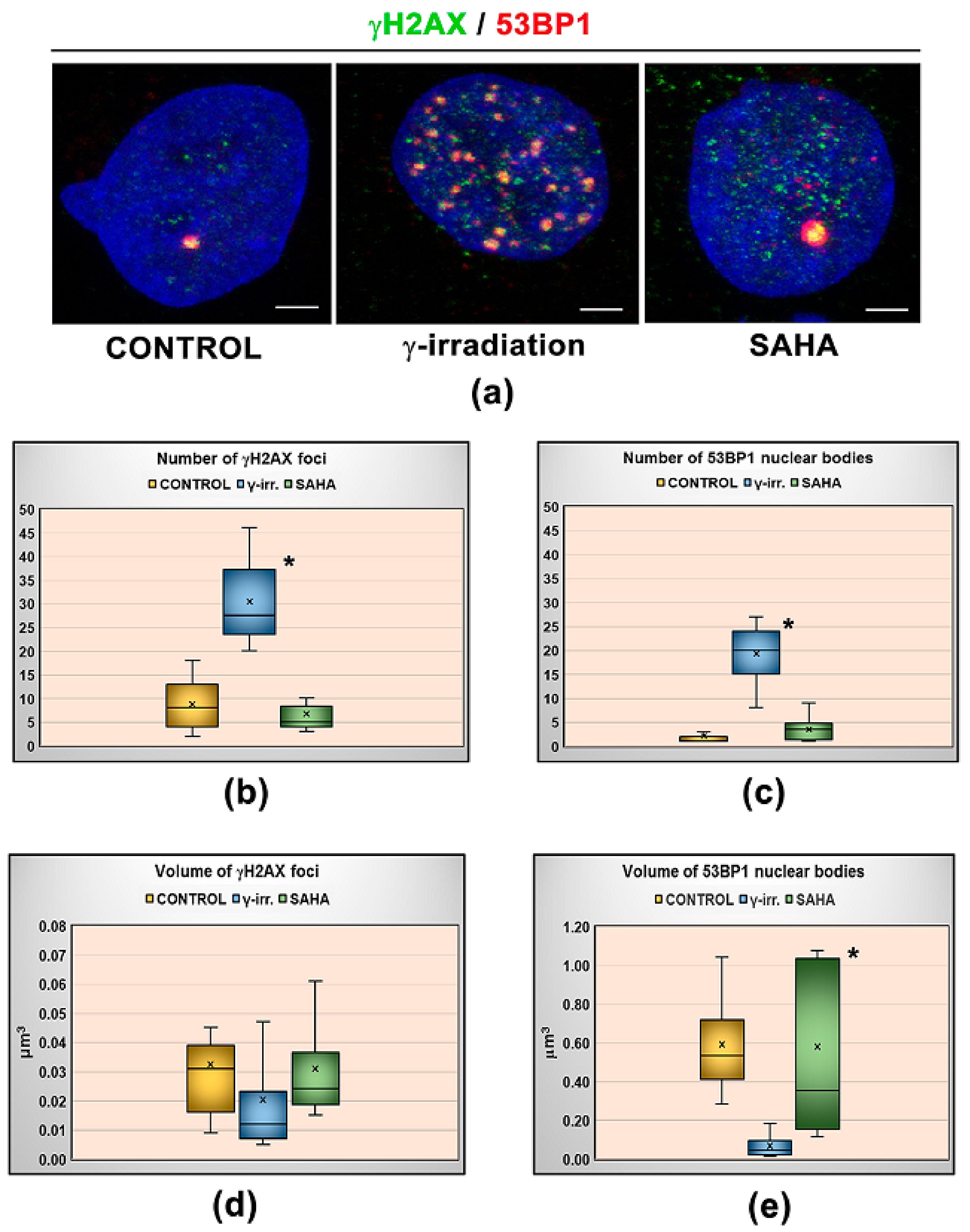
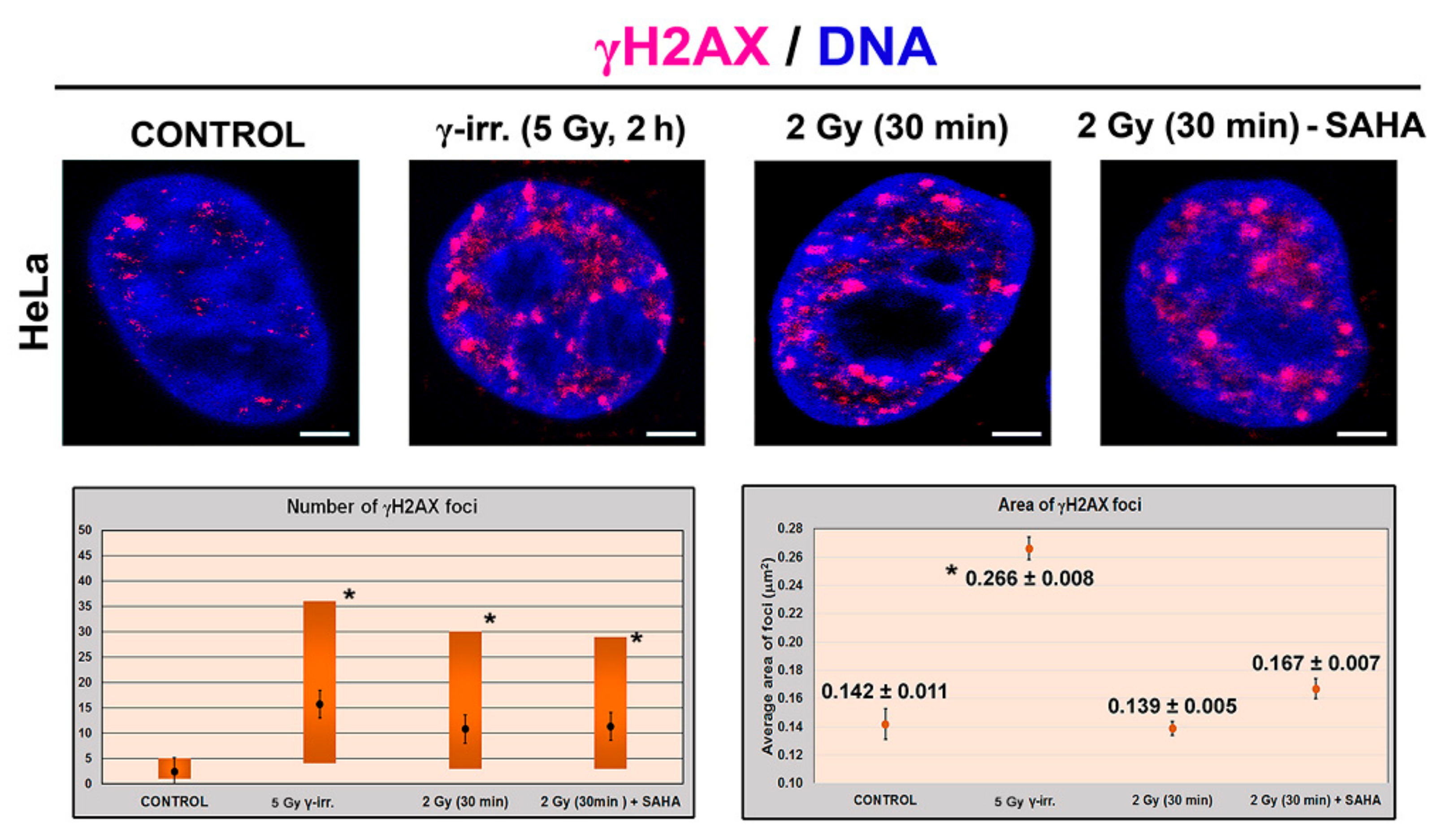
3.1. Distribution Pattern of HP1 Isoforms in the Compartment of the Nucleolus
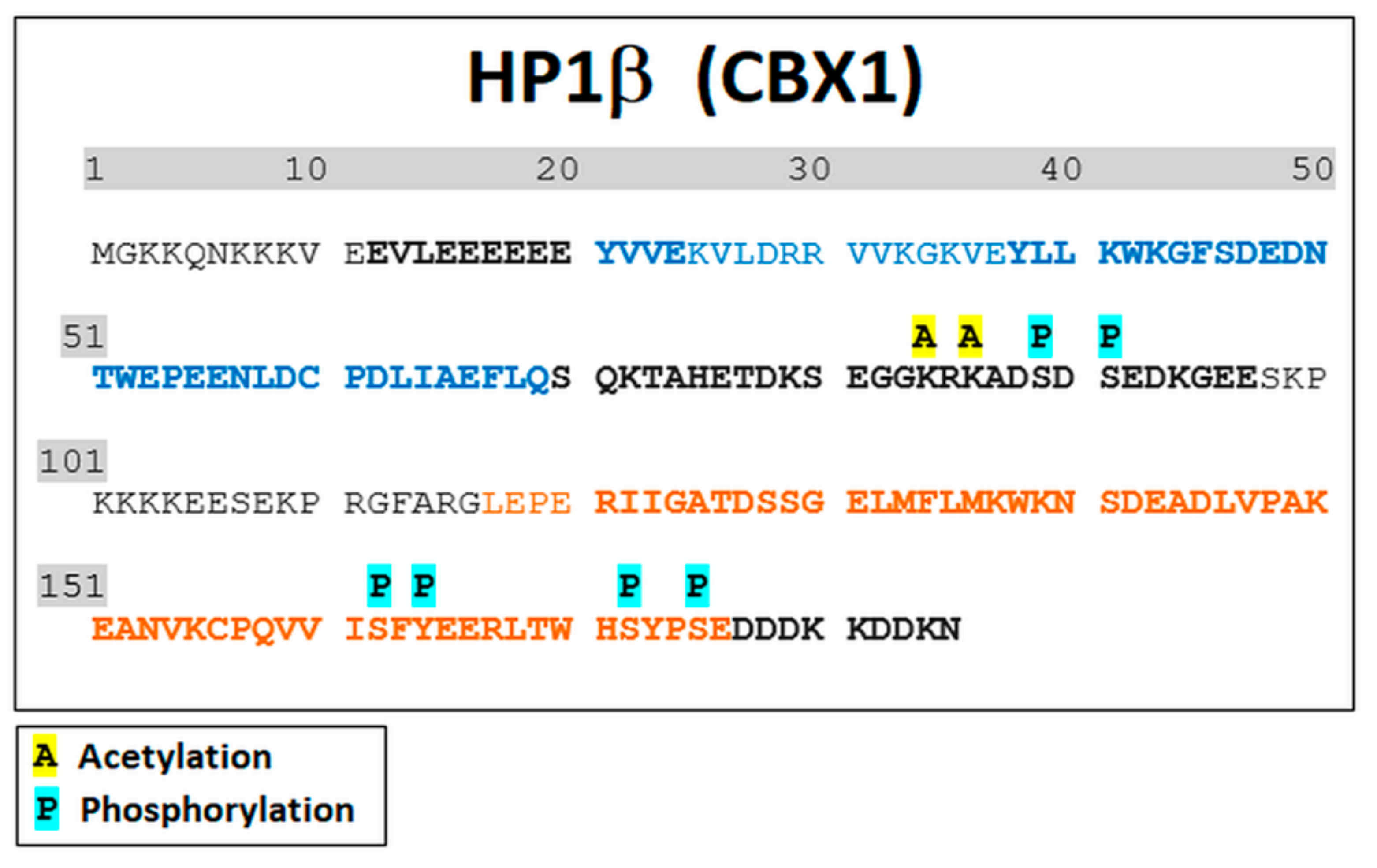
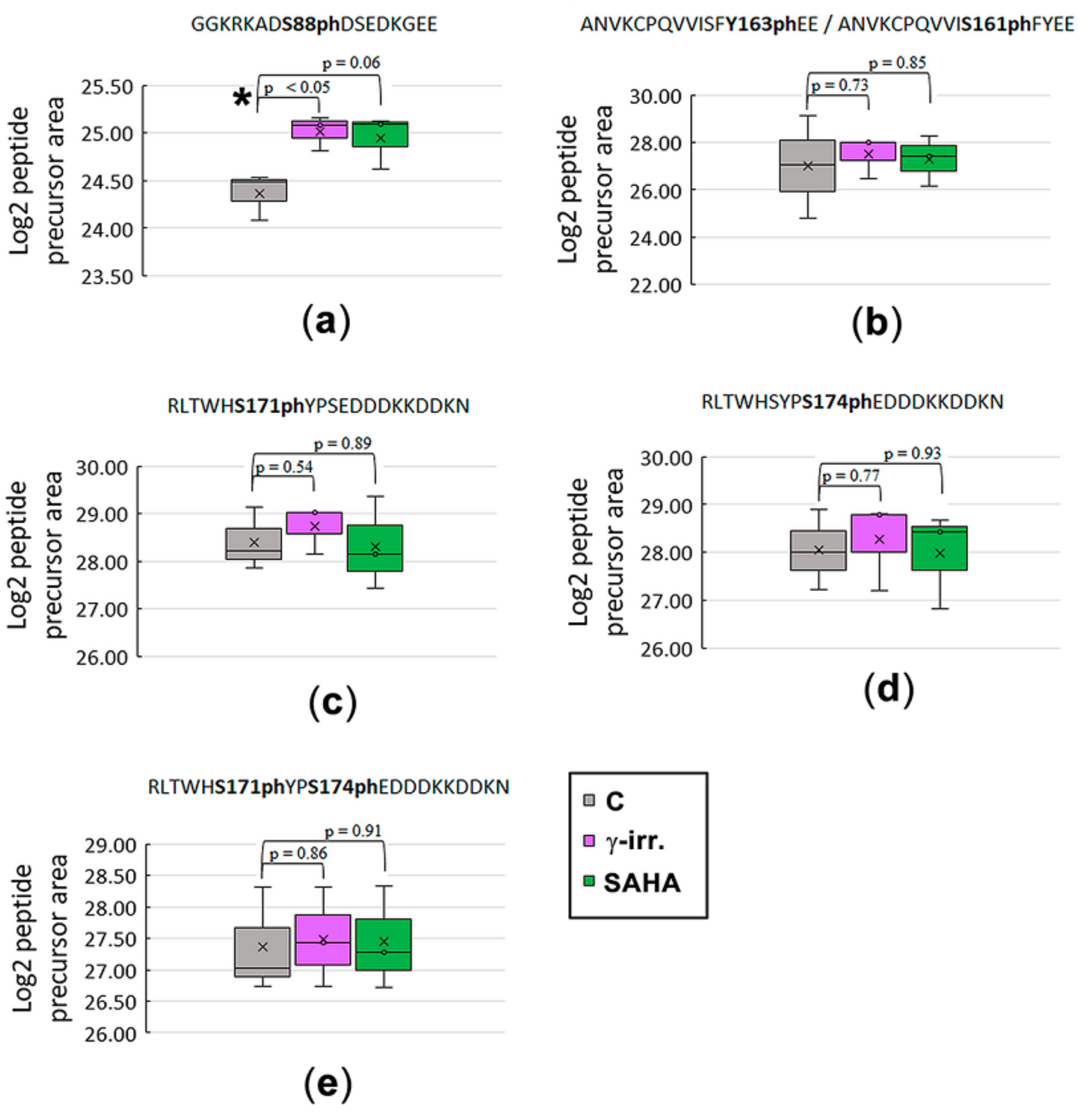
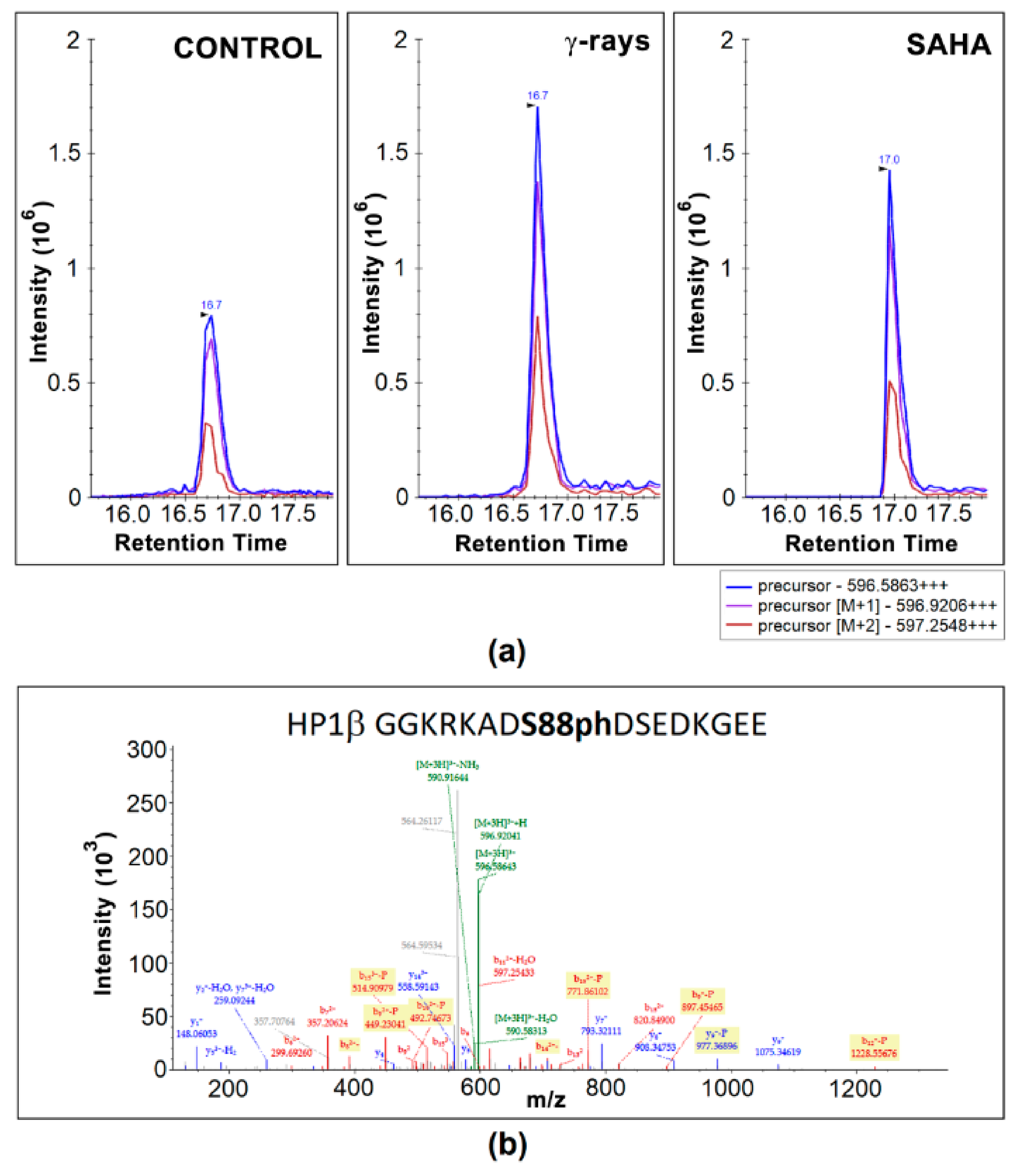
3.2. Mass Spectrometry Showed an Increased Level of HP1β Ser88 Phosphorylation Induced by γ-Irradiation
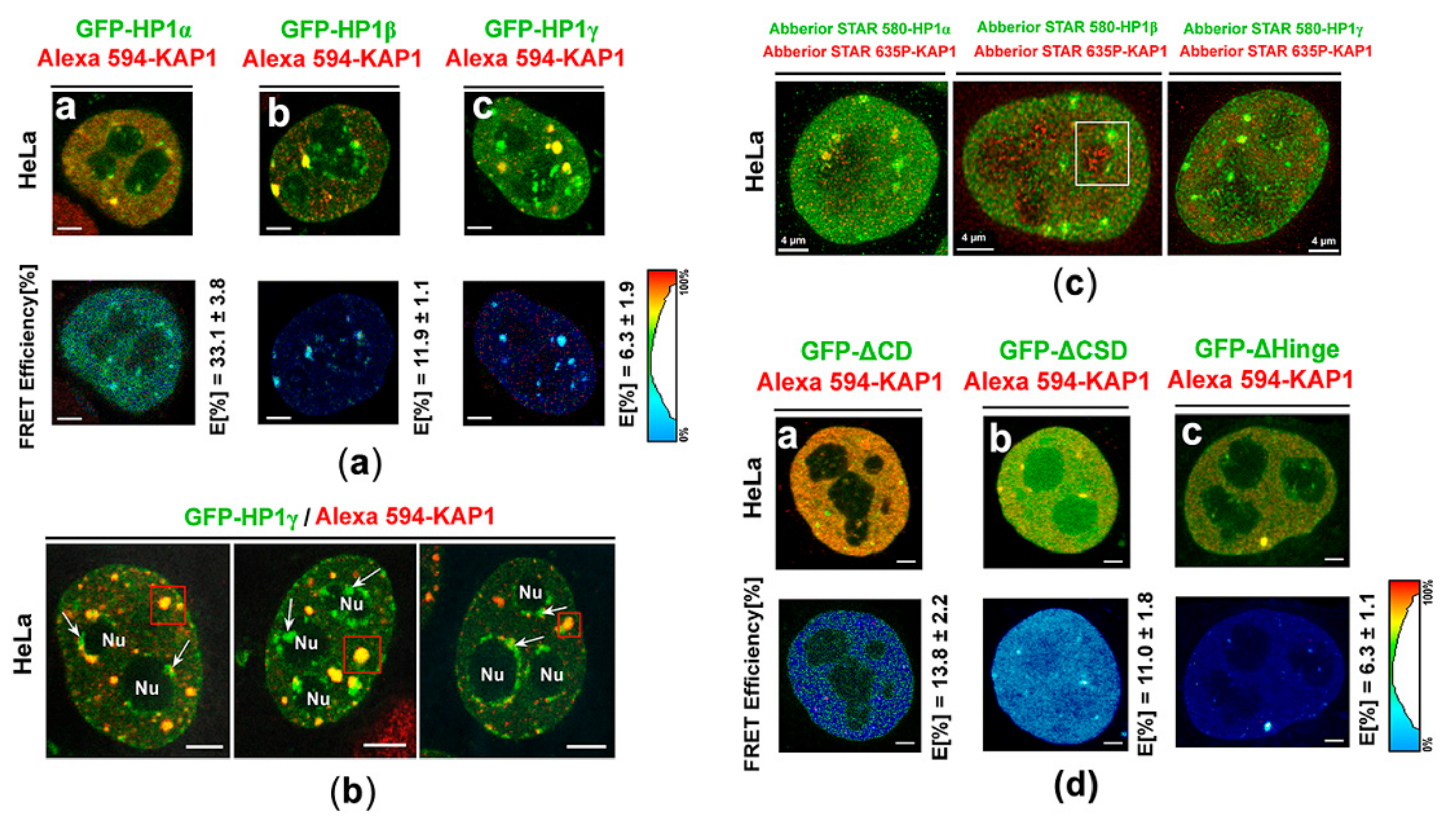
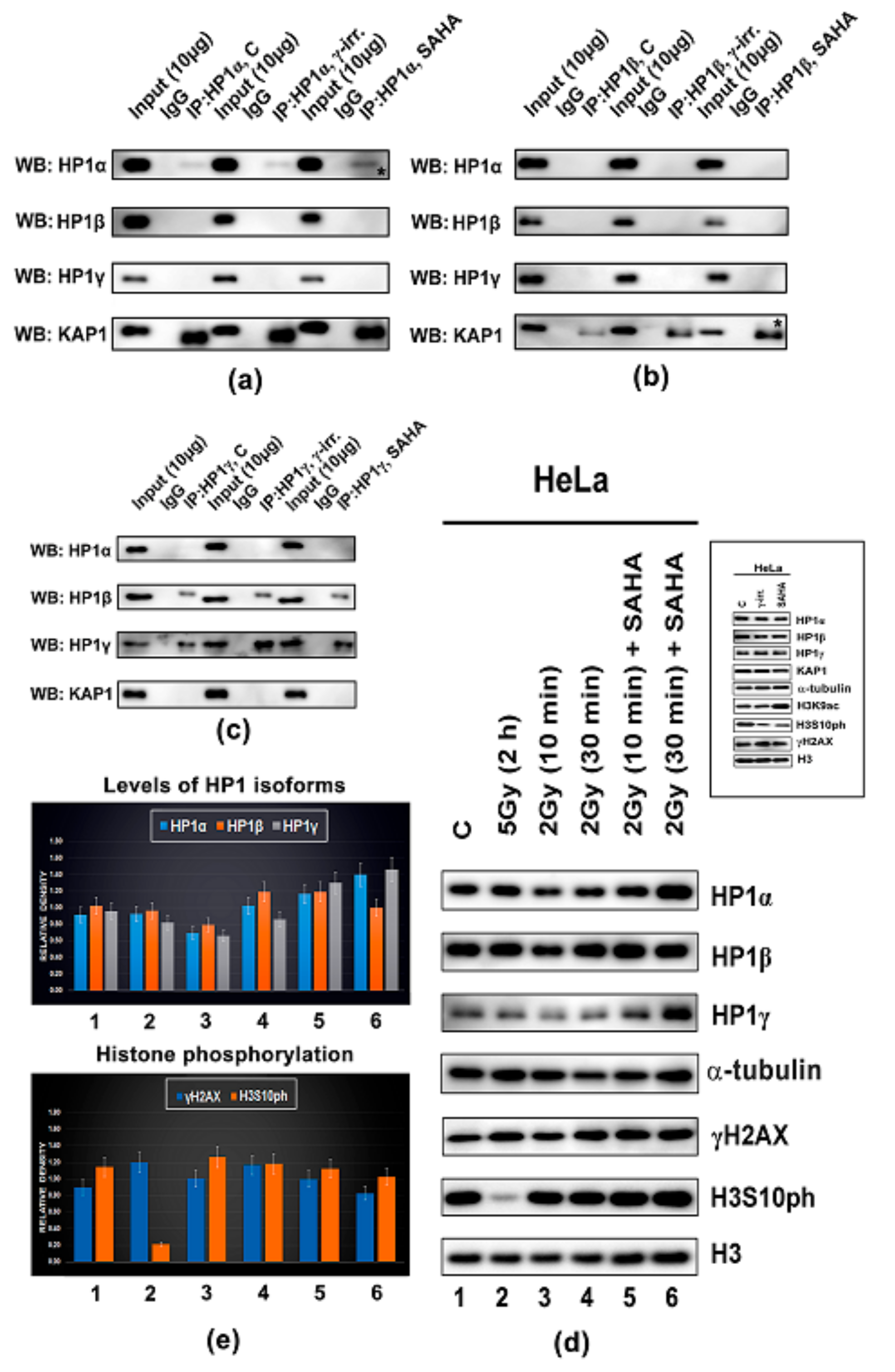
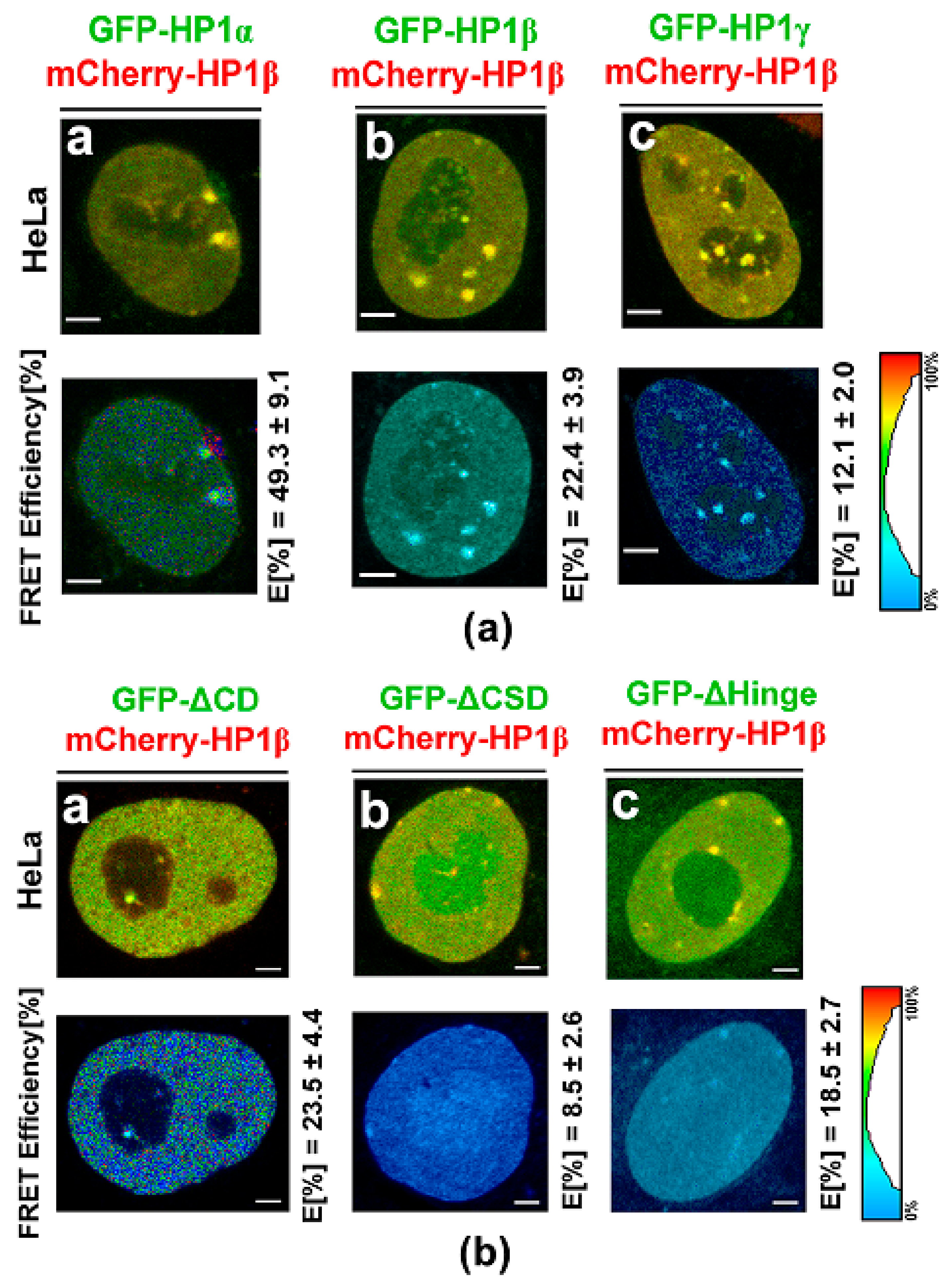
3.3. Protein–protein Interactions: H1β Forms Homodimers and This Interaction is Mediated via CSD Domain. Interaction Between HP1β and KAP1 is Strengthened by HDAC Inhibitor
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Grewal, S.I.; Jia, S. Heterochromatin revisited. Nat. Rev. Genet. 2007, 8, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.H.; Workman, J.L. The changing faces of hp1: From heterochromatin formation and gene silencing to euchromatic gene expression: Hp1 acts as a positive regulator of transcription. Bioessays 2011, 33, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Probst, A.V.; Dunleavy, E.; Almouzni, G. Epigenetic inheritance during the cell cycle. Nat. Rev. Mol. Cell Biol. 2009, 10, 192–206. [Google Scholar] [CrossRef] [PubMed]
- Bannister, A.J.; Zegerman, P.; Partridge, J.F.; Miska, E.A.; Thomas, J.O.; Allshire, R.C.; Kouzarides, T. Selective recognition of methylated lysine 9 on histone h3 by the hp1 chromo domain. Nature 2001, 410, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Martens, J.H.; O’Sullivan, R.J.; Braunschweig, U.; Opravil, S.; Radolf, M.; Steinlein, P.; Jenuwein, T. The profile of repeat-associated histone lysine methylation states in the mouse epigenome. Embo. J. 2005, 24, 800–812. [Google Scholar] [CrossRef] [PubMed]
- Noma, K.; Allis, C.D.; Grewal, S.I. Transitions in distinct histone h3 methylation patterns at the heterochromatin domain boundaries. Science 2001, 293, 1150–1155. [Google Scholar] [CrossRef] [PubMed]
- Suka, N.; Luo, K.; Grunstein, M. Sir2p and sas2p opposingly regulate acetylation of yeast histone h4 lysine16 and spreading of heterochromatin. Nat. Genet. 2002, 32, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Rea, S.; Eisenhaber, F.; O’Carroll, D.; Strahl, B.D.; Sun, Z.W.; Schmid, M.; Opravil, S.; Mechtler, K.; Ponting, C.P.; Allis, C.D.; et al. Regulation of chromatin structure by site-specific histone h3 methyltransferases. Nature 2000, 406, 593–599. [Google Scholar] [CrossRef]
- Schultz, D.C.; Ayyanathan, K.; Negorev, D.; Maul, G.G.; Rauscher, F.J., III. Setdb1: A novel kap-1-associated histone h3, lysine 9-specific methyltransferase that contributes to hp1-mediated silencing of euchromatic genes by krab zinc-finger proteins. Genes Dev. 2002, 16, 919–932. [Google Scholar] [CrossRef]
- Tachibana, M. [mammalian histone methyltransferases]. Seikagaku 2006, 78, 50–53. [Google Scholar]
- Tachibana, M.; Sugimoto, K.; Fukushima, T.; Shinkai, Y. Set domain-containing protein, g9a, is a novel lysine-preferring mammalian histone methyltransferase with hyperactivity and specific selectivity to lysines 9 and 27 of histone h3. J. Biol. Chem. 2001, 276, 25309–25317. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, M.; Sugimoto, K.; Nozaki, M.; Ueda, J.; Ohta, T.; Ohki, M.; Fukuda, M.; Takeda, N.; Niida, H.; Kato, H.; et al. G9a histone methyltransferase plays a dominant role in euchromatic histone h3 lysine 9 methylation and is essential for early embryogenesis. Genes Dev. 2002, 16, 1779–1791. [Google Scholar] [CrossRef] [PubMed]
- Canzio, D.; Chang, E.Y.; Shankar, S.; Kuchenbecker, K.M.; Simon, M.D.; Madhani, H.D.; Narlikar, G.J.; Al-Sady, B. Chromodomain-mediated oligomerization of hp1 suggests a nucleosome-bridging mechanism for heterochromatin assembly. Mol. Cell 2011, 41, 67–81. [Google Scholar] [CrossRef]
- Lachner, M.; O’Carroll, D.; Rea, S.; Mechtler, K.; Jenuwein, T. Methylation of histone h3 lysine 9 creates a binding site for hp1 proteins. Nature 2001, 410, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, A.L.; Oulad-Abdelghani, M.; Ortiz, J.A.; Remboutsika, E.; Chambon, P.; Losson, R. Heterochromatin formation in mammalian cells: Interaction between histones and hp1 proteins. Mol. Cell 2001, 7, 729–739. [Google Scholar] [CrossRef]
- Singh, P.B.; Miller, J.R.; Pearce, J.; Kothary, R.; Burton, R.D.; Paro, R.; James, T.C.; Gaunt, S.J. A sequence motif found in a drosophila heterochromatin protein is conserved in animals and plants. Nucleic Acids Res. 1991, 19, 789–794. [Google Scholar] [CrossRef] [PubMed]
- Fischle, W.; Wang, Y.; Jacobs, S.A.; Kim, Y.; Allis, C.D.; Khorasanizadeh, S. Molecular basis for the discrimination of repressive methyl-lysine marks in histone h3 by polycomb and hp1 chromodomains. Genes Dev. 2003, 17, 1870–1881. [Google Scholar] [CrossRef]
- Stewart, M.D.; Li, J.; Wong, J. Relationship between histone h3 lysine 9 methylation, transcription repression, and heterochromatin protein 1 recruitment. Mol. Cell Biol. 2005, 25, 2525–2538. [Google Scholar] [CrossRef]
- Aasland, R.; Stewart, A.F. The chromo shadow domain, a second chromo domain in heterochromatin-binding protein 1, hp1. Nucleic Acids Res. 1995, 23, 3168–3173. [Google Scholar] [CrossRef]
- Li, Y.; Kirschmann, D.A.; Wallrath, L.L. Does heterochromatin protein 1 always follow code? Proc. Natl. Acad. Sci. USA 2002, 99, 16462–16469. [Google Scholar] [CrossRef]
- Meehan, R.R.; Kao, C.F.; Pennings, S. Hp1 binding to native chromatin in vitro is determined by the hinge region and not by the chromodomain. Embo J. 2003, 22, 3164–3174. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Heyduk, T.; Allis, C.D.; Eissenberg, J.C. Heterochromatin protein 1 binds to nucleosomes and DNA in vitro. J. Biol. Chem. 2000, 275, 28332–28338. [Google Scholar]
- Badugu, R.; Yoo, Y.; Singh, P.B.; Kellum, R. Mutations in the heterochromatin protein 1 (hp1) hinge domain affect hp1 protein interactions and chromosomal distribution. Chromosoma 2005, 113, 370–384. [Google Scholar] [CrossRef] [PubMed]
- Koike, N.; Maita, H.; Taira, T.; Ariga, H.; Iguchi-Ariga, S.M. Identification of heterochromatin protein 1 (hp1) as a phosphorylation target by pim-1 kinase and the effect of phosphorylation on the transcriptional repression function of hp1(1). Febs. Lett. 2000, 467, 17–21. [Google Scholar] [CrossRef]
- Zhao, T.; Heyduk, T.; Eissenberg, J.C. Phosphorylation site mutations in heterochromatin protein 1 (hp1) reduce or eliminate silencing activity. J. Biol. Chem. 2001, 276, 9512–9518. [Google Scholar] [CrossRef]
- Lomberk, G.; Wallrath, L.; Urrutia, R. The heterochromatin protein 1 family. Genome Biol. 2006, 7, 228. [Google Scholar] [CrossRef] [PubMed]
- Dialynas, G.K.; Terjung, S.; Brown, J.P.; Aucott, R.L.; Baron-Luhr, B.; Singh, P.B.; Georgatos, S.D. Plasticity of hp1 proteins in mammalian cells. J. Cell Sci. 2007, 120, 3415–3424. [Google Scholar] [CrossRef][Green Version]
- Kwon, S.H.; Florens, L.; Swanson, S.K.; Washburn, M.P.; Abmayr, S.M.; Workman, J.L. Heterochromatin protein 1 (hp1) connects the fact histone chaperone complex to the phosphorylated ctd of rna polymerase ii. Genes Dev. 2010, 24, 2133–2145. [Google Scholar] [CrossRef]
- Lin, C.H.; Li, B.; Swanson, S.; Zhang, Y.; Florens, L.; Washburn, M.P.; Abmayr, S.M.; Workman, J.L. Heterochromatin protein 1a stimulates histone h3 lysine 36 demethylation by the drosophila kdm4a demethylase. Mol. Cell 2008, 32, 696–706. [Google Scholar] [CrossRef]
- Smothers, J.F.; Henikoff, S. The hinge and chromo shadow domain impart distinct targeting of hp1-like proteins. Mol. Cell Biol. 2001, 21, 2555–2569. [Google Scholar] [CrossRef]
- Hayakawa, T.; Haraguchi, T.; Masumoto, H.; Hiraoka, Y. Cell cycle behavior of human hp1 subtypes: Distinct molecular domains of hp1 are required for their centromeric localization during interphase and metaphase. J. Cell Sci. 2003, 116, 3327–3338. [Google Scholar] [CrossRef]
- Minc, E.; Allory, Y.; Worman, H.J.; Courvalin, J.C.; Buendia, B. Localization and phosphorylation of hp1 proteins during the cell cycle in mammalian cells. Chromosoma 1999, 108, 220–234. [Google Scholar] [CrossRef]
- Ryu, H.W.; Lee, D.H.; Florens, L.; Swanson, S.K.; Washburn, M.P.; Kwon, S.H. Analysis of the heterochromatin protein 1 (hp1) interactome in drosophila. J. Proteom. 2014, 102, 137–147. [Google Scholar] [CrossRef]
- Cheutin, T.; McNairn, A.J.; Jenuwein, T.; Gilbert, D.M.; Singh, P.B.; Misteli, T. Maintenance of stable heterochromatin domains by dynamic hp1 binding. Science 2003, 299, 721–725. [Google Scholar] [CrossRef]
- Legartova, S.; Jugova, A.; Stixova, L.; Kozubek, S.; Fojtova, M.; Zdrahal, Z.; Lochmanova, G.; Bartova, E. Epigenetic aspects of hp1 exchange kinetics in apoptotic chromatin. Biochimie 2013, 95, 167–179. [Google Scholar] [CrossRef]
- Yearim, A.; Gelfman, S.; Shayevitch, R.; Melcer, S.; Glaich, O.; Mallm, J.P.; Nissim-Rafinia, M.; Cohen, A.H.; Rippe, K.; Meshorer, E.; et al. Hp1 is involved in regulating the global impact of DNA methylation on alternative splicing. Cell Rep. 2015, 10, 1122–1134. [Google Scholar] [CrossRef]
- Luco, R.F.; Pan, Q.; Tominaga, K.; Blencowe, B.J.; Pereira-Smith, O.M.; Misteli, T. Regulation of alternative splicing by histone modifications. Science 2010, 327, 996–1000. [Google Scholar] [CrossRef]
- Sawyer, I.A.; Sturgill, D.; Dundr, M. Membraneless nuclear organelles and the search for phases within phases. Wiley Interdiscip Rev. RNA 2019, 10, e1514. [Google Scholar] [CrossRef]
- Yuan, X.; Feng, W.; Imhof, A.; Grummt, I.; Zhou, Y. Activation of rna polymerase i transcription by cockayne syndrome group b protein and histone methyltransferase g9a. Mol. Cell 2007, 27, 585–595. [Google Scholar] [CrossRef]
- Horakova, A.H.; Bartova, E.; Galiova, G.; Uhlirova, R.; Matula, P.; Kozubek, S. Suv39h-independent association of hp1 beta with fibrillarin-positive nucleolar regions. Chromosoma 2010, 119, 227–241. [Google Scholar] [CrossRef]
- Friedman, J.R.; Fredericks, W.J.; Jensen, D.E.; Speicher, D.W.; Huang, X.P.; Neilson, E.G.; Rauscher, F.J., III. Kap-1, a novel corepressor for the highly conserved krab repression domain. Genes Dev. 1996, 10, 2067–2078. [Google Scholar] [CrossRef]
- Sripathy, S.P.; Stevens, J.; Schultz, D.C. The kap1 corepressor functions to coordinate the assembly of de novo hp1-demarcated microenvironments of heterochromatin required for krab zinc finger protein-mediated transcriptional repression. Mol. Cell Biol. 2006, 26, 8623–8638. [Google Scholar] [CrossRef]
- Cammas, F.; Oulad-Abdelghani, M.; Vonesch, J.L.; Huss-Garcia, Y.; Chambon, P.; Losson, R. Cell differentiation induces TIF1-beta association with centromeric heterochromatin via an HP1 interaction. J. Cell Sci. 2002, 115, 3439–3448. [Google Scholar]
- Cammas, F.; Herzog, M.; Lerouge, T.; Chambon, P.; Losson, R. Association of the transcriptional corepressor TIF1beta with heterochromatin protein 1 (HP1): An essential role for progression through differentiation. Genes Dev. 2004, 18, 2147–2160. [Google Scholar] [CrossRef]
- Bartova, E.; Pachernik, J.; Kozubik, A.; Kozubek, S. Differentiation-specific association of hp1alpha and hp1beta with chromocentres is correlated with clustering of tif1beta at these sites. Histochem. Cell Biol. 2007, 127, 375–388. [Google Scholar] [CrossRef]
- White, D.; Rafalska-Metcalf, I.U.; Ivanov, A.V.; Corsinotti, A.; Peng, H.; Lee, S.C.; Trono, D.; Janicki, S.M.; Rauscher, F.J., III. The atm substrate kap1 controls DNA repair in heterochromatin: Regulation by hp1 proteins and serine 473/824 phosphorylation. Mol. Cancer Res. 2012, 10, 401–414. [Google Scholar] [CrossRef]
- Chang, C.W.; Chou, H.Y.; Lin, Y.S.; Huang, K.H.; Chang, C.J.; Hsu, T.C.; Lee, S.C. Phosphorylation at ser473 regulates heterochromatin protein 1 binding and corepressor function of tif1beta/kap1. Bmc Mol. Biol. 2008, 9, 61. [Google Scholar] [CrossRef]
- Hiragami-Hamada, K.; Shinmyozu, K.; Hamada, D.; Tatsu, Y.; Uegaki, K.; Fujiwara, S.; Nakayama, J. N-terminal phosphorylation of hp1{alpha} promotes its chromatin binding. Mol. Cell Biol. 2011, 31, 1186–1200. [Google Scholar] [CrossRef]
- Ayoub, N.; Jeyasekharan, A.D.; Bernal, J.A.; Venkitaraman, A.R. Hp1-beta mobilization promotes chromatin changes that initiate the DNA damage response. Nature 2008, 453, 682–686. [Google Scholar] [CrossRef]
- Luijsterburg, M.S.; Dinant, C.; Lans, H.; Stap, J.; Wiernasz, E.; Lagerwerf, S.; Warmerdam, D.O.; Lindh, M.; Brink, M.C.; Dobrucki, J.W.; et al. Heterochromatin protein 1 is recruited to various types of DNA damage. J. Cell Biol. 2009, 185, 577–586. [Google Scholar] [CrossRef]
- Sustackova, G.; Kozubek, S.; Stixova, L.; Legartova, S.; Matula, P.; Orlova, D.; Bartova, E. Acetylation-dependent nuclear arrangement and recruitment of bmi1 protein to uv-damaged chromatin. J. Cell Physiol. 2012, 227, 1838–1850. [Google Scholar] [CrossRef]
- Stixova, L.; Sehnalova, P.; Legartova, S.; Suchankova, J.; Hruskova, T.; Kozubek, S.; Sorokin, D.V.; Matula, P.; Raska, I.; Kovarik, A.; et al. Hp1beta-dependent recruitment of ubf1 to irradiated chromatin occurs simultaneously with cpds. Epigenetics Chromatin 2014, 7, 39. [Google Scholar] [CrossRef]
- Morgenstern, J.P.; Land, H. A series of mammalian expression vectors and characterisation of their expression of a reporter gene in stably and transiently transfected cells. Nucleic Acids Res. 1990, 18, 1068. [Google Scholar] [CrossRef][Green Version]
- Legartova, S.; Suchankova, J.; Krejci, J.; Kovarikova, A.; Bartova, E. Advanced confocal microscopy techniques to study protein-protein interactions and kinetics at DNA lesions. J. Vis. Exp. 2017. [Google Scholar] [CrossRef]
- LeRoy, G.; Weston, J.T.; Zee, B.M.; Young, N.L.; Plazas-Mayorca, M.D.; Garcia, B.A. Heterochromatin protein 1 is extensively decorated with histone code-like post-translational modifications. Mol. Cell Proteom. 2009, 8, 2432–2442. [Google Scholar] [CrossRef]
- Lakowicz, J.R.; Gryczynski, I.I.; Gryczynski, Z. High throughput screening with multiphoton excitation. J. Biomol. Screen 1999, 4, 355–362. [Google Scholar] [CrossRef][Green Version]
- Sillen, A.; Engelborghs, Y. The correct use of “average” fluorescence parameters. Photochem. Photobiol. 1998, 67, 475–486. [Google Scholar]
- Vizcaino, J.A.; Csordas, A.; Del-Toro, N.; Dianes, J.A.; Griss, J.; Lavidas, I.; Mayer, G.; Perez-Riverol, Y.; Reisinger, F.; Ternent, T.; et al. 2016 update of the pride database and its related tools. Nucleic Acids Res. 2016, 44, 11033. [Google Scholar] [CrossRef]
- Strasak, L.; Bartova, E.; Harnicarova, A.; Galiova, G.; Krejci, J.; Kozubek, S. H3k9 acetylation and radial chromatin positioning. J. Cell Physiol. 2009, 220, 91–101. [Google Scholar] [CrossRef]
- Santoro, R.; Grummt, I. Epigenetic mechanism of rrna gene silencing: Temporal order of norc-mediated histone modification, chromatin remodeling, and DNA methylation. Mol. Cell Biol. 2005, 25, 2539–2546. [Google Scholar] [CrossRef]
- Jugova, A.; Sustackova, G.; Legartova, S.; Stixova, L.; Kozubek, S.; Bartova, E. Effects of epigenetic-based anti-cancer drugs in leukaemia and multiple myeloma cells. Cell Biol. Int. 2011, 35, 1195–1203. [Google Scholar] [CrossRef]
- Morgenstern, H.; Greenland, S. Graphing ratio measures of effect. J. Clin. Epidemiol. 1990, 43, 539–542. [Google Scholar] [CrossRef]
- Lomberk, G.; Bensi, D.; Fernandez-Zapico, M.E.; Urrutia, R. Evidence for the existence of an hp1-mediated subcode within the histone code. Nat. Cell Biol. 2006, 8, 407–415. [Google Scholar] [CrossRef]
- Lechner, M.S.; Begg, G.E.; Speicher, D.W.; Rauscher, F.J., III. Molecular determinants for targeting heterochromatin protein 1-mediated gene silencing: Direct chromoshadow domain-kap-1 corepressor interaction is essential. Mol. Cell Biol. 2000, 20, 6449–6465. [Google Scholar] [CrossRef]
- Bartova, E.; Pachernik, J.; Harnicarova, A.; Kovarik, A.; Kovarikova, M.; Hofmanova, J.; Skalnikova, M.; Kozubek, M.; Kozubek, S. Nuclear levels and patterns of histone h3 modification and hp1 proteins after inhibition of histone deacetylases. J. Cell Sci. 2005, 118, 5035–5046. [Google Scholar] [CrossRef]
- Minc, E.; Allory, Y.; Courvalin, J.C.; Buendia, B. Immunolocalization of hp1 proteins in metaphasic mammalian chromosomes. Methods Cell Sci. 2001, 23, 171–174. [Google Scholar] [CrossRef]
- Minc, E.; Courvalin, J.C.; Buendia, B. Hp1gamma associates with euchromatin and heterochromatin in mammalian nuclei and chromosomes. Cytogenet Cell Genet. 2000, 90, 279–284. [Google Scholar] [CrossRef]
- Foltankova, V.; Legartova, S.; Kozubek, S.; Hofer, M.; Bartova, E. DNA-damage response in chromatin of ribosomal genes and the surrounding genome. Gene 2013, 522, 156–167. [Google Scholar] [CrossRef]
- Zarebski, M.; Wiernasz, E.; Dobrucki, J.W. Recruitment of heterochromatin protein 1 to DNA repair sites. Cytom. A 2009, 75, 619–625. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Legartová, S.; Lochmanová, G.; Zdráhal, Z.; Kozubek, S.; Šponer, J.; Krepl, M.; Pokorná, P.; Bártová, E. DNA Damage Changes Distribution Pattern and Levels of HP1 Protein Isoforms in the Nucleolus and Increases Phosphorylation of HP1β-Ser88. Cells 2019, 8, 1097. https://doi.org/10.3390/cells8091097
Legartová S, Lochmanová G, Zdráhal Z, Kozubek S, Šponer J, Krepl M, Pokorná P, Bártová E. DNA Damage Changes Distribution Pattern and Levels of HP1 Protein Isoforms in the Nucleolus and Increases Phosphorylation of HP1β-Ser88. Cells. 2019; 8(9):1097. https://doi.org/10.3390/cells8091097
Chicago/Turabian StyleLegartová, Soňa, Gabriela Lochmanová, Zbyněk Zdráhal, Stanislav Kozubek, Jiří Šponer, Miroslav Krepl, Pavlína Pokorná, and Eva Bártová. 2019. "DNA Damage Changes Distribution Pattern and Levels of HP1 Protein Isoforms in the Nucleolus and Increases Phosphorylation of HP1β-Ser88" Cells 8, no. 9: 1097. https://doi.org/10.3390/cells8091097
APA StyleLegartová, S., Lochmanová, G., Zdráhal, Z., Kozubek, S., Šponer, J., Krepl, M., Pokorná, P., & Bártová, E. (2019). DNA Damage Changes Distribution Pattern and Levels of HP1 Protein Isoforms in the Nucleolus and Increases Phosphorylation of HP1β-Ser88. Cells, 8(9), 1097. https://doi.org/10.3390/cells8091097





