Zebrafish in Inflammasome Research
Abstract
:1. Introduction
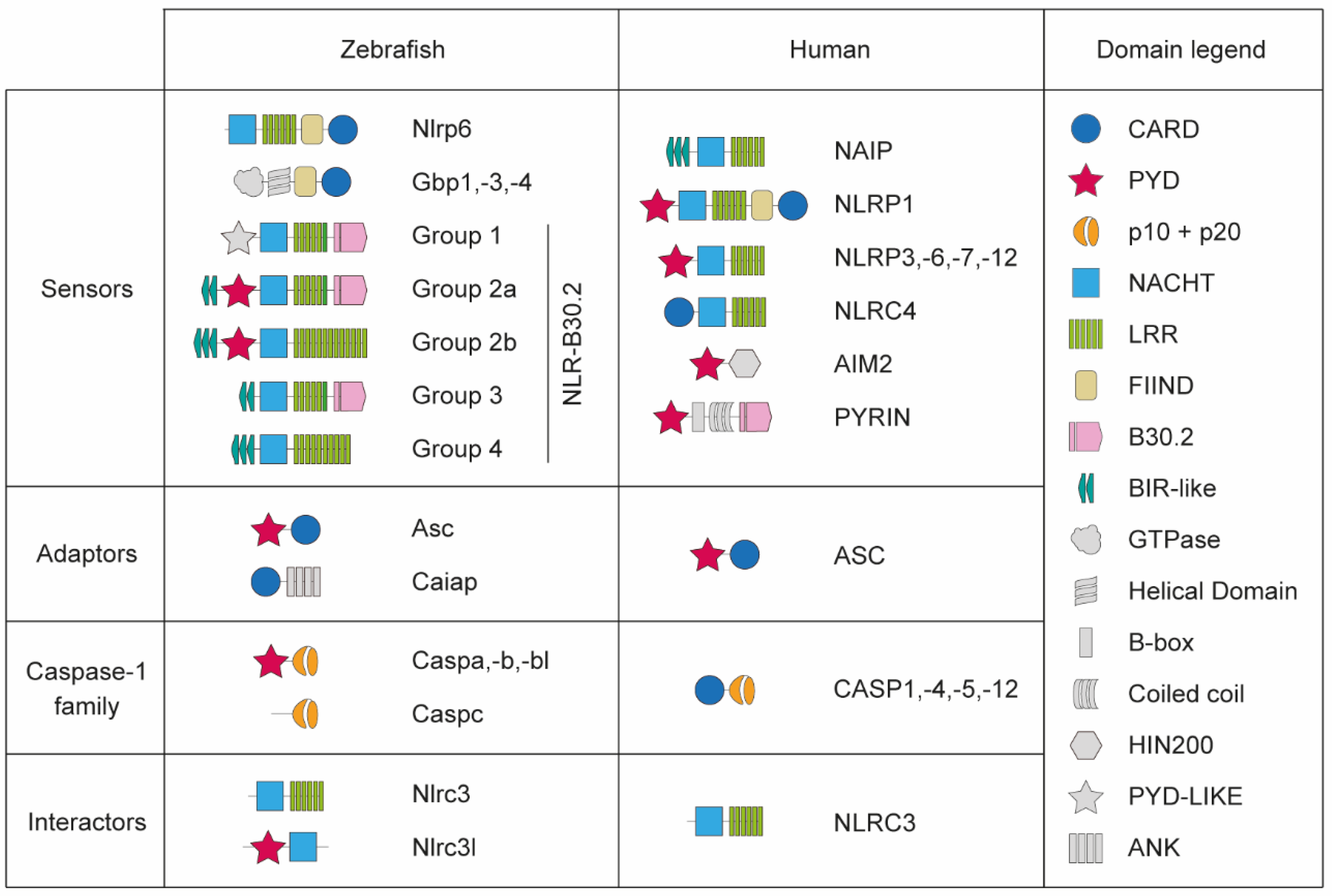
2. Evolutionary Conservation of Inflammasome Components in Zebrafish
2.1. Inflammasome Sensors
2.2. Inflammasome Adaptors
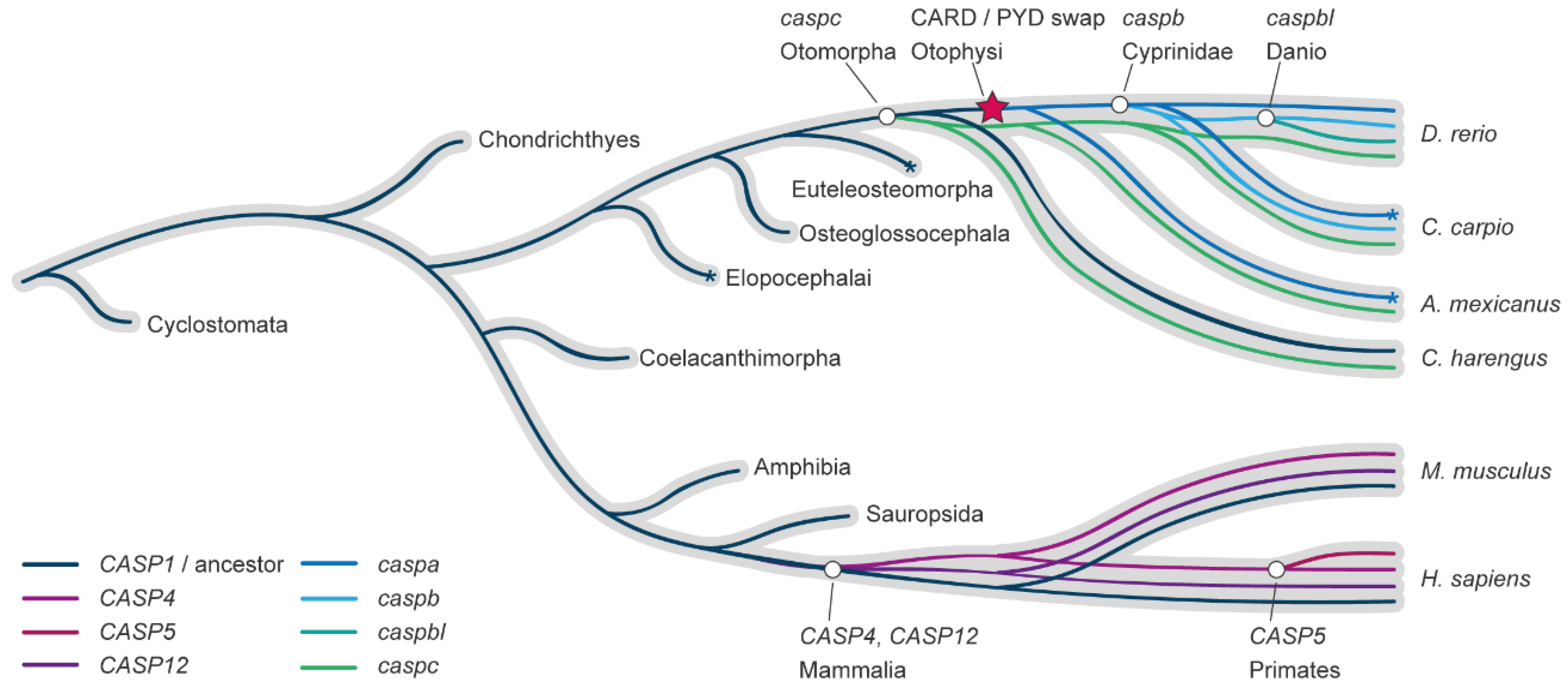
2.3. Zebrafish Pro-Inflammatory Caspases
2.4. Inflammasome Effectors
3. Zebrafish Models of Inflammasome Regulation in Health and Disease
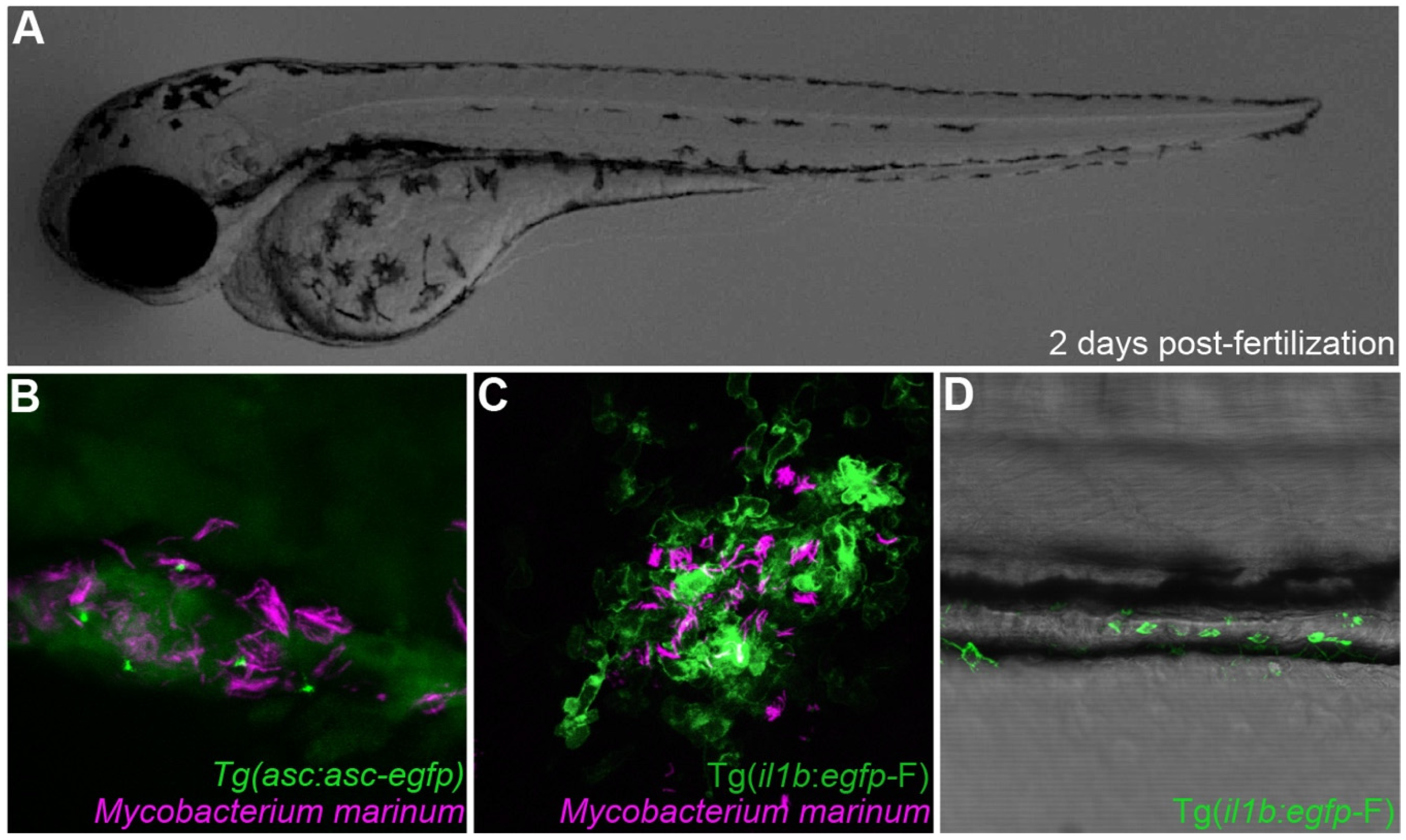
3.1. Inflammasome Function in Zebrafish Infectious Models
3.2. Inflammasome Function in Zebrafish Non-infectious Models
4. Concluding Remarks and Future Considerations
Supplementary Materials
Funding
Acknowledgments
Conflicts of Interest
References
- He, Y.; Hara, H.; Núñez, G. Mechanism and Regulation of NLRP3 Inflammasome Activation. Trends Biochem. Sci. 2016, 41, 1012–1021. [Google Scholar] [CrossRef] [Green Version]
- Lamkanfi, M.; Dixit, V.M. Mechanisms and Functions of Inflammasomes. Cell 2014, 157, 1013–1022. [Google Scholar] [CrossRef]
- Monteleone, M.; Stanley, A.C.; Chen, K.W.; Brown, D.L.; Bezbradica, J.S.; von Pein, J.B.; Holley, C.L.; Boucher, D.; Shakespear, M.R.; Kapetanovic, R.; et al. Interleukin-1β Maturation Triggers Its Relocation to the Plasma Membrane for Gasdermin-D-Dependent and -Independent Secretion. Cell Rep. 2018, 24, 1425–1433. [Google Scholar] [CrossRef]
- Heilig, R.; Dick, M.S.; Sborgi, L.; Meunier, E.; Hiller, S.; Broz, P. The Gasdermin-D pore acts as a conduit for IL-1β secretion in mice. Eur. J. Immunol. 2018, 48, 584–592. [Google Scholar] [CrossRef]
- Carty, M.; Kearney, J.; Shanahan, K.A.; Hams, E.; Sugisawa, R.; Connolly, D.; Doran, C.G.; Muñoz-Wolf, N.; Gürtler, C.; Fitzgerald, K.A.; et al. Cell Survival and Cytokine Release after Inflammasome Activation Is Regulated by the Toll-IL-1R Protein SARM. Immunity 2019, 50, 1412–1424.e6. [Google Scholar] [CrossRef]
- Mao, L.; Kitani, A.; Strober, W.; Fuss, I.J. The Role of NLRP3 and IL-1β in the Pathogenesis of Inflammatory Bowel Disease. Front. Immunol. 2018, 9, 2566. [Google Scholar] [CrossRef]
- Sharma, D.; Kanneganti, T.-D. The cell biology of inflammasomes: Mechanisms of inflammasome activation and regulation. J. Cell Biol. 2016, 213, 617–629. [Google Scholar] [CrossRef] [Green Version]
- Freeman, L.C.; Ting, J. The pathogenic role of the inflammasome in neurodegenerative diseases. J. Neurochem. 2016, 136, 29–38. [Google Scholar] [CrossRef]
- Rathinam, V.; Fitzgerald, K.A. Inflammasome Complexes: Emerging Mechanisms and Effector Functions. Cell 2016, 165, 792–800. [Google Scholar] [CrossRef] [Green Version]
- Malik, A.; Kanneganti, T.-D. Inflammasome activation and assembly at a glance. J. Cell Sci. 2017, 130, 3955–3963. [Google Scholar] [CrossRef] [Green Version]
- Monie, T.P. The Canonical Inflammasome: A Macromolecular Complex Driving Inflammation. Subcell Biochem. 2017, 83, 43–73. [Google Scholar]
- Ding, J.; Shao, F. SnapShot: The Noncanonical Inflammasome. Cell 2017, 168. [Google Scholar] [CrossRef]
- Zanoni, I.; Tan, Y.; Gioia, M.; Broggi, A.; Ruan, J.; Shi, J.; Donado, C.A.; Shao, F.; Wu, H.; Springstead, J.R.; et al. An endogenous caspase-11 ligand elicits interleukin-1 release from living dendritic cells. Science 2016, 352, 1232–1236. [Google Scholar] [CrossRef] [Green Version]
- Hara, H.; Seregin, S.S.; Yang, D.; Fukase, K.; Chamaillard, M.; Alnemri, E.S.; Inohara, N.; Chen, G.Y.; Núñez, G. The NLRP6 Inflammasome Recognizes Lipoteichoic Acid and Regulates Gram-Positive Pathogen Infection. Cell 2018, 175, 1654–1664.e14. [Google Scholar] [CrossRef]
- Shi, J.; Zhao, Y.; Wang, K.; Shi, X.; Wang, Y.; Huang, H.; Zhuang, Y.; Cai, T.; Wang, F.; Shao, F. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 2015, 526, 660–665. [Google Scholar] [CrossRef]
- Kayagaki, N.; Stowe, I.B.; Lee, B.L.; O’Rourke, K.; Anderson, K.; Warming, S.; Cuellar, T.; Haley, B.; Roose-Girma, M.; Phung, Q.T.; et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature 2015, 526, 666–671. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Z.; Ruan, J.; Pan, Y.; Magupalli, V.; Wu, H.; Lieberman, J. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature 2016, 535, 153–158. [Google Scholar] [CrossRef] [Green Version]
- Renshaw, S.A.; Trede, N.S. A model 450 million years in the making: Zebrafish and vertebrate immunity. Dis. Model Mech. 2012, 5, 38–47. [Google Scholar] [CrossRef]
- Torraca, V.; Masud, S.; Spaink, H.P.; Meijer, A.H. Macrophage-pathogen interactions in infectious diseases: New therapeutic insights from the zebrafish host model. Dis. Model Mech. 2014, 7, 785–797. [Google Scholar] [CrossRef]
- Yoshida, N.; Frickel, E.-M.; Mostowy, S. Macrophage–Microbe Interactions: Lessons from the Zebrafish Model. Front. Immunol. 2017, 8, 1703. [Google Scholar] [CrossRef]
- Henry, K.M.; Loynes, C.A.; Whyte, M.K.; Renshaw, S.A. Zebrafish as a model for the study of neutrophil biology. J. Leukocyte Biol. 2013, 94, 633–642. [Google Scholar] [CrossRef]
- Santoriello, C.; Zon, L.I. Hooked! Modeling human disease in zebrafish. J Clin Invest 2012, 122, 2337–2343. [Google Scholar] [CrossRef] [Green Version]
- Meijer, A.H. Protection and pathology in TB: Learning from the zebrafish model. Semin. Immunopathol. 2016, 38, 261–273. [Google Scholar] [CrossRef]
- Angosto, D.; Mulero, V. The zebrafish as a model to study the inflammasome. Inflammasome 2014, 1. [Google Scholar] [CrossRef]
- García-Moreno, D.; Tyrkalska, S.D.; Valera-Pérez, A.; Gómez-Abenza, E.; Oliva, A.B.; Mulero, V. The Zebrafish: A Research Model To Understand The Evolution Of Vertebrate Immunity. Fish Shellfish Immunol. 2019, 90, 215–222. [Google Scholar] [CrossRef]
- Ogryzko, N.V.; Renshaw, S.A.; Wilson, H.L. The IL-1 family in fish: Swimming through the muddy waters of inflammasome evolution. Dev. Comp. Immunol. 2014, 46, 53–62. [Google Scholar] [CrossRef]
- Kuri, P.; Schieber, N.L.; Thumberger, T.; Wittbrodt, J.; Schwab, Y.; Leptin, M. Dynamics of in vivo ASC speck formation. J. Cell Biol. 2017, 216, 2891–2909. [Google Scholar] [CrossRef]
- Vincent, W.; Freisinger, C.M.; Lam, P.; Huttenlocher, A.; Sauer, J. Macrophages mediate flagellin induced inflammasome activation and host defense in zebrafish. Cell Microbiol. 2016, 18, 591–604. [Google Scholar] [CrossRef]
- Varela, M.; van der Vaart, M.; Groenewoud, A.; Meijer, A.H. Extracellular mycobacterial DNA drives disease progression by triggering Caspase-11-dependent pyroptosis of infected macrophages. bioRxiv 2019, 514125. [Google Scholar] [CrossRef]
- Wang, T.; Yan, B.; Lou, L.; Lin, X.; Yu, T.; Wu, S.; Lu, Q.; Liu, W.; Huang, Z.; Zhang, M.; et al. Nlrc3-like is required for microglia maintenance in zebrafish. J. Genet. Genom. 2019, 46, 291–299. [Google Scholar] [CrossRef]
- Tyrkalska, S.D.; Pérez-Oliva, A.B.; Rodríguez-Ruiz, L.; Martínez-Morcillo, F.J.; Alcaraz-Pérez, F.; Martínez-Navarro, F.J.; Lachaud, C.; Ahmed, N.; Schroeder, T.; Pardo-Sánchez, I.; et al. Inflammasome Regulates Hematopoiesis through Cleavage of the Master Erythroid Transcription Factor GATA1. Immunity 2019, 51, 50–63. [Google Scholar] [CrossRef]
- Forn-Cuní, G.; Varela, M.; Pereiro, P.; Novoa, B.; Figueras, A. Conserved gene regulation during acute inflammation between zebrafish and mammals. Sci. Rep. 2017, 7, 41905. [Google Scholar] [CrossRef]
- Carradice, D.; Lieschke, G.J. Zebrafish in hematology: Sushi or science? Blood 2008, 111, 3331–3342. [Google Scholar] [CrossRef]
- Varela, M.; Romero, A.; Dios, S.; van der Vaart, M.; Figueras, A.; Meijer, A.H.; Novoa, B. Cellular Visualization of Macrophage Pyroptosis and I nterleukin-1β Release in a Viral Hemorrhagic Infection in Zebrafish Larvae. J. Virol. 2014, 88, 12026–12040. [Google Scholar] [CrossRef]
- Mostowy, S.; Boucontet, L.; Moya, M.J.; Sirianni, A.; Boudinot, P.; Hollinshead, M.; Cossart, P.; Herbomel, P.; Levraud, J.-P.; Colucci-Guyon, E. The Zebrafish as a New Model for the In Vivo Study of Shigella flexneri Interaction with Phagocytes and Bacterial Autophagy. PLoS Pathog. 2013, 9, e1003588. [Google Scholar] [CrossRef]
- Kenyon, A.; Gavriouchkina, D.; Zorman, J.; Napolitani, G.; Cerundolo, V.; Sauka-Spengler, T. Active nuclear transcriptome analysis reveals inflammasome-dependent mechanism for early neutrophil response to Mycobacterium marinum. Sci. Rep. 2017, 7, 6505. [Google Scholar] [CrossRef] [Green Version]
- Matty, M.A.; Knudsen, D.R.; Walton, E.M.; Beerman, R.W.; Cronan, M.R.; Pyle, C.J.; Hernandez, R.E.; Tobin, D.M. Potentiation of P2RX7 as a host-directed strategy for control of mycobacterial infection. Elife 2019, 8, e39123. [Google Scholar] [CrossRef]
- Mazon-Moya, M.J.; Willis, A.R.; Torraca, V.; Boucontet, L.; Shenoy, A.R.; Colucci-Guyon, E.; Mostowy, S. Septins restrict inflammation and protect zebrafish larvae from Shigella infection. PloS Pathog. 2017, 13, e1006467. [Google Scholar] [CrossRef]
- Tyrkalska, S.D.; Candel, S.; Angosto, D.; Gómez-Abellán, V.; Martín-Sánchez, F.; García-Moreno, D.; Zapata-Pérez, R.; Sánchez-Ferrer, Á.; Sepulcre, M.P.; Pelegrín, P.; et al. Neutrophils mediate Salmonella Typhimurium clearance through the GBP4 inflammasome-dependent production of prostaglandins. Nat. Commun. 2016, 7, 12077. [Google Scholar] [CrossRef] [Green Version]
- Tyrkalska, S.D.; Candel, S.; Pérez-Oliva, A.B.; Valera, A.; Alcaraz-Pérez, F.; García-Moreno, D.; Cayuela, M.L.; Mulero, V. Identification of an Evolutionarily Conserved Ankyrin Domain-Containing Protein, Caiap, Which Regulates Inflammasome-Dependent Resistance to Bacterial Infection. Front. Immunol. 2017, 8, 1375. [Google Scholar] [CrossRef] [Green Version]
- Masumoto, J.; Zhou, W.; Chen, F.F.; Su, F.; Kuwada, J.Y.; Hidaka, E.; Katsuyama, T.; Sagara, J.; Taniguchi, S.; Ngo-Hazelett, P.; et al. Caspy, a Zebrafish Caspase, Activated by ASC Oligomerization Is Required for Pharyngeal Arch Development. J. Biol. Chem. 2003, 278, 4268–4276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, D.; Zheng, X.; Chen, S.; Wang, Z.; Xu, W.; Tan, J.; Hu, T.; Hou, M.; Wang, W.; Gu, Z.; et al. Sensing of cytosolic LPS through caspy2 pyrin domain mediates noncanonical inflammasome activation in zebrafish. Nat. Commun. 2018, 9, 3052. [Google Scholar] [CrossRef] [PubMed]
- Varela, M.; Forn-Cuní, G.; Dios, S.; Figueras, A.; Novoa, B. Proinflammatory Caspase A Activation and an Antiviral State Are Induced by a Zebrafish Perforin after Possible Cellular and Functional Diversification from a Myeloid Ancestor. J. Innate Immun. 2016, 8, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Valera-Pérez, A.; Tyrkalska, S.D.; Viana, C.; Rojas-Fernández, A.; Pelegrín, P.; García-Moreno, D.; Pérez-Oliva, A.B.; Mulero, V. WDR90 is a new component of the NLRC4 inflammasome involved in Salmonella Typhimurium resistance. Dev. Comp. Immunol. 2019, 100, 103428. [Google Scholar] [CrossRef] [PubMed]
- Progatzky, F.; Sangha, N.J.; Yoshida, N.; McBrien, M.; Cheung, J.; Shia, A.; Scott, J.; Marchesi, J.R.; Lamb, J.R.; Bugeon, L.; et al. Dietary cholesterol directly induces acute inflammasome-dependent intestinal inflammation. Nat. Commun. 2014, 5, 5864. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Niethammer, P. Tissue Damage Signaling Is a Prerequisite for Protective Neutrophil Recruitment to Microbial Infection in Zebrafish. Immunity 2018, 48, 1006–1013. [Google Scholar] [CrossRef] [PubMed]
- Ogryzko, N.V.; Lewis, A.; Wilson, H.L.; Meijer, A.H.; Renshaw, S.A.; Elks, P.M. Hif-1α–Induced Expression of Il-1β Protects against Mycobacterial Infection in Zebrafish. J. Immunol. 2018, 202, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Nguyen-Chi, M.; Phan, Q.; Gonzalez, C.; Dubremetz, J.-F.; Levraud, J.-P.; Lutfalla, G. Transient infection of the zebrafish notochord with E. coli induces chronic inflammation. Dis. Model Mech. 2014, 7, 871–882. [Google Scholar] [CrossRef] [PubMed]
- Saraceni, P.R.; Romero, A.; Figueras, A.; Novoa, B. Establishment of Infection Models in Zebrafish Larvae (Danio rerio) to Study the Pathogenesis of Aeromonas hydrophila. Front. Microbiol. 2016, 7, 1219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mesureur, J.; Feliciano, J.R.; Wagner, N.; Gomes, M.C.; Zhang, L.; Blanco-Gonzalez, M.; van der Vaart, M.; O’Callaghan, D.; Meijer, A.H.; Vergunst, A.C. Macrophages, but not neutrophils, are critical for proliferation of Burkholderia cenocepacia and ensuing host-damaging inflammation. PLoS Pathog. 2017, 13, e1006437. [Google Scholar] [CrossRef] [PubMed]
- Ogryzko, N.V.; Hoggett, E.E.; Solaymani-Kohal, S.; Tazzyman, S.; Chico, T.J.; Renshaw, S.A.; Wilson, H.L. Zebrafish tissue injury causes upregulation of interleukin-1 and caspase-dependent amplification of the inflammatory response. Dis. Model Mech. 2014, 7, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Vojtech, L.N.; Scharping, N.; Woodson, J.C.; Hansen, J.D. Roles of Inflammatory Caspases during Processing of Zebrafish Interleukin-1β in Francisella noatunensis Infection. Infect. Immun. 2012, 80, 2878–2885. [Google Scholar] [CrossRef] [PubMed]
- Galindo-Villegas, J.; Montalban-Arques, A.; Liarte, S.; de Oliveira, S.; Pardo-Pastor, C.; Rubio-Moscardo, F.; Meseguer, J.; Valverde, M.A.; Mulero, V. TRPV4-Mediated Detection of Hyposmotic Stress by Skin Keratinocytes Activates Developmental Immunity. J. Immunol. 2016, 196, 738–749. [Google Scholar] [CrossRef] [PubMed]
- Meunier, E.; Broz, P. Evolutionary Convergence and Divergence in NLR Function and Structure. Trends Immunol. 2017, 38, 744–757. [Google Scholar] [CrossRef] [PubMed]
- Howe, K.; Schiffer, P.H.; Zielinski, J.; Wiehe, T.; Laird, G.K.; Marioni, J.C.; Soylemez, O.; Kondrashov, F.; Leptin, M. Structure and evolutionary history of a large family of NLR proteins in the zebrafish. Open Biol. 2016, 6, 160009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Gao, K.; Shao, T.; Fan, D.; Hu, C.; Sun, C.; Dong, W.; Lin, A.; Xiang, L.; Shao, J. Characterization of an NLRP1 Inflammasome from Zebrafish Reveals a Unique Sequential Activation Mechanism Underlying Inflammatory Caspases in Ancient Vertebrates. J. Immunol. 2018, 201, 1946–1966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Huang, Y.; Cao, X.; Yin, X.; Jin, X.; Liu, S.; Jiang, J.; Jiang, W.; Xiao, T.; Zhou, R.; et al. Functional and structural characterization of zebrafish ASC. FEBS J. 2018, 285, 2691–2707. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.-H.; Bradfield, C.J.; Park, E.-S.; MacMicking, J.D.; Chee, J.D.; Kumar, P. Interferon-induced guanylate-binding proteins in inflammasome activation and host defense. Nat. Immunol. 2016, 17, 481. [Google Scholar] [CrossRef] [PubMed]
- Jin, T.; Huang, M.; Smith, P.; Jiang, J.; Xiao, T.S. Structure of the caspase-recruitment domain from a zebrafish guanylate-binding protein. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2013, 69, 855–860. [Google Scholar] [CrossRef]
- Shenoy, A.R.; Wellington, D.A.; Kumar, P.; Kassa, H.; Booth, C.J.; Cresswell, P.; MacMicking, J.D. GBP5 Promotes NLRP3 Inflammasome Assembly and Immunity in Mammals. Science 2012, 336, 481–485. [Google Scholar] [CrossRef]
- Santos, J.; Dick, M.S.; Lagrange, B.; Degrandi, D.; Pfeffer, K.; Yamamoto, M.; Meunier, E.; Pelczar, P.; Henry, T.; Broz, P. LPS targets host guanylate-binding proteins to the bacterial outer membrane for non-canonical inflammasome activation. EMBO J. 2018, 37, e98089. [Google Scholar] [CrossRef] [PubMed]
- von Moltke, J.; Trinidad, N.J.; Moayeri, M.; Kintzer, A.F.; Wang, S.B.; van Rooijen, N.; Brown, C.R.; Krantz, B.A.; Leppla, S.H.; Gronert, K.; et al. Rapid induction of inflammatory lipid mediators by the inflammasome in vivo. Nature 2012, 490, 107–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahillioglu, A.; Sumbul, F.; Ozoren, N.; Haliloglu, T. Structural and Dynamics Aspects of ASC Speck Assembly. Structure 2014, 22, 1722–1734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stutz, A.; Horvath, G.L.; Monks, B.G.; Latz, E. ASC speck formation as a readout for inflammasome activation. Methods Mol Biol. 2013, 1040, 91–101. [Google Scholar] [PubMed]
- Dick, M.S.; Sborgi, L.; Rühl, S.; Hiller, S.; Broz, P. ASC filament formation serves as a signal amplification mechanism for inflammasomes. Nat. Commun. 2016, 7, 11929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spead, O.; Verreet, T.; Donelson, C.J.; Poulain, F.E. Characterization of the caspase family in zebrafish. PLoS ONE 2018, 13, e0197966. [Google Scholar] [CrossRef] [PubMed]
- Broz, P.; Monack, D.M. Noncanonical Inflammasomes: Caspase-11 Activation and Effector Mechanisms. PLoS Pathog. 2013, 9, e1003144. [Google Scholar] [CrossRef]
- Zou, J.; Secombes, C.J. The Function of Fish Cytokines. Biology 2016, 5, 23. [Google Scholar] [CrossRef]
- Huising, M.O.; Stet, R.J.; Savelkoul, H.; Verburg-van Kemenade, B.L. The molecular evolution of the interleukin-1 family of cytokines; IL-18 in teleost fish. Dev. Comp. Immunol. 2004, 28, 395–413. [Google Scholar] [CrossRef]
- Brun, N.R.; Koch, B.E.; Varela, M.; Peijnenburg, W.J.; Spaink, H.P.; Vijver, M.G. Nanoparticles induce dermal and intestinal innate immune system responses in zebrafish embryos. Environ. Sci. Nano 2018, 5, 904–916. [Google Scholar] [CrossRef]
- Ramakrishnan, L. The New Paradigm of Immunity to Tuberculosis. Adv. Exp. Med. Biol. 2013, 783, 251–266. [Google Scholar] [PubMed]
- Cronan, M.R.; Tobin, D.M. Fit for consumption: Zebrafish as a model for tuberculosis. Dis. Model Mech. 2014, 7, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.M.; Clay, H.; Lewis, J.L.; Ghori, N.; Herbomel, P.; Ramakrishnan, L. Real-Time Visualization of Mycobacterium-Macrophage Interactions Leading to Initiation of Granuloma Formation in Zebrafish Embryos. Immunity 2002, 17, 693–702. [Google Scholar] [CrossRef] [Green Version]
- Volkman, H.E.; Pozos, T.C.; Zheng, J.; Davis, M.J.; Rawls, J.F.; Ramakrishnan, L. Tuberculous Granuloma Induction via Interaction of a Bacterial Secreted Protein with Host Epithelium. Science 2010, 327, 466–469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, Y.; Chen, S.; Jiang, Z.; Wang, Z.; Tan, J.; Hu, T.; Wang, Q.; Zhou, X.; Zhang, Y.; Liu, Q.; et al. Dysregulated haemolysin promotes bacterial outer membrane vesicles-induced pyroptotic-like cell death in zebrafish. Cell Microbiol. 2019, 21, e13010. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.-Y.; Ichinohe, T. Response of host inflammasomes to viral infection. Trends Microbiol. 2015, 23, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Kanneganti, T.-D. Central roles of NLRs and inflammasomes in viral infection. Nat. Rev. Immunol. 2010, 10, 688. [Google Scholar] [CrossRef] [PubMed]
- Varela, M.; Figueras, A.; Novoa, B. Modelling viral infections using zebrafish: Innate immune response and antiviral research. Antivir. Res. 2017, 139, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Prajsnar, T.K.; Cunliffe, V.T.; Foster, S.J.; Renshaw, S.A. A novel vertebrate model of Staphylococcus aureus infection reveals phagocyte-dependent resistance of zebrafish to non-host specialized pathogens. Cell Microbiol. 2008, 10, 2312–2325. [Google Scholar] [CrossRef] [PubMed]
- Bruch-Bertani, J.; Uribe-Cruz, C.; Pasqualotto, A.; Longo, L.; Ayres, R.; Beskow, C.; Barth, A.; Lima-Morales, D.; Meurer, F.; Tayguara, G.; et al. Hepatoprotective Effect of Probiotic Lactobacillus rhamnosus GG Through the Modulation of Gut Permeability and Inflammasomes in a Model of Alcoholic Liver Disease in Zebrafish. J. Am. Coll. Nutr. 2019, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Shiau, C.E.; Monk, K.R.; Joo, W.; Talbot, W.S. An Anti-inflammatory NOD-like Receptor Is Required for Microglia Development. Cell Rep. 2013, 5, 1342–1352. [Google Scholar] [CrossRef] [Green Version]
- Artlett, C.M. Inflammasomes in wound healing and fibrosis. J. Pathol. 2013, 229, 157–167. [Google Scholar] [CrossRef]
- Doyle, S.; Ozaki, E.; Campbell, M. Targeting the NLRP3 inflammasome in chronic inflammatory diseases: Current perspectives. J. Inflamm. Res. 2015, 8, 15–27. [Google Scholar] [CrossRef]
- Renshaw, S.A.; Loynes, C.A.; Trushell, D.; Elworthy, S.; Ingham, P.W.; Whyte, M. A transgenic zebrafish model of neutrophilic inflammation. Blood 2006, 108, 3976–3978. [Google Scholar] [CrossRef]
- Roehl, H. Linking wound response and inflammation to regeneration in the zebrafish larval fin. Int. J. Dev. Biol. 2018, 62, 473–477. [Google Scholar] [CrossRef]
- Dai, W.; Wang, K.; Zheng, X.; Chen, X.; Zhang, W.; Zhang, Y.; Hou, J.; Liu, L. High fat plus high cholesterol diet lead to hepatic steatosis in zebrafish larvae: A novel model for screening anti-hepatic steatosis drugs. Nutr. Metab. 2015, 12, 42. [Google Scholar] [CrossRef]
- Brugman, S. The zebrafish as a model to study intestinal inflammation. Dev. Comp. Immunol. 2016, 64, 82–92. [Google Scholar] [CrossRef] [Green Version]
- Stoilova, B.; Vyas, P. The Inflammasome: More Than a Protective Innate Immune Mechanism. Immunity 2019, 51, 3–5. [Google Scholar] [CrossRef]
- Torraca, V.; Mostowy, S. Zebrafish Infection: From Pathogenesis to Cell Biology. Trends Cell Biol. 2018, 28, 143–156. [Google Scholar] [CrossRef]
| Readout | Assay | References |
|---|---|---|
| Cell death | TUNEL | [29,34,35] |
| Sytox Green | [36] | |
| Caspase activity | in situ caspase activity (FLICA) | [29,37,38] |
| Fluorometric assay (e.g., Z-YVAD-AFC) | [28,29,39,40,41,42,43,44] |
| Inflammasome Target/Aim. | Tool/Assay | References |
|---|---|---|
| Asc | Tg(asc:asc-egfp) | [27,29] |
| Tg(HSE:asc-mkate2) | [27] | |
| asc CRISPR mutant | [30] | |
| asc TALEN mutant | [37] | |
| asc Translation-blocking MO | [27,29,39,40,45] | |
| asc Splice-blocking MO | [39,45,46] | |
| Asc zebrafish antibody | [27] | |
| Caspa | caspa CRISPR mutant | [27,29] |
| caspa Translation-bloking MO | [28,29,41] | |
| Caspb | caspb Splice-blocking MO | [29,42] |
| caspb CRISP | [36] | |
| Il1b | TgBAC(il1b:egfp) | [47] |
| Tg(il1b:efgp-F) | [48,49,50] | |
| il1b CRISPR mutant | [47] | |
| il1b Splice-blocking mo | [29,39,45,47,48,50,51] | |
| Il1b antibody | [34,52] | |
| Il1b antibody | [53] | |
| Gsdmea | gsdmea Splice-blocking MO | [30] |
| Gsdmeb | gsdmeb Translation-blocking MO | [30] |
| gsdmeb Splice-blocking MO | [29] |
| Model | References | |
|---|---|---|
| Infectious | Aeromonas hydrophila | [49] |
| Burkholderia cenocepacia | [50] | |
| Edwarsiella piscicida | [42,75] | |
| Escherichia coli | [48] | |
| Francisella noatunensis | [52] | |
| Listeria monocytogenes | [28] | |
| Mycobacterium marinum | [29,36,47] | |
| Salmonella enterica serovar Typhimurium | [39,40,44] | |
| Shigella flexneri | [35,38] | |
| Staphylococcus aureus | [79] | |
| Spring viraemia of carp virus | [34] | |
| Non-infectious | Hematopoiesis | [31] |
| Hepatic inflammation | [80] | |
| Intestinal inflammation | [45] | |
| Microglia homeostasis | [30,81] | |
| Tail fin transection | [51] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Forn-Cuní, G.; Meijer, A.H.; Varela, M. Zebrafish in Inflammasome Research. Cells 2019, 8, 901. https://doi.org/10.3390/cells8080901
Forn-Cuní G, Meijer AH, Varela M. Zebrafish in Inflammasome Research. Cells. 2019; 8(8):901. https://doi.org/10.3390/cells8080901
Chicago/Turabian StyleForn-Cuní, Gabriel, Annemarie H. Meijer, and Monica Varela. 2019. "Zebrafish in Inflammasome Research" Cells 8, no. 8: 901. https://doi.org/10.3390/cells8080901
APA StyleForn-Cuní, G., Meijer, A. H., & Varela, M. (2019). Zebrafish in Inflammasome Research. Cells, 8(8), 901. https://doi.org/10.3390/cells8080901







