Therapeutic Targeting of the IGF Axis
Abstract
1. Introduction
2. Disease States Characterized by IGF Axis Activation
2.1. Cancer
2.2. Endocrine Disorders
2.2.1. Acromegaly
2.2.2. Diabetes
2.2.3. Thyroid Eye Disease
2.3. Skin Diseases
2.3.1. Psoriasis
2.3.2. Acne
2.4. Frailty and Lifespan
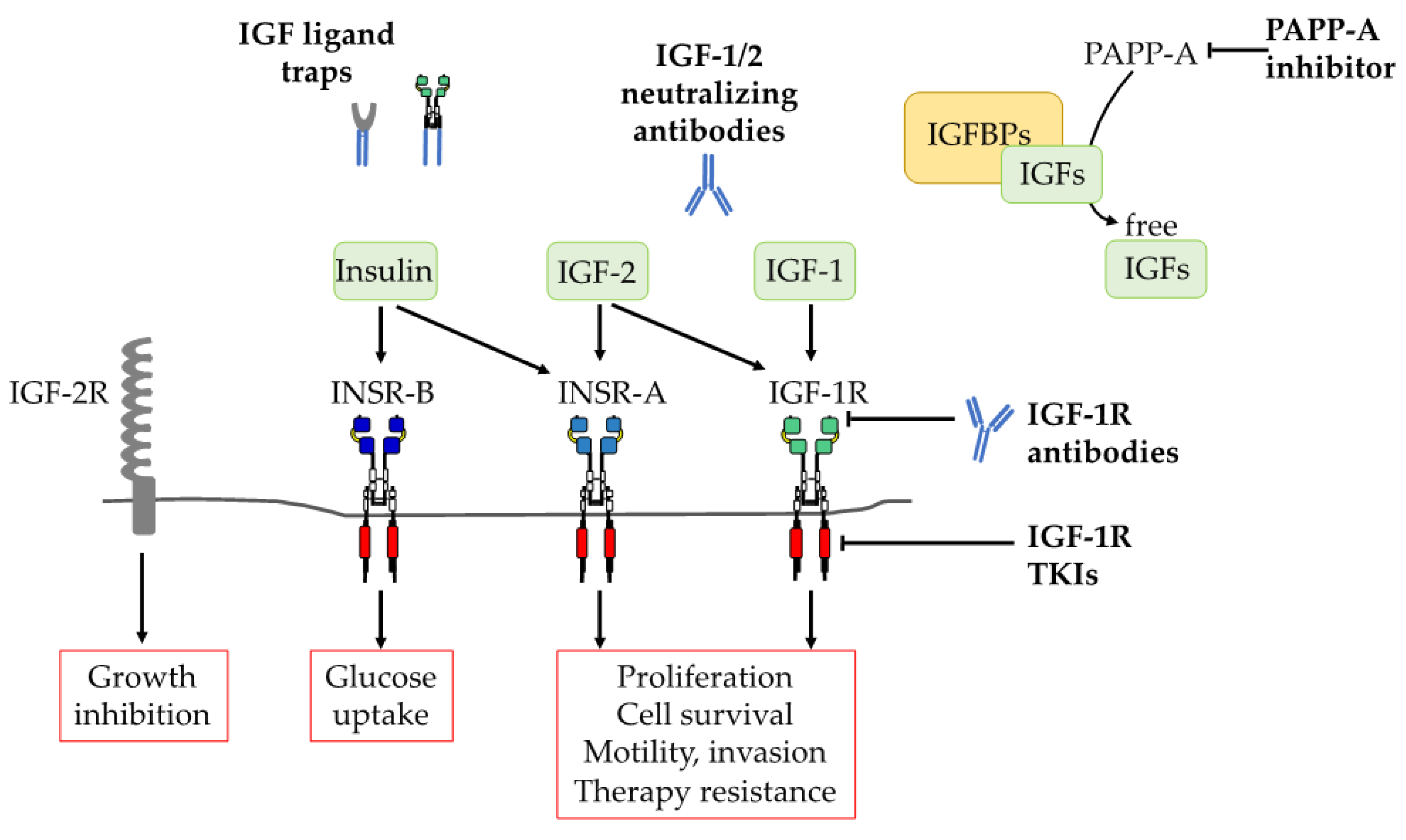
3. Therapeutic Strategies for Targeting IGF-1R in Cancer
3.1. Molecular Approaches
3.1.1. Antisense Oligonucleotides
3.1.2. siRNAs
3.1.3. Dominant Negative Receptors
3.2. Anti-IGF-1R Agents
3.2.1. IGF-1R Antibodies
3.2.2. Tyrosine Kinase Inhibitors (TKIs)
3.3. Targeting IGF Ligands
3.3.1. IGF Neutralizating Antibodies
3.3.2. IGF Ligand-TRAPs
3.3.3. Recombinant IGFBPs
3.3.4. PAPP-A Inhibition
3.4. Natural Products That Inhibit the IGF Axis
4. Therapeutic Use of IGF Axis Inhibitors: Current Status
4.1. Negative Trials of IGF-1R mABs and TKIs in Cancer Patients
4.2. Potential Grounds for Cautious Optimism
4.3. Repurposing IGF Axis Inhibitors for Non-Malignant Disorders
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Leroith, D.; Roberts, C.T. The insulin-like growth factor system and cancer. Cancer Lett. 2003, 195, 127–137. [Google Scholar] [CrossRef]
- Brzozowski, A.M.; Dodson, E.J.; Dodson, G.G.; Murshudov, G.N.; Verma, C.; Turkenburg, J.P.; De Bree, F.M.; Dauter, Z. Structural Origins of the Functional Divergence of Human Insulin-Like Growth Factor-I. and Insulin. Biochemistry. 2002, 41, 9389–9397. [Google Scholar] [CrossRef]
- De Meyts, P.; Whittaker, J. Structural biology of insulin and IGF1 receptors: Implications for drug design. Nat. Rev. Drug Discov. 2002, 1, 769–783. [Google Scholar] [CrossRef] [PubMed]
- Ullrich, A.; Gray, A.; Tam, A.; Yang-Feng, T.; Tsubokawa, M.; Collins, C.; Henzel, W.; Le Bon, T.; Kathuria, S.; Chen, E. Insulin-like growth factor I receptor primary structure: Comparison with insulin receptor suggests structural determinants that define functional specificity. EMBO J. 1986, 5, 2503–2512. [Google Scholar] [CrossRef] [PubMed]
- Adams, T.E.; Epa, V.C.; Garrett, T.P.J.; Ward, C.W. Structure and function of the type 1 insulin-like growth factor receptor. Cell. Mol. Life Sci. 2000, 57, 1050–1093. [Google Scholar] [CrossRef] [PubMed]
- Forbes, B.E.; Hartfield, P.J.; McNeil, K.A.; Surinya, K.H.; Milner, S.J.; Cosgrove, L.J.; Wallace, J.C. Characteristics of binding of insulin-like growth factor (IGF)-I and IGF-II analogues to the type 1 IGF receptor determined by BIAcore analysis. Eur. J. Biochem. 2002, 269, 961–968. [Google Scholar] [CrossRef] [PubMed]
- Denley, A.; Bonython, E.R.; Booker, G.W.; Cosgrove, L.J.; Forbes, B.; Ward, C.W.; Wallace, J.C. Structural Determinants for High-Affinity Binding of Insulin-Like Growth Factor II to Insulin Receptor (IR)-A, the Exon 11 Minus Isoform of the IR. Mol. Endocrinol. 2004, 18, 2502–2512. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Kong, G.K.-W.; Menting, J.G.; Margetts, M.B.; Delaine, C.A.; Jenkin, L.M.; Kiselyov, V.V.; De Meyts, P.; Forbes, B.E.; Lawrence, M.C. How ligand binds to the type 1 insulin-like growth factor receptor. Nat. Commun. 2018, 9, 821. [Google Scholar] [CrossRef] [PubMed]
- Kavran, J.M.; McCabe, J.M.; Byrne, P.O.; Connacher, M.K.; Wang, Z.; Ramek, A.; Sarabipour, S.; Shan, Y.; Shaw, D.E.; Hristova, K.; et al. How IGF-1 activates its receptor. eLife 2014, 3, e03772. [Google Scholar] [CrossRef] [PubMed]
- Chitnis, M.M.; Yuen, J.S.; Protheroe, A.S.; Pollak, M.; Macaulay, V.M. The Type 1 Insulin-Like Growth Factor Receptor Pathway. Clin. Cancer Res. 2008, 14, 6364–6370. [Google Scholar] [CrossRef] [PubMed]
- Pollak, M. The insulin and insulin-like growth factor receptor family in neoplasia: An update. Nat. Rev. Cancer 2012, 12, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhang, Z.; Tang, H.; Jiang, Z.; You, L.; Liao, Y. Crosstalk between IGF-1R and other tumor promoting pathways. Curr. Pharm. Des. 2014, 20, 2912–2921. [Google Scholar] [CrossRef] [PubMed]
- Cox, O.T.; O’Shea, S.; Tresse, E.; Bustamante-Garrido, M.; Kiran-Deevi, R.; O’Connor, R. IGF-1 Receptor and Adhesion Signaling: An Important Axis in Determining Cancer Cell Phenotype and Therapy Resistance. Front. Endocrinol. 2015, 6, 106. [Google Scholar] [CrossRef] [PubMed]
- De Meyts, P. The Insulin Receptor and Its Signal Transduction Network. Available online: https://www.ncbi.nlm.nih.gov/books/NBK378978/ (accessed on 4 July 2019).
- Sciacca, L.; Prisco, M.; Wu, A.; et al. Signaling differences from the A and B isoforms of the insulin receptor (IR) in 32D cells in the presence or absence of IR substrate-1. Endocrinology 2003, 144, 2650–2658. [Google Scholar] [CrossRef] [PubMed]
- Moller, D.E.; Yokota, A.; Caro, J.F.; Flier, J.S. Tissue-Specific Expression of Two Alternatively Spliced Insulin Receptor mRNAs in Man. Mol. Endocrinol. 1989, 3, 1263–1269. [Google Scholar] [CrossRef]
- Sciacca, L.; Costantino, A.; Pandini, G.; Mineo, R.; Frasca, F.; Scalia, P.; Sbraccia, P.; Goldfine, I.D.; Vigneri, R.; Belfiore, A. Insulin receptor activation by IGF-II in breast cancers: Evidence for a new autocrine/paracrine mechanism. Oncogene 1999, 18, 2471–2479. [Google Scholar] [CrossRef] [PubMed]
- Vella, V.; Pandini, G.; Sciacca, L.; Mineo, R.; Vigneri, R.; Pezzino, V.; Belfiore, A. A Novel Autocrine Loop Involving IGF-II and the Insulin Receptor Isoform-A Stimulates Growth of Thyroid Cancer. J. Clin. Endocrinol. Metab. 2002, 87, 245–254. [Google Scholar] [CrossRef]
- Belfiore, A.; Frasca, F.; Pandini, G.; Sciacca, L.; Vigneri, R. Insulin Receptor Isoforms and Insulin Receptor/Insulin-Like Growth Factor Receptor Hybrids in Physiology and Disease. Endocr. Rev. 2009, 30, 586–623. [Google Scholar] [CrossRef]
- Zapf, J.; Froesch, E. Insulin-Like Growth Factors/Somatomedins: Structure, Secretion, Biological Actions and Physiological Role. Horm. Res. 1986, 24, 121–130. [Google Scholar] [CrossRef]
- Salmon, W.D.; Daughaday, W.H. A hormonally controlled serum factor which stimulates sulfate incorporation by cartilage in vitro. J. Lab. Clin. Med. 1957, 49, 825–836. [Google Scholar]
- Yakar, S.; Pennisi, P.; Kim, C.H.; Zhao, H.; Toyoshima, Y.; Gavrilova, O.; Leroith, D. Studies involving the GH-IGF axis: Lessons from IGF-I and IGF-I receptor gene targeting mouse models. J. Endocrinol. Investig. 2005, 28, 19–22. [Google Scholar]
- Bach, L.A. IGF-binding proteins. J Mol Endocrinol. 2018, 61, T11–T28. [Google Scholar] [CrossRef]
- Collett-Solberg, P.F.; Cohen, P. The role of the insulin-like growth factor binding proteins and the IGFBP proteases in modulating IGF action. Endocrinol. Metab. Clin. North Am. 1996, 25, 591–614. [Google Scholar] [CrossRef]
- Forbes, B.E.; McCarthy, P.; Norton, R.S. Insulin-Like Growth Factor Binding Proteins: A Structural Perspective. Front. Endocrinol. 2012, 3, 38. [Google Scholar] [CrossRef]
- Baxter, R.C. IGF binding proteins in cancer: Mechanistic and clinical insights. Nat. Rev. Cancer 2014, 14, 329–341. [Google Scholar] [CrossRef]
- Bergman, D.; Halje, M.; Nordin, M.; Engström, W. Insulin-Like Growth Factor 2 in Development and Disease: A Mini-Review. Gerontology 2013, 59, 240–249. [Google Scholar] [CrossRef]
- Williams, C.; Rezgui, D.; Prince, S.N.; Zaccheo, O.J.; Foulstone, E.J.; Forbes, B.E.; Norton, R.S.; Crosby, J.; Hassan, A.B.; Crump, M.P. Structural Insights into the Interaction of Insulin-like Growth Factor 2 with IGF2R Domain 11. Structure 2007, 15, 1065–1078. [Google Scholar] [CrossRef]
- Liu, J.-P.; Baker, J.; Perkins, A.S.; Robertson, E.J.; Efstratiadis, A. Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r). Cell 1993, 75, 59–72. [Google Scholar] [CrossRef]
- Baker, J.; Liu, J.-P.; Robertson, E.J.; Efstratiadis, A. Role of insulin-like growth factors in embryonic and postnatal growth. Cell 1993, 75, 73–82. [Google Scholar] [CrossRef]
- Baumann, G. Genetic characterization of growth hormone deficiency and resistance: Implications for treatment with recombinant growth hormone. Am. J. PharmacoGenomics 2002, 2, 93–111. [Google Scholar] [CrossRef]
- Colao, A.; Grasso, L.F.S.; Giustina, A.; Melmed, S.; Chanson, P.; Pereira, A.M.; Pivonello, R. Acromegaly. Nat. Rev. Dis. Prim. 2019, 5, 20. [Google Scholar] [CrossRef]
- Guevara-Aguirre, J.; Balasubramanian, P.; Guevara-Aguirre, M.; Wei, M.; Madia, F.; Cheng, C.-W.; Hwang, D.; Martin-Montalvo, A.; Saavedra, J.; et al. Growth Hormone Receptor Deficiency is Associated With a Major Reduction in Pro-aging Signaling, Cancer and Diabetes in Humans. Sci. Transl. Med. 2011, 3, 70ra13. [Google Scholar] [CrossRef]
- Laron, Z. Lessons from 50 years of study of Laron syndrome. Endocr. Pract. 2015, 21, 1395–1402. [Google Scholar] [CrossRef]
- Woods, K.A.; Savage, M.O.; Clark, A.J.; Camacho-Hubner, C. Intrauterine Growth Retardation and Postnatal Growth Failure Associated with Deletion of the Insulin-Like Growth Factor I Gene. New Engl. J. Med. 1996, 335, 1363–1367. [Google Scholar] [CrossRef]
- Carvalho, J.A.R.; Stannard, B.; Boguszewski, M.C.S.; Sandrini, R.; Malozowski, S.N.; De Lacerda, L.; Werner, H.; Leroith, D.; Underwood, L.E. In vitro and in vivo responses to short-term recombinant human insulin-like growth factor-1 (IGF-I) in a severely growth-retarded girl with ring chromosome 15 and deletion of a single allele for the type 1 IGF receptor gene. Clin. Endocrinol. 1999, 51, 541–550. [Google Scholar]
- Denley, A.; Wang, C.C.; McNeil, K.A.; Walenkamp, M.J.E.; Van Duyvenvoorde, H.; Wit, J.M.; Wallace, J.C.; Norton, R.S.; Karperien, M.; Forbes, B.E. Structural and Functional Characteristics of the Val44Met Insulin-Like Growth Factor I Missense Mutation: Correlation with Effects on Growth and Development. Mol. Endocrinol. 2005, 19, 711–721. [Google Scholar] [CrossRef]
- Samani, A.A.; Zhang, D.; Brodt, P. The Role of the IGF-I Receptor in the Regulation of Matrix Metalloproteinases, Tumor Invasion and Metastasis. Horm. Metab. Res. 2003, 35, 802–808. [Google Scholar]
- Brodt, P.; Fallavollita, L.; Samani, A.A.; Zhang, D.; Khatib, A.-M. Cooperative Regulation of the Invasive and Metastatic Phenotypes by Different Domains of the Type I Insulin-like Growth Factor Receptor β Subunit. J. Boil. Chem. 2001, 276, 33608–33615. [Google Scholar] [CrossRef]
- Takeuchi, K.; Ito, F. Receptor tyrosine kinases and targeted cancer therapeutics. Boil. Pharm. Bull. 2011, 34, 1774–1780. [Google Scholar] [CrossRef]
- Nik-Zainal, S.; Davies, H.; Staaf, J.; Ramakrishna, M.; Glodzik, D.; Zou, X.; Martincorena, I.; Alexandrov, L.B.; Martin, S.; Wedge, D.C.; et al. Landscape of somatic mutations in 560 breast cancer whole genome sequences. Nature 2016, 534, 47–54. [Google Scholar] [CrossRef]
- Tarn, C.; Rink, L.; Merkel, E.; Flieder, D.; Pathak, H.; Koumbi, D.; Testa, J.R.; Eisenberg, B.; Von Mehren, M.; Godwin, A.K. Insulin-like growth factor 1 receptor is a potential therapeutic target for gastrointestinal stromal tumors. Proc. Natl. Acad. Sci. USA 2008, 105, 8387–8392. [Google Scholar] [CrossRef]
- Behjati, S.; Tarpey, P.S.; Haase, K.; Ye, H.; Young, M.D.; Alexandrov, L.B.; Farndon, S.J.; Collord, G.; Wedge, D.C.; Martincorena, I.; et al. Recurrent mutation of IGF signalling genes and distinct patterns of genomic rearrangement in osteosarcoma. Nat. Commun. 2017, 8, 15936. [Google Scholar] [CrossRef]
- Hu, C.K.; McCall, S.; Madden, J.; Huang, H.; Clough, R.; Jirtle, R.L.; Anscher, M.S. Loss of heterozygosity of M6P/IGF2R gene is an early event in the development of prostate cancer. Prostate Cancer Prostatic Dis. 2006, 9, 62–67. [Google Scholar] [CrossRef]
- Iida, Y.; Salomon, M.P.; Hata, K.; Tran, K.; Ohe, S.; Griffiths, C.F.; Hsu, S.C.; Nelson, N.; Hoon, D.S.B. Predominance of triple wild-type and IGF2R mutations in mucosal melanomas. BMC Cancer 2018, 18, 1054. [Google Scholar] [CrossRef]
- Cui, H.; Cruz-Correa, M.; Giardiello, F.M.; Hutcheon, D.F.; Kafonek, D.R.; Brandenburg, S.; Wu, Y.; He, X.; Powe, N.R.; Feinberg, A.P. Loss of IGF2 Imprinting: A Potential Marker of Colorectal Cancer Risk. Science 2003, 299, 1753–1755. [Google Scholar] [CrossRef]
- Riccio, A.; Sparago, A.; Verde, G.; De Crescenzo, A.; Citro, V.; Cubellis, M.V.; Ferrero, G.B.; Silengo, M.C.; Russo, S.; Larizza, L.; et al. Inherited and Sporadic Epimutations at the IGF2-H19 Locus in Beckwith-Wiedemann Syndrome and Wilms’ Tumor. Dev.Pancreas and Neonatal Diabetes 2009, 14, 1–9. [Google Scholar]
- Breuhahn, K.; Schirmacher, P. Reactivation of the insulin-like growth factor-II signaling pathway in human hepatocellular carcinoma. World J. Gastroenterol. 2008, 14, 1690–1698. [Google Scholar] [CrossRef][Green Version]
- Damaschke, N.A.; Yang, B.; Bhusari, S.; Avilla, M.; Zhong, W.; Blute, M.L.; Huang, W.; Jarrard, D.F. Loss of IGF2 gene Imprinting in Murine Prostate Promotes Widespread Neoplastic Growth. Cancer Res. 2017, 77, 5236–5247. [Google Scholar] [CrossRef]
- Werner, H. Tumor suppressors govern insulin-like growth factor signaling pathways: Implications in metabolism and cancer. Oncogene 2012, 31, 2703–2714. [Google Scholar] [CrossRef]
- Vidal, S.J.; Rodriguez-Bravo, V.; Quinn, S.A.; Rodriguez-Barrueco, R.; Lujambio, A.; Williams, E.; Sun, X.; De La Iglesia-Vicente, J.; Lee, A.; Readhead, B.; et al. A Targetable GATA2-IGF2 Axis Confers Aggressiveness in Lethal Prostate Cancer. Cancer Cell 2015, 27, 223–239. [Google Scholar] [CrossRef]
- Chitnis, M.M.; Lodhia, K.A.; Aleksic, T.; Gao, S.; Protheroe, AS.; Macaulay, VM. IGF-1R inhibition enhances radiosensitivity and delays double-strand break repair by both non-homologous end-joining and homologous recombination. Oncogene 2014, 33, 5262–5273. [Google Scholar] [CrossRef]
- Aleksic, T.; Verrill, C.; Bryant, R.J.; Han, C.; Worrall, A.R.; Brureau, L.; Larré, S.; Higgins, G.S.; Fazal, F.; Sabbagh, A.; et al. IGF-1R associates with adverse outcomes after radical radiotherapy for prostate cancer. Br. J. Cancer 2017, 117, 1600–1606. [Google Scholar] [CrossRef]
- Wu, J.D.; Odman, A.; Higgins, L.M.; Haugk, K.; Ludwig, D.L.; Vessella, R.; Plymate, S.R. In vivo Effects of the Human Type I Insulin-Like Growth Factor Receptor Antibody A12 on Androgen-Dependent and Androgen-Independent Xenograft Human Prostate Tumors. Clin. Cancer Res. 2005, 11, 3065–3074. [Google Scholar] [CrossRef]
- Quail, D.F.; Bowman, R.L.; Akkari, L.; Quick, M.L.; Schuhmacher, A.J.; Huse, J.T.; Holland, E.C.; Sutton, J.C.; Joyce, J.A. The tumor microenvironment underlies acquired resistance to CSF1R inhibition in gliomas. Science 2016, 352, aad3018. [Google Scholar] [CrossRef]
- Simpson, A.; Petnga, W.; Macaulay, V.M.; Weyer-Czernilofsky, U.; Bogenrieder, T. Insulin-Like Growth Factor (IGF) Pathway Targeting in Cancer: Role of the IGF Axis and Opportunities for Future Combination Studies. Target. Oncol. 2017, 12, 571–597. [Google Scholar] [CrossRef]
- Lee, J.S.; Kang, J.H.; Boo, H.J.; Hwang, SJ.; Hong, S.; Lee, SC.; Park, YJ.; Chung, TM.; Youn, H.; Mi Lee, S.; et al. STAT3-mediated IGF-2 secretion in the tumor microenvironment elicits innate resistance to anti-IGF-1R antibody. Nat. Commun. 2015, 6, 8499. [Google Scholar] [CrossRef]
- Unger, C.; Kramer, N.; Unterleuthner, D.; Scherzer, M.; Burian, A.; Rudisch, A.; Stadler, M.; Schlederer, M.; Lenhardt, D.; Riedl, A.; et al. Stromal-derived IGF2 promotes colon cancer progression via paracrine and autocrine mechanisms. Oncogene 2017, 36, 5341–5355. [Google Scholar] [CrossRef]
- Mutgan, A.C.; Besikcioglu, H.E.; Wang, S.; Friess, H.; Ceyhan, G.O.; Demir, I.E. Insulin/IGF-driven cancer cell-stroma crosstalk as a novel therapeutic target in pancreatic cancer. Mol. Cancer 2018, 17, 66. [Google Scholar] [CrossRef]
- Sehat, B.; Tofigh, A.; Lin, Y.; Trocmé, E.; Liljedahl, U.; Lagergren, J.; Larsson, O. SUMOylation Mediates the Nuclear Translocation and Signaling of the IGF-1 Receptor. Sci. Signal. 2010, 3, ra10. [Google Scholar] [CrossRef]
- Aleksic, T.; Chitnis, M.M.; Perestenko, O.V.; Gao, S.; Thomas, P.H.; Turner, G.D.; Protheroe, A.S.; Howarth, M.; Macaulay, V.M. Type 1 insulin-like growth factor receptor translocates to the nucleus of human tumor cells. Cancer Res. 2010, 70, 6412–6419. [Google Scholar] [CrossRef]
- Sarfstein, R.; Pasmanik-Chor, M.; Yeheskel, A.; Edry, L.; Shomron, N.; Warman, N.; Wertheimer, E.; Maor, S.; Shochat, L.; Werner, H. Insulin-like growth factor-I receptor (IGF-IR) translocates to nucleus and autoregulates IGF-IR gene expression in breast cancer cells. J. Biol. Chem. 2012, 287, 2766–2776. [Google Scholar] [CrossRef]
- Aleksic, T.; Gray, N.; Wu, X.; Rieunier, G.; Osher, E.; Mills, J.; Verrill, C.; Bryant, R.J.; Han, C.; Hutchinson, K.; et al. Nuclear IGF-1R interacts with regulatory regions of chromatin to promote RNA polymerase II recruitment and gene expression associated with advanced tumor stage. Cancer Res. 2018, 78, 3497–3509. [Google Scholar] [CrossRef]
- Vleugel, M.M.; Greijer, A.E.; Bos, R.; Van Der Wall, E.; Van Diest, P.J. c-Jun activation is associated with proliferation and angiogenesis in invasive breast cancer. Hum. Pathol. 2006, 37, 668–674. [Google Scholar] [CrossRef]
- Deng, Z.H.; Gomez, T.S.; Osborne, D.G.; Phillips-Krawczak, C.A.; Zhang, J.S.; Billadeau, D.D. Nuclear FAM21 participates in NF-kappaB-dependent gene regulation in pancreatic cancer cells. J. Cell Sci. 2015, 128, 373–384. [Google Scholar] [CrossRef]
- Steuerman, R.; Shevah, O.; Laron, Z. Congenital IGF1 deficiency tends to confer protection against post-natal development of malignancies. Eur. J. Endocrinol. 2011, 164, 485–489. [Google Scholar] [CrossRef]
- Allen, N.E.; Appleby, P.N.; Davey, G.K.; Key, T.J. Hormones and diet: Low insulin-like growth factor-I but normal bioavailable androgens in vegan men. Br. J. Cancer 2000, 83, 95–97. [Google Scholar] [CrossRef]
- Allen, N.E.; Appleby, P.N.; Davey, G.K.; Kaaks, R.; Rinaldi, S.; Key, T.J. The associations of diet with serum insulin-like growth factor I and its main binding proteins in 292 women meat-eaters, vegetarians, and vegans. Cancer Epidemiol. Biomark. Prev. 2002, 11, 1441–1448. [Google Scholar]
- Smith, W.J.; Underwood, L.E.; Clemmons, D.R. Effects of caloric or protein restriction on insulin-like growth factor-I (IGF-I) and IGF-binding proteins in children and adults. J. Clin. Endocrinol. Metab. 1995, 80, 443–449. [Google Scholar]
- Renehan, A.G.; Zwahlen, M.; Minder, C.; O’Dwyer, S.T.; Shalet, S.M.; Egger, M. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: Systematic review and meta-regression analysis. Lancet 2004, 363, 1346–1353. [Google Scholar] [CrossRef]
- Travis, R.C.; Appleby, P.N.; Martin, R.M.; Holly, J.M.; Albanes, D.; Black, A.; Bueno-De-Mesquita, H.; Chan, J.M.; Chen, C.; Chirlaque, M.-D.; et al. A meta-analysis of individual participant data reveals an association between circulating levels of IGF-I and prostate cancer risk. Cancer Res. 2016, 76, 2288–2300. [Google Scholar] [CrossRef]
- Ma, J.; Giovannucci, E.; Pollak, M.; Stampfer, M. RESPONSE: Re: Prospective Study of Colorectal Cancer Risk in Men and Plasma Levels of Insulin-Like Growth Factor (IGF)-I and IGF-Binding Protein-3. J. Natl. Cancer Inst. 1999, 91, 2052. [Google Scholar] [CrossRef][Green Version]
- Key, T.J.; Appleby, P.N.; Reeves, G.K.; Roddam, A.W. Insulin-like growth factor 1 (IGF1), IGF binding protein 3 (IGFBP3), and breast cancer risk: Pooled individual data analysis of 17 prospective studies. Lancet Oncol. 2010, 11, 530–542. [Google Scholar]
- Boguszewski, C.L.; Ayuk, J. MANAGEMENT OF ENDOCRINE DISEASE: Acromegaly and cancer: An old debate revisited. Eur. J. Endocrinol. 2016, 175, R147–R156. [Google Scholar] [CrossRef]
- Jawiarczyk-Przybyłowska, A.; Wojtczak, B.; Whitworth, J.; Sutkowski, K.; Bidlingmaier, M.; Korbonits, M.; Bolanowski, M. Acromegaly associated with GIST, non-small cell lung carcinoma, clear cell renal carcinoma, multiple myeloma, medulla oblongata tumour, adrenal adenoma, and follicular thyroid nodules. Endokrynol. Pol. 2019, 70, 213–217. [Google Scholar] [CrossRef]
- Rieunier, G.; Wu, X.; Macaulay, V.M.; Lee, A.V.; Weyer-Czernilofsky, U.; Bogenrieder, T. Bad to the Bone: The Role of the Insulin-Like Growth Factor Axis in Osseous Metastasis. Clin. Cancer Res. 2019, 25, 3479–3485. [Google Scholar] [CrossRef]
- Mazziotti, G.A.; Lania, A.G.; Canalis, E.; Lania, A. Management of endocrine disease: Bone disorders associated with acromegaly: Mechanisms and treatment. Eur. J. Endocrinol. 2019, 181, R45–R56. [Google Scholar] [CrossRef]
- Colao, A.; Grasso, L.F.; Di Somma, C.; Pivonello, R. Acromegaly and Heart Failure. Hear Fail. Clin. 2019, 15, 399–408. [Google Scholar] [CrossRef]
- Schneider, H.J.; Friedrich, N.; Klotsche, J.; Schipf, S.; Nauck, M.; Völzke, H.; Sievers, C.; Pieper, L.; März, W.; Wittchen, H.-U.; et al. Prediction of incident diabetes mellitus by baseline IGF1 levels. Eur. J. Endocrinol. 2011, 164, 223–229. [Google Scholar] [CrossRef]
- Friedrich, N.; Thuesen, B.; Jorgensen, T.; Juul, A.; Spielhagen, C.; Wallaschofksi, H.; Linneberg, A. The association between IGF-I and insulin resistance: A general population study in Danish adults. Diabetes Care 2012, 35, 768–773. [Google Scholar] [CrossRef]
- Smith, T.R.; Elmendorf, J.S.; David, T.S.; Turinsky, J. Growth hormone-induced insulin resistance: Role of the insulin receptor, IRS-1, GLUT-1, and GLUT-4. Am. J. Physiol. Metab. 1997, 272, 1071–1079. [Google Scholar] [CrossRef]
- Haywood, N.J.; Slater, T.A.; Matthews, C.J.; Wheatcroft, S.B. The insulin like growth factor and binding protein family: Novel therapeutic targets in obesity & diabetes. Mol. Metab. 2019, 19, 86–96. [Google Scholar] [CrossRef]
- Moses, A.C.; Young, S.C.; Morrow, L.A.; O’Brien, M.; Clemmons, D.R. Recombinant Human Insulin-Like Growth Factor I Increases Insulin Sensitivity and Improves Glycemic Control in Type II Diabetes. Diabetes 1996, 45, 91–100. [Google Scholar] [CrossRef]
- Kim, R.J.; Grimberg, A. POTENTIAL NON-GROWTH USES OF rhIGF-I. Growth, Genet. Horm. 2007, 23, 1–7. [Google Scholar]
- Morshed, S.A.; Davies, T.F. Graves’ Disease Mechanisms: The Role of Stimulating, Blocking, and Cleavage Region TSH Receptor Antibodies. Horm. Metab. Res. 2015, 47, 727–734. [Google Scholar] [CrossRef]
- Dolman, P.J. Evaluating Graves’ orbitopathy. Best Pract. Res. Clin. Endocrinol. Metab. 2012, 26, 229–248. [Google Scholar] [CrossRef]
- Smith, T.J.; Janssen, J. Insulin-like Growth Factor-I Receptor and Thyroid-Associated Ophthalmopathy. Endocr. Rev. 2019, 40, 236–267. [Google Scholar] [CrossRef]
- Tramontano, D.; Cushing, G.W.; Moses, A.C.; Ingbar, S.H. Insulin-like growth factor-i stimulates the growth of rat thyroid cells in culture and synergizes the stimulation of dna synthesis induced by tsh and graves′-igg. Endocrinol 1986, 119, 940–942. [Google Scholar] [CrossRef]
- Smith, T.J. Insulin-Like Growth Factor-I Regulation of Immune Function: A Potential Therapeutic Target in Autoimmune Diseases? Pharmacol. Rev. 2010, 62, 199–236. [Google Scholar] [CrossRef]
- Weightman, D.R.; Perros, P.; Sherif, I.H.; Kendall-Taylor, P. Autoantibodies to Igf-1 Binding Sites in Thyroid Associated Ophthalmopathy. Autoimmun 1993, 16, 251–257. [Google Scholar] [CrossRef]
- Pritchard, J.; Han, R.; Horst, N.; Cruikshank, W.W.; Smith, T.J. Immunoglobulin Activation of T Cell Chemoattractant Expression in Fibroblasts from Patients with Graves’ Disease Is Mediated Through the Insulin-Like Growth Factor I Receptor Pathway. J. Immunol. 2003, 170, 6348–6354. [Google Scholar] [CrossRef]
- Loos, U.; Minich, W.B. Detection of functionally different types of pathological autoantibodies against thyrotropin receptor in Graves’ patients sera by luminescent immunoprecipitation analysis. Exp. Clin. Endocrinol. Diabetes 2000, 108, 110–119. [Google Scholar]
- Martin, S.; Sirbu, A.; Betivoiu, M.; Florea, S.; Barbu, C.; Fica, S. IGF1 deficiency in newly diagnosed Graves’ disease patients. Hormes 2015, 14, 651–659. [Google Scholar]
- Ristow, H.J. Effect of insulin-like growth factor-I/somatomedin C on thymidine incorporation in cultured psoriatic keratinocytes after growth arrest in growth factor-free medium. Growth Regul. 1993, 3, 129–137. [Google Scholar]
- Hodak, E.; Gottlieb, A.B.; Anzilotti, M.; Krueger, J.G. The Insulin-like Growth Factor 1 Receptor Is Expressed by Epithelial Cells with Proliferative Potential in Human Epidermis and Skin Appendages: Correlation of Increased Expression with Epidermal Hyperplasia. J. Investig. Dermatol. 1996, 106, 564–570. [Google Scholar] [CrossRef]
- Xu, S.; Cwyfan-Hughes, S.C.; Van Der Stappen, J.W.; Sansom, J.; Burton, J.L.; Donnelly, M.; Holly, J.M.P. Altered Insulin-like Growth Factor–II (IGF-II) Level and IGF-Binding Protein–3 (IGFBP-3) Protease Activity in Interstitial Fluid Taken from the Skin Lesion of Psoriasis. J. Investig. Dermatol. 1996, 106, 109–112. [Google Scholar] [CrossRef][Green Version]
- Wraight, C.J.; Edmondson, S.R.; Fortune, D.W.; Varigos, G.; Werther, G.A. Expression of Insulin-Like Growth Factor Binding Protein-3 (IGFBP-3) in the Psoriatic Lesion. J. Investig. Dermatol. 1997, 108, 452–456. [Google Scholar] [CrossRef]
- Culig, Z. Androgen receptor cross-talk with cell signalling pathways. Growth Factors 2004, 22, 179–184. [Google Scholar] [CrossRef]
- Melnik, B.C.; John, S.M.; Schmitz, G. Over-stimulation of insulin/IGF-1 signaling by western diet may promote diseases of civilization: Lessons learnt from laron syndrome. Nutr. Metab. 2011, 8, 41. [Google Scholar] [CrossRef]
- Ben-Amitai, D.; Laron, Z. Effect of insulin-like growth factor-1 deficiency or administration on the occurrence of acne. J. Eur. Acad. Dermatol. Venereol 2011, 25, 950–954. [Google Scholar] [CrossRef]
- Danby, F.W. Nutrition and acne. Clin. Dermatol. 2010, 28, 598–604. [Google Scholar] [CrossRef]
- Rahaman, S.M.A.; De, D.; Handa, S.; Pal, A.; Sachdeva, N.; Ghosh, T.; Kamboj, P. Association of insulin-like growth factor (IGF)-1 gene polymorphisms with plasma levels of IGF-1 and acne severity. J. Am. Acad. Dermatol. 2016, 75, 768–773. [Google Scholar] [CrossRef]
- Smith, T.M.; Gilliland, K.; Clawson, G.A.; Thiboutot, D. IGF-1 induces SREBP-1 expression and lipogenesis in SEB-1 sebocytes via activation of the phosphoinositide 3-kinase/Akt pathway. J. Invest. Dermatol. 2008, 128, 1286–1293. [Google Scholar] [CrossRef]
- Kim, H.; Moon, S.Y.; Sohn, M.Y.; Lee, W.J. Insulin-Like Growth Factor-1 Increases the Expression of Inflammatory Biomarkers and Sebum Production in Cultured Sebocytes. Ann. Dermatol. 2017, 29, 20–25. [Google Scholar] [CrossRef]
- Mohamad, M.I.; Khater, M.S. Evaluation of insulin like growth factor-1 (IGF-1) level and its impact on muscle and bone mineral density in frail elderly male. Arch. Gerontol. Geriatr. 2015, 60, 124–127. [Google Scholar] [CrossRef]
- Doi, T.; Shimada, H.; Makizako, H.; Tsutsumimoto, K.; Hotta, R.; Nakakubo, S.; Suzuki, T. Insulin-like growth factor-1 related to disability among older adults. J. Gerontol. A. 2015, 71, 797–802. [Google Scholar] [CrossRef]
- Altintas, O.; Park, S.; Lee, S.J. The role of insulin/IGF-1 signaling in the longevity of model invertebrates, C. elegans and D. melanogaster. BMB Rep. 2016, 49, 81–92. [Google Scholar] [CrossRef]
- Holzenberger, M.; Dupont, J.; Ducos, B.; Leneuve, P.; Géloën, A.; Even, P.C.; Cervera, P.; Le Bouc, Y. IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature 2003, 421, 182–187. [Google Scholar] [CrossRef]
- Milman, S.; Atzmon, G.; Huffman, D.M.; Wan, J.; Crandall, J.P.; Cohen, P.; Barzilai, N. Low insulin-like growth factor-1 level predicts survival in humans with exceptional longevity. Aging Cell 2014, 13, 769–771. [Google Scholar] [CrossRef]
- Levine, M.E.; Suarez, J.A.; Brandhorst, S.; Balasubramanian, P.; Cheng, C.-W.; Madia, F.; Fontana, L.; Mirisola, M.G.; Guevara-Aguirre, J.; Wan, J.; et al. Low Protein Intake is Associated with a Major Reduction in IGF-1, Cancer, and Overall Mortality in the 65 and Younger but Not Older Population. Cell Metab. 2014, 19, 407–417. [Google Scholar] [CrossRef]
- Arteaga, C.L.; Kitten, L.J.; Coronado, E.B.; Jacobs, S.; Kull, F.C.; Allred, D.C.; Osborne, C.K. Blockade of the type I somatomedin receptor inhibits growth of human breast cancer cells in athymic mice. J. Clin. Investig. 1989, 84, 1418–1423. [Google Scholar] [CrossRef]
- Macaulay, V. Insulin-like growth factors and cancer. Br. J. Cancer 1992, 65, 311–320. [Google Scholar] [CrossRef]
- Li, R.; Pourpak, A.; Morris, S.W. Inhibition of the Insulin-like Growth Factor-1 Receptor (IGF1R) Tyrosine Kinase as a Novel Cancer Therapy Approach. J. Med. Chem. 2009, 52, 4981–5004. [Google Scholar] [CrossRef]
- Pillai, R.N.; Ramalingam, S.S. Inhibition of insulin-like growth factor receptor: End of a targeted therapy? Transl. Lung Cancer Res. 2013, 2, 14–22. [Google Scholar] [CrossRef]
- Beckwith, H.; Yee, D. Minireview: Were the IGF Signaling Inhibitors All Bad? Mol. Endocrinol. 2015, 29, 1549–1557. [Google Scholar] [CrossRef]
- Ekyalongo, R.C.; Yee, D. Revisiting the IGF-1R as a breast cancer target. NPG Precis. Oncol. 2017, 1, 59. [Google Scholar] [CrossRef]
- Qu, X.; Wu, Z.; Dong, W.; Zhang, T.; Wang, L.; Pang, Z.; Ma, W.; Du, J. Update of IGF-1 receptor inhibitor (ganitumab, dalotuzumab, cixutumumab, teprotumumab and figitumumab) effects on cancer therapy. Oncotarget 2017, 8, 29501–29518. [Google Scholar] [CrossRef]
- Trojan, J.; Johnson, T.; Rudin, S.; Tykocinski, M.; Ilan, J. Treatment and prevention of rat glioblastoma by immunogenic C6 cells expressing antisense insulin-like growth factor I RNA. Science 1993, 259, 94–97. [Google Scholar] [CrossRef]
- Resnicoff, M.; Sell, C.; Rubini, M.; Coppola, D.; Ambrose, D.; Baserga, R.; Rubin, R. Rat glioblastoma cells expressing an antisense RNA to the insulin-like growth factor-1 (IGF-1) receptor are nontumorigenic and induce regression of wild-type tumors. Cancer Res. 1994, 54, 2218–2222. [Google Scholar]
- Andrews, D.W.; Resnicoff, M.; Flanders, A.E.; Kenyon, L.; Curtis, M.; Merli, G.; Baserga, R.; Iliakis, G.; Aiken, R.D. Results of a Pilot Study Involving the Use of an Antisense Oligodeoxynucleotide Directed Against the Insulin-Like Growth Factor Type I Receptor in Malignant Astrocytomas. J. Clin. Oncol. 2001, 19, 2189–2200. [Google Scholar] [CrossRef]
- Wraight, C.J.; White, P.J.; Mckean, S.C.; Fogarty, R.D.; Venables, D.J.; Liepe, I.J.; Edmondson, S.R.; Werther, G.A. Reversal of epidermal hyperproliferation in psoriasis by insulin-like growth factor I receptor antisense oligonucleotides. Nat. Biotechnol. 2000, 18, 521–526. [Google Scholar] [CrossRef]
- Furukawa, J.; Wraight, C.J.; Freier, S.M.; Peralta, E.; Atley, L.M.; Monia, B.P.; Gleave, M.E.; Cox, M.E. Antisense oligonucleotide targeting of insulin-like growth factor-1 receptor (IGF-1R) in prostate cancer. Prostate 2010, 70, 206–218. [Google Scholar] [CrossRef]
- Fire, A.; Xu, S.; Montgomery, M.K.; Kostas, S.A.; Driver, S.E.; Mello, C.C. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 1998, 391, 806–811. [Google Scholar] [CrossRef]
- Elbashir, S.M.; Harborth, J.; Lendeckel, W.; Yalcin, A.; Weber, K.; Tuschl, T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 2001, 411, 494–498. [Google Scholar] [CrossRef]
- Castel, S.E.; Martienssen, R.A. RNA interference (RNAi) in the Nucleus: Roles for small RNA in transcription, epigenetics and beyond. Nat. Rev. Genet. 2013, 14, 100–112. [Google Scholar] [CrossRef]
- Bohula, E.A.; Salisbury, A.J.; Sohail, M.; Playford, M.P.; Riedemann, J.; Southern, E.M.; Macaulay, V.M. The Efficacy of Small Interfering RNAs Targeted to the Type 1 Insulin-like Growth Factor Receptor (IGF1R) Is Influenced by Secondary Structure in the IGF1R Transcript. J. Boil. Chem. 2003, 278, 15991–15997. [Google Scholar] [CrossRef]
- Niu, J.; Xu, Z.; Li, X.; Han, Z. siRNA-mediated type 1 insulin-like growth factor receptor silencing induces chemosensitization of a human liver cancer cell line with mutant P53. Cell Boil. Int. 2007, 31, 156–164. [Google Scholar] [CrossRef]
- Yuen, J.S.; Akkaya, E.; Wang, Y.; Takiguchi, M.; Peak, S.; Sullivan, M.; Protheroe, A.S.; Macaulay, V.M. Validation of the type 1 insulin-like growth factor receptor as a therapeutic target in renal cancer. Mol. Cancer. Ther. 2009, 8, 1448–1459. [Google Scholar] [CrossRef]
- Turney, B.W.; Kerr, M.; Chitnis, M.M.; Lodhia, K.; Wang, Y.; Riedemann, J.; Rochester, M.; Protheroe, A.S.; Brewster, S.F.; Macaulay, V.M. Depletion of the type 1 IGF receptor delays repair of radiation-induced DNA double strand breaks. Radiother. Oncol. 2012, 103, 402–409. [Google Scholar] [CrossRef]
- Zhao, H.; Gu, X. Silencing of insulin-like growth factor-1 receptor enhances the radiation sensitivity of human esophageal squamous cell carcinoma in vitro and in vivo. World J. Surg. Oncol. 2014, 12, 325. [Google Scholar] [CrossRef]
- Soutschek, J.; Akinc, A.; Bramlage, B.; Charisse, K.; Constien, R.; Donoghue, M.; Elbashir, S.; Geick, A.; Hadwiger, P.; Harborth, J.; et al. Therapeutic silencing of an endogenous gene by systemic administration of modified siRNAs. Nature 2004, 432, 173–178. [Google Scholar] [CrossRef]
- D’Ambrosio, C.; Ferber, A.; Resnicoff, M.; Baserga, R. A soluble insulin-like growth factor I receptor that induces apoptosis of tumor cells in vivo and inhibits tumorigenesis. Cancer Res. 1996, 56. [Google Scholar]
- Dunn, S.; Ehrlich, M.; Sharp, N.J.; Reiss, K.; Solomon, G.; Hawkins, R.; Baserga, R.; Barrett, J.C. A dominant negative mutant of the insulin-like growth factor-I receptor inhibits the adhesion, invasion, and metastasis of breast cancer. Cancer Res. 1998, 58, 3353–3361. [Google Scholar]
- Samani, A.A.; Chevet, E.; Fallavollita, L.; Galipeau, J.; Brodt, P. Loss of tumorigenicity and metastatic potential in carcinoma cells expressing the extracellular domain of the type 1 insulin-like growth factor receptor. Cancer Res. 2004, 64, 3380–3385. [Google Scholar] [CrossRef]
- Gan, Y.; Buckels, A.; Liu, Y.; Zhang, Y.; Paterson, A.J.; Jiang, J.; Zinn, K.R.; Frank, S.J. Human GH Receptor-IGF-1 Receptor Interaction: Implications for GH Signaling. Mol. Endocrinol. 2014, 28, 1841–1854. [Google Scholar] [CrossRef]
- Sachdev, D.; Hartell, J.S.; Lee, A.V.; Zhang, X.; Yee, D. A dominant negative type I insulin-like growth factor receptor inhibits metastasis of human cancer cells. J. Biol. Chem. 2004, 279, 5017–5024. [Google Scholar] [CrossRef]
- Min, Y.; Adachi, Y.; Yamamoto, H.; Imsumran, A.; Arimura, Y.; Endo, T.; Hinoda, Y.; Lee, C.-T.; Nadaf, S.; Carbone, D.P.; et al. Insulin-like growth factor I receptor blockade enhances chemotherapy and radiation responses and inhibits tumour growth in human gastric cancer xenografts. Gut 2005, 54, 591–600. [Google Scholar] [CrossRef]
- Fujita, M.; Ieguchi, K.; Cedano-Prieto, D.M.; Fong, A.; Wilkerson, C.; Chen, J.Q.; Wu, M.; Lo, S.-H.; Cheung, A.T.W.; Wilson, M.D.; et al. An Integrin Binding-defective Mutant of Insulin-like Growth Factor-1 (R36E/R37E IGF1) Acts as a Dominant-negative Antagonist of the IGF1 Receptor (IGF1R) and Suppresses Tumorigenesis but Still Binds to IGF1R. J. Boil. Chem. 2013, 288, 19593–19603. [Google Scholar] [CrossRef]
- Husain, S.R.; Han, J.; Au, P.; Shannon, K.; Puri, R.K. Gene therapy for cancer: Regulatory considerations for approval. Cancer Gene Ther. 2015, 22, 554–563. [Google Scholar] [CrossRef]
- Sachdev, D.; Li, S.-L.; Hartell, J.S.; Fujita-Yamaguchi, Y.; Miller, J.S.; Yee, D. A chimeric humanized single-chain antibody against the type I insulin-like growth factor (IGF) receptor renders breast cancer cells refractory to the mitogenic effects of IGF-I. Cancer Res. 2003, 63, 627–635. [Google Scholar]
- Sachdev, D.; Singh, R.; Fujita-Yamaguchi, Y.; Yee, D. Down-regulation of Insulin Receptor by Antibodies against the Type I Insulin-Like Growth Factor Receptor: Implications for Anti–Insulin-Like Growth Factor Therapy in Breast Cancer. Cancer Res. 2006, 66, 2391–2402. [Google Scholar] [CrossRef]
- Pandini, G.; Wurch, T.; Akla, B.; Corvaia, N.; Belfiore, A.; Goetsch, L. Functional responses and in vivo anti-tumour activity of h7C10: A humanised monoclonal antibody with neutralising activity against the insulin-like growth factor-1 (IGF-1) receptor and insulin/IGF-1 hybrid receptors. Eur. J. Cancer 2007, 43, 1318–1327. [Google Scholar] [CrossRef]
- Pollak, M.N.; Schernhammer, E.S.; Hankinson, S.E. Insulin-like growth factors and neoplasia. Nat. Rev. Cancer 2004, 4, 505–518. [Google Scholar] [CrossRef]
- Ulanet, D.B.; Ludwig, D.L.; Kahn, C.R.; Hanahan, D. Insulin receptor functionally enhances multistage tumor progression and conveys intrinsic resistance to IGF-1R targeted therapy. Proc. Natl. Acad. Sci. 2010, 107, 10791–10798. [Google Scholar] [CrossRef]
- Buck, E.; Gokhale, P.C.; Koujak, S.; Brown, E.; Eyzaguirre, A.; Tao, N.; Rosenfeld-Franklin, M.; Lerner, L.; Chiu, M.I.; Wild, R.; et al. Compensatory Insulin Receptor (IR) Activation on Inhibition of Insulin-Like Growth Factor-1 Receptor (IGF-1R): Rationale for Cotargeting IGF-1R and IR in Cancer. Mol. Cancer Ther. 2010, 9, 2652–2664. [Google Scholar] [CrossRef]
- Weinstein, D.; Sarfstein, R.; Laron, Z.; Werner, H. Insulin receptor compensates for IGF1R inhibition and directly induces mitogenic activity in prostate cancer cells. Endocr. Connect. 2014, 3, 24–35. [Google Scholar] [CrossRef]
- Forest, A.; Amatulli, M.; Ludwig, D.L.; Damoci, C.B.; Wang, Y.; Burns, C.A.; Donoho, G.P.; Zanella, N.; Fiebig, H.H.; Prewett, M.C.; et al. Intrinsic Resistance to Cixutumumab is Conferred by Distinct Isoforms of the Insulin Receptor. Mol. Cancer Res. 2015, 13, 1615–1626. [Google Scholar] [CrossRef]
- Bid, H.K.; Zhan, J.; Phelps, D.A.; Kurmasheva, R.T.; Houghton, P.J. Potent inhibition of angiogenesis by the IGF-1 receptor-targeting antibody SCH717454 is reversed by IGF-2. Mol. Cancer Ther. 2012, 11, 649–659. [Google Scholar] [CrossRef]
- Feng, Y.; Dimitrov, D.S. Antibody-based therapeutics against components of the IGF system. OncoImmunology 2012, 1, 1390–1391. [Google Scholar] [CrossRef]
- Veeken, J.; Oliveira, S.; Schiffelers, R.; Storm, G.; Henegouwen, P.; Roovers, R. Crosstalk Between Epidermal Growth Factor Receptor- and Insulin-Like Growth Factor-1 Receptor Signaling: Implications for Cancer Therapy. Curr. Cancer Drug Targets 2009, 9, 748–760. [Google Scholar] [CrossRef]
- Liu, R.; Tang, W.; Han, X.; Geng, R.; Wang, C.; Zhang, Z. Hepatocyte growth factor-induced mesenchymal-epithelial transition factor activation leads to insulin-like growth factor 1 receptor inhibitor unresponsiveness in gastric cancer cells. Oncol. Lett. 2018, 16, 5983–5991. [Google Scholar] [CrossRef]
- Cohen, B.D.; Baker, D.A.; Soderstrom, C.; Tkalcevic, G.; Rossi, A.M.; Miller, P.E.; Tengowski, M.W.; Wang, F.; Gualberto, A.; Beebe, J.S.; et al. Combination Therapy Enhances the Inhibition of Tumor Growth with the Fully Human Anti–Type 1 Insulin-Like Growth Factor Receptor Monoclonal Antibody CP-751,871. Clin. Cancer Res. 2005, 11, 2063–2073. [Google Scholar] [CrossRef]
- Lacy, M.Q.; Alsina, M.; Fonseca, R.; Paccagnella, M.L.; Melvin, C.L.; Yin, D.; Sharma, A.; Sarano, M.E.; Pollak, M.; Jagannath, S.; et al. Phase I, Pharmacokinetic and Pharmacodynamic Study of the Anti–Insulinlike Growth Factor Type 1 Receptor Monoclonal Antibody CP-751,871 in Patients with Multiple Myeloma. J. Clin. Oncol. 2008, 26, 3196–3203. [Google Scholar] [CrossRef]
- Pavlicek, A.; Lira, M.E.; Lee, N.V.; Ching, K.A.; Ye, J.; Cao, J.; Garza, S.J.; Hook, K.E.; Ozeck, M.; Shi, S.T.; et al. Molecular Predictors of Sensitivity to the Insulin-like Growth Factor 1 Receptor Inhibitor Figitumumab (CP-751,871). Mol. Cancer Ther. 2013, 12, 2929–2939. [Google Scholar] [CrossRef]
- De Bono, J.S.; Piulats, J.M.; Pandha, H.S.; Petrylak, D.P.; Saad, F.; Aparicio, L.M.A.; Sandhu, S.K.; Fong, P.; Gillessen, S.; Hudes, G.R.; et al. Phase II Randomized Study of Figitumumab plus Docetaxel and Docetaxel Alone with Crossover for Metastatic Castration-Resistant Prostate Cancer. Clin. Cancer Res. 2014, 20, 1925–1934. [Google Scholar] [CrossRef]
- Calvo, E.; Soria, J.C.; Ma, W.W.; Wang, T.; Bahleda, R.; Tolcher, A.W.; Gernhardt, D.; O’Connell, J.; Millham, R.; Giri, N.; et al. A Phase I Clinical Trial and Independent Patient-Derived Xenograft Study of Combined Targeted Treatment with Dacomitinib and Figitumumab in Advanced Solid Tumors. Clin. Cancer Res. 2017, 23, 1177–1185. [Google Scholar] [CrossRef]
- Tolcher, A.W.; Sarantopoulos, J.; Patnaik, A.; Papadopoulos, K.; Rodon, J.; Murphy, B.; Roth, B.; McCaffery, I.; Gorski, K.S.; Kaiser, B.; et al. Phase I, Pharmacokinetic, and Pharmacodynamic Study of AMG 479, a Fully Human Monoclonal Antibody to Insulin-Like Growth Factor Receptor 1. J. Clin. Oncol. 2009, 27, 5800–5807. [Google Scholar] [CrossRef]
- Beltran, P.J.; Chung, Y.-A.; Moody, G.; Mitchell, P.; Cajulis, E.; Vonderfecht, S.; Kendall, R.; Radinsky, R.; Calzone, F.J. Efficacy of Ganitumab (AMG 479), Alone and in Combination with Rapamycin, in Ewing’s and Osteogenic Sarcoma Models. J. Pharmacol. Exp. Ther. 2011, 337, 644–654. [Google Scholar] [CrossRef]
- Glisson, B.; Besse, B.; Dols, M.C.; Dubey, S.; Schupp, M.; Jain, R.; Jiang, Y.; Menon, H.; Nackaerts, K.; Orlov, S.; et al. A Randomized, Placebo-Controlled, Phase 1b/2 Study of Rilotumumab or Ganitumab in Combination with Platinum-Based Chemotherapy as First-Line Treatment for Extensive-Stage Small-Cell Lung Cancer. Clin. Lung Cancer 2017, 18, 615–625. [Google Scholar] [CrossRef]
- Kurzrock, R.; Patnaik, A.; Aisner, J.; Warren, T.; Leong, S.; Benjamin, R.; Eid, J.E.; Habben, K.; McCarthy, C.D.; Gore, L.; et al. A Phase I Study of Weekly R1507, A Human Monoclonal Antibody Insulin-like Growth Factor-I Receptor Antagonist, in Patients with Advanced Solid Tumors. Clin. Cancer Res. 2010, 16, 2458–2465. [Google Scholar] [CrossRef]
- Ramalingam, S.S.; Spigel, D.R.; Chen, D.; Steins, M.B.; Engelman, J.A.; Schneider, C.-P.; Novello, S.; Eberhardt, W.E.; Crino, L.; Habben, K.; et al. Randomized Phase II Study of Erlotinib in Combination With Placebo or R1507, a Monoclonal Antibody to Insulin-Like Growth Factor-1 Receptor, for Advanced-Stage Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2011, 29, 4574–4580. [Google Scholar] [CrossRef]
- Pappo, A.S.; Patel, S.R.; Crowley, J.; Reinke, D.K.; Kuenkele, K.-P.; Chawla, S.P.; Toner, G.C.; Maki, R.G.; Meyers, P.A.; Chugh, R.; et al. R1507, a Monoclonal Antibody to the Insulin-Like Growth Factor 1 Receptor, in Patients With Recurrent or Refractory Ewing Sarcoma Family of Tumors: Results of a Phase II Sarcoma Alliance for Research Through Collaboration Study. J. Clin. Oncol. 2011, 29, 4541–4547. [Google Scholar] [CrossRef]
- Pappo, A.S.; Vassal, G.; Crowley, J.J.; Bolejack, V.; Hogendoorn, P.C.W.; Chugh, R.; Ladanyi, M.; Grippo, J.F.; Dall, G.; Staddon, A.P.; et al. A phase 2 trial of R1507, a monoclonal antibody to the insulin-like growth factor-1 receptor (IGF-1R), in patients with recurrent or refractory rhabdomyosarcoma, osteosarcoma, synovial sarcoma, and other soft tissue sarcomas: Results of a Sarcoma Alliance. Cancer 2014, 120, 2448–2456. [Google Scholar] [CrossRef]
- Atzori, F.; Tabernero, J.; Cervantes, A.; Prudkin, L.; Andreu, J.; Rodríguez-Braun, E.; Domingo, A.; Guijarro, J.; Gamez, C.; Rodon, J.; et al. A Phase I Pharmacokinetic and Pharmacodynamic Study of Dalotuzumab (MK-0646), an Anti-Insulin-like Growth Factor-1 Receptor Monoclonal Antibody, in Patients with Advanced Solid Tumors. Clin. Cancer Res. 2011, 17, 6304–6312. [Google Scholar] [CrossRef]
- Brana, I.; Berger, R.; Golan, T.; Haluska, P.; Edenfield, J.; Fiorica, J.; Stephenson, J.; Martin, L.P.; Westin, S.; Hanjani, P.; et al. A parallel-arm phase I trial of the humanised anti-IGF-1R antibody dalotuzumab in combination with the AKT inhibitor MK-2206, the mTOR inhibitor ridaforolimus, or the NOTCH inhibitor MK-0752, in patients with advanced solid tumours. Br. J. Cancer 2014, 111, 1932–1944. [Google Scholar] [CrossRef]
- Huang, C.H.; Williamson, S.K.; Neupane, P.; Taylor, S.A.; Allen, A.; Smart, N.J.; Uypeckcuat, A.M.; Spencer, S.; Wick, J.; Smith, H.; et al. Impact Study: MK-0646 (Dalotuzumab), Insulin Growth Factor 1 Receptor Antibody Combined with Pemetrexed and Cisplatin in Stage IV Metastatic Non-squamous Lung Cancer. Front Oncol. 2016, 5, 301. [Google Scholar] [CrossRef]
- Sclafani, F.; Kim, T.Y.; Cunningham, D.; Kim, T.W.; Tabernero, J.; Schmoll, H.J.; Roh, J.K.; Kim, S.Y.; Park, Y.S.; Guren, T.K.; et al. A Randomized Phase II/III Study of Dalotuzumab in Combination With Cetuximab and Irinotecan in Chemorefractory, KRASWild-Type, Metastatic Colorectal Cancer. J. Natl. Cancer Inst. 2015, 107. [Google Scholar] [CrossRef]
- Rowinsky, E.K.; Youssoufian, H.; Tonra, J.R.; Solomon, P.; Burtrum, D.; Ludwig, D.L. IMC-A12, a Human IgG1 Monoclonal Antibody to the Insulin-Like Growth Factor I Receptor. Clin. Cancer Res. 2007, 13, 5549–5555. [Google Scholar] [CrossRef]
- Attias-Geva, Z.; Bentov, I.; Ludwig, D.L.; Fishman, A.; Bruchim, I.; Werner, H. Insulin-like growth factor-I receptor (IGF-IR) targeting with monoclonal antibody cixutumumab (IMC-A12) inhibits IGF-I action in endometrial cancer cells. Eur. J. Cancer 2011, 47, 1717–1726. [Google Scholar] [CrossRef]
- Malempati, S.; Weigel, B.; Ingle, A.M.; Ahern, C.H.; Carroll, J.M.; Roberts, C.T.; Reid, J.M.; Schmechel, S.; Voss, S.D.; Cho, S.Y.; et al. Phase I/II trial and pharmacokinetic study of cixutumumab in pediatric patients with refractory solid tumors and Ewing sarcoma: A report from the Children’s Oncology Group. J. Clin. Oncol. 2012, 30, 256–262. [Google Scholar] [CrossRef]
- Yu, E.Y.; Li, H.; Higano, C.S.; Agarwal, N.; Pal, S.K.; Alva, A.; Heath, E.I.; Lam, E.T.; Gupta, S.; Lilly, M.B.; et al. SWOG S0925: A Randomized Phase II Study of Androgen Deprivation Combined With Cixutumumab Versus Androgen Deprivation Alone in Patients With New Metastatic Hormone-Sensitive Prostate Cancer. Cli. Oncol. 2015, 33, 1601–1608. [Google Scholar] [CrossRef]
- Wang, Y.; Hailey, J.; Williams, D.; Lipari, P.; Malkowski, M.; Xie, L.; Li, G.; Saha, D.; Ling, W.L.W.; Cannon-Carlson, S.; et al. Inhibition of insulin-like growth factor-I receptor (IGF-IR) signaling and tumor cell growth by a fully human neutralizing anti-IGF-IR antibody. Mol. Cancer Ther. 2005, 4, 1214–1221. [Google Scholar] [CrossRef]
- Anderson, P.M.; Bielack, S.S.; Gorlick, R.G.; Skubitz, K.; Daw, N.C.; Herzog, C.E.; Monge, O.R.; Lassaletta, A.; Boldrini, E.; Pápai, Z.; et al. A phase II study of clinical activity of SCH 717454 (robatumumab) in patients with relapsed osteosarcoma and Ewing sarcoma. Pediatr. Blood Cancer 2016, 63, 1761–1770. [Google Scholar] [CrossRef]
- Fitzgerald, J.B.; Johnson, B.W.; Baum, J.; Adams, S.; Iadevaia, S.; Tang, J.; Rimkunas, V.; Xu, L.; Kohli, N.; Rennard, R.; et al. MM-141, an IGF-IR–and ErbB3-directed bispecific antibody, overcomes network adaptations that limit activity of IGF-IR inhibitors. Mol. Cancer Ther. 2014, 13, 410–425. [Google Scholar] [CrossRef]
- Kandasamy Hariharan, J.D.; Demarest, S.; Joseph, I.; Chu, P.; Graff, C.; Glaser, S.; Kramer-Stickland, K.; Peach, R.; Reff, M. BIIB022, a fully human nonglycosylated γ4P antibody targeting IGF-1R for cancer therapy. Mol Cancer Ther 2007, 6. [Google Scholar]
- Von Mehren, M.; Britten, C.D.; Pieslor, P.; Saville, W.; Vassos, A.; Harris, S.; Galluppi, G.R.; Darif, M.; Wainberg, Z.A.; Cohen, R.B.; et al. A phase 1, open-label, dose-escalation study of BIIB022 (anti-IGF-1R monoclonal antibody) in subjects with relapsed or refractory solid tumors. Investig. New Drugs 2014, 32, 518–525. [Google Scholar] [CrossRef]
- Gualberto, A.; Hixon, M.L.; Pollak, M. Reply: ‘Pre-treatment levels of circulating free IGF-1 identify NSCLC patients who derive clinical benefit from figitumumab’. Br. J. Cancer 2011, 105, 1467. [Google Scholar] [CrossRef][Green Version]
- Van Maldegem, A.M.; Bovee, J.V.; Peterse, E.F.; Hogendoorn, P.C.; Gelderblom, H. Ewing sarcoma: The clinical relevance of the insulin-like growth factor 1 and the poly-ADP-ribose-polymerase pathway. Eur. J. Cancer 2016, 53, 171–180. [Google Scholar] [CrossRef]
- Garcı́a-Echeverrı́a, C.; A. Pearson, M.; Marti, A.; Meyer, T.; Mestan, J.; Zimmermann, J.; Gao, J.; Brueggen, J.; Capraro, H.-G.; Cozens, R.; et al. In vivo antitumor activity of NVP-AEW541—A novel, potent, and selective inhibitor of the IGF-IR kinase. Cancer Cell 2004, 5, 231–239. [Google Scholar]
- Favelyukis, S.; Till, J.H.; Hubbard, S.; Miller, W.T. Structure and autoregulation of the insulin-like growth factor 1 receptor kinase. Nat. Genet. 2001, 8, 1058–1063. [Google Scholar]
- Mulvihill, M.J.; Cooke, A.; Rosenfeld-Franklin, M.; Buck, E.; Foreman, K.; Landfair, D.; O’Connor, M.; Pirritt, C.; Sun, Y.; Yao, Y.; et al. Discovery of OSI-906: A selective and orally efficacious dual inhibitor of the IGF-1 receptor and insulin receptor. Futur. Med. Chem. 2009, 1, 1153–1171. [Google Scholar] [CrossRef]
- Carboni, J.M.; Wittman, M.; Yang, Z.; Lee, F.; Greer, A.; Hurlburt, W.; Hillerman, S.; Cao, C.; Cantor, G.H.; Dell-John, J.; et al. BMS-754807, a small molecule inhibitor of insulin-like growth factor-1R/IR. Mol. Cancer Ther. 2009, 8, 3341–3349. [Google Scholar] [CrossRef]
- Weroha, S.J.; Haluska, P. IGF-1 Receptor Inhibitors in Clinical Trials—Early Lessons. J. Mammary Gland. Boil. Neoplasia 2008, 13, 471–483. [Google Scholar] [CrossRef]
- Leighl, N.B.; de Rizvi, N.A.; Lima, L.G., Jr.; Arpornwirat, W.; Rudin, C.M.; Chiappori, A.A.; Ahn, M.J.; Chow, L.Q.; Bazhenova, L.; Dechaphunkul, A. Phase 2 Study of Erlotinib in Combination With Linsitinib (OSI-906) or Placebo in Chemotherapy-Naive Patients With Non–Small-Cell Lung Cancer and Activating Epidermal Growth Factor Receptor Mutations. Clin. Lung Cancer 2017, 18, 34–42 e2. [Google Scholar] [CrossRef]
- Jones, R.L.; Kim, E.S.; Nava-Parada, P.; Alam, S.; Johnson, F.M.; Stephens, A.W.; Simantov, R.; Poondru, S.; Gedrich, R.; Lippman, S.M.; et al. Phase I study of intermittent oral dosing of the insulin-like growth factor-1 and insulin receptors inhibitor OSI-906 in patients with advanced solid tumors. Clin. Cancer Res. 2015, 21, 693–700. [Google Scholar] [CrossRef]
- Macaulay, V.M.; Middleton, M.R.; Eckhardt, S.G.; Rudin, C.M.; Juergens, R.A.; Gedrich, R.; Gogov, S.; McCarthy, S.; Poondru, S.; Stephens, A.W.; et al. Phase I Dose-Escalation Study of Linsitinib (OSI-906) and Erlotinib in Patients with Advanced Solid Tumors. Clin. Cancer Res. 2016, 22, 2897–2907. [Google Scholar] [CrossRef]
- Oza, A.; Kaye, S.; Van Tornout, J.; Sessa, C.; Gore, M.; Naumann, R.W.; Hirte, H.; Colombo, N.; Chen, J.; Gorla, S.; et al. Phase 2 study evaluating intermittent and continuous linsitinib and weekly paclitaxel in patients with recurrent platinum resistant ovarian epithelial cancer. Gynecol. Oncol. 2018, 149, 275–282. [Google Scholar] [CrossRef]
- Youngren, J.F.; Gable, K.; Penaranda, C.; Maddux, B.A.; Zavodovskaya, M.; Lobo, M.; Campbell, M.; Kerner, J.; Goldfine, I.D. Nordihydroguaiaretic Acid (NDGA) Inhibits the IGF-1 and c-erbB2/HER2/neu Receptors and Suppresses Growth in Breast Cancer Cells. Breast Cancer Res. Treat. 2005, 94, 37–46. [Google Scholar] [CrossRef]
- Cortes, J.; Paquette, R.; Talpaz, M.; Pinilla, J.; Asatiani, E.; Wetzler, M.; Lipton, J.H.; Kasap, C.; Bui, L.A.; Clary, D.O.; et al. Preliminary Clinical Activity in a Phase I Trial of the BCR-ABL/IGF- 1R/Aurora Kinase Inhibitor XL228 in Patients with Ph++ Leukemias with Either Failure to Multiple TKI Therapies or with T315I Mutation. Blood 2008, 112, 3232. [Google Scholar]
- Waraky, A.; Akopyan, K.; Parrow, V.; Strömberg, T.; Axelson, M.; Abrahmsén, L.; Lindqvist, A.; Larsson, O.; Aleem, E. Picropodophyllin causes mitotic arrest and catastrophe by depolymerizing microtubules via Insulin-like growth factor-1 receptor-independent mechanism. Oncotarget 2014, 5, 8379–8392. [Google Scholar] [CrossRef]
- Aiken, R.; Axelson, M.; Harmenberg, J.; Klockare, M.; Larsson, O.; Wassberg, C. Phase I clinical trial of AXL1717 for treatment of relapsed malignant astrocytomas: Analysis of dose and response. Oncotarget 2017, 8, 81501–81510. [Google Scholar] [CrossRef]
- Fassnacht, M.; Berruti, A.; Baudin, E.; Demeure, M.J.; Gilbert, J.; Haak, H.; Kroiss, M.I.; Quinn, D.; Hesseltine, E.; Ronchi, C.L.; et al. Linsitinib (OSI-906) versus placebo for patients with locally advanced or metastatic adrenocortical carcinoma: A double-blind, randomised, phase 3 study. Lancet Oncol. 2015, 16, 426–435. [Google Scholar] [CrossRef]
- Barata, P.; Cooney, M.; Tyler, A.; Wright, J.; Dreicer, R.; Garcia, J.A. A phase 2 study of OSI-906 (linsitinib, an insulin-like growth factor receptor-1 inhibitor) in patients with asymptomatic or mildly symptomatic (non-opioid requiring) metastatic castrate resistant prostate cancer (CRPC). Investig. New Drugs 2018, 36, 451–457. [Google Scholar] [CrossRef]
- Haluska, P.; Dhar, A.; Hou, X.; Huang, F.; Nuyten, D.S.A.; Park, J.; Brodie, A.H.; Ingle, J.N.; Carboni, J.M.; Gottardis, M.M.; et al. Phase II trial of the dual IGF-1R/IR inhibitor BMS-754807 with or without letrozole in aromatase inhibitor-resistant breast cancer. J. Clin. Oncol. 2011, 29, TPS111. [Google Scholar] [CrossRef]
- Toretsky, J.A.; Steinberg, S.M.; Thakar, M.; Counts, D.; Pironis, B.; Parente, C.; Eskenazi, A.; Helman, L.; Wexler, L.H. Insulin-like growth factor type 1 (IGF-1) and IGF binding protein-3 in patients with Ewing sarcoma family of tumors. Cancer 2001, 92, 2941–2947. [Google Scholar] [CrossRef]
- Girnita, L.; Del Prete, F.; Bartolazzi, A.; Larsson, O.; Axelson, M. Cyclolignans as Inhibitors of the Insulin-Like Growth Factor-1 Receptor and Malignant Cell Growth. Cancer Res. 2004, 64, 236–242. [Google Scholar] [CrossRef]
- Bergqvist, M.; Holgersson, G.; Bondarenko, I.; Grechanaya, E.; Maximovich, A.; Andor, G.; Klockare, M.; Thureson, M.; Jerling, M.; Harmenberg, J. Phase II randomized study of the IGF-1R pathway modulator AXL1717 compared to docetaxel in patients with previously treated, locally advanced or metastatic non-small cell lung cancer. Acta. Oncol. 2017, 56, 441–447. [Google Scholar] [CrossRef]
- Ryan, C.J.; Harzstark, A.H.; Rosenberg, J.; Lin, A.; Claros, C.; Goldfine, I.D.; Kerner, J.F.; Small, E.J. A pilot dose-escalation study of the effects of nordihydroguareacetic acid on hormone and prostate specific antigen levels in patients with relapsed prostate cancer. BJU Int. 2008, 101, 436–439. [Google Scholar] [CrossRef]
- Friedlander, T.W.; Weinberg, V.K.; Huang, Y.; Mi, J.T.; Formaker, C.G.; Small, E.J.; Harzstark, A.L.; Lin, A.M.; Fong, L.; Ryan, C.J. A phase II study of insulin-like growth factor receptor inhibition with nordihydroguaiaretic acid in men with non-metastatic hormone-sensitive prostate cancer. Oncol. Rep. 2012, 27, 3–9. [Google Scholar] [CrossRef][Green Version]
- Gao, J.; Chesebrough, J.W.; Cartlidge, S.A.; Ricketts, S.-A.; Incognito, L.; Veldman-Jones, M.; Blakey, D.C.; Tabrizi, M.; Jallal, B.; Trail, P.A.; et al. Dual IGF-I/II-Neutralizing Antibody MEDI-573 Potently Inhibits IGF Signaling and Tumor Growth. Cancer Res. 2011, 71, 1029–1040. [Google Scholar] [CrossRef]
- Haluska, P.; Menefee, M.; Plimack, E.R.; Rosenberg, J.; Northfelt, D.; Lavallee, T.; Shi, L.; Yu, X.-Q.; Burke, P.; Huang, J.; et al. Phase I Dose-Escalation Study of MEDI-573, a Bispecific, Antiligand Monoclonal Antibody against IGFI and IGFII, in Patients with Advanced Solid Tumors. Clin. Cancer Res. 2014, 20, 4747–4757. [Google Scholar] [CrossRef]
- Friedbichler, K.; Hofmann, M.H.; Kroez, M.; Ostermann, E.; Lamche, H.R.; Koessl, C.; Borges, E.; Pollak, M.N.; Adolf, G.; Adam, P.J. Pharmacodynamic and antineoplastic activity of BI 836845, a fully human IGF ligand-neutralizing antibody, and mechanistic rationale for combination with rapamycin. Mol. Cancer Ther. 2014, 13, 399–409. [Google Scholar] [CrossRef]
- Mireuta, M.; Birman, E.; Barmash, M.; Pollak, M. Quantification of Binding of IGF-1 to BI 836845, a Candidate Therapeutic Antibody Against IGF-1 and IGF-2, and Effects of This Antibody on IGF-1:IGFBP-3 Complexes In Vitro and in Male C57BL/6 Mice. Endocrinology 2014, 155, 703–715. [Google Scholar] [CrossRef]
- Hussain, S.A.; Maroto, P.; Climent, M.Á.; Bianchini, D.; Jones, R.H.; Lin, C.C.; Wang, S.S.; Dean, E.; Crossley, K.; Schlieker, L.; et al. Targeting IGF-1/2 with xentuzumab (Xe) plus enzalutamide (En) in metastatic castration-resistant prostate cancer (mCRPC) after progression on docetaxel chemotherapy (DCt) and abiraterone (Abi): Randomized phase II trial results. J. Clin. Oncol. 2019, 37, abstr–5030. [Google Scholar] [CrossRef]
- Crown, J.; Sablin, M.P.; Cortés, J.; Bergh, J.; Im, S.A.; Lu, Y.S.; Martínez, N.; Neven, P.; Lee, K.S.; Morales, S.; et al. Abstract P6-21-01: Xentuzumab (BI 836845), an insulin-like growth factor (IGF)-neutralizing antibody (Ab), combined with exemestane and everolimus in hormone receptor-positive (HR+) locally advanced/metastatic breast cancer (LA/mBC): Randomized phase 2 results. Cancer Res. 2019, 79, P6–21. [Google Scholar] [CrossRef]
- Zhang, X.H.-F.; Jin, X.; Malladi, S.; Zou, Y.; Wen, Y.H.; Brogi, E.; Smid, M.; Foekens, J.A.; Massagué, J. Selection of Bone Metastasis Seeds by Mesenchymal Signals in the Primary Tumor Stroma. Cell 2013, 154, 1060–1073. [Google Scholar] [CrossRef]
- Santarlasci, B.; Vaiani, M.; Messori, A. New Drugs for Rheumatoid Arthritis. New Engl. J. Med. 2004, 351, 937–938. [Google Scholar]
- Zhao, S.; Mysler, E.; Moots, R.J. Etanercept for the treatment of rheumatoid arthritis. Immunother 2018, 10, 433–445. [Google Scholar] [CrossRef]
- Holash, J.; Davis, S.; Papadopoulos, N.; Croll, S.D.; Ho, L.; Russell, M.; Boland, P.; Leidich, R.; Hylton, D.; Burova, E.; et al. VEGF-Trap: A VEGF blocker with potent antitumor effects. Proc. Natl. Acad. Sci. 2002, 99, 11393–11398. [Google Scholar] [CrossRef]
- Apte, R.S.; Chen, D.S.; Ferrara, N. VEGF in Signaling and Disease: Beyond Discovery and Development. Cell 2019, 176, 1248–1264. [Google Scholar] [CrossRef]
- Prince, S.N.; Foulstone, E.J.; Zaccheo, O.J.; Williams, C.; Hassan, A.B. Functional evaluation of novel soluble insulin-like growth factor (IGF)-II-specific ligand traps based on modified domain 11 of the human IGF2 receptor. Mol. Cancer Ther. 2007, 6, 607–617. [Google Scholar] [CrossRef]
- Frago, S.; Nicholls, R.D.; Strickland, M.; Hughes, J.; Williams, C.; Garner, L.; Surakhy, M.; MacLean, R.; Rezgui, D.; Prince, S.N.; et al. Functional evolution of IGF2:IGF2R domain 11 binding generates novel structural interactions and a specific IGF2 antagonist. Proc. Natl. Acad. Sci. 2016, 113, E2766–E2775. [Google Scholar] [CrossRef]
- Wang, N.; Rayes, R.F.; Elahi, S.M.; Lu, Y.; Hancock, M.A.; Massie, B.; Rowe, G.E.; Aomari, H.; Hossain, S.; Durocher, Y.; et al. The IGF-Trap: Novel Inhibitor of Carcinoma Growth and Metastasis. Mol. Cancer Ther. 2015, 14, 982–993. [Google Scholar] [CrossRef]
- Vaniotis, G.; Moffett, S.; Sulea, T.; Wang, N.; Elahi, S.M.; Lessard, E.; Baardsnes, J.; Perrino, S.; Durocher, Y.; Frystyk, J.; et al. Enhanced anti-metastatic bioactivity of an IGF-TRAP re-engineered to improve physicochemical properties. Sci. Rep. 2018, 8, 17361. [Google Scholar] [CrossRef]
- Lin, M.Z.; Marzec, K.A.; Martin, J.L.; Baxter, R.C. The role of insulin-like growth factor binding protein-3 in the breast cancer cell response to DNA-damaging agents. Oncogene 2014, 33, 85–96. [Google Scholar] [CrossRef]
- de Martin, J.L.; Silva, H.C.; Lin, M.Z.; Scott, C.D.; Baxter, R.C. Inhibition of insulin-like growth factor-binding protein-3 signaling through sphingosine kinase-1 sensitizes triple-negative breast cancer cells to EGF receptor blockade. Mol. Cancer Ther. 2014, 13, 316–328. [Google Scholar] [CrossRef]
- Jerome, L.; Alami, N.; Belanger, S.; Page, V.; Yu, Q.; Paterson, J.; Shiry, L.; Pegram, M.; Leyland-Jones, B. Recombinant Human Insulin-like Growth Factor Binding Protein 3 Inhibits Growth of Human Epidermal Growth Factor Receptor-2–Overexpressing Breast Tumors and Potentiates Herceptin Activity In vivo. Cancer Res. 2006, 66, 7245–7252. [Google Scholar] [CrossRef]
- Conover, C.A. Key Questions and Answers about Pregnancy-Associated Plasma Protein-A. Trends Endocrinol. Metab. 2012, 23, 242–249. [Google Scholar] [CrossRef]
- Becker, M.A.; Haluska, P.; Bale, L.K.; Oxvig, C.; Conover, C.A. A Novel Neutralizing Antibody Targeting Pregnancy-Associated Plasma Protein-A Inhibits Ovarian Cancer Growth and Ascites Accumulation in Patient Mouse Tumorgrafts. Mol. Cancer Ther. 2015, 14, 973–981. [Google Scholar] [CrossRef]
- Conover, C.A.; Bale, L.K.; Mader, J.R.; Mason, M.A.; Keenan, K.P.; Marler, R.J. Longevity and Age-Related Pathology of Mice Deficient in Pregnancy-Associated Plasma Protein-A. J Gerontol. Ser. A. 2010, 65, 590–599. [Google Scholar] [CrossRef]
- Conover, C.A. PAPP-A: A New Anti-Aging Target? Aging Cell 2010, 9, 942–946. [Google Scholar] [CrossRef]
- Mondal, S.; Bandyopadhyay, S.; Ghosh, M.K.; Mukhopadhyay, S.; Roy, S.; Mandal, C. Natural products: Promising resources for cancer drug discovery. Anti-Cancer Agents Med. Chem. 2012, 12, 49–75. [Google Scholar] [CrossRef]
- Sultana, N. Clinically useful anticancer, antitumor, and antiwrinkle agent, ursolic acid and related derivatives as medicinally important natural product. J. Enzym. Inhib. Med. Chem. 2011, 26, 616–642. [Google Scholar] [CrossRef]
- Amin, A.R.; Karpowicz, P.A.; Carey, T.E.; Arbiser, J.; Nahta, R.; Chen, Z.G.; Dong, J.T.; Kucuk, O.; Khan, G.N.; Huang, G.S.; et al. Evasion of anti-growth signaling: A key step in tumorigenesis and potential target for treatment and prophylaxis by natural compounds. Semin. Cancer Biol. 2015. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, T. Targeting cancer stem cells by curcumin and clinical applications. Cancer Lett. 2014, 346, 197–205. [Google Scholar] [CrossRef]
- Babcook, M.A.; Gupta, S. Apigenin Modulates Insulin-like Growth Factor Axis: Implications for Prevention and Therapy of Prostate Cancer. Curr. Drug Targets 2012.
- Jung, M.; Bu, S.Y.; Tak, K.-H.; Park, J.-E.; Kim, E. Anticarcinogenic effect of quercetin by inhibition of insulin-like growth factor (IGF)-1 signaling in mouse skin cancer. Nutr. Res. Pract. 2013, 7, 439–445. [Google Scholar] [CrossRef]
- Kim, H.; Park, J.; Tak, K.H.; Bu, S.Y.; Kim, E. Chemopreventive effects of curcumin on chemically induced mouse skin carcinogenesis in BK5.insulin-like growth factor-1 transgenic mice. In Vitro Cell Dev. Biol. Anim. 2014, 50, 883–892. [Google Scholar] [CrossRef]
- Hosseini, S.A.; Zand, H.; Cheraghpour, M. The Influence of Curcumin on the Downregulation of MYC, Insulin and IGF-1 Receptors: A Possible Mechanism Underlying the Anti-Growth and Anti-Migration in Chemoresistant Colorectal Cancer Cells. Medicine 2019, 55, 90. [Google Scholar] [CrossRef]
- DeMichele, A.; Yee, D.; Berry, D.A.; Albain, K.S.; Benz, C.C.; Boughey, J.; Buxton, M.; Chia, S.K.; Chien, A.J.; Chui, S.Y.; et al. The Neoadjuvant Model Is Still the Future for Drug Development in Breast Cancer. Clin. Cancer Res. 2015, 21, 2911–2915. [Google Scholar] [CrossRef]
- Lopez, J.S.; Banerji, U. Combine and conquer: Challenges for targeted therapy combinations in early phase trials. Nat. Rev. Clin. Oncol. 2017, 14, 57–66. [Google Scholar] [CrossRef]
- Zeng, X.; Sachdev, D.; Zhang, H.; Gaillard-Kelly, M.; Yee, D. Sequencing of type I insulin-like growth factor receptor inhibition affects chemotherapy response in vitro and in vivo. Clin. Cancer Res. 2009, 15, 2840–2849. [Google Scholar] [CrossRef]
- Ramcharan, R.; Aleksic, T.; Kamdoum, W.P.; Gao, S.; Pfister, S.X.; Tanner, J.; Bridges, E.; Asher, R.; Watson, A.J.; Margison, G.P.; et al. IGF-1R inhibition induces schedule-dependent sensitization of human melanoma to temozolomide. Oncotarget 2015, 6, 39877–39890. [Google Scholar] [CrossRef]
- Baserga, R. The decline and fall of the IGF-I receptor. J Cell Physiol 2013, 228, 675–679. [Google Scholar] [CrossRef]
- Olmos, D.; Postel-Vinay, S.; Molife, L.R.; Okuno, S.H.; Schuetze, S.M.; Paccagnella, M.L.; Batzel, G.N.; Yin, D.; Pritchard-Jones, K.; Judson, I.; et al. Safety, pharmacokinetics, and preliminary activity of the anti-IGF-1R antibody figitumumab (CP-751,871) in patients with sarcoma and Ewing’s sarcoma: A phase 1 expansion cohort study. Lancet Oncol. 2010, 11, 11–129. [Google Scholar] [CrossRef]
- Aleksic, T.; Browning, L.; Woodward, M.; Phillips, R.; Page, S.; Henderson, S.; Athanasou, N.; Ansorge, O.; Whitwell, D.; Pratap, S.; et al. Durable Response of Spinal Chordoma to Combined Inhibition of IGF-1R and EGFR. Front. Oncol. 2016, 6, 49. [Google Scholar] [CrossRef]
- Sanderson, M.P.; Hofmann, M.H.; Garin-Chesa, P.; Schweifer, N.; Wernitznig, A.; Fischer, S.; Jeschko, A.; Meyer, R.; Moll, J.; Pecina, T.; et al. The IGF1R/INSR Inhibitor BI 885578 Selectively Inhibits Growth of IGF2-Overexpressing Colorectal Cancer Tumors and Potentiates the Efficacy of Anti-VEGF Therapy. Mol. Cancer Ther. 2017, 16, 2223–2233. [Google Scholar] [CrossRef]
- Lee, H.; Kim, N.; Yoo, Y.J.; Kim, H.; Jeong, E.; Choi, S.; Moon, S.U.; Oh, S.H.; Mills, G.B.; Yoon, S.; et al. β-catenin/TCF activity regulates IGF-1R tyrosine kinase inhibitor sensitivity in colon cancer. Oncogene 2018, 37, 5466–5475. [Google Scholar] [CrossRef]
- Wu, J.; Chen, K.; Zhang, F.; Jin, J.; Zhang, N.; Li, D.; Ying, L.; Chen, W.; Yu, H.; Mao, W.; et al. Overcoming Linsitinib intrinsic resistance through inhibition of nuclear factor-κB signaling in esophageal squamous cell carcinoma. Cancer Med. 2017, 6, 1353–1361. [Google Scholar] [CrossRef]
- Chen, H.; Mester, T.; Raychaudhuri, N.; Kauh, C.Y.; Gupta, S.; Smith, T.J.; Douglas, R.S. Teprotumumab, an IGF-1R Blocking Monoclonal Antibody Inhibits TSH and IGF-1 Action in Fibrocytes. J. Clin. Endocrinol. Metab. 2014, 99, E1635–E1640. [Google Scholar] [CrossRef]
- Smith, T.J.; Kahaly, G.J.; Ezra, D.G.; Fleming, J.C.; Dailey, R.A.; Tang, R.A.; Harris, G.J.; Antonelli, A.; Salvi, M.; Goldberg, R.A.; et al. Teprotumumab for Thyroid-Associated Ophthalmopathy. New Engl. J. Med. 2017, 376, 1748–1761. [Google Scholar] [CrossRef]
- Smith, T.J.; Hegedus, L.; Douglas, R.S. Role of insulin-like growth factor-1 (IGF-1) pathway in the pathogenesis of Graves’ orbitopathy. Best Pract. Res. Clin. Endocrinol. Metab. 2012, 26, 291–302. [Google Scholar] [CrossRef]
- Longo, V.D.; Antebi, A.; Bartke, A.; Barzilai, N.; Brown-Borg, H.M.; Caruso, C.; Curiel, T.J.; De Cabo, R.; Franceschi, C.; Gems, D.; et al. Interventions to Slow Aging in Humans: Are We Ready? Aging Cell 2015, 14, 497–510. [Google Scholar] [CrossRef]
- Mao, K.; Quipildor, G.F.; Tabrizian, T.; Novaj, A.; Guan, F.; Walters, R.O.; Delahaye, F.; Hubbard, G.B.; Ikeno, Y.; Ejima, K.; et al. Late-life targeting of the IGF-1 receptor improves healthspan and lifespan in female mice. Nat. Commun. 2018, 9, 2394. [Google Scholar] [CrossRef]
| Antibody | Humanized | Class | Potency (IC50) IGF-1R | Potency (IC50) INSR | Clinical Trial Phase | References |
|---|---|---|---|---|---|---|
| Figitumumab (CP-751,871) | Fully human | IgG2a | 1.8 nM | N/A | III | [152,153,154,155,156] |
| Ganitumab (AMG 479) | Fully human | IgG1 | 2 nM | >50 nM | III | [157,158,159] |
| Teprotumumab (R1507) | Fully human | IgG1 | 0.5 nM | N/A | II | [160,161,162,163] |
| Dalotuzumab (MK-0646) | Humanized | IgG1 | 1 nM | N/A | III | [164,165,166,167] |
| Cixutumumab (IMC-A12) | Fully human | IgG1 | 0.6–1 nM | N/A | II | [168,169,170,171] |
| Robatumumab (SCH 717454) | Fully human | IgG1 | 2.7 nM | N/A | II | [172,173] |
| Istiratumab (MM-141) | Engineered human | IgG1 with 2 scFvs | 2 nM | Bispecific IGF-1R/ErbB3 | II | [174] |
| BIIB022 | Fully human | IgG4 | <10 nM | N/A | I | [175,176] |
| Drug Name | Mode of Inhibition | Potency (IC50) IGF-1R INSR | Additional Targets | Clinical Trial Phase | References | |
|---|---|---|---|---|---|---|
| Linsitinib (OSI-906) | ATP competitive | 35 nM | 75 nM | N/A | III | [145,181,184,185,186,187,192,193] |
| BMS-754807 | ATP competitive, | 1.8 nM | 1.7 nM | MET, RON, TrkA/B, AurA/B | II | [182,194] |
| XL-228 | ATP competitive | 1.6 nM | N/A | BCR-ABL, AurA, SRC, LYN | I | [189,195] |
| AXL1717 (Picropodophyllin) | Non-ATP competitive | 40 nM | N/A | Microtubules | II | [190,191,196,197] |
| Masoprocol (INSM-18, nordihydroguaiaretic acid) | Non-ATP competitive natural product of Larrea divaricata | 31 µM | N/A | HER2 | II | [188,198,199] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osher, E.; Macaulay, V.M. Therapeutic Targeting of the IGF Axis. Cells 2019, 8, 895. https://doi.org/10.3390/cells8080895
Osher E, Macaulay VM. Therapeutic Targeting of the IGF Axis. Cells. 2019; 8(8):895. https://doi.org/10.3390/cells8080895
Chicago/Turabian StyleOsher, Eliot, and Valentine M. Macaulay. 2019. "Therapeutic Targeting of the IGF Axis" Cells 8, no. 8: 895. https://doi.org/10.3390/cells8080895
APA StyleOsher, E., & Macaulay, V. M. (2019). Therapeutic Targeting of the IGF Axis. Cells, 8(8), 895. https://doi.org/10.3390/cells8080895




