Size Dependency of Circulation and Biodistribution of Biomimetic Nanoparticles: Red Blood Cell Membrane-Coated Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. RBC Ghost Deviation
2.3. Preparation of RBC-NPs with Different-Sized Polymeric Cores
2.4. Characterization of RBC-NPs
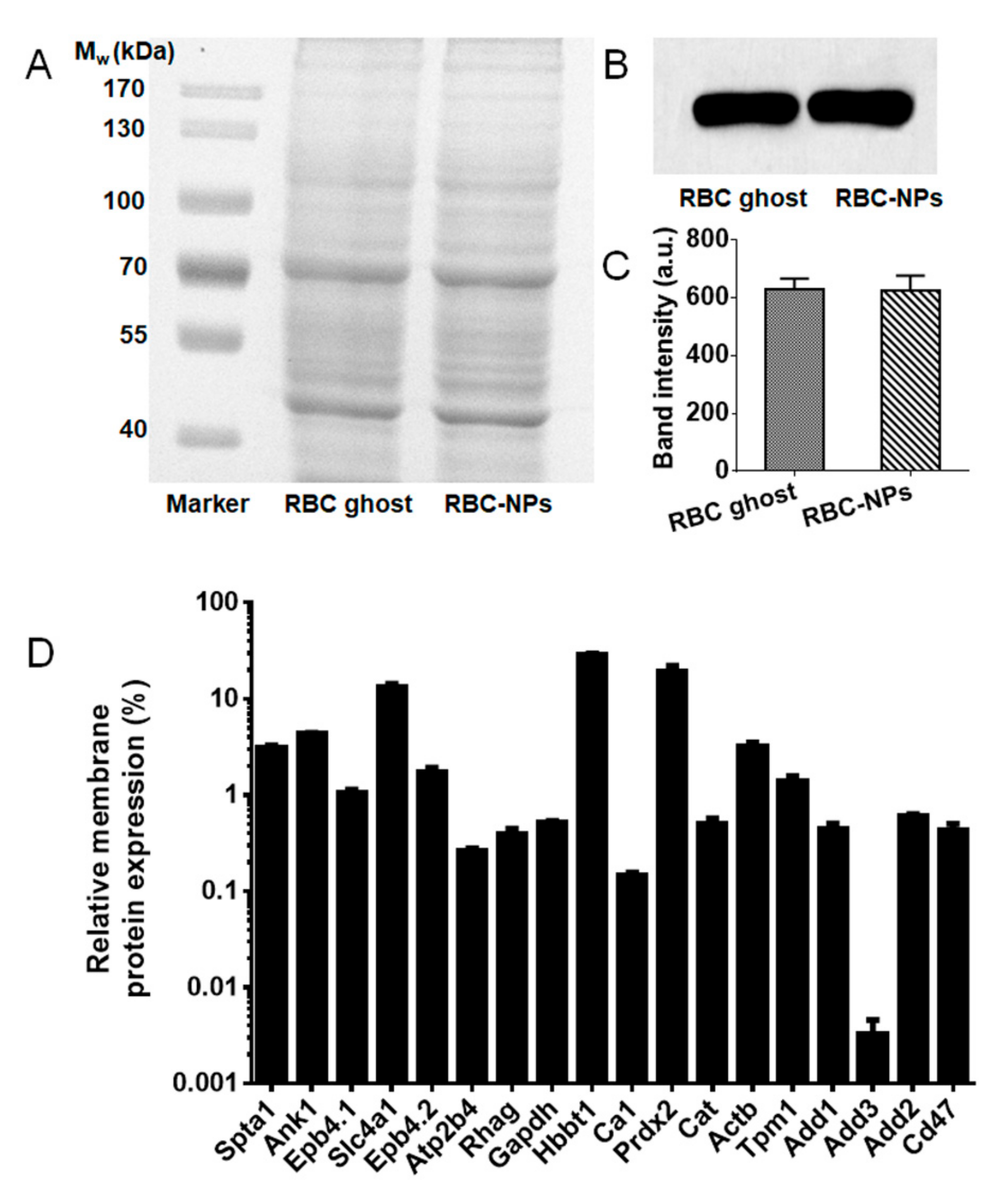
2.5. Membrane Protein Analysis of RBC-NPs
2.6. Cellular Uptake RBC-NPs by Macrophages
2.7. Filtration Test of RBC-NPs
2.8. Pharmacokinetics and Biodistribution of RBC-NPs
2.9. Immunogenic Response
2.10. Statistical Analysis
3. Results and Discussions
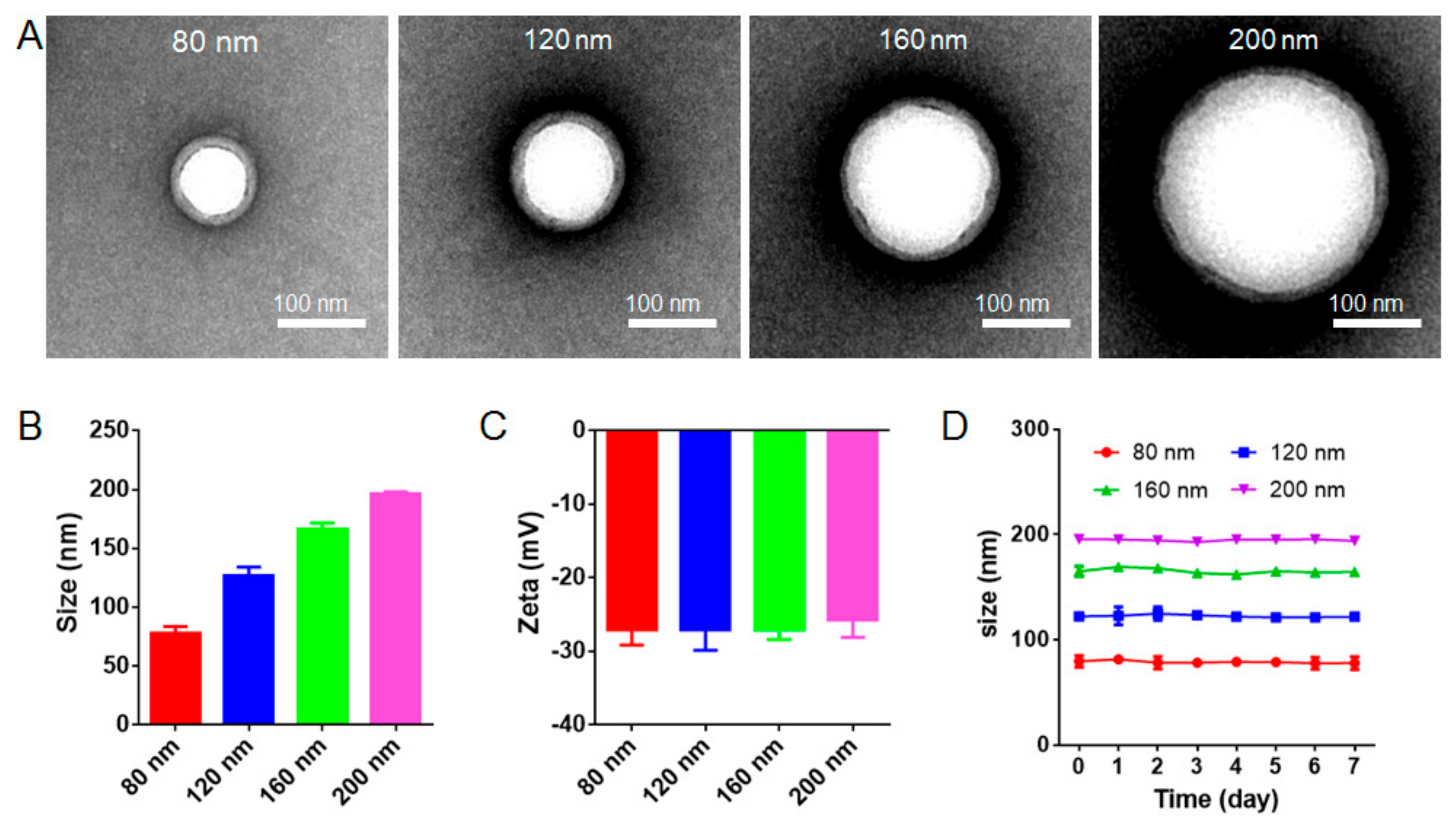
3.1. Characterization and Stability of RBC-NPs
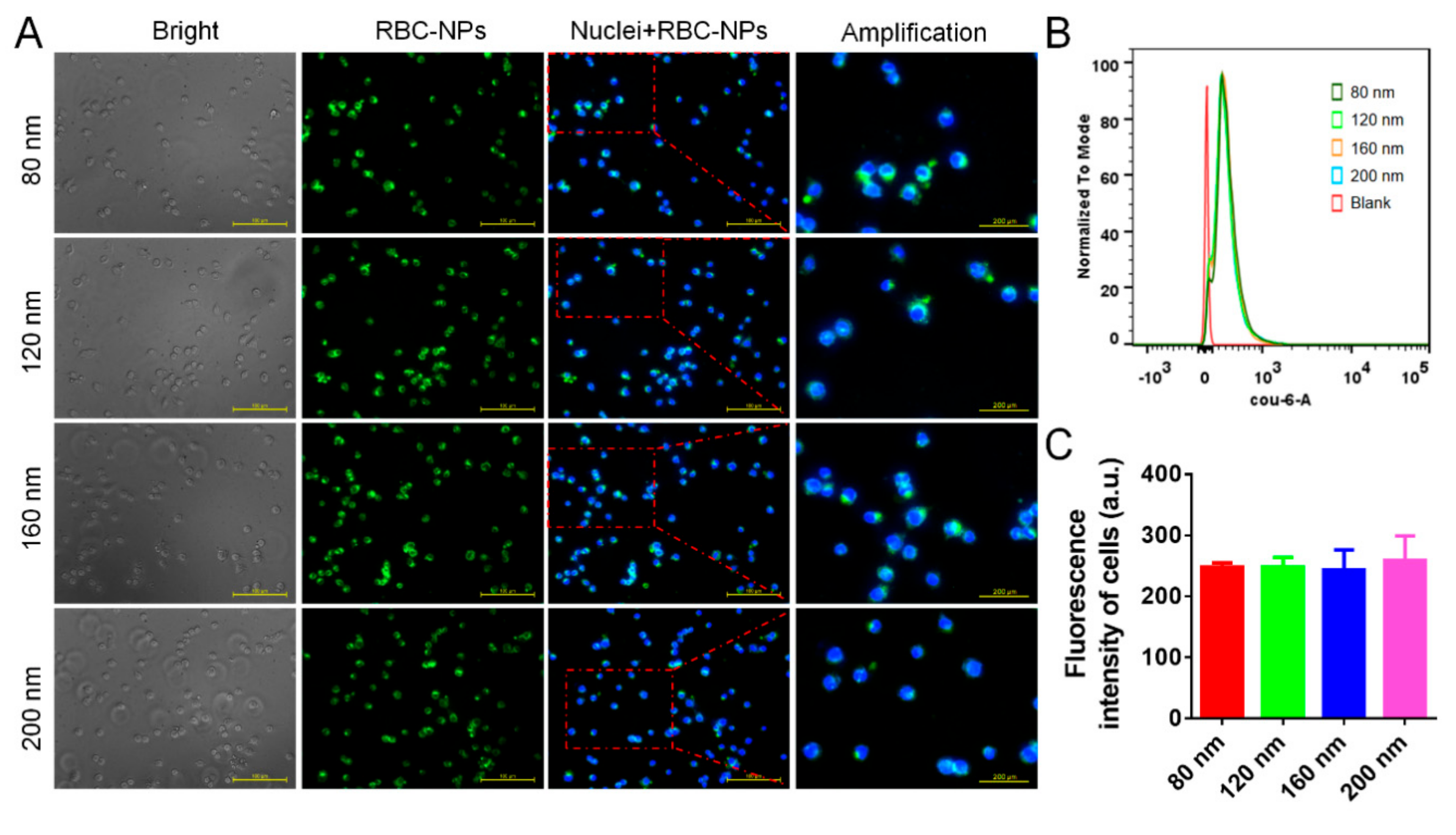
3.2. Cellular Uptake of RBC-NPs by Macrophages
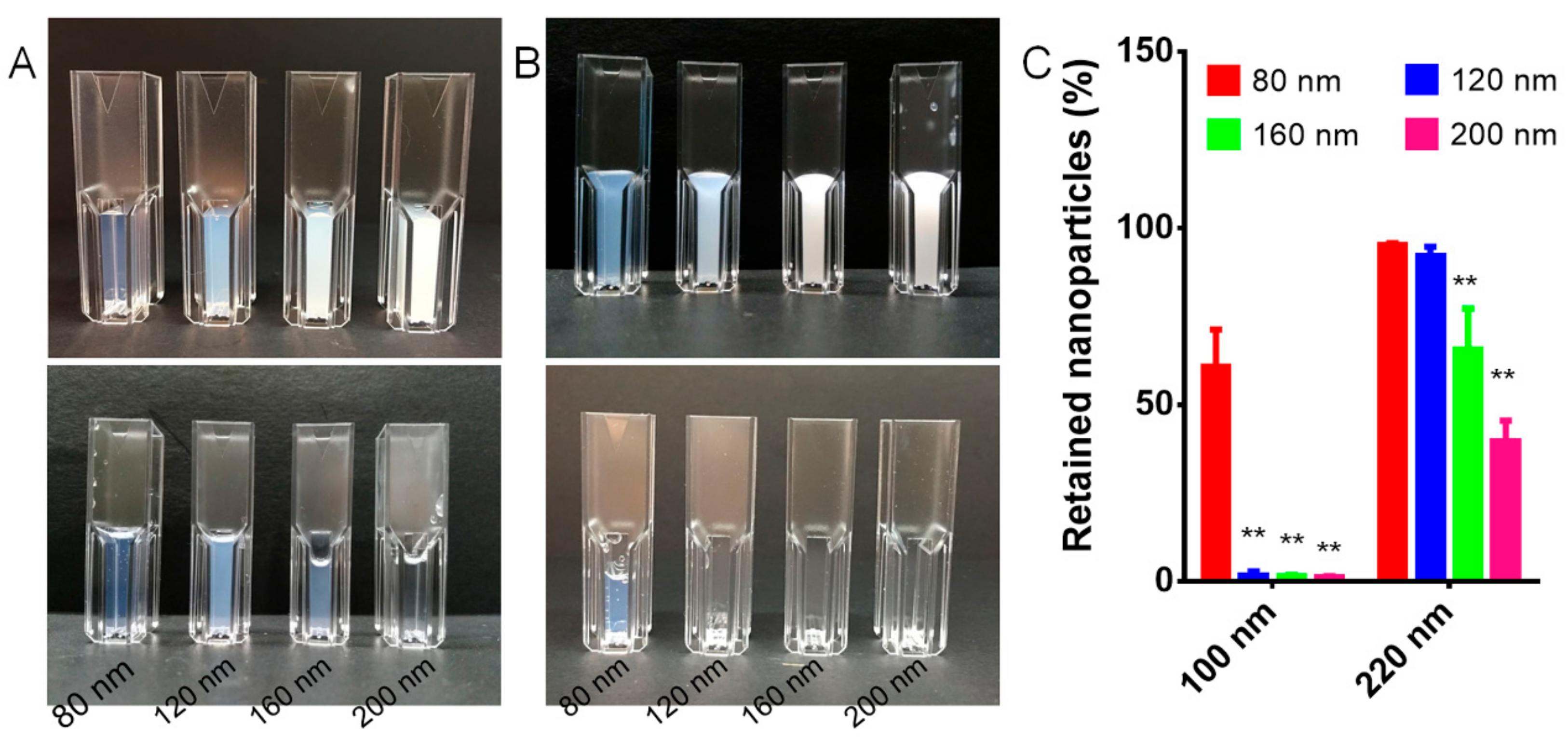
3.3. Filtration through Filter Membranes
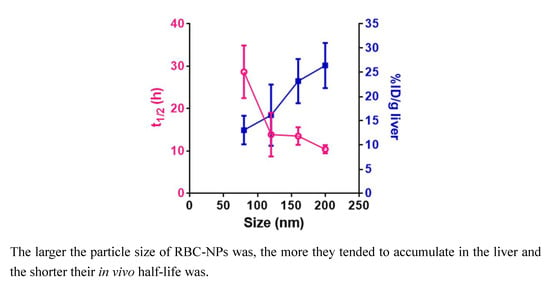
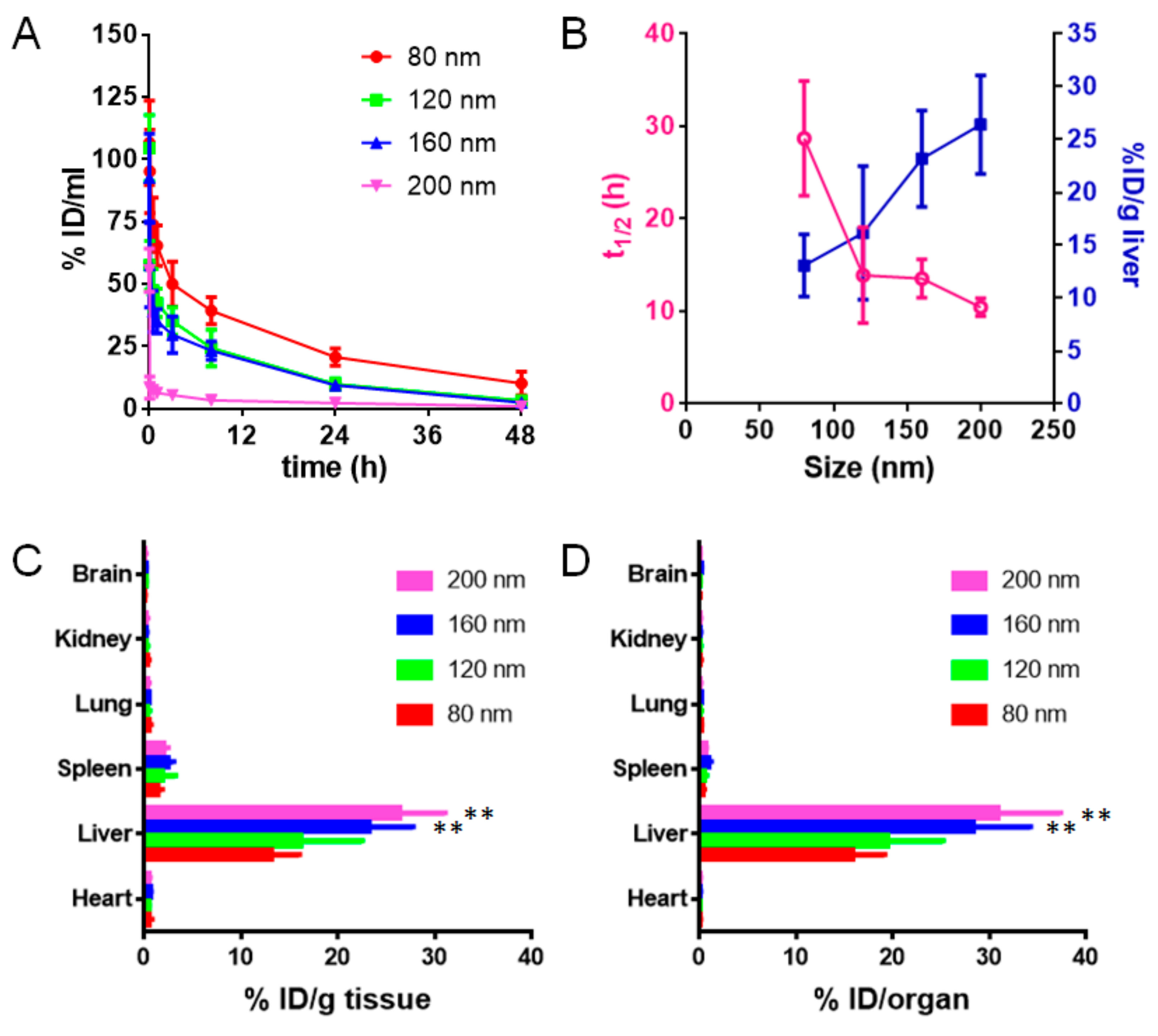
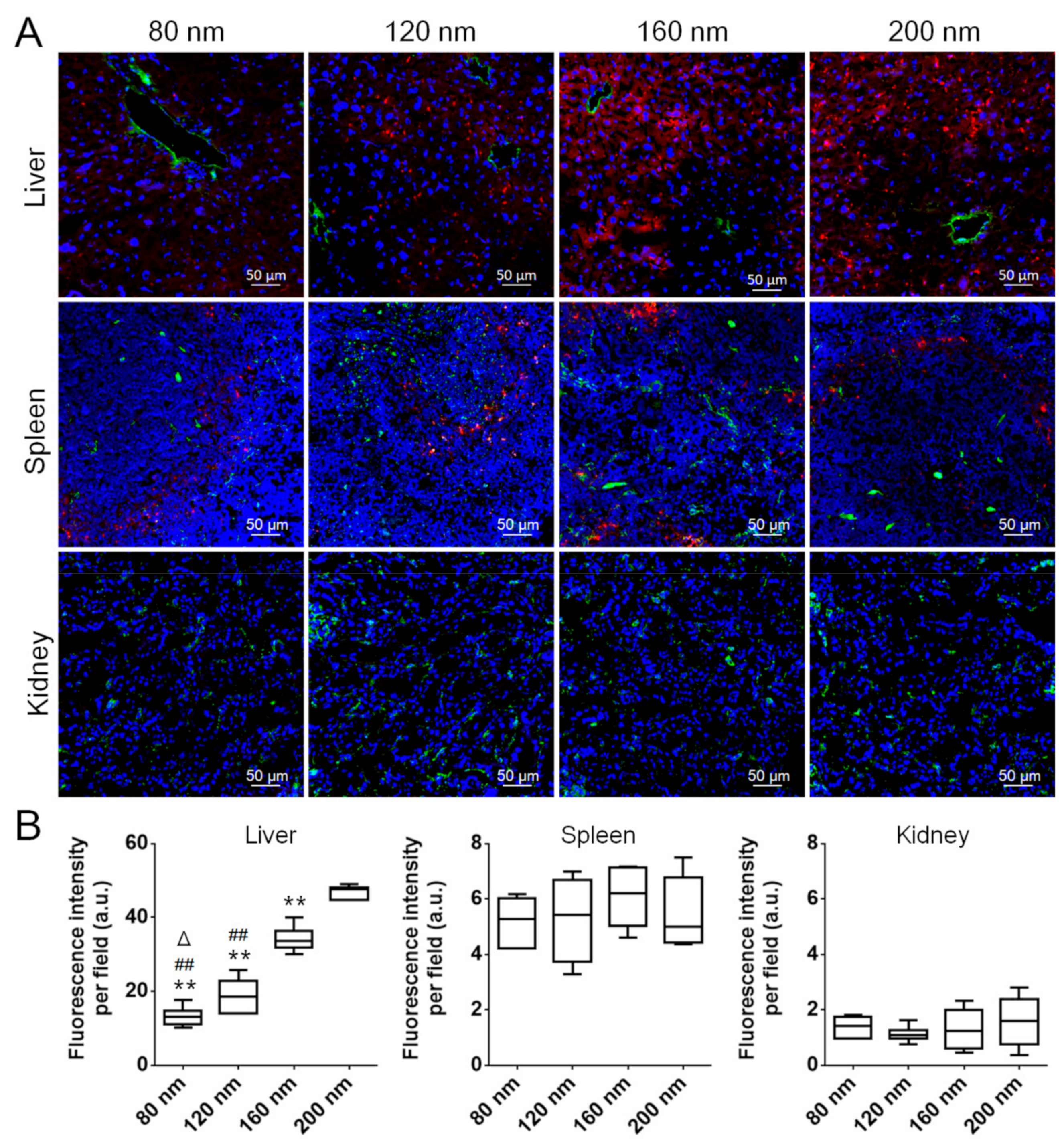
3.4. Pharmacokinetics and Biodistribution
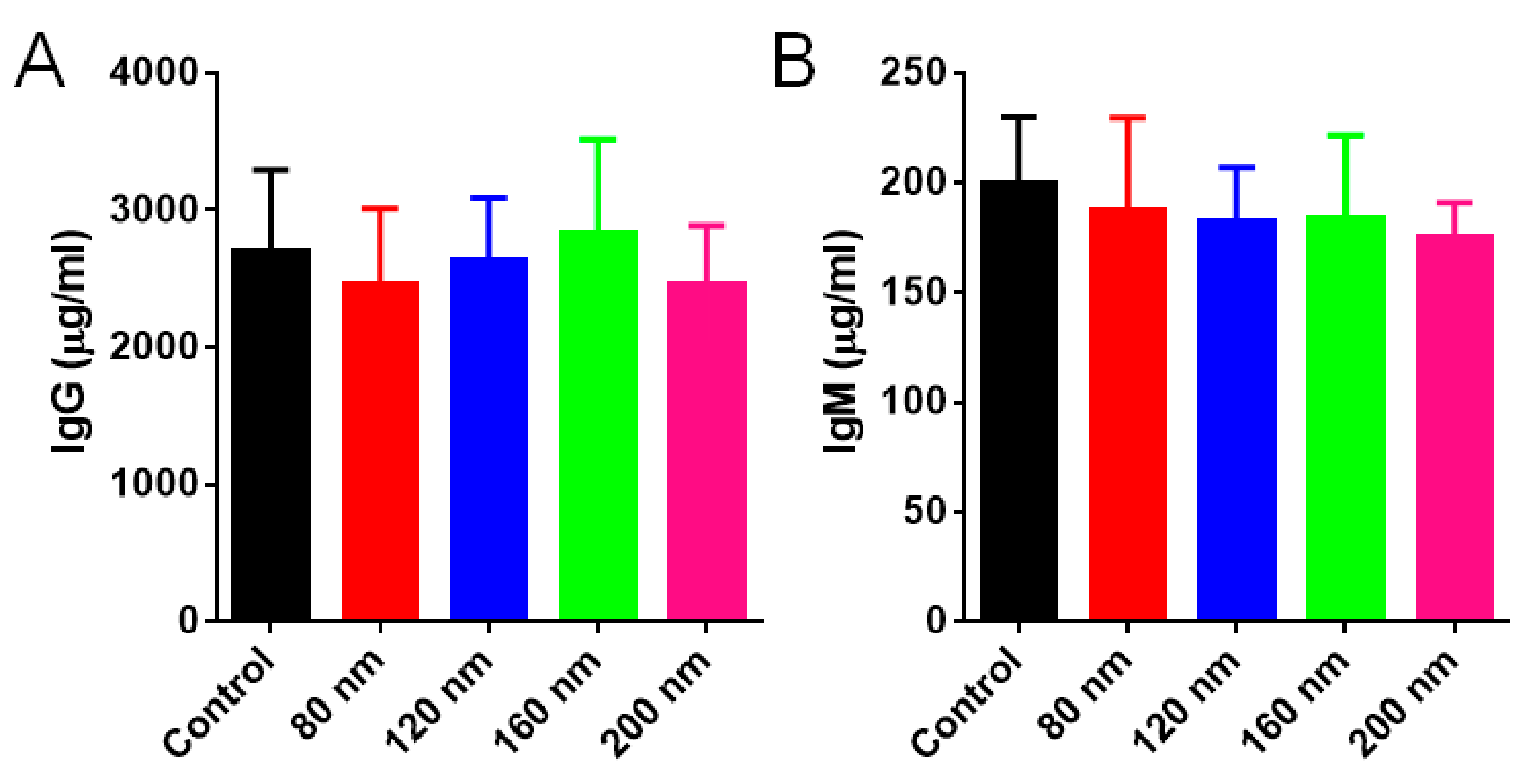
3.5. Immunogenic Response
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Li, S.D.; Huang, L. Pharmacokinetics and Biodistribution of Nanoparticles. Mol. Pharm. 2008, 5, 496. [Google Scholar] [CrossRef] [PubMed]
- Abu Lila, A.S.; Kiwada, H.; Ishida, T. The accelerated blood clearance (ABC) phenomenon: Clinical challenge and approaches to manage. J. Control. Release. Off. J. Control. Release Soc. 2013, 172, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, S.; Tavares, A.J.; Dai, Q.; Ohta, S.; Audet, J.; Dvorak, H.F.; Chan, W.C.W. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 2016, 1, 16014. [Google Scholar] [CrossRef]
- Maroof, H.; Islam, F.; Dong, L.; Ajjikuttira, P.; Gopalan, V.; McMillan, N.A.J.; Lam, A.K. Liposomal Delivery of miR-34b-5p Induced Cancer Cell Death in Thyroid Carcinoma. Cells 2018, 7, 265. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, N.; Grenier, P.; Mahmoudi, M.; Lima, E.M.; Appel, E.A.; Dormont, F.; Lim, J.M.; Karnik, R.; Langer, R.; Farokhzad, O.C. Mechanistic understanding of in vivo protein corona formation on polymeric nanoparticles and impact on pharmacokinetics. Nat. Commun. 2017, 8, 777. [Google Scholar] [CrossRef]
- Elward, K.; Gasque, P. “Eat me” and “don’t eat me” signals govern the innate immune response and tissue repair in the CNS: Emphasis on the critical role of the complement system. Mol. Immunol. 2003, 40, 85–94. [Google Scholar] [CrossRef]
- Hu, C.M.; Zhang, L.; Aryal, S.; Cheung, C.; Fang, R.H. Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proc. Natl. Acad Sci. USA 2011, 108, 10980–10985. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Shen, Q.; Huang, K.; Zheng, T.; Cheng, L.; Zhang, Z.; Yu, Y.; Liao, G.; Wang, X.; Li, C. Oriented Assembly of Cell-Mimicking Nanoparticles via a Molecular Affinity Strategy for Targeted Drug Delivery. Acs Nano 2019, 13, 5268–5277. [Google Scholar] [CrossRef]
- Rao, L.; Cai, B.; Bu, L.L.; Liao, Q.Q.; Guo, S.S.; Zhao, X.Z.; Dong, W.F.; Liu, W. Microfluidic Electroporation-Facilitated Synthesis of Erythrocyte Membrane-Coated Magnetic Nanoparticles for Enhanced Imaging-Guided Cancer Therapy. Acs Nano 2017, 11, 3496–3505. [Google Scholar] [CrossRef]
- Piao, J.G.; Wang, L.; Gao, F.; You, Y.Z.; Xiong, Y.; Yang, L. Erythrocyte membrane is an alternative coating to polyethylene glycol for prolonging the circulation lifetime of gold nanocages for photothermal therapy. Acs Nano 2014, 8, 10414–10425. [Google Scholar] [CrossRef]
- Jiang, Q.; Luo, Z.; Men, Y.; Yang, P.; Peng, H.; Guo, R.; Tian, Y.; Pang, Z.; Yang, W. Red blood cell membrane-camouflaged melanin nanoparticles for enhanced photothermal therapy. Biomaterials 2017, 143, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Zhang, B.; Shen, S.; Tuo, Y.; Luo, Z.; Hu, Y.; Pang, Z.; Jiang, X. Tumor Microenvironment Modulation by Cyclopamine Improved Photothermal Therapy of Biomimetic Gold Nanorods for Pancreatic Ductal Adenocarcinomas. Acs Appl. Mater. Interfaces 2017, 9, 31497–31508. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, H.; Liu, X.; Tian, Y.; Guo, H.; Jiang, T.; Luo, Z.; Jin, K.; Kuai, X.; Liu, Y.; et al. Enhanced photothermal therapy of biomimetic polypyrrole nanoparticles through improving blood flow perfusion. Biomater. 2017, 143, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Jin, K.; Luo, Z.; Zhang, B.; Pang, Z. Biomimetic nanoparticles for inflammation targeting. Acta Pharm. Sinica. B 2018, 8, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; He, Y.; Zhu, Y.; Jiang, L.; Zhang, S.; Qin, J.; Wu, Q.; Dai, W.; Shen, S.; Pang, Z.; et al. Route to Rheumatoid Arthritis by Macrophagederived Microvesicle-Coated Nanoparticles. Nano Lett. 2019, 19, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Xuan, M.; Shao, J.; Dai, L.; Li, J.; He, Q. Macrophage Cell Membrane Camouflaged Au Nanoshells for In Vivo Prolonged Circulation Life and Enhanced Cancer Photothermal Therapy. Acs Appl. Mater. Interfaces 2016, 8, 9610–9618. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cai, K.; Li, C.; Guo, Q.; Chen, Q.; He, X.; Liu, L.; Zhang, Y.; Lu, Y.; Chen, X.; et al. Macrophage-Membrane-Coated Nanoparticles for Tumor-Targeted Chemotherapy. Nano Lett. 2018, 18, 1908–1915. [Google Scholar] [CrossRef]
- Chen, Z.; Zhao, P.; Luo, Z.; Zheng, M.; Tian, H.; Gong, P.; Gao, G.; Pan, H.; Liu, L.; Ma, A.; et al. Cancer Cell Membrane-Biomimetic Nanoparticles for Homologous-Targeting Dual-Modal Imaging and Photothermal Therapy. Acs Nano 2016, 10, 10049–10057. [Google Scholar] [CrossRef]
- Rao, L.; Bu, L.L.; Cai, B.; Xu, J.H.; Li, A.; Zhang, W.F.; Sun, Z.J.; Guo, S.S.; Liu, W.; Wang, T.H.; et al. Cancer Cell Membrane-Coated Upconversion Nanoprobes for Highly Specific Tumor Imaging. Adv. Mater. (Deerfield Beachfla) 2016, 28, 3460–3466. [Google Scholar] [CrossRef]
- Fang, R.H.; Hu, C.M.; Luk, B.T.; Gao, W.; Copp, J.A.; Tai, Y.; O’Connor, D.E.; Zhang, L. Cancer cell membrane-coated nanoparticles for anticancer vaccination and drug delivery. Nano Lett. 2014, 14, 2181–2188. [Google Scholar] [CrossRef]
- Hu, C.M.; Fang, R.H.; Wang, K.C.; Luk, B.T.; Thamphiwatana, S.; Dehaini, D.; Nguyen, P.; Angsantikul, P.; Wen, C.H.; Kroll, A.V.; et al. Nanoparticle biointerfacing by platelet membrane cloaking. Natural 2015, 526, 118–121. [Google Scholar] [CrossRef]
- He, Y.; Li, R.; Liang, J.; Zhu, Y.; Zhang, S.; Zheng, Z.; Qin, J.; Pang, Z.; Wang, J. Drug targeting through platelet membrane-coated nanoparticles for the treatment of rheumatoid arthritis. Nano Res. 2018, 11, 6086–6101. [Google Scholar] [CrossRef]
- Song, Y.; Huang, Z.; Liu, X.; Pang, Z.; Chen, J.; Yang, H.; Zhang, N.; Cao, Z.; Liu, M.; Cao, J.; et al. Platelet membrane-coated nanoparticle-mediated targeting delivery of Rapamycin blocks atherosclerotic plaque development and stabilizes plaque in apolipoprotein E-deficient (ApoE(-/-)) mice. Nanomedicine 2019, 15, 13–24. [Google Scholar] [CrossRef]
- Wei, X.; Ying, M.; Dehaini, D.; Su, Y.; Kroll, A.V.; Zhou, J.; Gao, W.; Fang, R.H.; Chien, S.; Zhang, L. Nanoparticle Functionalization with Platelet Membrane Enables Multifactored Biological Targeting and Detection of Atherosclerosis. Acs Nano 2018, 12, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Sun, W.; Qian, C.; Bomba, H.N.; Xin, H.; Gu, Z. Relay Drug Delivery for Amplifying Targeting Signal and Enhancing Anticancer Efficacy. Adv. Mater. (Deerfield Beachfla) 2017, 29, 3. [Google Scholar] [CrossRef]
- Copp, J.A.; Fang, R.H.; Luk, B.T.; Hu, C.M.; Gao, W.; Zhang, K.; Zhang, L. Clearance of pathological antibodies using biomimetic nanoparticles. Proc. Natl. Acad Sci. USA 2014, 111, 13481–13486. [Google Scholar] [CrossRef]
- Fang, R.H.; Hu, B.T.; Hu, C.M.; Zhang, L. Engineered nanoparticles mimicking cell membranes for toxin neutralization. Adv. Drug Deliv. Rev. 2015, 90, 69–80. [Google Scholar] [CrossRef]
- Hu, C.M.; Copp, J.; Fang, R.H.; Luk, B.T.; Zhang, L. A biomimetic nanosponge that absorbs pore-forming toxins. Nat. Nanotechnol. 2013, 8, 336–340. [Google Scholar] [CrossRef]
- Pang, Z.; Hu, C.M.J.; Fang, R.H.; Luk, B.T.; Gao, W.; Fei, W.; Chuluun, E.; Angsantikul, P.; Thamphiwatana, S.; Lu, W. Detoxification of Organophosphate Poisoning Using Nanoparticle Bioscavengers. Acs Nano 2015, 9, 6450. [Google Scholar] [CrossRef]
- Wei, X.; Gao, J.; Wang, F.; Ying, M.; Angsantikul, P.; Kroll, A.V.; Zhou, J.; Gao, W.; Lu, W.; Fang, R.H.; et al. In Situ Capture of Bacterial Toxins for Antivirulence Vaccination. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef]
- Gao, W.; Fang, R.H.; Thamphiwatana, S.; Luk, B.T.; Li, J.; Angsantikul, P.; Zhang, Q.; Hu, C.M.; Zhang, L. Modulating antibacterial immunity via bacterial membrane-coated nanoparticles. Nano Lett. 2015, 15, 1403–1409. [Google Scholar] [CrossRef]
- Hu, C.M.; Fang, R.H.; Luk, B.T.; Zhang, L. Nanoparticle-detained toxins for safe and effective vaccination. Nat. Nanotechnol. 2013, 8, 933–938. [Google Scholar] [CrossRef]
- Wei, Y.; Quan, L.; Zhou, C.; Zhan, Q. Factors relating to the biodistribution & clearance of nanoparticles & their effects on in vivo application. Nanomedicine (Lond) 2018, 13, 1495–1512. [Google Scholar]
- Liu, R.; Yu, M.; Yang, X.; Umeshappa, C.S.; Hu, C.; Yu, W.; Qin, L.; Huang, Y.; Gao, H. Linear Chimeric Triblock Molecules Self-Assembled Micelles with Controllably Transformable Property to Enhance Tumor Retention for Chemo-Photodynamic Therapy of Breast Cancer. Adv. Fucntional Mater. 2019, 1808462. [Google Scholar] [CrossRef]
- Geng, Y.; Dalhaimer, P.; Cai, S.; Tsai, R.; Tewari, M.; Minko, T.; Discher, D.E. Shape effects of filaments versus spherical particles in flow and drug delivery. Nat. Nanotechnol. 2007, 2, 249–255. [Google Scholar] [CrossRef]
- Lee, C.; Hwang, H.S.; Lee, S.; Kim, B.; Kim, J.O.; Oh, K.T.; Lee, E.S.; Choi, H.G.; Yu, S.Y. Rabies Virus-Inspired Silica-Coated Gold Nanorods as a Photothermal Therapeutic Platform for Treating Brain Tumors. Adv. Mater. 2017, 29, 1605563. [Google Scholar] [CrossRef]
- Schleh, C.; Semmler-Behnke, M.; Lipka, J.; Wenk, A.; Hirn, S.; Schaffler, M.; Schmid, G.; Simon, U.; Kreyling, W.G. Size and surface charge of gold nanoparticles determine absorption across intestinal barriers and accumulation in secondary target organs after oral administration. Nanotoxicology 2012, 6, 36–46. [Google Scholar] [CrossRef]
- Alexis, F.; Pridgen, E.; Molnar, L.K.; Farokhzad, O.C. Factors Affecting the Clearance and Biodistribution of Polymeric Nanoparticles. Mol. Pharm. 2008, 5, 505. [Google Scholar] [CrossRef]
- Sarin, H. Physiologic upper limits of pore size of different blood capillary types and another perspective on the dual pore theory of microvascular permeability. J. Angiogenes Res. 2010, 2, 14. [Google Scholar] [CrossRef]
- Zhang, L.; Cao, Z.; Li, Y.; Ella-Menye, J.R.; Bai, T.S. Softer zwitterionic nanogels for longer circulation and lower splenic accumulation. Acs Nano 2012, 6, 6681–6686. [Google Scholar] [CrossRef]
- Villa, C.H.; Anselmo, A.C.; Mitragotri, S.; Muzykantov, V. Red blood cells: Supercarriers for drugs, biologicals, and nanoparticles and inspiration for advanced delivery systems. Adv. Drug Deliv. Rev. 2016, 106, 88–103. [Google Scholar] [CrossRef]
- Luk, B.T.; Hu, C.M.J.; Fang, R.H.; Dehaini, D.; Carpenter, C.; Gao, W.; Zhang, L. Interfacial Interactions between Natural RBC Membranes and Synthetic Polymeric Nanoparticles. Nanoscale 2014, 6, 2730–2737. [Google Scholar] [CrossRef]
- Waugh, R.E.; Sarelius, I.H. Effects of lost surface area on red blood cells and red blood cell survival in mice. Am J. Physiol. 1996, 271, C1847–C1852. [Google Scholar] [CrossRef]
- Oldenborg, P.A.; Zheleznyak, A.; Fang, Y.F.; Lagenaur, C.F.; Gresham, H.D.; Lindberg, F.P. Role of CD47 as a Marker of Self on Red Blood Cells. Science 2000, 288, 2051–2054. [Google Scholar] [CrossRef]
- He, Y.; Li, R.; Li, H.; Zhang, S.; Dai, W.; Wu, Q.; Jiang, L.; Zheng, Z.; Shen, S.; Chen, X.; et al. Erythroliposomes: Integrated Hybrid Nanovesicles Composed of Erythrocyte Membranes and Artificial Lipid Membranes for Pore-Forming Toxin Clearance. Acs Nano 2019, 13, 4148–4159. [Google Scholar] [CrossRef]
- Veillette, A.; Chen, J. SIRPalpha-CD47 Immune Checkpoint Blockade in Anticancer Therapy. Trends Immunol. 2018, 39, 173–184. [Google Scholar] [CrossRef]
- Liu, X.P.; Cron, K.; Deng, L.; Kline, J.; Frazier, W.A.; Xu, H.; Hua, P.; Fu, Y.X.; Meng, M.X. CD47 blockade triggers T cell–mediated destruction of immunogenic tumors. Nat. Med. 2015, 21, 1209. [Google Scholar] [CrossRef]
- Ayi, K.; Lu, Z.; Serghides, L.; Ho, J.M.; Finney, C.; Wang, J.C.; Liles, W.C.; Kain, K.C. CD47-SIRPα Interactions Regulate Macrophage Uptake of Plasmodium falciparum-Infected Erythrocytes and Clearance of Malaria In Vivo. Infect. Immun. 2016, 84, 2002. [Google Scholar] [CrossRef]
- Kojima, Y.; Volkmer, J.P.; Mckenna, K.; Civelek, M.; Lusis, A.J.; Miller, C.L.; Direnzo, D.; Nanda, V.; Ye, J.; Connolly, A.J. CD47-blocking antibodies restore phagocytosis and prevent atherosclerosis. Natural 2016, 536, 86. [Google Scholar] [CrossRef]
- Tsoi, K.M.; MacParland, S.A.; Ma, X.Z.; Spetzler, V.N.; Echeverri, J.; Ouyang, B.; Fadel, S.M.; Sykes, E.A.; Goldaracena, N.; Kaths, J.M.; et al. Mechanism of hard-nanomaterial clearance by the liver. Nat. Mater 2016, 15, 1212–1221. [Google Scholar] [CrossRef]
- Braet, F.; Muller, M.; Vekemans, K.; Wisse, E.; Le Couteur, D.G. Antimycin A-induced defenestration in rat hepatic sinusoidal endothelial cells. Hepatology (Baltim. Md.) 2003, 38, 394–402. [Google Scholar] [CrossRef]
- Fang, C.; Shi, B.; Pei, Y.Y.; Hong, M.H.; Wu, J.; Chen, H.Z. In vivo tumor targeting of tumor necrosis factor-alpha-loaded stealth nanoparticles: Effect of MePEG molecular weight and particle size. Eur. J. Pharm. Sci. 2006, 27, 27–36. [Google Scholar] [CrossRef]
- Merkel, T.J.; Chen, K.; Jones, S.W.; Pandya, A.A.; Tian, S.; Napier, M.E.; Zamboni, W.E.; DeSimone, J.M. The effect of particle size on the biodistribution of low-modulus hydrogel PRINT particles. J. Control. Release Off. J. Control. Release Soc. 2012, 162, 37–44. [Google Scholar] [CrossRef]
- Walkey, C.D.; Olsen, J.B.; Guo, H.; Emili, A.; Chan, W.C. Nanoparticle size and surface chemistry determine serum protein adsorption and macrophage uptake. J. Am. Chem. Soc. 2012, 134, 2139–2147. [Google Scholar] [CrossRef]
- Anselmo, A.C.; Zhang, M.; Kumar, S.; Vogus, D.R.; Menegatti, S.; Helgeson, M.E.; Mitragotri, S. Elasticity of nanoparticles influences their blood circulation, phagocytosis, endocytosis, and targeting. Acs Nano 2015, 9, 3169–3177. [Google Scholar] [CrossRef]
- Zhou, H.; Fan, Z.; Li, P.Y.; Deng, J.; Arhontoulis, D.C.; Li, C.Y.; Bowne, W.B.; Cheng, H. Dense and Dynamic Polyethylene Glycol Shells Cloak Nanoparticles from Uptake by Liver Endothelial Cells for Long Blood Circulation. Acs Nano 2018, 12, 10130–10141. [Google Scholar] [CrossRef]
- Qi, H.; Zhou, H.; Tang, Q.; Lee, J.Y.; Fan, Z.; Kim, S.; Staub, M.C.; Zhou, T.; Mei, S.; Han, L.; et al. Block copolymer crystalsomes with an ultrathin shell to extend blood circulation time. Nat. Commun. 2018, 9, 3005. [Google Scholar] [CrossRef]
- Rao, L.; Bu, L.L.; Xu, J.H.; Cai, B.; Yu, G.T.; Yu, X.; He, Z.; Huang, Q.; Li, A.; Guo, S.S.; et al. Red Blood Cell Membrane as a Biomimetic Nanocoating for Prolonged Circulation Time and Reduced Accelerated Blood Clearance. Small (Weinh. Der Bergstr. Ger.) 2015, 11, 6225–6236. [Google Scholar] [CrossRef]
| Groups | Core Size (nm) | Surface Area Per Core (μm2) | Mass Per Core (×10−9 μg) | Surface Area Per Milligram of Core (×1010 μm2) | RBC Ghost Required to Coat 1 Milligram of Core (μL) |
|---|---|---|---|---|---|
| 0 nm | 60 | 0.0113 | 0.136 | 8.33 | 138.9 |
| 120 nm | 100 | 0.0314 | 0.628 | 5.00 | 83.4 |
| 160 nm | 140 | 0.0615 | 1.72 | 3.57 | 59.6 |
| 200 nm | 160 | 0.0804 | 2.57 | 3.13 | 52.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Jin, K.; Luo, M.; Wang, X.; Zhu, X.; Liu, X.; Jiang, T.; Zhang, Q.; Wang, S.; Pang, Z. Size Dependency of Circulation and Biodistribution of Biomimetic Nanoparticles: Red Blood Cell Membrane-Coated Nanoparticles. Cells 2019, 8, 881. https://doi.org/10.3390/cells8080881
Li H, Jin K, Luo M, Wang X, Zhu X, Liu X, Jiang T, Zhang Q, Wang S, Pang Z. Size Dependency of Circulation and Biodistribution of Biomimetic Nanoparticles: Red Blood Cell Membrane-Coated Nanoparticles. Cells. 2019; 8(8):881. https://doi.org/10.3390/cells8080881
Chicago/Turabian StyleLi, Haichun, Kai Jin, Man Luo, Xuejun Wang, Xiaowen Zhu, Xianping Liu, Ting Jiang, Qin Zhang, Sheng Wang, and Zhiqing Pang. 2019. "Size Dependency of Circulation and Biodistribution of Biomimetic Nanoparticles: Red Blood Cell Membrane-Coated Nanoparticles" Cells 8, no. 8: 881. https://doi.org/10.3390/cells8080881
APA StyleLi, H., Jin, K., Luo, M., Wang, X., Zhu, X., Liu, X., Jiang, T., Zhang, Q., Wang, S., & Pang, Z. (2019). Size Dependency of Circulation and Biodistribution of Biomimetic Nanoparticles: Red Blood Cell Membrane-Coated Nanoparticles. Cells, 8(8), 881. https://doi.org/10.3390/cells8080881