Physical Exercise Affects Adipose Tissue Profile and Prevents Arterial Thrombosis in BDNF Val66Met Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Voluntary Physical Exercise (PE) Protocol
2.3. Arterial Thrombosis Model
2.4. Whole Blood Counts
2.5. Platelet–Leukocyte Aggregate Analysis
2.6. Cell Culture, Treatment, and Differentiation
2.7. Adipogenesis Evaluation by Flow Cytometry and Oil-Red-O
2.8. Quantitative Real-Time Polymerase Chain Reaction (RT-qPCR)
2.9. Adipose Tissue Histology and Quantification of Adipocyte Size and Number
2.10. Statistical Analysis
3. Results
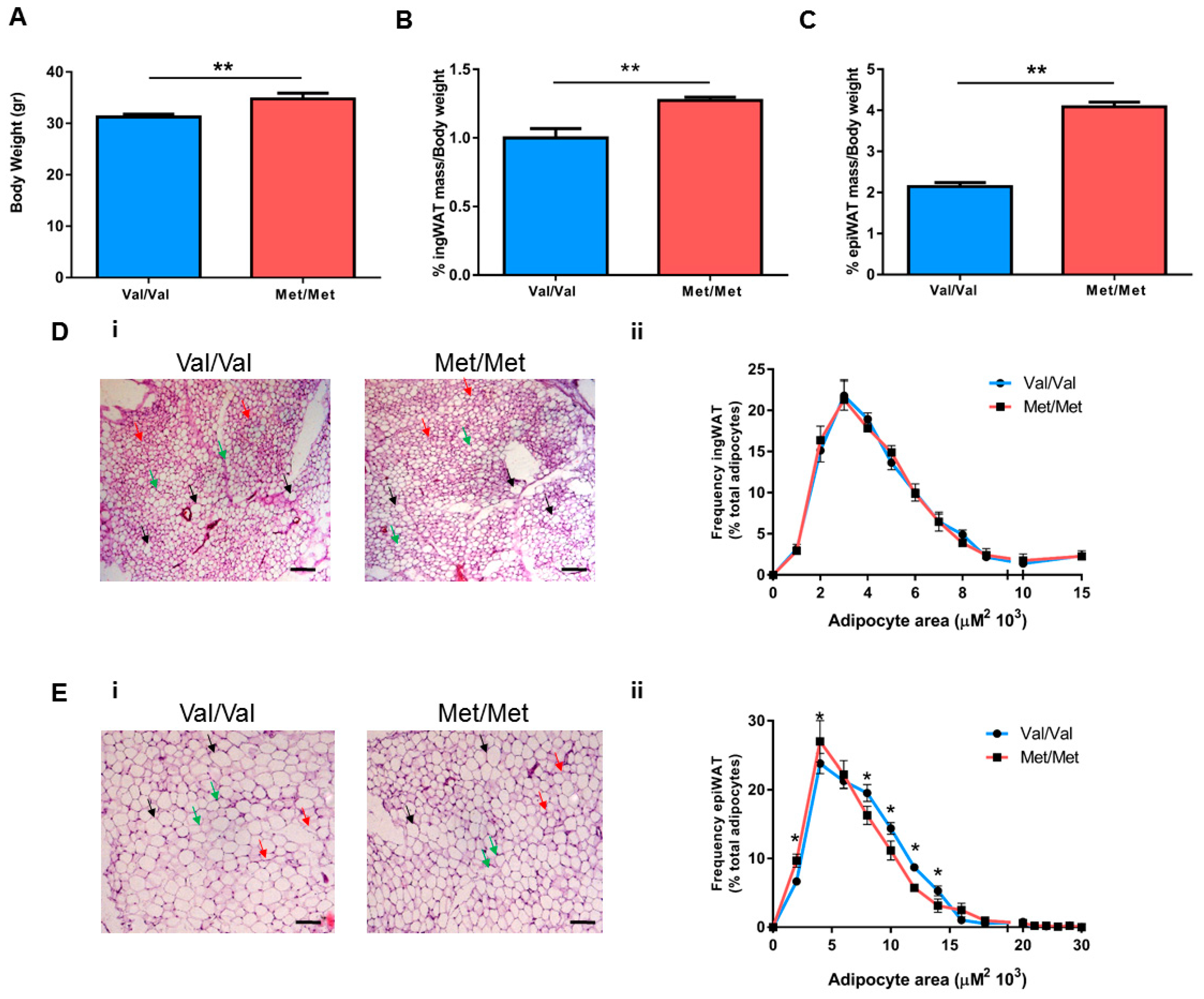
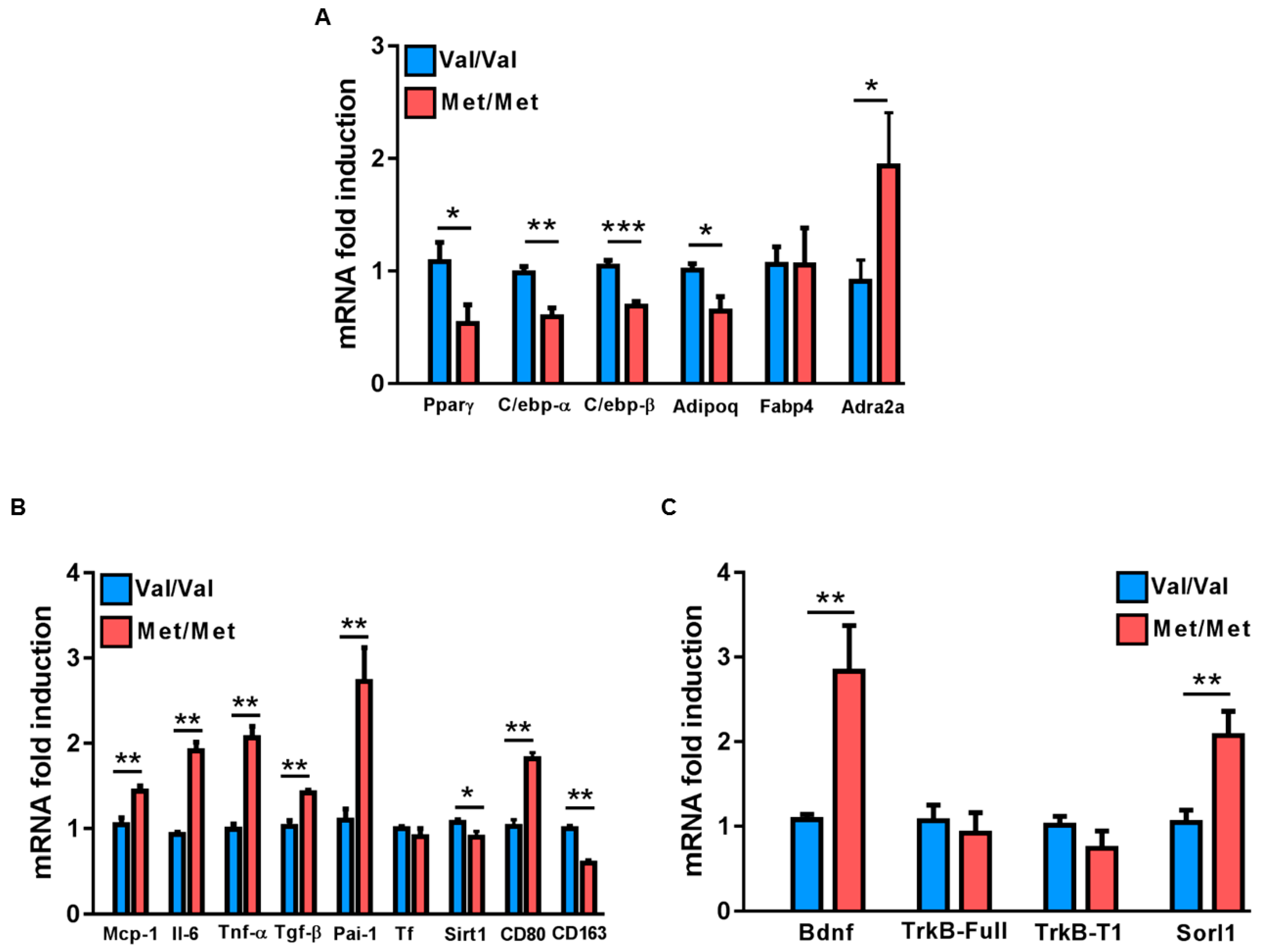
3.1. Characterization of the White Adipose Tissue Depots in BDNFMet/Met Mice
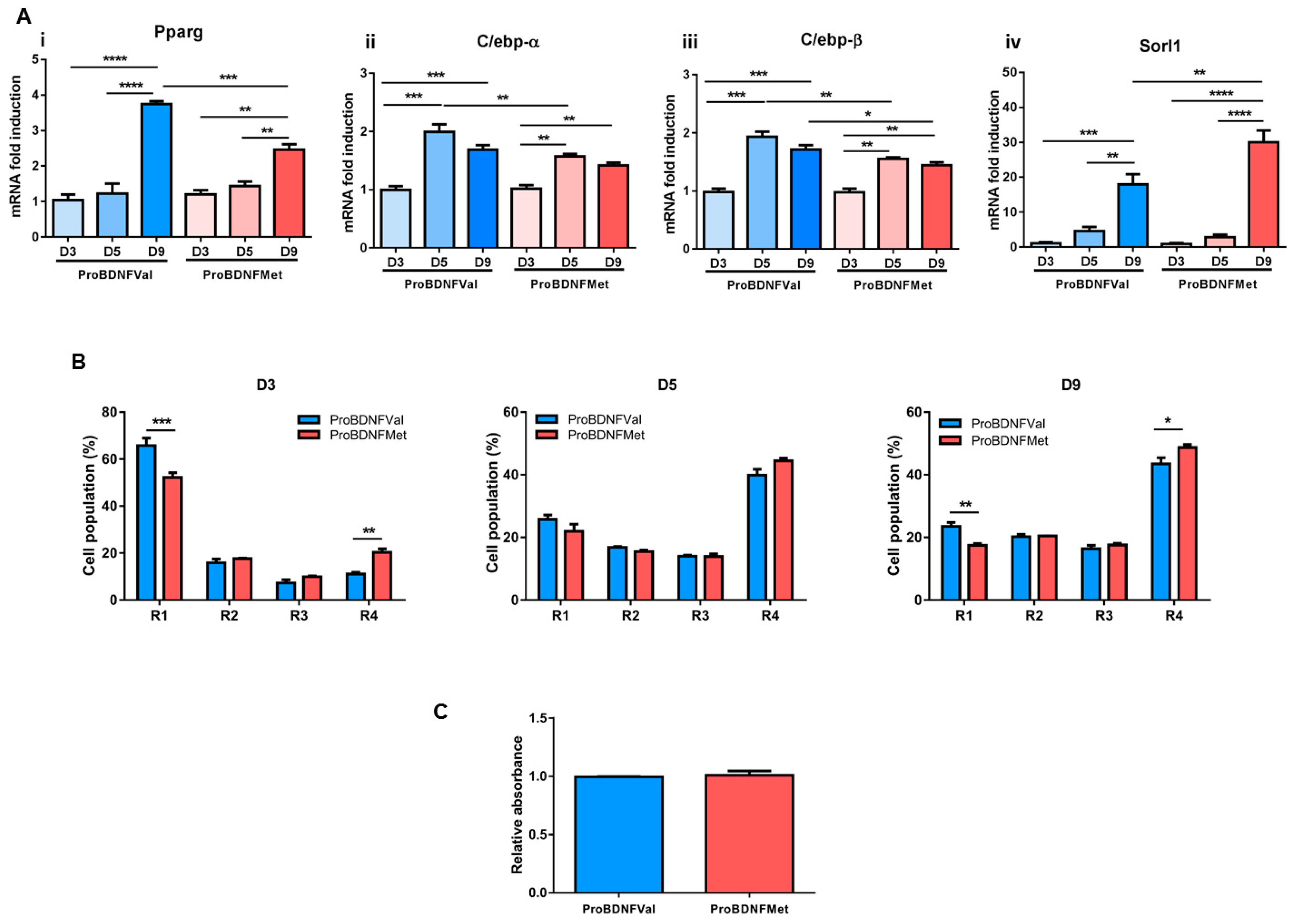
3.2. Evaluation of the Role of Mutant BDNF Val66Met Protein on Adipogenesis
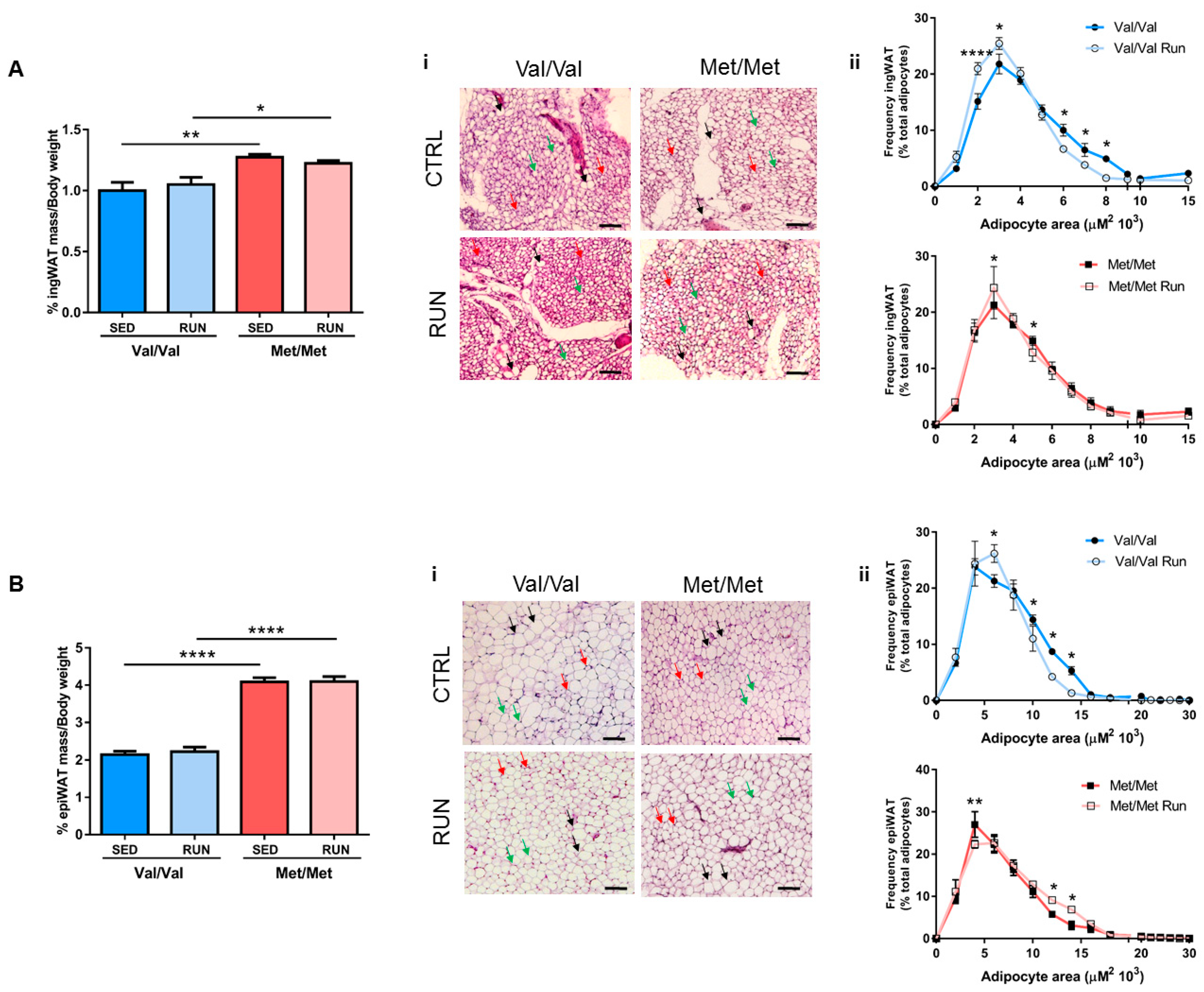
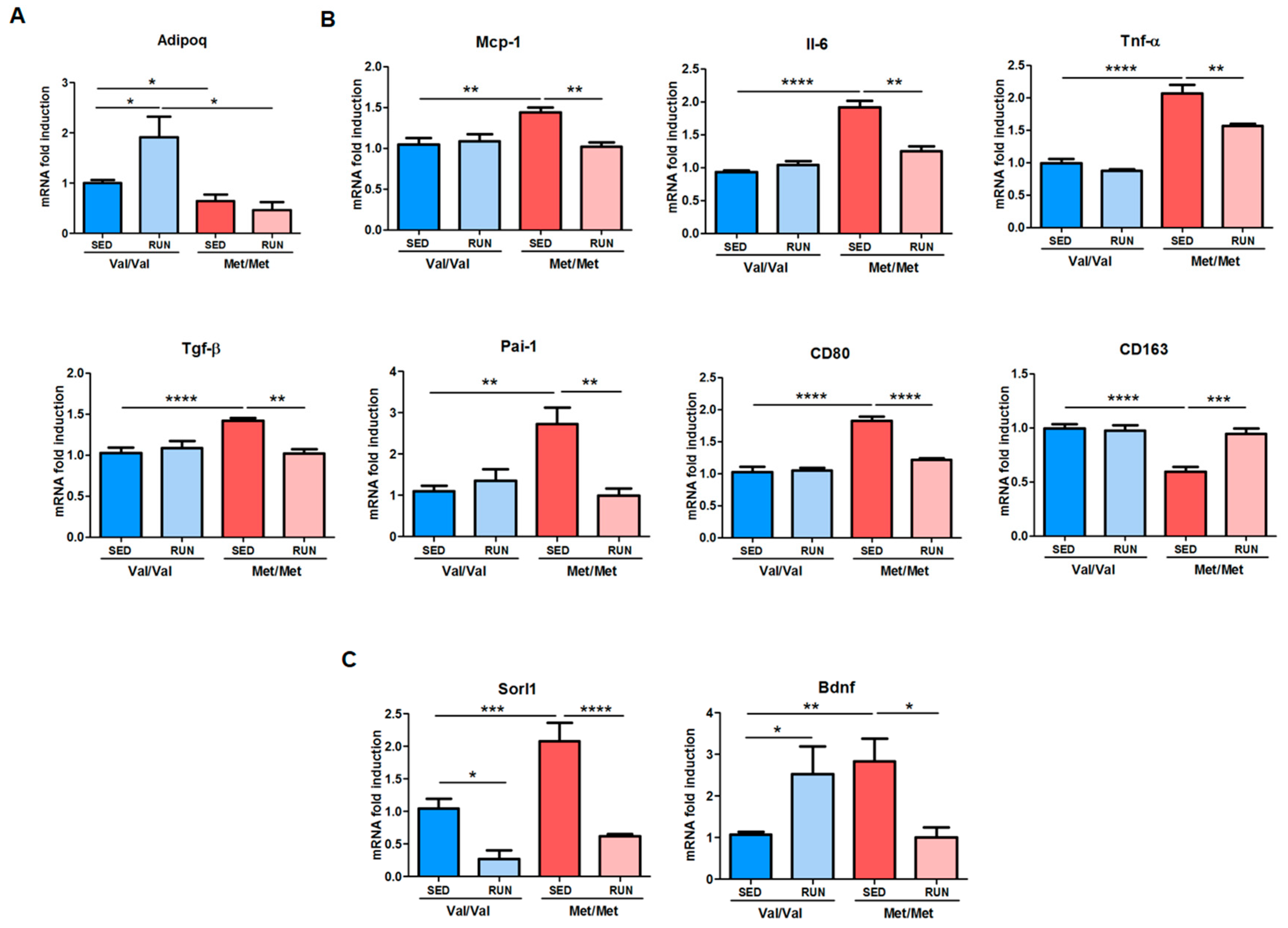
3.3. Effect of Physical Exercise (PE) on Adipose Tissue Phenotype of BDNF Val66Met Mice
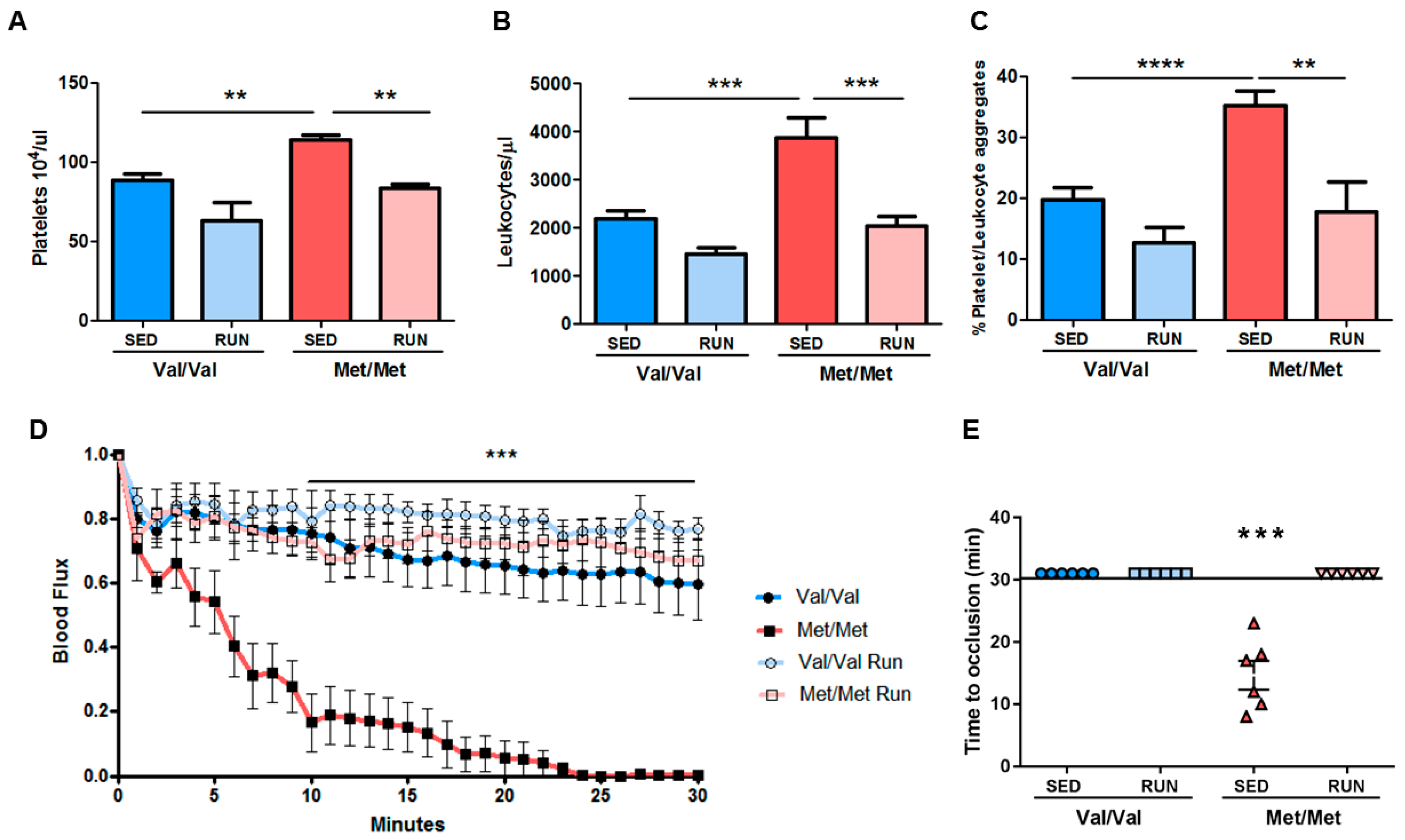
3.4. Effect of Physical Exercise (PE) on the Pro-Thrombotic Phenotype in BDNFMet/Met Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mathers, C.D.; Loncar, D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006, 3, e442. [Google Scholar] [CrossRef] [PubMed]
- Poirier, P.; Giles, T.D.; Bray, G.A.; Hong, Y.; Stern, J.S.; Pi-Sunyer, F.X.; Eckel, R.H.; American Heart Association; Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Obesity and cardiovascular disease: Pathophysiology, evaluation, and effect of weight loss: An update of the 1997 american heart association scientific statement on obesity and heart disease from the obesity committee of the council on nutrition, physical activity, and metabolism. Circulation 2006, 113, 898–918. [Google Scholar] [PubMed]
- Engeland, A.; Bjørge, T.; Søgaard, A.J.; Tverdal, A. Body mass index in adolescence in relation to total mortality: 32-year follow-up of 227,000 norwegian boys and girls. Am. J. Epidemiol. 2003, 157, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Must, A.; Jacques, P.F.; Dallal, G.E.; Bajema, C.J.; Dietz, W.H. Long-term morbidity and mortality of overweight adolescents. A follow-up of the harvard growth study of 1922 to 1935. N. Engl. J. Med. 1992, 327, 1350–1355. [Google Scholar] [CrossRef]
- Ortega, F.B.; Lavie, C.J.; Blair, S.N. Obesity and cardiovascular disease. Circ. Res. 2016, 118, 1752–1770. [Google Scholar] [CrossRef]
- Fain, J.N.; Madan, A.K.; Hiler, M.L.; Cheema, P.; Bahouth, S.W. Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans. Endocrinology 2004, 145, 2273–2282. [Google Scholar] [CrossRef]
- Carvalheira, J.B.; Qiu, Y.; Chawla, A. Blood spotlight on leukocytes and obesity. Blood 2013, 122, 3263–3267. [Google Scholar] [CrossRef]
- Ertek, S.; Cicero, A. Impact of physical activity on inflammation: Effects on cardiovascular disease risk and other inflammatory conditions. Arch. Med. Sci 2012, 8, 794–804. [Google Scholar] [CrossRef]
- Santilli, F.; Vazzana, N.; Liani, R.; Guagnano, M.T.; Davì, G. Platelet activation in obesity and metabolic syndrome. Obes Rev. 2012, 13, 27–42. [Google Scholar] [CrossRef]
- Piepoli, M.F.; Hoes, A.W.; Agewall, S.; Albus, C.; Brotons, C.; Catapano, A.L.; Cooney, M.T.; Corrà, U.; Cosyns, B.; Deaton, C.; et al. 2016 european guidelines on cardiovascular disease prevention in clinical practice: The sixth joint task force of the european society of cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of 10 societies and by invited experts)developed with the special contribution of the european association for cardiovascular prevention & rehabilitation (eacpr). Eur. Heart J. 2016, 37, 2315–2381. [Google Scholar]
- Bruunsgaard, H. Physical activity and modulation of systemic low-level inflammation. J. Leukoc Biol. 2005, 78, 819–835. [Google Scholar] [CrossRef]
- Schuler, G.; Adams, V.; Goto, Y. Role of exercise in the prevention of cardiovascular disease: Results, mechanisms, and new perspectives. Eur. Heart J. 2013, 34, 1790–1799. [Google Scholar] [CrossRef]
- Winzer, E.B.; Woitek, F.; Linke, A. Physical activity in the prevention and treatment of coronary artery disease. J. Am. Heart Assoc. 2018, 7. [Google Scholar] [CrossRef]
- You, T.; Arsenis, N.C.; Disanzo, B.L.; Lamonte, M.J. Effects of exercise training on chronic inflammation in obesity: Current evidence and potential mechanisms. Sports Med. 2013, 43, 243–256. [Google Scholar] [CrossRef]
- Montague, C.T.; Farooqi, I.S.; Whitehead, J.P.; Soos, M.A.; Rau, H.; Wareham, N.J.; Sewter, C.P.; Digby, J.E.; Mohammed, S.N.; Hurst, J.A.; et al. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature 1997, 387, 903–908. [Google Scholar] [CrossRef]
- Thaker, V.V. Genetic and epigenetic causes of obesity. Adolesc. Med. State Art Rev. 2017, 28, 379–405. [Google Scholar]
- Fairbrother, U.; Kidd, E.; Malagamuwa, T.; Walley, A. Genetics of severe obesity. Curr. Diab. Rep. 2018, 18, 85. [Google Scholar] [CrossRef]
- Rankinen, T.; Zuberi, A.; Chagnon, Y.C.; Weisnagel, S.J.; Argyropoulos, G.; Walts, B.; Pérusse, L.; Bouchard, C. The human obesity gene map: The 2005 update. Obesity (Silver Spring) 2006, 14, 529–644. [Google Scholar] [CrossRef]
- Monteleone, P.; Maj, M. Dysfunctions of leptin, ghrelin, bdnf and endocannabinoids in eating disorders: Beyond the homeostatic control of food intake. Psychoneuroendocrinology 2013, 38, 312–330. [Google Scholar] [CrossRef]
- Speliotes, E.K.; Willer, C.J.; Berndt, S.I.; Monda, K.L.; Thorleifsson, G.; Jackson, A.U.; Lango Allen, H.; Lindgren, C.M.; Luan, J.; Mägi, R.; et al. Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat. Genet. 2010, 42, 937–948. [Google Scholar] [CrossRef]
- Thorleifsson, G.; Walters, G.B.; Gudbjartsson, D.F.; Steinthorsdottir, V.; Sulem, P.; Helgadottir, A.; Styrkarsdottir, U.; Gretarsdottir, S.; Thorlacius, S.; Jonsdottir, I.; et al. Genome-wide association yields new sequence variants at seven loci that associate with measures of obesity. Nat. Genet. 2009, 41, 18–24. [Google Scholar] [CrossRef]
- Nakazato, M.; Hashimoto, K.; Shimizu, E.; Niitsu, T.; Iyo, M. Possible involvement of brain-derived neurotrophic factor in eating disorders. Iubmb. Life 2012, 64, 355–361. [Google Scholar] [CrossRef]
- Noble, E.E.; Billington, C.J.; Kotz, C.M.; Wang, C. The lighter side of bdnf. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 300, R1053–R1069. [Google Scholar] [CrossRef]
- Tsai, S.J. Critical issues in BDNF Val66Met Genetic Studies of Neuropsychiatric Disorders. Front. Mol. Neurosci. 2018, 11, 156. [Google Scholar] [CrossRef]
- Amadio, P.; Colombo, G.I.; Tarantino, E.; Gianellini, S.; Ieraci, A.; Brioschi, M.; Banfi, C.; Werba, J.P.; Parolari, A.; Lee, F.S.; et al. Bdnfval66met polymorphism: A potential bridge between depression and thrombosis. Eur. Heart J. 2017, 38, 1426–1435. [Google Scholar]
- Beckers, S.; Peeters, A.; Zegers, D.; Mertens, I.; Van Gaal, L.; Van Hul, W. Association of the bdnf val66met variation with obesity in women. Mol. Genet. Metab. 2008, 95, 110–112. [Google Scholar] [CrossRef]
- Wu, L.; Xi, B.; Zhang, M.; Shen, Y.; Zhao, X.; Cheng, H.; Hou, D.; Sun, D.; Ott, J.; Wang, X.; et al. Associations of six single nucleotide polymorphisms in obesity-related genes with bmi and risk of obesity in chinese children. Diabetes 2010, 59, 3085–3089. [Google Scholar] [CrossRef]
- Xi, B.; Cheng, H.; Shen, Y.; Chandak, G.R.; Zhao, X.; Hou, D.; Wu, L.; Wang, X.; Mi, J. Study of 11 bmi-associated loci identified in gwas for associations with central obesity in the chinese children. PLoS ONE 2013, 8, e56472. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, X.; Xi, B.; Shen, Y.; Wu, L.; Cheng, H.; Hou, D.; Mi, J. [impact of obesity-related gene polymorphism on risk of obesity and metabolic disorder in childhood]. Zhonghua Yu Fang Yi Xue Za Zhi 2014, 48, 776–783. [Google Scholar]
- Zhao, X.; Xi, B.; Shen, Y.; Wu, L.; Hou, D.; Cheng, H.; Mi, J. An obesity genetic risk score is associated with metabolic syndrome in chinese children. Gene 2014, 535, 299–302. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Jing, D.; Bath, K.G.; Ieraci, A.; Khan, T.; Siao, C.J.; Herrera, D.G.; Toth, M.; Yang, C.; McEwen, B.S.; et al. Genetic variant bdnf (val66met) polymorphism alters anxiety-related behavior. Science 2006, 314, 140–143. [Google Scholar] [CrossRef]
- Naoe, Y.; Shinkai, T.; Hori, H.; Fukunaka, Y.; Utsunomiya, K.; Sakata, S.; Matsumoto, C.; Shimizu, K.; Hwang, R.; Ohmori, O.; et al. No association between the brain-derived neurotrophic factor (bdnf) val66met polymorphism and schizophrenia in asian populations: Evidence from a case-control study and meta-analysis. Neurosci. Lett. 2007, 415, 108–112. [Google Scholar] [CrossRef]
- Shimizu, E.; Hashimoto, K.; Iyo, M. Ethnic difference of the bdnf 196g/a (val66met) polymorphism frequencies: The possibility to explain ethnic mental traits. Am. J. Med. Genet. B Neuropsychiatr Genet. 2004, 126B, 122–123. [Google Scholar] [CrossRef]
- Egan, M.F.; Kojima, M.; Callicott, J.H.; Goldberg, T.E.; Kolachana, B.S.; Bertolino, A.; Zaitsev, E.; Gold, B.; Goldman, D.; Dean, M.; et al. The bdnf val66met polymorphism affects activity-dependent secretion of bdnf and human memory and hippocampal function. Cell 2003, 112, 257–269. [Google Scholar] [CrossRef]
- Ieraci, A.; Madaio, A.I.; Mallei, A.; Lee, F.S.; Popoli, M. Brain-derived neurotrophic factor val66met human polymorphism impairs the beneficial exercise-induced neurobiological changes in mice. Neuropsychopharmacology 2016, 41, 3070–3079. [Google Scholar] [CrossRef]
- Ieraci, A.; Mallei, A.; Musazzi, L.; Popoli, M. Physical exercise and acute restraint stress differentially modulate hippocampal brain-derived neurotrophic factor transcripts and epigenetic mechanisms in mice. Hippocampus 2015, 25, 1380–1392. [Google Scholar] [CrossRef]
- Duman, C.H.; Schlesinger, L.; Russell, D.S.; Duman, R.S. Voluntary exercise produces antidepressant and anxiolytic behavioral effects in mice. Brain Res. 2008, 1199, 148–158. [Google Scholar] [CrossRef]
- Sartori, C.R.; Vieira, A.S.; Ferrari, E.M.; Langone, F.; Tongiorgi, E.; Parada, C.A. The antidepressive effect of the physical exercise correlates with increased levels of mature bdnf, and probdnf proteolytic cleavage-related genes, p11 and tpa. Neuroscience 2011, 180, 9–18. [Google Scholar] [CrossRef]
- Sandrini, L.; Ieraci, A.; Amadio, P.; Veglia, F.; Popoli, M.; Lee, F.S.; Tremoli, E.; Barbieri, S.S. Sub-chronic stress exacerbates the pro-thrombotic phenotype in bdnf. Int. J. Mol. Sci. 2018, 19, 3235. [Google Scholar] [CrossRef]
- Sandrini, L.; Ieraci, A.; Amadio, P.; Popoli, M.; Tremoli, E.; Barbieri, S.S. Apocynin prevents abnormal megakaryopoiesis and platelet activation induced by chronic stress. Oxid Med. Cell Longev. 2017, 2017, 9258937. [Google Scholar] [CrossRef]
- Beg, M.; Chauhan, P.; Varshney, S.; Shankar, K.; Rajan, S.; Saini, D.; Srivastava, M.N.; Yadav, P.P.; Gaikwad, A.N. A withanolide coagulin-l inhibits adipogenesis modulating wnt/beta-catenin pathway and cell cycle in mitotic clonal expansion. Phytomedicine 2014, 21, 406–414. [Google Scholar] [CrossRef]
- Biemann, R.; Fischer, B.; Bluher, M.; Navarrete Santos, A. Tributyltin affects adipogenic cell fate commitment in mesenchymal stem cells by a ppargamma independent mechanism. Chem. Biol. Interact. 2014, 214, 1–9. [Google Scholar] [CrossRef]
- Ruiz-Ojeda, F.J.; Ruperez, A.I.; Gomez-Llorente, C.; Gil, A.; Aguilera, C.M. Cell models and their application for studying adipogenic differentiation in relation to obesity: A review. Int. J. Mol. Sci. 2016, 17, 1040. [Google Scholar] [CrossRef]
- Baho, E.; Chattopadhyaya, B.; Lavertu-Jolin, M.; Mazziotti, R.; Awad, P.N.; Chehrazi, P.; Groleau, M.; Jahannault-Talignani, C.; Vaucher, E.; Ango, F.; et al. P75 neurotrophin receptor activation regulates the timing of the maturation of cortical parvalbumin interneuron connectivity and promotes juvenile-like plasticity in adult visual cortex. J. Neurosci. Off. J. Soc. Neurosci. 2019, 39, 4489–4510. [Google Scholar] [CrossRef]
- Guo, J.; Ji, Y.; Ding, Y.; Jiang, W.; Sun, Y.; Lu, B.; Nagappan, G. Bdnf pro-peptide regulates dendritic spines via caspase-3. Cell Death Dis. 2016, 7, e2264. [Google Scholar] [CrossRef]
- Mast, T.G.; Fadool, D.A. Mature and precursor brain-derived neurotrophic factor have individual roles in the mouse olfactory bulb. PLoS ONE 2012, 7, e31978. [Google Scholar] [CrossRef]
- Cheung, K.J.; Tzameli, I.; Pissios, P.; Rovira, I.; Gavrilova, O.; Ohtsubo, T.; Chen, Z.; Finkel, T.; Flier, J.S.; Friedman, J.M. Xanthine oxidoreductase is a regulator of adipogenesis and ppargamma activity. Cell Metab. 2007, 5, 115–128. [Google Scholar] [CrossRef]
- Lee, Y.H.; Chen, S.Y.; Wiesner, R.J.; Huang, Y.F. Simple flow cytometric method used to assess lipid accumulation in fat cells. J. Lipid Res. 2004, 45, 1162–1167. [Google Scholar] [CrossRef]
- Kraus, N.A.; Ehebauer, F.; Zapp, B.; Rudolphi, B.; Kraus, B.J.; Kraus, D. Quantitative assessment of adipocyte differentiation in cell culture. Adipocyte 2016, 5, 351–358. [Google Scholar] [CrossRef]
- Tarantino, E.; Amadio, P.; Squellerio, I.; Porro, B.; Sandrini, L.; Turnu, L.; Cavalca, V.; Tremoli, E.; Barbieri, S.S. Role of thromboxane-dependent platelet activation in venous thrombosis: Aspirin effects in mouse model. Pharm. Res. 2016, 107, 415–425. [Google Scholar] [CrossRef]
- Parlee, S.D.; Wang, Y.; Poirier, P.; Lapointe, M.; Martin, J.; Bastien, M.; Cianflone, K.; Goralski, K.B. Biliopancreatic diversion with duodenal switch modifies plasma chemerin in early and late post-operative periods. Obesity (Silver Spring) 2015, 23, 1201–1208. [Google Scholar] [CrossRef]
- Kernie, S.G.; Liebl, D.J.; Parada, L.F. Bdnf regulates eating behavior and locomotor activity in mice. EMBO J. 2000, 19, 1290–1300. [Google Scholar] [CrossRef]
- Lebrun, B.; Bariohay, B.; Moyse, E.; Jean, A. Brain-derived neurotrophic factor (bdnf) and food intake regulation: A minireview. Auton. Neurosci. 2006, 126–127. [Google Scholar] [CrossRef]
- Lyons, W.E.; Mamounas, L.A.; Ricaurte, G.A.; Coppola, V.; Reid, S.W.; Bora, S.H.; Wihler, C.; Koliatsos, V.E.; Tessarollo, L. Brain-derived neurotrophic factor-deficient mice develop aggressiveness and hyperphagia in conjunction with brain serotonergic abnormalities. Proc. Natl. Acad. Sci. USA 1999, 96, 15239–15244. [Google Scholar] [CrossRef]
- Rosas-Vargas, H.; Martínez-Ezquerro, J.D.; Bienvenu, T. Brain-derived neurotrophic factor, food intake regulation, and obesity. Arch. Med. Res. 2011, 42, 482–494. [Google Scholar] [CrossRef]
- Xu, H.; Barnes, G.T.; Yang, Q.; Tan, G.; Yang, D.; Chou, C.J.; Sole, J.; Nichols, A.; Ross, J.S.; Tartaglia, L.A.; et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Investig. 2003, 112, 1821–1830. [Google Scholar] [CrossRef]
- Yeo, G.S.; Connie Hung, C.C.; Rochford, J.; Keogh, J.; Gray, J.; Sivaramakrishnan, S.; O’Rahilly, S.; Farooqi, I.S. A de novo mutation affecting human trkb associated with severe obesity and developmental delay. Nat. Neurosci. 2004, 7, 1187–1189. [Google Scholar] [CrossRef]
- Choe, S.S.; Huh, J.Y.; Hwang, I.J.; Kim, J.I.; Kim, J.B. Adipose tissue remodeling: Its role in energy metabolism and metabolic disorders. Front. Endocrinol. (Lausanne) 2016, 7, 30. [Google Scholar] [CrossRef]
- Heilbronn, L.; Smith, S.R.; Ravussin, E. Failure of fat cell proliferation, mitochondrial function and fat oxidation results in ectopic fat storage, insulin resistance and type ii diabetes mellitus. Int. J. Obes. Relat. Metab. Disord. 2004, 28, S12–S21. [Google Scholar] [CrossRef]
- Jernås, M.; Palming, J.; Sjöholm, K.; Jennische, E.; Svensson, P.A.; Gabrielsson, B.G.; Levin, M.; Sjögren, A.; Rudemo, M.; Lystig, T.C.; et al. Separation of human adipocytes by size: Hypertrophic fat cells display distinct gene expression. FASEB J. 2006, 20, 1540–1542. [Google Scholar]
- Valet, P.; Grujic, D.; Wade, J.; Ito, M.; Zingaretti, M.C.; Soloveva, V.; Ross, S.R.; Graves, R.A.; Cinti, S.; Lafontan, M.; et al. Expression of human alpha 2-adrenergic receptors in adipose tissue of beta 3-adrenergic receptor-deficient mice promotes diet-induced obesity. J. Biol. Chem. 2000, 275, 34797–34802. [Google Scholar] [CrossRef]
- Lafontan, M.; Berlan, M.; Stich, V.; Crampes, F.; Rivière, D.; De Glisezinski, I.; Sengenes, C.; Galitzky, J. Recent data on the regulation of lipolysis by catecholamines and natriuretic peptides. Ann. Endocrinol. (Paris) 2002, 63, 86–90. [Google Scholar]
- Långberg, E.C.; Seed Ahmed, M.; Efendic, S.; Gu, H.F.; Östenson, C.G. Genetic association of adrenergic receptor alpha 2a with obesity and type 2 diabetes. Obesity (Silver Spring) 2013, 21, 1720–1725. [Google Scholar]
- Moro, C.; Polak, J.; Richterova, B.; Sengenès, C.; Pelikanova, T.; Galitzky, J.; Stich, V.; Lafontan, M.; Berlan, M. Differential regulation of atrial natriuretic peptide- and adrenergic receptor-dependent lipolytic pathways in human adipose tissue. Metabolism 2005, 54, 122–131. [Google Scholar] [CrossRef]
- Schmidt, V.; Schulz, N.; Yan, X.; Schürmann, A.; Kempa, S.; Kern, M.; Blüher, M.; Poy, M.N.; Olivecrona, G.; Willnow, T.E. Sorla facilitates insulin receptor signaling in adipocytes and exacerbates obesity. J. Clin. Investig. 2016, 126, 2706–2720. [Google Scholar] [CrossRef]
- Smith, E.N.; Chen, W.; Kähönen, M.; Kettunen, J.; Lehtimäki, T.; Peltonen, L.; Raitakari, O.T.; Salem, R.M.; Schork, N.J.; Shaw, M.; et al. Longitudinal genome-wide association of cardiovascular disease risk factors in the bogalusa heart study. PLoS Genet. 2010, 6, e1001094. [Google Scholar] [CrossRef]
- Ellulu, M.S.; Patimah, I.; Khaza’ai, H.; Rahmat, A.; Abed, Y. Obesity and inflammation: The linking mechanism and the complications. Arch. Med. Sci. 2017, 13, 851–863. [Google Scholar] [CrossRef]
- Berg, A.H.; Scherer, P.E. Adipose tissue, inflammation, and cardiovascular disease. Circ. Res. 2005, 96, 939–949. [Google Scholar] [CrossRef]
- Bodary, P.F. Links between adipose tissue and thrombosis in the mouse. Arter. Thromb. Vasc. Biol. 2007, 27, 2284–2291. [Google Scholar] [CrossRef]
- Odrowaz-Sypniewska, G. Markers of pro-inflammatory and pro-thrombotic state in the diagnosis of metabolic syndrome. Adv. Med. Sci. 2007, 52, 246–250. [Google Scholar]
- Davì, G.; Guagnano, M.T.; Ciabattoni, G.; Basili, S.; Falco, A.; Marinopiccoli, M.; Nutini, M.; Sensi, S.; Patrono, C. Platelet activation in obese women: Role of inflammation and oxidant stress. JAMA 2002, 288, 2008–2014. [Google Scholar] [CrossRef]
- Freedman, J.E.; Larson, M.G.; Tanriverdi, K.; O’Donnell, C.J.; Morin, K.; Hakanson, A.S.; Vasan, R.S.; Johnson, A.D.; Iafrati, M.D.; Benjamin, E.J. Relation of platelet and leukocyte inflammatory transcripts to body mass index in the framingham heart study. Circulation 2010, 122, 119–129. [Google Scholar] [CrossRef]
- Furuncuoğlu, Y.; Tulgar, S.; Dogan, A.N.; Cakar, S.; Tulgar, Y.K.; Cakiroglu, B. How obesity affects the neutrophil/lymphocyte and platelet/lymphocyte ratio, systemic immune-inflammatory index and platelet indices: A retrospective study. Eur. Rev. Med. Pharm. Sci. 2016, 20, 1300–1306. [Google Scholar]
- Vilahur, G.; Ben-Aicha, S.; Badimon, L. New insights into the role of adipose tissue in thrombosis. Cardiovasc. Res. 2017, 113, 1046–1054. [Google Scholar] [CrossRef]
- Skurk, T.; Alberti-Huber, C.; Herder, C.; Hauner, H. Relationship between adipocyte size and adipokine expression and secretion. J. Clin. Endocrinol. Metab. 2007, 92, 1023–1033. [Google Scholar] [CrossRef]
- Li, A.C.; Binder, C.J.; Gutierrez, A.; Brown, K.K.; Plotkin, C.R.; Pattison, J.W.; Valledor, A.F.; Davis, R.A.; Willson, T.M.; Witztum, J.L.; et al. Differential inhibition of macrophage foam-cell formation and atherosclerosis in mice by pparalpha, beta/delta, and gamma. J. Clin. Investig. 2004, 114, 1564–1576. [Google Scholar] [CrossRef]
- Okuno, A.; Tamemoto, H.; Tobe, K.; Ueki, K.; Mori, Y.; Iwamoto, K.; Umesono, K.; Akanuma, Y.; Fujiwara, T.; Horikoshi, H.; et al. Troglitazone increases the number of small adipocytes without the change of white adipose tissue mass in obese zucker rats. J. Clin. Investig. 1998, 101, 1354–1361. [Google Scholar] [CrossRef]
- Ricote, M.; Li, A.C.; Willson, T.M.; Kelly, C.J.; Glass, C.K. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature 1998, 391, 79–82. [Google Scholar] [CrossRef]
- Yamauchi, T.; Kamon, J.; Waki, H.; Murakami, K.; Motojima, K.; Komeda, K.; Ide, T.; Kubota, N.; Terauchi, Y.; Tobe, K.; et al. The mechanisms by which both heterozygous peroxisome proliferator-activated receptor gamma (ppargamma) deficiency and ppargamma agonist improve insulin resistance. J. Biol. Chem. 2001, 276, 41245–41254. [Google Scholar] [CrossRef]
- Bernhard, F.; Landgraf, K.; Klöting, N.; Berthold, A.; Büttner, P.; Friebe, D.; Kiess, W.; Kovacs, P.; Blüher, M.; Körner, A. Functional relevance of genes implicated by obesity genome-wide association study signals for human adipocyte biology. Diabetologia 2013, 56, 311–322. [Google Scholar] [CrossRef]
- Cancello, R.; Clément, K. Is obesity an inflammatory illness? Role of low-grade inflammation and macrophage infiltration in human white adipose tissue. BJOG 2006, 113, 1141–1147. [Google Scholar] [CrossRef]
- Lee, C.D.; Jackson, A.S.; Blair, S.N. Us weight guidelines: Is it also important to consider cardiorespiratory fitness? Int. J. Obes. Relat. Metab. Disord. 1998, 22, S2–S7. [Google Scholar]
- Polak, J.; Moro, C.; Klimcakova, E.; Hejnova, J.; Majercik, M.; Viguerie, N.; Langin, D.; Lafontan, M.; Stich, V.; Berlan, M. Dynamic strength training improves insulin sensitivity and functional balance between adrenergic alpha 2a and beta pathways in subcutaneous adipose tissue of obese subjects. Diabetologia 2005, 48, 2631–2640. [Google Scholar] [CrossRef]
- Stanford, K.I.; Goodyear, L.J. Exercise regulation of adipose tissue. Adipocyte 2016, 5, 153–162. [Google Scholar] [CrossRef]
- Woods, J.A.; Vieira, V.J.; Keylock, K.T. Exercise, inflammation, and innate immunity. Immunol. Allergy Clin. North. Am. 2009, 29, 381–393. [Google Scholar] [CrossRef]
- Bruun, J.M.; Helge, J.W.; Richelsen, B.; Stallknecht, B. Diet and exercise reduce low-grade inflammation and macrophage infiltration in adipose tissue but not in skeletal muscle in severely obese subjects. Am. J. Physiol. Endocrinol. Metab. 2006, 290, E961–E967. [Google Scholar] [CrossRef]
- Vieira, V.J.; Valentine, R.J.; Wilund, K.R.; Antao, N.; Baynard, T.; Woods, J.A. Effects of exercise and low-fat diet on adipose tissue inflammation and metabolic complications in obese mice. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E1164–E1171. [Google Scholar] [CrossRef]
- Goh, J.; Goh, K.P.; Abbasi, A. Exercise and adipose tissue macrophages: New frontiers in obesity research? Front. Endocrinol. (Lausanne) 2016, 7, 65. [Google Scholar] [CrossRef]
- Kjøbsted, R.; Hingst, J.R.; Fentz, J.; Foretz, M.; Sanz, M.N.; Pehmøller, C.; Shum, M.; Marette, A.; Mounier, R.; Treebak, J.T.; et al. Ampk in skeletal muscle function and metabolism. FASEB J. 2018, 32, 1741–1777. [Google Scholar] [CrossRef]
- Lira, V.A.; Benton, C.R.; Yan, Z.; Bonen, A. Pgc-1alpha regulation by exercise training and its influences on muscle function and insulin sensitivity. Am. J. Physiol. Endocrinol. Metab. 2010, 299, E145–E161. [Google Scholar] [CrossRef]
- Leal, L.G.; Lopes, M.A.; Batista, M.L. Physical exercise-induced myokines and muscle-adipose tissue crosstalk: A review of current knowledge and the implications for health and metabolic diseases. Front. Physiol. 2018, 9, 1307. [Google Scholar] [CrossRef]
- Kim, S.M.; Lun, M.; Wang, M.; Senyo, S.E.; Guillermier, C.; Patwari, P.; Steinhauser, M.L. Loss of white adipose hyperplastic potential is associated with enhanced susceptibility to insulin resistance. Cell Metab. 2014, 20, 1049–1058. [Google Scholar] [CrossRef]
- Rigamonti, A.; Brennand, K.; Lau, F.; Cowan, C.A. Rapid cellular turnover in adipose tissue. PLoS ONE 2011, 6, e17637. [Google Scholar] [CrossRef]
- White, U.; Ravussin, E. Dynamics of adipose tissue turnover in human metabolic health and disease. Diabetologia 2019, 62, 17–23. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sandrini, L.; Ieraci, A.; Amadio, P.; Zarà, M.; Mitro, N.; Lee, F.S.; Tremoli, E.; Barbieri, S.S. Physical Exercise Affects Adipose Tissue Profile and Prevents Arterial Thrombosis in BDNF Val66Met Mice. Cells 2019, 8, 875. https://doi.org/10.3390/cells8080875
Sandrini L, Ieraci A, Amadio P, Zarà M, Mitro N, Lee FS, Tremoli E, Barbieri SS. Physical Exercise Affects Adipose Tissue Profile and Prevents Arterial Thrombosis in BDNF Val66Met Mice. Cells. 2019; 8(8):875. https://doi.org/10.3390/cells8080875
Chicago/Turabian StyleSandrini, Leonardo, Alessandro Ieraci, Patrizia Amadio, Marta Zarà, Nico Mitro, Francis S. Lee, Elena Tremoli, and Silvia Stella Barbieri. 2019. "Physical Exercise Affects Adipose Tissue Profile and Prevents Arterial Thrombosis in BDNF Val66Met Mice" Cells 8, no. 8: 875. https://doi.org/10.3390/cells8080875
APA StyleSandrini, L., Ieraci, A., Amadio, P., Zarà, M., Mitro, N., Lee, F. S., Tremoli, E., & Barbieri, S. S. (2019). Physical Exercise Affects Adipose Tissue Profile and Prevents Arterial Thrombosis in BDNF Val66Met Mice. Cells, 8(8), 875. https://doi.org/10.3390/cells8080875






