Modulation of Agrin and RhoA Pathways Ameliorates Movement Defects and Synapse Morphology in MYO9A-Depleted Zebrafish
Abstract
:1. Introduction
2. Materials and Methods
2.1. Zebrafish Maintenance
2.2. CRISPR/Cas9-Mediated Knockdown in Zebrafish
2.3. NT1654 Treatment
2.4. Fasudil Treatment
2.5. Chorion Movements and Tactile Response Assay
2.6. Zebrafish Whole-Mount Immunofluorescence
2.7. NMJ Morphology Assessment
2.8. Statistical Analysis
3. Results
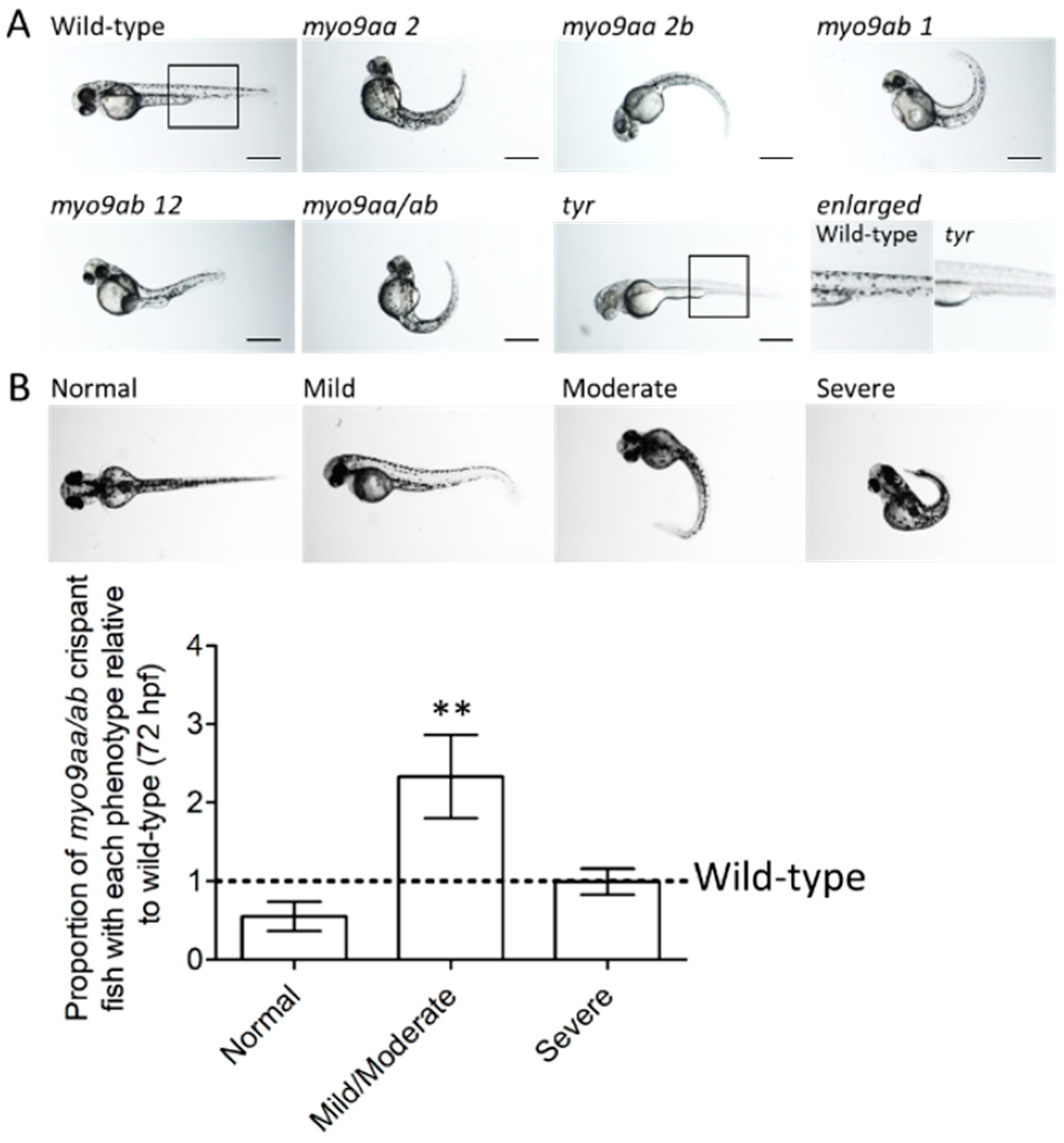
3.1. Myo9aa/ab Crispant Phenotype
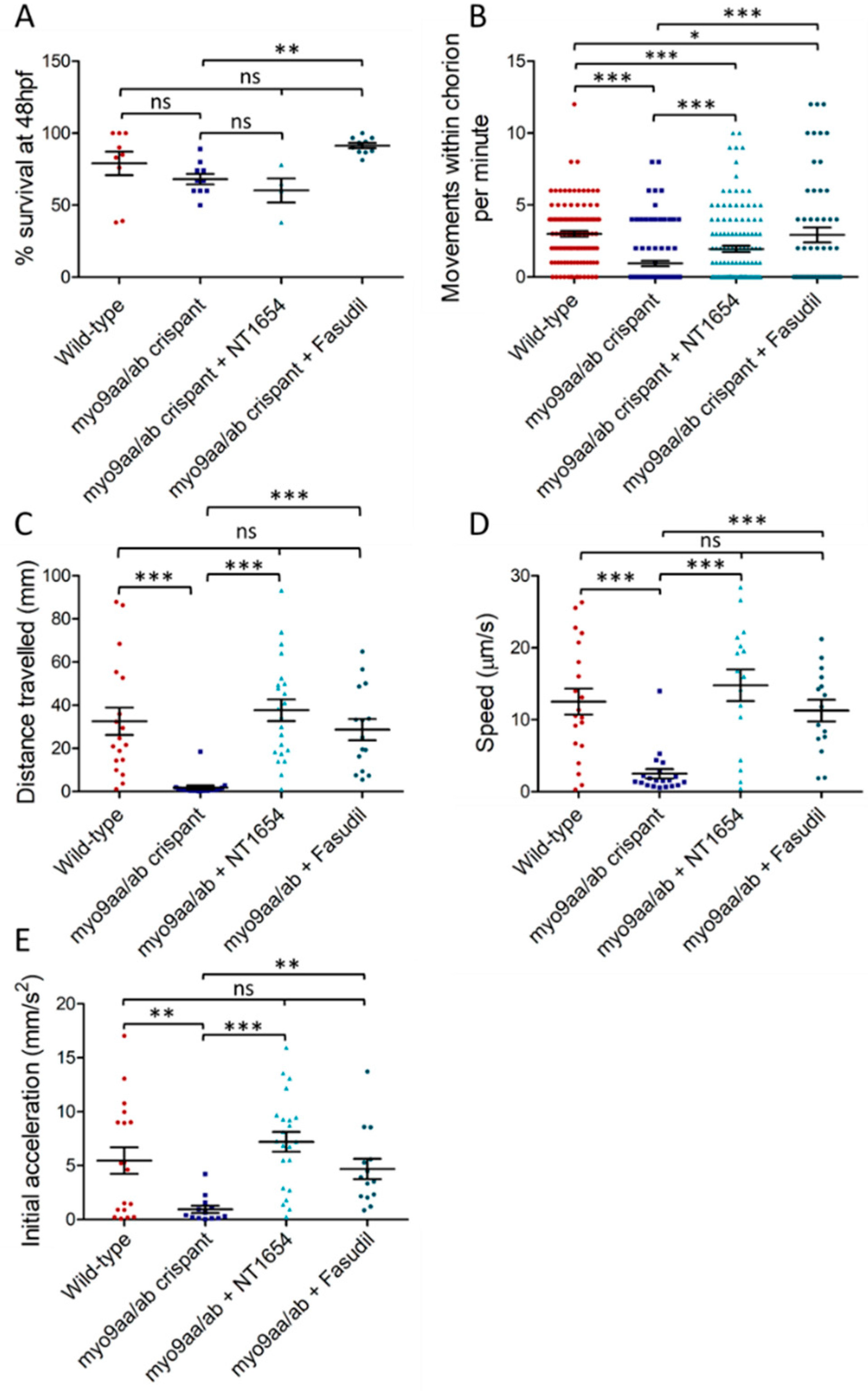
3.2. Myo9aa/ab Crispant Survival and Movement in the Presence of NT1654 and Fasudil
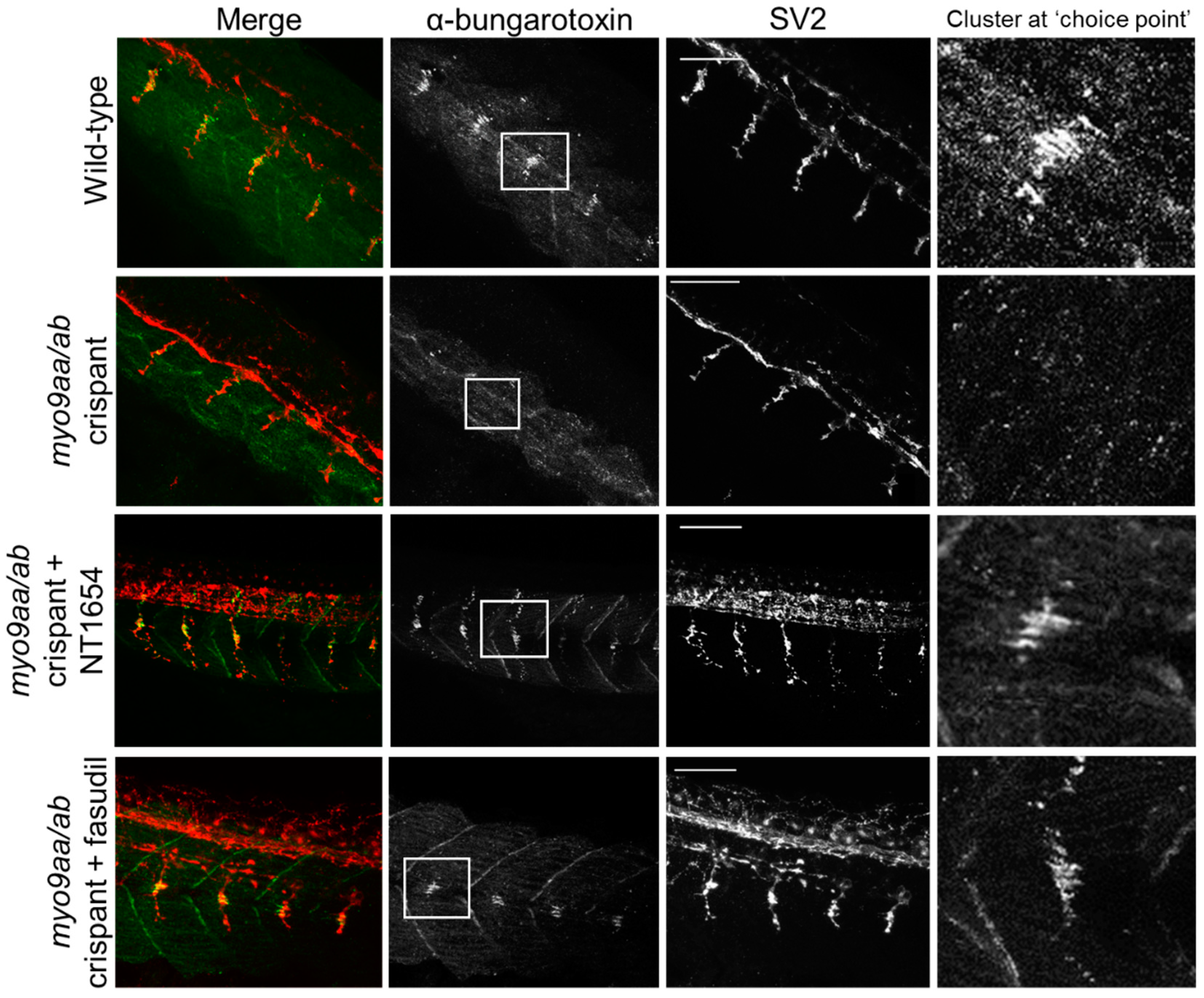
3.3. NMJ Morphology at 24 hpf
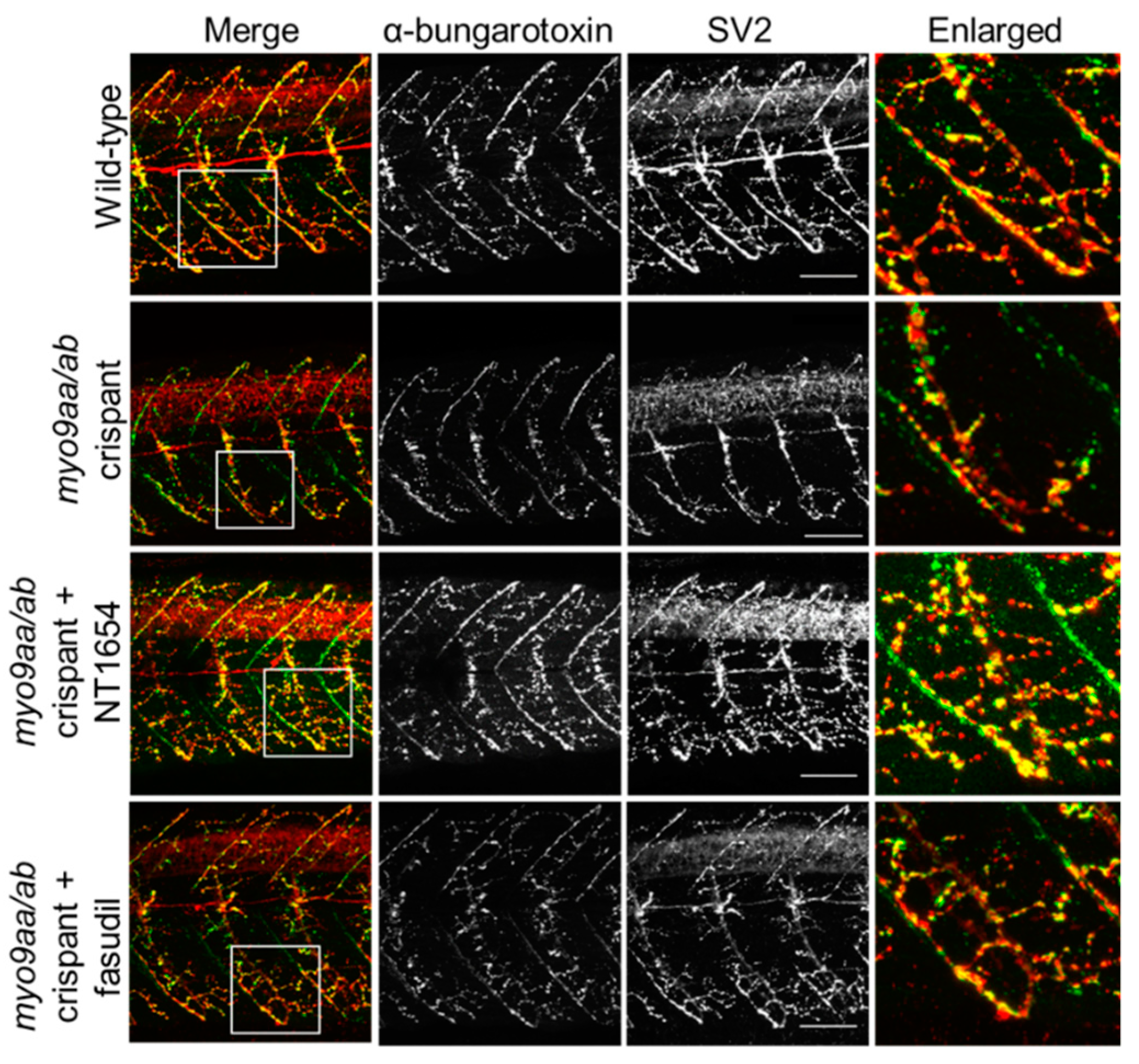
3.4. NMJ Morphology at 48 hpf
3.5. NMJ Morphology at 120 hpf
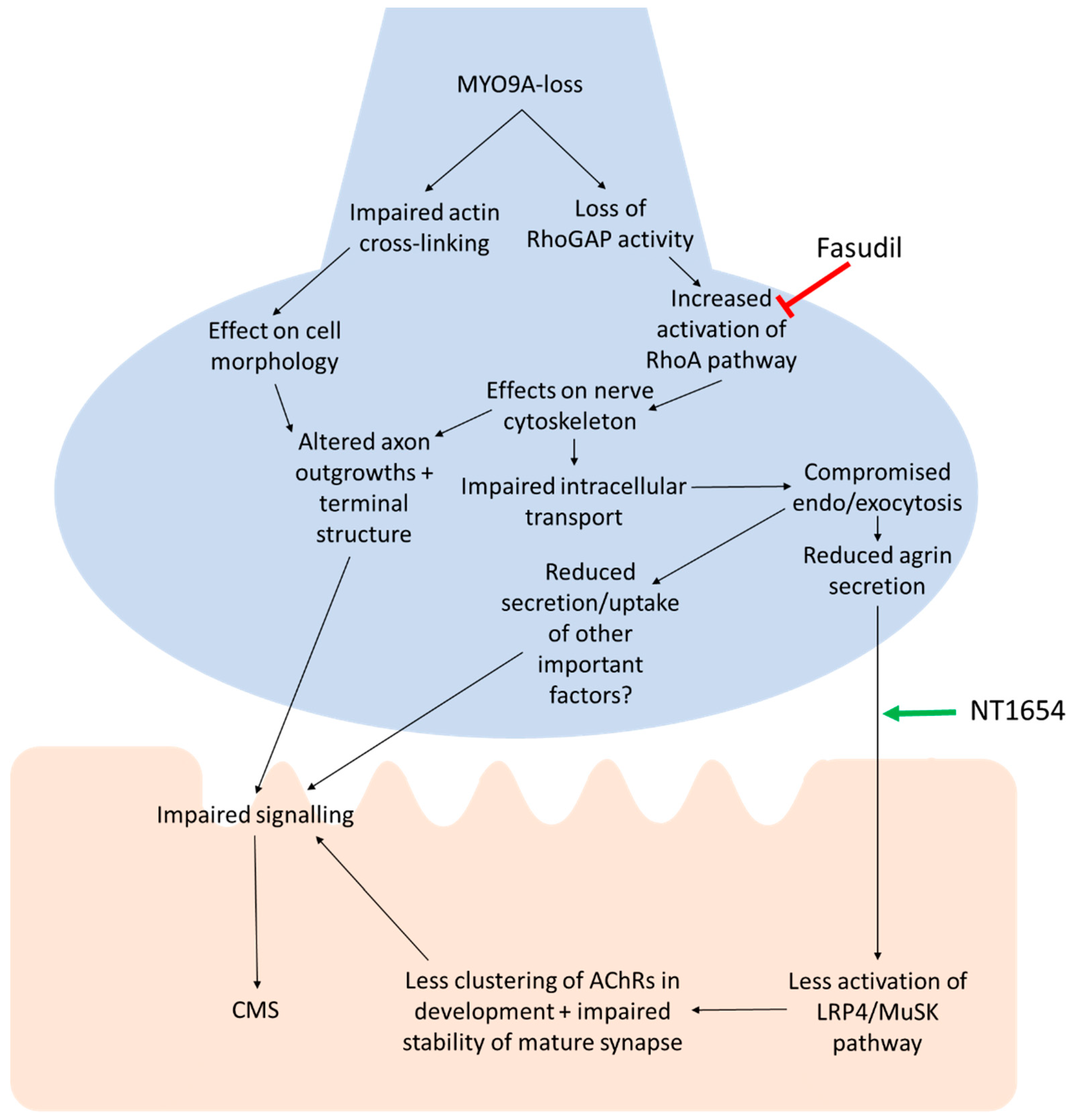
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Nicole, S.; Azuma, Y.; Bauche, S.; Eymard, B.; Lochmuller, H.; Slater, C. Congenital Myasthenic Syndromes or Inherited Disorders of Neuromuscular Transmission: Recent Discoveries and Open Questions. J. Neuromuscul. Dis. 2017, 4, 269–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McMacken, G.; Abicht, A.; Evangelista, T.; Spendiff, S.; Lochmuller, H. The Increasing Genetic and Phenotypical Diversity of Congenital Myasthenic Syndromes. Neuropediatrics 2017, 48, 294–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Connor, E.; Topf, A.; Muller, J.S.; Cox, D.; Evangelista, T.; Colomer, J.; Abicht, A.; Senderek, J.; Hasselmann, O.; Yaramis, A.; et al. Identification of mutations in the MYO9A gene in patients with congenital myasthenic syndrome. Brain 2016, 139, 2143–2153. [Google Scholar] [CrossRef] [PubMed]
- Abouhamed, M.; Grobe, K.; San, I.V.; Thelen, S.; Honnert, U.; Balda, M.S.; Matter, K.; Bahler, M. Myosin IXa regulates epithelial differentiation and its deficiency results in hydrocephalus. Mol. Biol. Cell 2009, 20, 5074–5085. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, E.; Topf, A.; Zahedi, R.P.; Spendiff, S.; Cox, D.; Roos, A.; Lochmuller, H. Clinical and research strategies for limb-girdle congenital myasthenic syndromes. Ann. N. Y. Acad. Sci. 2018, 1412, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Omelchenko, T.; Hall, A. Myosin-IXA regulates collective epithelial cell migration by targeting RhoGAP activity to cell-cell junctions. Curr. Biol. 2012, 22, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Bezakova, G.; Rabben, I.; Sefland, I.; Fumagalli, G.; Lomo, T. Neural agrin controls acetylcholine receptor stability in skeletal muscle fibers. Proc. Natl. Acad. Sci. USA 2001, 98, 9924–9929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gautam, M.; Noakes, P.G.; Moscoso, L.; Rupp, F.; Scheller, R.H.; Merlie, J.P.; Sanes, J.R. Defective neuromuscular synaptogenesis in agrin-deficient mutant mice. Cell 1996, 85, 525–535. [Google Scholar] [CrossRef]
- McMahan, U.J. The agrin hypothesis. Cold Spring Harb. Symp. Quant. Biol. 1990, 55, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Samuel, M.A.; Valdez, G.; Tapia, J.C.; Lichtman, J.W.; Sanes, J.R. Agrin and synaptic laminin are required to maintain adult neuromuscular junctions. PLoS ONE 2012, 7, e46663. [Google Scholar] [CrossRef] [PubMed]
- Hettwer, S.; Lin, S.; Kucsera, S.; Haubitz, M.; Oliveri, F.; Fariello, R.G.; Ruegg, M.A.; Vrijbloed, J.W. Injection of a soluble fragment of neural agrin (NT-1654) considerably improves the muscle pathology caused by the disassembly of the neuromuscular junction. PLoS ONE 2014, 9, e88739. [Google Scholar] [CrossRef] [PubMed]
- Kok, F.O.; Shin, M.; Ni, C.W.; Gupta, A.; Grosse, A.S.; van Impel, A.; Kirchmaier, B.C.; Peterson-Maduro, J.; Kourkoulis, G.; Male, I.; et al. Reverse genetic screening reveals poor correlation between morpholino-induced and mutant phenotypes in zebrafish. Dev. Cell 2015, 32, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Stainier, D.Y.R.; Raz, E.; Lawson, N.D.; Ekker, S.C.; Burdine, R.D.; Eisen, J.S.; Ingham, P.W.; Schulte-Merker, S.; Yelon, D.; Weinstein, B.M.; et al. Guidelines for morpholino use in zebrafish. PLoS Genet. 2017, 13, e1007000. [Google Scholar] [CrossRef] [PubMed]
- Varshney, G.K.; Carrington, B.; Pei, W.; Bishop, K.; Chen, Z.; Fan, C.; Xu, L.; Jones, M.; LaFave, M.C.; Ledin, J.; et al. A high-throughput functional genomics workflow based on CRISPR/Cas9-mediated targeted mutagenesis in zebrafish. Nat. Protoc. 2016, 11, 2357–2375. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Mateos, M.A.; Vejnar, C.E.; Beaudoin, J.D.; Fernandez, J.P.; Mis, E.K.; Khokha, M.K.; Giraldez, A.J. CRISPRscan: Designing highly efficient sgRNAs for CRISPR-Cas9 targeting in vivo. Nat. Methods 2015, 12, 982–988. [Google Scholar] [CrossRef] [PubMed]
- Jao, L.E.; Wente, S.R.; Chen, W. Efficient multiplex biallelic zebrafish genome editing using a CRISPR nuclease system. Proc. Natl. Acad. Sci. USA 2013, 110, 13904–13909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burger, A.; Lindsay, H.; Felker, A.; Hess, C.; Anders, C.; Chiavacci, E.; Zaugg, J.; Weber, L.M.; Catena, R.; Jinek, M.; et al. Maximizing mutagenesis with solubilized CRISPR-Cas9 ribonucleoprotein complexes. Development 2016, 143, 2025–2037. [Google Scholar] [CrossRef] [PubMed]
- Pelka, K.E.; Henn, K.; Keck, A.; Sapel, B.; Braunbeck, T. Size does matter—Determination of the critical molecular size for the uptake of chemicals across the chorion of zebrafish (Danio rerio) embryos. Aquat. Toxicol. 2017, 185, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Tinevez, J.Y.; Perry, N.; Schindelin, J.; Hoopes, G.M.; Reynolds, G.D.; Laplantine, E.; Bednarek, S.Y.; Shorte, S.L.; Eliceiri, K.W. TrackMate: An open and extensible platform for single-particle tracking. Methods 2017, 115, 80–90. [Google Scholar] [CrossRef]
- McMacken, G.; Cox, D.; Roos, A.; Muller, J.; Whittaker, R.; Lochmuller, H. The beta-adrenergic agonist salbutamol modulates neuromuscular junction formation in zebrafish models of human myasthenic syndromes. Hum. Mol. Genet. 2018, 27, 1556–1564. [Google Scholar] [CrossRef] [PubMed]
- Stauffer, W.; Sheng, H.; Lim, H.N. EzColocalization: An ImageJ plugin for visualizing and measuring colocalization in cells and organisms. Sci. Rep. 2018, 8, 15764. [Google Scholar] [CrossRef] [PubMed]
- Schulte-Merker, S.; Stainier, D.Y. Out with the old, in with the new: Reassessing morpholino knockdowns in light of genome editing technology. Development 2014, 141, 3103–3104. [Google Scholar] [CrossRef] [PubMed]
- Myers, P.Z.; Eisen, J.S.; Westerfield, M. Development and axonal outgrowth of identified motoneurons in the zebrafish. J. Neurosci. 1986, 6, 2278–2289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saint-Amant, L.; Drapeau, P. Time course of the development of motor behaviors in the zebrafish embryo. J. Neurobiol. 1998, 37, 622–632. [Google Scholar] [CrossRef]
- Sztal, T.E.; Ruparelia, A.A.; Williams, C.; Bryson-Richardson, R.J. Using Touch-evoked Response and Locomotion Assays to Assess Muscle Performance and Function in Zebrafish. J. Vis. Exp. 2016. [Google Scholar] [CrossRef]
- Panzer, J.A.; Gibbs, S.M.; Dosch, R.; Wagner, D.; Mullins, M.C.; Granato, M.; Balice-Gordon, R.J. Neuromuscular synaptogenesis in wild-type and mutant zebrafish. Dev. Biol. 2005, 285, 340–357. [Google Scholar] [CrossRef]
- Wood, S.J.; Slater, C.R. Safety factor at the neuromuscular junction. Prog. Neurobiol. 2001, 64, 393–429. [Google Scholar] [CrossRef]
- Thelen, S.; Abouhamed, M.; Ciarimboli, G.; Edemir, B.; Bahler, M. Rho GAP myosin IXa is a regulator of kidney tubule function. Am. J. Physiol. Renal. Physiol. 2015, 309, F501–F513. [Google Scholar] [CrossRef] [Green Version]
- Outtandy, P.; Russell, C.; Kleta, R.; Bockenhauer, D. Zebrafish as a model for kidney function and disease. Pediatr. Nephrol. 2019, 34, 751–762. [Google Scholar] [CrossRef]
- Kim, M.J.; Liu, I.H.; Song, Y.; Lee, J.A.; Halfter, W.; Balice-Gordon, R.J.; Linney, E.; Cole, G.J. Agrin is required for posterior development and motor axon outgrowth and branching in embryonic zebrafish. Glycobiology 2007, 17, 231–247. [Google Scholar] [CrossRef] [PubMed]
- Bogdanik, L.P.; Burgess, R.W. A valid mouse model of AGRIN-associated congenital myasthenic syndrome. Hum. Mol. Genet. 2011, 20, 4617–4633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.J.; Cotman, S.L.; Halfter, W.; Cole, G.J. The heparan sulfate proteoglycan agrin modulates neurite outgrowth mediated by FGF-2. J. Neurobiol. 2003, 55, 261–277. [Google Scholar] [CrossRef] [PubMed]
- Govek, E.E.; Newey, S.E.; Van Aelst, L. The role of the Rho GTPases in neuronal development. Genes. Dev. 2005, 19, 1–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bowerman, M.; Murray, L.M.; Boyer, J.G.; Anderson, C.L.; Kothary, R. Fasudil improves survival and promotes skeletal muscle development in a mouse model of spinal muscular atrophy. BMC Med. 2012, 10, 24. [Google Scholar] [CrossRef]
- Saczko-Brack, D.; Warchol, E.; Rogez, B.; Kross, M.; Heissler, S.M.; Sellers, J.R.; Batters, C.; Veigel, C. Self-organization of actin networks by a monomeric myosin. Proc. Natl. Acad. Sci. USA 2016, 113, E8387–E8395. [Google Scholar] [CrossRef] [Green Version]
- Tintignac, L.A.; Brenner, H.R.; Ruegg, M.A. Mechanisms Regulating Neuromuscular Junction Development and Function and Causes of Muscle Wasting. Physiol. Rev. 2015, 95, 809–852. [Google Scholar] [CrossRef] [Green Version]
- Boon, K.L.; Xiao, S.; McWhorter, M.L.; Donn, T.; Wolf-Saxon, E.; Bohnsack, M.T.; Moens, C.B.; Beattie, C.E. Zebrafish survival motor neuron mutants exhibit presynaptic neuromuscular junction defects. Hum. Mol. Genet. 2009, 18, 3615–3625. [Google Scholar] [CrossRef] [Green Version]
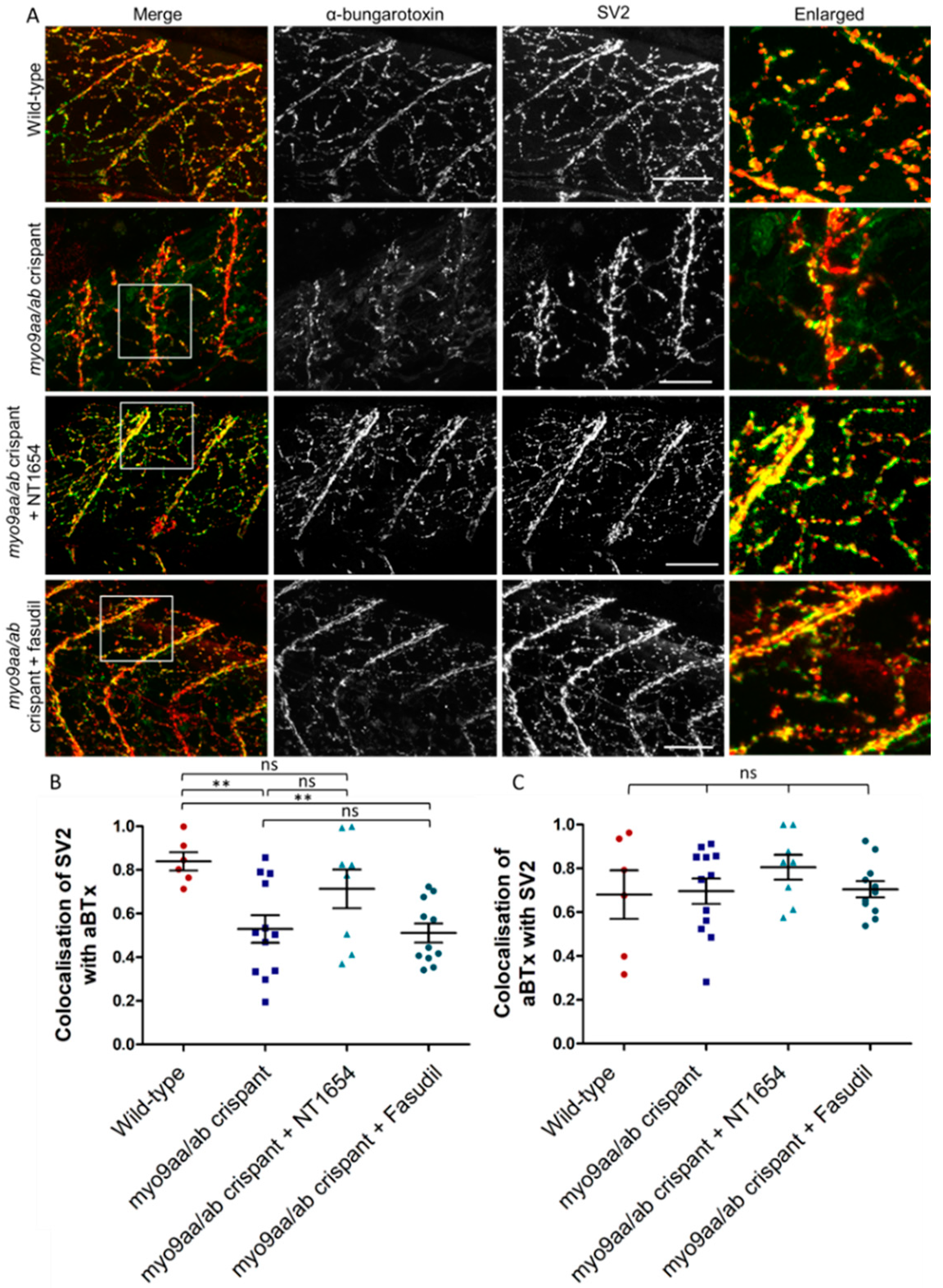
| Wild-Type | myo9aa/ab Crispant | myo9aa/ab Crispant + NT1654 | myo9aa/ab Crispant + Fasudil | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NMJ feature | Mean | SD | Mean | SD | P value (vs untreated WT) | Mean | SD | P value (vs untreated WT) | P value (vs untreated crispant) | Mean | SD | P value (vs untreated WT) | P value (vs untreated crispant) |
| Length of neuron outgrowth past choice point (µm) | 19.91 | 10.53 | 8.55 | 8.70 | ** | 24.15 | 9.46 | ns | ** | 16.95 | 9.64 | ns | ns |
| Average area of αBTx clusters (µm2) | 2.62 | 0.41 | 1.72 | 2.25 | ns | 6.10 | 1.84 | ** | *** | 2.22 | 1.36 | ns | ns |
| Total area of αBTx clusters per 100 µm2 (µm2) | 14.13 | 2.84 | 18.1 | 25.74 | ns | 28.6 | 7.16 | ns | ns | 14.51 | 9.81 | ns | ns |
| Number of αBTx clusters >20 µm2 | 5.68 | 3.01 | 3.2 | 4.19 | * | 9.5 | 3.08 | ns | ** | 3.6 | 3.78 | ns | ns |
| Proportion of somites with a choice point cluster | 0.97 | 0.08 | 0.32 | 0.41 | ** | 1 | 0 | ns | ** | 0.90 | 0.20 | ns | * |
| Size of myotomes (µm2) | 2863 | 800.6 | 1724 | 971.2 | ** | 1145 | 192.8 | *** | ns | 2722 | 604.1 | ns | * |
| Wild-Type | myo9aa/ab Crispant | myo9aa/ab Crispant + NT1654 | myo9aa/ab Crispant + Fasudil | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NMJ feature | Mean | SD | Mean | SD | P value (vs untreated WT) | Mean | SD | P value (vs untreated WT) | P value (vs untreated crispant) | Mean | SD | P value (vs untreated WT) | P value (vs untreated crispant) |
| No. of SV2 clusters per 100 µm2 | 3.72 | 0.85 | 2.53 | 1.74 | ** | 4.48 | 1.96 | ns | *** | 1.15 | 0.28 | *** | * |
| Avg area SV2 clusters (µm2) | 2.17 | 1.25 | 3.44 | 2.53 | ** | 1.77 | 0.89 | ns | ** | 3.99 | 2.02 | *** | ns |
| Total area of SV2 clusters per 100 µm2 (µm2) | 8.25 | 1.49 | 5.35 | 2.03 | ** | 6.83 | 1.75 | ns | ns | 4.24 | 1.12 | *** | ns |
| Number of SV2 clusters >20 µm2 | 2.10 | 1.86 | 1.47 | 1.12 | ns | 1.79 | 1.53 | ns | ns | 1.72 | 1.14 | ns | ns |
| % myosepta overlaid by motorneuron | 79.0 | 15.47 | 64.90 | 22.15 | ns | 71.54 | 23.68 | ns | ns | 81.50 | 14.35 | ns | ns |
| No. of αBTx clusters per 100 µm2 | 3.10 | 0.60 | 1.73 | 1.02 | *** | 2.63 | 0.63 | ns | * | 1.18 | 0.29 | *** | ns |
| Avg area αBTx clusters (µm2) | 1.64 | 0.46 | 3.12 | 2.27 | *** | 1.33 | 0.36 | ns | *** | 3.95 | 0.93 | *** | ** |
| Total area of αBTx clusters per 100 µm2 (µm2) | 8.00 | 1.96 | 4.38 | 2.1 | ** | 3.43 | 0.81 | ** | ns | 4.11 | 1.03 | ** | ns |
| Number of αBTx clusters >20 µm2 | 1.70 | 1.39 | 0.94 | 0.90 | ns | 0.64 | 0.93 | * | ns | 1.58 | 1.08 | ns | ns |
| Size of myotomes (µm2) | 5257 | 911 | 4574 | 1211 | ns | 4573 | 719 | ns | ns | 4646 | 1306 | ns | ns |
| Wild-Type | myo9aa/ab Crispant | myo9aa/ab Crispant + NT1654 | myo9aa/ab Crispant + Fasudil | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NMJ feature | Mean | SD | Mean | SD | P value (vs untreated WT) | Mean | SD | P value (vs untreated WT) | P value (vs untreated crispant) | Mean | SD | P value (vs untreated WT) | P value (vs untreated crispant) |
| No. of SV2 clusters per 100 µm2 | 3.44 | 0.63 | 5.14 | 1.55 | ns | 3.49 | 0.41 | ns | ns | 2.0 | 0.55 | * | *** |
| Avg area SV2 clusters (µm2) | 1.02 | 0.18 | 0.96 | 0.21 | ns | 1.21 | 0.19 | ns | * | 4.27 | 1.46 | *** | *** |
| Total area of SV2 clusters per 100 µm2 (µm2) | 4.35 | 2.74 | 4.72 | 1.22 | ns | 4.21 | 0.78 | ns | ns | 7.93 | 2.63 | ** | ns |
| Number of SV2 clusters >20 µm2 | 0.73 | 0.73 | 0.14 | 0.38 | ns | 0.65 | 0.48 | ns | ns | 5.05 | 3.96 | ** | *** |
| % myosepta overlaid by motorneuron | 100 | 0 | 90.7 | 17.0 | ns | 100 | 0 | ns | ns | 100 | 0 | ns | ns |
| No. of αBTx clusters per 100 µm2 | 4.39 | 0.94 | 5.68 | 1.28 | * | 7.09 | 3.04 | * | ns | 1.65 | 0.56 | *** | *** |
| Avg area αBTx clusters (µm2) | 1.18 | 0.13 | 0.82 | 0.23 | *** | 0.48 | 0.12 | *** | ** | 2.04 | 0.69 | *** | *** |
| Total area of αBTx clusters per 100 µm2 (µm2) | 6.23 | 3.85 | 5.28 | 1.46 | ns | 5.11 | 1.55 | ns | ns | 3.41 | 1.57 | * | ns |
| Number of αBTx clusters >20 µm2 | 1.22 | 1.30 | 0.15 | 0.24 | ns | 1.13 | 1.0 | ns | ns | 1.10 | 0.84 | ns | * |
| Size of myotomes (µm2) | 9977 | 1452 | 6256 | 1930 | ** | 8785 | 1302 | ns | ns | 6838 | 2185 | * | ns |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’Connor, E.; Cairns, G.; Spendiff, S.; Burns, D.; Hettwer, S.; Mäder, A.; Müller, J.; Horvath, R.; Slater, C.; Roos, A.; et al. Modulation of Agrin and RhoA Pathways Ameliorates Movement Defects and Synapse Morphology in MYO9A-Depleted Zebrafish. Cells 2019, 8, 848. https://doi.org/10.3390/cells8080848
O’Connor E, Cairns G, Spendiff S, Burns D, Hettwer S, Mäder A, Müller J, Horvath R, Slater C, Roos A, et al. Modulation of Agrin and RhoA Pathways Ameliorates Movement Defects and Synapse Morphology in MYO9A-Depleted Zebrafish. Cells. 2019; 8(8):848. https://doi.org/10.3390/cells8080848
Chicago/Turabian StyleO’Connor, Emily, George Cairns, Sally Spendiff, David Burns, Stefan Hettwer, Armin Mäder, Juliane Müller, Rita Horvath, Clarke Slater, Andreas Roos, and et al. 2019. "Modulation of Agrin and RhoA Pathways Ameliorates Movement Defects and Synapse Morphology in MYO9A-Depleted Zebrafish" Cells 8, no. 8: 848. https://doi.org/10.3390/cells8080848
APA StyleO’Connor, E., Cairns, G., Spendiff, S., Burns, D., Hettwer, S., Mäder, A., Müller, J., Horvath, R., Slater, C., Roos, A., & Lochmüller, H. (2019). Modulation of Agrin and RhoA Pathways Ameliorates Movement Defects and Synapse Morphology in MYO9A-Depleted Zebrafish. Cells, 8(8), 848. https://doi.org/10.3390/cells8080848





