MiR-200-3p Is Potentially Involved in Cell Cycle Arrest by Regulating Cyclin A during Aestivation in Apostichopus japonicus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Prediction of the miR-200-3p Target
2.3. RNA Extraction, Cyclin A cDNA Cloning and Sequence Analysis
2.4. Expression Level Analysis of miR-200-3p and AjCA
2.5. Dual-Luciferase Reporter Assays
2.6. Loss and Gain-Functional Analysis of miR-200-3p In Vivo
2.7. Statistics
3. Results
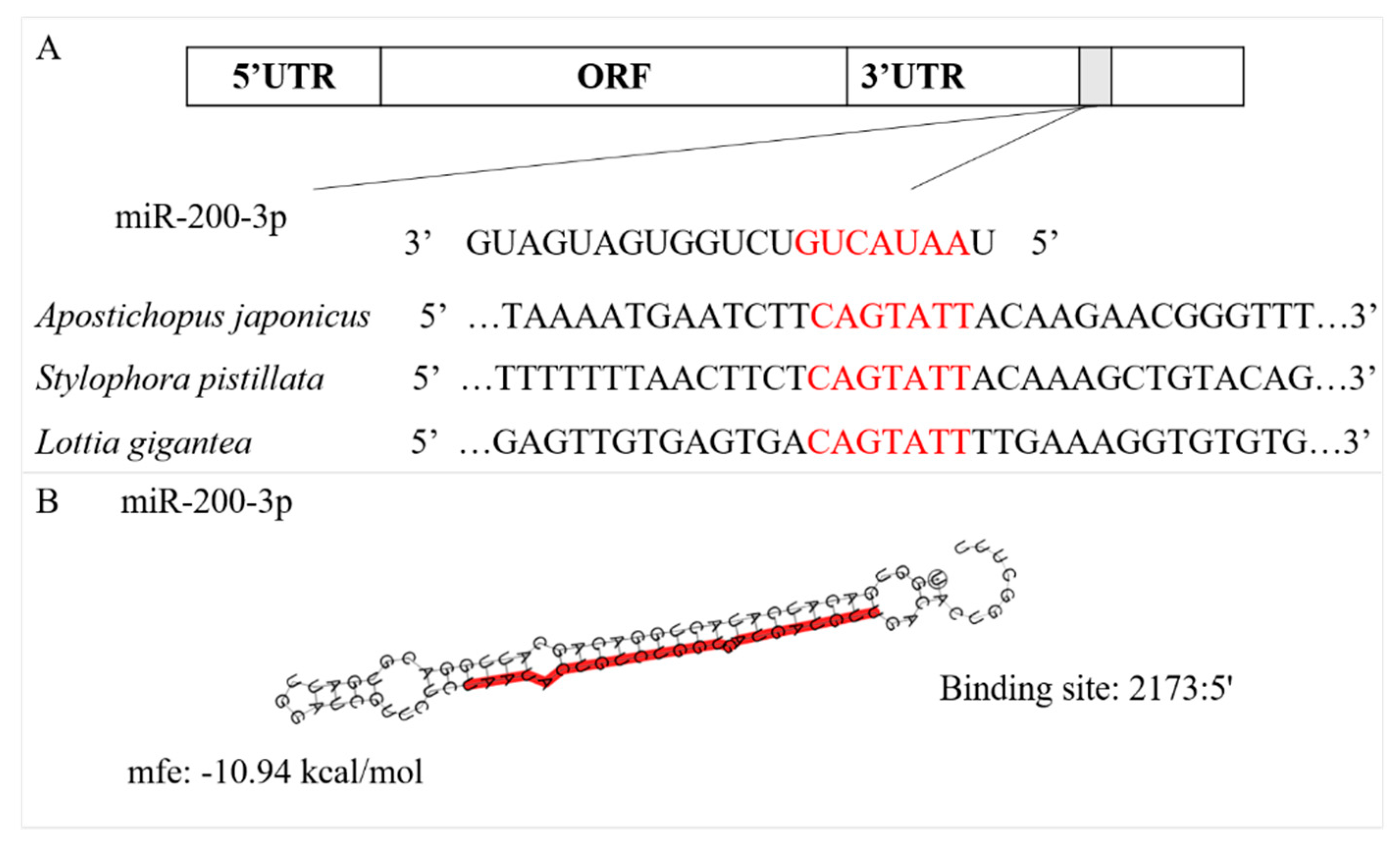
3.1. Sequence Characterization of AjCA and Target Identification of miR-200-3p
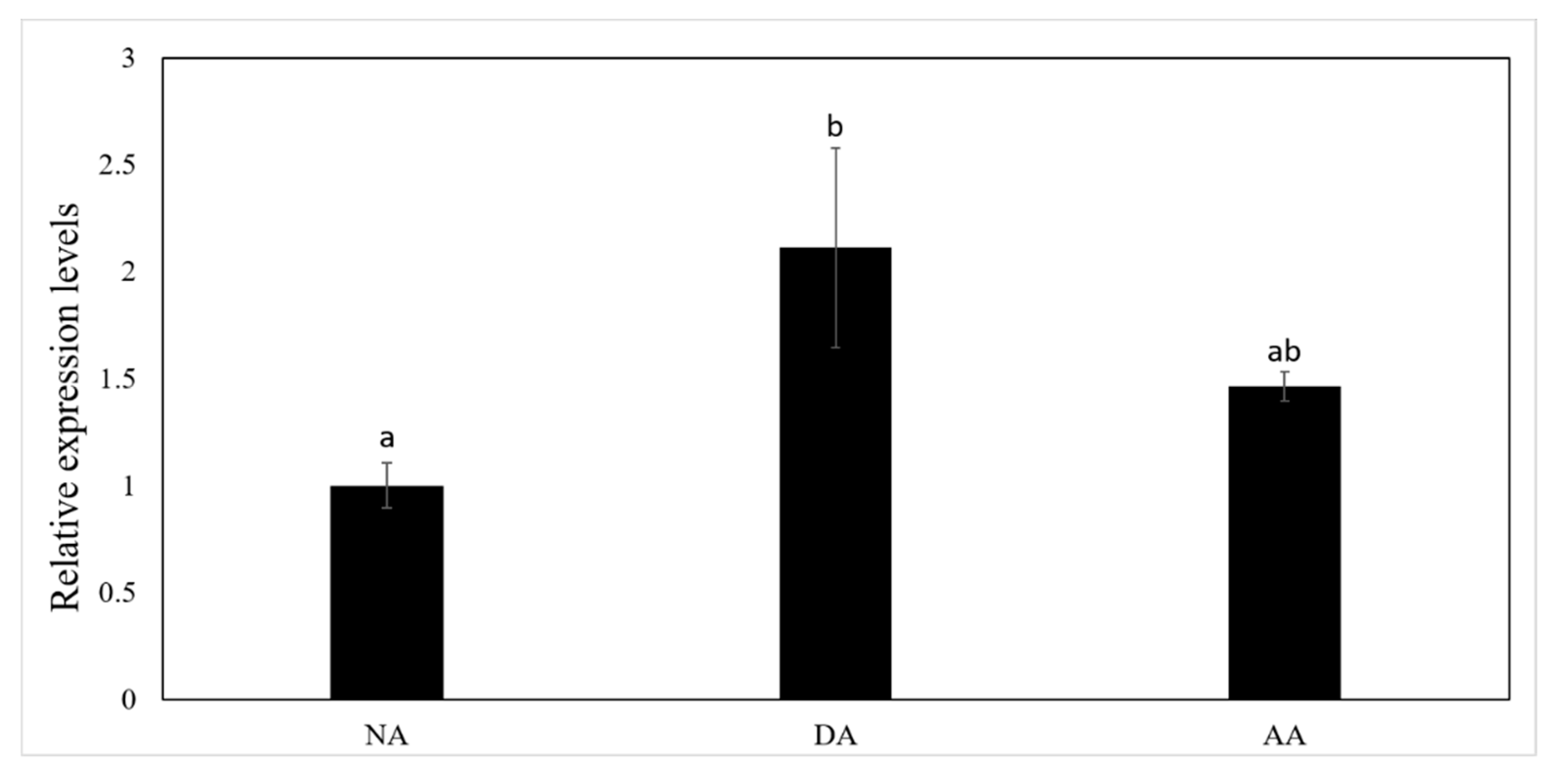
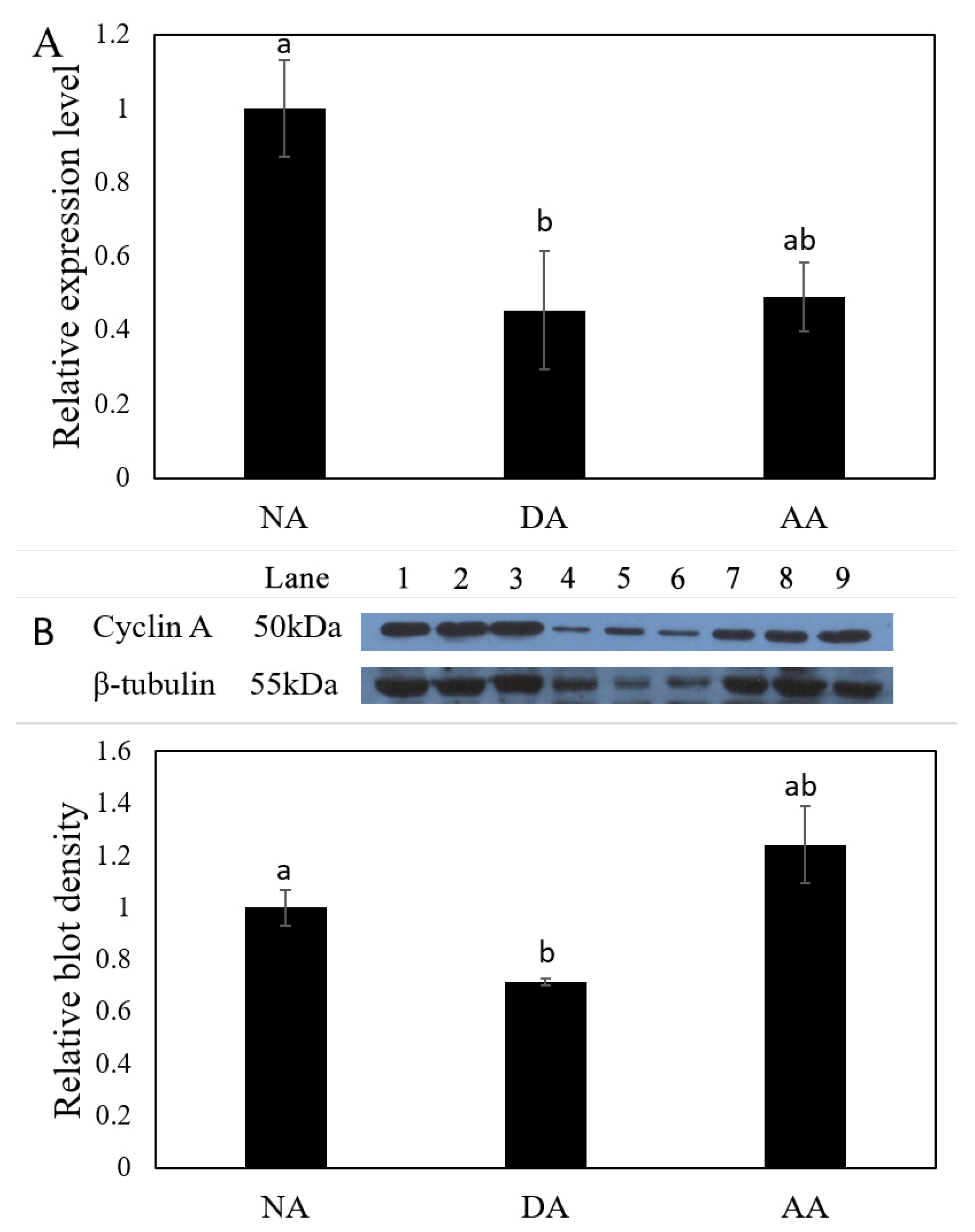
3.2. The Expression of miR-200-3p and AjCA Gene and the Production of AjCA Protein during the Whole Period of Aestivation
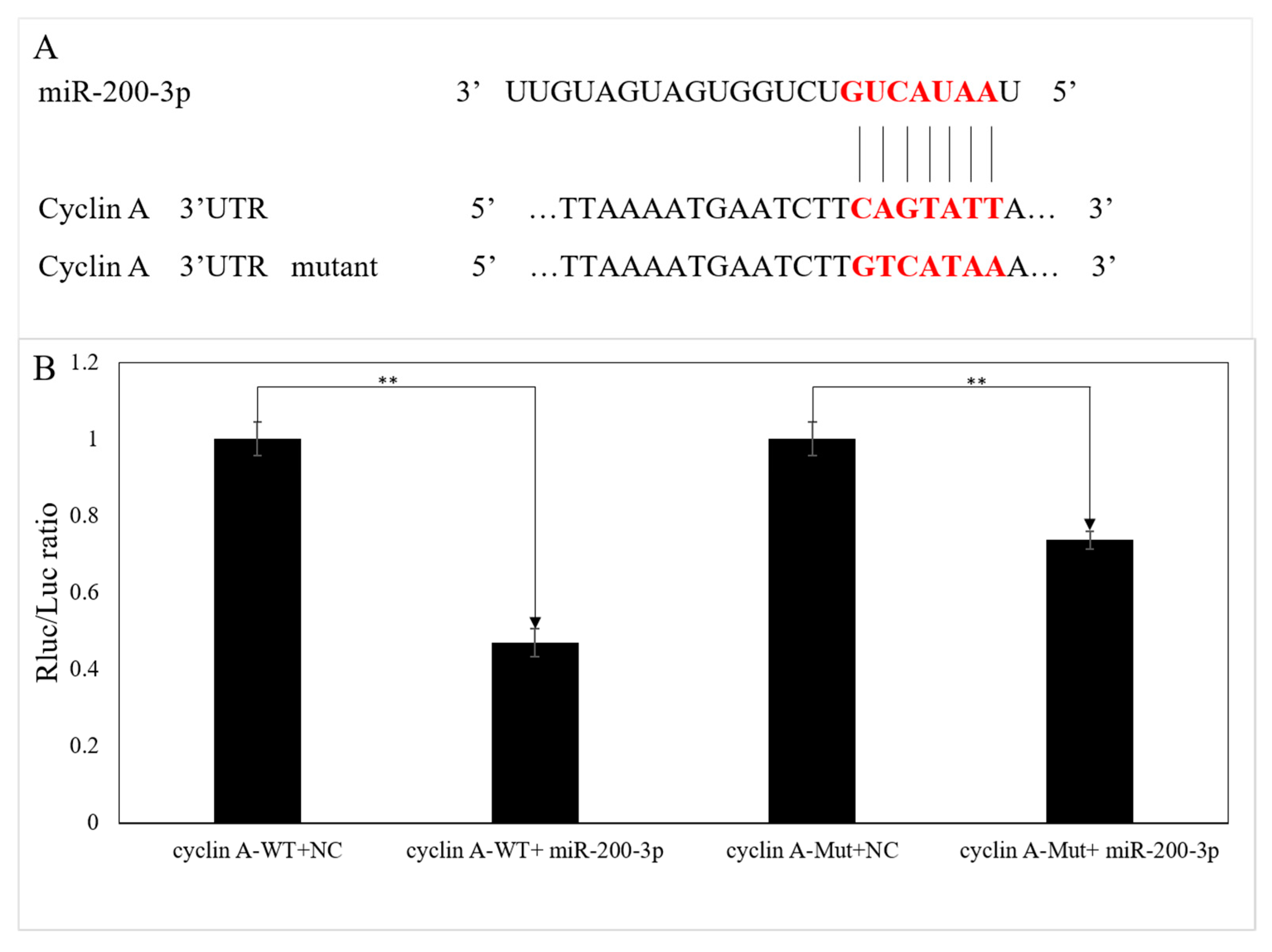
3.3. Validation of the Interaction between the 3′UTR of AjCA and miR-200-3p by Dual-Luciferase Reporter Assays
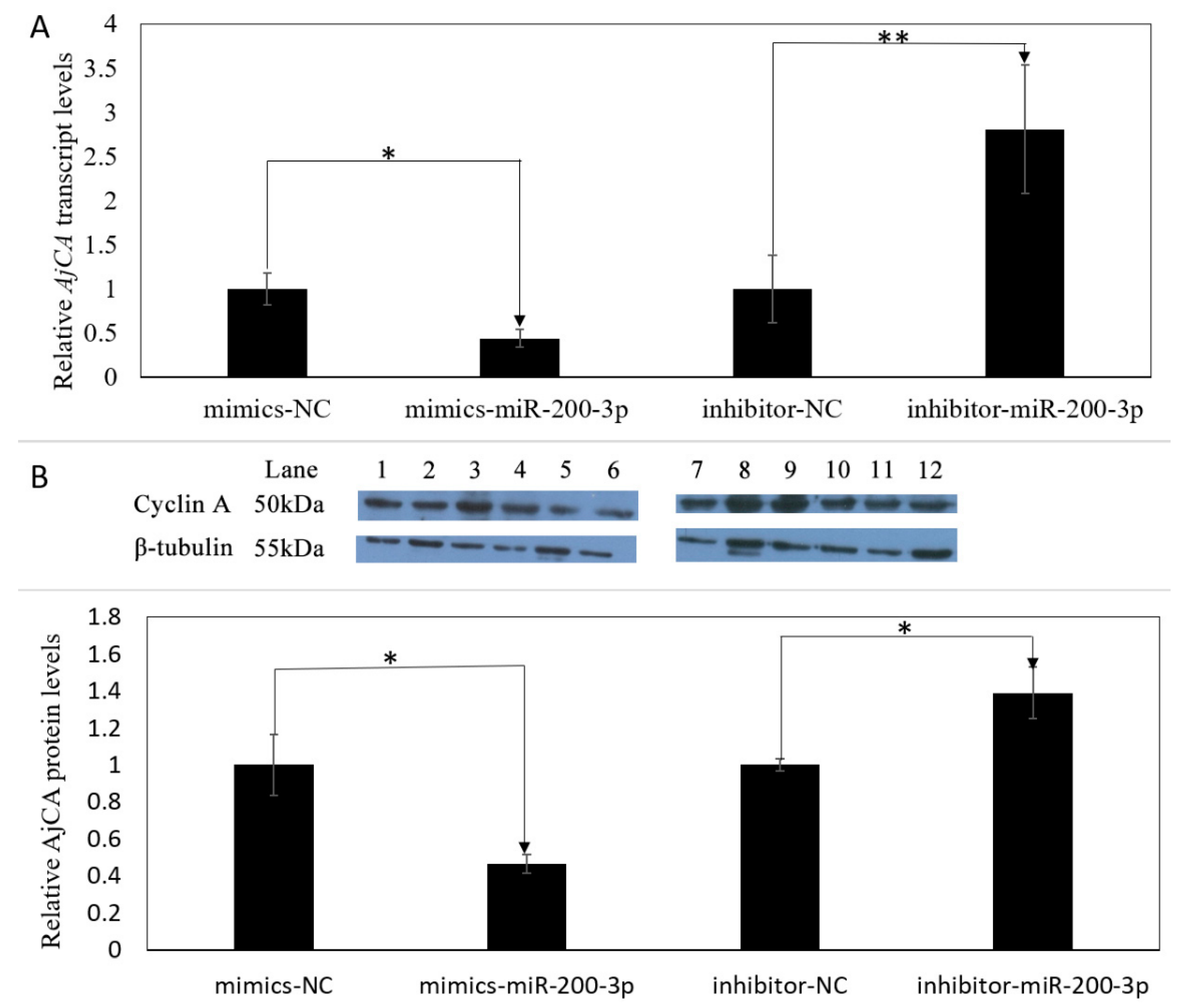
3.4. Gain and Loss of Function Analysis of miR-200-3p In Vivo
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liao, Y. Fauna Sinica: Phylum Echinodermata Class Holothuroidea; Science Press: Beijing, China, 1997; pp. 148–150. (In Chinese) [Google Scholar]
- Yang, H.; Zhou, Y.; Zhang, T.; Yuan, X.; Li, X.; Liu, Y.; Zhang, F. Metabolic characteristics of sea cucumber Apostichopus japonicus (Selenka) during aestivation. J. Exp. Mar. Biol. Ecol. 2006, 330, 505–510. [Google Scholar] [CrossRef]
- Yuan, X.; Yang, H.; Chen, M.; Gao, F. Research advances in aestivation of sea cucumber Apostichopus japonicus (Selenka): A review. Mar. Sci. 2007, 31, 88–90. [Google Scholar]
- Gao, F.; Yang, H.; Xu, Q.; Wang, F.; Liu, G.; German, D.P. Phenotypic plasticity of gut structure and function during periods of inactivity in Apostichopus japonicus. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2008, 150, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhang, X.; Liu, J.; Storey, K.B. High-throughput sequencing reveals differential expression of miRNAs in intestine from sea cucumber during aestivation. PLoS ONE 2013, 8, e76120. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yang, H.S.; Storey, K.B.; Chen, M.Y. RNA-Seq dependent transcriptional analysis unveils gene expression profile in the intestine of sea cucumber Apostichopus japonicus during aestivation, Comp. Biochem. Physiol. D 2014, 10, 30–43. [Google Scholar]
- Chen, M.; Storey, K.B. Large-scale identification and comparative analysis of miRNA expression profile in the respiratory tree of the sea cucumber Apostichopus japonicus during aestivation. Mar. Genom. 2014, 13, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Li, X.; Zhu, A.; Storey, K.B.; Sun, L.; Gao, T.; Wang, T. Understanding mechanism of sea cucumber Apostichopus japonicus aestivation: Insights from TMT-based proteomic study. Comp. Biochem. Physiol. Part D Genom. Proteom. 2016, 19, 78–89. [Google Scholar] [CrossRef]
- Chen, M.; Zhu, A.; Storey, K.B. Comparative phosphoproteomic analysis of intestinal phosphorylated proteins in active versus aestivating sea cucumbers. J. Proteom. 2016, 135, 141–150. [Google Scholar] [CrossRef]
- Zhu, A.; Chen, M.; Zhang, X.; Storey, K.B. Gene structure, expression, and DNA methylation characteristics of sea cucumber cyclin B gene during aestivation. Gene 2016, 594, 82–88. [Google Scholar] [CrossRef]
- Chen, M.; Wang, S.; Li, X.; Storey, K.B.; Zhang, X. The potential contribution of miRNA-200-3p to the fatty acid metabolism by regulating AjEHHADH during aestivation in sea cucumber. PeerJ 2018, 6, e5703. [Google Scholar] [CrossRef]
- Storey, K.B.; Storey, J.M. Putting life on ‘pause’—Molecular regulation of hypometabolism. J. Exp. Biol. 2007, 210, 1700–1714. [Google Scholar] [CrossRef] [PubMed]
- Ambros, V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 1993, 75, 843–854. [Google Scholar]
- Biggar, K.K.; Storey, K.B. Functional impact of microRNA regulation in models of extreme stress adaptation. J. Mol. Cell Biol. 2018, 10, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Hu, W.; Wang, H.; Lu, M.; Shao, C.; Menzel, C.; Yan, Z.; Li, Y.; Zhao, S.; Khaitovich, P.; et al. Genomic analysis of miRNAs in an extreme mammalian hibernator, the Arctic ground squirrel. Physiol. Genom. 2010, 42, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Biggar, K.K.; Storey, K.B. The emerging roles of microRNAs in the molecular responses of metabolic rate depression. J. Mol. Cell Biol. 2011, 3, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Kornfeld, S.F.; Biggar, K.K.; Storey, K.B. Differential expression of mature microRNAs involved in muscle maintenance of hibernating little brown bats, Myotis lucifugus: A model of muscle atrophy resistance. Genom. Proteom. Bioinform. 2012, 10, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Biggar, K.K.; Storey, K.B. Identification and expression of microRNA in the brain of hibernating bats, Myotis lucifugus. Gene 2014, 544, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Lang-Ouellette, D.; Morin, P.J. Differential expression of miRNAs with metabolic implications in hibernating thirteen-lined ground squirrels, Ictidomys tridecemlineatus. Mol. Cell. Biochem. 2014, 394, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Luu, B.E.; Biggar, K.K.; Wu, C.W.; Storey, K.B. Torpor-responsive expression of novel microRNAs regulating metabolism and other cellular pathways in the thirteen-lined ground squirrel, Ictidomys tridecemlineatus. FEBS Lett. 2016, 590, 3574–3582. [Google Scholar] [CrossRef] [PubMed]
- Hadj-Moussa, H.; Moggridge, J.A.; Luu, B.E.; Quintero-Galvis, J.F.; Gaitán-Espitia, J.D.; Nespolo, R.F.; Storey, K.B. The hibernating South American marsupial, Dromiciops gliroides, displays torpor-sensitive microRNA expression patterns. Sci. Rep. 2016, 6, 24627. [Google Scholar] [CrossRef]
- Biggar, K.K.; Storey, K.B. Perspectives in cell cycle regulation: Lessons from an anoxic vertebrate. Curr. Genom. 2009, 10, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.W.; Storey, K.B. Pattern of cellular quiescence over the hibernation cycle in liver of thirteen-lined ground squirrels. Cell Cycle 2012, 11, 1714–1726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swenson, K.; Farrell, K.M.; Ruderman, J.V. The clam embryo protein cyclin A induces entry into M phase and the resumption of meiosis in Xenopus oocytes. Cell 1986, 47, 861–870. [Google Scholar] [CrossRef]
- Kanakkanthara, A.; Jeganathan, K.B.; Limzerwala, J.F.; Baker, D.J.; Hamada, M.; Nam, H.J.; Deursen, W.H.; Hamada, N.; Naylor, R.M.; Becker, N.A.; et al. Cyclin A2 is an RNA binding protein that controls Mre11 mRNA translation. Science 2016, 353, 1549–1552. [Google Scholar] [CrossRef] [PubMed]
- Barlat, I.; Fesquet, D.; Brechot, C.; Henglein, B.; Dangeac, A.D.; Vie, A.; Blanchard, J.M. Loss of the G1-S control of cyclin A expression during tumoral progression of Chinese hamster lung fibroblasts. Cell Growth Differ. 1993, 4, 105–113. [Google Scholar] [PubMed]
- Gramantieri, L.; Ferracin, M.; Fornari, F.; Veronese, A.; Sabbioni, S.; Liu, C.G.; Calin, G.A.; Giovannini, C.; Ferrazzi, E.; Grazi, G.L.; et al. Cyclin G1 is a target of miR-122a, a microRNA frequently down-regulated in human hepatocellular carcinoma. Cancer Res. 2007, 67, 6092–6099. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhao, L.J.; Tan, Y.X.; Ren, H.; Qi, Z.T. MiR-138 induces cell cycle arrest by targeting cyclin D3 in hepatocellular carcinoma. Carcinogenesis 2012, 33, 1113–1120. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Zheng, C.; Ye, H.; Teng, Y.; Zheng, B.; Yang, X.; Zhang, J. MicroRNA-365 inhibits vascular smooth muscle cell proliferation through targeting cyclin D1. Int. J. Med. Sci. 2014, 11, 765–770. [Google Scholar] [CrossRef]
- Lines, K.E.; Newey, P.J.; Yates, C.J.; Stevenson, M.; Dyar, R.; Walls, G.V.; Bowl, M.R.; Thakker, R.V. miR-15a/miR-16-1 expression inversely correlates with cyclin D1 levels in Men1 pituitary NETs. J. Endourol. 2018, 240, 41–50. [Google Scholar] [CrossRef]
- Wang, S.S.; Li, X.K.; Chen, M.Y.; Storey, K.B.; Wang, T.M. A potential antiapoptotic regulation: The interaction of heat shock protein 70 and apoptosis-inducing factor mitochondrial 1 during heat stress and aestivation in sea cucumber. J. Exp. Zool. A Ecol. Integr. Physiol. 2018, 329, 103–111. [Google Scholar] [CrossRef]
- Obaya, A.J.; Sedivy, J.M. Regulation of cyclin-Cdk activity in mammalian cells. Cell. Mol. Life Sci. 2002, 59, 126–142. [Google Scholar] [CrossRef] [PubMed]
- Pagano, M.; Pepperkok, R.; Verde, F.; Ansorge, W.; Draetta, G. Cyclin A is required at two points in the human cell cycle. EMBO J. 1992, 11, 961–971. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.P.; William, C.E.; Jennifer, L.; Graham, J. Cell Biology, 3rd ed.; Elsevier: Philadelphia, PA, USA, 2017. [Google Scholar]
- Biggar, K.K.; Storey, K.B. Evidence for cell cycle suppression and microRNA regulation of cyclin D1 during anoxia exposure in turtles. Cell Cycle 2012, 11, 1705–1713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, X.; Wang, Z.; Fillmore, R.; Xi, Y. MiR-200, a new star miRNA in human cancer. Cancer Lett. 2014, 344, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Pautke, C.; Schieker, M.; Tischer, T.; Kolk, A.; Neth, P.; Mutschler, W.; Milz, S. Characterization of osteosarcoma cell lines MG-63, Saos-2 and U-2 OS in comparison to human osteoblasts. Anticancer Res. 2004, 24, 3743–3748. [Google Scholar] [PubMed]
- Xiao, Y.; Yan, W.; Lu, L.; Wang, Y.; Lu, W.; Cao, Y.; Cai, W. p38/p53/miR-200a-3p feedback loop promotes oxidative stress-mediated liver cell death. Cell Cycle. 2015, 14, 1548–1558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, L.; Chen, M.; Wang, T.; Liu, J.; Zhao, Y.; Yang, H. The division of different stages of aestivation and quantitative analysis of tissue layers thickness of anterior intestine in sea cucumber Apostichopus japonicus. Mar. Sci. 2012, 36, 1–5. [Google Scholar]
- Li, Y.; Wu, Y. MiR-200-3p inhibits tumor cell proliferation and induces apoptosis by upregulation of FOXO1 in osteosarcoma cells. Mol. Cell. Toxicol. 2018, 14, 73–78. [Google Scholar] [CrossRef]
- Storey, K.B.; Storey, J.M. Metabolic rate depression in animals: Transcriptional and translational controls. Biol. Rev. Camb. Philos. Soc. 2004, 79, 207–233. [Google Scholar] [CrossRef] [PubMed]

| Name | Primer Sequences (5′–3′) | Location | |
|---|---|---|---|
| RACE | AjCA-F1 | AGCTGGTCGGAACGGCAAGTGCA | 1269–1291 |
| AjCA-R1 | GATTGTCGGGACAGCCAGATCAAAGGAC | 1414–1441 | |
| qRT-PCR | miR-200-3p-F | TAATACTGTCTGGTGATGATG | |
| miR-200-3p-R | mRQ 3′primer | ||
| 5.8s-F | ATCACTCGGCTCGTGCGTC | ||
| 5.8s-R | GCCATTTGCGTTCGAATAAGT | ||
| AjCA-RT-F | TATCAAGGCCAGCGACGAAGGAG | 1461–1448 | |
| AjCA-RT-R | GGAGATGCAATGTGTGTCGAGCC | 1594–1616 | |
| β-actin-F | AAGGTTATGCTCTTCCTCACGCT | ||
| β-actin-R | GATGTCACGGACGATTTCACG | ||
| β-Tubulin-F | GAAAGCCTTACGACGGAACA | ||
| β-Tubulin-R | CACCACGTGGACTCAAAATG | ||
| In vivo | miR-200-3p mimics-F | UAAUACUGUCUGGUGAUGAUG | |
| miR-200-3p mimics-R | UCAUCACCAGACAGUAUUAUU | ||
| miR-200-3p mimics-NC-F | GAGAUGUUCAAUCGGGUAUUU | ||
| miR-200-3p mimics-NC-R | AUACCCGAUUGAACAUCUCUU | ||
| miR-200-3p inhibitor | CAUCAUCACCAGACAGUAUUA | ||
| miR-200-3p inhibitor-NC | GAAUUACAUGCACCACUCAAU | ||
| Dual-luciferase | AjCA-WT-F | GCGGCTCGAGGACATCTCCAAATTAAAGGA | 1806–1836 |
| AjCA-WT-R | AATGCGGCCGCGTAACACTTATGTACAAATG | 3010–3029 | |
| AjCA-MUT-F | TGAATCTTGTCATAAACAAGAACGGGTTTTTAC | 2164–2197 | |
| AjCA-MUT-R | GTTCTTGTTTATGACAAGATTCATTTTAAAAAC | 2155–2187 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Chen, M.; Yin, Y.; Storey, K.B. MiR-200-3p Is Potentially Involved in Cell Cycle Arrest by Regulating Cyclin A during Aestivation in Apostichopus japonicus. Cells 2019, 8, 843. https://doi.org/10.3390/cells8080843
Wang S, Chen M, Yin Y, Storey KB. MiR-200-3p Is Potentially Involved in Cell Cycle Arrest by Regulating Cyclin A during Aestivation in Apostichopus japonicus. Cells. 2019; 8(8):843. https://doi.org/10.3390/cells8080843
Chicago/Turabian StyleWang, Shanshan, Muyan Chen, Yingchao Yin, and Kenneth B. Storey. 2019. "MiR-200-3p Is Potentially Involved in Cell Cycle Arrest by Regulating Cyclin A during Aestivation in Apostichopus japonicus" Cells 8, no. 8: 843. https://doi.org/10.3390/cells8080843





