NK Cell Induced T Cell Anergy Depends on GRAIL Expression
Abstract
1. Introduction
2. Material and Methods
2.1. Animals
2.2. Reagents
2.3. In Vitro Preparation of HINT1/Hsp70 Complexes
2.4. EAE Induction and Pretreatment with HINT1/Hsp70 Complex
2.5. Magnetic Beads and FACS Cell Sorting
2.6. Flow Cytometry
2.7. CD4+ T Cell Proliferation Assay
2.8. In Vitro Cell Cocultures
2.9. Cell Transfection with siRNA or pcDNA Vector
2.10. Cell Cytotoxicity
2.11. Gel Electrophoresis and Western Blot
2.12. qRT-PCR
2.13. GRAIL Vector Construction
2.14. Statistical Methods
3. Results
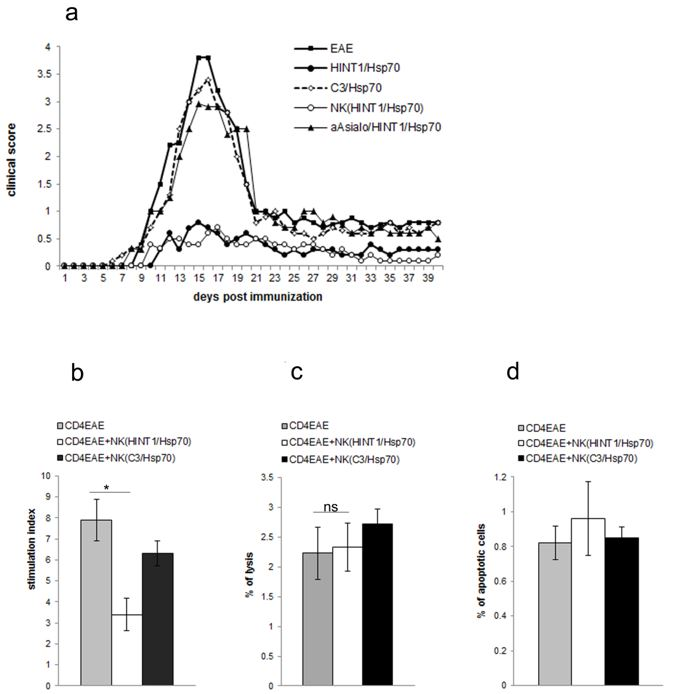
3.1. EAE Inhibition Induced by HINT1/Hsp70 Depends on NK Cells
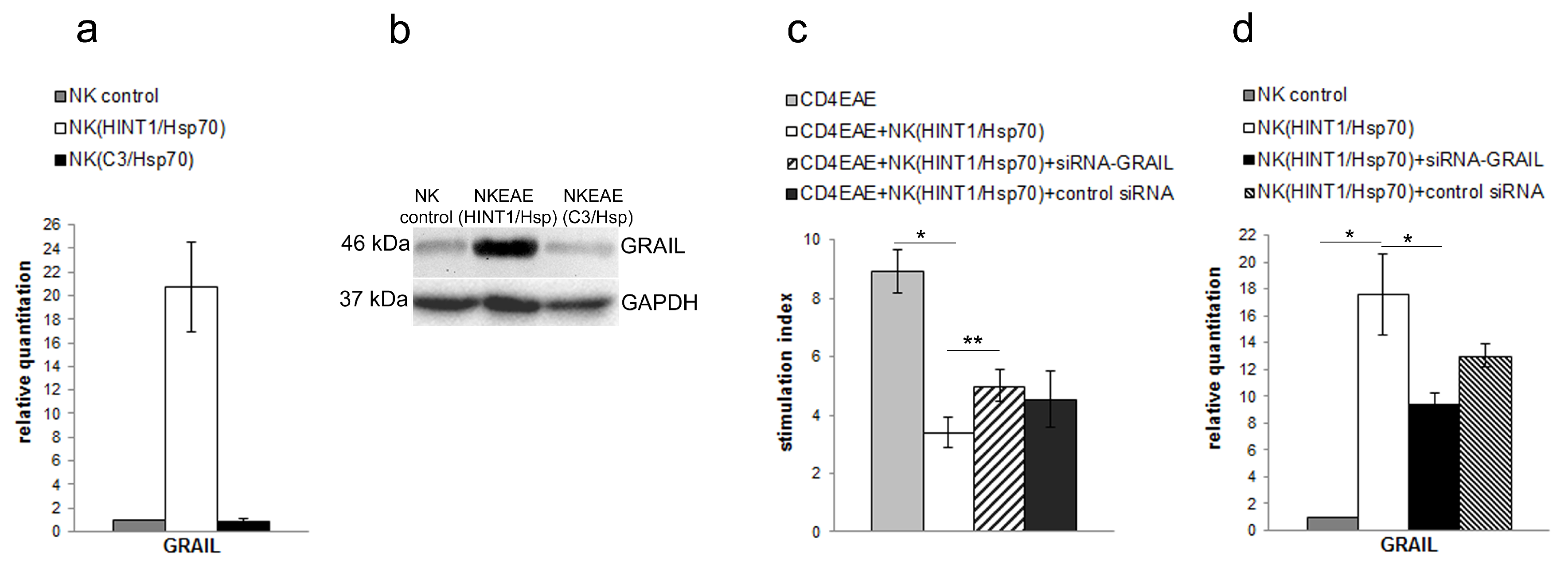
3.2. GRAIL Expression in CD4+ T Cells Is Indispensable for Inhibition of CD4+ T Cells Proliferation
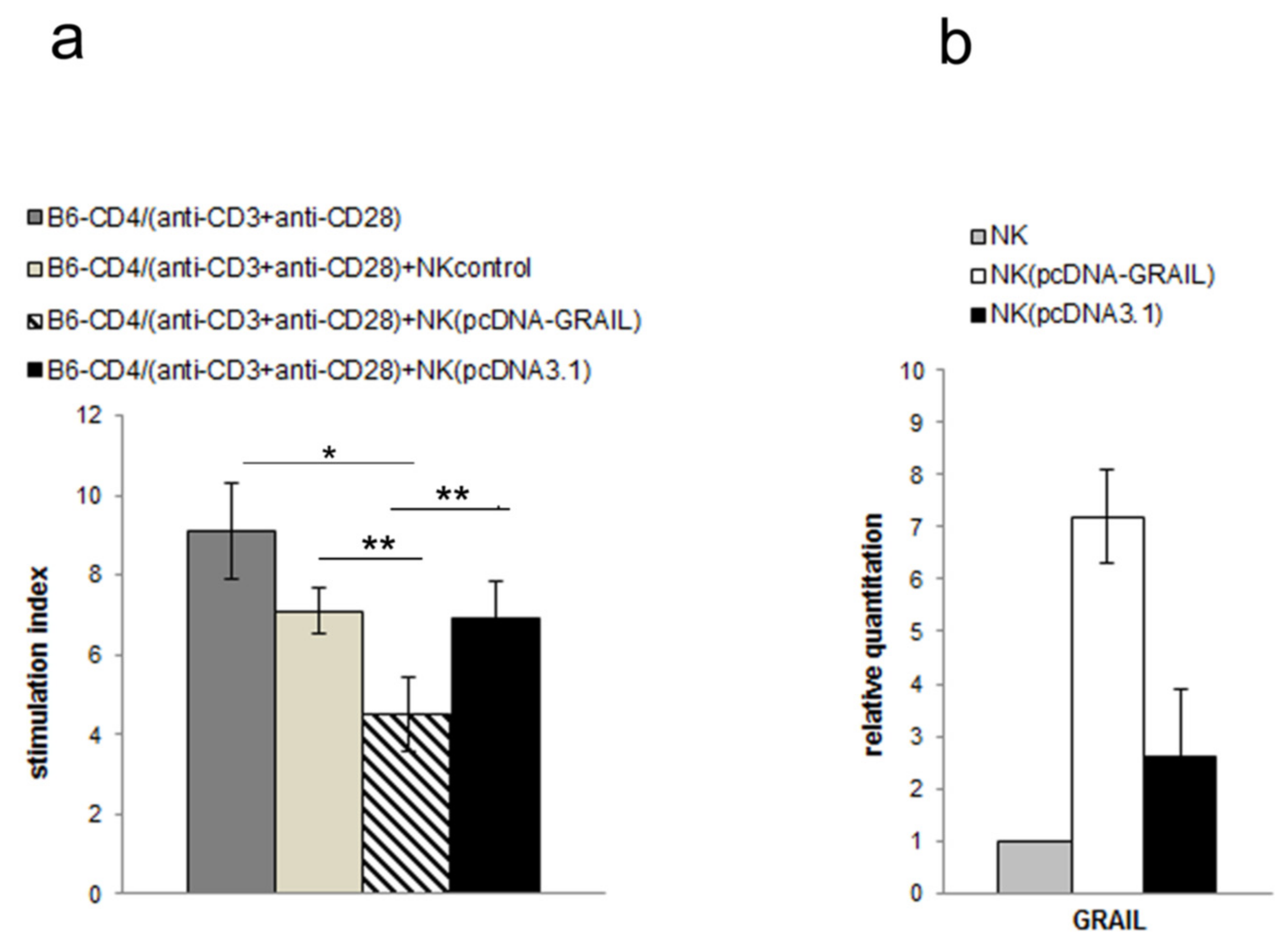
3.3. NK Cell Inhibitory Effect Depends on GRAIL Expression
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gianchecchi, E.; Delfino, D.V.; Fierabracci, A. NK cells in autoimmune diseases: Linking innate and adaptive immune responses. Autoimmun. Rev. 2018, 17, 142–154. [Google Scholar] [CrossRef] [PubMed]
- Dobson, R.; Giovannoni, G. Multiple sclerosis: A review. Eur. J. Neurol. 2019, 26, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Goverman, J. Autoimmune T cell responses in the central nervous system. Nat. Rev. Immunol. 2009, 9, 393–407. [Google Scholar] [CrossRef] [PubMed]
- Sloan-Lancaster, J.; Evavold, B.; Allen, P. Induction of T-cell anergy by altered T-cell-receptor ligand on live antigen-presenting cells. Nature 1993, 363, 156–159. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, M.; Schwartz, R. Antigen presentation by chemically modified splenocytes induces antigen-specific T cell unresponsiveness in vitro and in vivo. J. Exp. Med. 1987, 165, 302–319. [Google Scholar] [CrossRef] [PubMed]
- Anandasabapathy, N.; Ford, G.S.; Bloom, D.; Holness, C.; Paragas, V.; Seroogy, C.; Skrenta, H.; Hollenhorst, M.; Fathman, C.; Soares, L. GRAIL: An E3 ubiquitin ligase that inhibits cytokine gene transcription is expressed in anergic CD4+ T cells. Immunity 2003, 18, 457–535. [Google Scholar] [CrossRef]
- Shartner, J.; Simonson, W.T.; Wernimont, S.A.; Nettenstrom, L.M.; Huttenlocher, A.; Seroogy, C. Gene related to anergy in lymphocytes (GRAIL) expression in CD4+ T cells impairs actin cytoskeletal organization during T cell/antigen-presenting cell interactions. J. Biol. Chem. 2009, 284, 34674–34681. [Google Scholar] [CrossRef] [PubMed]
- Aziz, M.; Yang, W.-L.L.; Matsuo, S.; Sharma, A.; Zhou, M.; Wang, P. Upregulation of GRAIL is associated with impaired CD4 T cell proliferation in sepsis. J. Immunol. 2014, 192, 2305–2314. [Google Scholar] [CrossRef]
- Ichikawa, D.; Mizuno, M.; Yamamura, T.; Miyake, S. GRAIL (gene related to anergy in lymphocytes) regulates cytoskeletal reorganization through ubiquitination and degradation of Arp2/3 subunit 5 and coronin 1A. J. Biol. Chem. 2011, 286, 43465–43474. [Google Scholar] [CrossRef]
- Su, L.L.; Iwai, H.; Lin, J.T.; Fathman, C. The transmembrane E3 ligase GRAIL ubiquitinates and degrades CD83 on CD4 T cells. J. Immunol. 2009, 183, 438–444. [Google Scholar] [CrossRef]
- Nurieva, R.I.; Zheng, S.; Jin, W.; Chung, Y.; Zhang, Y.; Martinez, G.J.; Reynolds, J.M.; Wang, S.L.L.; Lin, X.; Sun, S.C.C.; et al. The E3 ubiquitin ligase GRAIL regulates T cell tolerance and regulatory T cell function by mediating T cell receptor-CD3 degradation. Immunity 2010, 32, 670–680. [Google Scholar] [CrossRef] [PubMed]
- Shih, C.C.C.; Liu, P.Y.Y.; Chen, J.H.H.; Liao, M.H.H.; Hsieh, C.M.M.; Ka, S.M.M.; Wu, C.C.C.; Lin, H.T.T.; Wu, T.H.H.; Chen, Y.C.C. Macrophage expression of E3 ubiquitin ligase Grail protects mice from lipopolysaccharide-induced hyperinflammation and organ injury. PLoS ONE 2018, 13, e0208279. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Liu, B.; Li, Z.; Wu, H.; Wang, P.; Zhao, K.; Jiang, G.; Zhang, L.; Gao, C. E3 ubiquitin ligase RNF128 promotes innate antiviral immunity through K63-linked ubiquitination of TBK1. Nat. Immunol. 2016, 17, 1342. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.C.; Chan, J.; Chiu, Y.L.L.; Liu, S.T.T.; Lozano, G.; Wang, S.L.L.; Ho, C.L.L.; Huang, S.M.M. Grail as a molecular determinant for the functions of the tumor suppressor p53 in tumorigenesis. Cell Death Differ. 2013, 20, 732–743. [Google Scholar] [CrossRef] [PubMed]
- Elkins, J.; Sheridan, J.; Amaravadi, L.; Riester, K.; Selmaj, K.; Bielekova, B.; Parr, E.; Giovannoni, G. CD56(bright) natural killer cells and response to daclizumab HYP in relapsing-remitting MS. Neurol. (R) Neuroimmunol. Neuroinflamma. 2015, 2, e65. [Google Scholar] [CrossRef] [PubMed]
- Bielekova, B.; Catalfamo, M.; Reichert-Scrivner, S.; Packer, A.; Cerna, M.; Waldmann, T.A.; McFarland, H.; Henkart, P.A.; Martin, R. Regulatory CD56(bright) natural killer cells mediate immunomodulatory effects of IL-2Ralpha-targeted therapy (daclizumab) in multiple sclerosis. Proc. Natl. Acad. Sci. USA 2006, 103, 5941–5946. [Google Scholar] [CrossRef] [PubMed]
- Putzki, N.; Baranwal, M.K.; Tettenborn, B.; Limmroth, V.; Kreuzfelder, E. Effects of natalizumab on circulating B cells, T regulatory cells and natural killer cells. Eur. Neurol. 2010, 63, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Saraste, M.; Irjala, H.; Airas, L. Expansion of CD56Bright natural killer cells in the peripheral blood of multiple sclerosis patients treated with interferon-beta. Neurol. Sci. Off. J. Ital. Neurol. Soc. Ital. Soc. Clin. Neurophysiol. 2007, 28, 121–126. [Google Scholar] [CrossRef]
- Nielsen, N.; Ødum, N.; Ursø, B.; Lanier, L.L.; Spee, P. Cytotoxicity of CD56(bright) NK cells towards autologous activated CD4+ T cells is mediated through NKG2D, LFA-1 and TRAIL and dampened via CD94/NKG2A. PLoS ONE 2012, 7, e31959. [Google Scholar] [CrossRef] [PubMed]
- Gross, C.C.; Schulte-Mecklenbeck, A.; Rünzi, A.; Kuhlmann, T.; Posevitz-Fejfár, A.; Schwab, N.; Schneider-Hohendorf, T.; Herich, S.; Held, K.; Konjević, M.; et al. Impaired NK-mediated regulation of T-cell activity in multiple sclerosis is reconstituted by IL-2 receptor modulation. Proc. Natl. Acad. Sci. USA 2016, 113, E2973–E2982. [Google Scholar] [CrossRef]
- Zhang, B.; Yamamura, T.; Kondo, T.; Fujiwara, M.; Tabira, T. Regulation of experimental autoimmune encephalomyelitis by natural killer (NK) cells. J. Exp. Med. 1997, 186, 1677–1687. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Campagnolo, D.; Liu, R.; Piao, W.; Shi, S.; Hu, B.; Xiang, R.; Zhou, Q.; Vollmer, T.; Kaer, L.; et al. Interleukin-2/interleukin-2 antibody therapy induces target organ natural killer cells that inhibit central nervous system inflammation. Ann. Neurol. 2011, 69, 721–734. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Liu, R.; Piao, W.; Zhou, Q.; Vollmer, T.L.; Campagnolo, D.I.; Xiang, R.; Cava, A.; Kaer, L.; Shi, F.D.D. Central nervous system (CNS)-resident natural killer cells suppress Th17 responses and CNS autoimmune pathology. J. Exp. Med. 2010, 207, 1907–1921. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Shi, F.D.D.; Jung, S.; Pien, G.C.; Wang, J.; Salazar-Mather, T.P.; He, T.T.; Weaver, J.T.; Ljunggren, H.-G.G.; Biron, C.A.; et al. The neuronal chemokine CX3CL1/fractalkine selectively recruits NK cells that modify experimental autoimmune encephalomyelitis within the central nervous system. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2006, 20, 896–905. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Li, D.; Han, R.; Zhang, C.; Jin, W.-N.N.; Wood, K.; Liu, Q.; Shi, F.-D.D.; Hao, J. Acetylcholine-producing NK cells attenuate CNS inflammation via modulation of infiltrating monocytes/macrophages. Proc. Natl. Acad. Sci. USA 2017, 114, E6202–E6211. [Google Scholar] [CrossRef] [PubMed]
- Galazka, G.; Jurewicz, A.; Domowicz, M.; Cannella, B.; Raine, C.S.; Selmaj, K. HINT1 peptide/Hsp70 complex induces NK-cell-dependent immunoregulation in a model of autoimmune demyelination. Eur. J. Immunol. 2014, 44, 3026–3044. [Google Scholar] [CrossRef] [PubMed]
- Skov, S.; Klausen, P.; Claesson, M. Ligation of major histocompatability complex (MHC) class I molecules on human T cells induces cell death through PI-3 kinase-induced c-Jun NH2-terminal kinase activity: A novel apoptotic pathway distinct from Fas-induced apoptosis. J. Cell Biol. 1997, 139, 1523–1531. [Google Scholar] [CrossRef] [PubMed]
- Galazka, G.; Jurewicz, A.; Orlowski, W.; Stasiolek, M.; Brosnan, C.F.; Raine, C.S.; Selmaj, K. EAE tolerance induction with Hsp70-peptide complexes depends on H60 and NKG2D activity. J. Immunol. 2007, 179, 4503–4512. [Google Scholar] [CrossRef] [PubMed]
- Galazka, G.; Stasiolek, M.; Walczak, A.; Jurewicz, A.; Zylicz, A.; Brosnan, C.F.; Raine, C.S.; Selmaj, K.W. Brain-derived heat shock protein 70-peptide complexes induce NK cell-dependent tolerance to experimental autoimmune encephalomyelitis. J. Immunol. 2006, 176, 1588–1599. [Google Scholar] [CrossRef]
- Kozela, E.; Juknat, A.; Kaushansky, N.; Ben-Nun, A.; Coppola, G.; Vogel, Z. Cannabidiol, a non-psychoactive cannabinoid, leads to EGR2-dependent anergy in activated encephalitogenic T cells. J. Neuroinflammation 2015, 12, 52. [Google Scholar] [CrossRef]
- Shi, F.-D.D.; Kaer, L. Reciprocal regulation between natural killer cells and autoreactive T cells. Nat. Rev. Immunol. 2006, 6, 751–760. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galazka, G.; Domowicz, M.; Ewiak-Paszynska, A.; Jurewicz, A. NK Cell Induced T Cell Anergy Depends on GRAIL Expression. Cells 2019, 8, 790. https://doi.org/10.3390/cells8080790
Galazka G, Domowicz M, Ewiak-Paszynska A, Jurewicz A. NK Cell Induced T Cell Anergy Depends on GRAIL Expression. Cells. 2019; 8(8):790. https://doi.org/10.3390/cells8080790
Chicago/Turabian StyleGalazka, Grazyna, Malgorzata Domowicz, Alicja Ewiak-Paszynska, and Anna Jurewicz. 2019. "NK Cell Induced T Cell Anergy Depends on GRAIL Expression" Cells 8, no. 8: 790. https://doi.org/10.3390/cells8080790
APA StyleGalazka, G., Domowicz, M., Ewiak-Paszynska, A., & Jurewicz, A. (2019). NK Cell Induced T Cell Anergy Depends on GRAIL Expression. Cells, 8(8), 790. https://doi.org/10.3390/cells8080790




