Naturally Occurring Nervonic Acid Ester Improves Myelin Synthesis by Human Oligodendrocytes
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Autoimmune Encephalomyelitis (EAE) Mouse Model
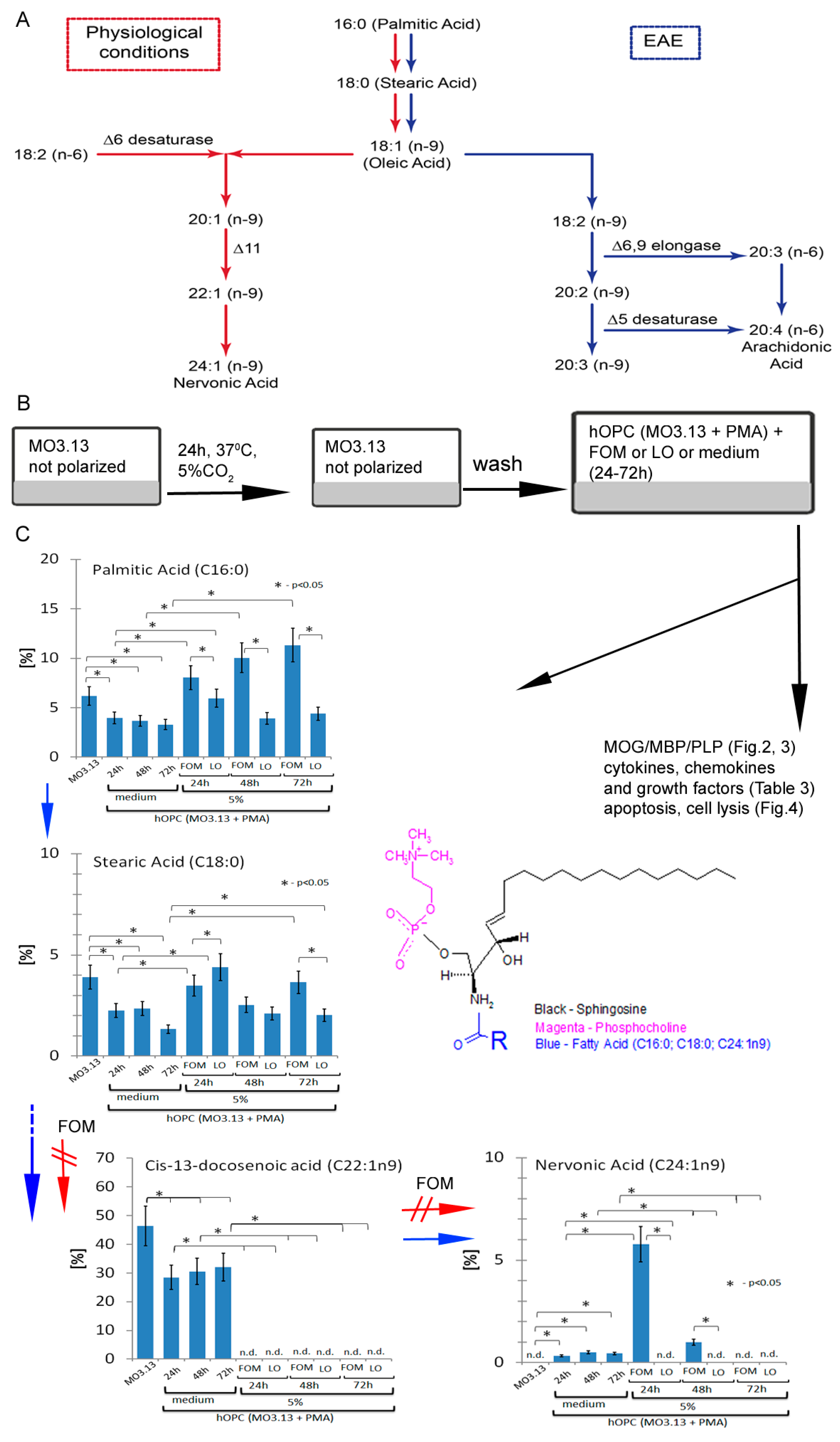
2.2. Human Cell Model of Progenitor Cell Differentiation to Mature Myelin-Producing Oligodendrocytes
2.3. hOPC Supplementation with Fish Oil Mixture
2.4. Lactate Dehydrogenase (LDH) Release Assay
2.5. Immunocytochemical Analysis (ICC)
2.6. RNA Isolation and Total mRNA Concentration Analysis
2.7. Quantitative Real-Time PCR (qRT-PCR)
2.8. Lipid Profiling for Free and Esterified Fatty Acids by Gas Chromatography-Mass Spectrometry (GC/MS)
2.9. Cytokine, Chemokine, and Growth Factor Analysis by Human Cytokine Multiple Profiling Assays
2.10. Statistics
3. Results
3.1. Dysregulated Lipid Composition in EAE Brain in Comparison to Healthy Mouse Brain
3.2. Maturating hOPCs Produce Nervonic Acid
3.3. hOPCs Incorporate and Metabolize Nervonic Acid
3.4. FOM Affects Maturing hOPCs Resulting in Increased Production of Myelin Proteins
3.5. FOM Affects hOPC Production of Cytokines, Chemokines and Growth Factors
3.6. Fish Oil Mixture Promotes hOPC Survival and Prolongs OL Lifespan
4. Discussion
5. Patents
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Palavicini, J.P.; Wang, C.; Chen, L.; Ahmar, S.; Higuera, J.D.; Dupree, J.L.; Han, X. Novel molecular insights into the critical role of sulfatide in myelin maintenance/function. J. Neurochem. 2016, 139, 40–54. [Google Scholar] [CrossRef] [PubMed]
- Prineas, J.W.; Connell, F. Remyelination in multiple sclerosis. Ann. Neurol. 1979, 5, 22–31. [Google Scholar] [CrossRef]
- Schwarz, S.; Leweling, H. Multiple sclerosis and nutrition. Mult. Scler. 2005, 11, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Xiang, M.; Alfvén, G.; Blennow, M.; Trygg, M.; Zetterström, R. Long-chain polyunsaturated fatty acids in human milk and brain growth during early infancy. Acta Paediatr. 2000, 89, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Bramow, S.; Frischer, J.M.; Lassmann, H.; Koch-Henriksen, N.; Lucchinetti, C.F.; Sørensen, P.S.; Laursen, H. Demyelination versus remyelination in progressive multiple sclerosis. Brain 2010, 133, 2983–2998. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Dreyfus, C.F. The role of growth factors as a therapeutic approach to demyelinating disease. Exp. Neurol. 2016, 283, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Mills, J.D.; Bailes, J.E.; Sedney, C.L.; Hutchins, H.; Sears, B. Omega-3 fatty acid supplementation and reduction of traumatic axonal injury in a rodent head injury model. J. Neurosurg. 2011, 114, 77–84. [Google Scholar] [CrossRef]
- Kong, W.; Yen, J.H.; Ganea, D. Docosahexaenoic acid prevents dendritic cell maturation, inhibits antigen-specific Th1/Th17 differentiation and suppresses experimental autoimmune encephalomyelitis. Brain Behav. Immun. 2011, 25, 872–882. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, H.; Pu, H.; Wang, G.; Li, W.; Leak, R.K.; Chen, J.; Liou, A.K.; Hu, X. n-3 PUFA supplementation benefits microglial responses to myelin pathology. Sci. Rep. 2014, 4, 7458. [Google Scholar] [CrossRef]
- Agranoff, B.W.; Goldberg, D. Diet and the geographical distribution of multiple sclerosis. Lancet 1974, 2, 1061–1066. [Google Scholar] [CrossRef]
- Jelinek, G.A.; Hadgkiss, E.J.; Weiland, T.J.; Pereira, N.G.; Marck, C.H.; van der Meer, D.M. Association of fish consumption and Ω 3 supplementation with quality of life, disability and disease activity in an international cohort of people with multiple sclerosis. Int. J. Neurosci. 2013, 123, 792–800. [Google Scholar] [CrossRef] [PubMed]
- Weinstock-Guttman, B.; Baier, M.; Park, Y.; Feichter, J.; Lee-Kwen, P.; Gallagher, E.; Venkatraman, J.; Meksawan, K.; Deinehert, S.; Pendergast, D.; et al. Low fat dietary intervention with omega-3 fatty acid supplementation in multiple sclerosis patients. Prostaglandins Leukot. Essent. Fat. Acids 2005, 73, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Dyall, S.C. Long-chain omega-3 fatty acids and the brain: A review of the independent and shared effects of EPA, DPA and DHA. Front. Aging Neurosci. 2015, 7, 52. [Google Scholar] [CrossRef] [PubMed]
- Riccio, P.; Rossano, R. Nutrition facts in multiple sclerosis. ASN Neuro 2015, 7. [Google Scholar] [CrossRef] [PubMed]
- Hoare, S.; Lithander, F.; van der Mei, I.; Ponsonby, A.L.; Lucas, R. Ausimmune Investigator Group. Higher intake of omega-3 polyunsaturated fatty acids is associated with a decreased risk of a first clinical diagnosis of central nervous system demyelination: Results from the Ausimmune Study. Mult. Scler 2016, 22, 884–892. [Google Scholar] [CrossRef] [PubMed]
- Siegert, E.; Paul, F.; Rothe, M.; Weylandt, K.H. The effect of omega-3 fatty acids on central nervous system remyelination in fat-1 mice. BMC Neurosci. 2017, 18, 19. [Google Scholar] [CrossRef] [PubMed]
- Salem, N., Jr.; Litman, B.; Kim, H.Y.; Gawrisch, K. Mechanism of action of docosahexaenoic acid in the nervous system. Lipids 2001, 36, 945–959. [Google Scholar] [CrossRef] [PubMed]
- Edmond, J. Essential polyunsaturated fatty acids and the barrier to the brain. J. Mol. Neurosci. 2001, 16, 181–193. [Google Scholar] [CrossRef]
- Ge, Y.; Wang, X.; Chen, Z.; Landman, N.; Lo, E.H.; Kang, J.X. Gene transfer of Caenorhabditis elegans n-3 fatty acid desaturase inhibits neuronal apoptosis. J. Neurochem. 2002, 82, 1360–1366. [Google Scholar] [CrossRef]
- Kawakita, E.; Hashimoto, M.; Shido, O. Docosahexaenoic acid promotes neurogenesis in vitro and in vivo. Neuroscience 2006, 139, 991–997. [Google Scholar] [CrossRef]
- Demar, J.C., Jr.; Ma, K.; Chang, L.; Bell, J.M.; Rapoport, S.I. Alpha-linolenic acid does not contribute appreciably to docosahexaenoic acid within brain phospholipids of adult rats fed a diet enriched in docosahexaenoic acid. J. Neurochem. 2005, 94, 1063–1076. [Google Scholar] [CrossRef] [PubMed]
- Ouellet, M.; Emond, V.; Chen, C.T.; Julien, C.; Bourasset, F.; Oddo, S. Diffusion of docosahexaenoic and eicosapentaenoic acids through the blood-brain barrier: an in situ cerebral perfusion study. Neurochem. Int. 2009, 55, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Martínez, M.; Mougan, I. Fatty acid composition of human brain phospholipids during normal development. J. Neurochem. 1998, 71, 2528–2533. [Google Scholar] [CrossRef] [PubMed]
- Amminger, G.P.; Schäfer, M.R.; Klier, C.M.; Slavik, J.M.; Holzer, I.; Holub, M.; Goldstone, S.; Whitford, T.J.; McGorry, P.D.; Berk, M. Decreased nervonic acid levels in erythrocyte membranes predict psychosis in help-seeking ultra-high-risk individuals. Mol. Psychiatry 2012, 17, 1150–1152. [Google Scholar] [CrossRef] [PubMed]
- Kageyama, Y.; Kasahara, T.; Nakamura, T.; Hattori, K.; Deguchi, Y.; Tani, M.; Kuroda, K.; Yoshida, S.; Goto, Y.I.; Inoue, K.; et al. Plasma Nervonic Acid Is a Potential Biomarker for Major Depressive Disorder: A Pilot Study. Int. J. Neuropsychopharmacol. 2018, 21, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Ntoumani, E.; Strandvik, B.; Sabel, K.G. Nervonic acid is much lower in donor milk than in milk from mothers delivering premature infants--of neglected importance? Prostaglandins Leukot. Essent. Fat. Acids 2013, 89, 241–244. [Google Scholar] [CrossRef]
- Buntinx, M.; Vanderlocht, J.; Hellings, N.; Vandenabeele, F.; Lambrichts, I.; Raus, J.; Ameloot, M.; Stinissen, P.; Steels, P. Characterization of three human oligodendroglial cell lines as a model to study oligodendrocyte injury: morphology and oligodendrocyte-specific gene expression. J. Neurocytol. 2003, 32, 25–38. [Google Scholar] [CrossRef]
- Dima, A.A.; Elliott, J.T.; Filliben, J.J.; Halter, M.; Peskin, A.; Bernal, J.; Kociolek, M.; Brady, M.C.; Tang, H.C.; Plant, A.L. Comparison of segmentation algorithms for fluorescence microscopy images of cells. Cytom. A 2011, 79, 545–559. [Google Scholar] [CrossRef]
- Franklin, R.J.M.; Ffrench-Constant, C. Regenerating CNS myelin—From mechanisms to experimental medicines. Nat. Rev. Neurosci. 2017, 18, 753–769. [Google Scholar] [CrossRef]
- Sassa, T.; Kihara, A. Metabolism of very long-chain Fatty acids: Genes and pathophysiology. Biomol. Ther. 2014, 22, 83–92. [Google Scholar] [CrossRef]
- Merrill, A.H., Jr.; Schmelz, E.M.; Wang, E.; Dillehay, D.L.; Rice, L.G.; Meredith, F.; Riley, R.T. Importance of sphingolipids and inhibitors of sphongolipid metabolism as components of animal diets. J. Nutr. 1997, 127, 830S–833S. [Google Scholar] [CrossRef]
- Poulos, A. Very long chain fatty acids in higher animal—A review. Lipids 1995, 30, 1–14. [Google Scholar] [CrossRef]
- Kim, W.S.; Hsiao, J.H.; Bhatia, S.; Glaros, E.N.; Don, A.S.; Tsuruoka, S.; Shannon Weickert, C.; Halliday, G.M. ABCA8 stimulates sphingomyelin production in oligodendrocytes. Biochem. J. 2013, 452, 401–410. [Google Scholar] [CrossRef]
- Isaac, G.; Bylund, D.; Månsson, J.E.; Markides, K.E.; Bergquist, J. Analysis of phosphatidylcholine and sphingomyelin molecular species from brain extracts using capillary liquid chromatography electrospray ionization mass spectrometry. J. Neurosci. Methods 2003, 128, 111–119. [Google Scholar] [CrossRef]
- Goldman, S.A.; Nedergaard, M.; Windrem, M.S. Glial progenitor cell-based treatment and modeling of neurological disease. Science 2012, 338, 491–495. [Google Scholar] [CrossRef]
- Bernardo, A.; Giammarco, M.L.; De Nuccio, C.; Ajmone-Cat, M.A.; Visentin, S.; De Simone, R.; Minghetti, L. Docosahexaenoic acid promotes oligodendrocyte differentiation via PPAR-γ signalling and prevents tumor necrosis factor-α-dependent maturational arrest. Biochim. Biophys. Acta Mol. Cell. Biol. Lipids 2017, 1862, 1013–1023. [Google Scholar] [CrossRef]
- Salvati, S.; Natali, F.; Attorri, L.; Di Benedetto, R.; Leonardi, F.; Di Biase, A.; Ferri, F.; Fortuna, S.; Lorenzini, P.; Sanchez, M.; et al. Eicosapentaenoic acid stimulates the expression of myelin proteins in rat brain. J. Neurosci. Res. 2008, 86, 776–784. [Google Scholar] [CrossRef]
- Guardiola-Diaz, H.M.; Ishii, A.; Bansal, R. Erk1/2 MAPK and mTOR signaling sequentially regulates progression through distinct stages of oligodendrocyte differentiation. Glia 2012, 60, 476–486. [Google Scholar] [CrossRef]
- Le, H.D.; Meisel, J.A.; de Meijer, V.E.; Gura, K.M.; Puder, M. The essentiality of arachidonic acid and docosahexaenoic acid. Prostaglandins Leukot. Essent. Fatty Acids 2009, 81, 165–170. [Google Scholar] [CrossRef]
- Fan, Y.; Meng, H.M.; Hu, G.R.; Li, F.L. Biosynthesis of nervonic acid and perspectives for its production by microalgae and other microorganisms. Appl. Microbiol. Biotechnol. 2018, 102, 3027–3035. [Google Scholar] [CrossRef]
- Desvergne, B.; Wahli, W. Peroxisome proliferator-activated receptors: Nuclear control of metabolism. Endocr. Rev. 1999, 20, 649–688. [Google Scholar]
- Burns, K.A.; Vanden Heuvel, J.P. Modulation of PPAR activity via phosphorylation. Biochim. Et Biophys. Acta 2007, 1771, 952–960. [Google Scholar] [CrossRef]
- Steinberg, G.R.; Kemp, B.E. AMPK in Health and Disease. Physiol. Rev. 2009, 89, 1025–1078. [Google Scholar] [CrossRef]
- Jing, H.; Yao, J.; Liu, X.; Fan, H.; Zhang, F.; Li, Z.; Tian, X.; Zhou, Y. Fish-oil emulsion (omega-3 polyunsaturated fatty acids) attenuates acute lung injury induced by intestinal ischemia-reperfusion through Adenosine 5’-monophosphate-activated protein kinase-sirtuin1 pathway. J. Surg. Res. 2014, 187, 252–261. [Google Scholar] [CrossRef]
- Katakura, M.; Hashimoto, M.; Okui, T.; Shahdat, H.M.; Matsuzaki, K.; Shido, O. Omega-3 polyunsaturated fatty acids enhance neuronal differentiation in cultured rat neural stem cells. Stem Cells Int. 2013, 490476. [Google Scholar] [CrossRef]
- Stańdo, M.; Lewkowicz, N. Omega-3 Polyunsaturated Fatty Acids as an Adjunct to Non-Surgical Treatment of Periodontitis. Eur. J. Lipid Sci. Technol. 2019, 121, 1800345. [Google Scholar] [CrossRef]
- Lewkowicz, P.; Tchórzewski, H. Anti-tumor activity of 1-O-alkylglycerols--the main component of shark liver oil. Pol. Merkur. Lekarski. 2012, 33, 353–356. [Google Scholar]
- Lewkowicz, P.; Banasik, M.; Głowacka, E.; Lewkowicz, N.; Tchórzewski, H. Effect of high doses of shark liver oil supplementation on T cell polarization and peripheral blood polymorphonuclear cell function. Pol. Merkur. Lekarski. 2005, 18, 686–692. [Google Scholar]
- Petković, F.; Castellano, B. The role of interleukin-6 in central nervous system demyelination. Neural Regen. Res. 2016, 11, 1922–1923. [Google Scholar] [CrossRef]
- Du, Y.; Dreyfus, C.F. Oligodendrocytes as providers of growth factors. J. Neurosci. Res. 2002, 68, 647–654. [Google Scholar] [CrossRef]
- Azin, M.; Mirnajafi-Zadeh, J.; Javan, M. Fibroblast Growth Factor-2 Enhanced The Recruitment of Progenitor Cells and Myelin Repair in Experimental Demyelination of Rat Hippocampal Formations. Cell J. 2015, 17, 540–556. [Google Scholar]
- Girolamo, F.; Coppola, C.; Ribatti, D.; Trojano, M. Angiogenesis in multiple sclerosis and experimental autoimmune encephalomyelitis. Acta Neuropathol. Commun. 2014, 2, 84. [Google Scholar] [CrossRef]
- Bozek, K.; Wei, Y.; Yan, Z.; Liu, X.; Xiong, J.; Sugimoto, M.; Tomita, M.; Pääbo, S.; Sherwood, C.C.; Hof, P.R.; et al. Organization and evolution of brain lipidome revealed by large-scale analysis of human, chimpanzee, macaque, and mouse tissues. Neuron 2015, 85, 695–702. [Google Scholar] [CrossRef]



| Lipid Acid | HC [%] | EAE [%] | |
|---|---|---|---|
| 1 | Myristic Acid (C14:0) | n.d. | 0.13 |
| 2 | Palmitic Acid (C16:0) | 39.48 | 35.14 * |
| 3 | Palmitoleic Acid (C16:1) | n.d. | 0.65 |
| 4 | Pentadecanoic Acid (C15:0) | n.d. | 0.58 |
| 5 | Stearic Acid (C18:0) | 18.85 | 18.06 |
| 6 | Oleic Acid (C18:1n9c) | 18.19 | 20.30 |
| 7 | Elaidic Acid (C18:1n9t) | 5.92 | 8.16 * |
| 8 | Linolelaidic Acid (C18:2n6t) | n.d. | 0.83 |
| 9 | cis-11-Eicosenoic Acid (C20:1n9) | n.d. | 0.66 |
| 10 | Arachidonic Acid (C20:4n6) | 1.74 | 5.56 * |
| 11 | cis-4,7,10,13,16,19-Docosahexaenoic Acid (C22:6n3) | 14.80 | 9.76 * |
| 12 | Nervonic Acid (C24:1n9) | 1.91 | n.d. * |
| Lipid Acid | MO3.13 Cells not Polarized | hOPCs (PMA) | hOPCs (PMA) + FOM | hOPCs (PMA) + LO | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 24 h | 48 h | 72 h | 24 h | 48 h | 72 h | 24 h | 48 h | 72 h | 24 h | 48 h | 72 h | ||
| 1 | Myristic acid (C14:0) | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 1.15# | 1.20# | 1.85# | n.d. | n.d. | 0.12 |
| 2 | Pentadecanoic acid (C15:0) | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 0.13 | n.d. | n.d. | n.d. | n.d. |
| 3 | Palmitic acid (C16:0) | 9.58 | 9.28 | 8.78 | 3.98 † | 3.66 † | 3.31 † | 8.04 # | 10.04 # | 11.32 # | 5.96 | 3.91 | 4.42 |
| 4 | Palmitoleic acid (C16:1) | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 2.48# | 3.55# | 3.2# | n.d. | n.d. | n.d. |
| 5 | Heptadeconoic acid (C17:0) | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 0.41 | 0.77 | n.d. | n.d. | n.d. | n.d. |
| 6 | Heptadeconoic acid, 16-methyl | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 0.10 | n.d. | n.d. | n.d. | n.d. |
| 7 | Cis-10-heptadecenoic acid (C17:1) | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 1.67# | n.d. | n.d. | n.d. | n.d. |
| 8 | Stearic acid (C18:0) | 4.89 | 5.33 | 4.09 | 1.05 † | 1.01 † | 1.34 † | 2.82 # | 2.53 # | 3.65 # | 4.44 # | 2.10 | 2.06 |
| 9 | Elaidic acid (C18:1n9t) | 0.7 | 3.89 | 2.57 | n.d. | n.d. | n.d. | 22.49 # | 23.12 # | 24.33 # | 17.04 # | 16.81 # | 16.34 # |
| 10 | Oleic Aaid (C18:1n9c) | 17.98 | 20.21 | 17.65 | 17.87 | 18.90 | 18.86 | n.d. # | n.d. # | n.d. # | n.d. # | n.d. # | 2.24 # |
| 11 | Linoleic acid (C18:2n6c) | 19.46 | 12.61 | 23.52 | 33.28 † | 30.58 † | 28.28 † | 8.46 # | 1.42 # | 3.47 # | 14.49 # | 28.14 | 25.93 |
| 12 | Linolelaidic acid (C18:2n6t) | n.d. | n.d. | 2.12 | n.d. | 3.18 | 3.78 | 0.58 # | 0.16 # | n.d. # | n.d. | 2.41 | 0.29 # |
| 13 | Gamma-Linolenic acid (C18:3n6) | n.d. | n.d. | n.d. | 3.4 † | 2.34 † | 2.38 † | n.d. # | n.d. # | n.d. # | n.d. # | n.d. # | 0.67 # |
| 14 | Hexadecanoic acid | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 0.03 | n.d. | n.d. | n.d. | n.d. |
| 15 | Alpha-Linolenic acid (C18:3n3) | n.d. | n.d. | 0.66 | 6.82 † | 5.48 † | 4.58 † | 2.72 # | 0.82 # | n.d. # | 54.71 # | 6.66 | 13.05 # |
| 16 | Stearidonic acid (C18:4n3) | n.d. | n.d. | n.d. | 0.13 | 0.18 | 0.18 | 0.821 | 1.89 | 3.54 | n.d. | n.d. | n.d. |
| 17 | Cis-11-Eicosenoic acid (C20:1n9) | 1.05 | 1.35 | 1.19 | 3.74 † | 2.85 † | 2.82 † | 5.11 | 5.46 # | 5.93 # | n.d # | 2.32 | 1.94 |
| 18 | Cis-11,14,17-Eicosatrienoic acid (C20:3n3) | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 0.27 |
| 19 | Cis-5,8,11,14,17-Eicosapentaenoic acid (C20:5n3) | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 9.82 # | 10.53 # | 12.57 # | n.d. | n.d. | n.d. |
| 20 | Cis-8,11,14-eicosatrienoic acid (C20:3n6) | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 0.05 | n.d. | n.d. | n.d. | n.d. |
| 21 | Arachidic acid (C20:0) | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 0.23 | 0.25 | n.d. | 0.64 | 0.51 |
| 22 | Arachidonic acid (C20:4n6) | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 0.45 | 0.94 | 1.21 # | n.d. | n.d. | n.d. |
| 23 | Behenic acid (C22:0) | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 0.09 | n.d. | n.d. | 1.74 # | 0.97 # |
| 24 | Cetoleic acid (C22:1n11) | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 16.02 # | 14.26 # | 13.04 # | 3.34 # | 35.22 # | 30.4 # |
| 25 | Docosapentaenoic acid | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 1.65 | n.d. | n.d. | n.d. | n.d. |
| 26 | Cis-13-docosenoic acid (C22:1n9) | 46.38 | 47.27 | 38.74 | 28.51 † | 30.58 † | 32.06 † | n.d. # | n.d. # | n.d. # | n.d. # | n.d. # | n.d. # |
| 27 | Cis-4,7,10,13,16,19-Docosahexaenoic acid (C22:6n3) | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 5.34 # | 9.18 # | 7.52 # | n.d. | n.d. | n.d. |
| 28 | Lignoceric acid (C24:0) | n.d. | n.d. | n.d. | 0.39 | 0.17 | 0.38 | n.d. | n.d. | n.d. | n.d. | n.d. | 0.26 |
| 29 | Nervonic acid (C24:1n9) | n.d. | n.d. | n.d. | 1.32 | 1.31 | 1.44 | 6.78 # | 1.63 # | n.d. # | n.d. # | n.d. # | n.d. # |
| 30 | Squalene | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 5.78 # | 9.05 # | 8.08 # | n.d. | n.d. | n.d. |
| Medium (48 h) | FOM (5%, 48 h) | LO (5%, 48 h) | ||||
|---|---|---|---|---|---|---|
| MO3.13 | hOPC | MO3.13 | hOPC | MO3.13 | hOPC | |
| IL-1β | 0.1 ± 0.04 | 0.3 ± 0.29 | 0.1 ± 0.06 | 0.2 ± 0.16 | 0.1 ± 0.05 | 0.2 ± 0.13 |
| IL-1ra | 30.1 ± 13.66 | 40.8 ± 17.97 † | 33.5 ± 17.59 | 40.0 ± 19.94 | 28.3 ± 12.00 | 37.2 ± 18.31 |
| IL-2 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| IL-4 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| IL-5 | 6.6 ± 2.90 | 17.3 ± 9.56 † | 6.5 ± 3.55 | 9.9 ± 3.92 # | 11.0 ± 5.44 | 16.0 ± 11.80 |
| IL-6 | 5.4 ± 0.99 | 22.9 ± 3.93 † | 4.0 ± 0.79 | 7.1 ± 1.02 # | 4.9 ± 1.11 | 19.6 ± 4.02 |
| IL-7 | 13.1 ± 9.81 | 24.5 ± 11.32 † | 10.6 ± 6.61 | 11.0± 5.58 # | 12.0 ± 10.82 | 21.7 ± 15.09 |
| IL-8 | 358.4 ± 52.62 | 1102.6 ± 167.59 † | 419.6 ± 59.55 | 651.2 ± 91.05 # | 535.6 ± 84.53 * | 1287.9 ± 192.67 |
| IL-9 | 6.1 ± 2.98 | 13.3 ± 9.33 † | 6.1 ± 2.11 | 9.8± 5.57 | 8.1 ± 3.97 | 12.4 ± 9.11 |
| IL-10 | 6.6 ± 2.04 | 8.1 ± 3.98 | 6.4 ± 2.62 | 6.2 ± 2.77 | 6.3 ± 2.96 | 5.7 ± 3.99 |
| IL-12 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| IL-13 | 1.1 ± 0.88 | 1.7 ± 0.55 | 1.3 ± 0.42 | 1.6 ± 0.71 | 1.2 ± 0.51 | 1.4 ± 0.56 |
| IL-15 | 36.4 ± 16.82 | 58.5 ± 17.07 † | 20.7 ± 12.77 * | 9.7 ± 7.88 # | 42.7 ± 21.29 | 53.2 ± 28.67 |
| IL-17A | 4.8 ± 2.95 | 7.1 ± 3.56 † | 4.5 ± 2.11 | 4.1 ± 1.46 # | 4.1 ± 3.34 | 7.3 ± 3.86 |
| Eotaxin | 54.4 ± 21.33 | 90.9 ± 32.78 † | 36.6 ± 16.73 * | 49.0 ± 20.55 # | 31.4 ± 20.76 * | 72.3 ± 22.51 |
| FGF-2 | n.d. | 18.6 ± 4.33 † | 16.9 ± 3.21 * | 40.9± 6.81 # | 14.2 ± 2.77 * | 18.5 ± 2.80 |
| G-CSF | 95.9 ± 46.15 | 132.1 ± 52.82 † | 94.0 ± 26.82 | 62.0 ± 12.90 # | 90.1 ± 26.80 | 78.3 ± 21.91 # |
| GM-CSF | n.d. | 0.6 ± 0.46 | n.d. | 0.6 ± 0.29 | n.d. | 0.8 ± 0.60 |
| IFN-γ | 50.2 ± 12.96 | 110.1 ± 25.44 † | 44.9 ± 22.39 | 54.4 ± 24.86 # | 39.5 ± 15.91 | 72.7 ± 22.55 # |
| IP-10 | 33.4 ± 18.80 | 53.4 ± 21.96 † | 44.9 ± 24.62 | 43.7 ± 22,95 | 40.2 ± 18.49 | 46.8 ± 21.88 |
| MCP-1 | 1175.4 ± 201.60 | 2105.6 ± 399.63 † | 857.8 ± 142.18 * | 968.2 ± 199.33 # | 976.9 ± 247.78 | 1567.0 ± 451.83 |
| MIP-1α | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| PDGF-bb | 56.7 ± 17.97 | 82.7 ± 23.55 † | 53.5 ± 10.04 | 79.4 ± 12.18 | 53.6 ± 19.76 | 79.6 ± 21.68 |
| MIP-1β | 2.8 ± 1.13 | 6.9 ± 2.99 † | 4.3 ± 2.98 | 5.2 ± 3.08 | 3.6 ± 1.83 | 6.0 ± 2.75 |
| RANTES | 16.4 ± 9.33 | 28.3± 9.74 † | 15.4 ± 10.59 | 14.2 ± 9.44 # | 16.1 ± 8.07 | 23.7 ± 12.94 |
| TNF | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| VEGF | 99.2 ± 21.69 | 139.6 ± 43.80 † | 119.6 ± 28.75 | 227.9 ± 69.43 # | 110.3 ± 41.54 | 140.0 ± 59.43 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lewkowicz, N.; Piątek, P.; Namiecińska, M.; Domowicz, M.; Bonikowski, R.; Szemraj, J.; Przygodzka, P.; Stasiołek, M.; Lewkowicz, P. Naturally Occurring Nervonic Acid Ester Improves Myelin Synthesis by Human Oligodendrocytes. Cells 2019, 8, 786. https://doi.org/10.3390/cells8080786
Lewkowicz N, Piątek P, Namiecińska M, Domowicz M, Bonikowski R, Szemraj J, Przygodzka P, Stasiołek M, Lewkowicz P. Naturally Occurring Nervonic Acid Ester Improves Myelin Synthesis by Human Oligodendrocytes. Cells. 2019; 8(8):786. https://doi.org/10.3390/cells8080786
Chicago/Turabian StyleLewkowicz, Natalia, Paweł Piątek, Magdalena Namiecińska, Małgorzata Domowicz, Radosław Bonikowski, Janusz Szemraj, Patrycja Przygodzka, Mariusz Stasiołek, and Przemysław Lewkowicz. 2019. "Naturally Occurring Nervonic Acid Ester Improves Myelin Synthesis by Human Oligodendrocytes" Cells 8, no. 8: 786. https://doi.org/10.3390/cells8080786
APA StyleLewkowicz, N., Piątek, P., Namiecińska, M., Domowicz, M., Bonikowski, R., Szemraj, J., Przygodzka, P., Stasiołek, M., & Lewkowicz, P. (2019). Naturally Occurring Nervonic Acid Ester Improves Myelin Synthesis by Human Oligodendrocytes. Cells, 8(8), 786. https://doi.org/10.3390/cells8080786





