The Cell Wall Hydrolytic NlpC/P60 Endopeptidases in Mycobacterial Cytokinesis: A Structural Perspective
Abstract
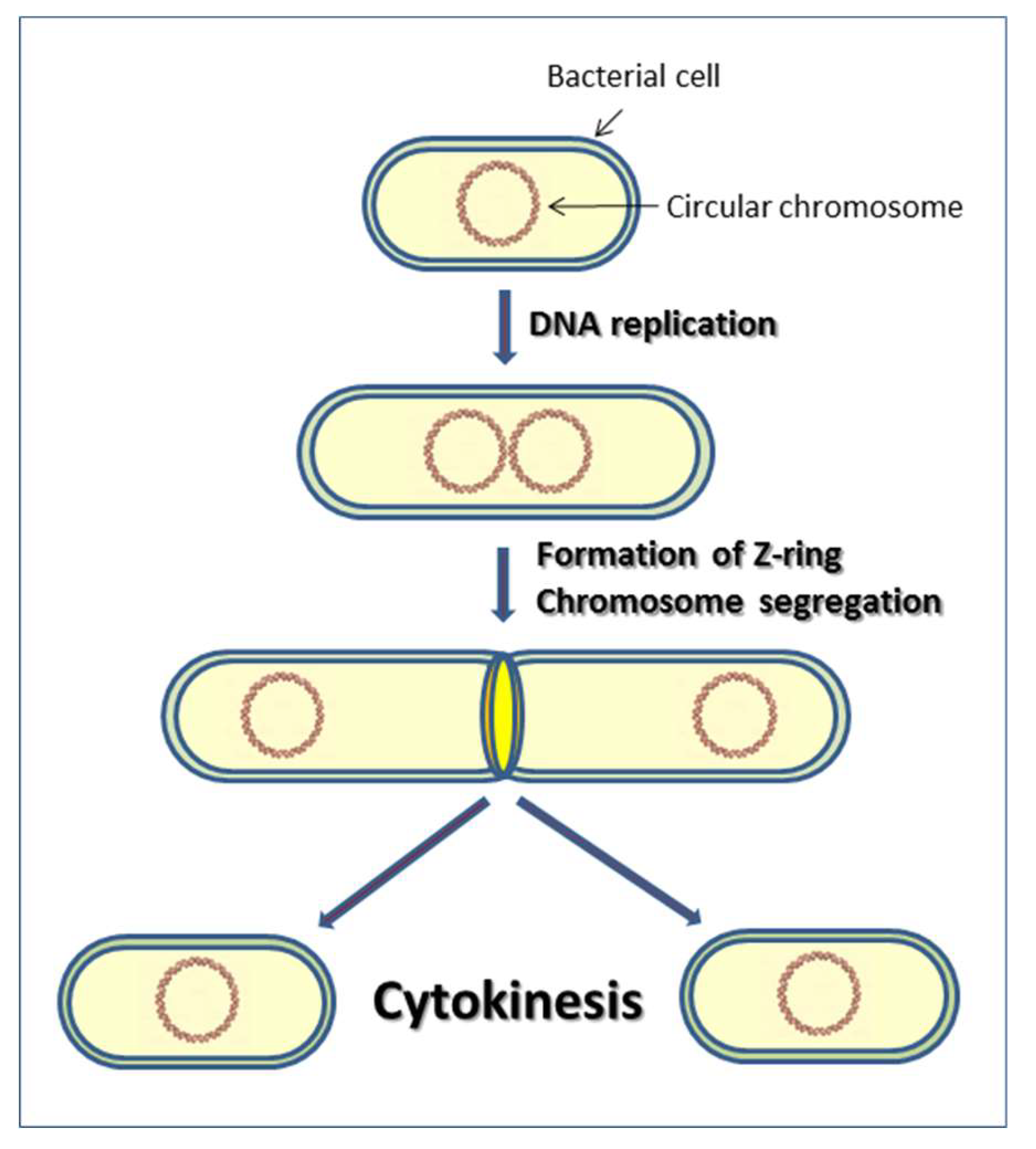
1. Introduction to Cytokinesis in Bacteria
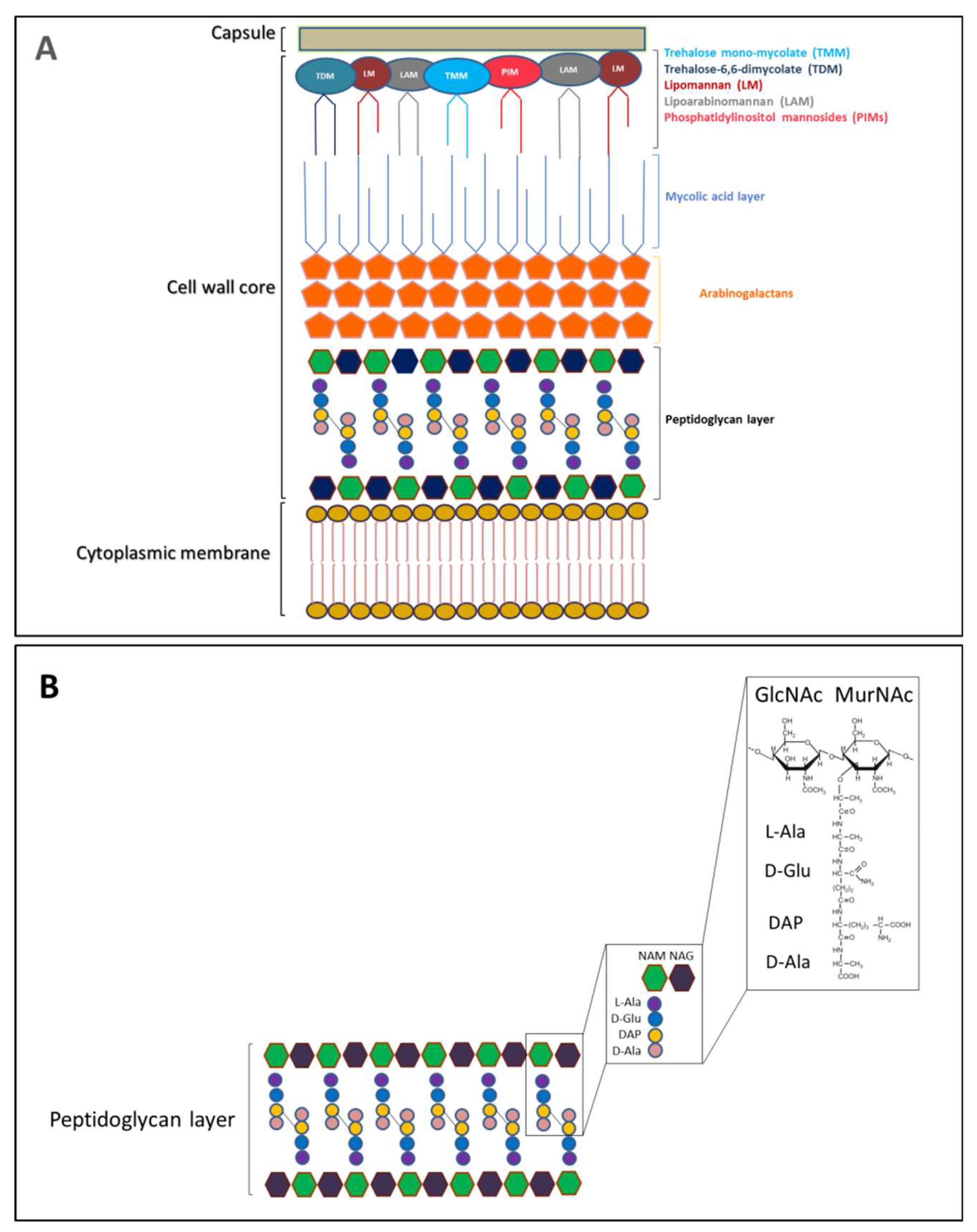
2. The Peptidoglycan Nature of The Septal Ring
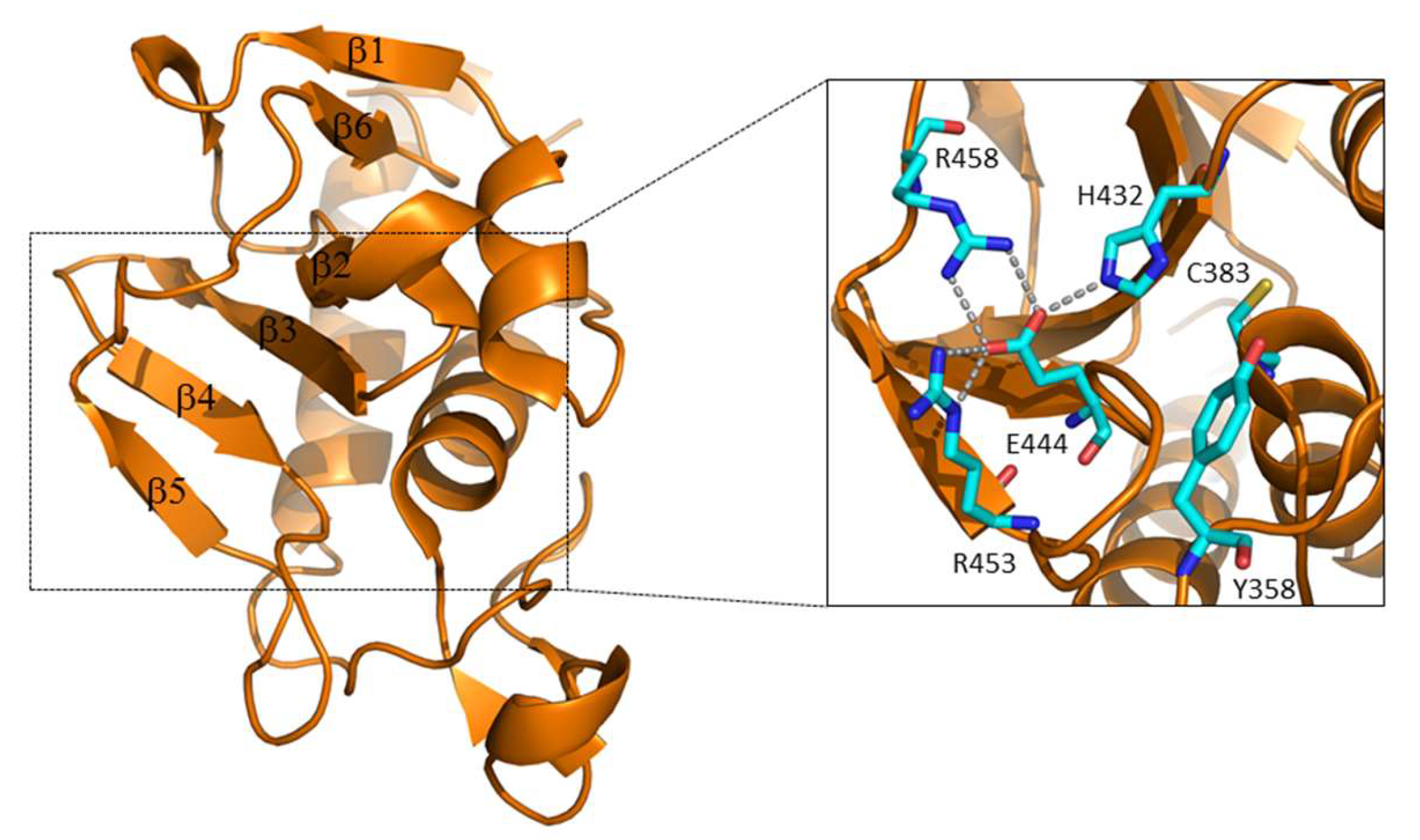
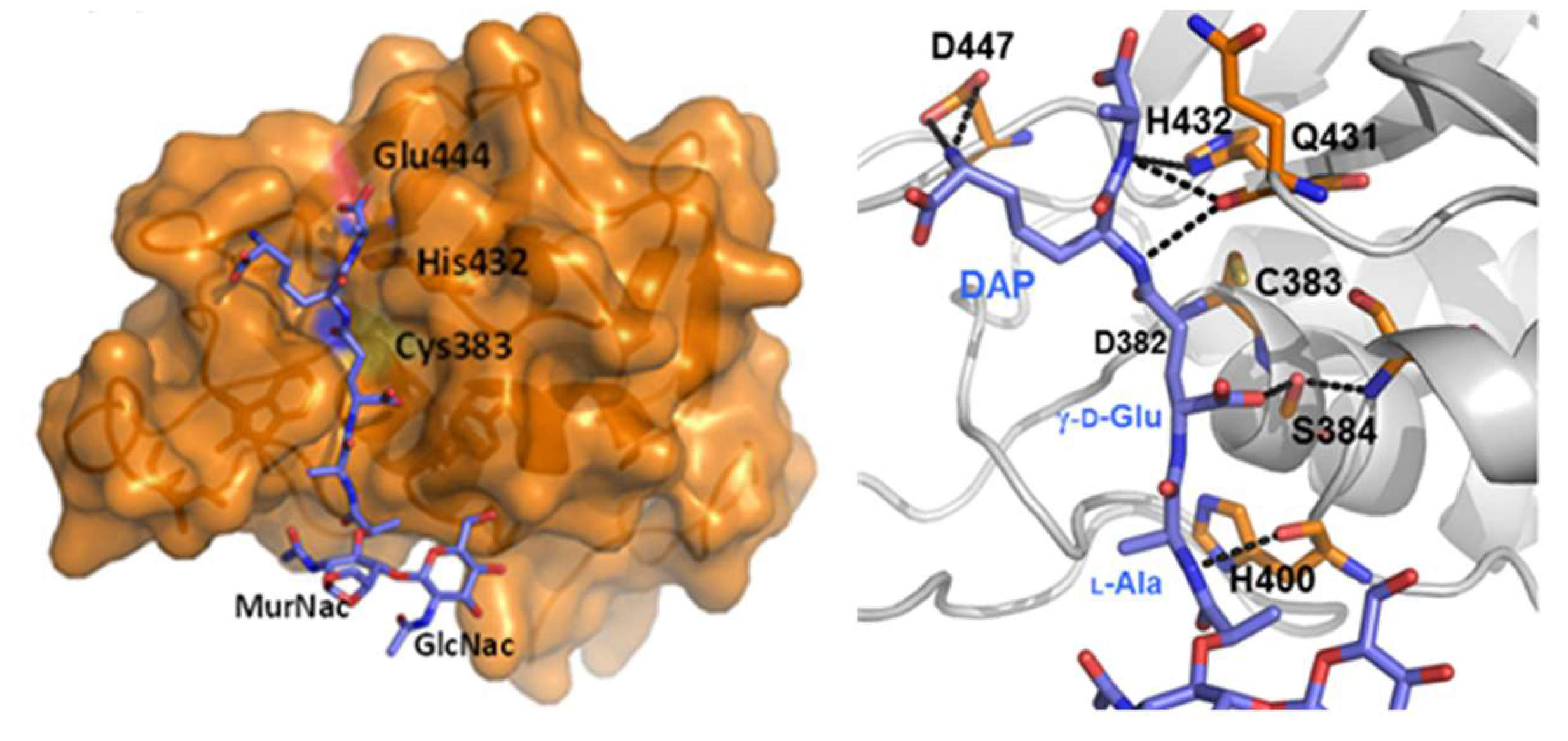
3. Septal Ring Degradation and Daughter Cell Division
3.1. The Regulation of RipA Through MarP
3.2. Regulation of RipA Via Protein-Protein Interactions
4. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AG | arabinogalactans |
| AnhydroGMDP | N-acetylglucosaminyl-β(1→4)-N-acetyl-1,6-anhydromuramoyl-l-alanyl-d-isoglutamate |
| CHAP | Cys, His, Asp Peptidase |
| DAP | meso-diaminopimelic acid |
| Fts | filamenting temperature-sensitive |
| GlcNAc | β−(1-4)-linked-N-acetylglucosamine |
| LM | lipomannan |
| LAM | lipoarabinomannan |
| MA | mycolic acids |
| MurNAc | N-acetylmuramic acid |
| PDB | Protein Databank |
| PGN | peptidoglycan |
| PIMs | phosphatidylinositol mannosides |
| Rip | resuscitation-promoting factor interacting protein |
| Rpf | resuscitation-promoting factor |
| SP | signal peptide |
| STPK | Serine/Threonine Protein Kinase |
| TAT | Twin-Arginine Translocation |
References
- Errington, J.; Daniel, R.A.; Scheffers, D.J. Cytokinesis in bacteria. Microbiol. Mol. Biol. Rev. 2003, 67, 52–65. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Guo, K.K. Exploration of cell division times during bacterial cytokinesis. Phys. Chem. Chem. Phys. 2017, 19, 32038–32046. [Google Scholar] [CrossRef] [PubMed]
- Dajkovic, A.; Lutkenhaus, J. Z ring as executor of bacterial cell division. J. Mol. Microbiol. Biotechnol. 2006, 11, 140–151. [Google Scholar] [CrossRef]
- Lutkenhaus, J.F.; Wolf-Watz, H.; Donachie, W.D. Organization of genes in the ftsA-envA region of the Escherichia coli genetic map and identification of a new fts locus (ftsZ). J. Bacteriol. 1980, 142, 615–620. [Google Scholar] [PubMed]
- Lariviere, P.J.; Mahone, C.R.; Santiago-Collazo, G.; Howell, M.; Daitch, A.K.; Zeinert, R.; Chien, P.; Brown, P.J.B.; Goley, E.D. An Essential Regulator of Bacterial Division Links FtsZ to Cell Wall Synthase Activation. Curr. Biol. 2019, 29, 1460–1470. [Google Scholar] [CrossRef] [PubMed]
- Pazos, M.; Peters, K.; Casanova, M.; Palacios, P.; VanNieuwenhze, M.; Breukink, E.; Vicente, M.; Vollmer, W. Z-ring membrane anchors associate with cell wall synthases to initiate bacterial cell division. Nat. Commun. 2018, 9, 5090. [Google Scholar] [CrossRef] [PubMed]
- Vega, D.E.; Margolin, W. Direct Interaction between the Two Z Ring Membrane Anchors FtsA and ZipA. J. Bacteriol. 2019, 201, e00579-18. [Google Scholar] [CrossRef] [PubMed]
- Lutkenhaus, J.; Pichoff, S.; Du, S.S. Bacterial cytokinesis: From Z ring to divisome. Cytoskeleton 2012, 69, 778–790. [Google Scholar] [CrossRef]
- den Blaauwen, T.; Luirink, J. Checks and Balances in Bacterial Cell Division. MBio 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Turner, D.J.; Portman, I.; Dafforn, T.R.; Rodger, A.; Roper, D.I.; Smith, C.J.; Turner, M.S. The mechanics of FtsZ fibers. Biophys. J. 2012, 102, 731–738. [Google Scholar] [CrossRef] [PubMed]
- Leung, A.K.W.; White, E.L.; Ross, L.J.; Reynolds, R.C.; DeVito, J.A.; Borhani, D.W. Structure of Mycobacterium tuberculosis FtsZ reveals unexpected, G protein-like conformational switches. J. Mol. Biol. 2004, 342, 953–970. [Google Scholar] [CrossRef] [PubMed]
- Margolin, W. FtsZ and the division of prokaryotic cells and organelles. Nat. Rev. Mol. Cell Biol. 2005, 6, 862–871. [Google Scholar] [CrossRef] [PubMed]
- Perez, A.J.; Cesbron, Y.; Shaw, S.L.; Bazan Villicana, J.; Tsui, H.-C.T.; Boersma, M.J.; Ye, Z.A.; Tovpeko, Y.; Dekker, C.; Holden, S.; et al. Movement dynamics of divisome proteins and PBP2x:FtsW in cells of Streptococcus pneumoniae. Proc. Natl. Acad. Sci. USA 2019, 116, 3211–3220. [Google Scholar] [CrossRef]
- Mir, M.A.; Arumugam, M.; Mondal, S.; Rajeswari, H.S.; Ramakumar, S.; Ajitkumar, P. Mycobacterium tuberculosis Cell Division Protein, FtsE, is an ATPase in Dimeric Form. Protein J. 2015, 34, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Rued, B.E.; Alcorlo, M.; Edmonds, K.A.; Martinez-Caballero, S.; Straume, D.; Fu, Y.; Bruce, K.E.; Wu, H.W.; Havarstein, L.S.; Hermoso, J.A.; et al. Structure of the Large Extracellular Loop of FtsX and Its Interaction with the Essential Peptidoglycan Hydrolase PcsB in Streptococcus pneumoniae. MBio 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.C.; Peters, N.T.; Parzych, K.R.; Uehara, T.; Markovski, M.; Bernhardt, T.G. An ATP-binding cassette transporter-like complex governs cell-wall hydrolysis at the bacterial cytokinetic ring. Proc. Natl. Acad. Sci. USA 2011, 108, E1052–E1060. [Google Scholar] [CrossRef] [PubMed]
- Bartual, S.G.; Straume, D.; Stamsas, G.A.; Munoz, I.G.; Alfonso, C.; Martinez-Ripoll, M.; Havarstein, L.S.; Hermoso, J.A. Structural basis of PcsB-mediated cell separation in Streptococcus pneumoniae. Nat. Commun. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Kumar, S.; Kumar, D.; Mishra, A.; Dewangan, R.P.; Shrivastava, P.; Ramachandran, S.; Taneja, B. The structure of Rv3717 reveals a novel amidase from Mycobacterium tuberculosis. Acta Crystallogr. D Biol. Crystallogr. 2013, 69, 2543–2554. [Google Scholar] [CrossRef] [PubMed]
- Prigozhin, D.M.; Mavrici, D.; Huizar, J.P.; Vansell, H.J.; Alber, T. Structural and biochemical analyses of Mycobacterium tuberculosis N-acetylmuramyl-L-alanine amidase Rv3717 point to a role in peptidoglycan fragment recycling. J. Biol. Chem. 2013, 288, 31549–31555. [Google Scholar] [CrossRef]
- Ruggiero, A.; Marasco, D.; Squeglia, F.; Soldini, S.; Pedone, E.; Pedone, C.; Berisio, R. Structure and Functional Regulation of RipA, a Mycobacterial Enzyme Essential for Daughter Cell Separation. Structure 2010, 18, 1184–1190. [Google Scholar] [CrossRef] [PubMed]
- Hett, E.C.; Chao, M.C.; Deng, L.L.; Rubin, E.J. A mycobacterial enzyme essential for cell division synergizes with resuscitation-promoting factor. PLoS Pathog. 2008, 4. [Google Scholar] [CrossRef] [PubMed]
- Chao, M.C.; Kieser, K.J.; Minami, S.; Mavrici, D.; Aldridge, B.B.; Fortune, S.M.; Alber, T.; Rubin, E.J. Protein complexes and proteolytic activation of the cell wall hydrolase RipA regulate septal resolution in mycobacteria. PLoS Pathog. 2013, 9, e1003197. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, A.; Marchant, J.; Squeglia, F.; Makarov, V.; De Simone, A.; Berisio, R. Molecular determinants of inactivation of the resuscitation promoting factor B from Mycobacterium tuberculosis. J. Biomol. Struct. Dyn. 2013, 31, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, A.; Squeglia, F.; Romano, M.; Vitagliano, L.; De Simone, A.; Berisio, R. The structure of Resuscitation promoting factor B from M. tuberculosis reveals unexpected ubiquitin-like domains. Bba-Gen. Subj. 2016, 1860, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, A.; Tizzano, B.; Pedone, E.; Pedone, C.; Wilmanns, M.; Berisio, R. Crystal Structure of the Resuscitation-Promoting Factor (Delta DUF)RpfB from M. tuberculosis. J. Mol. Biol. 2009, 385, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Alderwick, L.J.; Harrison, J.; Lloyd, G.S.; Birch, H.L. The Mycobacterial Cell Wall-Peptidoglycan and Arabinogalactan. Cold Spring Harbor Perspect. Med. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Sarmiento, M.E.; Alvarez, N.; Chin, K.L.; Bigi, F.; Tirado, Y.; Garcia, M.A.; Anis, F.Z.; Norazmi, M.N.; Acosta, A. Tuberculosis vaccine candidates based on mycobacterial cell envelope components. Tuberculosis 2019, 115, 26–41. [Google Scholar] [CrossRef]
- Brennan, P.J. Structure, function, and biogenesis of the cell wall of Mycobacterium tuberculosis. Tuberculosis 2003, 83, 91–97. [Google Scholar] [CrossRef]
- Mishra, A.K.; Driessen, N.N.; Appelmelk, B.J.; Besra, G.S. Lipoarabinomannan and related glycoconjugates: Structure, biogenesis and role in Mycobacterium tuberculosis physiology and host-pathogen interaction. Fems Microbiol. Rev. 2011, 35, 1126–1157. [Google Scholar] [CrossRef]
- Glickman, M.S.; Cox, J.S.; Jacobs, W.R. A novel mycolic acid cyclopropane synthetase is required for cording, persistence, and virulence of Mycobacterium tuberculosis. Mol. Cell 2000, 5, 717–727. [Google Scholar] [CrossRef]
- Dubnau, E.; Chan, J.; Raynaud, C.; Mohan, V.P.; Laneelle, M.A.; Yu, K.M.; Quemard, A.; Smith, I.; Daffe, M. Oxygenated mycolic acids are necessary for virulence of Mycobacterium tuberculosis in mice. Mol. Microbiol. 2000, 36, 630–637. [Google Scholar] [CrossRef] [PubMed]
- Takayama, K.; Wang, C.; Besra, G.S. Pathway to synthesis and processing of mycolic acids in Mycobacterium tuberculosis. Clin. Microbiol. Rev. 2005, 18, 81–101. [Google Scholar] [CrossRef] [PubMed]
- Kallenius, G.; Correia-Neves, M.; Buteme, H.; Hamasur, B.; Svenson, S.B. Lipoarabinomannan, and its related glycolipids, induce divergent and opposing immune responses to Mycobacterium tuberculosis depending on structural diversity and experimental variations. Tuberculosis 2016, 96, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Lemassu, A.; Daffe, M. Structural Features of the Exocellular Polysaccharides of Mycobacterium-Tuberculosis. Biochem. J. 1994, 297, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Ortalomagne, A.; Dupont, M.A.; Lemassu, A.; Andersen, A.B.; Gounon, P.; Daffe, M. Molecular Composition of the Outermost Capsular Material of the Tubercle Bacillus. Microbiology 1995, 141, 1609–1620. [Google Scholar] [CrossRef]
- Daffe, M.; Etienne, G. The capsule of Mycobacterium tuberculosis and its implications for pathogenicity. Tubercle Lung Dis. 1999, 79, 153–169. [Google Scholar] [CrossRef]
- Esposito, C.; Cantisani, M.; D’Auria, G.; Falcigno, L.; Pedone, E.; Galdiero, S.; Berisio, R. Mapping key interactions in the dimerization process of HBHA from Mycobacterium tuberculosis, insights into bacterial agglutination. FEBS Lett. 2012, 586, 659–667. [Google Scholar] [CrossRef]
- Esposito, C.; Carullo, P.; Pedone, E.; Graziano, G.; Del Vecchio, P.; Berisio, R. Dimerisation and structural integrity of Heparin Binding Hemagglutinin A from Mycobacterium tuberculosis: Implications for bacterial agglutination. FEBS Lett. 2010, 584, 1091–1096. [Google Scholar] [CrossRef]
- Esposito, C.; Marasco, D.; Delogu, G.; Pedone, E.; Berisio, R. Heparin-binding hemagglutinin HBHA from Mycobacterium tuberculosis affects actin polymerisation. Biochem. Biophys. Res. Commun. 2011, 410, 339–344. [Google Scholar] [CrossRef]
- Squeglia, F.; Ruggiero, A.; De Simone, A.; Berisio, R. A structural overview of mycobacterial adhesins: Key biomarkers for diagnostics and therapeutics. Protein Sci. 2018, 27, 369–380. [Google Scholar] [CrossRef]
- Kieser, K.J.; Rubin, E.J. How sisters grow apart: Mycobacterial growth and division. Nat. Rev. Microbiol. 2014, 12, 550–562. [Google Scholar] [CrossRef] [PubMed]
- Squeglia, F.; Ruggiero, A.; Berisio, R. Chemistry of Peptidoglycan in Mycobacterium tuberculosis Life Cycle: An off-the-wall Balance of Synthesis and Degradation. Chem.-Eur. J. 2018, 24, 2533–2546. [Google Scholar] [CrossRef] [PubMed]
- Squeglia, F.; Romano, M.; Ruggiero, A.; Berisio, R. Molecular Players in Tuberculosis Drug Development: Another Break in the Cell Wall. Curr. Med. Chem. 2017, 24, 3954–3969. [Google Scholar] [CrossRef] [PubMed]
- Lederer, E.; Adam, A.; Ciorbaru, R.; Petit, J.F.; Wietzerbin, J. Cell walls of Mycobacteria and related organisms; chemistry and immunostimulant properties. Mol. Cell. Biochem. 1975, 7, 87–104. [Google Scholar] [CrossRef] [PubMed]
- Layec, S.; Decaris, B.; Leblond-Bourget, N. Diversity of Firmicutes peptidoglycan hydrolases and specificities of those involved in daughter cell separation. Res. Microbiol. 2008, 159, 507–515. [Google Scholar] [CrossRef]
- Layec, S.; Gerard, J.; Legue, V.; Chapot-Chartier, M.P.; Courtin, P.; Borges, F.; Decaris, B.; Leblond-Bourget, N. The CHAP domain of Cse functions as an endopeptidase that acts at mature septa to promote Streptococcus thermophilus cell separation. Mol. Microbiol. 2009, 71, 1205–1217. [Google Scholar] [CrossRef] [PubMed]
- Rossi, P.; Aramini, J.M.; Xiao, R.; Chen, C.X.; Nwosu, C.; Owens, L.A.; Maglaqui, M.; Nair, R.; Fischer, M.; Acton, T.B.; et al. Structural elucidation of the Cys-His-Glu-Asn proteolytic relay in the secreted CHAP domain enzyme from the human pathogen Staphylococcus saprophyticus. Proteins 2009, 74, 515–519. [Google Scholar] [CrossRef]
- Baranowski, C.; Rego, E.H.; Rubin, E.J. The Dream of a Mycobacterium. Microbiol. Spectr. 2019, 7. [Google Scholar] [CrossRef]
- Squeglia, F.; Ruggiero, A.; Romano, M.; Vitagliano, L.; Berisio, R. Mutational and structural study of RipA, a key enzyme in Mycobacterium tuberculosis cell division: Evidence for the L-to-D inversion of configuration of the catalytic cysteine. Acta Crystallogr. D 2014, 70, 2295–2300. [Google Scholar] [CrossRef]
- Steiner, E.M.; Lyngso, J.; Guy, J.E.; Bourenkov, G.; Lindqvist, Y.; Schneider, T.R.; Pedersen, J.S.; Schneider, G.; Schnell, R. The structure of the N-terminal module of the cell wall hydrolase RipA and its role in regulating catalytic activity. Proteins-Struct. Funct. Bioinform. 2018, 86, 912–923. [Google Scholar] [CrossRef]
- Both, D.; Schneider, G.; Schnell, R. Peptidoglycan Remodeling in Mycobacterium tuberculosis: Comparison of Structures and Catalytic Activities of RipA and RipB. J. Mol. Biol. 2011, 413, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Both, D.; Steiner, E.M.; Izumi, A.; Schneider, G.; Schnell, R. RipD (Rv1566c) from Mycobacterium tuberculosis: Adaptation of an NlpC/p60 domain to a non-catalytic peptidoglycan-binding function. Biochem. J. 2014, 457, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Squeglia, F.; Romano, M.; Ruggiero, A.; Vitagliano, L.; De Simone, A.; Berisio, R. Carbohydrate Recognition by RpfB from Mycobacterium tuberculosis Unveiled by Crystallographic and Molecular Dynamics Analyses. Biophys. J. 2013, 104, 2530–2539. [Google Scholar] [CrossRef] [PubMed]
- Chauviac, F.X.; Robertson, G.; Quay, D.H.X.; Bagneris, C.; Dumas, C.; Henderson, B.; Ward, J.; Keep, N.H.; Cohen-Gonsaud, M. The RpfC (Rv1884) atomic structure shows high structural conservation within the resuscitation-promoting factor catalytic domain. Acta Crystallogr. F 2014, 70, 1022–1026. [Google Scholar] [CrossRef] [PubMed]
- Maione, V.; Ruggiero, A.; Russo, L.; De Simone, A.; Pedone, P.V.; Malgieri, G.; Berisio, R.; Isernia, C. NMR Structure and Dynamics of the Resuscitation Promoting Factor RpfC Catalytic Domain. PLoS ONE 2015, 10. [Google Scholar] [CrossRef]
- Mavrici, D.; Prigozhin, D.M.; Alber, T. Mycobacterium tuberculosis RpfE crystal structure reveals a positively charged catalytic cleft. Protein Sci. 2014, 23, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Mavrici, D.; Marakalala, M.J.; Holton, J.M.; Prigozhin, D.M.; Gee, C.L.; Zhang, Y.J.J.; Rubin, E.J.; Alber, T. Mycobacterium tuberculosis FtsX extracellular domain activates the peptidoglycan hydrolase, RipC. Proc. Natl. Acad. Sci. USA 2014, 111, 8037–8042. [Google Scholar] [CrossRef]
- Hett, E.C.; Rubin, E.J. Bacterial growth and cell division: A mycobacterial perspective. Microbiol. Mol. Biol. Rev. 2008, 72, 126–156. [Google Scholar] [CrossRef]
- Fukushima, T.; Kitajima, T.; Yamaguchi, H.; Ouyang, Q.; Furuhata, K.; Yamamoto, H.; Shida, T.; Sekiguchi, J. Identification and characterization of novel cell wall hydrolase CwlT: A two-domain autolysin exhibiting n-acetylmuramidase and DL-endopeptidase activities. J. Biol. Chem. 2008, 283, 11117–11125. [Google Scholar] [CrossRef]
- Aramini, J.M.; Rossi, P.; Huang, Y.J.; Zhao, L.; Jiang, M.; Maglaqui, M.; Xiao, R.; Locke, J.; Nair, R.; Rost, B.; et al. Solution NMR structure of the NlpC/P60 domain of lipoprotein Spr from Escherichia coli: Structural evidence for a novel cysteine peptidase catalytic triad. Biochemistry 2008, 47, 9715–9717. [Google Scholar] [CrossRef]
- Botella, H.; Vaubourgeix, J.; Lee, M.H.; Song, N.; Xu, W.Z.; Makinoshima, H.; Glickman, M.S.; Ehrt, S. Mycobacterium tuberculosis protease MarP activates a peptidoglycan hydrolase during acid stress. Embo J. 2017, 36, 536–548. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro-Guimaraes, M.L.; Tempone, A.J.; Amaral, J.J.; Nery, J.A.; Gomes Antunes, S.L.; Pessolani, M.C. Expression analysis of proteases of Mycobacterium leprae in human skin lesions. Microb. Pathog. 2007, 43, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Small, J.L.; O’Donoghue, A.J.; Boritsch, E.C.; Tsodikov, O.V.; Knudsen, G.M.; Vandal, O.; Craik, C.S.; Ehrt, S. Substrate specificity of MarP, a periplasmic protease required for resistance to acid and oxidative stress in Mycobacterium tuberculosis. J. Biol. Chem. 2013, 288, 12489–12499. [Google Scholar] [CrossRef] [PubMed]
- Sonnhammer, E.L.; von Heijne, G.; Krogh, A. A hidden Markov model for predicting transmembrane helices in protein sequences. Proc. Int. Conf. Intell. Syst. Mol. Biol. 1998, 6, 175–182. [Google Scholar] [PubMed]
- Biswas, T.; Small, J.; Vandal, O.; Odaira, T.; Deng, H.; Ehrt, S.; Tsodikov, O.V. Structural insight into serine protease Rv3671c that Protects M. tuberculosis from oxidative and acidic stress. Structure 2010, 18, 1353–1363. [Google Scholar] [CrossRef] [PubMed]
- Vandal, O.H.; Roberts, J.A.; Odaira, T.; Schnappinger, D.; Nathan, C.F.; Ehrt, S. Acid-susceptible mutants of Mycobacterium tuberculosis share hypersusceptibility to cell wall and oxidative stress and to the host environment. J. Bacteriol. 2009, 191, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Hett, E.C.; Chao, M.C.; Steyn, A.J.; Fortune, S.M.; Deng, L.L.; Rubin, E.J. A partner for the resuscitation-promoting factors of Mycobacterium tuberculosis. Mol. Microbiol. 2007, 66, 658–668. [Google Scholar] [CrossRef]
- Hett, E.C.; Chao, M.C.; Rubin, E.J. Interaction and modulation of two antagonistic cell wall enzymes of mycobacteria. PLoS Pathog. 2010, 6, e1001020. [Google Scholar] [CrossRef]
- Bhuwan, M.; Arora, N.; Sharma, A.; Khubaib, M.; Pandey, S.; Chaudhuri, T.K.; Hasnain, S.E.; Ehtesham, N.Z. Interaction of Mycobacterium tuberculosis Virulence Factor RipA with Chaperone MoxR1 Is Required for Transport through the TAT Secretion System. MBio 2016, 7. [Google Scholar] [CrossRef]
- Mukamolova, G.V.; Turapov, O.A.; Young, D.I.; Kaprelyants, A.S.; Kell, D.B.; Young, M. A family of autocrine growth factors in Mycobacterium tuberculosis. Mol Microbiol 2002, 46, 623–635. [Google Scholar] [CrossRef]
- Telkov, M.V.; Demina, G.R.; Voloshin, S.A.; Salina, E.G.; Dudik, T.V.; Stekhanova, T.N.; Mukamolova, G.V.; Kazaryan, K.A.; Goncharenko, A.V.; Young, M.; et al. Proteins of the Rpf (resuscitation promoting factor) family are peptidoglycan hydrolases. Biochemistry 2006, 71, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Kaprelyants, A.S.; Mukamolova, G.V.; Ruggiero, A.; Makarov, V.A.; Demina, G.R.; Shleeva, M.O.; Potapov, V.D.; Shramko, P.A. Resuscitation-Promoting Factors (RPF): In Search of Inhibitors. Protein Pept. Lett. 2012, 19, 1026–1034. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, S.H. Tuberculosis: Deadly combination. Nature 2008, 453, 295–296. [Google Scholar] [CrossRef] [PubMed]
- Keep, N.H.; Ward, J.M.; Cohen-Gonsaud, M.; Henderson, B. Wake up! Peptidoglycan lysis and bacterial non-growth states. Trends Microbiol. 2006, 14, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Mukamolova, G.V.; Kaprelyants, A.S.; Young, D.I.; Young, M.; Kell, D.B. A bacterial cytokine. Proc. Natl. Acad. Sci. USA 1998, 95, 8916–8921. [Google Scholar] [CrossRef] [PubMed]
- Davies, A.P.; Dhillon, A.P.; Young, M.; Henderson, B.; McHugh, T.D.; Gillespie, S.H. Resuscitation-promoting factors are expressed in Mycobacterium tuberculosis-infected human tissue. Tuberculosis 2008, 88, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Kana, B.D.; Gordhan, B.G.; Downing, K.J.; Sung, N.; Vostroktunova, G.; Machowski, E.E.; Tsenova, L.; Young, M.; Kaprelyants, A.; Kaplan, G.; et al. The resuscitation-promoting factors of Mycobacterium tuberculosis are required for virulence and resuscitation from dormancy but are collectively dispensable for growth in vitro. Mol. Microbiol. 2008, 67, 672–684. [Google Scholar] [CrossRef]
- Wu, X.; Yang, Y.; Han, Y.; Zhang, J.; Liang, Y.; Li, H.; Li, B.; Wang, L. Effect of recombinant Rv1009 protein on promoting the growth of Mycobacterium tuberculosis. J. Appl. Microbiol. 2008, 105, 1121–1127. [Google Scholar] [CrossRef]
- Russell-Goldman, E.; Xu, J.; Wang, X.; Chan, J.; Tufariello, J.M. A Mycobacterium tuberculosis Rpf double-knockout strain exhibits profound defects in reactivation from chronic tuberculosis and innate immunity phenotypes. Infect. Immun. 2008, 76, 4269–4281. [Google Scholar] [CrossRef]
- Tufariello, J.M.; Mi, K.; Xu, J.; Manabe, Y.C.; Kesavan, A.K.; Drumm, J.; Tanaka, K.; Jacobs, W.R., Jr.; Chan, J. Deletion of the Mycobacterium tuberculosis resuscitation-promoting factor Rv1009 gene results in delayed reactivation from chronic tuberculosis. Infect. Immun. 2006, 74, 2985–2995. [Google Scholar] [CrossRef]
- Cohen-Gonsaud, M.; Barthe, P.; Bagneris, C.; Henderson, B.; Ward, J.; Roumestand, C.; Keep, N.H. The structure of a resuscitation-promoting factor domain from Mycobacterium tuberculosis shows homology to lysozymes. Nat. Struct. Mol. Biol. 2005, 12, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Finley, D.; Ulrich, H.D.; Sommer, T.; Kaiser, P. The ubiquitin-proteasome system of Saccharomyces cerevisiae. Genetics 2012, 192, 319–360. [Google Scholar] [CrossRef] [PubMed]
- Nikitushkin, V.D.; Demina, G.R.; Shleeva, M.O.; Guryanova, S.V.; Ruggiero, A.; Berisio, R.; Kaprelyants, A.S. A product of RpfB and RipA joint enzymatic action promotes the resuscitation of dormant mycobacteria. FEBS J. 2015, 282, 2500–2511. [Google Scholar] [CrossRef] [PubMed]
- Berisio, R.; Squeglia, F.; Ruggiero, A.; Petraccone, L.; Stellato, M.I.; Del Vecchio, P. Differential thermodynamic behaviours of the extra-cellular regions of two Ser/Thr PrkC kinases revealed by calorimetric studies. Biochim. Biophys. Acta 2015, 1854, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Calvanese, L.; Falcigno, L.; Squeglia, F.; D’Auria, G.; Berisio, R. PASTA in Penicillin Binding Proteins and Serine/Threonine Kinases: A Recipe of Structural, Dynamic and Binding Properties. Curr. Med. Chem. 2017, 24, 4038–4056. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, A.; De Simone, P.; Smaldone, G.; Squeglia, F.; Berisio, R. Bacterial Cell Division Regulation by Ser/Thr Kinases: A Structural Perspective. Curr. Protein Pept. Sci. 2012, 13, 756–766. [Google Scholar] [CrossRef]
- Ruggiero, A.; Squeglia, F.; Marasco, D.; Marchetti, R.; Molinaro, A.; Berisio, R. X-ray structural studies of the entire extracellular region of the serine/threonine kinase PrkC from Staphylococcus aureus. Biochem. J. 2011, 435, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Squeglia, F.; Marchetti, R.; Ruggiero, A.; Lanzetta, R.; Marasco, D.; Dworkin, J.; Petoukhov, M.; Molinaro, A.; Berisio, R.; Silipo, A. Chemical basis of peptidoglycan discrimination by PrkC, a key kinase involved in bacterial resuscitation from dormancy. J. Am. Chem. Soc. 2011, 133, 20676–20679. [Google Scholar] [CrossRef]
- Squeglia, F.; Ruggiero, A.; Berisio, R. Exit from Mycobacterial Dormancy: A Structural Perspective. Curr. Med. Chem. 2015, 22, 1698–1709. [Google Scholar] [CrossRef]
- Kieser, K.J.; Boutte, C.C.; Kester, J.C.; Baer, C.E.; Barczak, A.K.; Meniche, X.; Chao, M.C.; Rego, E.H.; Sassetti, C.M.; Fortune, S.M.; et al. Phosphorylation of the Peptidoglycan Synthase PonA1 Governs the Rate of Polar Elongation in Mycobacteria. PLoS Pathog. 2015, 11, e1005010. [Google Scholar] [CrossRef]
- Gustine, J.N.; Au, M.B.; Haserick, J.R.; Hett, E.C.; Rubin, E.J.; Gibson, F.C., 3rd; Deng, L.L. Cell Wall Hydrolytic Enzymes Enhance Antimicrobial Drug Activity Against Mycobacterium. Curr. Microbiol. 2019, 76, 398–409. [Google Scholar] [CrossRef] [PubMed]

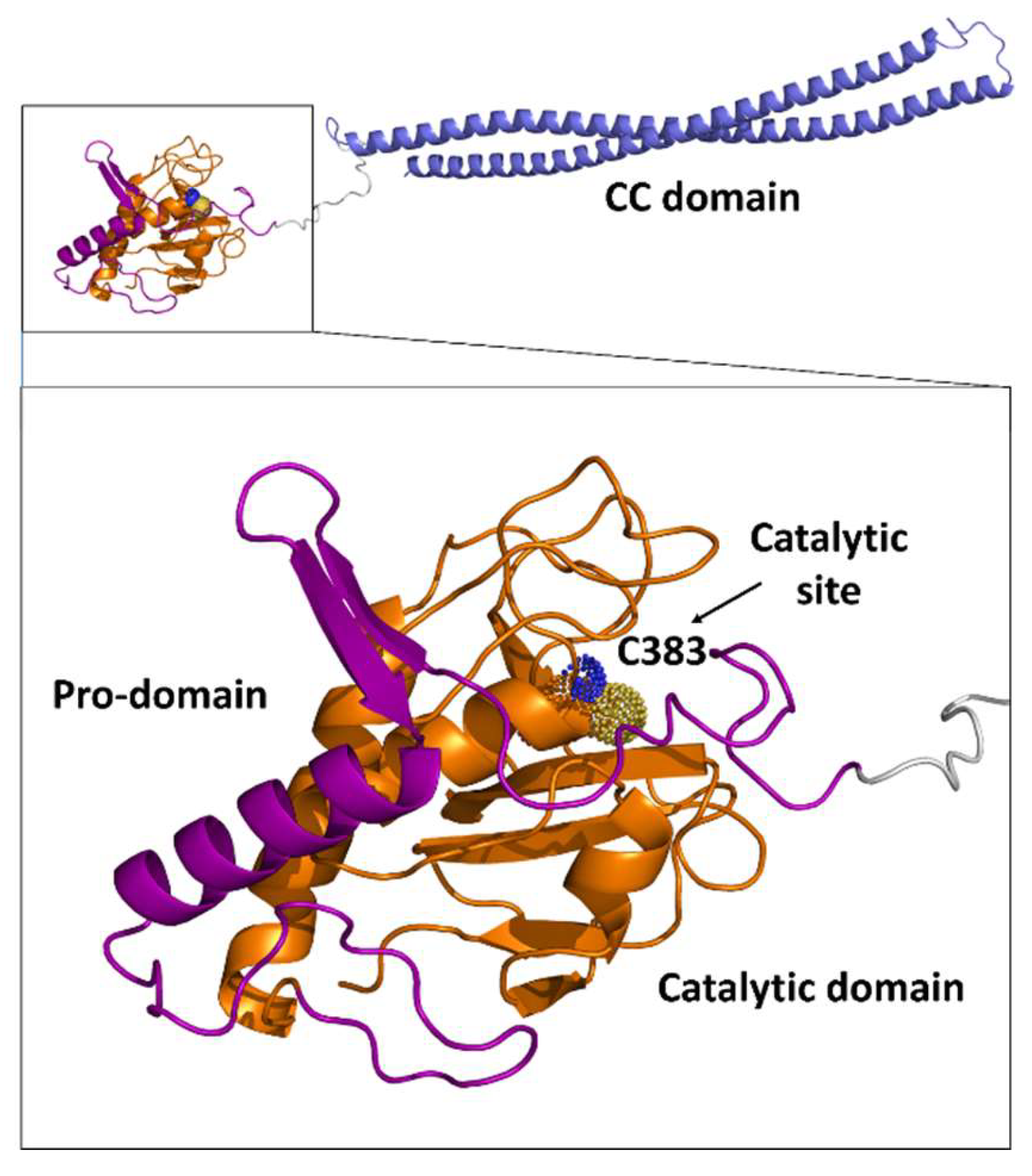
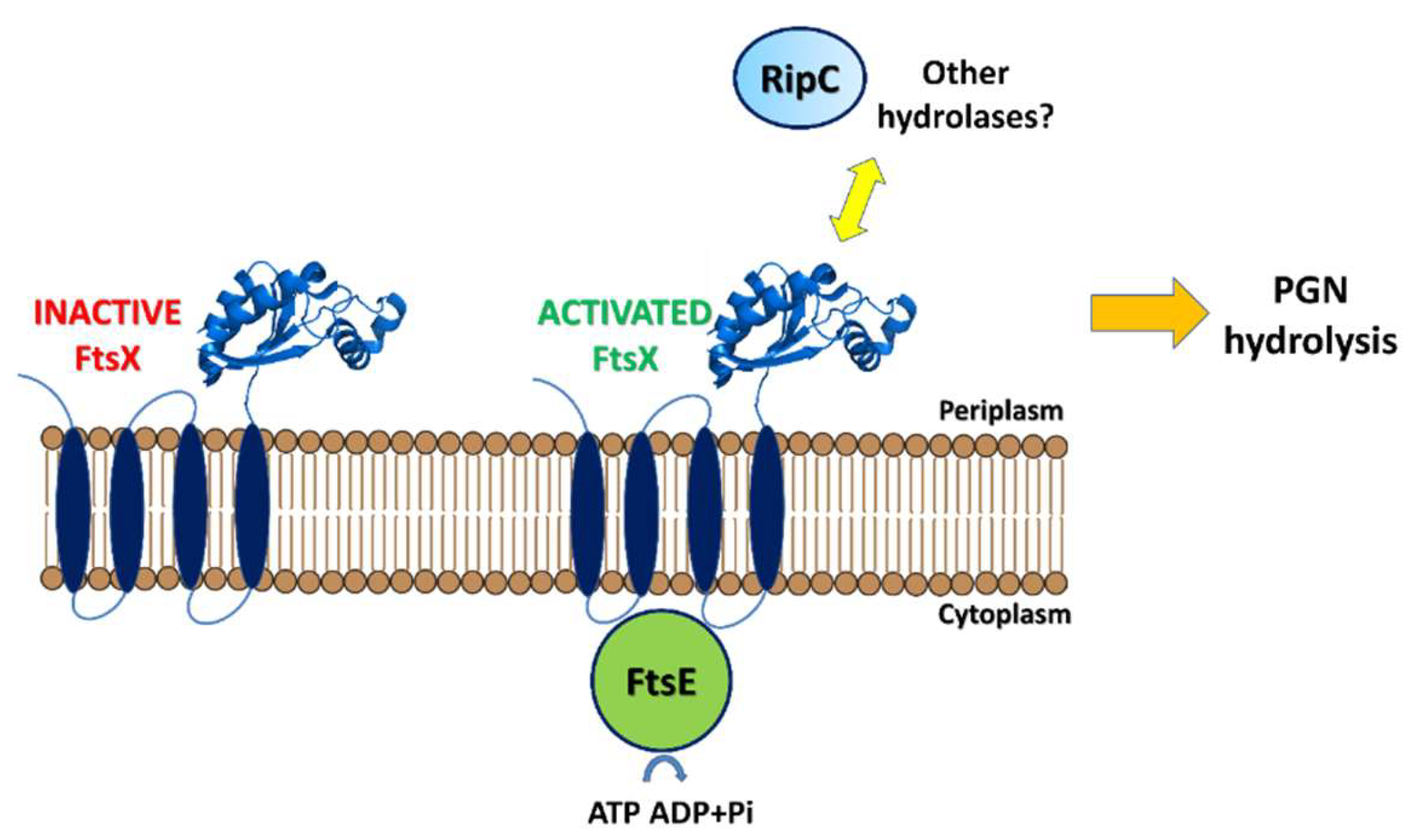
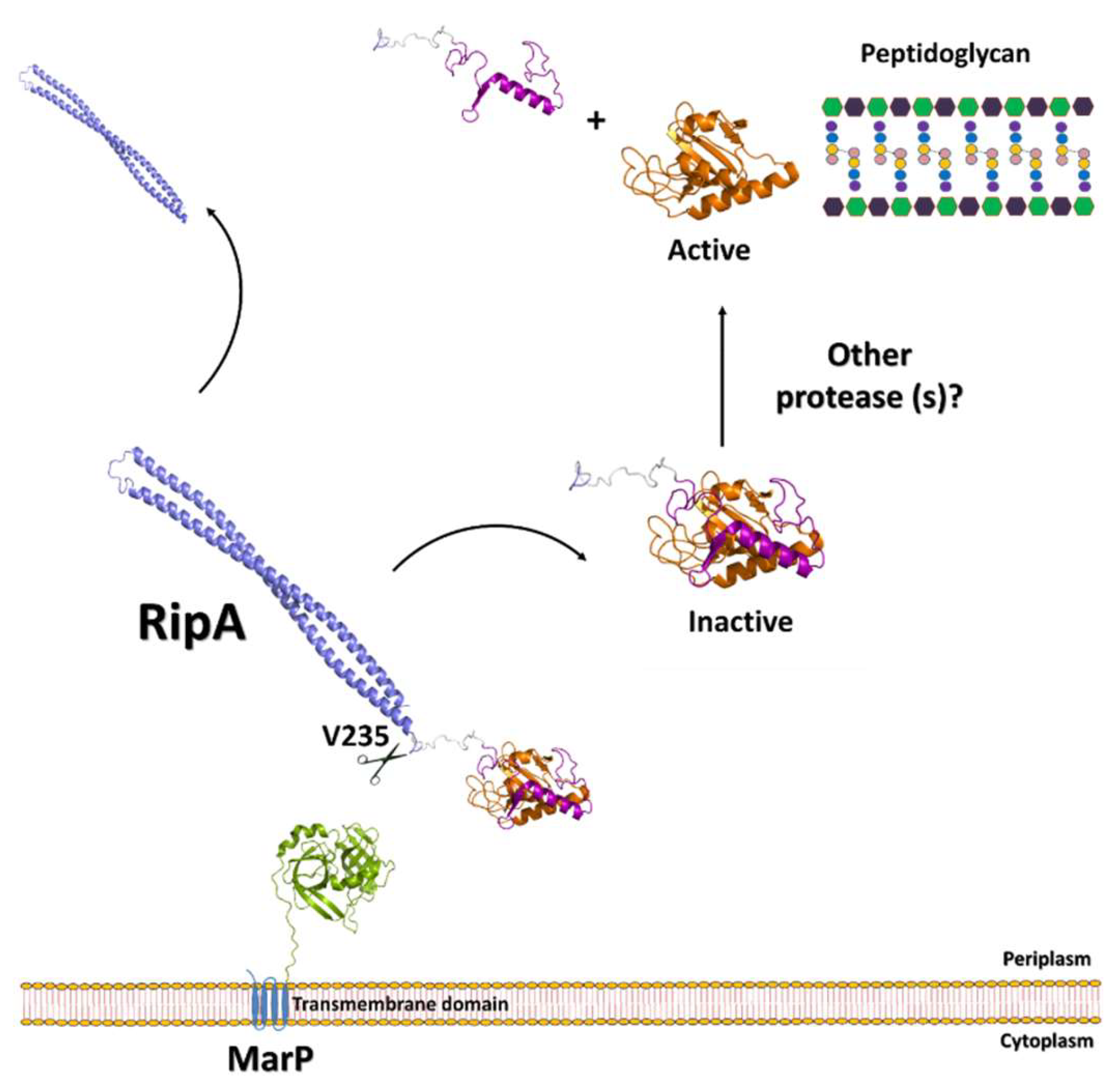
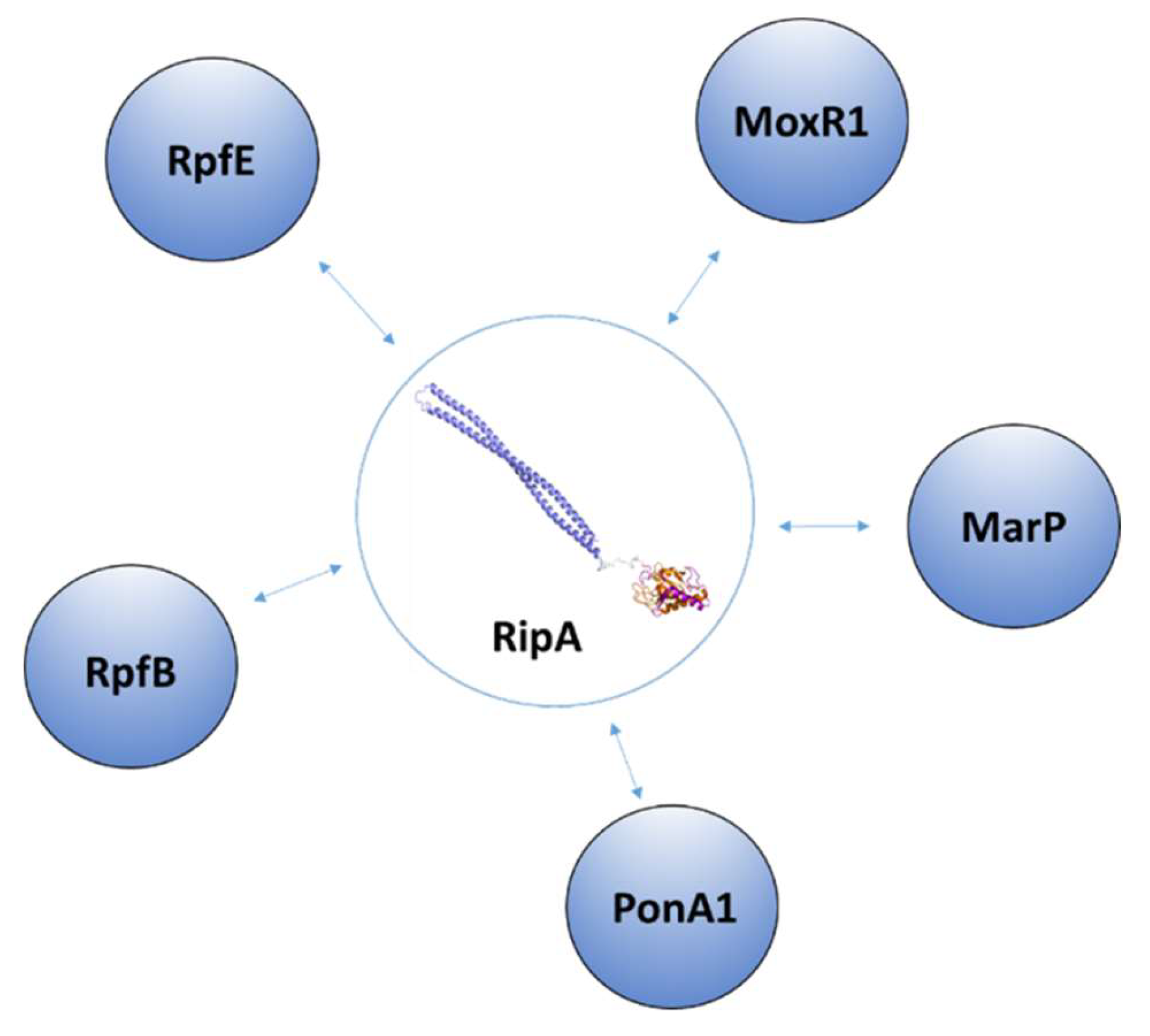
| Protein | Code | Fragment | PDB code | Ref |
|---|---|---|---|---|
| RipA | Rv1477 | 263−472 | 3ne0,4q4g, 4q4n,4q4t | [20,49] |
| 40−240 | 6ewy | [50] | ||
| RipB | Rv1478 | 30−241 | 3pbi | [51] |
| RipD | Rv1566c | 38−169 | 4jxb | [52] |
| 38−182 | 4lj1 | [52] | ||
| RpfB | Rv1009 | 194−362 | 3eo5 | [25] |
| 282−362 | 4emn,4kl7,4kpm | [23,53] | ||
| 115−362 | 5e27 | [24] | ||
| RpfC | Rv1884c | 68−159 | 4ow1 | [54] |
| 68−146 | 2n5z | [55] | ||
| RpfE | Rv2450c | 98−172 | 4cge | [56] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Squeglia, F.; Moreira, M.; Ruggiero, A.; Berisio, R. The Cell Wall Hydrolytic NlpC/P60 Endopeptidases in Mycobacterial Cytokinesis: A Structural Perspective. Cells 2019, 8, 609. https://doi.org/10.3390/cells8060609
Squeglia F, Moreira M, Ruggiero A, Berisio R. The Cell Wall Hydrolytic NlpC/P60 Endopeptidases in Mycobacterial Cytokinesis: A Structural Perspective. Cells. 2019; 8(6):609. https://doi.org/10.3390/cells8060609
Chicago/Turabian StyleSqueglia, Flavia, Miguel Moreira, Alessia Ruggiero, and Rita Berisio. 2019. "The Cell Wall Hydrolytic NlpC/P60 Endopeptidases in Mycobacterial Cytokinesis: A Structural Perspective" Cells 8, no. 6: 609. https://doi.org/10.3390/cells8060609
APA StyleSqueglia, F., Moreira, M., Ruggiero, A., & Berisio, R. (2019). The Cell Wall Hydrolytic NlpC/P60 Endopeptidases in Mycobacterial Cytokinesis: A Structural Perspective. Cells, 8(6), 609. https://doi.org/10.3390/cells8060609







