The Role of Micronutrients in the Infection and Subsequent Response to Hepatitis C Virus
Abstract
1. Introduction
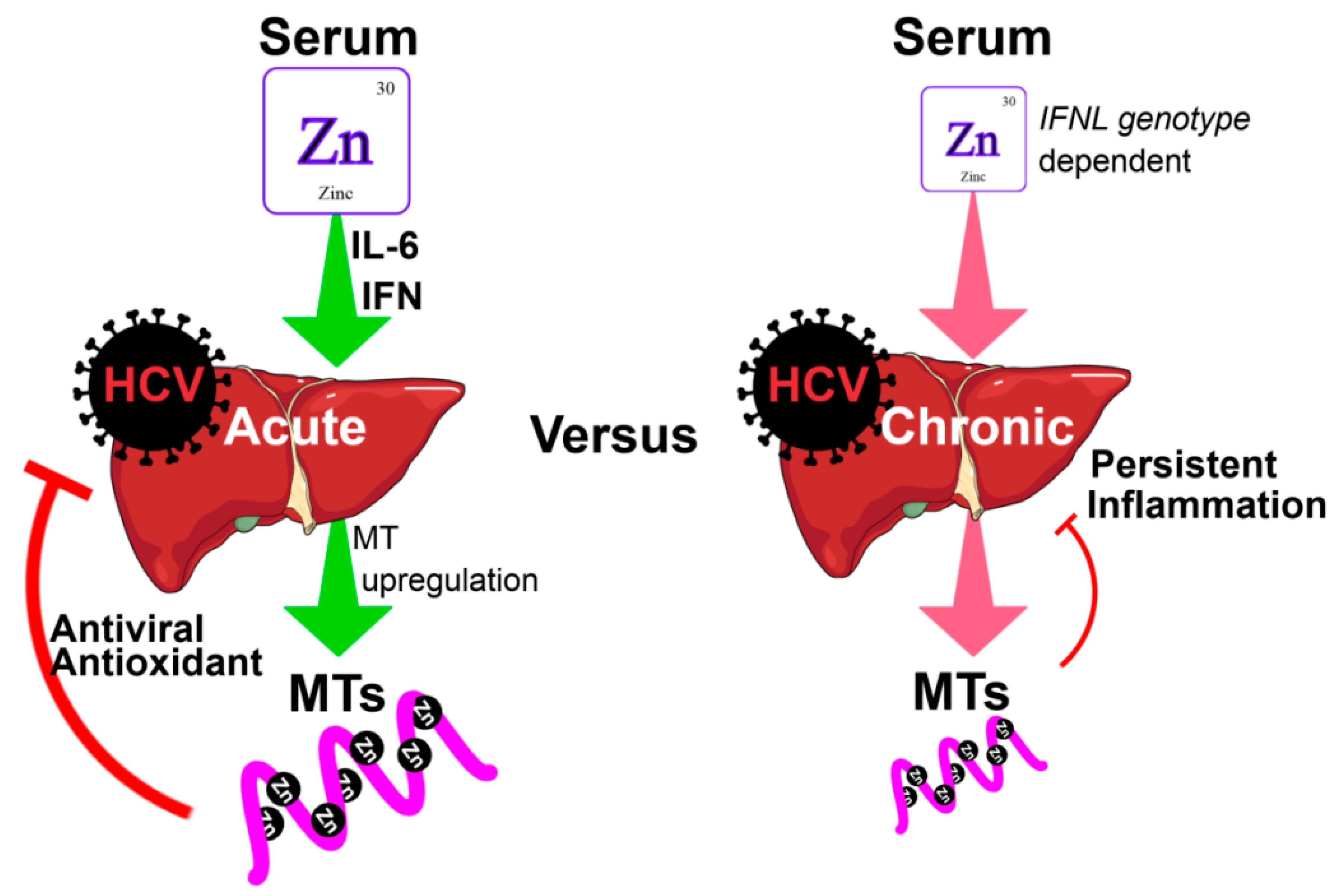
2. Zinc
2.1. Zinc Deficiency in HCV
2.2. The Effect of Zinc on HCV Pathogenesis, Immune Response and Treatment
3. Iron
3.1. Iron Excess in HCV
3.2. The Effect of Iron on HCV Pathogenesis, Immune Response and Treatment
4. Selenium
4.1. Selenium Deficiency in HCV
4.2. The Effect of Selenium on HCV Pathogenesis, Immune Response and Treatment
5. Copper
5.1. Copper Excess in HCV
5.2. The Effect of Copper on HCV Pathogenesis, Immune Response and Treatment
6. Vitamin A
6.1. Vitamin A Deficiency in HCV
6.2. The Effect of Vitamin A on HCV Pathogenesis, Immune Response and Treatment
7. Vitamin B12
The Effect of Vitamin B12 on HCV Pathogenesis, Immune Response and Treatment
8. Vitamin D
8.1. Vitamin D Deficiency in HCV
8.2. The effect of Vitamin D on HCV Pathogenesis, Immune Response and Treatment
9. Vitamin E
The Effect of Vitamin E on HCV Pathogenesis, Immune Response and Treatment
10. Conclusions
Funding
Conflicts of Interest
References
- McMillan, D.C.; Maguire, D.; Talwar, D. Relationship between nutritional status and the systemic inflammatory response: Micronutrients. Proc. Nutr. Soc. 2019, 78, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Bailey, R.L.; West, K.P., Jr.; Black, R.E. The epidemiology of global micronutrient deficiencies. Ann. Nutr. Metabol. 2015, 66, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Howson, C.P.; Kennedy, E.T.; Horwitz, A. Prevention of Micronutrient Deficiencies: Tools for Policymakers and Public Health Workers; Committee on Micronutrient Deficiencies, Board on International Health, Food and Nutrition Board: Washington, DC, USA, 1998. [Google Scholar]
- Rashed, M.N. The role of trace elements on hepatitis virus infections: A review. J. Trace Element. Med. Biol. 2011, 25, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Robertson, B.; Myers, G.; Howard, C.; Brettin, T.; Bukh, J.; Gaschen, B.; Gojobori, T.; Maertens, G.; Mizokami, M.; Nainan, O. Classification, nomenclature, and database development for hepatitis C virus (HCV) and related viruses: Proposals for standardization. Arch. Virol. 1998, 143, 2493–2503. [Google Scholar] [CrossRef] [PubMed]
- Alter, M.J. HCV routes of transmission: What goes around comes around. Semin. Liver Dis. 2011, 31, 340–346. [Google Scholar] [CrossRef]
- Fischer, R.; Baumert, T.; Blum, H.E. Hepatitis C virus infection and apoptosis. World J. Gastroenterol. 2007, 13, 4865. [Google Scholar] [CrossRef] [PubMed]
- Sy, T.; Jamal, M.M. Epidemiology of hepatitis C virus (HCV) infection. Int. J. Med. Sci. 2006, 3, 41. [Google Scholar] [CrossRef]
- Mills, C.F. Zinc in Human Biology; Springer: Berlin, Germany, 2013; pp. 1–4. [Google Scholar]
- Lee, Y.S.; Jeong, W.I. Retinoic acids and hepatic stellate cells in liver disease. J. Gastroenterol. Hepatol.y 2012, 27, 75–79. [Google Scholar] [CrossRef]
- Falasca, K.; Ucciferri, C.; Dalessandro, M.; Zingariello, P.; Mancino, P.; Petrarca, C.; Pizzigallo, E.; Conti, P.; Vecchiet, J. Cytokine patterns correlate with liver damage in patients with chronic hepatitis B and C. Ann. Clin. Lab. Sci. 2006, 36, 144–150. [Google Scholar]
- Chen, S.L.; Morgan, T.R. The natural history of hepatitis C virus (HCV) infection. Int. J. Med. Sci. 2006, 3, 47. [Google Scholar] [CrossRef]
- Read, S.A.; Parnell, G.; Booth, D.; Douglas, M.W.; George, J.; Ahlenstiel, G. The antiviral role of zinc and metallothioneins in hepatitis C infection. J. Viral Hepat. 2018, 25, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Maret, W. Metallothionein redox biology in the cytoprotective and cytotoxic functions of zinc. Exp. Gerontol. 2008, 43, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Sciavolino, P.J.; Vilček, J. Regulation of metallothionein gene expression by TNF-α and IFN-β in human fibroblasts. Cytokine 1995, 7, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Peres, W.; Chaves, G.; Gonçalves, J.; Ramalho, A.; Coelho, H. Vitamin A deficiency in patients with hepatitis C virus-related chronic liver disease. Br. J. Nutr. 2011, 106, 1724–1731. [Google Scholar] [CrossRef] [PubMed]
- Backstedt, D.; Pedersen, M.; Choi, M.; Seetharam, A. 25-Vitamin D levels in chronic hepatitis C infection: Association with cirrhosis and sustained virologic response. Ann. Gastroenterol. 2017, 30, 344. [Google Scholar] [CrossRef] [PubMed]
- Ko, Y.-L.; Morihara, D.; Shibata, K.; Yamauchi, R.; Fukuda, H.; Kunimoto, H.; Takata, K.; Tanaka, T.; Inomata, S.; Yokoyama, K. Factors attenuating zinc deficiency improvement in direct-acting antiviral agent-treated chronic hepatitis C virus infection. Nutrients 2018, 10, 1620. [Google Scholar] [CrossRef]
- Hayashi, F.; Momoki, C.; Yuikawa, M.; Simotani, Y.; Kawamura, E.; Hagihara, A.; Fujii, H.; Kobayashi, S.; Iwai, S.; Morikawa, H. Nutritional status in relation to lifestyle in patients with compensated viral cirrhosis. World J. Gastroenterol. 2012, 18, 5759. [Google Scholar] [CrossRef]
- Ismail, F.W.; Khan, R.A.; Kamani, L.; Wadalawala, A.A.; Shah, H.A.; Hamid, S.; Jafri, W. Nutritional status in patients with hepatitis C. J. College Phys. Surg. Pakistan 2012, 22, 139. [Google Scholar]
- Pennington, J.; Young, B. Iron, zinc, copper, manganese, selenium, and iodine in foods from the United States total diet study. J.Food Compos. Anal. 1990, 3, 166–184. [Google Scholar] [CrossRef]
- Olza, J.; Aranceta-Bartrina, J.; González-Gross, M.; Ortega, R.; Serra-Majem, L.; Varela-Moreiras, G.; Gil, Á. Reported dietary intake and food sources of zinc, selenium, and vitamins A, E and C in the Spanish population: Findings from the ANIBES study. Nutrients 2017, 9, 697. [Google Scholar] [CrossRef]
- Andreini, C.; Banci, L.; Bertini, I.; Rosato, A. Counting the zinc-proteins encoded in the human genome. J. Proteome Res. 2006, 5, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Tuerk, M.J.; Fazel, N. Zinc deficiency. Curr. Opin. Gastroenterol. 2009, 25, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Fraker, P.J.; King, L.E. Reprogramming of the immune system during zinc deficiency. Ann. Rev. Nutr. 2004, 24, 277–298. [Google Scholar] [CrossRef] [PubMed]
- Overbeck, S.; Rink, L.; Haase, H. Modulating the immune response by oral zinc supplementation: A single approach for multiple diseases. Arch. Immunol. Et Ther. Exp. 2008, 56, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.S.; Oberleas, D. Binding of zinc to amino acids and serum proteins in vitro. Translat. Res. 1970, 76, 416–425. [Google Scholar]
- Prasad, A.S. Clinical, endocrinological and biochemical effects of zinc deficiency. Clin. Endocrinol. Metabol. 1985, 14, 567–589. [Google Scholar] [CrossRef]
- Lichten, L.A.; Cousins, R.J. Mammalian zinc transporters: Nutritional and physiologic regulation. Annu. Rev. Nutr. 2009, 29, 153–176. [Google Scholar] [CrossRef] [PubMed]
- Grüngreiff, K.; Reinhold, D. Zinc: A complementary factor in the treatment of chronic hepatitis C? Mol. Med. Rep. 2010, 3, 371–375. [Google Scholar] [CrossRef]
- Gupta, S.H.H.; Read, S.; Wijaya, R.; George, J.; Ahlenstiel, G. The effect of fibrosis and direct-acting antiviral therapy on serum zinc levels in chronic hepatitis C infection. J. Gastroenterol. Hepatol. 2018, 33, 34–81. [Google Scholar]
- Grüngreiff, K.; Reinhold, D.; Ansorge, S. Serum concentrations of sIL-2R, IL-6, TGF-β1, neopterin, and zinc in chronic hepatitis C patients treated with interferon-alpha. Cytokine 1999, 11, 1076–1080. [Google Scholar] [CrossRef]
- Keeling, P.; Ruse, W.; Bull, J.; Hannigan, B.; Thompson, R. Direct measurement of the hepatointestinal extraction of zinc in cirrhosis and hepatitis. Clin. Sci. 1981, 61, 441–444. [Google Scholar] [CrossRef] [PubMed]
- Capocaccia, L.; Merli, M.; Piat, C.; Servi, R.; Zullo, A.; Riggio, O. Zinc and other trace elements in liver cirrhosis. Italian J. Gastroenterol. 1991, 23, 386–391. [Google Scholar]
- Cacciarelli, T.V.; Martinez, O.M.; Gish, R.G.; Villanueva, J.C.; Krams, S.M. Immunoregulatory cytokines in chronic hepatitis C virus infection: Pre-and posttreatment with interferon alfa. Hepatology 1996, 24, 6–9. [Google Scholar] [CrossRef]
- Beck, F.W.; Kaplan, J.; Fine, N.; Handschu, W.; Prasad, A.S. Decreased expression of CD73 (ecto-5′-nucleotidase) in the CD8+ subset is associated with zinc deficiency in human patients. J. Lab. Clin. Med. 1997, 130, 147–156. [Google Scholar] [CrossRef]
- Tapazoglou, E.; Prasad, A.S.; Hill, G.; Brewer, G.J.; Kaplan, J. Decreased natural killer cell activity in patients with zinc deficiency with sickle cell disease. J. Lab. Clin. Med. 1985, 105, 19–22. [Google Scholar] [PubMed]
- Beck, F.; Prasad, A.; Kaplan, J.; Fitzgerald, J.; Brewer, G. Changes in cytokine production and T cell subpopulations in experimentally induced zinc-deficient humans. Am. J. Physiol. Endocrinol. Metabol. 1997, 272, E1002–E1007. [Google Scholar] [CrossRef]
- Kaltenberg, J.; Plum, L.M.; Ober-Blöbaum, J.L.; Hönscheid, A.; Rink, L.; Haase, H. Zinc signals promote IL-2-dependent proliferation of T cells. Eur. J. Immunol. 2010, 40, 1496–1503. [Google Scholar] [CrossRef]
- Maywald, M.; Rink, L. Zinc supplementation induces CD4+ CD25+ Foxp3+ antigen-specific regulatory T cells and suppresses IFN-γ production by upregulation of Foxp3 and KLF-10 and downregulation of IRF-1. Eur. J. Nutr. 2017, 56, 1859–1869. [Google Scholar] [CrossRef]
- Chandra, R.K. Excessive intake of zinc impairs immune responses. JAMA 1984, 252, 1443–1446. [Google Scholar] [CrossRef]
- Yuasa, K.; Naganuma, A.; Sato, K.; Ikeda, M.; Kato, N.; Takagi, H.; Mori, M. Zinc is a negative regulator of hepatitis C virus RNA replication. Liver Int. 2006, 26, 1111–1118. [Google Scholar] [CrossRef]
- Read, S.A.; O’Connor, K.S.; Suppiah, V.; Ahlenstiel, C.L.; Obeid, S.; Cook, K.M.; Cunningham, A.; Douglas, M.W.; Hogg, P.J.; Booth, D. Zinc is a potent and specific inhibitor of IFN-λ3 signalling. Nat. Commun. 2017, 8, 15245. [Google Scholar] [CrossRef] [PubMed]
- Carrera, G.; Paternain, J.L.; Carrere, N.; Folch, J.; Courtade-Saïdi, M.; Orfila, C.; Vinel, J.P.; Alric, L.; Pipy, B. Hepatic metallothionein in patients with chronic hepatitis C: Relationship with severity of liver disease and response to treatment. Am. J. Gastroenterol. 2003, 98, 1142. [Google Scholar] [PubMed]
- Matsuoka, S.; Matsumura, H.; Nakamura, H.; Oshiro, S.; Arakawa, Y.; Hayashi, J.; Sekine, N.; Nirei, K.; Yamagami, H.; Ogawa, M. Zinc supplementation improves the outcome of chronic hepatitis C and liver cirrhosis. J. Clin. Biochem. Nutr. 2009, 45, 292–303. [Google Scholar] [CrossRef] [PubMed]
- Takagi, H.; Nagamine, T.; Abe, T.; Takayama, H.; Sato, K.; Otsuka, T.; Kakizaki, S.; Hashimoto, Y.; Matsumoto, T.; Kojima, A. Zinc supplementation enhances the response to interferon therapy in patients with chronic hepatitis C. J. Viral Hepat. 2001, 8, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Nagamine, T.; Takagi, H.; Hashimoto, Y.; Takayama, H.; Shimoda, R.; Nomura, N.; Suzuki, K.; Mori, M.; Nakajima, K. The possible role of zinc and metallothionein in the liver on the therapeutic effect of IFN-α to hepatitis C patients. Biol. Trace Elem. Res. 1997, 58, 65. [Google Scholar] [CrossRef] [PubMed]
- Read, S.A.; Tay, E.S.; Shahidi, M.; O’Connor, K.S.; Booth, D.R.; George, J.; Douglas, M.W. Hepatitis C virus driven AXL expression suppresses the hepatic type I interferon response. PLoS ONE 2015, 10, e0136227. [Google Scholar] [CrossRef] [PubMed]
- Sarasin-Filipowicz, M.; Oakeley, E.J.; Duong, F.H.; Christen, V.; Terracciano, L.; Filipowicz, W.; Heim, M.H. Interferon signaling and treatment outcome in chronic hepatitis C. Proc. Nat. Acad. Sci. USA 2008, 105, 7034–7039. [Google Scholar] [CrossRef]
- Lieu, P.T.; Heiskala, M.; Peterson, P.A.; Yang, Y. The roles of iron in health and disease. Mol. Asp. Med. 2001, 22, 1–87. [Google Scholar] [CrossRef]
- Winter, W.E.; Bazydlo, L.A.; Harris, N.S. The molecular biology of human iron metabolism. Lab. Med. 2014, 45, 92–102. [Google Scholar] [CrossRef]
- Fraga, C.G. Relevance, essentiality and toxicity of trace elements in human health. Mol. Asp. Med. 2005, 26, 235–244. [Google Scholar] [CrossRef]
- Gordeuk, V.R.; Bacon, B.R.; Brittenham, G.M. Iron overload: Causes and consequences. Annu. Rev. Nutr. 1987, 7, 485–508. [Google Scholar] [CrossRef] [PubMed]
- Hezode, C.; Cazeneuve, C.; Coue, O.; Roudot-Thoraval, F.; Lonjon, I.; Bastie, A.; Duvoux, C.; Pawlotsky, J.M.; Zafrani, E.S.; Amselem, S.; et al. Liver iron accumulation in patients with chronic active hepatitis C: Prevalence and role of hemochromatosis gene mutations and relationship with hepatic histological lesions. J. Hepatol. 1999, 31, 979–984. [Google Scholar] [CrossRef]
- Riggio, O.; Montagnese, F.; Fiore, P.; Folino, S.; Giambartolomei, S.; Gandin, C.; Merlis, M.; Quinti, I.; Violante, N.; Caroli, S. Iron overload in patients with chronic viral hepatitis: How common is it? Am. J. Gastroenterol. 1997, 92, 1298–1301. [Google Scholar] [PubMed]
- Fabris, C.; Toniutto, P.; Scott, C.A.; Falleti, E.; Avellini, C.; Del Forno, M.; Mattiuzzo, M.; Branca, B.; Pirisi, M. Serum iron indices as a measure of iron deposits in chronic hepatitis C. Clin. Chim. Acta 2001, 304, 49–55. [Google Scholar] [CrossRef]
- Metwally, M.A.; Zein, C.O.; Zein, N.N. Clinical significance of hepatic iron deposition and serum iron values in patients with chronic hepatitis C infection. Am. J. Gastroenterol. 2004, 99, 286. [Google Scholar] [CrossRef]
- Arber, N.; Konikoff, F.M.; Moshkowitz, M.; Baratz, M.; Hallak, A.; Santo, M.; Halpern, Z.; Weiss, H.; Gilat, T. Increased Serum iron and iron saturation without liver iron accumulation distinguish chronic hepatitis-C from other chronic liver-diseases. Dig. Dis. Sci. 1994, 39, 2656–2659. [Google Scholar] [CrossRef] [PubMed]
- Nishina, S.; Hino, K.; Korenaga, M.; Vecchi, C.; Pietrangelo, A.; Mizukami, Y.; Furutani, T.; Sakai, A.; Okuda, M.; Hidaka, I.; et al. Hepatitis C virus-induced reactive oxygen species raise hepatic iron level in mice by reducing hepcidin transcription. Gastroenterology 2008, 134, 226–238. [Google Scholar] [CrossRef]
- Miura, K.; Taura, K.; Kodama, Y.; Schnabl, B.; Brenner, D.A. Hepatitis C virus-induced oxidative stress suppresses hepcidin expression through increased histone deacetylase activity. Hepatology 2008, 48, 1420–1429. [Google Scholar] [CrossRef]
- Abboud, S.; Haile, D.J. A novel mammalian iron-regulated protein involved in intracellular iron metabolism. J. Biol. Chem. 2000, 275, 19906–19912. [Google Scholar] [CrossRef]
- Nemeth, E.; Tuttle, M.S.; Powelson, J.; Vaughn, M.B.; Donovan, A.; Ward, D.M.; Ganz, T.; Kaplan, J. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 2004, 306, 2090–2093. [Google Scholar] [CrossRef]
- Fujita, N.; Sugimoto, R.; Takeo, M.; Urawa, N.; Mifuji, R.; Tanaka, H.; Kobayashi, Y.; Iwasa, M.; Watanabe, S.; Adachi, Y. Hepcidin expression in the liver: Relatively low level in patients with chronic hepatitis C. Mol. Med. 2007, 13, 97. [Google Scholar] [CrossRef] [PubMed]
- Kakizaki, S.; Takagi, H.; Horiguchi, N.; Toyoda, M.; Takayama, H.; Nagamine, T.; Mori, M.; Kato, N. Iron enhances hepatitis C virus replication in cultured human hepatocytes. Liver 2000, 20, 125–128. [Google Scholar] [CrossRef] [PubMed]
- Fillebeen, C.; Pantopoulos, K. Iron inhibits replication of infectious hepatitis C virus in permissive Huh7.5.1 cells. J. Hepatol. 2010, 53, 995–999. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liu, Y.; An, D.; Diao, H.; Xu, W.; He, X.; Sun, R.; Wei, L.; Li, L. Regulation of hepatitis C virus translation initiation by iron: Role of eIF3 and La protein. Virus Res. 2012, 167, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Fillebeen, C.; Rivas-Estilla, A.M.; Bisaillon, M.; Ponka, P.; Muckenthaler, M.; Hentze, M.W.; Koromilas, A.E.; Pantopoulos, K. Iron inactivates the RNA polymerase NS5B and suppresses subgenomic replication of hepatitis C virus. J. Biol. Chem. 2005, 280, 9049–9057. [Google Scholar] [CrossRef] [PubMed]
- Bassett, S.E.; Di Bisceglie, A.M.; Bacon, B.R.; Sharp, R.M.; Govindarajan, S.; Hubbard, G.B.; Brasky, K.M.; Lanford, R.E. Effects of iron loading on pathogenicity in hepatitis C virus–infected chimpanzees. Hepatology 1999, 29, 1884–1892. [Google Scholar] [CrossRef] [PubMed]
- Meneghini, R. Iron homeostasis, oxidative stress, and DNA damage. Free Radic. Biol. Med. 1997, 23, 783–792. [Google Scholar] [CrossRef]
- Fong, T.-L.; Han, S.H.; Tsai, N.C.; Morgan, T.R.; Mizokami, M.; QianP, D.; Phan, C.; Goad, K.; Redeker, A.G. A pilot randomized, controlled trial of the effect of iron depletion on long-term response to α-interferon in patients with chronic hepatitis C. J. Hepatol. 1998, 28, 369–374. [Google Scholar] [CrossRef]
- Sartori, M.; Andorno, S.; Rigamonti, C.; Boldorini, R. Chronic hepatitis C treated with phlebotomy alone: Biochemical and histological outcome. Digest. Liver Dis. 2001, 33, 157–162. [Google Scholar] [CrossRef]
- Yano, M.; Hayashi, H.; Wakusawa, S.; Sanae, F.; Takikawa, T.; Shiono, Y.; Arao, M.; Ukai, K.; Ito, H.; Watanabe, K. Long term effects of phlebotomy on biochemical and histological parameters of chronic hepatitis C. Am. J. Gastroenterol. 2002, 97, 133. [Google Scholar] [CrossRef]
- Fargion, S.; Fracanzani, A.L.; Rossini, A.; Borzio, M.; Riggio, O.; Belloni, G.; Bissoli, F.; Ceriani, R.; Ballarè, M.; Massari, M. Iron reduction and sustained response to interferon-α therapy in patients with chronic hepatitis C: Results of an Italian multicenter randomized study. Am. J. Gastroenterol. 2002, 97, 1204–1210. [Google Scholar] [PubMed]
- Barton, A.L.; Banner, B.F.; Cable, E.E.; Bonkovsky, H.L. Distribution of iron in the liver predicts the response of chronic hepatitis C infection to interferon therapy. Am. J. Clin. Pathol. 1995, 103, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Sievert, W.; Pianko, S.; Warner, S.; Bowden, S.; Simpson, I.; Bowden, D.; Locarnini, S. Hepatic iron overload does not prevent a sustained virological response to interferon-α therapy: A long term follow-up study in hepatitis C-infected patients with β thalassemia major. Am. J. Gastroenterol. 2002, 97, 982. [Google Scholar] [CrossRef]
- Di Bisceglie, A.M.; Bonkovsky, H.L.; Chopra, S.; Flamm, S.; Reddy, R.K.; Grace, N.; Killenberg, P.; Hunt, C.; Tamburro, C.; Tavill, A.S. Iron reduction as an adjuvant to interferon therapy in patients with chronic hepatitis C who have previously not responded to interferon: A multicenter, prospective, randomized, controlled trial. Hepatology 2000, 32, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Goldhaber, S.B. Trace element risk assessment: Essentiality vs. toxicity. Regulat. Toxicol. Pharmacol. 2003, 38, 232–242. [Google Scholar] [CrossRef]
- Rotruck, J.T.; Pope, A.L.; Ganther, H.E.; Swanson, A.; Hafeman, D.G.; Hoekstra, W. Selenium: Biochemical role as a component of glutathione peroxidase. Science 1973, 179, 588–590. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, P.R.; Berry, M.J. The influence of selenium on immune responses. Mol. Nutr. Food Res. 2008, 52, 1273–1280. [Google Scholar] [CrossRef]
- Khan, M.S.; Dilawar, S.; Ali, I.; Rauf, N. The possible role of selenium concentration in hepatitis B and C patients. Saudi J. Gastroenterol. 2012, 18, 106. [Google Scholar] [CrossRef]
- Harrison, I.; Littlejohn, D.; Fell, G.S. Distribution of selenium in human blood plasma and serum. Analyst 1996, 121, 189–194. [Google Scholar] [CrossRef]
- Takahashi, K.; Cohen, H.J. Selenium-dependent glutathione peroxidase protein and activity: Immunological investigations on cellular and plasma enzymes. Blood 1986, 68, 640–645. [Google Scholar]
- Åkesson, B.; Bellew, T.; Burk, R.F. Purification of selenoprotein P from human plasma. Biochim. Biophys. Acta Protein Struct. Mol. Enzymol. 1994, 1204, 243–249. [Google Scholar] [CrossRef]
- Li, G.; Wang, F.; Kang, D.; Li, C. Keshan disease: An endemic cardiomyopathy in China. Human Pathol. 1985, 16, 602–609. [Google Scholar] [CrossRef]
- Moreno-Reyes, R.; Suetens, C.; Mathieu, F.; Begaux, F.; Zhu, D.; Rivera, M.T.; Boelaert, M.; Nève, J.; Perlmutter, N.; Vanderpas, J. Kashin–Beck osteoarthropathy in rural Tibet in relation to selenium and iodine status. N. Engl. J. Med. 1998, 339, 1112–1120. [Google Scholar] [CrossRef] [PubMed]
- Bettinger, D.; Schultheiss, M.; Hennecke, N.; Panther, E.; Knüppel, E.; Blum, H.E.; Thimme, R.; Spangenberg, H.C. Selenium levels in patients with hepatitis C virus-related chronic hepatitis, liver cirrhosis, and hepatocellular carcinoma: A pilot study. Hepatology 2013, 57, 2543–2544. [Google Scholar] [CrossRef] [PubMed]
- Himoto, T.; Yoneyama, H.; Kurokohchi, K.; Inukai, M.; Masugata, H.; Goda, F.; Haba, R.; Watababe, S.; Kubota, S.; Senda, S. Selenium deficiency is associated with insulin resistance in patients with hepatitis C virus–related chronic liver disease. Nutr. Res. 2011, 31, 829–835. [Google Scholar] [CrossRef] [PubMed]
- González-Reimers, E.; Martín-González, M.C.; Alemán-Valls, M.R.; de la Vega-Prieto, M.J.; Galindo-Martín, L.; Abreu-González, P.; Santolaria-Fernández, F. Relative and combined effects of chronic alcohol consumption and HCV infection on serum zinc, copper, and selenium. Biol. Trace Elem. Res. 2009, 132, 75. [Google Scholar] [CrossRef] [PubMed]
- Jablonska-Kaszewska, I.; Swiatkowska-Stodulska, R.; Lukasiak, J.; Dejneka, W.; Dorosz, A.; Dabrowska, E.; Falkiewicz, B. Serum selenium levels in alcoholic liver disease. Med. Sci. Monit. 2003, 9 (Suppl. 3), 15–18. [Google Scholar]
- Thuluvath, P.; Triger, D. Selenium in chronic liver disease. J. Hepatol. 1992, 14, 176–182. [Google Scholar] [CrossRef]
- Zhang, W.; Cox, A.G.; Taylor, E.W. Hepatitis C virus encodes a selenium-dependent glutathione peroxidase gene. Medizinische Klinik 1999, 94, 2. [Google Scholar] [CrossRef]
- Zhang, W.; Ramanathan, C.; Nadimpalli, R.; Bhat, A.; Cox, A.; Taylor, E.W. Selenium-dependent glutathione peroxidase modules encoded by RNA viruses. Biol. Trace Elem. Res. 1999, 70, 97–116. [Google Scholar] [CrossRef]
- Berkson, B.M. A conservative triple antioxidant approach to the treatment of hepatitis C. Medizinische Klinik 1999, 94, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Morbitzer, M.; Herget, T. Expression of gastrointestinal glutathione peroxidase is inversely correlated to the presence of hepatitis C virus subgenomic RNA in human liver cells. J. Biol. Chem. 2005, 280, 8831–8841. [Google Scholar] [CrossRef] [PubMed]
- Ko, W.-S.; Guo, C.-H.; Yeh, M.-S.; Lin, L.-Y.; Hsu, G.-S.W.; Chen, P.-C.; Luo, M.-C.; Lin, C.-Y. Blood micronutrient, oxidative stress, and viral load in patients with chronic hepatitis C. World J. Gastroenterol. 2005, 11, 4697. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.-W.; Horng, I.-S.; Hsu, K.-H.; Chiang, Y.-C.; Liaw, Y.F.; Chen, C.-J. Plasma selenium levels and risk of hepatocellular carcinoma among men with chronic hepatitis virus infection. Am. J. Epidemiol. 1999, 150, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Parola, M.; Pinzani, M.; Casini, A.; Albano, E.; Poli, G.; Gentilini, A.; Gentilini, P.; Dianzani, M.U. Stimulation of lipid peroxidation or 4-hydroxynonenal treatment increases procollagen α1 (I) gene expression in human liver fat-storing cells. Biochem. Biophys. Res. Commun. 1993, 194, 1044–1050. [Google Scholar] [CrossRef] [PubMed]
- Groenbaek, K.; Friis, H.; Hansen, M.; Ring-Larsen, H.; Krarup, H.B. The effect of antioxidant supplementation on hepatitis C viral load, transaminases and oxidative status: A randomized trial among chronic hepatitis C virus-infected patients. Eur. J. Gastroenterol. Hepatol. 2006, 18, 985–989. [Google Scholar] [CrossRef]
- Ghayour-Mobarhan, M.; Taylor, A.; New, S.; Lamb, D.; Ferns, G. Determinants of serum copper, zinc and selenium in healthy subjects. Ann. Clin. Biochem. 2005, 42, 364–375. [Google Scholar] [CrossRef]
- Gibson, R.S. Principles of Nutritional Assessment; Oxford University Press: New York, NY, USA, 2005; pp. 697–711. [Google Scholar]
- Johnson, M.A.; Fischer, J.G.; Kays, S.E. Is copper an antioxidant nutrient? Crit. Rev. Food Sci. Nutr. 1992, 32, 1–31. [Google Scholar] [CrossRef]
- Uauy, R.; Olivares, M.; Gonzalez, M. Essentiality of copper in humans. Am. J. Clin. Nutr. 1998, 67, 952S–959S. [Google Scholar] [CrossRef]
- Paterson, C.R.; Burns, J. Copper deficiency in infancy. J. Clin. Biochem. Nutr. 1988, 4, 175–190. [Google Scholar] [CrossRef]
- Eck, P.C.; Wilson, L. Copper Toxicity; Eck Institute of Applied Nutrition and Bioenergetics Ltd.: Feniks, AZ, USA, 1989; pp. 1–16. [Google Scholar]
- Arain, S.A.; Kazi, T.G.; Afridi, H.I.; Talpur, F.N.; Mughal, M.A.; Shah, F.; Arain, S.S.; Panhwar, A.H. Estimation of copper and iron burden in biological samples of various stages of hepatitis C and liver cirrhosis patients. Biol. Trace Elem. Res. 2014, 160, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.H.; Chen, P.C.; Ko, W.S. Status of essential trace minerals and oxidative stress in viral hepatitis C patients with nonalcoholic fatty liver disease. Int. J. Med. Sci. 2013, 10, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Cesur, S.; Cebeci, S.A.; Kavas, G.O.; Yılmaz, N.; Buyukkagnici, D.I. Serum copper and zinc concentrations in patients with chronic hepatitis C. J. Infect. 2005, 51, 35–37. [Google Scholar] [CrossRef] [PubMed]
- Hatano, R.; Ebara, M.; Fukuda, H.; Yoshikawa, M.; Sugiura, N.; Kondo, F.; Yukawa, M.; Saisho, H. Accumulation of copper in the liver and hepatic injury in chronic hepatitis C. J. Gastroenterol. Hepatol. 2000, 15, 786–791. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.T.; Rui, M.; Ueda, J.; Ozawa, T. Production of hydroxyl radicals by copper-containing metallothionein: Roles as prooxidant. Toxicol. Appl. Pharmacol. 1996, 141, 231–237. [Google Scholar] [CrossRef]
- Sakurai, H.; Fukudome, A.; Tawa, R.; Kito, M.; Takeshima, S.; Kimura, M.; Otaki, N.; Nakajima, K.; Hagino, T.; Kawano, K. Unusual accumulation of copper related to induction of metallothionein in the liver of LEC rats. Biochem. Biophys. Res. Commun. 1992, 184, 1393–1397. [Google Scholar] [CrossRef]
- Tao, T.Y.; Gitlin, J.D. Hepatic copper metabolism: Insights from genetic disease. Hepatology 2003, 37, 1241–1247. [Google Scholar] [CrossRef]
- Jorquera, F.; Monte, M.J.; Guerra, J.; Sanchez-Campos, S.; Merayo, J.A.; Olcoz, J.L.; Gonzalez-Gallego, J.; Marin, J.J. Usefulness of combined measurement of serum bile acids and ferritin as additional prognostic markers to predict failure to reach sustained response to antiviral treatment in chronic hepatitis C. J. Gastroenterol. Hepatol. 2005, 20, 547–554. [Google Scholar] [CrossRef]
- Shils, M.E.; Shike, M. Modern Nutrition in Health and Disease; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2006; pp. 286–299. [Google Scholar]
- Uriu-Adams, J.Y.; Keen, C.L. Copper, oxidative stress, and human health. Mol. Aspects Med. 2005, 26, 268–298. [Google Scholar] [CrossRef]
- Barbaro, G.; Di Lorenzo, G.; Ribersani, M.; Soldini, M.; Giancaspro, G.; Bellomo, G.; Belloni, G.; Grisorio, B.; Barbarini, G. Serum ferritin and hepatic glutathione concentrations in chronic hepatitis C patients related to the hepatitis C virus genotype. J. Hepatol. 1999, 30, 774–782. [Google Scholar] [CrossRef]
- Borkow, G.; Zhou, S.S.; Page, T.; Gabbay, J. A novel anti-influenza copper oxide containing respiratory face mask. PLoS ONE 2010, 5, e11295. [Google Scholar] [CrossRef] [PubMed]
- Sunada, K.; Minoshima, M.; Hashimoto, K. Highly efficient antiviral and antibacterial activities of solid-state cuprous compounds. J. Hazard. Mater. 2012, 235, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Tjoa, V.; Fan, H.M.; Tan, H.R.; Sayle, D.C.; Olivo, M.; Mhaisalkar, S.; Wei, J.; Sow, C.H. Reduced graphene oxide conjugated Cu2O nanowire mesocrystals for high-performance NO2 gas sensor. J. Am. Chem. Soc. 2012, 134, 4905–4917. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Srivastava, M.; Roychoudhury, A.; Lee, D.W.; Lee, S.H.; Malhotra, B. Bienzyme-functionalized monodispersed biocompatible cuprous oxide/chitosan nanocomposite platform for biomedical application. J. Phys. Chem. B 2012, 117, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Hang, X.; Peng, H.; Song, H.; Qi, Z.; Miao, X.; Xu, W. Antiviral activity of cuprous oxide nanoparticles against Hepatitis C Virus in vitro. J. Virol. Methods 2015, 222, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Bang, B.R.; Saito, T. Cellular retinoic acid-binding proteins regulation of hepatitis C virus infection. J. Hepatol. 2018, 68 (Suppl. 1), S780. [Google Scholar] [CrossRef]
- Bitetto, D.; Bortolotti, N.; Falleti, E.; Vescovo, S.; Fabris, C.; Fattovich, G.; Cussigh, A.; Cmet, S.; Fornasiere, E.; Ceriani, E. Vitamin A deficiency is associated with hepatitis C virus chronic infection and with unresponsiveness to interferon-based antiviral therapy. Hepatology 2013, 57, 925–933. [Google Scholar] [CrossRef] [PubMed]
- Yadav, D.; Hertan, H.I.; Schweitzer, P.; Norkus, E.; Pitchumoni, C. Serum and liver micronutrient antioxidants and serum oxidative stress in patients with chronic hepatitis C. Am. J. Gastroenterol. 2002, 97, 2634. [Google Scholar] [CrossRef]
- Bang, B.R.; Li, M.; Tsai, K.N.; Aoyagi, H.; Lee, S.A.; Machida, K.; Aizaki, H.; Jung, J.U.; Ou, J.J.; Saito, T. Regulation of hepatitis C virus infection by cellular retinoic acid binding proteins through the modulation of lipid droplet abundance. J. Virol. 2019, 93. [Google Scholar] [CrossRef]
- Hamamoto, S.; Fukuda, R.; Ishimura, N.; Rumi, M.A.; Kazumori, H.; Uchida, Y.; Kadowaki, Y.; Ishihara, S.; Kinoshita, Y. 9-cis retinoic acid enhances the antiviral effect of interferon on hepatitis C virus replication through increased expression of type I interferon receptor. J. Lab. Clin. Med. 2003, 141, 58–66. [Google Scholar] [CrossRef]
- Böcher, W.O.; Wallasch, C.; Höhler, T.; Galle, P.R. All-trans retinoic acid for treatment of chronic hepatitis C. Liver Int. 2008, 28, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.Z.; Sarkar, D.; Emdad, L.; Barral, P.M.; Fisher, P.B. Central role of interferon regulatory factor-1 (IRF-1) in controlling retinoic acid inducible gene-I (RIG-I) expression. J. Cell. Physiol. 2007, 213, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Kast, R.E. Potential for all-trans retinoic acid [tretinoin] to enhance interferon-alpha treatment response in chronic myelogenous leukemia, melanoma, myeloma, and renal cell carcinoma. Cancer Biol. Ther. 2008, 7, 1515–1519. [Google Scholar] [CrossRef] [PubMed]
- Penniston, K.L.; Tanumihardjo, S.A. The acute and chronic toxic effects of vitamin A–. Am. J. Clin. Nutr. 2006, 83, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Hunt, A.; Harrington, D.; Robinson, S. Vitamin B12 deficiency. BMJ 2014, 349, 5226. [Google Scholar] [CrossRef] [PubMed]
- Stabler, S.P. Vitamin B12 deficiency. N. Engl. J. Med. 2013, 368, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Langan, R.C.; Zawistoski, K.J. Update on Vitamin B 12 Deficiency. Am. Family Phys. 2011, 83, 1–6. [Google Scholar]
- Beck, W. Biological and medical aspects of vitamin B12. In Dolphin D (ed) Vitamin B12; Wiley: New York, NY, USA, 1982; Volume 2, pp. 1–30. [Google Scholar]
- Lukavsky, P.J. Structure and function of HCV IRES domains. Virus Res. 2009, 139, 166–171. [Google Scholar] [CrossRef]
- Sussman, D.; Nix, J.C.; Wilson, C. The structural basis for molecular recognition by the vitamin B 12 RNA aptamer. Nat. Struct. Mol. Biol. 2000, 7, 53. [Google Scholar] [CrossRef]
- Lott, W.B.; Takyar, S.S.; Tuppen, J.; Crawford, D.H.G.; Harrison, M.; Sloots, T.P.; Gowans, E.J. Vitamin B12 and hepatitis C: Molecular biology and human pathology. Proc. Nat. Acad. Sci. USA 2001, 98, 4916–4921. [Google Scholar] [CrossRef]
- Rosenberg, P.; Hagen, K. Serum B12 levels predict response to treatment with interferon and ribavirin in patients with chronic HCV infection. J. Viral. Hepat. 2011, 18, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Mechie, N.C.; Goralzcyk, A.D.; Reinhardt, L.; Mihm, S.; Amanzada, A. Association of serum vitamin B12 levels with stage of liver fibrosis and treatment outcome in patients with chronic hepatitis C virus genotype 1 infection: A retrospective study. BMC Res. Notes 2015, 8, 260. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Arendt, J.F.; Nexo, E. Cobalamin related parameters and disease patterns in patients with increased serum cobalamin levels. PLoS ONE 2012, 7, e45979. [Google Scholar] [CrossRef] [PubMed]
- Borgia, G.; Gentile, I.; Fortunato, G.; Borrelli, F.; Borelli, S.; De Caterina, M.; Di Taranto, M.D.; Simone, M.; Borgia, F.; Viola, C. Homocysteine levels and sustained virological response to pegylated-interferon α2b plus ribavirin therapy for chronic hepatitis C: A prospective study. Liver Int. 2009, 29, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Savage, D.G.; Lindenbaum, J.; Stabler, S.P.; Allen, R.H. Sensitivity of serum methylmalonic acid and total homocysteine determinations for diagnosing cobalamin and folate deficiencies. Am. J. Med. 1994, 96, 239–246. [Google Scholar] [CrossRef]
- Mokhtare, M.; Zeidabadi, A.D.; Bahardoust, M.; Safari, S.; Barati, M.; Agah, S.; Motavaf, M. The efficacy of adding vitamin B12 to pegylated interferon and ribavirin treatment in Hepatitis C virus patients regarding the host and viral prognostic factors. Biomed. Res. Ther. 2019, 6, 3016–3026. [Google Scholar] [CrossRef]
- Rocco, A.; Compare, D.; Coccoli, P.; Esposito, C.; Di Spirito, A.; Barbato, A.; Strazzullo, P.; Nardone, G. Vitamin B12 supplementation improves rates of sustained viral response in patients chronically infected with hepatitis C virus. Gut 2013, 62, 766–773. [Google Scholar] [CrossRef] [PubMed]
- Verstuyf, A.; Carmeliet, G.; Bouillon, R.; Mathieu, C. Vitamin D: A pleiotropic hormone. Kidney Int. 2010, 78, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Mora, J.R.; Iwata, M.; Von Andrian, U.H. Vitamin effects on the immune system: Vitamins A and D take centre stage. Nat. Rev. Immunol. 2008, 8, 685. [Google Scholar] [CrossRef]
- Jin, C.-N.; Chen, J.-D.; Sheng, J.-F. Vitamin D deficiency in hepatitis C virus infection: What is old? what is new? Eur. J. Gastroenterol. Hepatol. 2018, 30, 741–746. [Google Scholar] [CrossRef]
- Bitetto, D.; Fabris, C.; Fornasiere, E.; Pipan, C.; Fumolo, E.; Cussigh, A.; Bignulin, S.; Cmet, S.; Fontanini, E.; Falleti, E.; et al. Vitamin D supplementation improves response to antiviral treatment for recurrent hepatitis C. Transpl. Int. 2011, 24, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Ladero, J.M.; Torrejón, M.J.; Sánchez-Pobre, P.; Suárez, A.; Cuenca, F.; de la Orden, V.; Devesa, M.J.; Rodrigo, M.; Estrada, V.; López-Alonso, G. Vitamin D deficiency and vitamin D therapy in chronic hepatitis C. Ann. Hepatol. 2015, 12, 199–204. [Google Scholar] [CrossRef]
- Melo-Villar, L.; Lampe, E.; de Almeida, A.J.; de, P.S.L.; Lewis-Ximenez, L.L.; Miguel, J.C.; Del Campo, J.A.; Ranchal, I.; Villela-Nogueira, C.A.; Romero-Gomez, M. Hypovitaminosis D and its relation to demographic and laboratory data among hepatitis C patients. Ann. Hepatol. 2015, 14, 457–463. [Google Scholar] [CrossRef]
- Weintraub, S.J.; Fleckenstein, J.F.; Marion, T.N.; Madey, M.A.; Mahmoudi, T.M.; Schechtman, K.B. Vitamin D and the racial difference in the genotype 1 chronic hepatitis C treatment response. Am. J. Clin. Nutr. 2012, 96, 1025–1031. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Petta, S.; Cammà, C.; Scazzone, C.; Tripodo, C.; Di Marco, V.; Bono, A.; Cabibi, D.; Licata, G.; Porcasi, R.; Marchesini, G. Low vitamin D serum level is related to severe fibrosis and low responsiveness to interferon-based therapy in genotype 1 chronic hepatitis C. Hepatology 2010, 51, 1158–1167. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, J.P.S.; Liberatti, L.S.; Barros, F.E.N.; Kallaur, A.P.; Lozovoy, M.A.B.; Scavuzzi, B.M.; Panis, C.; Reiche, E.M.V.; Dichi, I.; Simão, A.N.C. Profile of oxidative stress markers is dependent on vitamin D levels in patients with chronic hepatitis C. Nutrition 2016, 32, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Gal-Tanamy, M.; Bachmetov, L.; Ravid, A.; Koren, R.; Erman, A.; Tur-Kaspa, R.; Zemel, R. Vitamin D: An innate antiviral agent suppressing hepatitis C virus in human hepatocytes. Hepatology 2011, 54, 1570–1579. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, T.; Kato, T.; Sugiyama, N.; Tasaka-Fujita, M.; Murayama, A.; Masaki, T.; Wakita, T.; Imawari, M. 25-hydroxyvitamin D3 suppresses hepatitis C virus production. Hepatology 2012, 56, 1231–1239. [Google Scholar] [CrossRef]
- Lange, C.M.; Gouttenoire, J.; Duong, F.H.; Morikawa, K.; Heim, M.H.; Moradpour, D. Vitamin D receptor and Jak-STAT signaling crosstalk results in calcitriol-mediated increase of hepatocellular response to IFN-alpha. J. Immunol. 2014, 192, 6037–6044. [Google Scholar] [CrossRef]
- Nimer, A.; Mouch, A. Vitamin D improves viral response in hepatitis C genotype 2-3 naïve patients. World J. Gastroenterol. 2012, 18, 800. [Google Scholar] [CrossRef]
- Abdelsalam, A.; Rashed, L.; Salman, T.; Hammad, L.; Sabry, D. Molecular assessment of vitamin D receptor polymorphism as a valid predictor to the response of interferon/ribavirin-based therapy in Egyptian patients with chronic hepatitis C. J. Digest. Dis. 2016, 17, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Atsukawa, M.; Tsubota, A.; Shimada, N.; Abe, H.; Kondo, C.; Itokawa, N.; Nakagawa, A.; Iwakiri, K.; Kawamoto, C.; Aizawa, Y. Serum 25 (OH) D3 levels affect treatment outcomes for telaprevir/peg-interferon/ribavirin combination therapy in genotype 1b chronic hepatitis C. Digest. Liver Dis. 2014, 46, 738–743. [Google Scholar] [CrossRef] [PubMed]
- Lange, C.M.; Bibert, S.; Kutalik, Z.; Burgisser, P.; Cerny, A.; Dufour, J.-F.; Geier, A.; Gerlach, T.J.; Heim, M.H.; Malinverni, R. A genetic validation study reveals a role of vitamin D metabolism in the response to interferon-alfa-based therapy of chronic hepatitis C. PLoS ONE 2012, 7, e40159. [Google Scholar] [CrossRef] [PubMed]
- Esmat, G.; El Raziky, M.; Elsharkawy, A.; Sabry, D.; Hassany, M.; Ahmed, A.; Assem, N.; El Kassas, M.; Doss, W. Impact of vitamin D supplementation on sustained virological response in chronic hepatitis C genotype 4 patients treated by pegylated interferon/ribavirin. J. Interferon Cytokine Res. 2015, 35, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Abu-Mouch, S.; Fireman, Z.; Jarchovsky, J.; Zeina, A.-R.; Assy, N. Vitamin D supplementation improves sustained virologic response in chronic hepatitis C (genotype 1)-naïve patients. World J. Gastroenterol. 2011, 17, 5184. [Google Scholar] [CrossRef] [PubMed]
- Behera, M.K.; Shukla, S.K.; Dixit, V.K.; Nath, P.; Abhilash, V.; Asati, P.K.; Jain, A.K. Effect of vitamin D supplementation on sustained virological response in genotype 1/4 chronic hepatitis C treatment-naïve patients from India. Indian J. Med. Res. 2018, 148, 200. [Google Scholar] [CrossRef]
- Von Herbay, A.; Stahl, W.; Niederau, C.; Sies, H. Vitamin E improves the aminotransferase status of patients suffering from viral hepatitis C: A randomized, double-blind, placebo-controlled study. Free Radic. Res. 1997, 27, 599–605. [Google Scholar] [CrossRef]
- Herbay, A.V.; Stahl, W.; Niederau, C.; Laar, J.V.; Strohmeyer, G.; Sies, H. Diminished plasma levels of vitamin E in patients with severe viral hepatitis. Free Radic. Res. 1996, 25, 461–466. [Google Scholar] [CrossRef]
- Mezes, M.; Par, A.; Nemeth, P.; Javor, T. Studies of the blood lipid peroxide status and vitamin E levels in patients with chronic active hepatitis and alcoholic liver disease. Int. J. Clin. Pharmacol. Res. 1986, 6, 333–338. [Google Scholar]
- Bunchorntavakul, C.; Wootthananont, T.; Atsawarungruangkit, A. Effects of vitamin E on chronic hepatitis C genotype 3: A randomized, double-blind, placebo-controlled study. J. Med. Assoc. Thai. 2014, 97, S31–S40. [Google Scholar]
- Falasca, K.; Ucciferri, C.; Mancino, P.; Vitacolonna, E.; De Tullio, D.; Pizzigallo, E.; Conti, P.; Vecchiet, J. Treatment with silybin-vitamin E-phospholipid complex in patients with hepatitis C infection. J. Med. Virol. 2008, 80, 1900–1906. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, S.; Yamada, G.; Niiyama, G.; Kawanaka, M.; Togawa, K.; Sho, M.; Ito, T.; Sasagawa, T.; Okita, M.; Nakamura, H. Effect of vitamin E on serum aminotransferase and thioredoxin levels in patients with viral hepatitis C. Free Radic. Res. 2003, 37, 781–785. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations. Undernourishment Around the World, The State of Food Insecurity in the World; United Nations: Rome, Italy, 2004. [Google Scholar]
- Alter, M.J. Epidemiology of hepatitis C virus infection. World J. Gastroenterol. 2007, 13, 2436. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Zhao, L.; Ren, M.; Deng, M.; Li, C. Zinc mediated hepatic stellate cell collagen synthesis reduction through TGF-β signaling pathway inhibition. Int. J. Clin. Exp. Med. 2015, 8, 20463. [Google Scholar] [PubMed]
- Takahashi, M.; Saito, H.; Higashimoto, M.; Hibi, T. Possible inhibitory effect of oral zinc supplementation on hepatic fibrosis through downregulation of TIMP-1: A pilot study. Hepatol. Res. 2007, 37, 405–409. [Google Scholar] [CrossRef]
- Himoto, T.; Hosomi, N.; Nakai, S.; Deguchi, A.; Kinekawa, F.; Matsuki, M.; Yachida, M.; Masaki, T.; Kurokochi, K.; Watanabe, S. Efficacy of zinc administration in patients with hepatitis C virus-related chronic liver disease. Scand. J. Gastroenterol. 2007, 42, 1078–1087. [Google Scholar] [CrossRef]
- Marchesini, G.; Bugianesi, E.; Ronchi, M.; Flamia, R.; Thomaseth, K.; Pacini, G. Zinc supplementation improves glucose disposal in patients with cirrhosis. Metabolism 1998, 47, 792–798. [Google Scholar] [CrossRef]
- Georgopoulou, U.; Dimitriadis, A.; Foka, P.; Karamichali, E.; Mamalaki, A. Hepcidin and the iron enigma in HCV infection. Virulence 2014, 5, 465–476. [Google Scholar] [CrossRef]
| Micronutrient | Mechanism of Deficiency/Excess | Role in HCV Life Cycle | Role in Tissue Damage / Fibrosis | Role in Treatment Response |
|---|---|---|---|---|
| Zinc (Deficiency) | Acute HCV stimulates IL-6 induction, stimulating hepatocyte uptake of zinc via the Zip14 zinc transporter [29]. | Zinc is a negative regulator of HCV replication in genome-length RNA-replicating cells [13,42]. | Zinc inhibits proliferation and collagen synthesis in HSCs by increasing matrix metalloproteinase 13 [171]. Promotes apoptosis of HSCs and reduces type-IV collagen [172]. Inhibits IFN-λ3 [43]. | Zinc supplementation reduces HCV replication in vitro, improves response to IFN-α [42,45,46] and results in higher rate of HCV clearance [173,174]. |
| Iron (Excess) | Through HCV protein-mediated oxidative stress, hepcidin is lowered, increasing FPN-mediated iron absorption [175]. | Iron promotes HCV via initiation factor 3, La proteins, and binding of cellular factors to HCV IRES [66]. Inhibits NS5B polymerase activity [67]. | Iron deposition in the liver of HCV patients triggers reactive oxygen species, inducing lipid peroxidation and mitochondrial dysfunction [68,69]. Iron is associated with a higher prevalence of HCC in HCV patients [69]. | Phlebotomy modestly increases SVR [73]. In IFN therapy non-responders, reduces AST and necro-inflammation [76]. |
| Selenium (Deficiency) | Possible malabsorption [87,90] or viral sequestration [93]. | HCV inhibits expression of selenium-dependent GPx, promoting intracellular HCV propagation and higher viral loads [94,95]. | Selenium levels decline in proportion with hepatic fibrosis [87] and result in accumulation of lipid peroxides. This leads to expression of VEGF and IL-8, accelerating the growth of HCC [96]. | Selenium with alpha-lipoic acid and silymarin improves ALT [93]. When given with vitamin E and ascorbic acid, it does not affect ALT or HCV RNA viral load [98]. |
| Copper (Excess) | Hepatic copper-MT accumulation contribute to copper overload [108]. Reduction in biliary copper secretion [112] | Cuprous oxide nanoparticles (CO-NPs) inhibit the infectivity of HCV in vitro [120]. | Hepatic copper increases with hepatic fibrosis and correlates positively with type IV collagen [108]. MT-bound copper stimulates hydroxyl radicals in rats, driving liver damage and fibrosis [109]. | No data available. |
| Vitamin A (Deficiency) | No clear mechanism identified. | HCV replication is up-regulated in cells expressing CRABP1 via lipid droplet formation [125]. ATRA reduces viral replication [94]. | Diminished liver retinol levels are found in CHC patients with moderate to severe fibrosis compared to those with mild fibrosis [123] | ATRA mediates retinoic acid-inducible gene-I [128], leading to transcription of type-1 IFNs, enhancing the effect of IFN-α on HCV [126,129]. |
| Vitamin B12 (Deficiency) | No clear mechanism identified. | HCV may use a virally encoded protein or cellular factor, such as B12, that targets HCV IRES to regulate translation [137]. | No clear association identified. | Vitamin B12 supplementation with pegylated-IFN-α and ribavirin therapy improves SVR [143,144]. |
| Vitamin D (Deficiency) | No clear mechanism identified. | Vitamin D3 inhibits HCV replication via expression of IFN-β [155]. No direct antiviral effect [157]. | Low vitamin D levels in CHC are linked to severe fibrosis [153] and higher levels of nitric oxide metabolites [154]. | Vitamin D predicts response in HCV-2/3 patients from Europe [153] and Asia [158], and in HCV-4 patients from Africa [159]. Studies in HCV-1 found no association [149,161,162]. |
| Vitamin E (Deficiency) | No clear mechanism identified. | No clear association identified. | No clear association identified. | High-dose vitamin E results in reductions in ALT and AST [165,168]. In combination with silybin–phospholipid, vitamin E reduced IFN-γ, TNF-α and IL-6, suggesting an anti-inflammatory effect [169]. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gupta, S.; Read, S.A.; Shackel, N.A.; Hebbard, L.; George, J.; Ahlenstiel, G. The Role of Micronutrients in the Infection and Subsequent Response to Hepatitis C Virus. Cells 2019, 8, 603. https://doi.org/10.3390/cells8060603
Gupta S, Read SA, Shackel NA, Hebbard L, George J, Ahlenstiel G. The Role of Micronutrients in the Infection and Subsequent Response to Hepatitis C Virus. Cells. 2019; 8(6):603. https://doi.org/10.3390/cells8060603
Chicago/Turabian StyleGupta, Sunil, Scott A. Read, Nicholas A. Shackel, Lionel Hebbard, Jacob George, and Golo Ahlenstiel. 2019. "The Role of Micronutrients in the Infection and Subsequent Response to Hepatitis C Virus" Cells 8, no. 6: 603. https://doi.org/10.3390/cells8060603
APA StyleGupta, S., Read, S. A., Shackel, N. A., Hebbard, L., George, J., & Ahlenstiel, G. (2019). The Role of Micronutrients in the Infection and Subsequent Response to Hepatitis C Virus. Cells, 8(6), 603. https://doi.org/10.3390/cells8060603





