Melanin and Neuromelanin Fluorescence Studies Focusing on Parkinson’s Disease and Its Inherent Risk for Melanoma
Abstract
:1. Introduction
2. Material and Methods
2.1. Introductory Overview
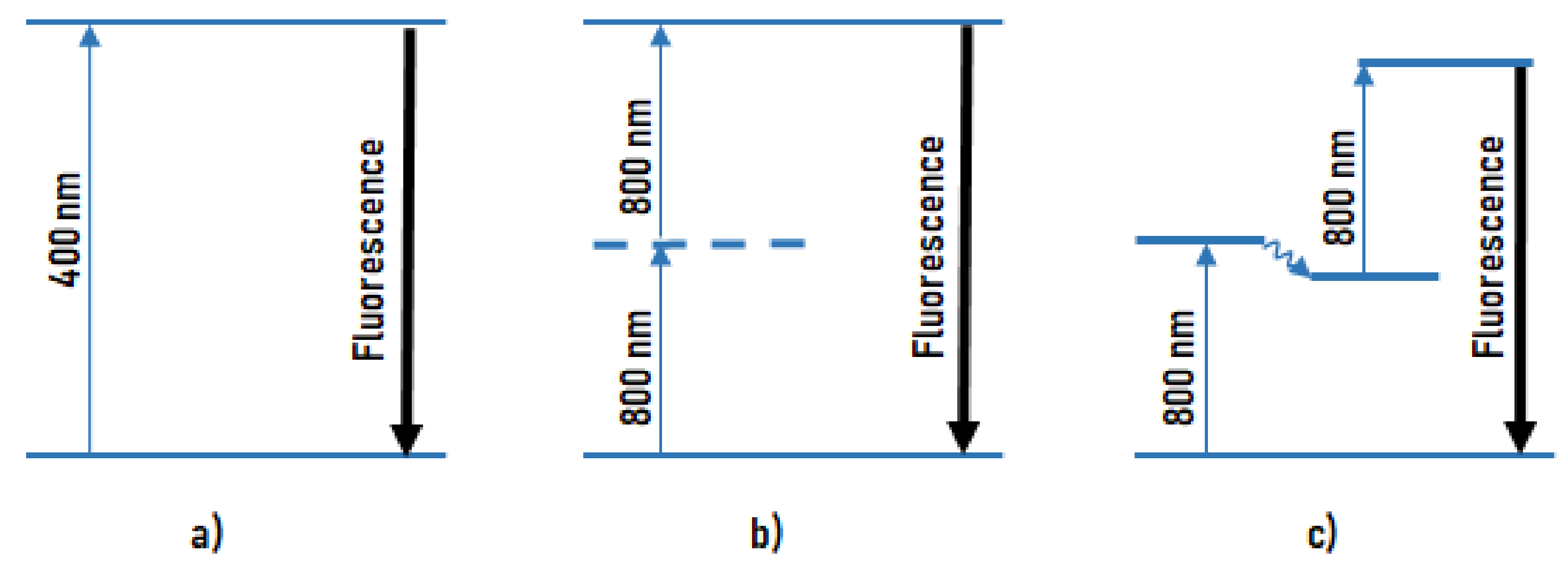
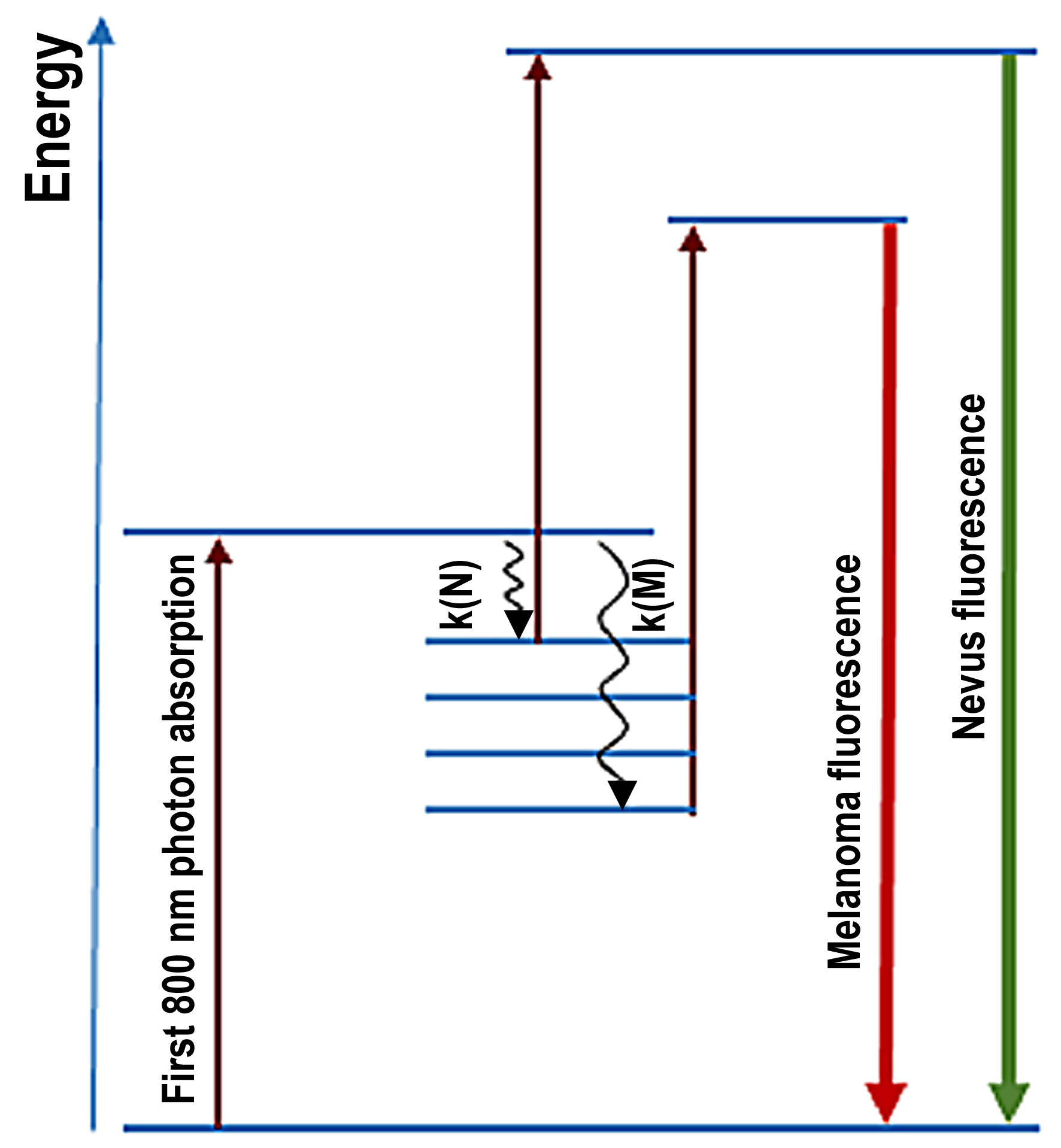
2.2. Melanin Fluorescence
2.3. Skin of PD Patients of Caucasian Origin
2.4. Postmortem Neuromelanin of Substantia Nigra
3. Results
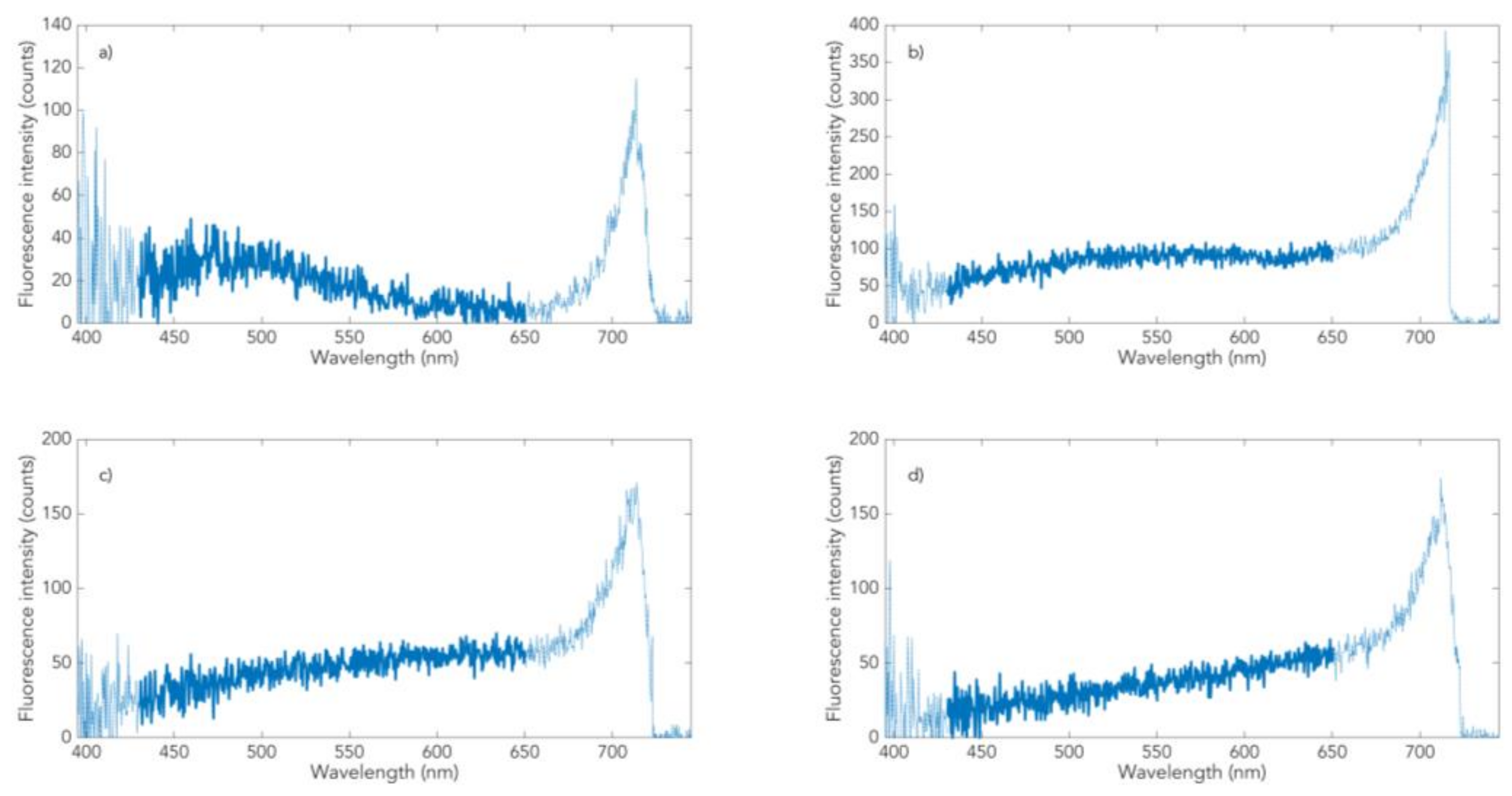
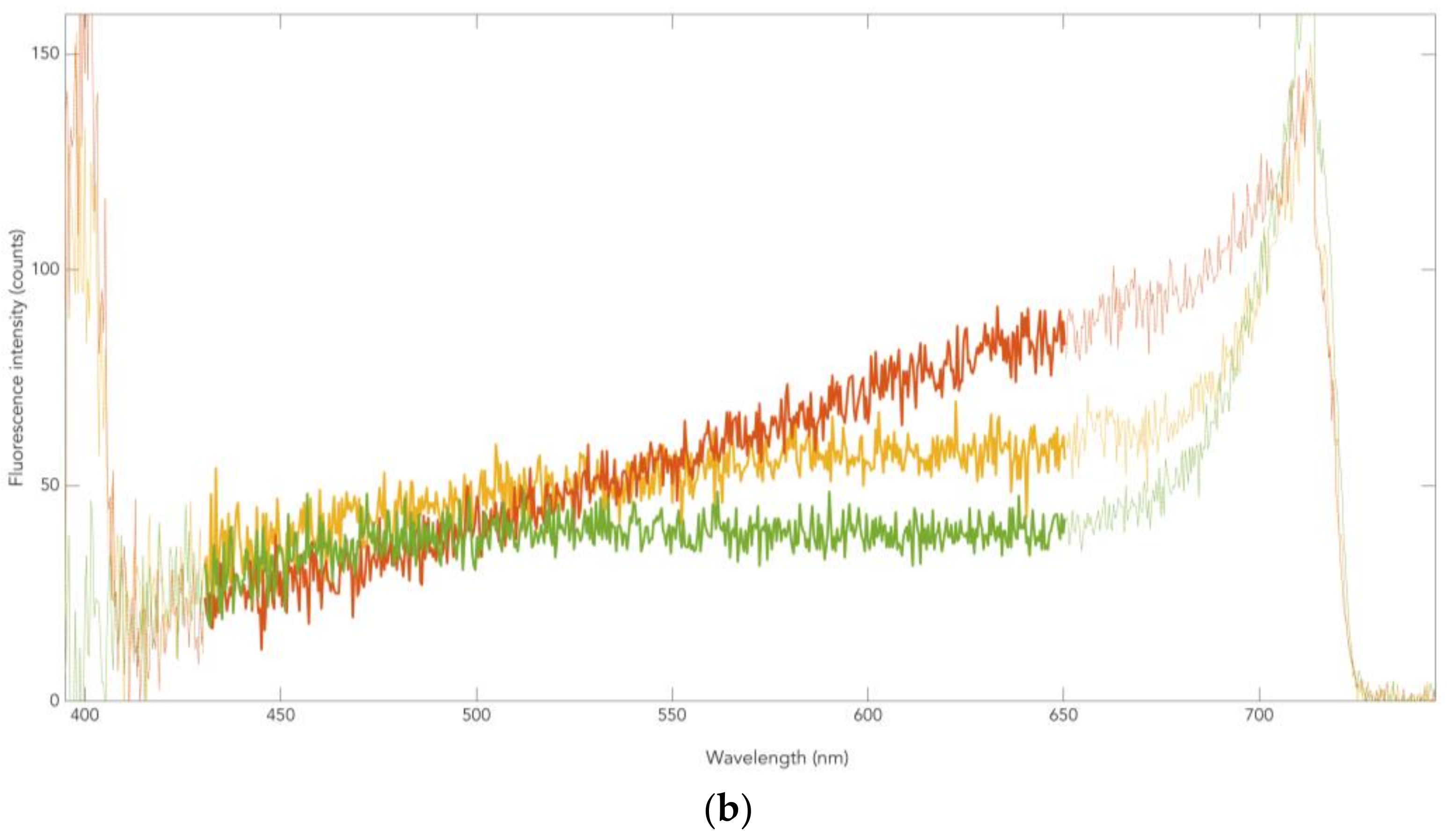
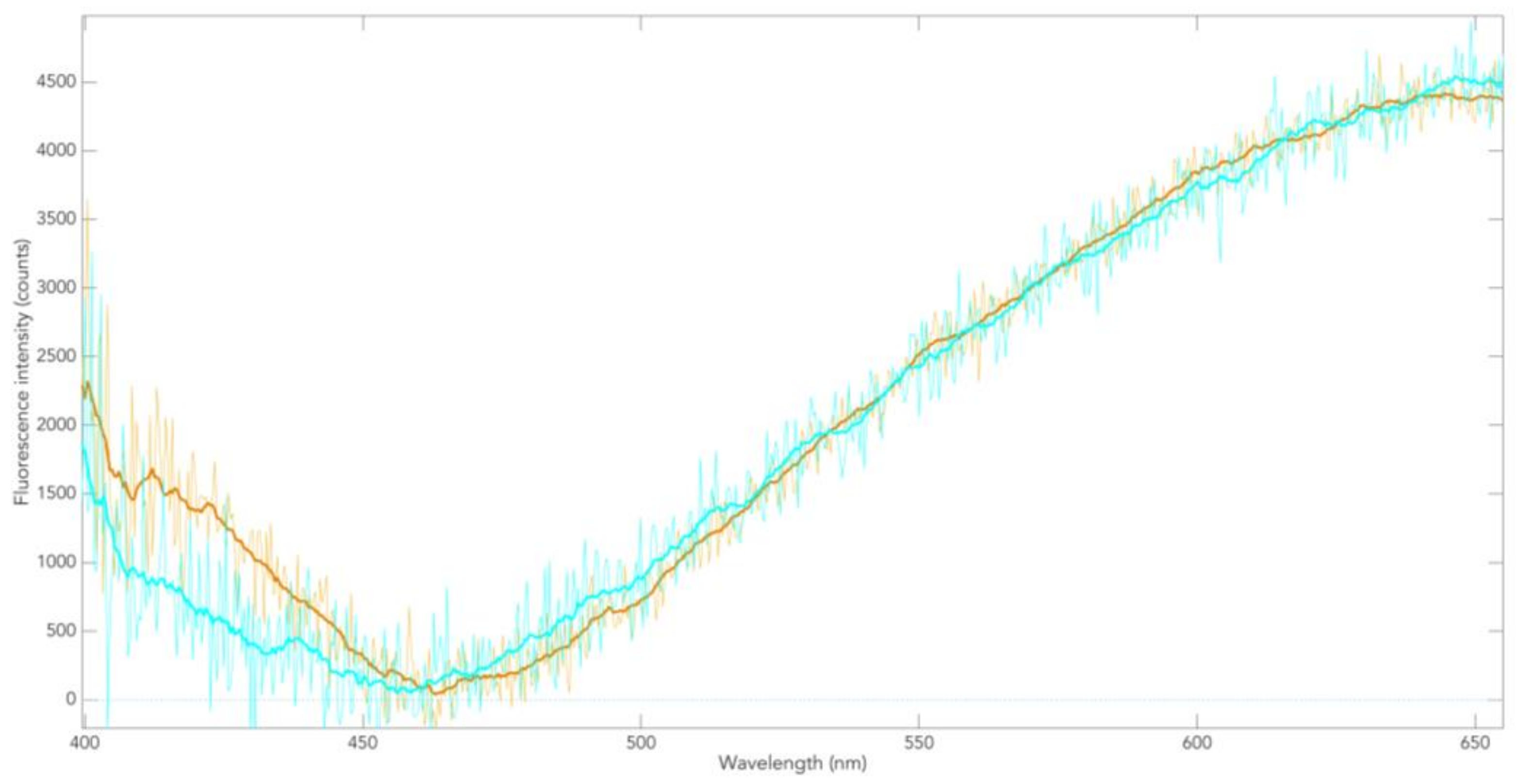
3.1. Skin Pigmentation of PD Patients and Controls
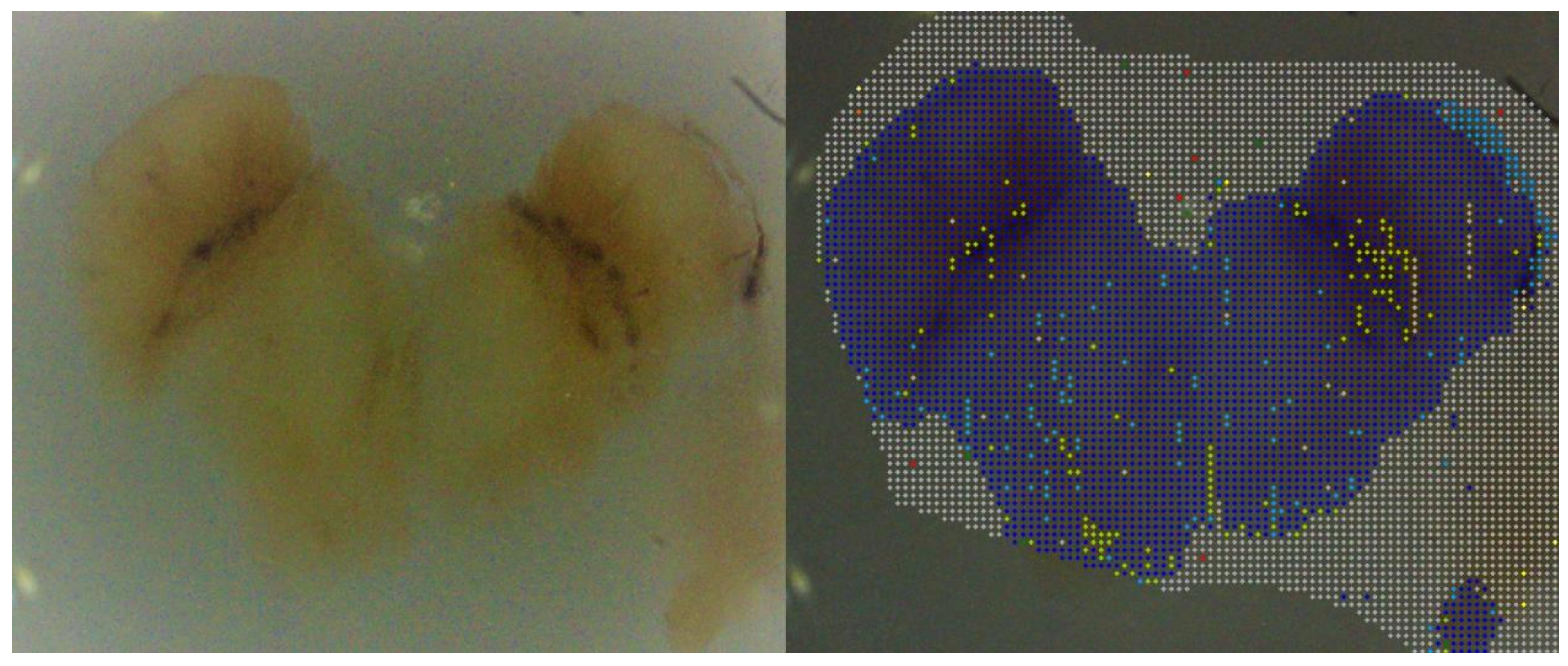
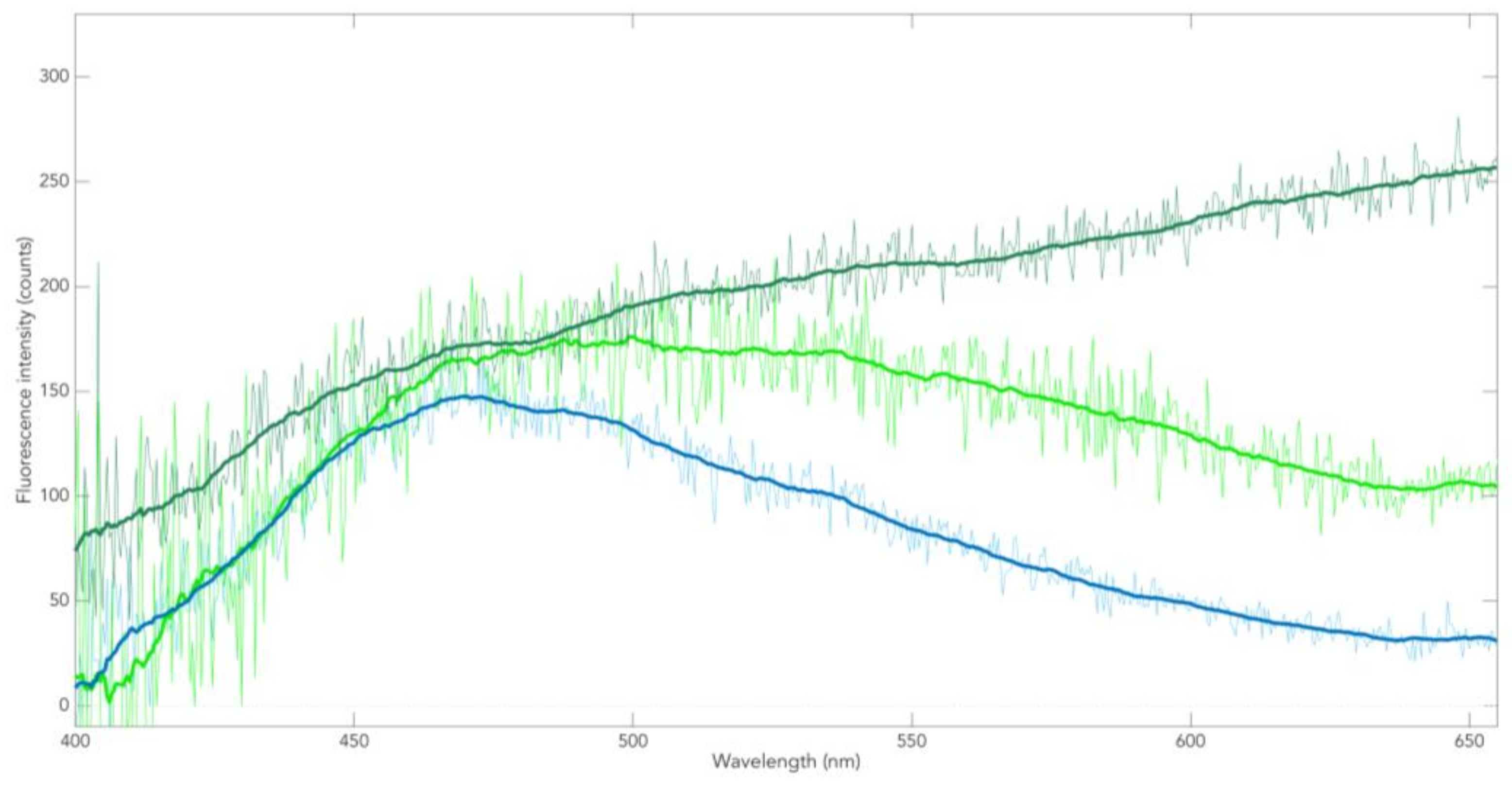
3.2. Neuromelanin Fluorescence of SNpc from PD and Controls
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bertoni, J.M.; Arlette, J.P.; Fernandez, H.H.; Fitzer-Attas, C.; Frei, K.; Hassan, M.N.; Isaacson, S.H.; Lew, M.F.; Molho, E.; Ondo, W.G.; et al. Increased Melanoma Risk in Parkinson Disease: A Prospective Clinicopathological Study. Arch Neurol. 2010, 67, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Disse, M.; Reich, H.; Lee, PK.; Schram, SS. A Review of the Association between Parkinson Disease and Malignant Melanoma. Dermatol. Surg. 2016, 42, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Dalvin, L.A.; Damento, G.M.; Yawn, B.P.; Abbott, B.A.; Hodge, D.O.; Pulido, J.S. Parkinson Disease and Melanoma: Confirming and Reexamining an Association. Mayo Clin. Proc. 2017, 92, 1070–1079. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Simon, K.C.; Han, J.; Schwarzschild, M.A.; Ascherio, A. Family History of Melanoma and Parkinson Disease Risk. Neurology 2009, 73, 1286–1291. [Google Scholar] [CrossRef] [PubMed]
- Raimondi, S.; Sera, F.; Gandini, S.; Iodice, S.; Caini, S.; Maisonneuve, P.; Fargnoli, M.C. Mc1r Variants, Melanoma and Red Hair Color Phenotype: A Meta-Analysis. Int. J. Cancer 2008, 122, 2753–2760. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Chan, P. Interaction between Neuromelanin and Alpha-Synuclein in Parkinson’s Disease. Biomolecules 2015, 5, 1122–1142. [Google Scholar] [CrossRef]
- Elincx-Benizri, S.; Inzelberg, R.; Greenbaum, L.; Cohen, O.S.; Yahalom, G.; Laitman, Y.; Djaldetti, R.; Orlev, Y.; Scope, A.; Azizi, E.; et al. The Melanocortin 1 Receptor (Mc1r) Variants Do Not Account for the Co-Occurrence of Parkinson’s Disease and Malignant Melanoma. J. Mol. Neurosci. 2014, 54, 820–825. [Google Scholar] [CrossRef]
- Chen, X.; Chen, H.; Cai, W.; Maguire, M.; Ya, B.; Zuo, F.; Logan, R.; Li, H.; Robinson, K.; Vanderburg, C.R.; et al. The Melanoma-Linked "Redhead" Mc1r Influences Dopaminergic Neuron Survival. Ann. Neurol. 2017, 81, 395–406. [Google Scholar] [CrossRef]
- Spillantini, M.G.; Schmidt, M.L.; Lee, V.M.; Trojanowski, J.Q.; Jakes, R.; Goedert, M.G. Alpha-Synuclein in Lewy Bodies. Nature 1997, 388, 839–840. [Google Scholar] [CrossRef]
- Halliday, G.M.; Ophof, A.; Broe, M.; Jensen, P.H.; Kettle, E.; Fedorow, H.; Cartwright, M.I.; Griffiths, F.M.; Shepherd, C.E.; Double, K.L.M. Alpha-Synuclein Redistributes to Neuromelanin Lipid in the Substantia Nigra Early in Parkinson’s Disease. Brain 2005, 128, 2654–2664. [Google Scholar] [CrossRef]
- Matsuo, Y.; Kamitani, T. Parkinson’s Disease-Related Protein, Alpha-Synuclein, in Malignant Melanoma. PLoS ONE 2010, 5, e10481. [Google Scholar] [CrossRef] [PubMed]
- Ikemoto, K.; Nagatsu, I.; Ito, S.A.; King, R.; Nishimura, A.; Nagatsu, T. Does Tyrosinase Exist in Neuromelanin-Pigmented Neurons in the Human Substantia Nigra? Neurosci. Lett. 1998, 253, 198–200. [Google Scholar] [CrossRef]
- Welinder, C.; Jönsson, G.B.; Ingvar, C.; Lundgren, L.; Baldetorp, B.; Olsson, H.; Breslin, T.; Rezeli, M.; Jansson, B.; Fehniger, T.E.; et al. Analysis of Alpha-Synuclein in Malignant Melanoma - Development of a Srm Quantification Assay. PLoS ONE 2014, 9, e110804. [Google Scholar] [CrossRef] [PubMed]
- Donadio, V.; Incensi, A.; Piccinini, C.; Cortelli, P.; Giannoccaro, M.P.; Baruzzi, A.; Liguori, R. Skin Nerve Misfolded Alpha-Synuclein in Pure Autonomic Failure and Parkinson Disease. Ann. Neurol. 2016, 79, 306–316. [Google Scholar] [CrossRef] [PubMed]
- Doppler, K.; Jentschke, H.-M.; Schulmeyer, L.; Vadasz, D.; Janzen, A.; Luster, M.; Höffken, H.; Mayer, G.; Brumberg, J.; Booij, J.; et al. Dermal Phospho-Alpha-Synuclein Deposits Confirm Rem Sleep Behaviour Disorder as Prodromal Parkinson’s Disease. Acta Neuropathol. 2017, 133, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Inzelberg, R.; Samuels, Y.; Azizi, E.; Qutob, N.; Inzelberg, L.; Domany, E.; Schechtman, E.; Friedman, E. Parkinson Disease (Park) Genes Are Somatically Mutated in Cutaneous Melanoma. Neurol. Genet. 2016, 2, e70. [Google Scholar] [CrossRef]
- Cesari, R.; Martin, E.S.; Calin, G.A.; Pentimalli, F.; Bichi, R.; McAdams, H.; Trapasso, F.; Drusco, A.; Shimizu, M.; Masciullo, V.; et al. Parkin, a Gene Implicated in Autosomal Recessive Juvenile Parkinsonism, Is a Candidate Tumor Suppressor Gene on Chromosome 6q25-Q27. Proc. Natl. Acad. Sci. USA 2003, 100, 5956–5961. [Google Scholar] [CrossRef]
- Hu, H.H.; Kannengiesser, C.; Lesage, S.; André, J.; Mourah, S.; Michel, L.; Descamps, V.; Basset-Seguin, N.; Bagot, M.; Bensussan, A.; et al. Parkin Inactivation Links Parkinson’s Disease to Melanoma. J. Natl. Cancer Inst. 2016, 108. [Google Scholar] [CrossRef]
- Leupold, D.; Giering, H.G. Dermatofluoroskopie; Stolz, W., Haenssle, H., Sattler, E., Welzel, J., Eds.; Thieme Verlag: Stuttgart, Germany, 2017. [Google Scholar]
- Leupold, D.; Scholz, M.; Stankovic, G.; Reda, J.; Buder, S.; Eichhorn, R.; Wessler, G.; Stücker, M.; Hoffmann, K.; Bauer, J.; et al. The Stepwise Two-Photon Excited Melanin Fluorescence Is a Unique Diagnostic Tool for the Detection of Malignant Transformation in Melanocytes. Pigment. Cell Melanoma Res. 2011, 24, 438–445. [Google Scholar] [CrossRef]
- Haining, R.L.; Jones, T.M.; Hernandez, A. Saturation Binding of Nicotine to Synthetic Neuromelanin Demonstrated by Fluorescence Spectroscopy. Neurochem. Res. 2016, 41, 3356–3363. [Google Scholar] [CrossRef]
- Bush, W.D.; Garguilo, J.; Zucca, F.A.; Bellei, C.; Nemanich, R.J.; Edwards, G.S.; Zecca, L.; Simon, J.D. Neuromelanins Isolated from Different Regions of the Human Brain Exhibit a Common Surface Photoionization Threshold. Photochem. Photobiol. 2009, 85, 387–390. [Google Scholar] [CrossRef] [PubMed]
- Forschner, A.; Keim, U.; Hofmann, M.; Spänkuch, I.; Lomberg, D.; Weide, B.; Tampouri, I.; Eigentler, T.; Fink, C.; Garbe, C.; et al. Diagnostic Accuracy of Dermatofluoroscopy in Cutaneous Melanoma Detection: Results of a Prospective Multicentre Clinical Study in 476 Pigmented Lesions. Br. J. Dermatol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Leupold, D.; Scholz, M.; Stankovic, G.; Pfeifer, L.; Giering, H.-G.; Buder, S.; Bauer, J.; Dummer, R.; Garbe, C. Uniform Spectral Fingerprint of the Different Melanoma Subtypes: Diagnostic Utility and Mechanistic Implications. In Proceedings of the 11th International Congress of the Society for Melanoma Research, Zurich, Switzerland, 13–16 November 2014. [Google Scholar]
- Scholz, M.; Leupold, D.; Szyc, L.; Stankovic, G.; Pfeifer, L.; Buder, S.; Giering, H.-G. Follow-up Atypical Melanocytic Lesions with Dermato Fluoroscopy: Rapid Malignant Degeneration, Stable State or Repair. In Proceedings of the 16th World Congress on Cancers of the Skin, Vienna, Austria, 31 August–3 September 2016. [Google Scholar]
- Fitzpatrick, T.B. The Validity and Practicality of Sun-Reactive Skin Types I through Vi. Arch. Dermatol. 1988, 124, 869–871. [Google Scholar] [CrossRef] [PubMed]
- Double, K.L.; Halliday, G.M. New Face of Neuromelanin. J. Neural. Transm. Suppl. 2006, 119–123. [Google Scholar]
- Chen, X.; Feng, D.; Schwarzschild, MA.; Gao, X. Red Hair, Mc1r Variants, and Risk for Parkinson’s Disease - a Meta-Analysis. Ann. Clin. Transl. Neurol. 2017, 4, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Mitra, D.; Luo, X.; Morgan, A.; Wang, J.; Hoang, M.P.; Lo, J.; Guerrero, C.R.; Lennerz, J.K.; Mihm, M.C.; Wargo, J.A.; et al. An Ultraviolet-Radiation-Independent Pathway to Melanoma Carcinogenesis in the Red Hair/Fair Skin Background. Nature 2012, 491, 449–453. [Google Scholar] [CrossRef] [PubMed]
- Rumpf, J.; Schirmer, M.; Fricke, C.; Weise, D.; Wagner, J.A.; Šimon, J.; Classen, J. Light Pigmentation Phenotype Is Correlated with Increased Substantia Nigra Echogenicity. Mov. Disord. 2015, 30, 1848–1852. [Google Scholar] [CrossRef]
- Pan, T.; Li, X.; Jankovic, J. The Association between Parkinson’s Disease and Melanoma. Int. J. Cancer 2011, 128, 2251–2260. [Google Scholar] [CrossRef]

| Patient | Age | Gender | Size of Nevi (mm) | Location of Nevi | Number of Spectra/cl.1-cl.2-Fault |
|---|---|---|---|---|---|
| 1 | 62 | F | 3.0 × 2.0 | Sole | 160/0-0-3 |
| 2.4 × 3.8 | Right lower leg | 240/0-0-0 | |||
| 2 | 39 | F | 4.0 × 3.8 | Abdomen | 364/2-1-4 |
| 3 | 72 | F | 3.2 × 3.8 | Right temporal | 291/1-2-3 |
| 4 | 78 | M | 3.8 × 4.0 | Chest wall | 345/0-2-9 |
| 5 | 50 | M | 4.6 × 4.0 | Right shoulder | 350/0-3-7 |
| 3.6 × 4.2 | Right upper leg | 381/0-3-10 | |||
| 6 | 52 | M | 3.8 × 3.6 | Right foot | 310/2-1-8 |
| 5.4 × 2.6 | Back | 464/0-3-9 | |||
| 7 | 67 | M | 4.4 × 3.6 | Abdomen | 392/1-3-8 |
| 8 | 75 | M | 4.5 × 3.0 | Left shoulder | 310/0-3-10 |
| 9 | 75 | M | 2.2 × 3.0 | Abdomen | 158/1-2-2 |
| 10 | 78 | M | 4.4 × 4.4 | Left shoulder | 494/2-1-14 |
| 11 | 67 | M | 4.6 × 3.6 | Back | 453/0-3-10 |
| 12 | 61 | M | 3.5 × 3.5 | Chest wall | 278/0-3-6 |
| 13 | 35 | M | 2.8 × 3.8 | Right temporal | 275/2-1-1 |
| 4.6 × 4.8 | Back | 442/2-1-9 | |||
| 14 | 53 | M | 3.0 × 4.0 | Chest wall | 250/0-0-0 |
| 1.6 × 1.8 | Left upper leg | 77/0-3-8 |
| Group | Age | Gender | PMI (h) | DD (years) |
|---|---|---|---|---|
| 1 | 77 | F | 9.5 | -- |
| 1 | 83 | M | 15 | -- |
| 1 | 76 | M | 9 | -- |
| 1 | 85 | M | 12.5 | -- |
| 1 | 71 | F | 16 | -- |
| 1 | 84 | F | 19 | -- |
| 1 | 79 | M | 10 | -- |
| 1 | 83 | M | 12.5 | -- |
| 1 | 88 | F | 13 | -- |
| 1 | 75 | F | 17 | -- |
| 2 | 83 | M | 9.5 | 15 |
| 2 | 77 | M | 13.5 | 9 |
| 2 | 85 | M | 17 | 12 |
| 2 | 72 | M | 16.5 | 6 |
| 2 | 83 | F | 12 | 13 |
| 2 | 90 | M | 19.5 | 22 |
| 2 | 74 | M | 15.5 | 9 |
| 2 | 76 | F | 12 | 10 |
| 2 | 88 | F | 9.5 | 21 |
| 2 | 81 | M | 13.5 | 17 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leupold, D.; Szyc, L.; Stankovic, G.; Strobel, S.; Völker, H.-U.; Fleck, U.; Müller, T.; Scholz, M.; Riederer, P.; Monoranu, C.-M. Melanin and Neuromelanin Fluorescence Studies Focusing on Parkinson’s Disease and Its Inherent Risk for Melanoma. Cells 2019, 8, 592. https://doi.org/10.3390/cells8060592
Leupold D, Szyc L, Stankovic G, Strobel S, Völker H-U, Fleck U, Müller T, Scholz M, Riederer P, Monoranu C-M. Melanin and Neuromelanin Fluorescence Studies Focusing on Parkinson’s Disease and Its Inherent Risk for Melanoma. Cells. 2019; 8(6):592. https://doi.org/10.3390/cells8060592
Chicago/Turabian StyleLeupold, Dieter, Lukasz Szyc, Goran Stankovic, Sabrina Strobel, Hans-Ullrich Völker, Ulrike Fleck, Thomas Müller, Matthias Scholz, Peter Riederer, and Camelia-Maria Monoranu. 2019. "Melanin and Neuromelanin Fluorescence Studies Focusing on Parkinson’s Disease and Its Inherent Risk for Melanoma" Cells 8, no. 6: 592. https://doi.org/10.3390/cells8060592
APA StyleLeupold, D., Szyc, L., Stankovic, G., Strobel, S., Völker, H.-U., Fleck, U., Müller, T., Scholz, M., Riederer, P., & Monoranu, C.-M. (2019). Melanin and Neuromelanin Fluorescence Studies Focusing on Parkinson’s Disease and Its Inherent Risk for Melanoma. Cells, 8(6), 592. https://doi.org/10.3390/cells8060592







