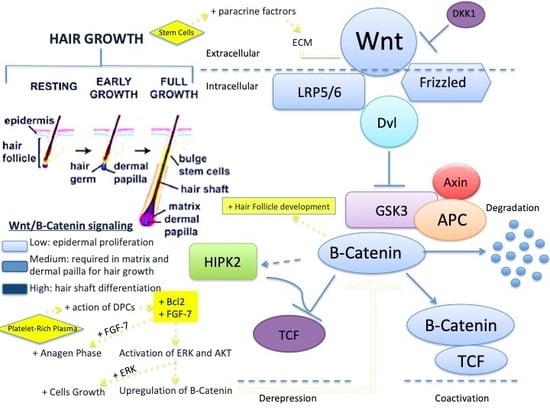
Advances in Regenerative Stem Cell Therapy in Androgenic Alopecia and Hair Loss: Wnt Pathway, Growth-Factor, and Mesenchymal Stem Cell Signaling Impact Analysis on Cell Growth and Hair Follicle Development
Abstract
1. Introduction
2. Hair Loss and Androgenic Alopecia: Bio-Molecular Pathway Disorder
3. SCs Use in HF Regeneration
4. FDA and European Rules Regarding Use of Adipose Derived-Stromal Vascular Cells (AD-SVFs) and Human Follicle Mesenchymal Stem Cells (HF-MSCs)
4.1. EMA/CAT Recommendations on Minimal Manipulation
4.2. Italian Rules Regarding Platelet-Rich Plasma Use
5. Cell and Growth Factor Sources
Bio-Molecular Pathway of Stem Cells and Growth Factors That Improve Hair Regrowth
- Hepatocyte growth factor (HGF) and HGF activator (discharged by DPC) enhance the proliferation of follicular ECs;
- EGF improves the activity and growth of follicle outer-root sheath cells by activation of Wnt/β-catenin flagging;
- b-FGF improves the advancement of hair follicles;
- Interleukein-6 (IL-6) is involved in WIHN through STAT3 enactment;
- VEGF improves peri-follicular angiogenesis;
- TGF-β stimulates the signaling pathways that manage the HC;
- IGF-1 improves the migration, survival, and proliferation of HF cells;
- IGFBP-1 to -6 manages the IGF-1 effect and its connection with extracellular matrix proteins at the HF level;
- BMP maintains the DPC phenotype (fundamental for stimulation of HFSCs);
- BMPR1a maintains the proper identity of the DPCs (basic for explicit DPC work);
- M-CSF is involved in wound-induced hair growth;
- M-CSFR is involved in wound-induced hair growth;
- PDGF and PDGFR-β/-α64 up-regulate the genes associated with HF separation, induction, and control of anagen. PDGF and its receptors are fundamental for follicular improvement;
- Wnt3a is involved in HF advancement through β-catenin flagging;
- PGE2 stimulates anagen in HF;
- PGF2α and analogs enhance change from telogen to anagen;
- BIO (GSK-3 inhibitor);
- PGE2 or hindrance of PGD2 or PGD2 receptor D2/GPR4477 enhances follicle regeneration; and
- Iron and l-lysine95 (still under examination).
6. Clinical Intra-Surgical Applications of AD-SVFs in Hair Loss and Androgenic Alopecia
6.1. AD-SVFs Regenerative Mechanisms
6.2. Adipose Tissue, Adipocyte, and AD-SVFs Potential Roles in Hair Loss
7. Clinical Intra-Surgical Application of PRP in HL and AGA
- (1)
- Leukocyte-poor PRP (LP-PRP) or pure platelet-rich plasma (P-PRP): Suspension without leukocytes and with a low-density fibrin after induction;
- (2)
- PRP and leukocyte (L-PRP): Suspensions with leukocytes and a low-density fibrin after induction (the largest of the commercial packages);
- (3)
- Leukocyte-poor platelet-rich fibrin (LP-PRF) or pure platelet-rich fibrin (P-PRF): Suspension without leukocytes and a high-density fibrin;
- (4)
- Leukocytes and platelet rich fibrin (L-PRF) or second-era PRP products are arrangements with leukocytes and a high fibrin density.
8. Clinical Intra-Surgical Application of HFSCs in Hair Loss and Androgenic Alopecia
Hair Follicles and HF-MSCs Regenerative Mechanisms in Hair Loss and Androgenic Alopecia
9. Studies on Using Stem Cells from Wharton’s Jelly
10. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Coelho, M.B.; Cabral, J.M.; Karp, J.M. Intraoperative Stem Cell Therapy. Annu. Rev. Biomed. Eng. 2012, 14, 325–349. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Owczarczyk-Saczonek, A.; Krajewska-Włodarczyk, M.; Kruszewska, A.; Banasiak, Ł; Placek, W.; Maksymowicz, W.; Wojtkiewicz, J. Therapeutic Potential of Stem Cells in Follicle Regeneration. Stem Cells Int. 2018, 5, 1049641. [Google Scholar]
- Alsantali, A.; Shapiro, J. Androgens and hair loss. Curr. Opin. Endocrinol. Diabetes Obes. 2009, 16, 246–253. [Google Scholar] [CrossRef]
- Price, V.H. Treatment of hair loss. N. Engl. J. Med. 1999, 341, 964–973. [Google Scholar] [CrossRef] [PubMed]
- Garcovich, S.; Gentile, P.; Bielli, A.; Scioli, M.G.; Orlandi, A.; Cervelli, V.; Gentile, P.; Bielli, A.; Scioli, M.G.; Orlandi, A.; et al. The effect of platelet-rich plasma in hair regrowth: A randomized placebo-controlled trial. Stem Cells Transl. Med. 2015, 4, 317–1323. [Google Scholar]
- Gentile, P.; Cole, J.P.; Cole, M.A.; Garcovich, S.; Bielli, A.; Scioli, M.G.; Orlandi, A.; Insalaco, C.; Cervelli, V.; Piva, T. Evaluation of Not-Activated and Activated PRP in Hair Loss Treatment: Role of Growth Factor and Cytokine Concentrations Obtained by Different Collection Systems. Int. J. Mol. Sci. 2017, 18, 408. [Google Scholar] [CrossRef] [PubMed]
- Garza, L.A.; Yang, C.-C.; Zhao, T.; Blatt, H.B.; Lee, M.; He, H.; Stanton, D.C.; Carrasco, L.; Spiegel, J.H.; Tobias, J.W.; et al. Bald scalp in men with androgenetic alopecia retains hair follicle stem cells but lacks CD200-rich and CD34-positive hair follicle progenitor cells. J. Clin. Invest. 2011, 121, 613–622. [Google Scholar] [CrossRef]
- Ohyama, M.; Terunuma, A.; Tock, C.L.; Radonovich, M.F.; Pise-Masison, C.A.; Hopping, S.B.; Brady, J.N.; Udey, M.C.; Vogel, J.C. Characterization and isolation of stem cell-enriched human hair follicle bulge cells. J. Clin. Invest. 2006, 116, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, P.; Youssef, K.K.; Abbasalizadeh, S.; Baharvand, H.; Aghdami, N. Human hair reconstruction: Close, but yet so far. Stem Cells Dev. 2016, 25, 1767–1779. [Google Scholar] [CrossRef] [PubMed]
- Elmaadawi, I.H.; Mohamed, B.M.; Ibrahim, Z.A.S.; Abdou, S.M.; El Attar, Y.A.; Youssef, A.; Shamloula, M.M.; Taha, A.; Metwally, H.G.; El Afandy, M.M.; et al. Stem cell therapy as a novel therapeutic intervention for resistant cases of alopecia areata and androgenetic alopecia. J. Dermatol. Treat. 2018, 29, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Rompolas, P.; Greco, V. Stem cell dynamics in the hair follicle niche. Semin. Cell Dev. Biol. 2014, 25-26, 34–42. [Google Scholar] [CrossRef]
- Means, A.L.; Xu, Y.; Zhao, A.; Ray, K.C.; Gu, G. CK19CreERT knockin mouse line allows for conditional DNA recombination in epithelial cells in multiple endodermal organs. Genesis 2008, 46, 318–323. [Google Scholar] [CrossRef]
- Jaks, V.; Barker, N.; Kasper, M.; Van Es, J.H.; Snippert, H.J.; Clevers, H.; Toftgard, R. Lgr5 marks cycling, yet long-lived, hair follicle stem cells. Nat. Genet. 2008, 40, 1291–1299. [Google Scholar] [CrossRef]
- Guasch, G. The epithelial stem cell niche in skin. Biology and engineering of stem cell niches. Elsevier Inc. 2017, 127–143. [Google Scholar]
- Hoeck, J.D.; Biehs, B.; Kurtovaetal, A.V. Stem cell plasticity enables hair regeneration following Lgr5+ cell loss. Nat. Cell Biol. 2017, 19, 666–676. [Google Scholar] [CrossRef]
- Snippert, H.J.; Haegebarth, A.; Kasper, M.; Jaks, V.; Van Es, J.H.; Barker, N.; Van De Wetering, M.; Born, M.V.D.; Begthel, H.; Vries, R.G.; et al. Lgr6 marks stem cells in the hair follicle that generate all cell lineages of the skin. Science 2010, 327, 1385–1389. [Google Scholar] [CrossRef]
- Jensen, K.B.; Collins, C.A.; Nascimento, E.; Tan, D.W.; Frye, M.; Itami, S.; Watt, F.M. Lrig1 expression defines a distinct multipotent stem cell population in mammalian epidermis. Cell Stem Cell 2009, 4, 427–439. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, M.; Aiba, S.; Kurosawa, M.; Tagami, H. Ber-EP4 antigen is a marker for a cell population related to the secondary hair germ. Exp. Dermatol. 2004, 13, 401–405. [Google Scholar] [CrossRef]
- Purba, T.S.; Haslam, I.S.; Poblet, E.; Jiménez, F.; Gandarillas, A.; Izeta, A.; Paus, R. Human epithelial hair follicle stem cells and their progeny: Current state of knowledge, the widening gap in translational research and future challenges. BioEssays 2014, 36, 513–525. [Google Scholar] [CrossRef]
- Inoue, K.; Aoi, N.; Sato, T.; Yamauchi, Y.; Suga, H.; Eto, H.; Kato, H.; Araki, J.; Yoshimura, K. Differential expression of stem-cell-associated markers in human hair follicle epithelial cells. Lab. Invest. 2009, 89, 844–856. [Google Scholar] [CrossRef]
- Kissling, S.; Wenzel, E.; Huth, A.; Hoffmann, R.; McElwee, K.J.; Mc Elwee, K.J. Cultured peribulbar dermal sheath cells can induce hair follicle development and contribute to the dermal sheath and dermal papilla. J. Invest. Dermatol. 2003, 121, 1267–1275. [Google Scholar]
- Lee, S.-H.; Yoon, J.; Shin, S.H.; Zahoor, M.; Kim, H.J.; Park, P.J.; Park, W.-S.; Min, D.S.; Kim, H.-Y.; Choi, K.-Y. Valproic acid induces hair regeneration in murine model and activates alkaline phosphatase activity in human dermal papilla cells. PLoS ONE 2012, 7, e34152. [Google Scholar] [CrossRef] [PubMed]
- Sellheyer, K.; Krahl, D. PHLDA1 (TDAG51) is a follicular stem cell marker and differentiates between morphoeic basal cell carcinoma and desmoplastic trichoepithelioma. Br. J. Dermatol. 2011, 164, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; E Kling, R.; Ravuri, S.K.; E Kokai, L.; Rubin, J.P.; Chai, J.-K.; Marra, K.G. A review of adipocyte lineage cells and dermal papilla cells in hair follicle regeneration. J. Tissue Eng. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Rendl, M.; Lewis, L.; Fuchs, E. Molecular dissection of mesenchymal-epithelial interactions in the hair follicle. PLoS Biol. 2005, 3, e331. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Hamazaki, T.S.; Ohnuma, K.; Tamaki, K.; Asashima, M.; Okochi, H. Isolation of murine hair-inducing cells using the cell surface marker prominin-1/CD133. J. Invest. Dermatol. 2007, 127, 1052–1060. [Google Scholar] [CrossRef]
- Li, J.; Jiang, T.X.; Chuong, C.M. Many paths to alopecia via compromised regeneration of hair follicle stem cells. J. Invest. Dermatol. 2013, 133, 1450–1452. [Google Scholar] [CrossRef]
- Turksen, K. Tissue Specific Stem Cell Niche; Springer: Basel, Switzerland, 2015. [Google Scholar]
- Greco, V.; Chen, T.; Rendl, M.; Schöber, M.; Pasolli, H.A.; Stokes, N.; Cruz-Racelis, J.D.; Fuchs, E. A two-step mechanism for stem cell activation during hair regeneration. Cell Stem Cell 2009, 4, 155–169. [Google Scholar] [CrossRef] [PubMed]
- Rompolas, P.; Mesa, K.R.; Greco, V. Spatial organization within a niche as a determinant of stem-cell fate. Nature 2013, 502, 513–518. [Google Scholar] [CrossRef]
- Oh, J.W.; Kloepper, J.; Langanetal, E.A. A guide to studying human hair follicle cycling in vivo. J. Invest. Dermatol. 2016, 136, 34–44. [Google Scholar] [CrossRef]
- Garza, L.A.; Liu, Y.; Yang, Z.; Alagesan, B.; Lawson, J.A.; Norberg, S.M.; Loy, D.E.; Zhao, T.; Blatt, H.B.; Stanton, D.C.; et al. Prostaglandin D2 inhibits hair growth and is elevated in bald scalp of men with androgenetic alopecia. Sci. Transl. Med. 2012, 4, 126ra34. [Google Scholar] [CrossRef]
- Halloy, J.; Bernard, B.A.; Loussouarn, G.; Goldbeter, A. Modeling the dynamics of human hair cycles by a follicular automaton. Proc. Natl. Acad. Sci. USA 2000, 97, 8328–8333. [Google Scholar] [CrossRef]
- Nakamura, M.; Schneider, M.R.; Schmidt-Ullrich, R.; Paus, R. Mutant laboratory mice with abnormalities in hair follicle morphogenesis, cycling, and/or structure: An update. J. Dermatol. Sci. 2013, 69, 6–29. [Google Scholar] [CrossRef]
- Christoph, T.; Müller-Röver, S.; Audringetal, H. Thehuman hair follicle immune system: Cellular composition and immune privilege. Br. J. Dermatol. 2000, 142, 862–873. [Google Scholar] [CrossRef] [PubMed]
- Tanimura, S.; Tadokoro, Y.; Inomata, K.; Binh, N.T.; Nishie, W.; Yamazaki, S.; Nakauchi, H.; Tanaka, Y.; McMillan, J.R.; Sawamura, D.; et al. Hair follicle stem cells provide a functional niche for melanocyte stem cells. Cell Stem Cell. 2011, 8, 177–187. [Google Scholar] [CrossRef]
- Deschene, E.R.; Myung, P.; Rompolasetal, P. β-catenin activation regulates tissue growth non-cell autonomously in the hair stem cell niche. Science 2014, 343, 1353–1356. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, H.; Jing, J.; Yu, L.; Wu, X.; Lu, Z. Regulation of hair follicle development by exosomes derived from dermal papilla cells. Biochem. Biophys. Res. Commun. 2018, 500, 325–332. [Google Scholar] [CrossRef]
- Paus, R.; Nickoloff, B.; Ito, T. A hairy’ privilege. Trends Immunol. 2005, 26, 32–40. [Google Scholar] [CrossRef]
- Gentile, P.; Scioli, M.G.; Bielli, A.; Orlandi, A.; Cervelli, V. Stem cells from human hair follicles: First mechanical isolation for immediate autologous clinical use in androgenetic alopecia and hair loss. Stem Cell Invest. 2017, 4, 58. [Google Scholar] [CrossRef]
- Asakawa, K.; Toyoshima, K.-E.; Ishibashi, N.; Tobe, H.; Iwadate, A.; Kanayama, T.; Hasegawa, T.; Nakao, K.; Toki, H.; Noguchi, S.; et al. Hair organ regeneration via the bioengineered hair follicular unit transplantation. Sci. Rep. 2012, 2, 424. [Google Scholar] [CrossRef] [PubMed]
- Balañá, M.E.; Charreau, H.E.; Leiroós, G.J. Epidermal stem cells and skin tissue engineering in hair follicle regeneration. World J. Stem Cells 2015, 7, 711–727. [Google Scholar] [CrossRef]
- Campagnoli, C. Identification of mesenchymal stem/progenitor cells in human first-trimester fetal blood, liver, and bone marrow. Blood 2001, 98, 2396–2402. [Google Scholar] [CrossRef] [PubMed]
- Baksh, D.; Davies, J.E.; Zandstra, P.W. Adult human bone marrow–derived mesenchymal progenitor cells are capable of adhesion-independent survival and expansion. Exp. Hematol. 2003, 31, 723–732. [Google Scholar] [CrossRef]
- Muschler, G.F.; Nitto, H.; Boehm, C.A.; Easley, K.A. Age- and gender-related changes in the cellularity of human bone marrow and the prevalence of osteoblastic progenitors. J. Orthop. Res. 2001, 19, 117–125. [Google Scholar] [CrossRef]
- Muschler, G.F.; Boehm, C.; Easley, K. Aspiration to obtain osteoblast progenitor cells from human bone marrow:the influence of aspiration volume. JBJS 1997, 79, 1699–1709. [Google Scholar] [CrossRef]
- Peng, L.; Jia, Z.; Yin, X.; Zhang, X.; Liu, Y.; Chen, P.; Ma, K.; Zhou, C. Comparative analysis of mesenchymal stem cells from bone marrow, cartilage, and adipose tissue. Stem Cells Dev. 2008, 17, 761–774. [Google Scholar] [CrossRef]
- Kern, S.; Eichler, H.; Stoeve, J.; Klüter, H.; Bieback, K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells 2006, 24, 1294–1301. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, H.; Muneta, T.; Nimura, A.; Yokoyama, A.; Koga, H.; Sekiya, I. Comparison of rat mesenchymal stem cells derived from bone marrow, synovium, periosteum, adipose tissue, and muscle. Cell Tissue Res. 2007, 327, 449–462. [Google Scholar] [CrossRef]
- Rebelatto, C.K.; Aguiar, A.M.; Moretão, M.P.; Senegaglia, A.C.; Hansen, P.; Barchiki, F.; Oliveira, J.; Martins, J.; Kuligovski, C.; Mansur, F.; et al. Dissimilar differentiation of mesenchymal stem cells from bone marrow, umbilical cord blood, and adipose tissue. Exp. Biol. Med. 2008, 233, 901–913. [Google Scholar] [CrossRef]
- Zuk, P.A.; Zhu, M.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001, 7, 211–228. [Google Scholar] [CrossRef]
- Oedayrajsingh-Varma, M.; Van Ham, S.; Knippenberg, M.; Helder, M.; Klein-Nulend, J.; Schouten, T.; Ritt, M.; Van Milligen, F. Adipose tissue–derived mesenchymal stem cell yield and growth characteristics are affected by the tissue-harvesting procedure. Cytotherapy 2006, 8, 166–177. [Google Scholar] [CrossRef]
- Cole, J.P.; Cole, M.A.; Insalaco, C.; Cervelli, V.; Gentile, P. Alopecia and platelet-derived therapies. Stem Cell Invest. 2017, 4, 88. [Google Scholar] [CrossRef][Green Version]
- Gimble, J.M.; Bunnell, B.A.; Chiu, E.S.; Guilak, F. Concise review: Adipose-derived stromal vascular fraction cells and stem cells: let’s not get lost in translation. Stem Cells 2011, 29, 749–754. [Google Scholar] [CrossRef]
- Cervelli, V.; Gentile, P.; Scioli, M.G.; Grimaldi, M.; Casciani, C.U.; Spagnoli, L.G.; Orlandi, A. Application of platelet-rich plasma in plastic surgery: Clinical and in vitro evaluation. Tissue Eng. Part. C Methods 2009, 15, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Cervelli, V.; Bocchini, I.; Di Pasquali, C.; De Angelis, B.; Cervelli, G.; Curcio, C.B.; Orlandi, A.; Scioli, M.G.; Tati, E.; Delogu, P.; et al. P.R.L. platelet rich lipotransfert: Our experience and current state of art in the combined use of fat and PRP. Biomed. Res. Int. 2013, 2013, 434191. [Google Scholar] [CrossRef]
- Kapur, S.K.; Katz, A.J. Review of the adipose derived stem cell secretome. Biochimie 2013, 95, 2222–2228. [Google Scholar] [CrossRef]
- Cervelli, V.; Scioli, M.G.; Gentile, P.; Doldo, E.; Bonanno, E.; Spagnoli, L.G.; Orlandi, A. Platelet-rich plasma greatly potentiates insulin-induced adipogenic differentiation of human adipose-derived stem cells through a serine/threonine kinase Akt-dependent mechanism and promotes clinical fat graft maintenance. Stem Cell Trasl. Med. 2012, 1, 206–220. [Google Scholar] [CrossRef] [PubMed]
- Festa, E.; Fretz, J.; Berry, R.; Schmidt, B.; Rodeheffer, M.; Horowitz, M.; Horsley, V. Adipocyte lineage cells contribute to the skin stem cell niche to drive hair cycling. Cell 2011, 146, 761–771. [Google Scholar] [CrossRef]
- Perez-Meza, D.; Ziering, C.; Sforza, M.; Krishnan, G.; Ball, E.; Daniels, E.; Perez-Meza, V.A.P.B.D. Views: Hair follicle growth by stromal vascular fraction-enhanced adipose transplantation in baldness. Stem Cells Cloning 2017, 10, 1–10. [Google Scholar] [PubMed]
- Fukuoka, H.; Suga, H. Hair Regeneration Treatment Using Adipose-Derived Stem Cell Conditioned Medium: Follow-up with Trichograms. Eplasty 2015, 15, e10. [Google Scholar] [PubMed]
- Zanzottera, F.; Lavezzari, E.; Trovato, L.; Icardi, A.; Graziano, A. Adipose derived stem cells and growth factors applied on hair transplantation. Follow-up of clinical outcome. J. Cosmet. Dermatol. Sci. Appl. 2014, 4, 268–274. [Google Scholar] [CrossRef]
- Fukuoka, H.; Narita, K.; Suga, H. Hair Regeneration Therapy: Application of Adipose-Derived Stem Cells. Curr. Stem Cell Res. Ther. 2017, 12, 531–534. [Google Scholar] [CrossRef]
- Shin, H.; Won, C.H.; Chung, W.K.; Park, B.-S. Up-to-date clinical trials of hair regeneration using conditioned media of adipose-derived stem cells in male and female pattern hair loss. Curr. Stem Cell Res. Ther. 2017, 12, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Won, C.H.; Yoo, H.G.; Kwon, O.S.; Sung, M.Y.; Kang, Y.J.; Chung, J.H.; Park, B.S.; Sung, J.-H.; Kim, W.S.; Kim, K.H. Hair growth promoting effects of adipose tissue-derived stem cells. J. Dermatol. Sci. 2010, 57, 134–137. [Google Scholar] [CrossRef]
- Zhu, M.; Zhou, Z.; Chen, Y.; Schreiber, R.; Ransom, J.T.; Fraser, J.K.; Hedrick, M.H.; Pinkernell, K.; Kuo, H.-C. Supplementation of fat grafts with adipose-derived regenerative cells improves long-term graft retention. Ann. Plastic Sur. 2010, 64, 222–228. [Google Scholar] [CrossRef]
- Zimber, M.P.; Ziering, C.; Zeigler, F.; Hubka, M.; Mansbridge, J.N.; Baumgartner, M.; Hubka, K.; Kellar, R.; Perez-Meza, D.; Sadick, N.; et al. Hair regrowth following a Wnt- and follistatin containing treatment: Safety and efficacy in a first-in-man phase 1 clinical trial. J. Drugs Dermatol. 2011, 10, 1308–1312. [Google Scholar]
- Zhao, W.; Schäfer, S.; Choi, J.; Yamanaka, Y.J.; Lombardi, M.L.; Bose, S.; Carlson, A.L.; Phillips, J.A.; Teo, W.; Droujinine, I.A.; et al. Cell-surface sensors for real-time probing of cellular environments. Nat. Nanotechnol. 2011, 6, 524–531. [Google Scholar] [CrossRef]
- Sarkar, D.; Vemula, P.K.; Zhao, W.; Gupta, A.; Karnik, R.; Karp, J.M. Engineered mesenchymal stem cells with self-assembled vesicles for systemic cell targeting. Biomaterials 2010, 31, 5266–5274. [Google Scholar] [CrossRef]
- Plikus, M.V.; Mayer, J.A.; De La Cruz, D.; Baker, R.E.; Maini, P.K.; Maxson, R.; Chuong, C.-M. Cyclic dermal BMP signalling regulates stem cell activation during hair regeneration. Nature 2008, 451, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Yi, R. Concise review: Mechanisms of quiescent hair follicle stem cell regulation. Stem Cells 2017, 35, 2323–2330. [Google Scholar] [CrossRef]
- Mohyeldin, A.; Garzón-Muvdi, T.; Quiñones-Hinojosa, A. Oxygen in stem cell biology: A critical component of the stem cell niche. Cell Stem Cell 2010, 7150–7161. [Google Scholar] [CrossRef]
- Gay, D.; Kwon, O.; Zhang, Z.; Spata, M.; Plikus, M.V.; Holler, P.D.; Ito, M.; Yang, Z.; Treffeisen, E.; Kim, C.D.; et al. Fgf9 from dermal γδ T cells induces hair follicle neogenesis after wounding. Nat. Med. 2013, 19, 916–923. [Google Scholar] [CrossRef]
- Castellana, D.; Paus, R.; Perez-Moreno, M. Macrophages contribute to the cyclic activation of adult hair follicle stem cells. PLoS Biol. 2014, 12, e1002002. [Google Scholar] [CrossRef]
- Osaka, N.; Takahashi, T.; Murakami, S.; Matsuzawa, A.; Noguchi, T.; Fujiwara, T.; Aburatani, H.; Moriyama, K.; Takeda, K.; Ichijo, H. ASK1-dependent recruitment and activation of macrophages induce hair growth in skin wounds. J. Cell Biol. 2007, 176, 903–909. [Google Scholar] [CrossRef]
- Ali, N.; Zirak, B.; Rodriguez, R.S.; Pauli, M.L.; Truong, H.-A.; Lai, K.; Ahn, R.; Corbin, K.; Lowe, M.M.; Scharschmidt, T.C.; et al. Regulatory T cells in skin facilitate epithelial stem cell differentiation. Cell 2017, 169, 1119–1129. [Google Scholar] [CrossRef]
- Gaur, M.; Dobke, M.; Lunyak, V. Mesenchymal stem cells from adipose tissue in clinical applications for dermatological indications and skin aging. Int. J. Mol. Sci. 2017, 18. [Google Scholar] [CrossRef]
- Lee, P.; Sadick, N.S.; Diwan, A.H.; Zhang, P.S.; Liu, J.S.; Prieto, V.G.; Zhu, C.-C.; Zhu, C. Expression of androgen receptor coactivator ARA70/ELE1 in androgenic alopecia. J. Cutan. Pathol. 2005, 32, 567–571. [Google Scholar] [CrossRef]
- Gentile, P.; Scioli, M.G.; Bielli, A.; Cervelli, V. Concise Review: The Use of Adipose-Derived Stromal Vascular Fraction Cells and Platelet Rich Plasma in Regenerative Plastic Surgery. Stem Cells 2017, 35, 117–134. [Google Scholar] [CrossRef]
- Huang, C.-F.; Chang, Y.-J.; Hsueh, Y.-Y.; Huang, C.-W.; Wang, D.-H.; Huang, T.-C.; Wu, Y.-T.; Su, F.-C.; Hughes, M.; Chuong, C.-M.; et al. Assembling composite dermal papilla spheres with adipose-derived stem cells to enhance hair follicle induction. Sci. Rep. 2016, 6, 2016. [Google Scholar]
- Yang, Y.; Choi, H.; Seon, M.; Cho, D.; Bang, S.I. LL-37 stimulates the functions of adipose-derived stromal/stem cells via early growth response 1 and the MAPK pathway. Stem Cell Res. Ther. 2016, 7, 58. [Google Scholar] [CrossRef]
- Kang, B.M.; Kwack, M.H.; Kim, M.K.; Kim, J.C.; Sung, Y.K. Sphere formation increases the ability of cultured human dermal papilla cells to induce hair follicles from mouse epidermal cells in a reconstitution assay. J. Invest. Dermatol. 2012, 132, 237–239. [Google Scholar] [CrossRef]
- Seo, C.H.; Kwack, M.H.; Lee, S.-H.; Kim, M.K.; Kim, J.C.; Sung, Y.K. Poor capability of 3D-cultured adipose-derived stem cells to induce hair follicles in contrast to 3D-cultured dermal papilla cells. Ann. Dermatol. 2016, 28, 662–665. [Google Scholar] [CrossRef] [PubMed]
- Ramdasi, S.; Tiwari, S.K. Human mesenchymal stem cell-derived conditioned media for hair regeneration applications. J. Stem Cells 2016, 11, 201–211. [Google Scholar]
- Misago, N.; Toda, S.; Sugihara, H.; Kohda, H.; Narisawa, Y. Proliferation and differentiation of organoid hair follicle cells co-cultured with fat cells in collagen gel matrix culture. Br. J. Dermatol. 1998, 139, 40–48. [Google Scholar] [CrossRef]
- Jong, M.C.; Gijbels, M.J.; E Dahlmans, V.; Gorp, P.J.; Koopman, S.J.; Ponec, M.; Hofker, M.H.; Havekes, L.M. Hyperlipidemia and cutaneous abnormalities in transgenic mice overexpressing human apolipoprotein C1. J. Clin. Invest. 1998, 101, 145–152. [Google Scholar] [CrossRef]
- Park, B.-S.; Kim, W.-S.; Choi, J.-S.; Kim, H.-K.; Won, J.-H.; Ohkubo, F.; Fukuoka, H. Hair growth stimulated by conditioned medium of adipose-derived stem cells is enhanced by hypoxia: Evidence of increased growth factor secretion. BioMed Res. Int. 2010, 31, 34. [Google Scholar] [CrossRef]
- Metcalf, K.B.; Mandelbaum, B.R.; McIlwraith, C.W. Application of Platelet-Rich Plasma to Disorders of the Knee Joint. Cartilage 2013, 4, 295–312. [Google Scholar] [CrossRef]
- Dhurat, R.; Sukesh, M. Principles and Methods of Preparation of Platelet-Rich Plasma: A Review and Author’s Perspective. J. Cutan. Aesthet. Surg. 2014, 7, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Gobbi, A.; Karnatzikos, G.; Mahajan, V.; Malchira, S. Platelet-rich plasma treatment in symptomatic patients with knee osteoarthritis: Preliminary results in a group of active patients. Sports Health 2012, 4, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.H.; Kim, W.; Roh, Y.H.; Park, K.U. Comparison of the Cellular Composition and Cytokine-Release Kinetics of Various Platelet-Rich Plasma Preparations. Am. J. Sports Med. 2015, 43, 3062–3070. [Google Scholar] [CrossRef]
- Afifi, L.; Maranda, E.L.; Zarei, M.; Delcanto, G.M.; Falto-Aizpurua, L.; Kluijfhout, W.P.; Jimenez, J.J. Low-level laser therapy as a treatment for androgenetic alopecia. Lasers Surg. Med. 2017, 49, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.E.; Hardy, C.; Ridgway, J. Keloid management: A retrospective case review on a new approach using surgical excision, platelet-rich plasma, and in-office superficial photon X-ray radiation therapy. Adv. Skin Wound Care 2016, 29, 303–307. [Google Scholar] [CrossRef]
- Naik, A.R.; Ramesh, A.V.; Dwarkanath, C.D.; Naik, M.S.; Chinnappa, A.B. Use of autologous platelet rich plasma to treat gingival recession in esthetic periodontal surgery. J. Indian Soc. Periodontol. 2013, 17, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Cervelli, V.; Lucarini, L.; Spallone, D.; Palla, L.; Colicchia, G.M.; Gentile, P.; De Angelis, B. Use of platelet-rich plasma and hyaluronic acid in the loss of substance with bone exposure. Adv. Skin Wound Care 2011, 24, 176–181. [Google Scholar] [CrossRef]
- Nicoli, F.; Balzani, A.; Lazzeri, D.; Gentile, P.; Chilgar, R.M.; Di Pasquali, C.; Nicoli, M.; Bocchini, I.; Agovino, A.; Cervelli, V. Severe hidradenitis suppurativa treatment using platelet-rich plasma gel and Hyalomatrix. Int. Wound J. 2015, 12, 338–343. [Google Scholar] [CrossRef]
- Klosova, H.; Stetinsky, J.; Bryjová, I.; Hledík, S.; Klein, L. Objective evaluation of the effect of autologous platelet concentrate on post-operative scarring in deep burns. Burns 2013, 39, 1263–1276. [Google Scholar] [CrossRef]
- Motolese, A.; Vignati, F.; Antelmi, A.; Satumi, V. Effectiveness of platelet-rich plasma in healing necrobiosis lipoidica diabeticorum ulcers. Clin. Exp. Dermatol. 2015, 40, 39–41. [Google Scholar] [CrossRef]
- Gawdat, H.I.; Hegazy, R.A.; Fawzy, M.M.; Fathy, M. Autologous platelet rich plasma: Topical versus intradermal after fractional ablative carbon dioxide laser treatment of atrophic acne scars. Dermatol. Surg. 2014, 40, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Zucker, M.B.; Nachmias, V.T. Platelet activation. Arteriosclerosis 1985, 5, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Uebel, C.O.; da Silva, J.B.; Cantarelli, D.; Martins, P. The role of platelet plasma growth factors in male pattern baldness surgery. Plast. Reconstr. Surg. 2006, 118, 1458–1466. [Google Scholar] [CrossRef] [PubMed]
- Cervelli, V.; Garcovich, S.; Bielli, A.; Cervelli, G.; Curcio, B.C.; Scioli, M.G.; Orlandi, A.; Gentile, P. The effect of autologous activated platelet rich plasma (AA-PRP) injection on pattern hair loss: Clinical and histomorphometric evaluation. BioMed Res. Int. 2014, 2014, 760709. [Google Scholar] [CrossRef]
- Gkini, M.A.; Kouskoukis, A.E.; Tripsianis, G.; Rigopoulos, D.; Kouskoukis, K. Study of platelet-rich plasma injections in the treatment of androgenetic alopecia through a one-year period. J. Cutan. Aesthet. Surg. 2014, 7, 213–219. [Google Scholar] [CrossRef]
- Hodak, E.; Gottlieb, A.B.; Anzilotti, M.; Krueger, J.G. The insulin-like growth factor 1 receptor is expressed by epithelial cells with proliferative potential in human epidermis and skin appendages: Correlation of increased expression with epidermal hyperplasia. J. Investig. Dermatol. 1996, 106, 564–570. [Google Scholar] [CrossRef] [PubMed]
- Ristow, H.J.; Messmer, T.O. Basic fibroblast growth-factor and insulin-like growth factor-I are strong mitogens for cultured mouse keratinocytes. J. Cell Physiol. 1988, 137, 277–284. [Google Scholar] [CrossRef]
- Matsumoto, K.; Hashimoto, K.; Hashiro, M.; Yoshimasa, H.; Yoshikawa, K. Modulation of growth and differentiation in normal human keratinocytes by transforming growth-factor-beta. J. Cell Physiol. 1990, 145, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Shipley, G.D.; Pittelkow, M.R.; Wille, J.J.; E Scott, R.; Moses, H.L. Reversible inhibition of normal human prokeratinocyte proliferation by type-beta transforming growth factor-growth inhibitor in serum-free medium. Cancer Res. 1986, 46, 2068–2071. [Google Scholar]
- Born, G.V.R. Aggregation of blood platelets by adenosine diphosphate and its reversal. Nature 1962, 194, 927–929. [Google Scholar] [CrossRef] [PubMed]
- Kiso, M.; Hamazaki, T.S.; Itoh, M.; Kikuchi, S.; Nakagawa, H.; Okochi, H. Synergistic effect of PDGF and FGF2 for cell proliferation and hair inductive activity in murine vibrissal dermal papilla in vitro. J. Dermatol. Sci. 2015, 79, 110–118. [Google Scholar] [CrossRef]
- Pierce, G.F.; A Mustoe, T.; Lingelbach, J.; Masakowski, V.R.; Griffin, G.L.; Senior, R.M.; Deuel, T.F. Platelet-derived growth-factor and transforming growth factor-beta enhance tissue-repair activities by unique mechanisms. J. Cell Biol. 1989, 109, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.J.; Choi, H.-I.; Choi, D.-K.; Sohn, K.-C.; Seo, Y.-J.; Lee, J.-H.; Lee, Y.; Choi, H.; Sohn, K.; Im, M.; et al. Autologous platelet-rich plasma: A potential therapeutic tool for promoting hair growth. Dermatol. Surg. 2012, 38, 1040–1046. [Google Scholar] [CrossRef]
- Robinson, M.J.; Cobb, M.H. Mitogen-activated protein kinase pathways. Curr. Opin. Cell Biol. 1997, 9, 180–186. [Google Scholar] [CrossRef]
- Lichtenberger, B.M.; Mastrogiannaki, M.; Watt, F.M. Epidermal β -catenin activation remodels the dermis via paracrine signalling to distinct fibroblast lineages. Nat. Commun. 2016, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Singh, N.; Glazer, R.I. Role of AKT1 in 17 β -estradiol-and insulin-like growth factor 1 (IGF-1)-dependent proliferation and prevention of apoptosis in MCF-7 breast carcinoma cells. Biochem. Pharmacol. 1999, 58, 425–430. [Google Scholar] [CrossRef]
- Yang, J.; Zhao, S.L.; Yang, X.L.; Zhang, H.; Zheng, P.; Wu, H. Inhibition of B-cell apoptosis is mediated through increased expression of Bcl-2 in patients with rheumatoid arthritis. Int. J. Rheum. Dis. 2016, 19, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Gentile, P.; Garcovich, S.; Scioli, M.G.; Bielli, A.; Orlandi, A.; Cervelli, V. Mechanical and Controlled PRP Injections in Patients Affected by Androgenetic Alopecia. J. Vis. Exp. 2018, 27, 131. [Google Scholar] [CrossRef]
- Hamberg, M.; Svensson, J.; Samuelsson, B. Thromboxanes—New group of biologically-active compounds derived from prostaglandin endoperoxides. Proc. Natl. Acad. Sci. USA 1975, 72, 2994–2998. [Google Scholar] [CrossRef]
- Liu, X.; Bai, T.; Li, M.; Li, L.; Chi, G.; Liu, J.Y.; Zhang, X.; Wang, Y.; Gao, Y.; Xu, H.; et al. Maintenance of high proliferation and multipotent potential of human hair follicle-derived mesenchymal stem cells by growth factors. Int. J. Mol. Med. 2013, 31, 913–921. [Google Scholar]
- Yu, H.; Fang, D.; Kumar, S.M.; Li, L.; Nguyen, T.K.; Acs, G.; Herlyn, M.; Xu, X. Isolation of a novel population of multipotent adult stem cells from human hair follicles. Am. J. Pathol. 2006, 168, 1879–1888. [Google Scholar] [CrossRef]
- Cotsarelis, G.; Sun, T.T.; Lavker, R.M. Label-retaining cells reside in the bulge area of pilosebaceous unit: Implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell 1990, 61, 1329–1337. [Google Scholar] [CrossRef]
- Tumbar, T.; Guasch, G.; Greco, V.; Blanpain, C.; Lowry, W.E.; Rendl, M.; Fuchs, E. Defining the epithelial stem cell niche in skin. Science 2004, 303, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Morris, R.J.; Liu, Y.; Marles, L.; Yang, Z.; Trempus, C.; Li, S.; Lin, J.S.; A Sawicki, J.; Cotsarelis, G. Capturing and profiling adult hair follicle stem cells. Nat. Biotechnol. 2004, 22, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Taylor, G.; Lehrer, M.S.; Jensen, P.J.; Sun, T.-T.; Lavker, R.M. Involvement of follicular stem cells in forming not only the follicle but also the epidermis. Cell 2000, 102, 451–461. [Google Scholar] [CrossRef]
- Pesce, M.; Scholer, H.R. Oct-4: Gatekeeper in the beginnings of mammalian development. Stem Cells 2001, 19, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Alonso, L.; Fuchs, E. The hair cycle. J. Cell Sci. 2006, 119, 391–393. [Google Scholar] [CrossRef]
- Blanpain, C.; Lowry, W.E.; Geoghegan, A.; Polak, L.; Fuchs, E. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell 2004, 118, 635–648. [Google Scholar] [CrossRef]
- Botchkarev, V.A.; Kishimoto, J. Molecular control of epithelial-mesenchymal interactions during hair follicle cycling. J. Investig. Dermatol. Symp. Proc. 2003, 8, 46–55. [Google Scholar] [CrossRef]
- Roh, C.; Tao, Q.; Lyle, S. Dermal papilla-induced hair differentiation of adult epithelial stem cells from human skin. Physiol. Genet. 2004, 19, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.C.; Pasolli, H.A.; Fuchs, E. Dynamics between stem cells, niche, and progeny in the hair follicle. Cell 2011, 144, 92–105. [Google Scholar] [CrossRef]
- Reynolds, A.J.; Lawrence, C.; Cserhalmi-Friedman, P.B.; Christiano, A.M.; Jahoda, C.A.B. Trans-gender induction of hair follicles. Nature 1999, 402, 33–34. [Google Scholar] [CrossRef]
- Jahoda, C.A. Induction of follicle formation and hair growth by vibrissa dermal papillae implanted into rat ear wounds: Vibrissa-type fibres are specified. Development 1992, 115, 1103–1109. [Google Scholar]
- Kalabusheva, E.; Terskikh, V.; Vorotelyak, E. Hair Germ Model In Vitro via Human Postnatal Keratinocyte-Dermal Papilla Interactions: Impact of Hyaluronic Acid. Stem Cells Int. 2017, 2017. [Google Scholar] [CrossRef]
- Talavera-Adame, D.; Newman, D.; Newman, D. Conventional and novel stem cell-based therapies for androgenic alopecia. Stem Cells Cloning 2017, 10, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.Y.; Sennett, R.; Rezza, A.; Clavel, C.; Grisanti, L.; Zemla, R.; Najam, S.; Rendl, M. Wnt/β-catenin signaling in dermal condensates is required for hair follicle formation. Dev. Biol. 2014, 385, 179–188. [Google Scholar] [CrossRef]
- Huelsken, J.; Vogel, R.; Erdmann, B.; Cotsarelis, G.; Birchmeier, W. Beta-Catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell 2001, 105, 533–545. [Google Scholar] [CrossRef]
- Pirastu, N.; Joshi, P.K.; De Vries, P.S.; Cornelis, M.C.; McKeigue, P.M.; Keum, N.; Franceschini, N.; Colombo, M.; Giovannucci, E.L.; Spiliopoulou, A.; et al. GWAS for male-pattern baldness identifies 71 susceptibility loci explaining 38% of the risk. Nat. Commun. 2017, 8, 1584. [Google Scholar] [CrossRef]
- Richardson, S.M.; Kalamegam, G.; Pushparaj, P.N.; Matta, C.; Memic, A.; Khademhosseini, A.; Mobasheri, R.; Poletti, F.L.; Hoyland, J.A.; Mobasheri, A. Mesenchymal stem cells in regenerative medicine: Focus on articular cartilage and intervertebral disc regeneration. Methods 2016, 99, 69–80. [Google Scholar] [CrossRef]
- Sabapathy, V.; Sundaram, B.; Vm, S.; Mankuzhy, P.; Kumar, S. Human Wharton’s jelly mesenchymal stem cells plasticity augments scar-free skin wound healing with hair growth. PLoS ONE 2014, 9, e93726. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, Q.; Wang, Z.; Tong, H.; Ma, L.; Zhang, Y.; Shan, F.; Meng, Y.; Yuan, Z. Comparative analysis of human mesenchymal stem cells from fetal-bone marrow, adipose tissue, and Warton’s jelly as sources of cell immunomodulatory therapy. Hum. Vaccines Immunother. 2015, 12, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Jadalannagari, S.; Converse, G.; McFall, C.; Buse, E.; Filla, M.; Villar, M.T.; Artigues, A.; Mellot, A.J.; Wang, J.; Detamore, M.S.; et al. Decellularized Wharton’s jelly from human umbilical cord as a novel 3D scaffolding material for tissue engineering applications. PLoS ONE 2017, 12, e0172098. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gentile, P.; Garcovich, S. Advances in Regenerative Stem Cell Therapy in Androgenic Alopecia and Hair Loss: Wnt Pathway, Growth-Factor, and Mesenchymal Stem Cell Signaling Impact Analysis on Cell Growth and Hair Follicle Development. Cells 2019, 8, 466. https://doi.org/10.3390/cells8050466
Gentile P, Garcovich S. Advances in Regenerative Stem Cell Therapy in Androgenic Alopecia and Hair Loss: Wnt Pathway, Growth-Factor, and Mesenchymal Stem Cell Signaling Impact Analysis on Cell Growth and Hair Follicle Development. Cells. 2019; 8(5):466. https://doi.org/10.3390/cells8050466
Chicago/Turabian StyleGentile, Pietro, and Simone Garcovich. 2019. "Advances in Regenerative Stem Cell Therapy in Androgenic Alopecia and Hair Loss: Wnt Pathway, Growth-Factor, and Mesenchymal Stem Cell Signaling Impact Analysis on Cell Growth and Hair Follicle Development" Cells 8, no. 5: 466. https://doi.org/10.3390/cells8050466
APA StyleGentile, P., & Garcovich, S. (2019). Advances in Regenerative Stem Cell Therapy in Androgenic Alopecia and Hair Loss: Wnt Pathway, Growth-Factor, and Mesenchymal Stem Cell Signaling Impact Analysis on Cell Growth and Hair Follicle Development. Cells, 8(5), 466. https://doi.org/10.3390/cells8050466