Systemic Dysfunction of Osteoblast Differentiation in Adipose-Derived Stem Cells from Patients with Multiple Myeloma
Abstract
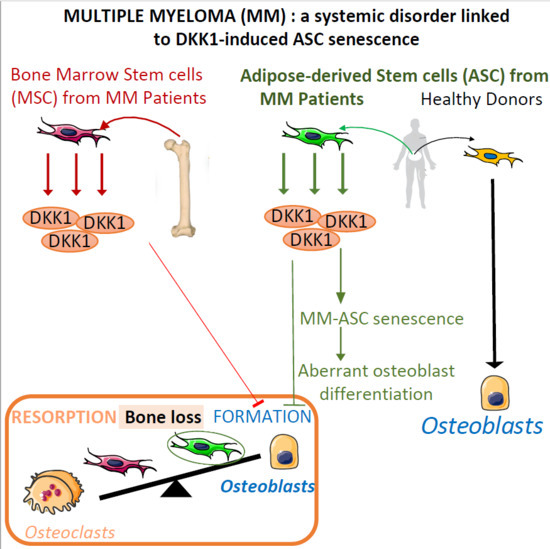
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Cell Sample Collection
2.3. Mesenchymal Stromal Cell Isolation and Culture
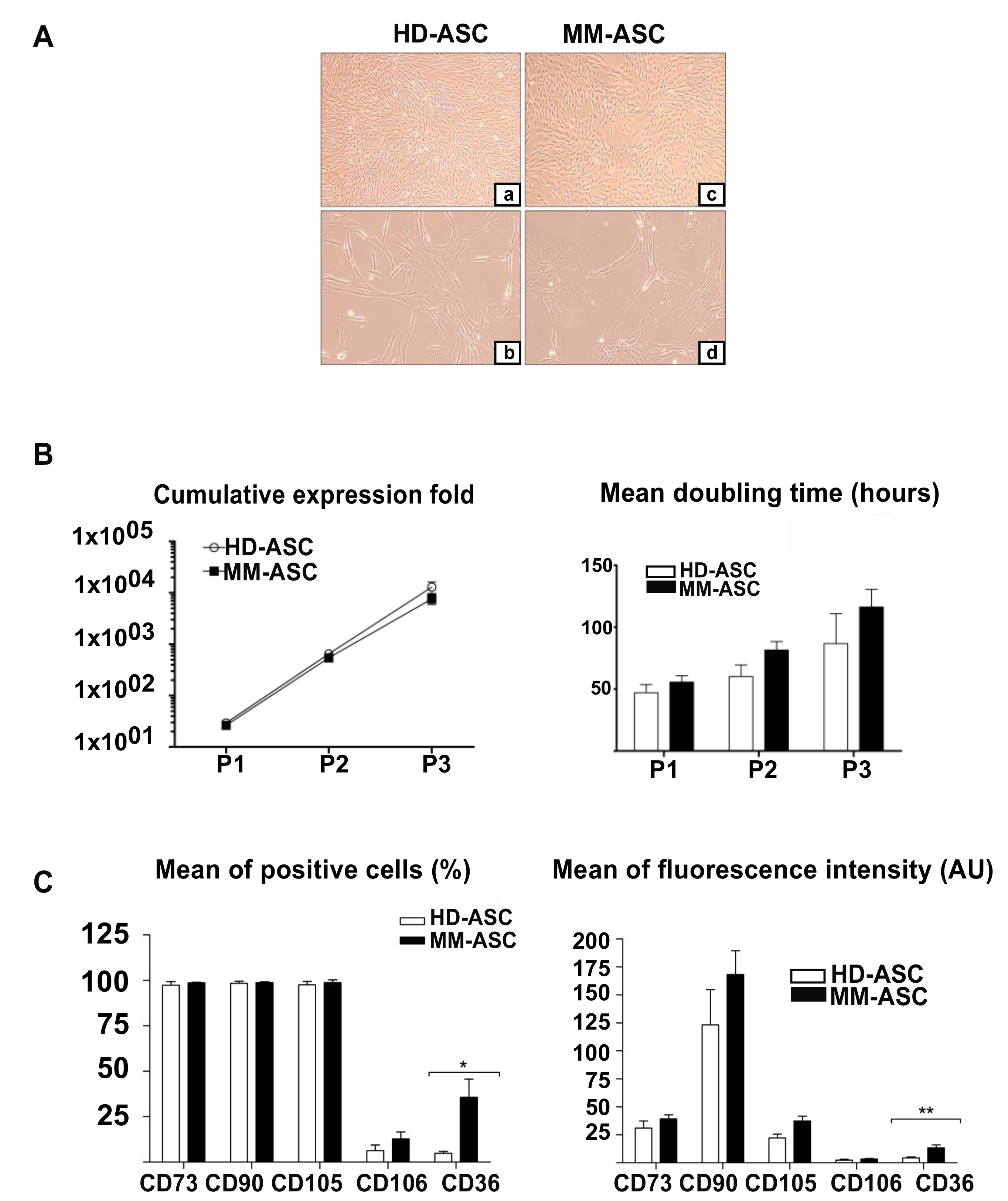
2.4. Mesenchymal Stromal Cell Immunophenotyping
2.5. Cell Differentiation
2.6. Cell Senescence
2.7. Western Blotting
2.8. Statistical Analyses
3. Results
3.1. ASC from Healthy Donors and MM Patients are Comparable with Respect to Morphology, Phenotype and Proliferative Capacity
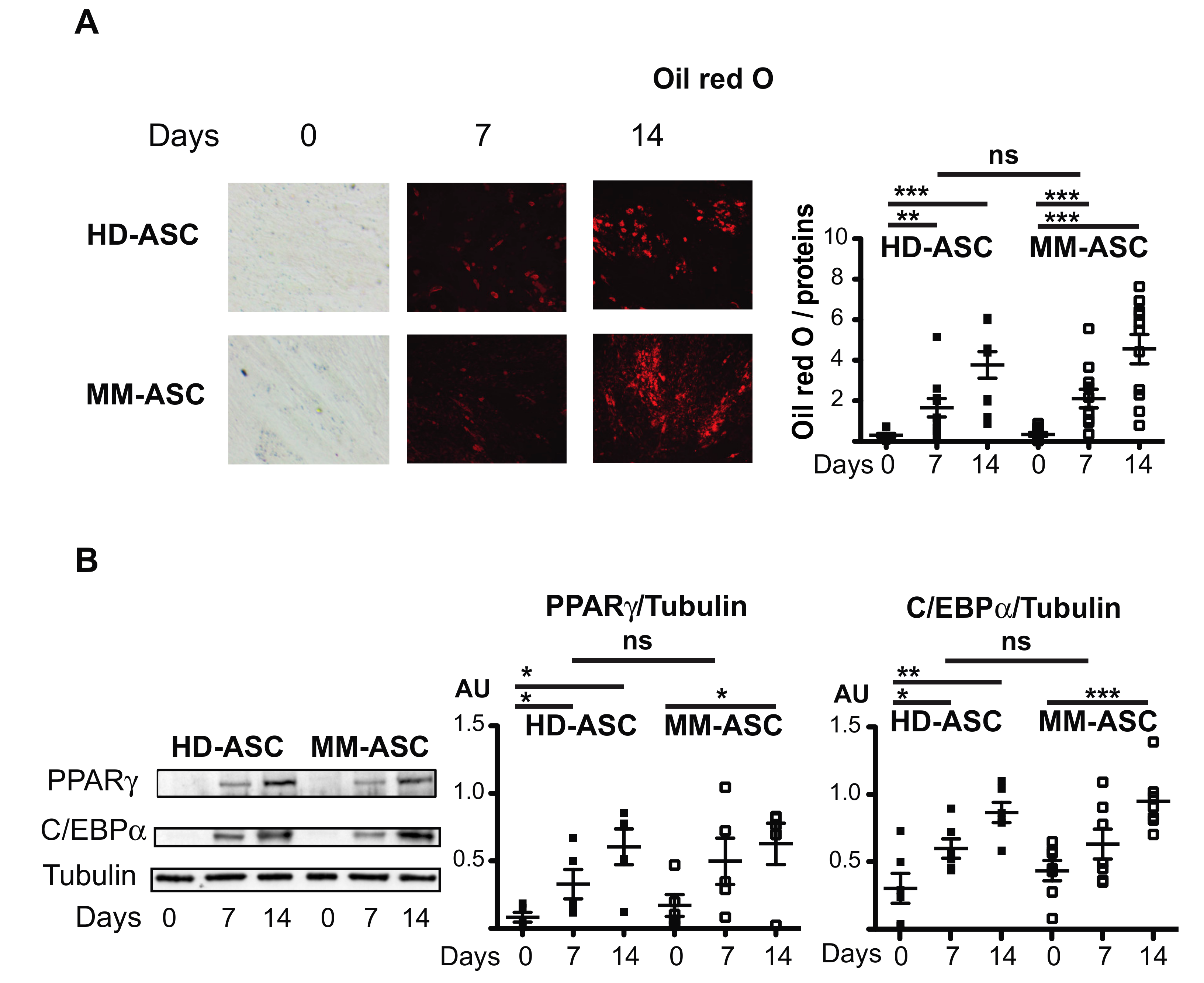
3.2. ASC from MM Patients are Capable of Normal Adipocyte Differentiation
3.3. ASC from MM Patients Display Defective Osteoblast Differentiation
3.4. ASC from MM Patients Exhibit a Senescent Phenotype
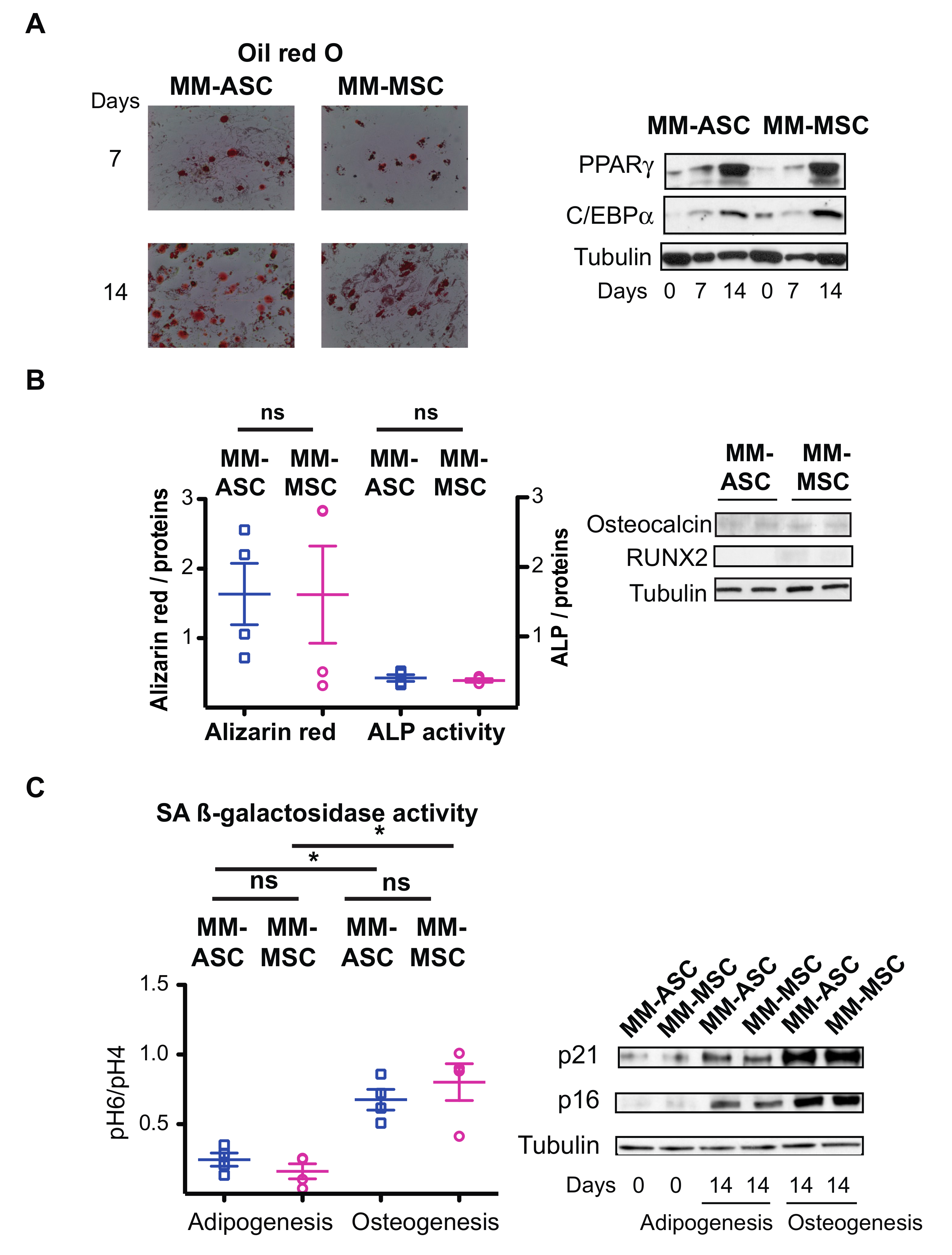
3.5. ASC and MSC from MM Patients Present Comparable Defects
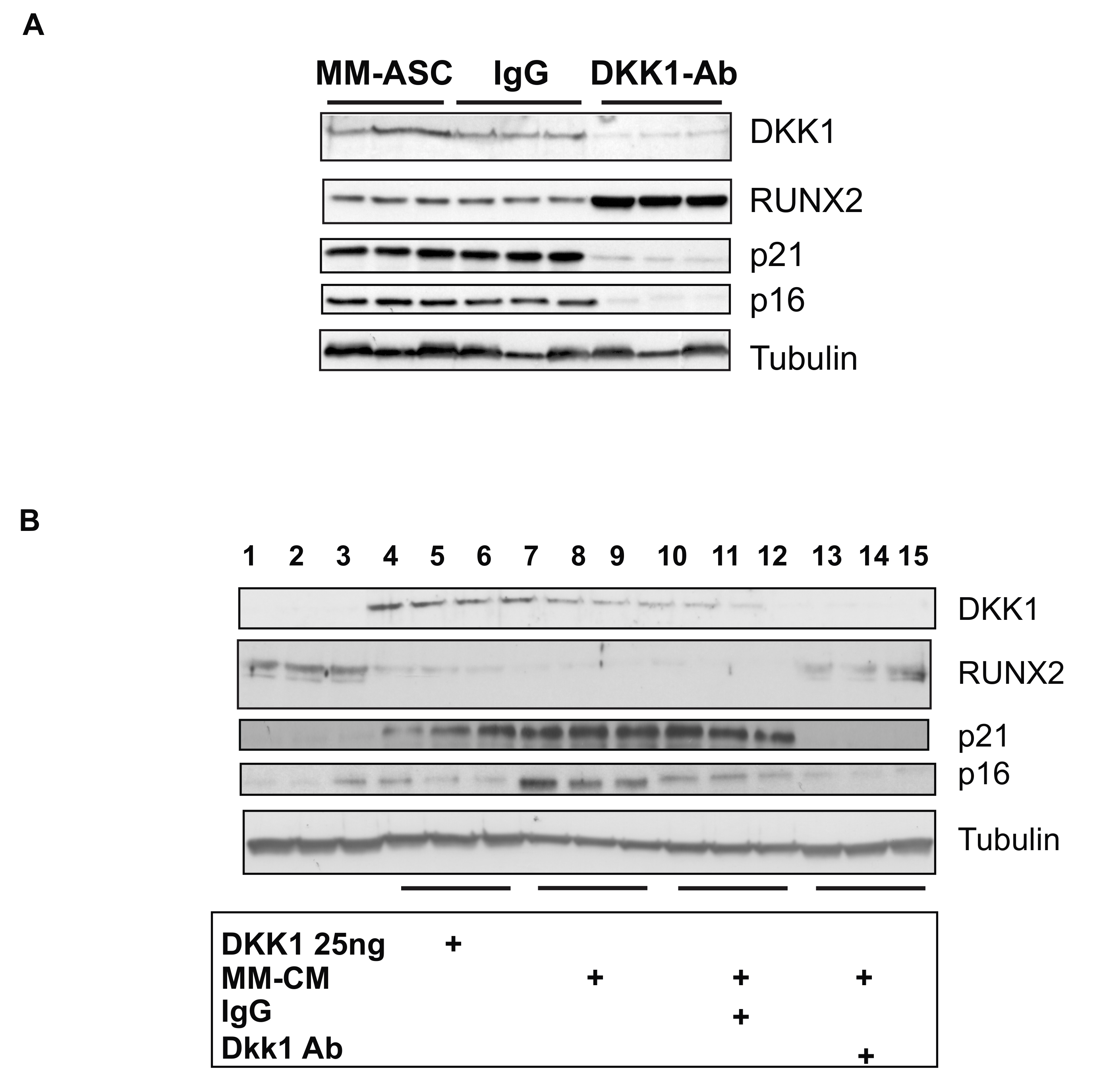
3.6. DKK1 Inhibition Rescues RUNX2 Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Ethics Approval and Consent to Participate
Conflicts of Interest
References
- Kumar, S.K.; Rajkumar, V.; Kyle, R.A.; van Duin, M.; Sonneveld, P.; Mateos, M.V.; Gay, F.; Anderson, K.C. Multiple myeloma. Nat. Rev. Dis. Primers. 2017, 3, 17046. [Google Scholar] [CrossRef] [PubMed]
- Mansour, A.; Wakkach, A.; Blin-Wakkach, C. Emerging Roles of Osteoclasts in the Modulation of Bone Microenvironment and Immune Suppression in Multiple Myeloma. Front. Immunol. 2017, 8, 954. [Google Scholar] [CrossRef]
- Callander, N.S.; Roodman, G.D. Myeloma bone disease. Semin. Hematol. 2001, 38, 276–285. [Google Scholar] [CrossRef]
- Giuliani, N.; Rizzoli, V.; Roodman, G.D. Multiple myeloma bone disease: Pathophysiology of osteoblast inhibition. Blood 2006, 108, 3992–3996. [Google Scholar] [CrossRef] [PubMed]
- Terpos, E.; Ntanasis-Stathopoulos, I.; Gavriatopoulou, M.; Dimopoulos, M.A. Pathogenesis of bone disease in multiple myeloma: From bench to bedside. Blood Cancer J. 2018, 8, 7. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.E.; Rogers, M.J.; Halasy, J.M.; Luckman, S.P.; Hughes, D.E.; Masarachia, P.J.; Wesolowski, G.; Russell, R.G.; Rodan, G.A.; Reszka, A.A. Alendronate mechanism of action: geranylgeraniol, an intermediate in the mevalonate pathway, prevents inhibition of osteoclast formation, bone resorption, and kinase activation in vitro. Proc. Natl. Acad. Sci. USA 1999, 96, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Lee, O.L.; Horvath, N.; Lee, C.; Joshua, D.; Ho, J.; Szer, J.; Quach, H.; Spencer, A.; Harrison, S.; Mollee, P.; et al. Bisphosphonate guidelines for treatment and prevention of myeloma bone disease. Intern. Med. J. 2017, 47, 938–951. [Google Scholar] [CrossRef]
- Corre, J.; Mahtouk, K.; Attal, M.; Gadelorge, M.; Huynh, A.; Fleury-Cappellesso, S.; Danho, C.; Laharrague, P.; Klein, B.; Reme, T.; et al. Bone marrow mesenchymal stem cells are abnormal in multiple myeloma. Leukemia 2007, 21, 1079–1088. [Google Scholar] [CrossRef] [PubMed]
- Arnulf, B.; Lecourt, S.; Soulier, J.; Ternaux, B.; Lacassagne, M.N.; Crinquette, A.; Dessoly, J.; Sciaini, A.K.; Benbunan, M.; Chomienne, C.; et al. Phenotypic and functional characterization of bone marrow mesenchymal stem cells derived from patients with multiple myeloma. Leukemia 2007, 21, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Garderet, L.; Mazurier, C.; Chapel, A.; Ernou, I.; Boutin, L.; Holy, X.; Gorin, N.C.; Lopez, M.; Doucet, C.; Lataillade, J.J. Mesenchymal stem cell abnormalities in patients with multiple myeloma. Leuk. Lymphoma 2007, 48, 2032–2041. [Google Scholar] [CrossRef]
- Bafico, A.; Liu, G.; Yaniv, A.; Gazit, A.; Aaronson, S.A. Novel mechanism of Wnt signalling inhibition mediated by Dickkopf-1 interaction with LRP6/Arrow. Nat. Cell Biol. 2001, 3, 683–686. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; De Veirman, K.; De Becker, A.; Vanderkerken, K.; Van Riet, I. Mesenchymal stem cells in multiple myeloma: A therapeutical tool or target? Leukemia 2018, 32, 1500–1514. [Google Scholar] [CrossRef] [PubMed]
- Gimble, J.M.; Katz, A.J.; Bunnell, B.A. Adipose-derived stem cells for regenerative medicine. Circ. Res. 2007, 100, 1249–1260. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Mizuno, H.; Tobita, M.; Uysal, A.C. Concise review: Adipose-derived stem cells as a novel tool for future regenerative medicine. Stem Cells 2012, 30, 804–810. [Google Scholar] [CrossRef]
- Bourin, P.; Bunnell, B.A.; Casteilla, L.; Dominici, M.; Katz, A.J.; March, K.L.; Redl, H.; Rubin, J.P.; Yoshimura, K.; Gimble, J.M. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: A joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy 2013, 15, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Secunda, R.; Vennila, R.; Mohanashankar, A.M.; Rajasundari, M.; Jeswanth, S.; Surendran, R. Isolation, expansion and characterisation of mesenchymal stem cells from human bone marrow, adipose tissue, umbilical cord blood and matrix: A comparative study. Cytotechnology 2015, 67, 793–807. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Heydarkhan-Hagvall, S.; Hedrick, M.; Benhaim, P.; Zuk, P. Manual isolation of adipose-derived stem cells from human lipoaspirates. J. Vis. Exp. 2013, e50585. [Google Scholar] [CrossRef]
- Gorwood, J.; Bourgeois, C.; Mantecon, M.; Atlan, M.; Pourcher, V.; Pourcher, G.; Le Grand, R.; Desjardins, D.; Feve, B.; Lambotte, O.; et al. Impact of HIV/SIV infection and viral proteins on adipose tissue fibrosis and adipogenesis. AIDS 2019, 33, 953–964. [Google Scholar] [CrossRef]
- Lagathu, C.; Eustace, B.; Prot, M.; Frantz, D.; Gu, Y.; Bastard, J.P.; Maachi, M.; Azoulay, S.; Briggs, M.; Caron, M.; et al. Some HIV antiretrovirals increase oxidative stress and alter chemokine, cytokine or adiponectin production in human adipocytes and macrophages. Antivir. Ther. 2007, 12, 489–500. [Google Scholar] [PubMed]
- Cotter, E.J.; Chew, N.; Powderly, W.G.; Doran, P.P. HIV type 1 alters mesenchymal stem cell differentiation potential and cell phenotype ex vivo. AIDS Res. Hum. Retroviruses 2011, 27, 187–199. [Google Scholar] [CrossRef]
- Dimri, G.P.; Lee, X.; Basile, G.; Acosta, M.; Scott, G.; Roskelley, C.; Medrano, E.E.; Linskens, M.; Rubelj, I.; Pereira-Smith, O.; et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl. Acad. Sci. USA 1995, 92, 9363–9367. [Google Scholar] [CrossRef] [PubMed]
- Caron, M.; Auclairt, M.; Vissian, A.; Vigouroux, C.; Capeau, J. Contribution of mitochondrial dysfunction and oxidative stress to cellular premature senescence induced by antiretroviral thymidine analogues. Antivir. Ther. 2008, 13, 27–38. [Google Scholar] [PubMed]
- Silbermann, R.; Roodman, G.D. Myeloma bone disease: Pathophysiology and management. J. Bone Oncol. 2013, 2, 59–69. [Google Scholar] [CrossRef]
- Li, B.; Shi, M.; Li, J.; Zhang, H.; Chen, B.; Chen, L.; Gao, W.; Giuliani, N.; Zhao, R.C. Elevated tumor necrosis factor-alpha suppresses TAZ expression and impairs osteogenic potential of Flk-1+ mesenchymal stem cells in patients with multiple myeloma. Stem Cells Dev. 2007, 16, 921–930. [Google Scholar] [CrossRef]
- Tian, E.; Zhan, F.; Walker, R.; Rasmussen, E.; Ma, Y.; Barlogie, B.; Shaughnessy, J.D., Jr. The role of the Wnt-signaling antagonist DKK1 in the development of osteolytic lesions in multiple myeloma. N. Engl. J. Med. 2003, 349, 2483–2494. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Volat, F.; Sandhow, L.; Galitzky, J.; Nguyen, T.; Esteve, D.; Astrom, G.; Mejhert, N.; Ledoux, S.; Thalamas, C.; et al. CD36 Is a Marker of Human Adipocyte Progenitors with Pronounced Adipogenic and Triglyceride Accumulation Potential. Stem Cells 2017, 35, 1799–1814. [Google Scholar] [CrossRef]
- Baglio, S.R.; Rooijers, K.; Koppers-Lalic, D.; Verweij, F.J.; Perez Lanzon, M.; Zini, N.; Naaijkens, B.; Perut, F.; Niessen, H.W.; Baldini, N.; et al. Human bone marrow- and adipose-mesenchymal stem cells secrete exosomes enriched in distinctive miRNA and tRNA species. Stem Cell Res. Ther. 2015, 6, 127. [Google Scholar] [CrossRef]
- Gao, J.; Yang, T.; Han, J.; Yan, K.; Qiu, X.; Zhou, Y.; Fan, Q.; Ma, B. MicroRNA expression during osteogenic differentiation of human multipotent mesenchymal stromal cells from bone marrow. J. Cell Biochem. 2011, 112, 1844–1856. [Google Scholar] [CrossRef] [PubMed]
- Aval, S.F.; Zarghami, N.; Alizadeh, E.; Mohammadi, S.A. The effect of ketorolac and triamcinolone acetonide on adipogenic and hepatogenic differentiation through miRNAs 16/15/195: Possible clinical application in regenerative medicine. Biomed. Pharmacother. 2018, 97, 675–683. [Google Scholar] [CrossRef]
- Fakhry, M.; Hamade, E.; Badran, B.; Buchet, R.; Magne, D. Molecular mechanisms of mesenchymal stem cell differentiation towards osteoblasts. World J. Stem Cells 2013, 5, 136–148. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yuan, X.; Shi, H.; Wu, L.; Qian, H.; Xu, W. Exosomes in cancer: Small particle, big player. J. Hematol. Oncol. 2015, 8, 83. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.; Garcia, M.; Weng, L.; Jung, X.; Murakami, J.L.; Hu, X.; McDonald, T.; Lin, A.; Kumar, A.R.; DiGiusto, D.L.; et al. Acute myeloid leukemia transforms the bone marrow niche into a leukemia-permissive microenvironment through exosome secretion. Leukemia 2018, 32, 575–587. [Google Scholar] [CrossRef]
- Pinzone, J.J.; Hall, B.M.; Thudi, N.K.; Vonau, M.; Qiang, Y.W.; Rosol, T.J.; Shaughnessy, J.D., Jr. The role of Dickkopf-1 in bone development, homeostasis, and disease. Blood 2009, 113, 517–525. [Google Scholar] [CrossRef]
- Gustafson, B.; Eliasson, B.; Smith, U. Thiazolidinediones increase the wingless-type MMTV integration site family (WNT) inhibitor Dickkopf-1 in adipocytes: A link with osteogenesis. Diabetologia 2010, 53, 536–540. [Google Scholar] [CrossRef]
- Veld, J.; O’Donnell, E.K.; Reagan, M.R.; Yee, A.J.; Torriani, M.; Rosen, C.J.; Bredella, M.A. Abdominal adipose tissue in MGUS and multiple myeloma. Skeletal. Radiol. 2016, 45, 1277–1283. [Google Scholar] [CrossRef]
- Chong, M.; Yin, T.; Chen, R.; Xiang, H.; Yuan, L.; Ding, Y.; Pan, C.C.; Tang, Z.; Alexander, P.B.; Li, Q.J.; et al. CD36 initiates the secretory phenotype during the establishment of cellular senescence. EMBO Rep. 2018, 19. [Google Scholar] [CrossRef] [PubMed]
- Oshima, T.; Abe, M.; Asano, J.; Hara, T.; Kitazoe, K.; Sekimoto, E.; Tanaka, Y.; Shibata, H.; Hashimoto, T.; Ozaki, S.; et al. Myeloma cells suppress bone formation by secreting a soluble Wnt inhibitor, sFRP-2. Blood 2005, 106, 3160–3165. [Google Scholar] [CrossRef]
- Kwack, M.H.; Kim, M.K.; Kim, J.C.; Sung, Y.K. Dickkopf 1 promotes regression of hair follicles. J. Invest. Dermatol. 2012, 132, 1554–1560. [Google Scholar] [CrossRef] [PubMed]
- Lyros, O.; Rafiee, P.; Nie, L.; Medda, R.; Jovanovic, N.; Schmidt, J.; Mackinnon, A.; Venu, N.; Shaker, R. Dickkopf-1, the Wnt antagonist, is induced by acidic pH and mediates epithelial cellular senescence in human reflux esophagitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2014, 306, G557–G574. [Google Scholar] [CrossRef][Green Version]
- Rani, S.; Chauhan, R.; Parsad, D.; Kumar, R. Effect of Dickkopf1 on the senescence of melanocytes: In vitro study. Arch. Dermatol. Res. 2018, 310, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Bajada, S.; Marshall, M.J.; Wright, K.T.; Richardson, J.B.; Johnson, W.E. Decreased osteogenesis, increased cell senescence and elevated Dickkopf-1 secretion in human fracture non union stromal cells. Bone 2009, 45, 726–735. [Google Scholar] [CrossRef]


| HD-ASC | MM-ASC | |
|---|---|---|
| CD45 | <1% | <1% |
| CD14 | <1% | <1% |
| HLA-DR | <1% | <1% |
| CD11b | <1% | <1% |
| CD19 | <1% | <1% |
| CD34 (%) | 5.6 ± 2.4 | 2.8 ± 0.9 |
| CD34 MFI (AU) | 2.1 ± 0.9 | 4.2 ± 2.05 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Béréziat, V.; Mazurier, C.; Auclair, M.; Ferrand, N.; Jolly, S.; Marie, T.; Kobari, L.; Toillon, I.; Delhommeau, F.; Fève, B.; et al. Systemic Dysfunction of Osteoblast Differentiation in Adipose-Derived Stem Cells from Patients with Multiple Myeloma. Cells 2019, 8, 441. https://doi.org/10.3390/cells8050441
Béréziat V, Mazurier C, Auclair M, Ferrand N, Jolly S, Marie T, Kobari L, Toillon I, Delhommeau F, Fève B, et al. Systemic Dysfunction of Osteoblast Differentiation in Adipose-Derived Stem Cells from Patients with Multiple Myeloma. Cells. 2019; 8(5):441. https://doi.org/10.3390/cells8050441
Chicago/Turabian StyleBéréziat, Véronique, Christelle Mazurier, Martine Auclair, Nathalie Ferrand, Séverine Jolly, Tiffany Marie, Ladan Kobari, Indira Toillon, François Delhommeau, Bruno Fève, and et al. 2019. "Systemic Dysfunction of Osteoblast Differentiation in Adipose-Derived Stem Cells from Patients with Multiple Myeloma" Cells 8, no. 5: 441. https://doi.org/10.3390/cells8050441
APA StyleBéréziat, V., Mazurier, C., Auclair, M., Ferrand, N., Jolly, S., Marie, T., Kobari, L., Toillon, I., Delhommeau, F., Fève, B., Larsen, A. K., Sabbah, M., & Garderet, L. (2019). Systemic Dysfunction of Osteoblast Differentiation in Adipose-Derived Stem Cells from Patients with Multiple Myeloma. Cells, 8(5), 441. https://doi.org/10.3390/cells8050441