Telomere Length and Risk of Major Adverse Cardiac Events and Cancer in Obstructive Sleep Apnea Patients
Abstract
1. Introduction
2. Materials and Methods
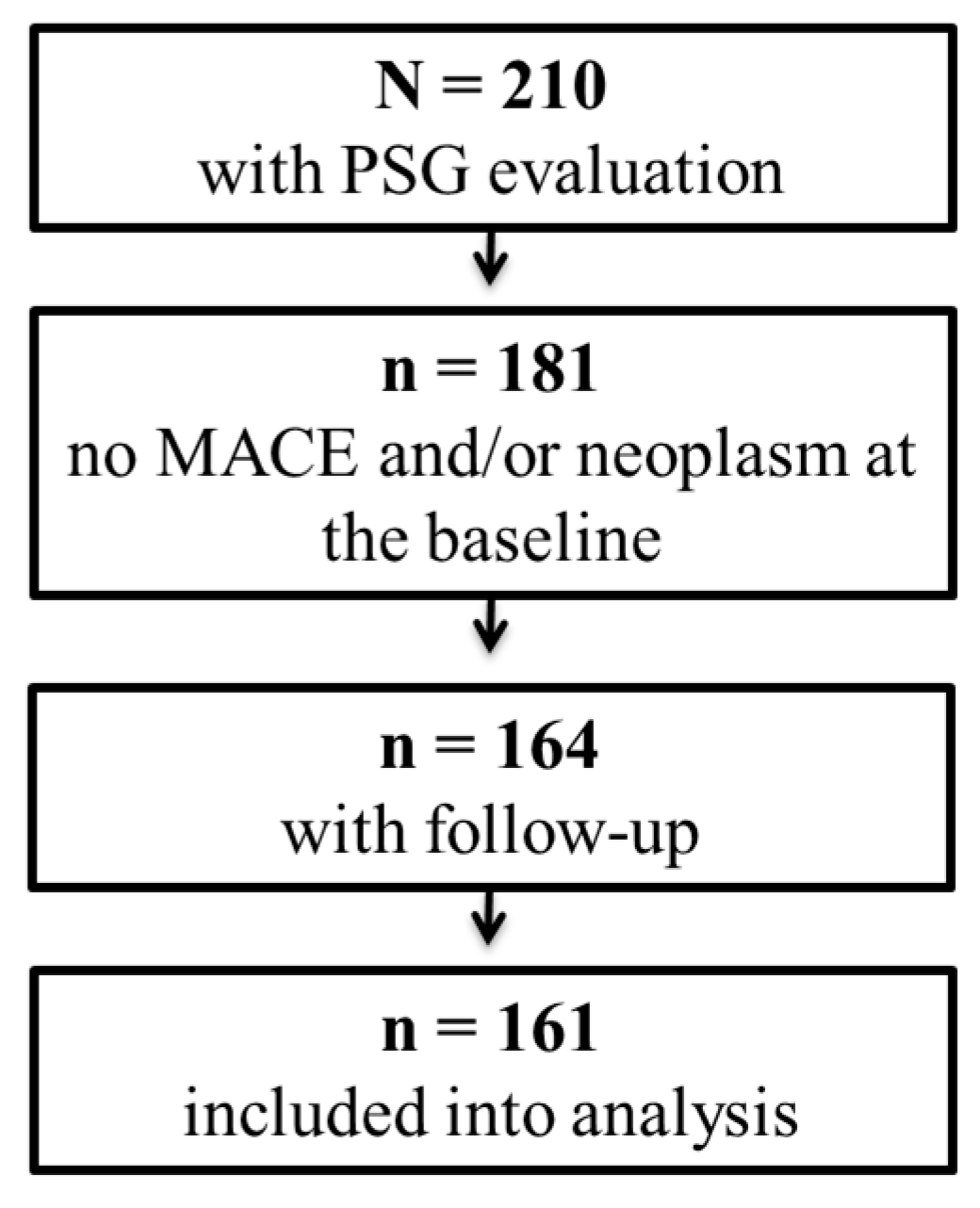
2.1. Study Population
2.2. Clinical Follow-Up and Outcomes
2.3. Polysomnography Examination
2.4. Leukocyte Telomere Length Measurements
2.5. Statistical Analysis
3. Results
3.1. Population Characteristic
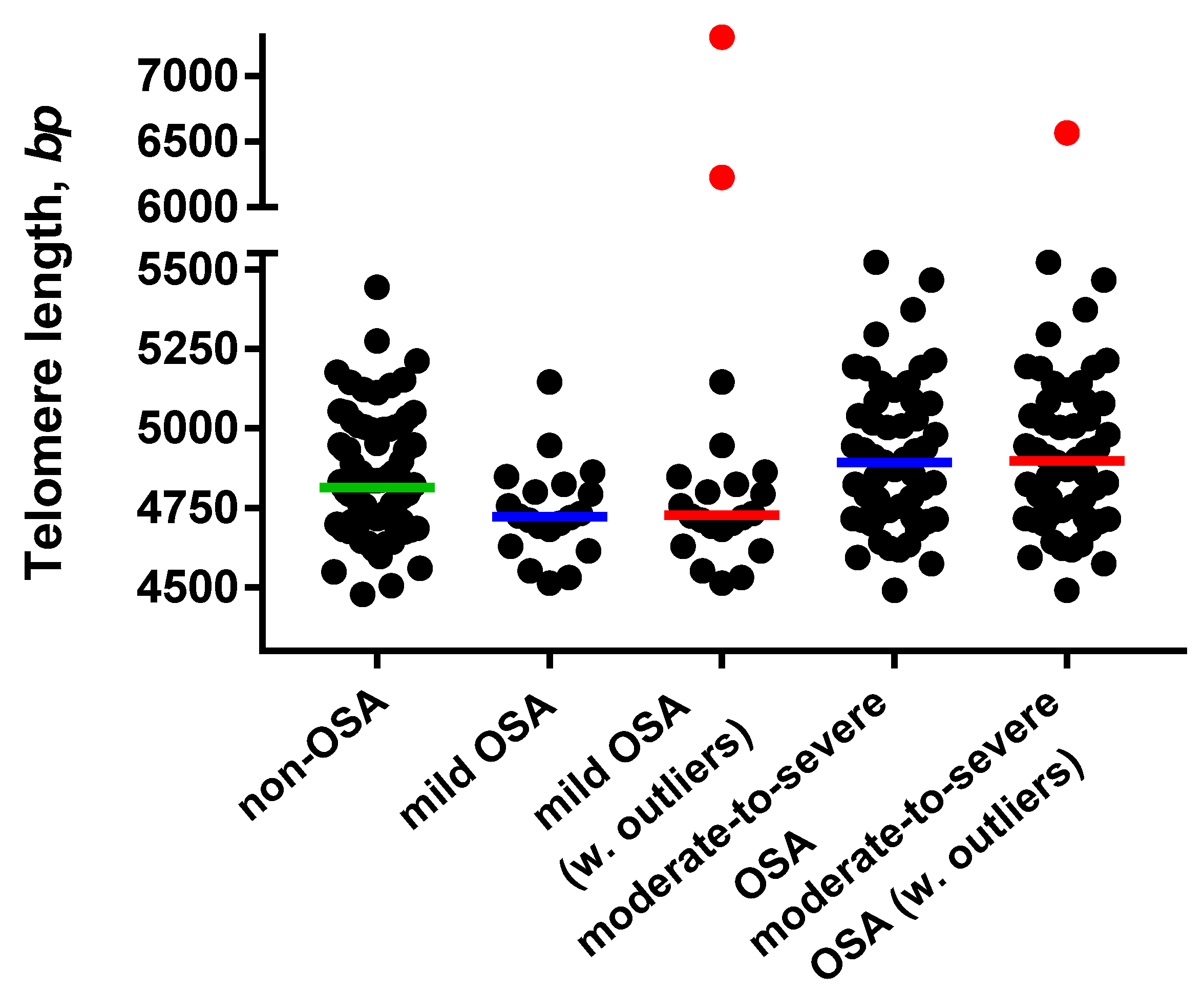
3.2. Telomere Length
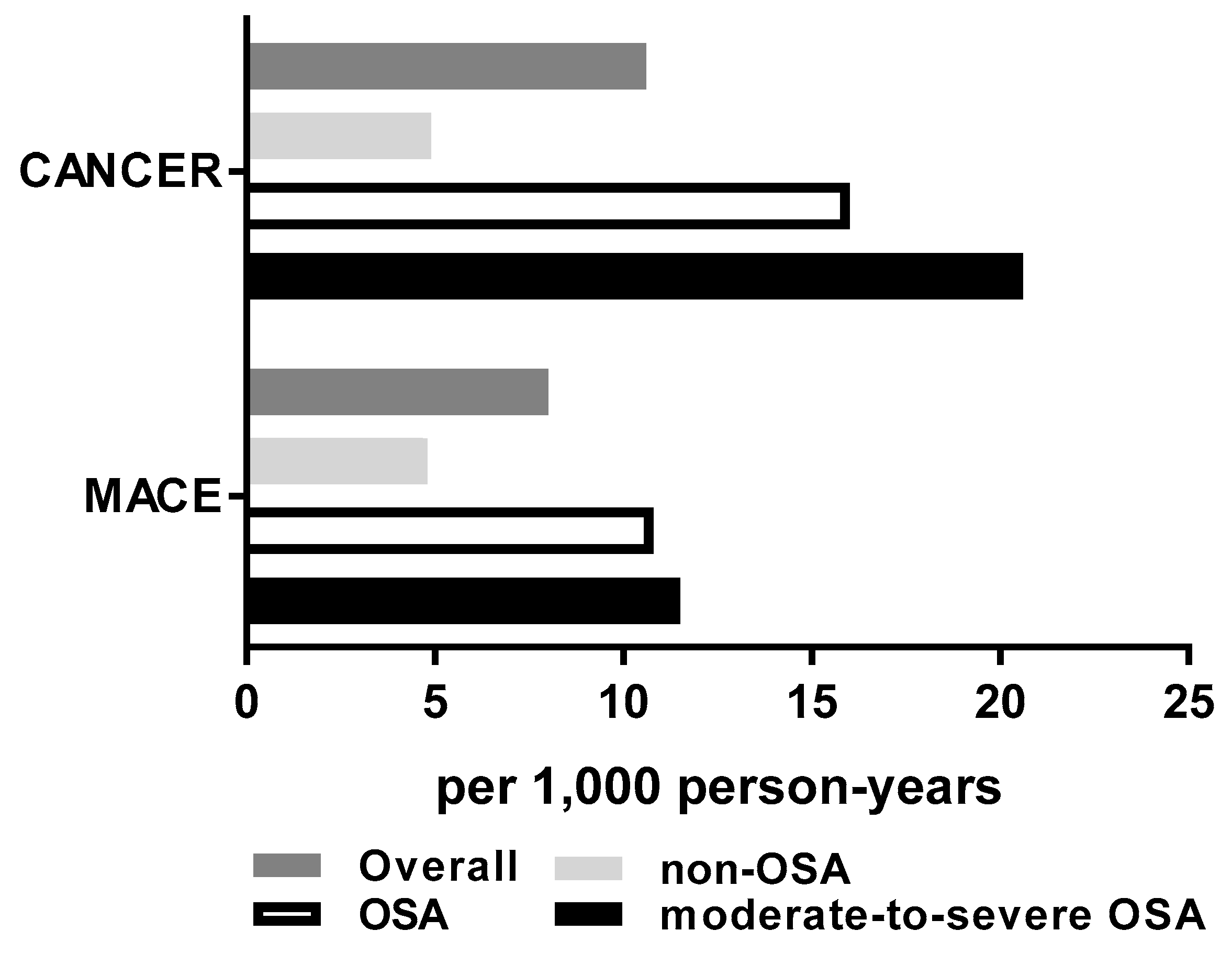
3.3. MACE and Cancer Risk
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Weir, H.K. Heart Disease and Cancer Deaths—Trends and Projections in the United States, 1969–2020. Prev. Chronic Dis. 2016, 13, 160211. [Google Scholar] [CrossRef] [PubMed]
- Somers, V.K.; White, D.P.; Amin, R.; Abraham, W.T.; Costa, F.; Culebras, A.; Daniels, S.; Floras, J.S.; Hunt, C.E.; Olson, L.J.; et al. Sleep apnea and cardiovascular disease: An American Heart Association/American College Of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council On Cardiovascular Nursing. In collaboration with the National Heart, Lung, and Blood Institute National Center on Sleep Disorders Research (National Institutes of Health). Circulation 2008, 118, 1080–1111. [Google Scholar]
- Senaratna, C.V.; Perret, J.L.; Lodge, C.J.; Lowe, A.J.; Campbell, B.E.; Matheson, M.C.; Hamilton, G.S.; Dharmage, S.C. Prevalence of obstructive sleep apnea in the general population: A systematic review. Sleep Med. Rev. 2017, 34, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Martínez-García, M.Á.; Campos-Rodriguez, F.; Barbé, F. Cancer and OSA: Current Evidence From Human Studies. Chest 2016, 150, 451–463. [Google Scholar] [CrossRef] [PubMed]
- Masoudkabir, F.; Sarrafzadegan, N.; Gotay, C.; Ignaszewski, A.; Krahn, A.D.; Davis, M.K.; Franco, C.; Mani, A. Cardiovascular disease and cancer: Evidence for shared disease pathways and pharmacologic prevention. Atherosclerosis 2017, 263, 343–351. [Google Scholar] [CrossRef]
- Kendzerska, T.; Leung, R.S.; Hawker, G.; Tomlinson, G.; Gershon, A.S. Obstructive sleep apnea and the prevalence and incidence of cancer. CMAJ 2014, 186, 985–992. [Google Scholar] [CrossRef] [PubMed]
- Koene, R.J.; Prizment, A.E.; Blaes, A.; Konety, S.H. Shared Risk Factors in Cardiovascular Disease and Cancer. Circulation 2016, 133, 1104–1114. [Google Scholar] [CrossRef]
- Huang, P.; Zhou, J.; Zhao, X.; Chen, S.; Zou, C.; Li, J. The Association between Obstructive Sleep Apnea and Shortened Telomere Length: A Systematic Review and Meta-analysis. Sleep Med. 2018, 48, 107–112. [Google Scholar] [CrossRef]
- Blackburn, E.H.; Epel, E.S.; Lin, J. Human telomere biology: A contributory and interactive factor in aging, disease risks, and protection. Science 2015, 350, 1193–1198. [Google Scholar] [CrossRef]
- Shammas, M.A. Telomeres, lifestyle, cancer, and aging. Curr. Opin. Clin. Nutr. Metab. Care 2011, 14, 28–34. [Google Scholar] [CrossRef]
- Bekaert, S.; De Meyer, T.; Van Oostveldt, P. Telomere attrition as ageing biomarker. Anticancer Res. 2005, 25, 3011–3021. [Google Scholar]
- Haussmann, M.F.; Heidinger, B.J. Telomere dynamics may link stress exposure and ageing across generations. Biol. Lett. 2015, 11, 20150396. [Google Scholar] [CrossRef]
- Oeseburg, H.; de Boer, R.A.; van Gilst, W.H.; van der Harst, P. Telomere biology in healthy aging and disease. Pflugers Arch 2010, 459, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Yeh, J.-K.; Wang, C.-Y. Telomeres and Telomerase in Cardiovascular Diseases. Genes 2016, 7, 58. [Google Scholar] [CrossRef]
- Fuster, J.J.; Andrés, V. Telomere Biology and Cardiovascular Disease. Circ. Res. 2006, 99, 1167–1180. [Google Scholar] [CrossRef]
- D’Mello, M.J.J.; Ross, S.A.; Briel, M.; Anand, S.S.; Gerstein, H.; Paré, G. Association between shortened leukocyte telomere length and cardiometabolic outcomes: Systematic review and meta-analysis. Circ. Cardiovasc. Genet. 2015, 8, 82–90. [Google Scholar] [CrossRef]
- Haycock, P.C.; Heydon, E.E.; Kaptoge, S.; Butterworth, A.S.; Thompson, A.; Willeit, P. Leucocyte telomere length and risk of cardiovascular disease: Systematic review and meta-analysis. BMJ 2014, 349, g4227. [Google Scholar] [CrossRef]
- Zhu, X.; Han, W.; Xue, W.; Zou, Y.; Xie, C.; Du, J.; Jin, G. The association between telomere length and cancer risk in population studies. Sci. Rep. 2016, 6, 22243. [Google Scholar] [CrossRef]
- Tempaku, P.F.; Tufik, S. The paradigm of obstructive sleep apnea in aging: Interactions with telomere length. Sleep Med. 2018, 48, 155–156. [Google Scholar] [CrossRef]
- Huzen, J.; Wong, L.S.M.; van Veldhuisen, D.J.; Samani, N.J.; Zwinderman, A.H.; Codd, V.; Cawthon, R.M.; Benus, G.F.J.D.; van der Horst, I.C.C.; Navis, G.; et al. Telomere length loss due to smoking and metabolic traits. J. Intern. Med. 2014, 275, 155–163. [Google Scholar] [CrossRef]
- Aviv, A.; Chen, W.; Gardner, J.P.; Kimura, M.; Brimacombe, M.; Cao, X.; Srinivasan, S.R.; Berenson, G.S. Leukocyte telomere dynamics: Longitudinal findings among young adults in the Bogalusa Heart Study. Am. J. Epidemiol. 2009, 169, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Weischer, M.; Bojesen, S.E.; Nordestgaard, B.G. Telomere Shortening Unrelated to Smoking, Body Weight, Physical Activity, and Alcohol Intake: 4,576 General Population Individuals with Repeat Measurements 10 Years Apart. PLoS Genet. 2014, 10. [Google Scholar] [CrossRef]
- Polonis, K.; Somers, V.K.; Becari, C.; Covassin, N.; Schulte, P.J.; Druliner, B.R.; Johnson, R.A.; Narkiewicz, K.; Boardman, L.A.; Singh, P. Moderate-to-severe obstructive sleep apnea is associated with telomere lengthening. Am. J. Physiol. Heart Circ. Physiol. 2017, 313, H1022–H1030. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, S.; Bhattacharjee, R.; Khalyfa, A.; Kheirandish-Gozal, L.; Gozal, D. Leukocyte telomere length and plasma catestatin and myeloid-related protein 8/14 concentrations in children with obstructive sleep apnea. Chest 2010, 138, 91–99. [Google Scholar] [CrossRef]
- Kip, K.E.; Hollabaugh, K.; Marroquin, O.C.; Williams, D.O. The problem with composite end points in cardiovascular studies: The story of major adverse cardiac events and percutaneous coronary intervention. J. Am. Coll. Cardiol. 2008, 51, 701–707. [Google Scholar] [CrossRef]
- Rechtschaffen, A.; Kales, A. A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects; University of California: Los Angeles, CA, USA, 1968. [Google Scholar]
- Bonnet, M.H.; Carley, D.W.; Carskadon, M.A.; Easton, P.A. EEG arousals: Scoring rules and examples: A preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep 1992, 15, 173–184. [Google Scholar]
- Cawthon, R.M. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002, 30, e47. [Google Scholar] [CrossRef]
- Boardman, L.A.; Johnson, R.A.; Viker, K.B.; Hafner, K.A.; Jenkins, R.B.; Riegert-Johnson, D.L.; Smyrk, T.C.; Litzelman, K.; Seo, S.; Gangnon, R.E.; et al. Correlation of Chromosomal Instability, Telomere Length and Telomere Maintenance in Microsatellite Stable Rectal Cancer: A Molecular Subclass of Rectal Cancer. PLoS ONE 2013, 8, e80015. [Google Scholar] [CrossRef] [PubMed]
- Islami, F.; Goding Sauer, A.; Miller, K.D.; Siegel, R.L.; Fedewa, S.A.; Jacobs, E.J.; McCullough, M.L.; Patel, A.V.; Ma, J.; Soerjomataram, I.; et al. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA Cancer J. Clin. 2018, 68, 31–54. [Google Scholar] [CrossRef]
- Palamaner Subash Shantha, G.; Kumar, A.A.; Cheskin, L.J.; Pancholy, S.B. Association between sleep-disordered breathing, obstructive sleep apnea, and cancer incidence: A systematic review and meta-analysis. Sleep Med. 2015, 16, 1289–1294. [Google Scholar] [CrossRef]
- Martínez-García, M.Á.; Campos-Rodríguez, F.; Farré, R. Sleep apnoea and cancer: Current insights and future perspectives. Eur. Respir. J. 2012, 40, 1315–1317. [Google Scholar] [CrossRef]
- Gozal, D.; Farré, R.; Nieto, F.J. Obstructive sleep apnea and cancer: Epidemiologic links and theoretical biological constructs. Sleep Med. Rev. 2016, 27, 43–55. [Google Scholar] [CrossRef]
- Sillah, A.; Watson, N.F.; Schwartz, S.M.; Gozal, D.; Phipps, A.I. Sleep apnea and subsequent cancer incidence. Cancer Causes Control 2018, 29, 987–994. [Google Scholar] [CrossRef]
- Chen, J.-C.; Hwang, J.-H. Sleep apnea increased incidence of primary central nervous system cancers: A nationwide cohort study. Sleep Med. 2014, 15, 749–754. [Google Scholar] [CrossRef]
- Chang, W.-P.; Liu, M.-E.; Chang, W.-C.; Yang, A.C.; Ku, Y.-C.; Pai, J.-T.; Lin, Y.-W.; Tsai, S.-J. Sleep apnea and the subsequent risk of breast cancer in women: A nationwide population-based cohort study. Sleep Med. 2014, 15, 1016–1020. [Google Scholar] [CrossRef]
- Nieto, F.J.; Peppard, P.E.; Young, T.; Finn, L.; Hla, K.M.; Farré, R. Sleep-disordered breathing and cancer mortality: Results from the Wisconsin Sleep Cohort Study. Am. J. Respir. Crit. Care Med. 2012, 186, 190–194. [Google Scholar] [CrossRef]
- Campos-Rodriguez, F.; Martinez-Garcia, M.A.; Martinez, M.; Duran-Cantolla, J.; de la Peña, M.; Masdeu, M.J.; Gonzalez, M.; del Campo, F.; Gallego, I.; Marin, J.M.; et al. Association between obstructive sleep apnea and cancer incidence in a large multicenter Spanish cohort. Am. J. Respir. Crit. Care Med. 2013, 187, 99–105. [Google Scholar] [CrossRef]
- Marshall, N.S.; Wong, K.K.H.; Cullen, S.R.J.; Knuiman, M.W.; Grunstein, R.R. Sleep apnea and 20-year follow-up for all-cause mortality, stroke, and cancer incidence and mortality in the Busselton Health Study cohort. J. Clin. Sleep Med. 2014, 10, 355–362. [Google Scholar] [CrossRef]
- Hunyor, I.; Cook, K.M. Models of intermittent hypoxia and obstructive sleep apnea: Molecular pathways and their contribution to cancer. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 315, R669–R687. [Google Scholar] [CrossRef]
- Alqawi, O.; Wang, H.P.; Espiritu, M.; Singh, G. Chronic hypoxia promotes an aggressive phenotype in rat prostate cancer cells. Free Radic. Res. 2007, 41, 788–797. [Google Scholar] [CrossRef]
- Almendros, I.; Montserrat, J.M.; Ramírez, J.; Torres, M.; Duran-Cantolla, J.; Navajas, D.; Farré, R. Intermittent hypoxia enhances cancer progression in a mouse model of sleep apnoea. Eur. Respir. J. 2012, 39, 215–217. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Sceneay, J.; Gödde, N.; Kinwel, T.; Ham, S.; Thompson, E.W.; Humbert, P.O.; Möller, A. Intermittent hypoxia induces a metastatic phenotype in breast cancer. Oncogene 2018, 37, 4214. [Google Scholar] [CrossRef] [PubMed]
- Cabezas, E.; Pérez-Warnisher, M.T.; Troncoso, M.F.; Gómez, T.; Melchor, R.; Pinillos, E.J.; El Hachem, A.; Gotera, C.; Rodríguez, P.; Mahillo Fernández, I.; et al. Sleep Disordered Breathing Is Highly Prevalent in Patients with Lung Cancer: Results of the Sleep Apnea in Lung Cancer Study. Respiration 2019, 97, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Warnisher, M.T.; Cabezas, E.; Troncoso, M.F.; Gómez, T.; Melchor, R.; Pinillos, E.J.; El Hachem, A.; Gotera, C.; Rodriguez, P.; Mahíllo, I.; et al. Sleep disordered breathing and nocturnal hypoxemia are very prevalent in a lung cancer screening population and may condition lung cancer screening findings: Results of the prospective Sleep Apnea In Lung Cancer Screening (SAILS) study. Sleep Med. 2019, 54, 181–186. [Google Scholar] [CrossRef]
- Martínez-García, M.-Á.; Martorell-Calatayud, A.; Nagore, E.; Valero, I.; Selma, M.J.; Chiner, E.; Landete, P.; Montserrat, J.-M.; Carrera, C.; Pérez-Gil, A.; et al. Association between sleep disordered breathing and aggressiveness markers of malignant cutaneous melanoma. Eur. Respir. J. 2014, 43, 1661–1668. [Google Scholar] [CrossRef]
- Martínez-García, M.A.; Campos-Rodriguez, F.; Durán-Cantolla, J.; de la Peña, M.; Masdeu, M.J.; González, M.; Del Campo, F.; Serra, P.C.; Valero-Sánchez, I.; Ferrer, M.J.S.; et al. Obstructive sleep apnea is associated with cancer mortality in younger patients. Sleep Med. 2014, 15, 742–748. [Google Scholar] [CrossRef]
- Wai, L.K. Telomeres, Telomerase, and Tumorigenesis—A Review. MedGenMed 2004, 6, 19. [Google Scholar]
- Aviv, A.; Anderson, J.J.; Shay, J.W. Mutations, Cancer and the Telomere Length Paradox. Trends Cancer 2017, 3, 253–258. [Google Scholar] [CrossRef]
- Haycock, P.C.; Burgess, S.; Nounu, A.; Zheng, J.; Okoli, G.N.; Bowden, J.; Wade, K.H.; Timpson, N.J.; Evans, D.M.; Willeit, P.; et al. Association Between Telomere Length and Risk of Cancer and Non-Neoplastic Diseases: A Mendelian Randomization Study. JAMA Oncol. 2017, 3, 636–651. [Google Scholar] [CrossRef]
- Anderson, C.J.; Hoare, S.F.; Ashcroft, M.; Bilsland, A.E.; Keith, W.N. Hypoxic regulation of telomerase gene expression by transcriptional and post-transcriptional mechanisms. Oncogene 2006, 25, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Guan, J.-Z.; Guan, W.-P.; Maeda, T.; Makino, N. Different levels of hypoxia regulate telomere length and telomerase activity. Aging Clin. Exp. Res. 2012, 24, 213–217. [Google Scholar] [CrossRef]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef]
| Variable | Non-OSA n = 88 | OSA n = 73 | P-Value |
|---|---|---|---|
| Telomere length, bp | 4842 ± 180 | 4865 ± 226 | 0.482 |
| Age, years | 35.6 ± 11.0 | 47.3 ± 11.9 | <0.001 |
| Systolic BP, mmHg | 124.1 ± 15.3 | 131.7 ± 15.3 | 0.003 |
| Diastolic BP, mmHg | 73.1 ± 9.8 | 76.4 ± 9.1 | 0.030 |
| Heart rate, beats/min | 69.6 ± 11.3 | 73.9 ± 14.0 | 0.044 |
| Body mass index, kg/m2 | 26.9 ± 5.1 | 33.0 ± 7.7 | <0.001 |
| Male sex, n (%) | 59 (67%) | 64 (87%) | 0.002 |
| Diabetes mellitus, n (%) | 1 (1%) | 0 (0%) | 1 |
| Dyslipidemia, n (%) | 6 (7%) | 13 (18%) | 0.031 |
| Hypertension, n (%) | 7 (8%) | 17 (23%) | 0.010 |
| Current smoking, n (%) | 7 (8%) | 5 (7%) | 0.775 |
| Race/Ethnicity, n (%) | |||
| African American | 3 (3%) | 0 (0%) | 0.252 |
| Asian | 18 (20%) | 3 (4%) | 0.002 |
| Pacific Islander | 1 (1%) | 0 (0%) | 1.0 |
| White | 56 (64%) | 67 (92%) | <0.001 |
| Hispanic/Latino * | 7 (8%) | 3 (4%) | 0.314 |
| Unknown | 3 (3%) | 0 (0%) | 0.252 |
| Education, n (%) | |||
| High school graduate | 9 (10%) | 17 (23%) | 0.025 |
| Collage graduate/degree | 42 (48%) | 44 (60%) | 0.112 |
| Post graduate degree | 34 (39%) | 11 (15%) | <0.001 |
| Unknown | 3 (3%) | 1 (1%) | 0.627 |
| Apnea-hypopnea index, n/h | 1.0 ± 1.3 | 33.9 ± 27.3 | <0.001 |
| Minimum blood O2 saturation, % | 90.1 ± 5.2 | 81.8 ± 9.2 | <0.001 |
| Univariable Model | Multivariable Model 1 | Multivariable Model 2 | ||||
|---|---|---|---|---|---|---|
| MACE | HR (95% CI) | P-Value | HR (95% CI) | P-Value | HR (95% CI) | P-Value |
| OSA vs. non-OSA | 1.87 (0.57–6.15) | 0.301 | 0.96 (0.27–3.48) | 0.954 | 0.51 (0.12–2.19) | 0.368 |
| TL, per 100 bp | 1.02 (0.79–1.29) | 0.877 | 1.01 (0.78–1.28) | 0.905 | 1.05 (0.81–1.34) | 0.705 |
| Age, yrs | 1.07 (1.03–1.12) | 0.002 | 1.07 (1.02–1.12) | 0.003 | 1.07 (1.02–1.13) | 0.003 |
| BMI, kg/m2 | 1.07 (0.99–1.13) | 0.052 | 1.08 (1.00–1.15) | 0.041 | ||
| Male sex | 2.75 (0.36–21.20) | 0.333 | 3.92 (0.47–32.64) | 0.205 | ||
| CANCER | ||||||
| OSA vs. non-OSA | 3.30 (1.08–10.03) | 0.036 | 1.65 (0.50–5.41) | 0.409 | 1.73 (0.44–6.72) | 0.428 |
| TL, per 100 bp | 1.15 (0.93–1.39) | 0.175 | 1.18 (0.96–1.43) | 0.109 | 1.21 (0.98–1.48) | 0.068 |
| Age, yrs | 1.08 (1.04–1.12) | <0.001 | 1.07 (1.03–1.12) | <0.001 | 1.07 (1.03–1.12) | <0.001 |
| BMI, kg/m2 | 1.05 (1.00–1.10) | 0.032 | 1.03 (0.96–1.09) | 0.422 | ||
| Male sex | 0.58 (0.21–1.63) | 0.301 | 0.45 (0.13–1.54) | 0.204 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polonis, K.; Sompalli, S.; Becari, C.; Xie, J.; Covassin, N.; Schulte, P.J.; Druliner, B.R.; Johnson, R.A.; Narkiewicz, K.; Boardman, L.A.; et al. Telomere Length and Risk of Major Adverse Cardiac Events and Cancer in Obstructive Sleep Apnea Patients. Cells 2019, 8, 381. https://doi.org/10.3390/cells8050381
Polonis K, Sompalli S, Becari C, Xie J, Covassin N, Schulte PJ, Druliner BR, Johnson RA, Narkiewicz K, Boardman LA, et al. Telomere Length and Risk of Major Adverse Cardiac Events and Cancer in Obstructive Sleep Apnea Patients. Cells. 2019; 8(5):381. https://doi.org/10.3390/cells8050381
Chicago/Turabian StylePolonis, Katarzyna, Sreeja Sompalli, Christiane Becari, Jiang Xie, Naima Covassin, Phillip J Schulte, Brooke R Druliner, Ruth A Johnson, Krzysztof Narkiewicz, Lisa A Boardman, and et al. 2019. "Telomere Length and Risk of Major Adverse Cardiac Events and Cancer in Obstructive Sleep Apnea Patients" Cells 8, no. 5: 381. https://doi.org/10.3390/cells8050381
APA StylePolonis, K., Sompalli, S., Becari, C., Xie, J., Covassin, N., Schulte, P. J., Druliner, B. R., Johnson, R. A., Narkiewicz, K., Boardman, L. A., Singh, P., & Somers, V. K. (2019). Telomere Length and Risk of Major Adverse Cardiac Events and Cancer in Obstructive Sleep Apnea Patients. Cells, 8(5), 381. https://doi.org/10.3390/cells8050381





