Humoral Response to the HIV-1 Envelope V2 Region in a Thai Early Acute Infection Cohort
Abstract
1. Introduction
2. Materials and Methods
2.1. RV217 Plasma Samples
2.2. Surface Plasmon Resonance Assay (SPR)
2.3. Viral Genome Sequencing
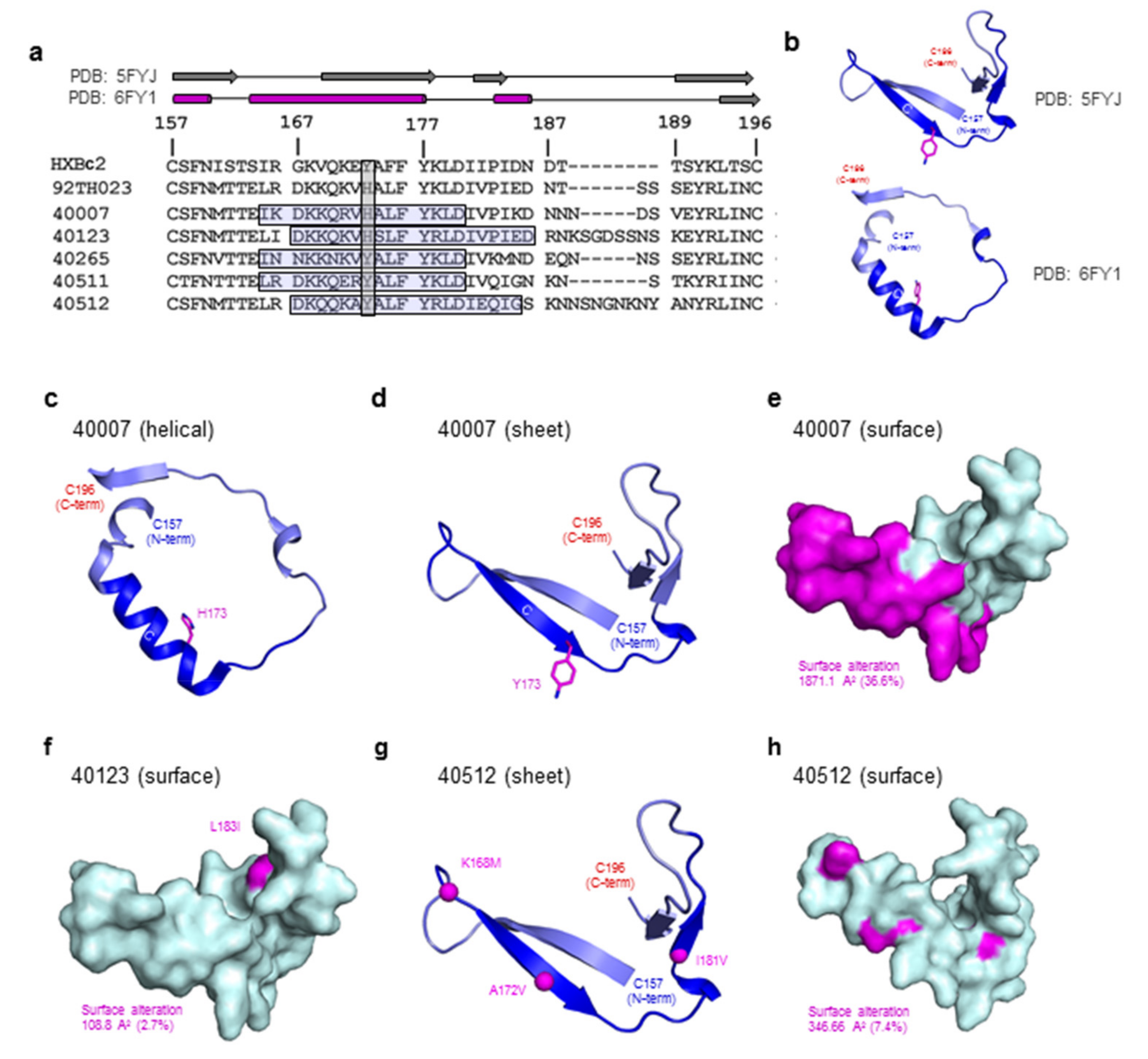
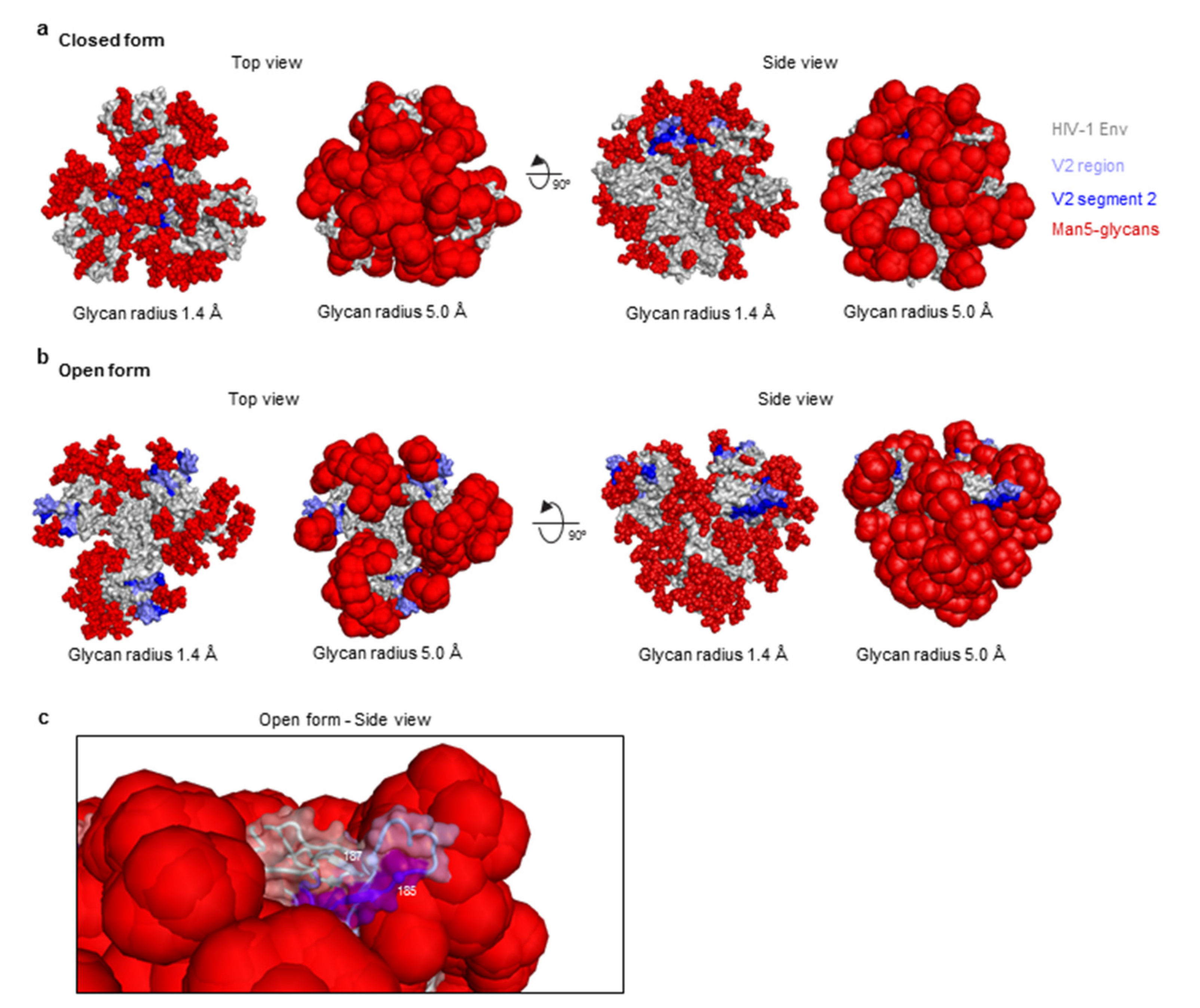
2.4. Structural Modeling and Analysis
2.5. Statistical Analysis
3. Results
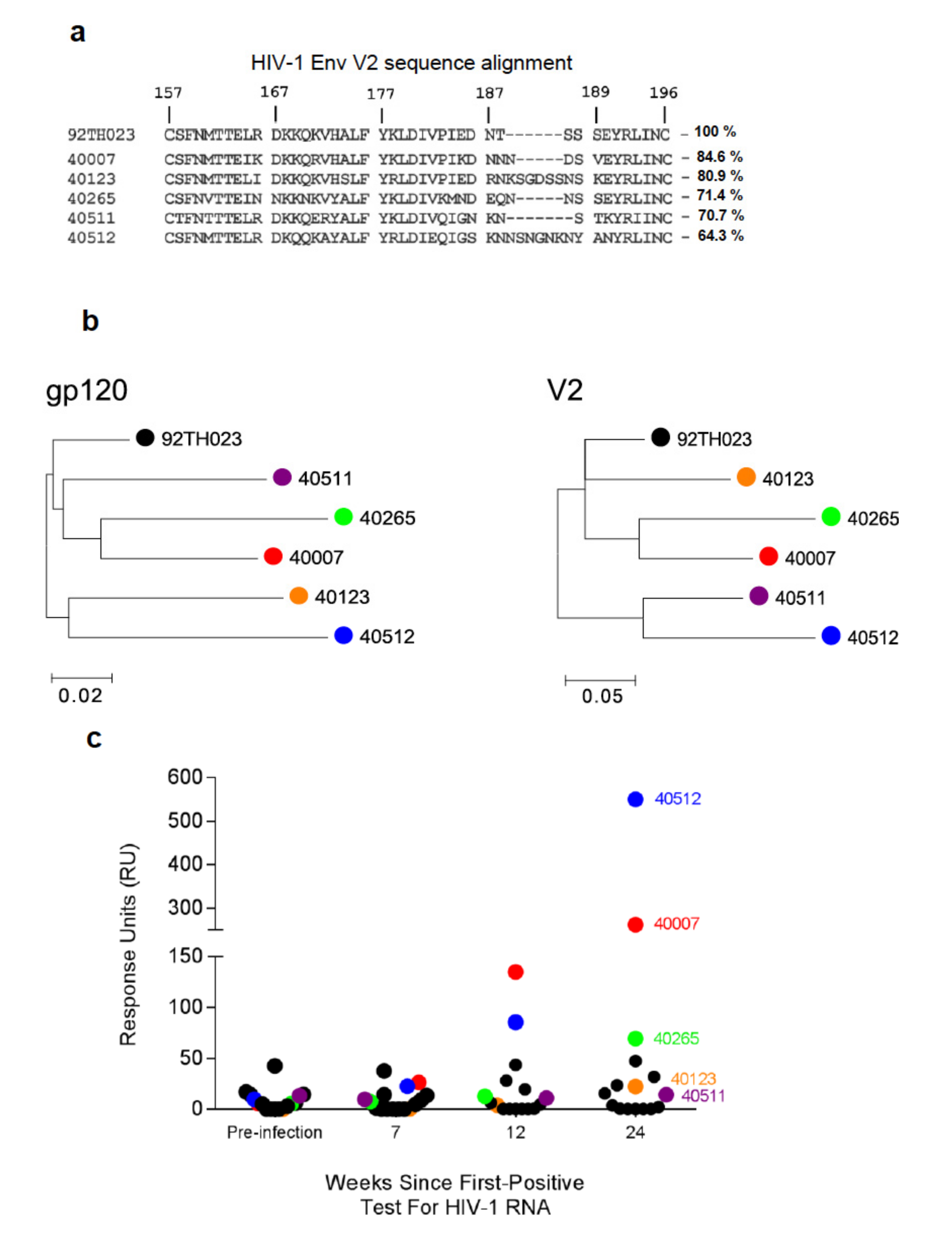
3.1. V2-Specific Antibodies in Acute HIV-1 Infection
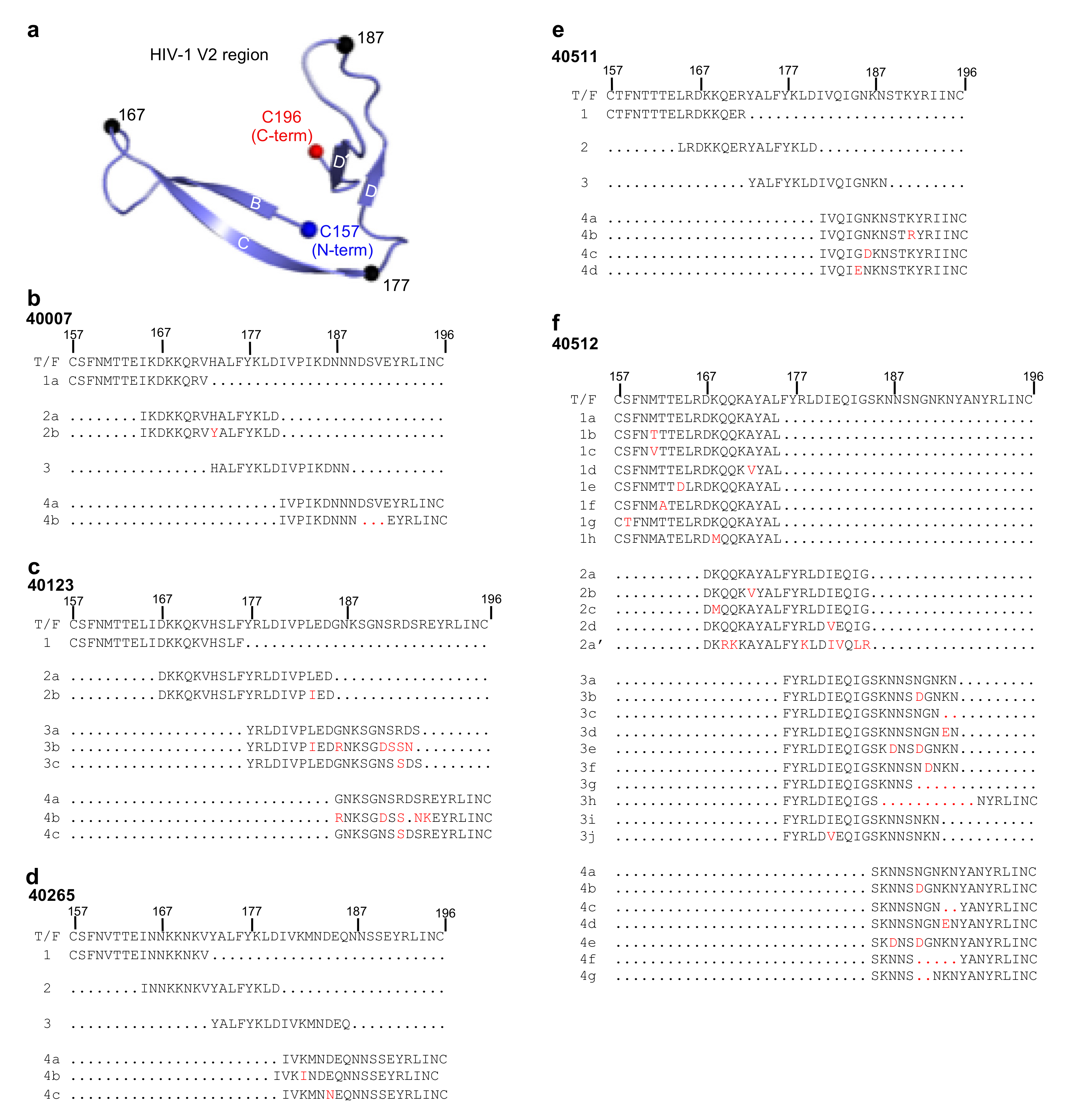
3.2. Single Genome Analysis of Longitudinal HIV-1 Env Sequences
3.3. Design of HIV-1 V2 Peptides Based on Viral SGA Sequences
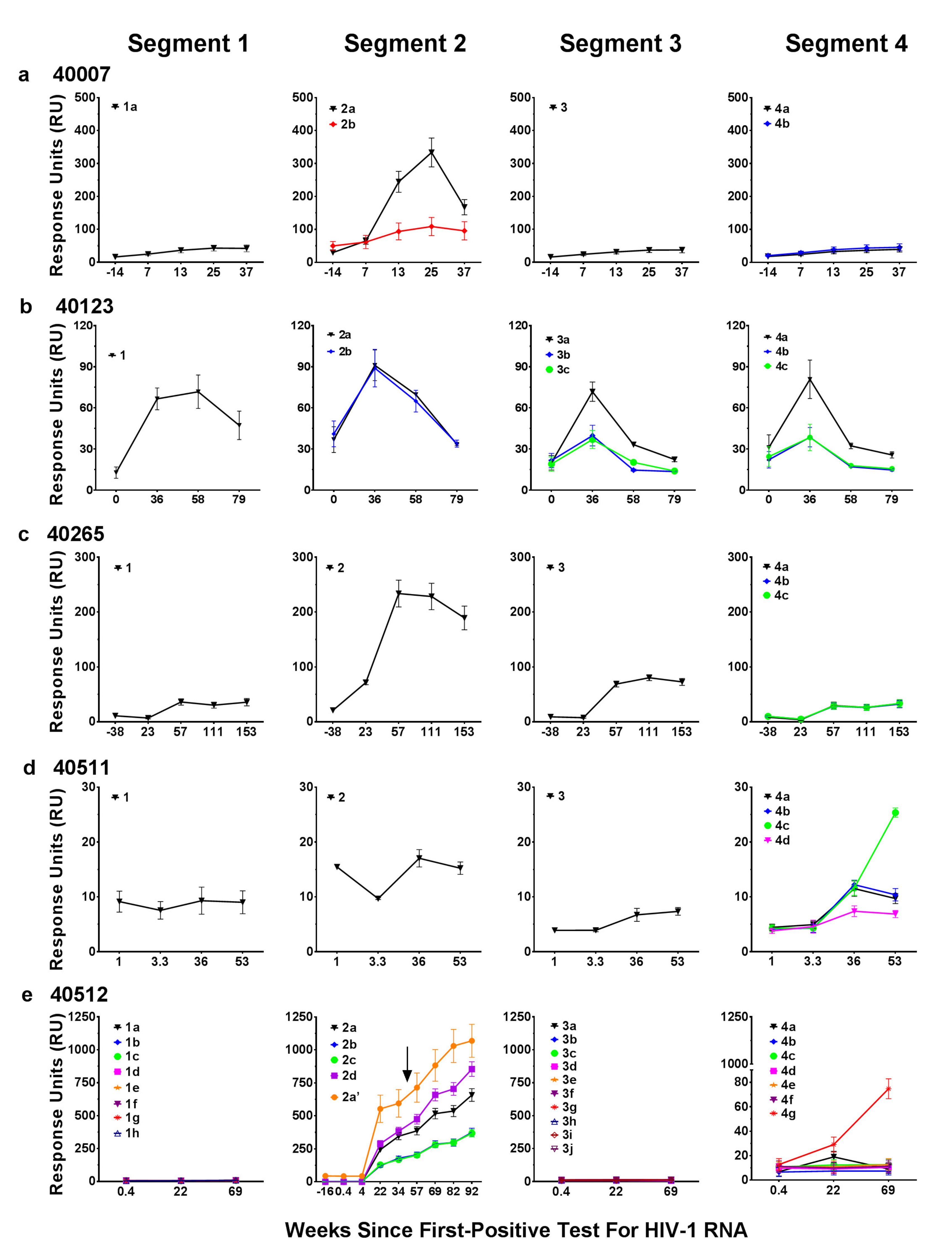
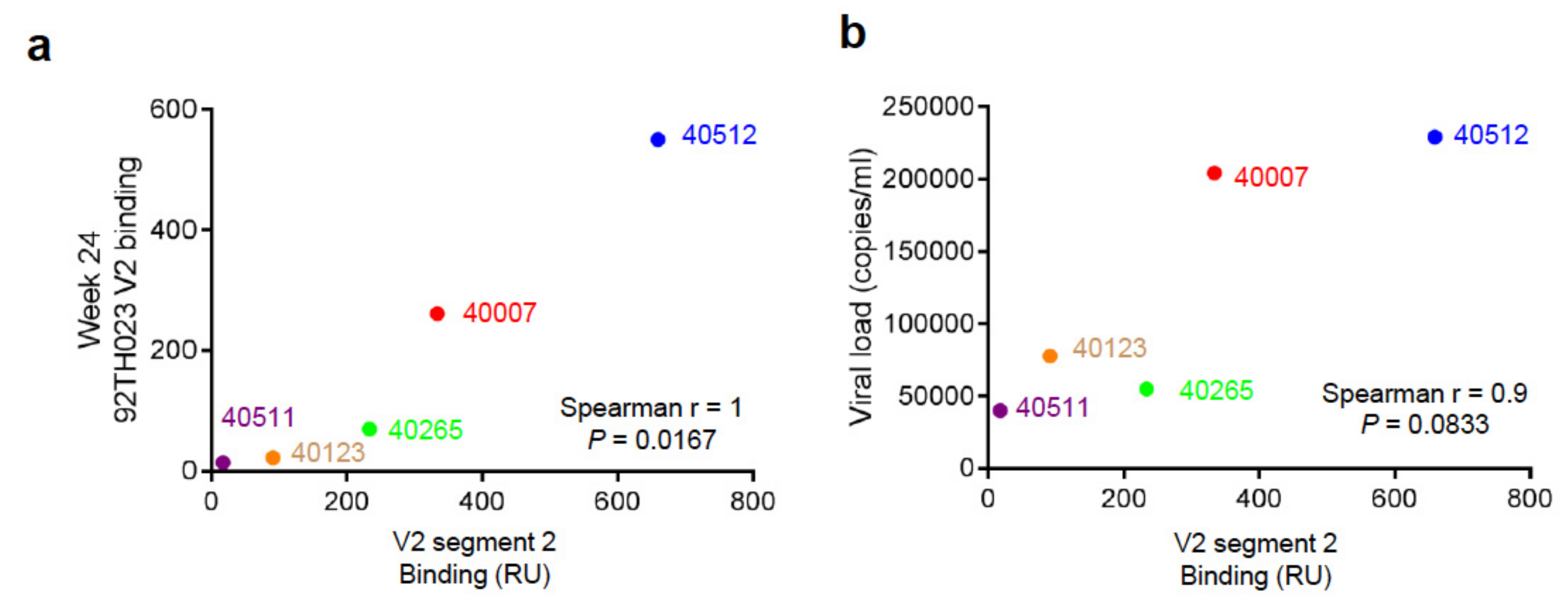
3.4. Binding Responses to V2 Region Peptides
3.5. Structure Modeling of the HIV-1 V2 Region
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Disclaimer
References
- Rerks-Ngarm, S.; Pitisuttithum, P.; Nitayaphan, S.; Kaewkungwal, J.; Chiu, J.; Paris, R.; Premsri, N.; Namwat, C.; de Souza, M.; Adams, E.; et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N. Engl. J. Med. 2009, 361, 2209–2220. [Google Scholar] [CrossRef]
- Robb, M.L.; Rerks-Ngarm, S.; Nitayaphan, S.; Pitisuttithum, P.; Kaewkungwal, J.; Kunasol, P.; Khamboonruang, C.; Thongcharoen, P.; Morgan, P.; Benenson, M.; et al. Risk behaviour and time as covariates for efficacy of the HIV vaccine regimen ALVAC-HIV (vCP1521) and AIDSVAX B/E:a post-hoc analysis of the Thai phase 3 efficacy trial RV 144. Lancet Infect. Dis. 2012, 12, 531–537. [Google Scholar] [CrossRef]
- Haynes, B.F.; Gilbert, P.B.; McElrath, M.J.; Zolla-Pazner, S.; Tomaras, G.D.; Alam, S.M.; Evans, D.T.; Montefiori, D.C.; Karnasuta, C.; Sutthent, R.; et al. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N. Engl. J. Med. 2012, 366, 1275–1286. [Google Scholar] [CrossRef] [PubMed]
- Pazner, S.; deCamp, A.; Gilbert, P.B.; Williams, C.; Yates, N.L.; Williams, W.T.; Howington, R.; Fong, Y.; Morris, D.E.; Soderberg, K.A.; et al. Vaccine-induced IgG antibodies to V1V2 regions of multiple HIV-1 subtypes correlate with decreased risk of HIV-1 infection. PLOS ONE 2014, 9, e87572. [Google Scholar] [CrossRef]
- Rolland, M.; Edlefsen, P.T.; Larsen, B.B.; Tovanabutra, S.; Sanders-Buell, E.; Hertz, T.; deCamp, A.C.; Carrico, C.; Menis, S.; Magaret, C.A.; et al. Increased HIV-1 vaccine efficacy against viruses with genetic signatures in Env V2. Nature 2012, 490, 417–420. [Google Scholar] [CrossRef] [PubMed]
- Karasavvas, N.; Billings, E.; Rao, M.; Williams, C.; Zolla-Pazner, S.; Bailer, R.T.; Koup, R.A.; Madnote, S.; Arworn, D.; Shen, X.; et al. The Thai Phase III HIV Type 1 Vaccine trial (RV144) regimen induces antibodies that target conserved regions within the V2 loop of gp120. AIDS Res. Hum. Retroviruses 2012, 28, 1444–1457. [Google Scholar] [CrossRef]
- Derdeyn, C.A.; Decker, J.M.; Bibollet-Ruche, F.; Mokili, J.L.; Muldoon, M.; Denham, S.A.; Heil, M.L.; Kasolo, F.; Musonda, R.; Hahn, B.H.; et al. Envelope-constrained neutralization-sensitive HIV-1 after heterosexual transmission. Science 2004, 303, 2019–2022. [Google Scholar] [CrossRef] [PubMed]
- Chohan, B.; Lang, D.; Sagar, M.; Korber, B.; Lavreys, L.; Richardson, B.; Overbaugh, J. Selection for human immunodeficiency virus type 1 envelope glycosylation variants with shorter V1-V2 loop sequences occurs during transmission of certain genetic subtypes and may impact viral RNA levels. J. Virol. 2005, 79, 6528–6531. [Google Scholar] [CrossRef]
- Sagar, M.; Wu, X.; Lee, S.; Overbaugh, J. Human immunodeficiency virus type 1 V1-V2 envelope loop sequences expand and add glycosylation sites over the course of infection, and these modifications affect antibody neutralization sensitivity. J. Virol. 2006, 80, 9586–9598. [Google Scholar] [CrossRef] [PubMed]
- Zolla-Pazner, S.; Cardozo, T. Structure-function relationships of HIV-1 envelope sequence-variable regions refocus vaccine design. Nat. Rev. Immunol. 2010, 10, 527–535. [Google Scholar] [CrossRef] [PubMed]
- O’Rourke, S.M.; Sutthent, R.; Phung, P.; Mesa, K.A.; Frigon, N.L.; To, B.; Horthongkham, N.; Limoli, K.; Wrin, T.; Berman, P.W. Glycans flanking the hypervariable connecting peptide between the A and B strands of the V1/V2 domain of HIV-1 gp120 confer resistance to antibodies that neutralize CRF01_AE viruses. PLoS ONE 2015, 10, e0119608. [Google Scholar] [CrossRef]
- Smith, S.A.; Burton, S.L.; Kilembe, W.; Lakhi, S.; Karita, E.; Price, M.; Allen, S.; Hunter, E.; Derdeyn, C.A. Diversification in the HIV-1 Envelope Hyper-variable Domains V2, V4, and V5 and Higher Probability of Transmitted/Founder Envelope Glycosylation Favor the Development of Heterologous Neutralization Breadth. PLoS Pathog. 2016, 12, e1005989. [Google Scholar] [CrossRef]
- Ashokkumar, M.; Nesakumar, M.; Cheedarla, N.; Vidyavijayan, K.K.; Babu, H.; Tripathy, S.P.; Hanna, L.E. Molecular Characteristics of the Envelope of Vertically Transmitted HIV-1 Strains from Infants with HIV Infection. AIDS Res. Hum. Retrovir. 2017, 33, 796–806. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Ma, L.; Feng, Y.; Hu, J.; Ni, N.; Ruan, Y.; Shao, Y. Generation and Characterization of HIV-1 Transmitted and Founder Virus Consensus Sequence from Intravenous Drug Users in Xinjiang, China. AIDS Res. Hum. Retrovir. 2017, 33, 610–613. [Google Scholar] [CrossRef]
- Wibmer, C.K.; Richardson, S.I.; Yolitz, J.; Cicala, C.; Arthos, J.; Moore, P.L.; Morris, L. Common helical V1V2 conformations of HIV-1 Envelope expose the alpha4beta7 binding site on intact virions. Nat. Commun. 2018, 9, 4489. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.D.; Pancera, M.; Acharya, P.; Georgiev, I.S.; Crooks, E.T.; Gorman, J.; Joyce, M.G.; Guttman, M.; Ma, X.; Narpala, S.; et al. Crystal structure, conformational fixation and entry-related interactions of mature ligand-free HIV-1 Env. Nat. Struct. Mol. Biol. 2015, 22, 522–531. [Google Scholar] [CrossRef]
- Lyumkis, D.; Julien, J.P.; de Val, N.; Cupo, A.; Potter, C.S.; Klasse, P.J.; Burton, D.R.; Sanders, R.W.; Moore, J.P.; Carragher, B.; et al. Cryo-EM structure of a fully glycosylated soluble cleaved HIV-1 envelope trimer. Science 2013, 342, 1484–1490. [Google Scholar] [CrossRef]
- Julien, J.P.; Cupo, A.; Sok, D.; Stanfield, R.L.; Lyumkis, D.; Deller, M.C.; Klasse, P.J.; Burton, D.R.; Sanders, R.W.; Moore, J.P.; et al. Crystal structure of a soluble cleaved HIV-1 envelope trimer. Science 2013, 342, 1477–1483. [Google Scholar] [CrossRef] [PubMed]
- McLellan, J.S.; Pancera, M.; Carrico, C.; Gorman, J.; Julien, J.P.; Khayat, R.; Louder, R.; Pejchal, R.; Sastry, M.; Dai, K.; et al. Structure of HIV-1 gp120 V1/V2 domain with broadly neutralizing antibody PG9. Nature 2011, 480, 336–343. [Google Scholar] [CrossRef]
- Gorman, J.; Soto, C.; Yang, M.M.; Davenport, T.M.; Guttman, M.; Bailer, R.T.; Chambers, M.; Chuang, G.Y.; DeKosky, B.J.; Doria-Rose, N.A.; et al. Structures of HIV-1 Env V1V2 with broadly neutralizing antibodies reveal commonalities that enable vaccine design. Nat. Struct. Mol. Biol. 2016, 23, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Cale, E.M.; Gorman, J.; Radakovich, N.A.; Crooks, E.T.; Osawa, K.; Tong, T.; Li, J.; Nagarajan, R.; Ozorowski, G.; Ambrozak, D.R.; et al. Virus-like Particles Identify an HIV V1V2 Apex-Binding Neutralizing Antibody that Lacks a Protruding Loop. Immunity 2017, 46, 777–791.e10. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Gristick, H.B.; Scharf, L.; West, A.P.; Galimidi, R.P.; Seaman, M.S.; Freund, N.T.; Nussenzweig, M.C.; Bjorkman, P.J. Asymmetric recognition of HIV-1 Envelope trimer by V1V2 loop-targeting antibodies. eLife 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Pancera, M.; Zhou, T.; Druz, A.; Georgiev, I.S.; Soto, C.; Gorman, J.; Huang, J.; Acharya, P.; Chuang, G.Y.; Ofek, G.; et al. Structure and immune recognition of trimeric pre-fusion HIV-1 Env. Nature 2014, 514, 455–461. [Google Scholar] [CrossRef]
- Wang, H.; Cohen, A.A.; Galimidi, R.P.; Gristick, H.B.; Jensen, G.J.; Bjorkman, P.J. Cryo-EM structure of a CD4-bound open HIV-1 envelope trimer reveals structural rearrangements of the gp120 V1V2 loop. Proc. Natl. Acad. Sci. USA 2016, 113, E7151–E7158. [Google Scholar] [CrossRef]
- Wang, H.; Barnes, C.O.; Yang, Z.; Nussenzweig, M.C.; Bjorkman, P.J. Partially Open HIV-1 Envelope Structures Exhibit Conformational Changes Relevant for Coreceptor Binding and Fusion. Cell Host Microbe 2018, 24, 579–592. [Google Scholar] [CrossRef] [PubMed]
- Pancera, M.; Shahzad-Ul-Hussan, S.; Doria-Rose, N.A.; McLellan, J.S.; Bailer, R.T.; Dai, K.; Loesgen, S.; Louder, M.K.; Staupe, R.P.; Yang, Y.; et al. Structural basis for diverse N-glycan recognition by HIV-1-neutralizing V1-V2-directed antibody PG16. Nat. Struct. Mol. Biol. 2013, 20, 804–813. [Google Scholar] [CrossRef]
- Lee, J.H.; Andrabi, R.; Su, C.Y.; Yasmeen, A.; Julien, J.P.; Kong, L.; Wu, N.C.; McBride, R.; Sok, D.; Pauthner, M.; et al. A Broadly Neutralizing Antibody Targets the Dynamic HIV Envelope Trimer Apex via a Long, Rigidified, and Anionic beta-Hairpin Structure. Immunity 2017, 46, 690–702. [Google Scholar] [CrossRef] [PubMed]
- Gorny, M.K.; Pan, R.; Williams, C.; Wang, X.H.; Volsky, B.; O’Neal, T.; Spurrier, B.; Sampson, J.M.; Li, L.; Seaman, M.S.; et al. Functional and immunochemical cross-reactivity of V2-specific monoclonal antibodies from HIV-1-infected individuals. Virology 2012, 427, 198–207. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liao, H.X.; Bonsignori, M.; Alam, S.M.; McLellan, J.S.; Tomaras, G.D.; Moody, M.A.; Kozink, D.M.; Hwang, K.K.; Chen, X.; Tsao, C.Y.; et al. Vaccine induction of antibodies against a structurally heterogeneous site of immune pressure within HIV-1 envelope protein variable regions 1 and 2. Immunity 2013, 38, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Wiehe, K.; Easterhoff, D.; Luo, K.; Nicely, N.I.; Bradley, T.; Jaeger, F.H.; Dennison, S.M.; Zhang, R.; Lloyd, K.E.; Stolarchuk, C.; et al. Antibody light-chain-restricted recognition of the site of immune pressure in the RV144 HIV-1 vaccine trial is phylogenetically conserved. Immunity 2014, 41, 909–918. [Google Scholar] [CrossRef]
- Aiyegbo, M.S.; Shmelkov, E.; Dominguez, L.; Goger, M.; Battacharya, S.; deCamp, A.C.; Gilbert, P.B.; Berman, P.W.; Cardozo, T. Peptide Targeted by Human Antibodies Associated with HIV Vaccine-Associated Protection Assumes a Dynamic alpha-Helical Structure. PLOS ONE 2017, 12, e0170530. [Google Scholar] [CrossRef] [PubMed]
- Pollara, J.; Bonsignori, M.; Moody, M.A.; Liu, P.; Alam, S.M.; Hwang, K.K.; Gurley, T.C.; Kozink, D.M.; Armand, L.C.; Marshall, D.J.; et al. HIV-1 vaccine-induced C1 and V2 Env-specific antibodies synergize for increased antiviral activities. J. Virol. 2014, 88, 7715–7726. [Google Scholar] [CrossRef]
- Peachman, K.K.; Karasavvas, N.; Chenine, A.L.; McLinden, R.; Rerks-Ngarm, S.; Jaranit, K.; Nitayaphan, S.; Pitisuttithum, P.; Tovanabutra, S.; Zolla-Pazner, S.; et al. Identification of New Regions in HIV-1 gp120 Variable 2 and 3 Loops that Bind to alpha4beta7 Integrin Receptor. PLOS ONE 2015, 10, e0143895. [Google Scholar] [CrossRef][Green Version]
- Robb, M.L.; Eller, L.A.; Kibuuka, H.; Rono, K.; Maganga, L.; Nitayaphan, S.; Kroon, E.; Sawe, F.K.; Sinei, S.; Sriplienchan, S.; et al. Prospective Study of Acute HIV-1 Infection in Adults in East Africa and Thailand. N. Engl. J. Med. 2016, 374, 2120–2130. [Google Scholar] [CrossRef]
- Eller, M.A.; Goonetilleke, N.; Tassaneetrithep, B.; Eller, L.A.; Costanzo, M.C.; Johnson, S.; Betts, M.R.; Krebs, S.J.; Slike, B.M.; Nitayaphan, S.; et al. Expansion of Inefficient HIV-Specific CD8 T Cells during Acute Infection. J. Virol. 2016, 90, 4005–4016. [Google Scholar] [CrossRef]
- Baden, L.R.; Walsh, S.R.; Seaman, M.S.; Cohen, Y.Z.; Johnson, J.A.; Licona, J.H.; Filter, R.D.; Kleinjan, J.A.; Gothing, J.A.; Jennings, J.; et al. First-in-Human Randomized, Controlled Trial of Mosaic HIV-1 Immunogens Delivered via a Modified Vaccinia Ankara Vector. J. Infect. Dis. 2018, 218, 633–644. [Google Scholar] [CrossRef]
- Rao, M.; Onkar, S.; Peachman, K.K.; White, Y.; Trinh, H.V.; Jobe, O.; Zhou, Y.; Dawson, P.; Eller, M.A.; Matyas, G.R.; et al. Liposome-Encapsulated Human Immunodeficiency Virus-1 gp120 Induces Potent V1V2-Specific Antibodies in Humans. J. Infect. Dis. 2018, 218, 1541–1550. [Google Scholar] [CrossRef]
- Vaccari, M.; Fourati, S.; Gordon, S.N.; Brown, D.R.; Bissa, M.; Schifanella, L.; Silva de Castro, I.; Doster, M.N.; Galli, V.; Omsland, M.; et al. HIV vaccine candidate activation of hypoxia and the inflammasome in CD14(+) monocytes is associated with a decreased risk of SIVmac251 acquisition. Nat. Med. 2018, 24, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Trinh, H.V.; Linton, C.E.; Tani, C.; Norais, N.; Martinez-Guzman, D.; Ramesh, P.; Sun, Y.; Situ, F.; Karaca-Griffin, S.; et al. Generation and characterization of a bivalent protein boost for future clinical trials: HIV-1 subtypes CR01_AE and B gp120 antigens with a potent adjuvant. PLOS ONE 2018, 13, e0194266. [Google Scholar] [CrossRef]
- Singh, S.; Ramirez-Salazar, E.G.; Doueiri, R.; Valentin, A.; Rosati, M.; Hu, X.; Keele, B.F.; Shen, X.; Tomaras, G.D.; Ferrari, G.; et al. Control of Heterologous Simian Immunodeficiency Virus SIVsmE660 Infection by DNA and Protein Coimmunization Regimens Combined with Different Toll-Like-Receptor-4-Based Adjuvants in Macaques. J. Virol. 2018, 92. [Google Scholar] [CrossRef] [PubMed]
- Kijak, G.H.; Sanders-Buell, E.; Chenine, A.L.; Eller, M.A.; Goonetilleke, N.; Thomas, R.; Leviyang, S.; Harbolick, E.A.; Bose, M.; Pham, P.; et al. Rare HIV-1 transmitted/founder lineages identified by deep viral sequencing contribute to rapid shifts in dominant quasispecies during acute and early infection. PLoS Pathog. 2017, 13, e1006510. [Google Scholar] [CrossRef]
- Salazar-Gonzalez, J.F.; Bailes, E.; Pham, K.T.; Salazar, M.G.; Guffey, M.B.; Keele, B.F.; Derdeyn, C.A.; Farmer, P.; Hunter, E.; Allen, S.; et al. Deciphering human immunodeficiency virus type 1 transmission and early envelope diversification by single-genome amplification and sequencing. J. Virol. 2008, 82, 3952–3970. [Google Scholar] [CrossRef] [PubMed]
- Spurrier, B.; Sampson, J.; Gorny, M.K.; Zolla-Pazner, S.; Kong, X.P. Functional implications of the binding mode of a human conformation-dependent V2 monoclonal antibody against HIV. J. Virol. 2014, 88, 4100–4112. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trinh, H.V.; Gohain, N.; Pham, P.T.; Hamlin, C.; Song, H.; Sanders-Buell, E.; Bose, M.; Eller, L.A.; Tovanabutra, S.; Michael, N.L.; et al. Humoral Response to the HIV-1 Envelope V2 Region in a Thai Early Acute Infection Cohort. Cells 2019, 8, 365. https://doi.org/10.3390/cells8040365
Trinh HV, Gohain N, Pham PT, Hamlin C, Song H, Sanders-Buell E, Bose M, Eller LA, Tovanabutra S, Michael NL, et al. Humoral Response to the HIV-1 Envelope V2 Region in a Thai Early Acute Infection Cohort. Cells. 2019; 8(4):365. https://doi.org/10.3390/cells8040365
Chicago/Turabian StyleTrinh, Hung V., Neelakshi Gohain, Peter T. Pham, Christopher Hamlin, Hongshuo Song, Eric Sanders-Buell, Meera Bose, Leigh A. Eller, Sodsai Tovanabutra, Nelson L. Michael, and et al. 2019. "Humoral Response to the HIV-1 Envelope V2 Region in a Thai Early Acute Infection Cohort" Cells 8, no. 4: 365. https://doi.org/10.3390/cells8040365
APA StyleTrinh, H. V., Gohain, N., Pham, P. T., Hamlin, C., Song, H., Sanders-Buell, E., Bose, M., Eller, L. A., Tovanabutra, S., Michael, N. L., Robb, M. L., Joyce, M. G., & Rao, M. (2019). Humoral Response to the HIV-1 Envelope V2 Region in a Thai Early Acute Infection Cohort. Cells, 8(4), 365. https://doi.org/10.3390/cells8040365




