Adult Hippocampal Neurogenesis in Different Taxonomic Groups: Possible Functional Similarities and Striking Controversies
Abstract
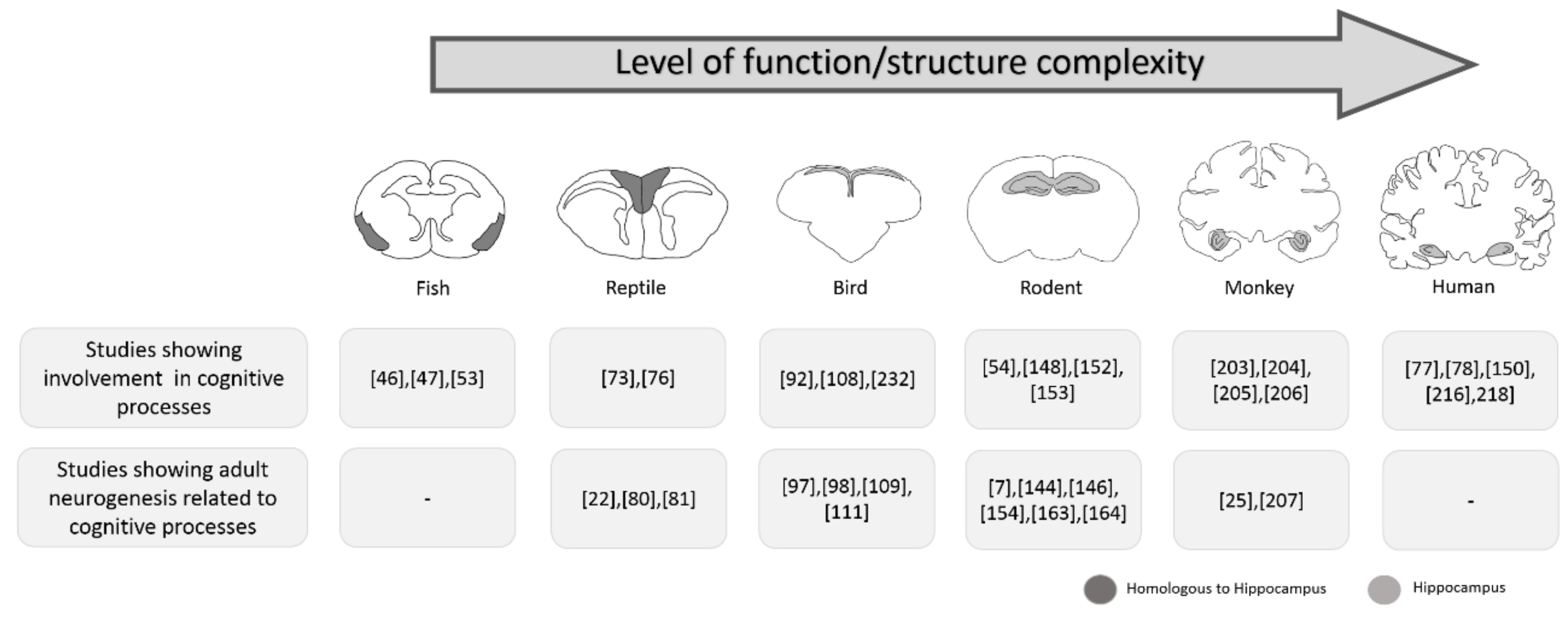
1. Introduction
2. Adult Neurogenesis in Fish
3. Adult Neurogenesis in Reptiles
4. Adult Neurogenesis in Birds
5. Adult Neurogenesis in Rodents
6. Adult Neurogenesis in Nonhuman Primates
7. Adult Neurogenesis in Humans
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Altman, J. Autoradiographic study of degenerative and regenerative proliferation of neuroglia cells with tritiated thymidine. Exp. Neurol. 1962, 5, 302–318. [Google Scholar] [CrossRef]
- Altman, J.; Das, G.D. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J. Comp. Neurol. 1965, 124, 319–335. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, M.S.; Hinds, J.W. Neurogenesis in the adult rat: Electron microscopic analysis of light radioautographs. Science 1977, 197, 1092–1094. [Google Scholar] [CrossRef] [PubMed]
- Goldman, S.A.; Nottebohm, F. Neuronal production, migration, and differentiation in a vocal control nucleus of the adult female canary brain. Proc. Natl. Acad. Sci. USA 1983, 80, 2390–2394. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, B.A.; Weiss, S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science 1992, 255, 1707–1710. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, B.M.; Moreira, F.A.; Massensini, A.R.; Moraes, M.F.; Pereira, G.S. Enriched environment increases neurogenesis and improves social memory persistence in socially isolated adult mice. Hippocampus 2014, 24, 239–248. [Google Scholar] [CrossRef] [PubMed]
- van Praag, H.; Kempermann, G.; Gage, F.H. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat. Neurosci. 1999, 2, 266–270. [Google Scholar] [CrossRef]
- Ma, C.L.; Ma, X.T.; Wang, J.J.; Liu, H.; Chen, Y.F.; Yang, Y. Physical exercise induces hippocampal neurogenesis and prevents cognitive decline. Behav. Brain Res. 2017, 317, 332–339. [Google Scholar] [CrossRef]
- Schoenfeld, T.J.; McCausland, H.C.; Morris, H.D.; Padmanaban, V.; Cameron, H.A. Stress and Loss of Adult Neurogenesis Differentially Reduce Hippocampal Volume. Biol. Psychiatry 2017, 82, 914–923. [Google Scholar] [CrossRef]
- Villeda, S.A.; Luo, J.; Mosher, K.I.; Zou, B.; Britschgi, M.; Bieri, G.; Stan, T.M.; Fainberg, N.; Ding, Z.; Eggel, A.; et al. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature 2011, 477, 90–94. [Google Scholar] [CrossRef]
- Ekdahl, C.T.; Claasen, J.H.; Bonde, S.; Kokaia, Z.; Lindvall, O. Inflammation is detrimental for neurogenesis in adult brain. Proc. Natl. Acad. Sci. USA 2003, 100, 13632–13637. [Google Scholar] [CrossRef]
- Fernandes, C.; Rocha, N.B.; Rocha, S.; Herrera-Solis, A.; Salas-Pacheco, J.; Garcia-Garcia, F.; Murillo-Rodriguez, E.; Yuan, T.F.; Machado, S.; Arias-Carrion, O. Detrimental role of prolonged sleep deprivation on adult neurogenesis. Front. Cell. Neurosci. 2015, 9, 140. [Google Scholar] [CrossRef] [PubMed]
- Kempermann, G. What the Bomb Said About the Brain. Science 2013, 340, 1180–1181. [Google Scholar] [CrossRef] [PubMed]
- Vivar, C.; van Praag, H. Functional circuits of new neurons in the dentate gyrus. Front. Neural Circuits 2013, 7, 15. [Google Scholar] [CrossRef] [PubMed]
- Grandel, H.; Kaslin, J.; Ganz, J.; Wenzel, I.; Brand, M. Neural stem cells and neurogenesis in the adult zebrafish brain: Origin, proliferation dynamics, migration and cell fate. Dev. Biol. 2006, 295, 263–277. [Google Scholar] [CrossRef] [PubMed]
- Maruska, K.P.; Carpenter, R.E.; Fernald, R.D. Characterization of cell proliferation throughout the brain of the African cichlid fish Astatotilapia burtoni and its regulation by social status. J. Comp. Neurol. 2012, 520, 3471–3491. [Google Scholar] [CrossRef] [PubMed]
- Ernst, A.; Frisen, J. Adult neurogenesis in humans- common and unique traits in mammals. PLoS Biol. 2015, 13, e1002045. [Google Scholar] [CrossRef]
- Paredes, M.F.; Sorrells, S.F.; Garcia-Verdugo, J.M.; Alvarez-Buylla, A. Brain size and limits to adult neurogenesis. J. Comp. Neurol. 2016, 524, 646–664. [Google Scholar] [CrossRef]
- Amrein, I.; Lipp, H.P. Adult hippocampal neurogenesis of mammals: Evolution and life history. Biol. Lett. 2009, 5, 141–144. [Google Scholar] [CrossRef]
- Lieberwirth, C.; Pan, Y.; Liu, Y.; Zhang, Z.; Wang, Z. Hippocampal adult neurogenesis: Its regulation and potential role in spatial learning and memory. Brain Res. 2016, 1644, 127–140. [Google Scholar] [CrossRef]
- Zupanc, G.K.; Hinsch, K.; Gage, F.H. Proliferation, migration, neuronal differentiation, and long-term survival of new cells in the adult zebrafish brain. J. Comp. Neurol. 2005, 488, 290–319. [Google Scholar] [CrossRef] [PubMed]
- LaDage, L.D.; Maged, R.M.; Forney, M.V.; Roth, T.C., 2nd; Sinervo, B.; Pravosudov, V.V. Interaction between territoriality, spatial environment, and hippocampal neurogenesis in male side-blotched lizards. Behav. Neurosci. 2013, 127, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Meskenaite, V.; Krackow, S.; Lipp, H.P. Age-Dependent Neurogenesis and Neuron Numbers within the Olfactory Bulb and Hippocampus of Homing Pigeons. Front. Behav. Neurosci. 2016, 10, 126. [Google Scholar] [CrossRef] [PubMed]
- Kornack, D.R.; Rakic, P. Continuation of neurogenesis in the hippocampus of the adult macaque monkey. Proc. Natl. Acad. Sci. USA 1999, 96, 5768–5773. [Google Scholar] [CrossRef] [PubMed]
- Ngwenya, L.B.; Heyworth, N.C.; Shwe, Y.; Moore, T.L.; Rosene, D.L. Age-related changes in dentate gyrus cell numbers, neurogenesis, and associations with cognitive impairments in the rhesus monkey. Front. Syst. Neurosci. 2015, 9, 102. [Google Scholar] [CrossRef] [PubMed]
- Boldrini, M.; Fulmore, C.A.; Tartt, A.N.; Simeon, L.R.; Pavlova, I.; Poposka, V.; Rosoklija, G.B.; Stankov, A.; Arango, V.; Dwork, A.J.; et al. Human Hippocampal Neurogenesis Persists throughout Aging. Cell Stem Cell 2018, 22, 589–599. [Google Scholar] [CrossRef] [PubMed]
- Spalding, K.L.; Bergmann, O.; Alkass, K.; Bernard, S.; Salehpour, M.; Huttner, H.B.; Bostrom, E.; Westerlund, I.; Vial, C.; Buchholz, B.A.; et al. Dynamics of hippocampal neurogenesis in adult humans. Cell 2013, 153, 1219–1227. [Google Scholar] [CrossRef]
- Amrein, I.; Dechmann, D.K.; Winter, Y.; Lipp, H.P. Absent or low rate of adult neurogenesis in the hippocampus of bats (Chiroptera). PLoS ONE 2007, 2, e455. [Google Scholar] [CrossRef]
- Patzke, N.; Spocter, M.A.; Karlsson, K.A.E.; Bertelsen, M.F.; Haagensen, M.; Chawana, R.; Streicher, S.; Kaswera, C.; Gilissen, E.; Alagaili, A.N.; et al. In contrast to many other mammals, cetaceans have relatively small hippocampi that appear to lack adult neurogenesis. Brain Struct. Funct. 2015, 220, 361–383. [Google Scholar] [CrossRef]
- Sorrells, S.F.; Paredes, M.F.; Cebrian-Silla, A.; Sandoval, K.; Qi, D.; Kelley, K.W.; James, D.; Mayer, S.; Chang, J.; Auguste, K.I.; et al. Human hippocampal neurogenesis drops sharply in children to undetectable levels in adults. Nature 2018, 555, 377–381. [Google Scholar] [CrossRef]
- Olivera-Pasilio, V.; Peterson, D.A.; Castello, M.E. Spatial distribution and cellular composition of adult brain proliferative zones in the teleost, Gymnotus omarorum. Front. Neuroanat. 2014, 8, 88. [Google Scholar] [CrossRef] [PubMed]
- Ekstrom, P.; Johnsson, C.M.; Ohlin, L.M. Ventricular proliferation zones in the brain of an adult teleost fish and their relation to neuromeres and migration (secondary matrix) zones. J. Comp. Neurol. 2001, 436, 92–110. [Google Scholar] [CrossRef] [PubMed]
- Zupanc, G.K. Adult neurogenesis and neuronal regeneration in the brain of teleost fish. J. Physiol. Paris 2008, 102, 357–373. [Google Scholar] [CrossRef] [PubMed]
- Kuroyanagi, Y.; Okuyama, T.; Suehiro, Y.; Imada, H.; Shimada, A.; Naruse, K.; Takeda, H.; Kubo, T.; Takeuchi, H. Proliferation zones in adult medaka (Oryzias latipes) brain. Brain Res. 2010, 1323, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Sirbulescu, R.F.; Zupanc, G.K. Spinal cord repair in regeneration-competent vertebrates: Adult teleost fish as a model system. Brain Res. Rev. 2011, 67, 73–93. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, E.; Mouriec, K.; Anglade, I.; Menuet, A.; Le Page, Y.; Gueguen, M.M.; Marmignon, M.H.; Brion, F.; Pakdel, F.; Kah, O. Identification of aromatase-positive radial glial cells as progenitor cells in the ventricular layer of the forebrain in zebrafish. J. Comp. Neurol. 2007, 501, 150–167. [Google Scholar] [CrossRef] [PubMed]
- Ganz, J.; Kaslin, J.; Hochmann, S.; Freudenreich, D.; Brand, M. Heterogeneity and Fgf dependence of adult neural progenitors in the zebrafish telencephalon. Glia 2010, 58, 1345–1363. [Google Scholar] [CrossRef]
- Marz, M.; Chapouton, P.; Diotel, N.; Vaillant, C.; Hesl, B.; Takamiya, M.; Lam, C.S.; Kah, O.; Bally-Cuif, L.; Strahle, U. Heterogeneity in progenitor cell subtypes in the ventricular zone of the zebrafish adult telencephalon. Glia 2010, 58, 870–888. [Google Scholar] [CrossRef]
- Hinsch, K.; Zupanc, G.K. Generation and long-term persistence of new neurons in the adult zebrafish brain: A quantitative analysis. Neuroscience 2007, 146, 679–696. [Google Scholar] [CrossRef]
- Cameron, H.A.; McKay, R.D. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J. Comp. Neurol. 2001, 435, 406–417. [Google Scholar] [CrossRef]
- Herculano-Houzel, S.; Lent, R. Isotropic fractionator: A simple, rapid method for the quantification of total cell and neuron numbers in the brain. J. Neurosci. 2005, 25, 2518–2521. [Google Scholar] [CrossRef]
- Kaslin, J.; Kroehne, V.; Benato, F.; Argenton, F.; Brand, M. Development and specification of cerebellar stem and progenitor cells in zebrafish: From embryo to adult. Neural Dev. 2013, 8, 9. [Google Scholar] [CrossRef] [PubMed]
- Duran, E.; Ocana, F.M.; Broglio, C.; Rodriguez, F.; Salas, C. Lateral but not medial telencephalic pallium ablation impairs the use of goldfish spatial allocentric strategies in a “hole-board” task. Behav. Brain Res. 2010, 214, 480–487. [Google Scholar] [CrossRef]
- Broglio, C.; Rodriguez, F.; Gomez, A.; Arias, J.L.; Salas, C. Selective involvement of the goldfish lateral pallium in spatial memory. Behav. Brain Res. 2010, 210, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Exposito, B.; Gomez, A.; Martin-Monzon, I.; Reiriz, M.; Rodriguez, F.; Salas, C. Goldfish hippocampal pallium is essential to associate temporally discontiguous events. Neurobiol. Learn. Mem. 2017, 139, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Uceda, S.; Ocana, F.M.; Martin-Monzon, I.; Rodriguez-Exposito, B.; Duran, E.; Rodriguez, F. Spatial learning-related changes in metabolic brain activity contribute to the delimitation of the hippocampal pallium in goldfish. Behav. Brain Res. 2015, 292, 403–408. [Google Scholar] [CrossRef]
- Ocana, F.M.; Uceda, S.; Arias, J.L.; Salas, C.; Rodriguez, F. Dynamics of Goldfish Subregional Hippocampal Pallium Activity throughout Spatial Memory Formation. Brain Behav. Evol. 2017, 90, 154–170. [Google Scholar] [CrossRef]
- Ganz, J.; Kroehne, V.; Freudenreich, D.; Machate, A.; Geffarth, M.; Braasch, I.; Kaslin, J.; Brand, M. Subdivisions of the adult zebrafish pallium based on molecular marker analysis. F1000Research 2014, 3, 308. [Google Scholar] [CrossRef]
- Sison, M.; Gerlai, R. Associative learning in zebrafish (Danio rerio) in the plus maze. Behav. Brain Res. 2010, 207, 99–104. [Google Scholar] [CrossRef]
- Pittman, J.T.; Lott, C.S. Startle response memory and hippocampal changes in adult zebrafish pharmacologically-induced to exhibit anxiety/depression-like behaviors. Physiol. Behav. 2014, 123, 174–179. [Google Scholar] [CrossRef]
- Vargas, R.; Thorsteinsson, H.; Karlsson, K.A. Spontaneous neural activity of the anterodorsal lobe and entopeduncular nucleus in adult zebrafish: A putative homologue of hippocampal sharp waves. Behav. Brain Res. 2012, 229, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Burt de Perera, T.; Holbrook, R.I.; Davis, V. The Representation of Three-Dimensional Space in Fish. Front. Behav. Neurosci. 2016, 10, 40. [Google Scholar] [CrossRef] [PubMed]
- Elliott, S.B.; Harvey-Girard, E.; Giassi, A.C.; Maler, L. Hippocampal-like circuitry in the pallium of an electric fish: Possible substrates for recursive pattern separation and completion. J. Comp. Neurol. 2017, 525, 8–46. [Google Scholar] [CrossRef] [PubMed]
- Rolls, E.T. The mechanisms for pattern completion and pattern separation in the hippocampus. Front. Syst. Neurosci. 2013, 7, 74. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, R.; Strahle, U.; Scholpp, S. Neurogenesis in zebrafish - from embryo to adult. Neural Dev. 2013, 8, 3. [Google Scholar] [CrossRef]
- Sandquist, E.J.; Essner, J.J.; Sakaguchi, D.S. Xenotransplantation of adult hippocampal neural progenitors into the developing zebrafish for assessment of stem cell plasticity. PLoS ONE 2018, 13, e0198025. [Google Scholar] [CrossRef] [PubMed]
- Font, E.; Desfilis, E.; Perez-Canellas, M.M.; Garcia-Verdugo, J.M. Neurogenesis and neuronal regeneration in the adult reptilian brain. Brain Behav. Evol. 2001, 58, 276–295. [Google Scholar] [CrossRef]
- Perez-Canellas, M.M.; Font, E.; Garcia-Verdugo, J.M. Postnatal neurogenesis in the telencephalon of turtles: Evidence for nonradial migration of new neurons from distant proliferative ventricular zones to the olfactory bulbs. Brain Res. Dev. Brain Res. 1997, 101, 125–137. [Google Scholar] [CrossRef]
- Font, E.; Desfilis, E.; Perez-Canellas, M.; Alcantara, S.; Garcia-Verdugo, J.M. 3-Acetylpyridine-induced degeneration and regeneration in the adult lizard brain: A qualitative and quantitative analysis. Brain Res. 1997, 754, 245–259. [Google Scholar] [CrossRef]
- Lopez-Garcia, C.; Molowny, A.; Garcia-Verdugo, J.M.; Ferrer, I. Delayed postnatal neurogenesis in the cerebral cortex of lizards. Brain Res. 1988, 471, 167–174. [Google Scholar] [CrossRef]
- Lopez-Garcia, C.; Molowny, A.; Garcia-Verdugo, J.M.; Martinez-Guijarro, F.J.; Bernabeu, A. Late generated neurons in the medial cortex of adult lizards send axons that reach the Timm-reactive zones. Brain Res. Dev. Brain Res. 1990, 57, 249–254. [Google Scholar] [CrossRef]
- Perez-Sanchez, F.; Molowny, A.; Garcia-Verdugo, J.M.; Lopez-Garcia, C. Postnatal neurogenesis in the nucleus sphericus of the lizard, Podarcis hispanica. Neurosci. Lett. 1989, 106, 71–75. [Google Scholar] [CrossRef]
- Garcia-Verdugo, J.M.; Llahi, S.; Ferrer, I.; Lopez-Garcia, C. Postnatal neurogenesis in the olfactory bulbs of a lizard. A tritiated thymidine autoradiographic study. Neurosci. Lett. 1989, 98, 247–252. [Google Scholar] [CrossRef]
- Perez-Canellas, M.M.; Garcia-Verdugo, J.M. Adult neurogenesis in the telencephalon of a lizard: A [3H]thymidine autoradiographic and bromodeoxyuridine immunocytochemical study. Brain Res. Dev. Brain Res. 1996, 93, 49–61. [Google Scholar] [CrossRef]
- Lopez-Garcia, C.; Molowny, A.; Nacher, J.; Ponsoda, X.; Sancho-Bielsa, F.; Alonso-Llosa, G. The lizard cerebral cortex as a model to study neuronal regeneration. An. Acad. Bras. Cienc. 2002, 74, 85–104. [Google Scholar] [CrossRef] [PubMed]
- Ngwenya, A.; Patzke, N.; Herculano-Houzel, S.; Manger, P.R. Potential Adult Neurogenesis in the Telencephalon and Cerebellar Cortex of the Nile Crocodile Revealed with Doublecortin Immunohistochemistry. Anat. Rec. 2018, 301, 659–672. [Google Scholar] [CrossRef]
- Marchioro, M.; Nunes, J.M.; Ramalho, A.M.; Molowny, A.; Perez-Martinez, E.; Ponsoda, X.; Lopez-Garcia, C. Postnatal neurogenesis in the medial cortex of the tropical lizard Tropidurus hispidus. Neuroscience 2005, 134, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Luis de la Iglesia, J.A.; Lopez-Garcia, C. A Golgi study of the principal projection neurons of the medial cortex of the lizard Podarcis hispanica. J. Comp. Neurol. 1997, 385, 528–564. [Google Scholar] [CrossRef]
- de la Iglesia, J.A.; Martinez-Guijarro, F.I.; Lopez-Garcia, C. Neurons of the medial cortex outer plexiform layer of the lizard Podarcis hispanica: Golgi and immunocytochemical studies. J. Comp. Neurol. 1994, 341, 184–203. [Google Scholar] [CrossRef]
- Srivastava, U.C.; Maurya, R.C.; Chand, P. Cyto-architecture and neuronal types of the dorsomedial cerebral cortex of the common Indian wall lizard, Hemidactylus flaviviridis. Arch. Ital. Biol. 2009, 147, 21–35. [Google Scholar]
- Ladage, L.D.; Roth, T.C.; Cerjanic, A.M.; Sinervo, B.; Pravosudov, V.V. Spatial memory: Are lizards really deficient? Biol. Lett. 2012, 8, 939–941. [Google Scholar] [CrossRef] [PubMed]
- Tosches, M.A.; Yamawaki, T.M.; Naumann, R.K.; Jacobi, A.A.; Tushev, G.; Laurent, G. Evolution of pallium, hippocampus, and cortical cell types revealed by single-cell transcriptomics in reptiles. Science 2018, 360, 881–888. [Google Scholar] [CrossRef] [PubMed]
- Lopez, J.C.; Vargas, J.P.; Gomez, Y.; Salas, C. Spatial and non-spatial learning in turtles: The role of medial cortex. Behav. Brain Res. 2003, 143, 109–120. [Google Scholar] [CrossRef]
- Bailey, D.J.; Wade, J.; Saldanha, C.J. Hippocampal lesions impair spatial memory performance, but not song—A developmental study of independent memory systems in the zebra finch. Dev. Neurobiol. 2009, 69, 491–504. [Google Scholar] [CrossRef] [PubMed]
- Broadbent, N.J.; Squire, L.R.; Clark, R.E. Reversible hippocampal lesions disrupt water maze performance during both recent and remote memory tests. Learn. Mem. 2006, 13, 187–191. [Google Scholar] [CrossRef]
- Holding, M.L.; Frazier, J.A.; Taylor, E.N.; Strand, C.R. Experimentally altered navigational demands induce changes in the cortical forebrain of free-ranging northern pacific rattlesnakes (Crotalus o. oreganus). Brain Behav. Evol. 2012, 79, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Maguire, E.A.; Nannery, R.; Spiers, H.J. Navigation around London by a taxi driver with bilateral hippocampal lesions. Brain 2006, 129, 2894–2907. [Google Scholar] [CrossRef]
- Maguire, E.A.; Woollett, K.; Spiers, H.J. London taxi drivers and bus drivers: A structural MRI and neuropsychological analysis. Hippocampus 2006, 16, 1091–1101. [Google Scholar] [CrossRef]
- Macedo-Lima, M.; Freire, M.A.; de Carvalho Pimentel, H.; Rodrigues Ferreira Lins, L.C.; Amador de Lucena Medeiros, K.A.; Viola, G.G.; Dos Santos, J.R.; Marchioro, M. Characterization of NADPH Diaphorase- and Doublecortin-Positive Neurons in the Lizard Hippocampal Formation. Brain Behav. Evol. 2016, 88, 222–234. [Google Scholar] [CrossRef]
- Powers, A.S. Plasticity and Adult Neurogenesis in Amphibians and Reptiles: More Questions than Answers. Brain Behav. Evol. 2016, 87, 175–183. [Google Scholar] [CrossRef]
- Delgado-Gonzalez, F.J.; Alonso-Fuentes, A.; Delgado-Fumero, A.; Garcia-Verdugo, J.M.; Gonzalez-Granero, S.; Trujillo-Trujillo, C.M.; Damas-Hernandez, M.C. Seasonal differences in ventricular proliferation of adult Gallotia galloti lizards. Brain Res. 2008, 1191, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Buylla, A.; Theelen, M.; Nottebohm, F. Birth of projection neurons in the higher vocal center of the canary forebrain before, during, and after song learning. Proc. Natl. Acad. Sci. USA 1988, 85, 8722–8726. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Buylla, A.; Theelen, M.; Nottebohm, F. Mapping of radial glia and of a new cell type in adult canary brain. J. Neurosci. 1988, 8, 2707–2712. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Buylla, A.; Nottebohm, F. Migration of young neurons in adult avian brain. Nature 1988, 335, 353–354. [Google Scholar] [CrossRef] [PubMed]
- Brenowitz, E.A.; Larson, T.A. Neurogenesis in the adult avian song-control system. Cold Spring Harb. Perspect. Biol. 2015, 7, a019000. [Google Scholar] [CrossRef] [PubMed]
- de Morais Magalhaes, N.G.; Guerreiro Diniz, C.; Guerreiro Diniz, D.; Pereira Henrique, E.; Correa Pereira, P.D.; Matos Moraes, I.A.; Damasceno de Melo, M.A.; Sherry, D.F.; Wanderley Picanco Diniz, C. Hippocampal neurogenesis and volume in migrating and wintering semipalmated sandpipers (Calidris pusilla). PLoS ONE 2017, 12, e0179134. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Buylla, A.; Theelen, M.; Nottebohm, F. Proliferation “hot spots” in adult avian ventricular zone reveal radial cell division. Neuron 1990, 5, 101–109. [Google Scholar] [CrossRef]
- Alvarez-Buylla, A.; Garcia-Verdugo, J.M.; Mateo, A.S.; Merchant-Larios, H. Primary neural precursors and intermitotic nuclear migration in the ventricular zone of adult canaries. J. Neurosci. 1998, 18, 1020–1037. [Google Scholar] [CrossRef]
- Scott, B.B.; Gardner, T.; Ji, N.; Fee, M.S.; Lois, C. Wandering neuronal migration in the postnatal vertebrate forebrain. J. Neurosci. 2012, 32, 1436–1446. [Google Scholar] [CrossRef]
- Mazengenya, P.; Bhagwandin, A.; Manger, P.R.; Ihunwo, A.O. Putative Adult Neurogenesis in Old World Parrots: The Congo African Grey Parrot (Psittacus erithacus) and Timneh Grey Parrot (Psittacus timneh). Front. Neuroanat. 2018, 12, 7. [Google Scholar] [CrossRef]
- Kirn, J.R.; Fishman, Y.; Sasportas, K.; Alvarez-Buylla, A.; Nottebohm, F. Fate of new neurons in adult canary high vocal center during the first 30 days after their formation. J. Comp. Neurol. 1999, 411, 487–494. [Google Scholar] [CrossRef]
- Sherry, D.F.; Hoshooley, J.S. Seasonal hippocampal plasticity in food-storing birds. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2010, 365, 933–943. [Google Scholar] [CrossRef] [PubMed]
- Gahr, M.; Leitner, S.; Fusani, L.; Rybak, F. What is the adaptive role of neurogenesis in adult birds? Prog. Brain Res. 2002, 138, 233–254. [Google Scholar] [CrossRef] [PubMed]
- Robertson, B.A.; Rathbone, L.; Cirillo, G.; D’Eath, R.B.; Bateson, M.; Boswell, T.; Wilson, P.W.; Dunn, I.C.; Smulders, T.V. Food restriction reduces neurogenesis in the avian hippocampal formation. PLoS ONE 2017, 12, e0189158. [Google Scholar] [CrossRef] [PubMed]
- Barnea, A.; Nottebohm, F. Seasonal recruitment of hippocampal neurons in adult free-ranging black-capped chickadees. Proc. Natl. Acad. Sci. USA 1994, 91, 11217–11221. [Google Scholar] [CrossRef] [PubMed]
- Barkan, S.; Roll, U.; Yom-Tov, Y.; Wassenaar, L.I.; Barnea, A. Possible linkage between neuronal recruitment and flight distance in migratory birds. Sci. Rep. 2016, 6, 21983. [Google Scholar] [CrossRef]
- LaDage, L.D.; Roth, T.C., 2nd; Fox, R.A.; Pravosudov, V.V. Ecologically relevant spatial memory use modulates hippocampal neurogenesis. Proc. Biol. Sci. 2010, 277, 1071–1079. [Google Scholar] [CrossRef]
- Patel, S.N.; Clayton, N.S.; Krebs, J.R. Spatial learning induces neurogenesis in the avian brain. Behav. Brain Res. 1997, 89, 115–128. [Google Scholar] [CrossRef]
- Thompson, C.K.; Brenowitz, E.A. Neurogenesis in an adult avian song nucleus is reduced by decreasing caspase-mediated apoptosis. J. Neurosci. 2009, 29, 4586–4591. [Google Scholar] [CrossRef]
- Walton, C.; Pariser, E.; Nottebohm, F. The zebra finch paradox: Song is little changed, but number of neurons doubles. J. Neurosci. 2012, 32, 761–774. [Google Scholar] [CrossRef]
- Striedter, G.F. Evolution of the hippocampus in reptiles and birds. J. Comp. Neurol. 2016, 524, 496–517. [Google Scholar] [CrossRef] [PubMed]
- Barnea, A.; Pravosudov, V. Birds as a model to study adult neurogenesis: Bridging evolutionary, comparative and neuroethological approaches. Eur. J. Neurosci. 2011, 34, 884–907. [Google Scholar] [CrossRef] [PubMed]
- Puelles, L.; Kuwana, E.; Puelles, E.; Bulfone, A.; Shimamura, K.; Keleher, J.; Smiga, S.; Rubenstein, J.L. Pallial and subpallial derivatives in the embryonic chick and mouse telencephalon, traced by the expression of the genes Dlx-2, Emx-1, Nkx-2.1, Pax-6, and Tbr-1. J. Comp. Neurol. 2000, 424, 409–438. [Google Scholar] [CrossRef]
- Belgard, T.G.; Montiel, J.F.; Wang, W.Z.; Garcia-Moreno, F.; Margulies, E.H.; Ponting, C.P.; Molnar, Z. Adult pallium transcriptomes surprise in not reflecting predicted homologies across diverse chicken and mouse pallial sectors. Proc. Natl. Acad. Sci. USA 2013, 110, 13150–13155. [Google Scholar] [CrossRef] [PubMed]
- Abellan, A.; Desfilis, E.; Medina, L. Combinatorial expression of Lef1, Lhx2, Lhx5, Lhx9, Lmo3, Lmo4, and Prox1 helps to identify comparable subdivisions in the developing hippocampal formation of mouse and chicken. Front. Neuroanat. 2014, 8, 59. [Google Scholar] [CrossRef] [PubMed]
- Atoji, Y.; Sarkar, S.; Wild, J.M. Proposed homology of the dorsomedial subdivision and V-shaped layer of the avian hippocampus to Ammon’s horn and dentate gyrus, respectively. Hippocampus 2016, 26, 1608–1617. [Google Scholar] [CrossRef] [PubMed]
- Atoji, Y.; Wild, J.M. Anatomy of the avian hippocampal formation. Rev. Neurosci. 2006, 17, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Mouritsen, H.; Heyers, D.; Gunturkun, O. The Neural Basis of Long-Distance Navigation in Birds. Annu. Rev. Physiol. 2016, 78, 133–154. [Google Scholar] [CrossRef]
- Hall, Z.J.; Delaney, S.; Sherry, D.F. Inhibition of cell proliferation in black-capped chickadees suggests a role for neurogenesis in spatial learning. Dev. Neurobiol. 2014, 74, 1002–1010. [Google Scholar] [CrossRef]
- Pravosudov, V.V.; Smulders, T.V. Integrating ecology, psychology and neurobiology within a food-hoarding paradigm. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2010, 365, 859–867. [Google Scholar] [CrossRef]
- Guitar, N.A.; Sherry, D.F. Decreased Neurogenesis Increases Spatial Reversal Errors in Chickadees (Poecile atricapillus). Dev. Neurobiol. 2018, 78, 1206–1217. [Google Scholar] [CrossRef] [PubMed]
- Kokoeva, M.V.; Yin, H.; Flier, J.S. Evidence for constitutive neural cell proliferation in the adult murine hypothalamus. J. Comp. Neurol. 2007, 505, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.; Sumners, C.; Moore, J.; Huentelman, M.J.; Deng, J.; Gelband, C.H.; Shaw, G. Characterization of mitotic neurons derived from adult rat hypothalamus and brain stem. J. Neurophysiol. 2002, 87, 1076–1085. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.F.; Arias-Carrion, O. Adult neurogenesis in the hypothalamus: Evidence, functions, and implications. CNS Neurol. Disord. Drug Targets 2011, 10, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Jhaveri, D.J.; Tedoldi, A.; Hunt, S.; Sullivan, R.; Watts, N.R.; Power, J.M.; Bartlett, P.F.; Sah, P. Evidence for newly generated interneurons in the basolateral amygdala of adult mice. Mol. Psychiatry 2018, 23, 521–532. [Google Scholar] [CrossRef] [PubMed]
- Palmer, T.D.; Ray, J.; Gage, F.H. FGF-2-responsive neuronal progenitors reside in proliferative and quiescent regions of the adult rodent brain. Mol. Cell. Neurosci. 1995, 6, 474–486. [Google Scholar] [CrossRef]
- Dayer, A.G.; Cleaver, K.M.; Abouantoun, T.; Cameron, H.A. New GABAergic interneurons in the adult neocortex and striatum are generated from different precursors. J. Cell Biol. 2005, 168, 415–427. [Google Scholar] [CrossRef]
- Lie, D.C.; Dziewczapolski, G.; Willhoite, A.R.; Kaspar, B.K.; Shults, C.W.; Gage, F.H. The adult substantia nigra contains progenitor cells with neurogenic potential. J. Neurosci. 2002, 22, 6639–6649. [Google Scholar] [CrossRef]
- Zhao, B.; Zhong, M.; Jin, K. Neurogenesis and neurodegenerative diseases in human. Panminerva Med. 2008, 50, 55–64. [Google Scholar]
- Kohler, S.J.; Williams, N.I.; Stanton, G.B.; Cameron, J.L.; Greenough, W.T. Maturation time of new granule cells in the dentate gyrus of adult macaque monkeys exceeds six months. Proc. Natl. Acad. Sci. USA 2011, 108, 10326–10331. [Google Scholar] [CrossRef]
- Schmidt-Hieber, C.; Jonas, P.; Bischofberger, J. Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature 2004, 429, 184–187. [Google Scholar] [CrossRef] [PubMed]
- Ohkawa, N.; Saitoh, Y.; Tokunaga, E.; Nihonmatsu, I.; Ozawa, F.; Murayama, A.; Shibata, F.; Kitamura, T.; Inokuchi, K. Spine formation pattern of adult-born neurons is differentially modulated by the induction timing and location of hippocampal plasticity. PLoS ONE 2012, 7, e45270. [Google Scholar] [CrossRef] [PubMed]
- Jungenitz, T.; Beining, M.; Radic, T.; Deller, T.; Cuntz, H.; Jedlicka, P.; Schwarzacher, S.W. Structural homo- and heterosynaptic plasticity in mature and adult newborn rat hippocampal granule cells. Proc. Natl. Acad. Sci. USA 2018, 115, E4670–E4679. [Google Scholar] [CrossRef] [PubMed]
- Ninkovic, J.; Mori, T.; Gotz, M. Distinct modes of neuron addition in adult mouse neurogenesis. J. Neurosci. 2007, 27, 10906–10911. [Google Scholar] [CrossRef] [PubMed]
- Imayoshi, I.; Sakamoto, M.; Ohtsuka, T.; Kageyama, R. Continuous neurogenesis in the adult brain. Dev. Growth Differ. 2009, 51, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Snyder, J.S.; Choe, J.S.; Clifford, M.A.; Jeurling, S.I.; Hurley, P.; Brown, A.; Kamhi, J.F.; Cameron, H.A. Adult-born hippocampal neurons are more numerous, faster maturing, and more involved in behavior in rats than in mice. J. Neurosci. 2009, 29, 14484–14495. [Google Scholar] [CrossRef]
- Recabal, A.; Caprile, T.; Garcia-Robles, M.L.A. Hypothalamic Neurogenesis as an Adaptive Metabolic Mechanism. Front. Neurosci. 2017, 11, 190. [Google Scholar] [CrossRef]
- Sakamoto, M.; Ieki, N.; Miyoshi, G.; Mochimaru, D.; Miyachi, H.; Imura, T.; Yamaguchi, M.; Fishell, G.; Mori, K.; Kageyama, R.; et al. Continuous postnatal neurogenesis contributes to formation of the olfactory bulb neural circuits and flexible olfactory associative learning. J. Neurosci. 2014, 34, 5788–5799. [Google Scholar] [CrossRef]
- Sakamoto, M.; Kageyama, R.; Imayoshi, I. The functional significance of newly born neurons integrated into olfactory bulb circuits. Front. Neurosci. 2014, 8, 121. [Google Scholar] [CrossRef]
- Lazarov, O.; Mattson, M.P.; Peterson, D.A.; Pimplikar, S.W.; van Praag, H. When neurogenesis encounters aging and disease. Trends Neurosci. 2010, 33, 569–579. [Google Scholar] [CrossRef]
- Bernal, G.M.; Peterson, D.A. Neural stem cells as therapeutic agents for age-related brain repair. Aging Cell 2004, 3, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Olariu, A.; Cleaver, K.M.; Cameron, H.A. Decreased neurogenesis in aged rats results from loss of granule cell precursors without lengthening of the cell cycle. J. Comp. Neurol. 2007, 501, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Lugert, S.; Basak, O.; Knuckles, P.; Haussler, U.; Fabel, K.; Gotz, M.; Haas, C.A.; Kempermann, G.; Taylor, V.; Giachino, C. Quiescent and active hippocampal neural stem cells with distinct morphologies respond selectively to physiological and pathological stimuli and aging. Cell Stem Cell 2010, 6, 445–456. [Google Scholar] [CrossRef] [PubMed]
- Encinas, J.M.; Michurina, T.V.; Peunova, N.; Park, J.H.; Tordo, J.; Peterson, D.A.; Fishell, G.; Koulakov, A.; Enikolopov, G. Division-coupled astrocytic differentiation and age-related depletion of neural stem cells in the adult hippocampus. Cell Stem Cell 2011, 8, 566–579. [Google Scholar] [CrossRef] [PubMed]
- Boschen, K.E.; Klintsova, A.Y. Neurotrophins in the Brain: Interaction With Alcohol Exposure During Development. Vitam. Horm. 2017, 104, 197–242. [Google Scholar] [CrossRef] [PubMed]
- Borsini, A.; Zunszain, P.A.; Thuret, S.; Pariante, C.M. The role of inflammatory cytokines as key modulators of neurogenesis. Trends Neurosci. 2015, 38, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.C.; Huddleston, D.E.; Brickman, A.M.; Sosunov, A.A.; Hen, R.; McKhann, G.M.; Sloan, R.; Gage, F.H.; Brown, T.R.; Small, S.A. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc. Natl. Acad. Sci. USA 2007, 104, 5638–5643. [Google Scholar] [CrossRef] [PubMed]
- van Praag, H. Neurogenesis and exercise: Past and future directions. Neuromol. Med. 2008, 10, 128–140. [Google Scholar] [CrossRef]
- Kempermann, G. Activity Dependency and Aging in the Regulation of Adult Neurogenesis. Cold Spring Harb. Perspect. Biol. 2015, 7, a018929. [Google Scholar] [CrossRef]
- Gould, E.; Beylin, A.; Tanapat, P.; Reeves, A.; Shors, T.J. Learning enhances adult neurogenesis in the hippocampal formation. Nat. Neurosci. 1999, 2, 260–265. [Google Scholar] [CrossRef]
- Deng, W.; Aimone, J.B.; Gage, F.H. New neurons and new memories: How does adult hippocampal neurogenesis affect learning and memory? Nat. Rev. Neurosci. 2010, 11, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Vivar, C.; Potter, M.C.; Choi, J.; Lee, J.Y.; Stringer, T.P.; Callaway, E.M.; Gage, F.H.; Suh, H.; van Praag, H. Monosynaptic inputs to new neurons in the dentate gyrus. Nat. Commun. 2012, 3, 1107. [Google Scholar] [CrossRef] [PubMed]
- Kee, N.; Teixeira, C.M.; Wang, A.H.; Frankland, P.W. Preferential incorporation of adult-generated granule cells into spatial memory networks in the dentate gyrus. Nat. Neurosci. 2007, 10, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Creer, D.J.; Romberg, C.; Saksida, L.M.; van Praag, H.; Bussey, T.J. Running enhances spatial pattern separation in mice. Proc. Natl. Acad. Sci. USA 2010, 107, 2367–2372. [Google Scholar] [CrossRef] [PubMed]
- Vivar, C.; Peterson, B.D.; van Praag, H. Running rewires the neuronal network of adult-born dentate granule cells. Neuroimage 2016, 131, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Tronel, S.; Belnoue, L.; Grosjean, N.; Revest, J.M.; Piazza, P.V.; Koehl, M.; Abrous, D.N. Adult-born neurons are necessary for extended contextual discrimination. Hippocampus 2012, 22, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Ikrar, T.; Guo, N.; He, K.; Besnard, A.; Levinson, S.; Hill, A.; Lee, H.K.; Hen, R.; Xu, X.; Sahay, A. Adult neurogenesis modifies excitability of the dentate gyrus. Front. Neural Circuits 2013, 7, 204. [Google Scholar] [CrossRef] [PubMed]
- Treves, A.; Rolls, E.T. Computational constraints suggest the need for two distinct input systems to the hippocampal CA3 network. Hippocampus 1992, 2, 189–199. [Google Scholar] [CrossRef]
- Marr, D. Simple memory: A theory for archicortex. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1971, 262, 23–81. [Google Scholar] [CrossRef]
- Yassa, M.A.; Stark, C.E. Pattern separation in the hippocampus. Trends Neurosci. 2011, 34, 515–525. [Google Scholar] [CrossRef]
- Rolls, E.T. Pattern separation, completion, and categorisation in the hippocampus and neocortex. Neurobiol. Learn. Mem. 2016, 129, 4–28. [Google Scholar] [CrossRef] [PubMed]
- O'Reilly, R.C.; McClelland, J.L. Hippocampal conjunctive encoding, storage, and recall: Avoiding a trade-off. Hippocampus 1994, 4, 661–682. [Google Scholar] [CrossRef] [PubMed]
- Rolls, E.T. A theory of hippocampal function in memory. Hippocampus 1996, 6, 601–620. [Google Scholar] [CrossRef]
- Clelland, C.D.; Choi, M.; Romberg, C.; Clemenson, G.D., Jr.; Fragniere, A.; Tyers, P.; Jessberger, S.; Saksida, L.M.; Barker, R.A.; Gage, F.H.; et al. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science 2009, 325, 210–213. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.J.; Kitamura, T.; Saitoh, Y.; Ohkawa, N.; Kondo, T.; Inokuchi, K. Adult Neurogenesis Conserves Hippocampal Memory Capacity. J. Neurosci. 2018, 38, 6854–6863. [Google Scholar] [CrossRef] [PubMed]
- Rakic, P. Limits of neurogenesis in primates. Science 1985, 227, 1054–1056. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.; Rampon, C.; Tang, Y.P.; Shrom, D.; Jin, J.; Kyin, M.; Sopher, B.; Miller, M.W.; Ware, C.B.; Martin, G.M.; et al. Deficient neurogenesis in forebrain-specific presenilin-1 knockout mice is associated with reduced clearance of hippocampal memory traces. Neuron 2001, 32, 911–926. [Google Scholar] [CrossRef]
- Becker, S. A computational principle for hippocampal learning and neurogenesis. Hippocampus 2005, 15, 722–738. [Google Scholar] [CrossRef]
- Weisz, V.I.; Argibay, P.F. A putative role for neurogenesis in neuro-computational terms: Inferences from a hippocampal model. Cognition 2009, 112, 229–240. [Google Scholar] [CrossRef]
- Van der Borght, K.; Wallinga, A.E.; Luiten, P.G.; Eggen, B.J.; Van der Zee, E.A. Morris water maze learning in two rat strains increases the expression of the polysialylated form of the neural cell adhesion molecule in the dentate gyrus but has no effect on hippocampal neurogenesis. Behav. Neurosci. 2005, 119, 926–932. [Google Scholar] [CrossRef]
- Valero, J.; Mastrella, G.; Neiva, I.; Sanchez, S.; Malva, J.O. Long-term effects of an acute and systemic administration of LPS on adult neurogenesis and spatial memory. Front. Neurosci. 2014, 8, 83. [Google Scholar] [CrossRef] [PubMed]
- Bizon, J.L.; Gallagher, M. Production of new cells in the rat dentate gyrus over the lifespan: Relation to cognitive decline. Eur. J. Neurosci. 2003, 18, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Jessberger, S.; Clark, R.E.; Broadbent, N.J.; Clemenson, G.D., Jr.; Consiglio, A.; Lie, D.C.; Squire, L.R.; Gage, F.H. Dentate gyrus-specific knockdown of adult neurogenesis impairs spatial and object recognition memory in adult rats. Learn. Mem. 2009, 16, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Gradari, S.; Perez-Domper, P.; Butler, R.G.; Martinez-Cue, C.; de Polavieja, G.G.; Trejo, J.L. The relationship between behavior acquisition and persistence abilities: Involvement of adult hippocampal neurogenesis. Hippocampus 2016, 26, 857–874. [Google Scholar] [CrossRef] [PubMed]
- Ambrogini, P.; Orsini, L.; Mancini, C.; Ferri, P.; Ciaroni, S.; Cuppini, R. Learning may reduce neurogenesis in adult rat dentate gyrus. Neurosci. Lett. 2004, 359, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Pham, K.; McEwen, B.S.; Ledoux, J.E.; Nader, K. Fear learning transiently impairs hippocampal cell proliferation. Neuroscience 2005, 130, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Olariu, A.; Cleaver, K.M.; Shore, L.E.; Brewer, M.D.; Cameron, H.A. A natural form of learning can increase and decrease the survival of new neurons in the dentate gyrus. Hippocampus 2005, 15, 750–762. [Google Scholar] [CrossRef]
- Dobrossy, M.D.; Drapeau, E.; Aurousseau, C.; Le Moal, M.; Piazza, P.V.; Abrous, D.N. Differential effects of learning on neurogenesis: Learning increases or decreases the number of newly born cells depending on their birth date. Mol. Psychiatry 2003, 8, 974–982. [Google Scholar] [CrossRef]
- Lemaire, V.; Tronel, S.; Montaron, M.F.; Fabre, A.; Dugast, E.; Abrous, D.N. Long-lasting plasticity of hippocampal adult-born neurons. J. Neurosci. 2012, 32, 3101–3108. [Google Scholar] [CrossRef]
- Tronel, S.; Charrier, V.; Sage, C.; Maitre, M.; Leste-Lasserre, T.; Abrous, D.N. Adult-born dentate neurons are recruited in both spatial memory encoding and retrieval. Hippocampus 2015, 25, 1472–1479. [Google Scholar] [CrossRef]
- So, J.H.; Huang, C.; Ge, M.; Cai, G.; Zhang, L.; Lu, Y.; Mu, Y. Intense Exercise Promotes Adult Hippocampal Neurogenesis But Not Spatial Discrimination. Front. Cell. Neurosci. 2017, 11, 13. [Google Scholar] [CrossRef] [PubMed]
- Germain, J.; Bruel-Jungerman, E.; Grannec, G.; Denis, C.; Lepousez, G.; Giros, B.; Francis, F.; Nosten-Bertrand, M. Doublecortin knockout mice show normal hippocampal-dependent memory despite CA3 lamination defects. PLoS ONE 2013, 8, e74992. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, K.; Sloan, S.A.; Bennett, M.L.; Scholze, A.R.; O’Keeffe, S.; Phatnani, H.P.; Guarnieri, P.; Caneda, C.; Ruderisch, N.; et al. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J. Neurosci. 2014, 34, 11929–11947. [Google Scholar] [CrossRef] [PubMed]
- Taupin, P. BrdU immunohistochemistry for studying adult neurogenesis: Paradigms, pitfalls, limitations, and validation. Brain Res. Rev. 2007, 53, 198–214. [Google Scholar] [CrossRef] [PubMed]
- Vellema, M.; Hertel, M.; Urbanus, S.L.; Van der Linden, A.; Gahr, M. Evaluating the predictive value of doublecortin as a marker for adult neurogenesis in canaries (Serinus canaria). J. Comp. Neurol. 2014, 522, 1299–1315. [Google Scholar] [CrossRef] [PubMed]
- Shors, T.J.; Townsend, D.A.; Zhao, M.; Kozorovitskiy, Y.; Gould, E. Neurogenesis may relate to some but not all types of hippocampal-dependent learning. Hippocampus 2002, 12, 578–584. [Google Scholar] [CrossRef] [PubMed]
- Gould, E.; Reeves, A.J.; Fallah, M.; Tanapat, P.; Gross, C.G.; Fuchs, E. Hippocampal neurogenesis in adult Old World primates. Proc. Natl. Acad. Sci. USA 1999, 96, 5263–5267. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.M.; Cai, Y.; Chu, Y.; Chen, E.Y.; Feng, J.C.; Luo, X.G.; Xiong, K.; Struble, R.G.; Clough, R.W.; Patrylo, P.R.; et al. Doublecortin-expressing cells persist in the associative cerebral cortex and amygdala in aged nonhuman primates. Front. Neuroanat. 2009, 3, 17. [Google Scholar] [CrossRef]
- Gould, E.; Reeves, A.J.; Graziano, M.S.; Gross, C.G. Neurogenesis in the neocortex of adult primates. Science 1999, 286, 548–552. [Google Scholar] [CrossRef]
- Jabes, A.; Lavenex, P.B.; Amaral, D.G.; Lavenex, P. Quantitative analysis of postnatal neurogenesis and neuron number in the macaque monkey dentate gyrus. Eur. J. Neurosci. 2010, 31, 273–285. [Google Scholar] [CrossRef]
- Rao, M.S.; Hattiangady, B.; Shetty, A.K. The window and mechanisms of major age-related decline in the production of new neurons within the dentate gyrus of the hippocampus. Aging Cell 2006, 5, 545–558. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Teng, E.M.; Summers, R.G., Jr.; Ming, G.L.; Gage, F.H. Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J. Neurosci. 2006, 26, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Ngwenya, L.B.; Peters, A.; Rosene, D.L. Maturational sequence of newly generated neurons in the dentate gyrus of the young adult rhesus monkey. J. Comp. Neurol. 2006, 498, 204–216. [Google Scholar] [CrossRef] [PubMed]
- Bunk, E.C.; Stelzer, S.; Hermann, S.; Schafers, M.; Schlatt, S.; Schwamborn, J.C. Cellular organization of adult neurogenesis in the Common Marmoset. Aging Cell 2011, 10, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Daynac, M.; Morizur, L.; Chicheportiche, A.; Mouthon, M.A.; Boussin, F.D. Age-related neurogenesis decline in the subventricular zone is associated with specific cell cycle regulation changes in activated neural stem cells. Sci. Rep. 2016, 6, 21505. [Google Scholar] [CrossRef] [PubMed]
- Seress, L. Comparative anatomy of the hippocampal dentate gyrus in adult and developing rodents, non-human primates and humans. Prog. Brain Res. 2007, 163, 23–41. [Google Scholar] [CrossRef] [PubMed]
- Small, S.A.; Chawla, M.K.; Buonocore, M.; Rapp, P.R.; Barnes, C.A. Imaging correlates of brain function in monkeys and rats isolates a hippocampal subregion differentially vulnerable to aging. Proc. Natl. Acad. Sci. USA 2004, 101, 7181–7186. [Google Scholar] [CrossRef]
- Murray, E.A.; Bussey, T.J.; Hampton, R.R.; Saksida, L.M. The parahippocampal region and object identification. Ann. N. Y. Acad. Sci. 2000, 911, 166–174. [Google Scholar] [CrossRef]
- Suzuki, W.A.; Eichenbaum, H. The neurophysiology of memory. Ann. N. Y. Acad. Sci. 2000, 911, 175–191. [Google Scholar] [CrossRef]
- Brown, M.W.; Aggleton, J.P. Recognition memory: What are the roles of the perirhinal cortex and hippocampus? Nat. Rev. Neurosci. 2001, 2, 51–61. [Google Scholar] [CrossRef]
- Milner, B.; Klein, D. Loss of recent memory after bilateral hippocampal lesions: Memory and memories-looking back and looking forward. J. Neurol. Neurosurg. Psychiatry 2016, 87, 230. [Google Scholar] [CrossRef] [PubMed]
- Eichenbaum, H. The hippocampus and declarative memory: Cognitive mechanisms and neural codes. Behav. Brain Res. 2001, 127, 199–207. [Google Scholar] [CrossRef]
- Opris, I.; Santos, L.M.; Gerhardt, G.A.; Song, D.; Berger, T.W.; Hampson, R.E.; Deadwyler, S.A. Distributed encoding of spatial and object categories in primate hippocampal microcircuits. Front. Neurosci. 2015, 9, 317. [Google Scholar] [CrossRef]
- Lee, C.H.; Ryu, J.; Lee, S.H.; Kim, H.; Lee, I. Functional cross-hemispheric shift between object-place paired associate memory and spatial memory in the human hippocampus. Hippocampus 2016, 26, 1061–1077. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.A.; Nathanson, J.; Franjic, D.; Shim, S.; Dalley, R.A.; Shapouri, S.; Smith, K.A.; Sunkin, S.M.; Bernard, A.; Bennett, J.L.; et al. Conserved molecular signatures of neurogenesis in the hippocampal subgranular zone of rodents and primates. Development 2013, 140, 4633–4644. [Google Scholar] [CrossRef] [PubMed]
- Karten, Y.J.; Olariu, A.; Cameron, H.A. Stress in early life inhibits neurogenesis in adulthood. Trends Neurosci. 2005, 28, 171–172. [Google Scholar] [CrossRef] [PubMed]
- Perera, T.D.; Lu, D.; Thirumangalakudi, L.; Smith, E.L.; Yaretskiy, A.; Rosenblum, L.A.; Kral, J.G.; Coplan, J.D. Correlations between hippocampal neurogenesis and metabolic indices in adult nonhuman primates. Neural Plast. 2011, 2011, 1–6. [Google Scholar] [CrossRef]
- Coe, C.L.; Kramer, M.; Czeh, B.; Gould, E.; Reeves, A.J.; Kirschbaum, C.; Fuchs, E. Prenatal stress diminishes neurogenesis in the dentate gyrus of juvenile rhesus monkeys. Biol. Psychiatry 2003, 54, 1025–1034. [Google Scholar] [CrossRef]
- Cinini, S.M.; Barnabe, G.F.; Galvao-Coelho, N.; de Medeiros, M.A.; Perez-Mendes, P.; Sousa, M.B.; Covolan, L.; Mello, L.E. Social isolation disrupts hippocampal neurogenesis in young non-human primates. Front. Neurosci. 2014, 8, 45. [Google Scholar] [CrossRef]
- Kozareva, D.A.; O’Leary, O.F.; Cryan, J.F.; Nolan, Y.M. Deletion of TLX and social isolation impairs exercise-induced neurogenesis in the adolescent hippocampus. Hippocampus 2018, 28, 3–11. [Google Scholar] [CrossRef]
- Taffe, M.A.; Kotzebue, R.W.; Crean, R.D.; Crawford, E.F.; Edwards, S.; Mandyam, C.D. Long-lasting reduction in hippocampal neurogenesis by alcohol consumption in adolescent nonhuman primates. Proc. Natl. Acad. Sci. USA 2010, 107, 11104–11109. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.E.; Squire, L.R. Similarity in form and function of the hippocampus in rodents, monkeys, and humans. Proc. Natl. Acad. Sci. USA 2013, 110 (Suppl. 2), 10365–10370. [Google Scholar] [CrossRef]
- Jutras, M.J.; Buffalo, E.A. Recognition memory signals in the macaque hippocampus. Proc. Natl. Acad. Sci. USA 2010, 107, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Bachevalier, J.; Nemanic, S.; Alvarado, M.C. The influence of context on recognition memory in monkeys: Effects of hippocampal, parahippocampal and perirhinal lesions. Behav. Brain Res. 2015, 285, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Rueckemann, J.W.; Buffalo, E.A. Spatial Responses, Immediate Experience, and Memory in the Monkey Hippocampus. Curr. Opin. Behav. Sci. 2017, 17, 155–160. [Google Scholar] [CrossRef]
- Burke, S.N.; Wallace, J.L.; Hartzell, A.L.; Nematollahi, S.; Plange, K.; Barnes, C.A. Age-associated deficits in pattern separation functions of the perirhinal cortex: A cross-species consensus. Behav. Neurosci. 2011, 125, 836–847. [Google Scholar] [CrossRef] [PubMed]
- Aizawa, K.; Ageyama, N.; Yokoyama, C.; Hisatsune, T. Age-dependent alteration in hippocampal neurogenesis correlates with learning performance of macaque monkeys. Exp. Anim. 2009, 58, 403–407. [Google Scholar] [CrossRef]
- Eriksson, P.S.; Perfilieva, E.; Bjork-Eriksson, T.; Alborn, A.M.; Nordborg, C.; Peterson, D.A.; Gage, F.H. Neurogenesis in the adult human hippocampus. Nat. Med. 1998, 4, 1313–1317. [Google Scholar] [CrossRef]
- Sanai, N.; Nguyen, T.; Ihrie, R.A.; Mirzadeh, Z.; Tsai, H.H.; Wong, M.; Gupta, N.; Berger, M.S.; Huang, E.; Garcia-Verdugo, J.M.; et al. Corridors of migrating neurons in the human brain and their decline during infancy. Nature 2011, 478, 382–386. [Google Scholar] [CrossRef]
- Ernst, A.; Alkass, K.; Bernard, S.; Salehpour, M.; Perl, S.; Tisdale, J.; Possnert, G.; Druid, H.; Frisen, J. Neurogenesis in the striatum of the adult human brain. Cell 2014, 156, 1072–1083. [Google Scholar] [CrossRef]
- Inta, D.; Lang, U.E.; Borgwardt, S.; Meyer-Lindenberg, A.; Gass, P. Adult neurogenesis in the human striatum: Possible implications for psychiatric disorders. Mol. Psychiatry 2016, 21, 446–447. [Google Scholar] [CrossRef] [PubMed]
- Curtis, M.A.; Kam, M.; Nannmark, U.; Anderson, M.F.; Axell, M.Z.; Wikkelso, C.; Holtas, S.; van Roon-Mom, W.M.; Bjork-Eriksson, T.; Nordborg, C.; et al. Human neuroblasts migrate to the olfactory bulb via a lateral ventricular extension. Science 2007, 315, 1243–1249. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, F.; Liu, Y.Y.; Zhao, C.H.; You, Y.; Wang, L.; Zhang, J.; Wei, B.; Ma, T.; Zhang, Q.; et al. Identification and characterization of neuroblasts in the subventricular zone and rostral migratory stream of the adult human brain. Cell Res. 2011, 21, 1534–1550. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, O.; Liebl, J.; Bernard, S.; Alkass, K.; Yeung, M.S.; Steier, P.; Kutschera, W.; Johnson, L.; Landen, M.; Druid, H.; et al. The age of olfactory bulb neurons in humans. Neuron 2012, 74, 634–639. [Google Scholar] [CrossRef] [PubMed]
- Knoth, R.; Singec, I.; Ditter, M.; Pantazis, G.; Capetian, P.; Meyer, R.P.; Horvat, V.; Volk, B.; Kempermann, G. Murine features of neurogenesis in the human hippocampus across the lifespan from 0 to 100 years. PLoS ONE 2010, 5, e8809. [Google Scholar] [CrossRef] [PubMed]
- Bakker, A.; Kirwan, C.B.; Miller, M.; Stark, C.E. Pattern separation in the human hippocampal CA3 and dentate gyrus. Science 2008, 319, 1640–1642. [Google Scholar] [CrossRef] [PubMed]
- Lacy, J.W.; Yassa, M.A.; Stark, S.M.; Muftuler, L.T.; Stark, C.E. Distinct pattern separation related transfer functions in human CA3/dentate and CA1 revealed using high-resolution fMRI and variable mnemonic similarity. Learn. Mem. 2011, 18, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Baker, S.; Vieweg, P.; Gao, F.; Gilboa, A.; Wolbers, T.; Black, S.E.; Rosenbaum, R.S. The Human Dentate Gyrus Plays a Necessary Role in Discriminating New Memories. Curr. Biol. 2016, 26, 2629–2634. [Google Scholar] [CrossRef]
- Berron, D.; Schutze, H.; Maass, A.; Cardenas-Blanco, A.; Kuijf, H.J.; Kumaran, D.; Duzel, E. Strong Evidence for Pattern Separation in Human Dentate Gyrus. J. Neurosci. 2016, 36, 7569–7579. [Google Scholar] [CrossRef]
- Paredes, M.F.; Sorrells, S.F.; Cebrian-Silla, A.; Sandoval, K.; Qi, D.; Kelley, K.W.; James, D.; Mayer, S.; Chang, J.; Auguste, K.I.; et al. Does Adult Neurogenesis Persist in the Human Hippocampus? Cell Stem Cell 2018, 23, 780–781. [Google Scholar] [CrossRef]
- Boekhoorn, K.; Joels, M.; Lucassen, P.J. Increased proliferation reflects glial and vascular-associated changes, but not neurogenesis in the presenile Alzheimer hippocampus. Neurobiol. Dis. 2006, 24, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Jung, J.; Lee, H.J.; Kim, J.C.; Wang, H.; Kim, S.H.; Shin, T.; Moon, C. Differences in immunoreactivities of Ki-67 and doublecortin in the adult hippocampus in three strains of mice. Acta Histochem. 2009, 111, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Kempermann, G.; Gage, F.H.; Aigner, L.; Song, H.; Curtis, M.A.; Thuret, S.; Kuhn, H.G.; Jessberger, S.; Frankland, P.W.; Cameron, H.A.; et al. Human Adult Neurogenesis: Evidence and Remaining Questions. Cell Stem Cell 2018, 23, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Lucassen, P.J.; Toni, N.; Kempermann, G.; Frisen, J.; Gage, F.H.; Swaab, D.F. Limits to human neurogenesis-really? Mol. Psychiatry 2019. [Google Scholar] [CrossRef] [PubMed]
- Tartt, A.N.; Fulmore, C.A.; Liu, Y.; Rosoklija, G.B.; Dwork, A.J.; Arango, V.; Hen, R.; Mann, J.J.; Boldrini, M. Considerations for Assessing the Extent of Hippocampal Neurogenesis in the Adult and Aging Human Brain. Cell Stem Cell 2018, 23, 782–783. [Google Scholar] [CrossRef] [PubMed]
- Snyder, J.S. Questioning human neurogenesis. Nature 2018, 555, 315–316. [Google Scholar] [CrossRef] [PubMed]
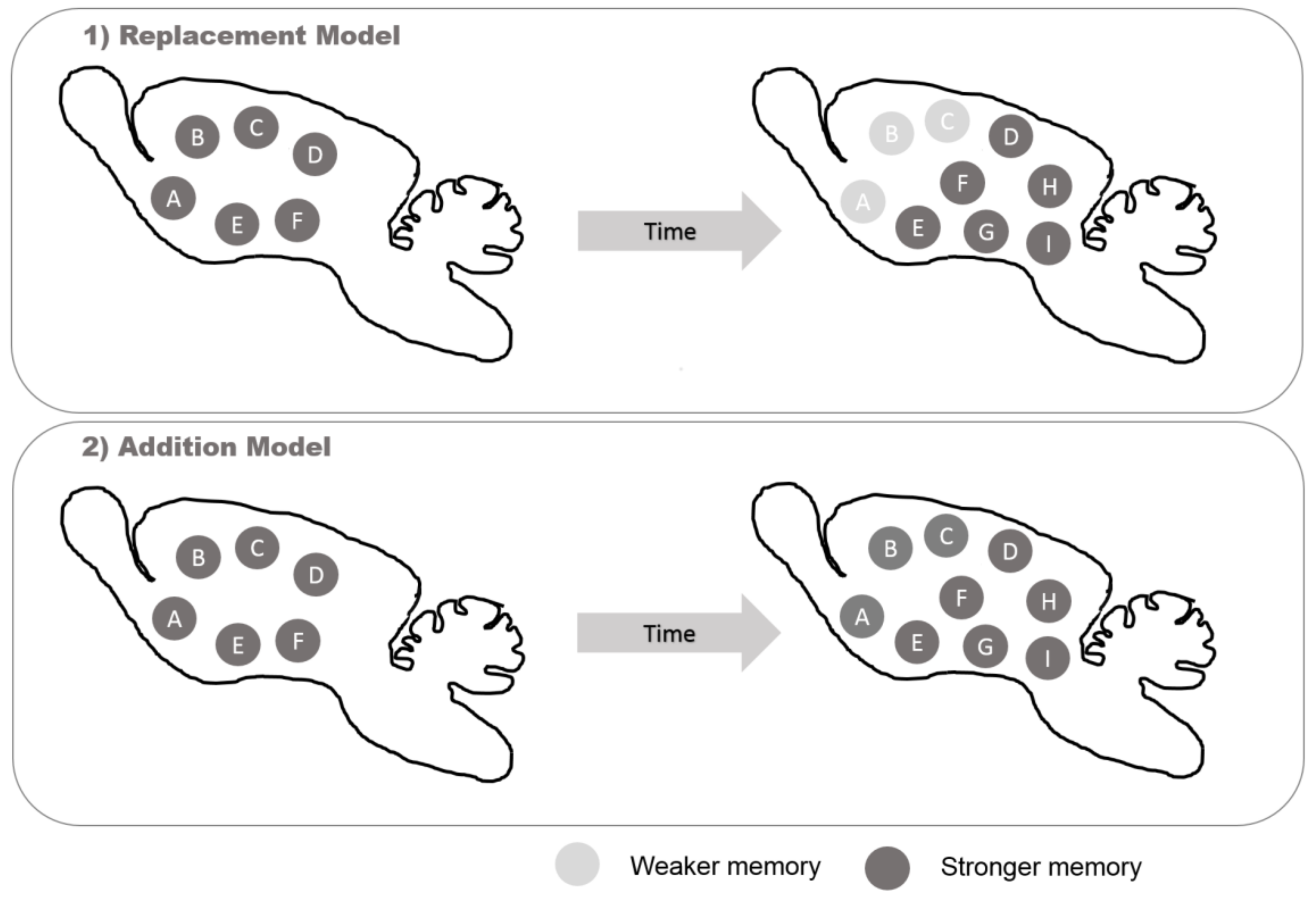
- Kempermann, G. New neurons for ‘survival of the fittest’. Nat. Rev. Neurosci. 2012, 13, 727–736. [Google Scholar] [CrossRef]
- La Rosa, C.; Parolisi, R.; Palazzo, O.; Levy, F.; Meurisse, M.; Bonfanti, L. Clusters of DCX+ cells “trapped” in the subcortical white matter of early postnatal Cetartiodactyla (Tursiops truncatus, Stenella coeruloalba and Ovis aries). Brain Struct. Funct. 2018, 223, 3613–3632. [Google Scholar] [CrossRef]
- Piumatti, M.; Palazzo, O.; La Rosa, C.; Crociara, P.; Parolisi, R.; Luzzati, F.; Levy, F.; Bonfanti, L. Non-Newly Generated, “Immature” Neurons in the Sheep Brain Are Not Restricted to Cerebral Cortex. J. Neurosci. 2018, 38, 826–842. [Google Scholar] [CrossRef]
- Hofman, M.A. Evolution of the human brain: When bigger is better. Front. Neuroanat. 2014, 8, 15. [Google Scholar] [CrossRef]
- Bedard, A.; Cossette, M.; Levesque, M.; Parent, A. Proliferating cells can differentiate into neurons in the striatum of normal adult monkey. Neurosci. Lett. 2002, 328, 213–216. [Google Scholar] [CrossRef]
- Sherry, D.F.; Jacobs, L.F.; Gaulin, S.J. Spatial memory and adaptive specialization of the hippocampus. Trends Neurosci. 1992, 15, 298–303. [Google Scholar] [CrossRef]
- Dennis, C.V.; Suh, L.S.; Rodriguez, M.L.; Kril, J.J.; Sutherland, G.T. Human adult neurogenesis across the ages: An immunohistochemical study. Neuropathol. Appl. Neurobiol. 2016, 42, 621–638. [Google Scholar] [CrossRef] [PubMed]
- Walton, N.M.; Shin, R.; Tajinda, K.; Heusner, C.L.; Kogan, J.H.; Miyake, S.; Chen, Q.; Tamura, K.; Matsumoto, M. Adult neurogenesis transiently generates oxidative stress. PLoS ONE 2012, 7, e35264. [Google Scholar] [CrossRef] [PubMed]
- Batista, C.M.; Mariano, E.D.; Barbosa, B.J.; Morgalla, M.; Marie, S.K.; Teixeira, M.J.; Lepski, G. Adult neurogenesis and glial oncogenesis: When the process fails. Biomed. Res. Int. 2014, 2014, 438639. [Google Scholar] [CrossRef] [PubMed]
- Tello-Ramos, M.C.; Branch, C.L.; Kozlovsky, D.Y.; Pitera, A.M.; Pravosudov, V.V. Spatial memory and cognitive flexibility trade-offs: To be or not to be flexible, that is the question. Anim. Behav. 2018, 147, 129–136. [Google Scholar] [CrossRef]
- Cavallucci, V.; Fidaleo, M.; Pani, G. Neural Stem Cells and Nutrients: Poised between Quiescence and Exhaustion. Trends Endocrinol. Metab. 2016, 27, 756–769. [Google Scholar] [CrossRef] [PubMed]
- Beckervordersandforth, R. Mitochondrial Metabolism-Mediated Regulation of Adult Neurogenesis. Brain Plast. 2017, 3, 73–87. [Google Scholar] [CrossRef]
- Wosiski-Kuhn, M.; Stranahan, A.M. Transient increases in dendritic spine density contribute to dentate gyrus long-term potentiation. Synapse 2012, 66, 661–664. [Google Scholar] [CrossRef]
- Eilam-Stock, T.; Serrano, P.; Frankfurt, M.; Luine, V. Bisphenol-A impairs memory and reduces dendritic spine density in adult male rats. Behav. Neurosci. 2012, 126, 175–185. [Google Scholar] [CrossRef]
- Koleske, A.J. Molecular mechanisms of dendrite stability. Nat. Rev. Neurosci. 2013, 14, 536–550. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Augusto-Oliveira, M.; Arrifano, G.P.F.; Malva, J.O.; Crespo-Lopez, M.E. Adult Hippocampal Neurogenesis in Different Taxonomic Groups: Possible Functional Similarities and Striking Controversies. Cells 2019, 8, 125. https://doi.org/10.3390/cells8020125
Augusto-Oliveira M, Arrifano GPF, Malva JO, Crespo-Lopez ME. Adult Hippocampal Neurogenesis in Different Taxonomic Groups: Possible Functional Similarities and Striking Controversies. Cells. 2019; 8(2):125. https://doi.org/10.3390/cells8020125
Chicago/Turabian StyleAugusto-Oliveira, Marcus, Gabriela P. F. Arrifano, João O. Malva, and Maria Elena Crespo-Lopez. 2019. "Adult Hippocampal Neurogenesis in Different Taxonomic Groups: Possible Functional Similarities and Striking Controversies" Cells 8, no. 2: 125. https://doi.org/10.3390/cells8020125
APA StyleAugusto-Oliveira, M., Arrifano, G. P. F., Malva, J. O., & Crespo-Lopez, M. E. (2019). Adult Hippocampal Neurogenesis in Different Taxonomic Groups: Possible Functional Similarities and Striking Controversies. Cells, 8(2), 125. https://doi.org/10.3390/cells8020125






