Recent Advances in EPAC-Targeted Therapies: A Biophysical Perspective
Abstract
1. Introduction
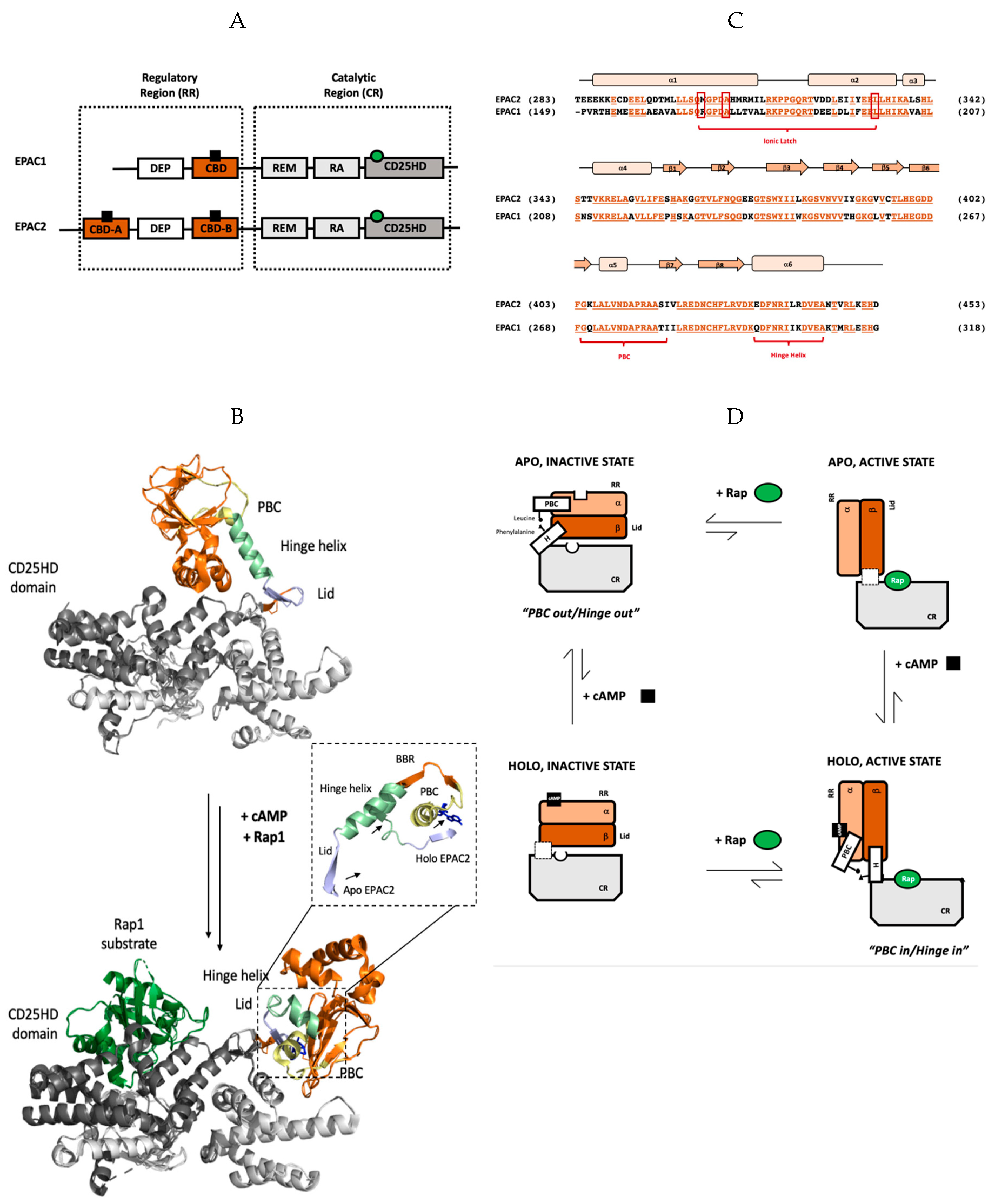
2. cAMP-Dependent Regulation of EPAC Function
3. The Discovery of EPAC-Specific Inhibitors
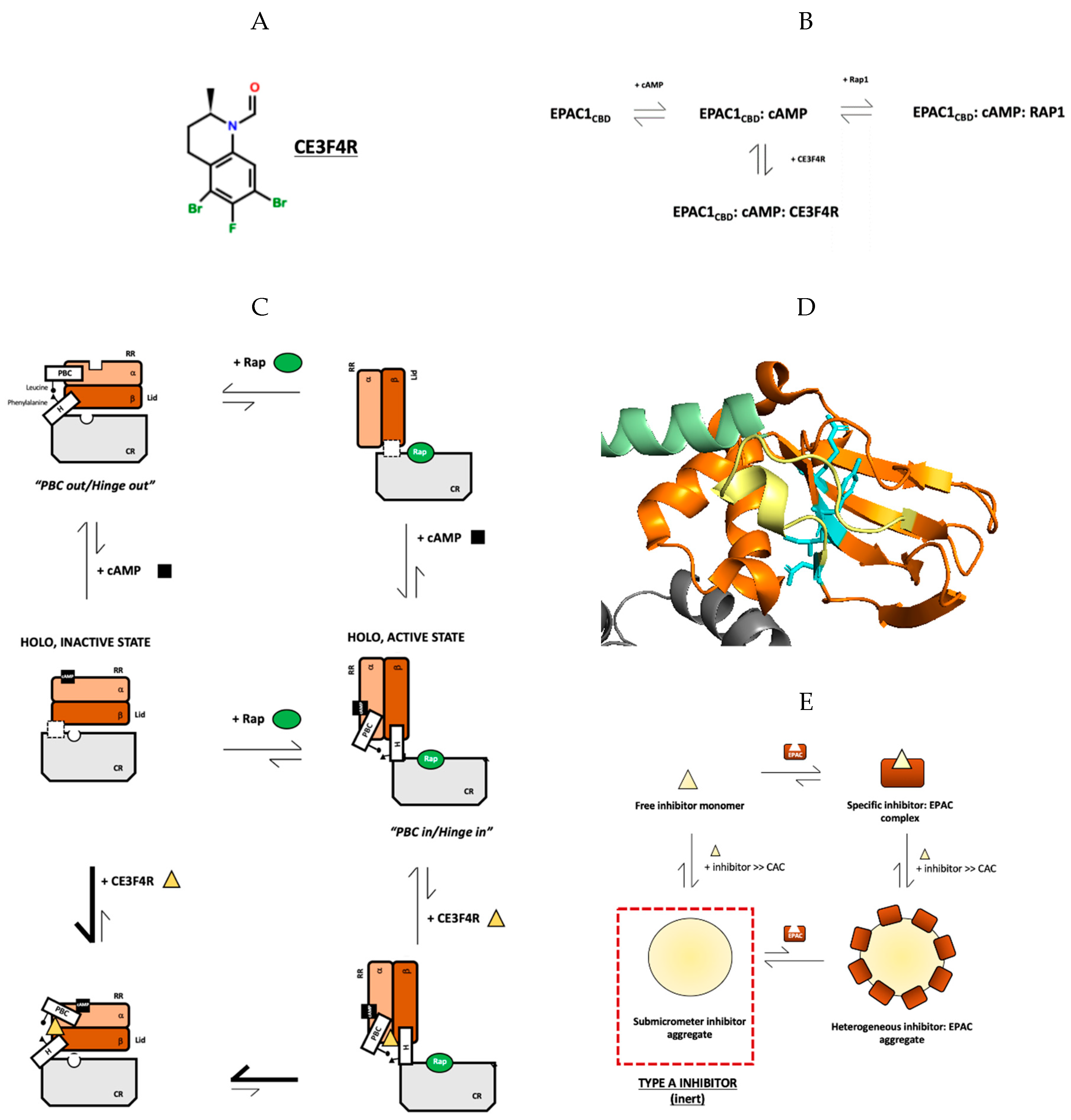
4. Specific and Non-Specific Inhibition of EPAC1 by CE3F4R
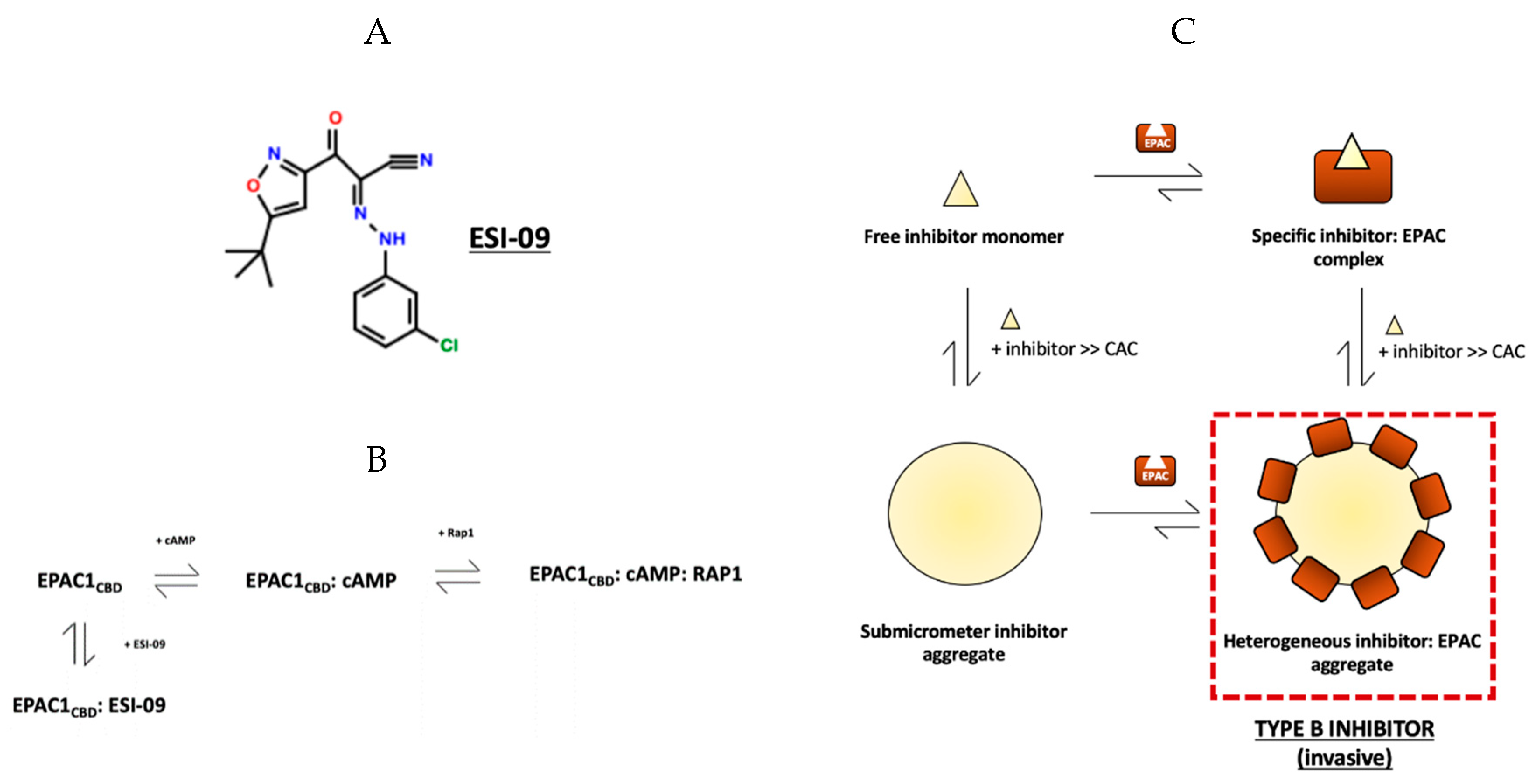
5. Specific and Non-Specific Inhibition of EPAC1 by ESI-09
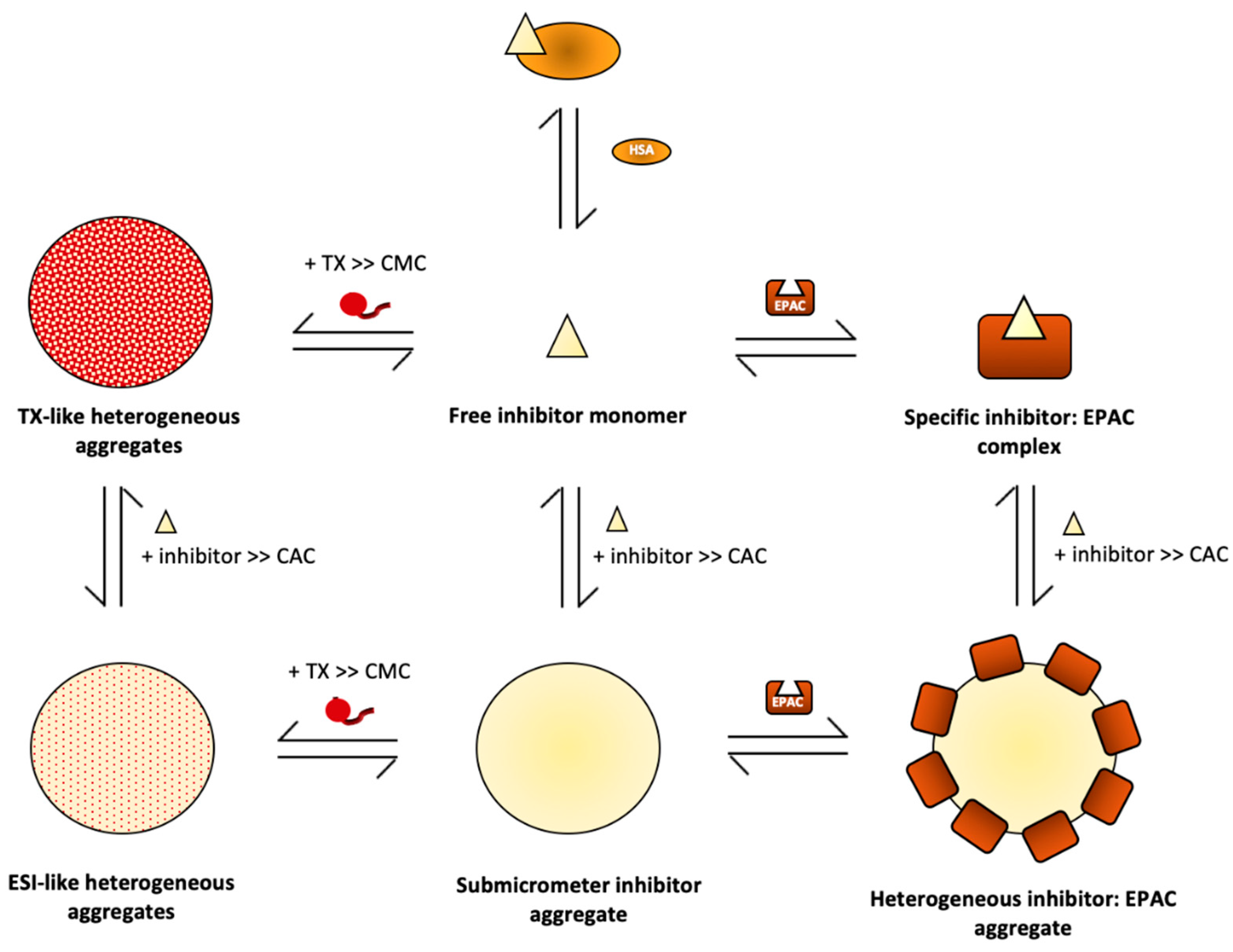
6. Attenuation of Aggregation-Based Inhibition Caused by EPAC-Specific Inhibitors
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviation | Description |
| ABI | aggregation-prone inhibition |
| BBR | base-binding region |
| BSA | bovine serum albumin |
| CAC | critical aggregation concentration |
| cAMP | cyclic adenosine monophosphate |
| CBD | cAMP binding domain |
| CD25HD | classic CD25 homology domain |
| CHESPA | chemical shift projection analyses |
| CMC | critical micellar concentration |
| CNG | cyclic nucleotide gated channels |
| CR | catalytic region |
| DLS | dynamic light scattering |
| EPAC | exchange protein directly activated by cAMP |
| ESI | EPAC-specific inhibitor |
| GTP | guanosine triphosphate |
| GDP | guanosine diphosphate |
| HCN | hyperpolarization activated cyclic nucleotide gated channels |
| HSA | human serum albumin |
| HSQC | heteronuclear single quantum coherence |
| HTS | high-throughput screen |
| IL | ionic latch |
| Kd | dissociation constant |
| PBC | phosphate-binding cassette |
| PKA | cAMP-dependent protein kinase A |
| Popdc | Popeye domain containing proteins |
| PRE | paramagnetic relaxation enhancement |
| RA | Ras association domain |
| REM | Ras-exchange motif |
| RR | regulatory region |
| SLAPSTIC | spin-labels attached to protein side chain |
| SPR | surface plasmon resonance |
| STD | saturation transfer difference |
| TEM | transmission electron microscopy |
| TX | Triton X100 |
References
- Berman, H.M.; Ten Eyck, L.F.; Goodsell, D.S.; Haste, N.M.; Kornev, A.; Taylor, S.S. The cAMP binding domain: An ancient signaling module. Proc. Natl. Acad. Sci. 2005, 102, 45–50. [Google Scholar] [CrossRef]
- Berdeaux, R.; Stewart, R. cAMP signaling in skeletal muscle adaptation: Hypertrophy, metabolism, and regeneration. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E1–E17. [Google Scholar] [CrossRef]
- Berthet, J.; Rall, T.W.; Sutherland, E.W. The relationship of epinephrine and glucagon to liver phosphorylase. IV. Effect of epinephrine and glucagon on the reactivation of phosphorylase in liver homogenates. J. Biol. Chem. 1957, 224, 463–475. [Google Scholar] [PubMed]
- Ravnskjaer, K.; Madiraju, A.; Montminy, M. Role of the cAMP pathway in glucose and lipid metabolism. Handb. Exp. Pharmacol. 2015, 233, 29–49. [Google Scholar]
- Spirli, C.; Mariotti, V.; Villani, A.; Fabris, L.; Fiorotto, R.; Strazzabosco, M. Adenylyl cyclase 5 links changes in calcium homeostasis to cAMP-dependent cyst growth in polycystic liver disease. J. Hepatol. 2017, 66, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Insel, P.A.; Zhang, L.; Murray, F.; Yokouchi, H.; Zambon, A.C. Cyclic AMP is both a pro-apoptotic and anti-apoptotic second messenger. Acta Physiol. 2012, 204, 277. [Google Scholar] [CrossRef]
- Serezani, C.H.; Ballinger, M.N.; Aronoff, D.M.; Peters-Golden, M. Cyclic AMP: Master regulator of innate immune cell function. Am. J. Respir. Cell Mol. Biol. 2008, 39, 127–132. [Google Scholar] [CrossRef]
- Rangarajan, S.; Enserink, J.M.; Kuiperij, H.B.; de Rooij, J.; Price, L.S.; Schwede, F.; Bos, J.L. Cyclic AMP induces integrin-mediated cell adhesion through Epac and Rap1 upon stimulation of the β2-adrenergic receptor. J. Cell Biol. 2003, 160, 487. [Google Scholar] [CrossRef]
- Tengholm, A.; Gylfe, E. cAMP signalling in insulin and glucagon secretion. Diabetes, Obes. Metab. 2017, 19, 42–53. [Google Scholar] [CrossRef]
- Zambon, A.C.; Zhang, L.; Minovitsky, S.; Kanter, J.R.; Prabhakar, S.; Salomonis, N.; Vranizan, K.; Dubchak, I.; Conklin, B.R.; Insel, P.A. Gene expression patterns define key transcriptional events in cell-cycle regulation by cAMP and protein kinase A. Proc. Natl. Acad. Sci. 2005, 102, 8561–8566. [Google Scholar] [CrossRef]
- Cohen, P. Protein kinases — The major drug targets of the twenty-first century? Nat. Rev. Drug Discov. 2002, 1, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Biel, M. Cyclic nucleotide-regulated cation channels. J. Biol. Chem. 2009, 284, 9017–9021. [Google Scholar] [CrossRef] [PubMed]
- Biel, M.; Michalakis, S. Cyclic nucleotide-gated channels. In cGMP: Generators, Effectors and Therapeutic Implications; Springer: Berlin/Heidelberg, Germany, 2009; pp. 111–136. [Google Scholar]
- Schindler, R.F.R.; Brand, T. The Popeye domain containing protein family – A novel class of cAMP effectors with important functions in multiple tissues. Prog. Biophys. Mol. Biol. 2016, 120, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Krähling, A.M.; Alvarez, L.; Debowski, K.; Van, Q.; Gunkel, M.; Irsen, S.; Al-Amoudi, A.; Strünker, T.; Kremmer, E.; Krause, E.; et al. CRIS—A novel cAMP-binding protein controlling spermiogenesis and the development of flagellar bending. PLoS Genet. 2013, 9, e1003960. [Google Scholar] [CrossRef] [PubMed]
- de Rooij, J.; Zwartkruis, F.J.T.; Verheijen, M.H.G.; Cool, R.H.; Nijman, S.M.B.; Wittinghofer, A.; Bos, J.L. Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature 1998, 396, 474–477. [Google Scholar] [CrossRef]
- Kawasaki, H.; Springett, G.M.; Mochizuki, N.; Toki, S.; Nakaya, M.; Matsuda, M.; Housman, D.E.; Graybiel, A.M. A family of cAMP-binding proteins that directly activate Rap1. Science 1998, 282, 2275–2279. [Google Scholar] [CrossRef]
- Schmidt, M.; Dekker, F.J.; Maarsingh, H. Exchange protein directly activated by cAMP (epac): A multidomain cAMP mediator in the regulation of diverse biological functions. Pharmacol. Rev. 2013, 65, 670–709. [Google Scholar] [CrossRef]
- Yokoyama, U.; Minamisawa, S.; Quan, H.; Akaike, T.; Jin, M.; Otsu, K.; Ulucan, C.; Wang, X.; Baljinnyam, E.; Takaoka, M.; et al. Epac1 is upregulated during neointima formation and promotes vascular smooth muscle cell migration. Am. J. Physiol. Circ. Physiol. 2008, 295, H1547–H1555. [Google Scholar] [CrossRef]
- Yokoyama, U.; Minamisawa, S.; Quan, H.; Akaike, T.; Suzuki, S.; Jin, M.; Jiao, Q.; Watanabe, M.; Otsu, K.; Iwasaki, S.; et al. Prostaglandin E 2 -activated Epac promotes neointimal formation of the rat ductus arteriosus by a process distinct from that of cAMP-dependent protein kinase A. J. Biol. Chem. 2008, 283, 28702–28709. [Google Scholar] [CrossRef]
- Zhang, C.-L.; Katoh, M.; Shibasaki, T.; Minami, K.; Sunaga, Y.; Takahashi, H.; Yokoi, N.; Iwasaki, M.; Miki, T.; Seino, S. The cAMP sensor Epac2 is a direct target of antidiabetic sulfonylurea drugs. Science 2009, 325, 607–610. [Google Scholar] [CrossRef]
- Zhong, N. cAMP acts on exchange protein activated by cAMP/cAMP-regulated guanine nucleotide exchange protein to regulate transmitter release at the crayfish neuromuscular junction. J. Neurosci. 2005, 25, 208–214. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ouyang, M.; Zhang, L.; Zhu, J.J.; Schwede, F.; Thomas, S.A. Epac signaling is required for hippocampus-dependent memory retrieval. Proc. Natl. Acad. Sci. 2008, 105, 11993–11997. [Google Scholar] [CrossRef] [PubMed]
- Almahariq, M.; Tsalkova, T.; Mei, F.C.; Chen, H.; Zhou, J.; Sastry, S.K.; Schwede, F.; Cheng, X. A Novel EPAC-specific inhibitor suppresses pancreatic cancer cell migration and invasion. Mol. Pharmacol. 2013, 83, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Gupta, S.; Dabral, S.; Singh, S.; Sehrawat, S. Role of exchange protein directly activated by cAMP (EPAC1) in breast cancer cell migration and apoptosis. Mol. Cell. Biochem. 2017, 430, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Métrich, M.; Lucas, A.; Gastineau, M.; Samuel, J.-L.; Heymes, C.; Morel, E.; Lezoualc’h, F. Epac mediates β-adrenergic receptor–induced cardiomyocyte hypertrophy. Circ. Res. 2008, 102, 959–965. [Google Scholar] [CrossRef]
- Barker, G.; Parnell, E.; van Basten, B.; Buist, H.; Adams, D.; Yarwood, S. The potential of a novel class of EPAC-selective agonists to combat cardiovascular inflammation. J. Cardiovasc. Dev. Dis. 2017, 4, 22. [Google Scholar] [CrossRef]
- Suzuki, S.; Yokoyama, U.; Abe, T.; Kiyonari, H.; Yamashita, N.; Kato, Y.; Kurotani, R.; Sato, M.; Okumura, S.; Ishikawa, Y. Differential roles of Epac in regulating cell death in neuronal and myocardial cells. J. Biol. Chem. 2010, 285, 24248–24259. [Google Scholar] [CrossRef]
- Tao, X.; Mei, F.; Agrawal, A.; Peters, C.J.; Ksiazek, T.G.; Cheng, X.; Tseng, C.-T.K. Blocking of exchange proteins directly activated by cAMP leads to reduced replication of middle east respiratory syndrome coronavirus. J. Virol. 2014, 88, 3902. [Google Scholar] [CrossRef]
- Dawn, A.; Singh, S.; More, K.R.; Siddiqui, F.A.; Pachikara, N.; Ramdani, G.; Langsley, G.; Chitnis, C.E. The central role of cAMP in regulating plasmodium falciparum merozoite invasion of human erythrocytes. PLoS Pathog. 2014, 10, e1004520. [Google Scholar] [CrossRef]
- Lezoualc’h, F.; Fazal, L.; Laudette, M.; Conte, C. Cyclic AMP sensor EPAC proteins and their role in cardiovascular function and disease. Circ. Res. 2016, 118, 881–897. [Google Scholar] [CrossRef]
- Robichaux, W.G.; Cheng, X. Intracellular cAMP sensor EPAC: Physiology, pathophysiology, and therapeutics development. Physiol. Rev. 2018, 98, 919–1053. [Google Scholar] [CrossRef] [PubMed]
- Breckler, M.; Berthouze, M.; Laurent, A.-C.; Crozatier, B.; Morel, E.; Lezoualc’h, F. Rap-linked cAMP signaling Epac proteins: Compartmentation, functioning and disease implications. Cell. Signal. 2011, 23, 1257–1266. [Google Scholar] [CrossRef] [PubMed]
- McGovern, S.L.; Helfand, B.T.; Feng, B.; Shoichet, B.K. A specific mechanism of nonspecific inhibition. J. Med. Chem. 2003, 46, 4265–4272. [Google Scholar] [CrossRef] [PubMed]
- McGovern, S.L.; Caselli, E.; Grigorieff, N.; Shoichet, B.K. A common mechanism underlying promiscuous inhibitors from virtual and high-throughput screening. J. Med. Chem. 2002, 45, 1712–1722. [Google Scholar] [CrossRef]
- Coan, K.E.D.; Maltby, D.A.; Burlingame, A.L.; Shoichet, B.K. Promiscuous aggregate-based inhibitors promote enzyme unfolding. J. Med. Chem. 2009, 52, 2067–2075. [Google Scholar] [CrossRef]
- Feng, B.Y.; Shelat, A.; Doman, T.N.; Guy, R.K.; Shoichet, B.K. High-throughput assays for promiscuous inhibitors. Nat. Chem. Biol. 2005, 1, 146–148. [Google Scholar] [CrossRef]
- Singhmar, P.; Huo, X.; Li, Y.; Dougherty, P.M.; Mei, F.; Cheng, X.; Heijnen, C.J.; Kavelaars, A. An orally active Epac inhibitor reverses mechanical allodynia and loss of intraepidermal nerve fibers in a mouse model of chemotherapy-induced peripheral neuropathy. Pain 2018, 159, 884. [Google Scholar] [CrossRef]
- Sukhanova, I.F.; Kozhevnikova, L.M.; Mironova, G.Y.; Avdonin, P.V. The Epac protein inhibitor ESI-09 eliminates the tonic phase of aorta contraction induced by endogenic vasoconstrictors in rats. Biol. Bull. 2017, 44, 179–186. [Google Scholar] [CrossRef]
- Owen, S.C.; Doak, A.K.; Wassam, P.; Shoichet, M.S.; Shoichet, B.K. Colloidal aggregation affects the efficacy of anticancer drugs in cell culture. ACS Chem. Biol. 2012, 7, 1429–1435. [Google Scholar] [CrossRef]
- Ganesh, A.N.; Donders, E.N.; Shoichet, B.K.; Shoichet, M.S. Colloidal aggregation: From screening nuisance to formulation nuance. Nano Today 2018, 19, 188–200. [Google Scholar] [CrossRef]
- Seidler, J.; McGovern, S.L.; Doman, T.N.; Shoichet, B.K. Identification and prediction of promiscuous aggregating inhibitors among known drugs. J. Med. Chem. 2003, 46, 4477–4486. [Google Scholar] [CrossRef] [PubMed]
- Habig, M.; Blechschmidt, A.; Dressler, S.; Hess, B.; Patel, V.; Billich, A.; Ostermeier, C.; Beer, D.; Klumpp, M. Efficient elimination of nonstoichiometric enzyme inhibitors from HTS hit lists. J. Biomol. Screen. 2009, 14, 679–689. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Coan, K.E.D.; Shoichet, B.K. Stability and equilibria of promiscuous aggregates in high protein milieus. Mol. Biosyst. 2007, 3, 208. [Google Scholar] [CrossRef] [PubMed]
- Boulton, S.; Selvaratnam, R.; Blondeau, J.-P.; Lezoualc’h, F.; Melacini, G. Mechanism of selective enzyme inhibition through uncompetitive regulation of an allosteric agonist. J. Am. Chem. Soc. 2018, 140, 9624–9637. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Chen, H.; Boulton, S.; Mei, F.; Ye, N.; Melacini, G.; Zhou, J.; Cheng, X. Biochemical and pharmacological characterizations of ESI-09 based EPAC inhibitors: Defining the ESI-09 “therapeutic window. ” Sci. Rep. 2015, 5, 9344. [Google Scholar] [CrossRef]
- Boulton, S.; Selvaratnam, R.; Ahmed, R.; Van, K.; Cheng, X.; Melacini, G. Mechanisms of specific versus nonspecific interactions of aggregation-prone inhibitors and attenuators. J. Med. Chem. 2019, 62, 5063–5079. [Google Scholar] [CrossRef]
- Rehmann, H.; Das, J.; Knipscheer, P.; Wittinghofer, A.; Bos, J.L. Structure of the cyclic-AMP-responsive exchange factor Epac2 in its auto-inhibited state. Nature 2006, 439, 625–628. [Google Scholar] [CrossRef]
- Rehmann, H.; Arias-Palomo, E.; Hadders, M.A.; Schwede, F.; Llorca, O.; Bos, J.L. Structure of Epac2 in complex with a cyclic AMP analogue and RAP1B. Nature 2008, 455, 124–127. [Google Scholar] [CrossRef]
- Niimura, M.; Miki, T.; Shibasaki, T.; Fujimoto, W.; Iwanaga, T.; Seino, S. Critical role of the N-terminal cyclic AMP-binding domain of Epac2 in its subcellular localization and function. J. Cell. Physiol. 2009, 219, 652–658. [Google Scholar] [CrossRef]
- Das, R.; Mazhab-Jafari, M.T.; Chowdhury, S.; SilDas, S.; Selvaratnam, R.; Melacini, G. Entropy-driven cAMP-dependent allosteric control of inhibitory interactions in exchange proteins directly activated by cAMP. J. Biol. Chem. 2008, 283, 19691–19703. [Google Scholar] [CrossRef]
- Yu, S.; Fan, F.; Flores, S.C.; Mei, F.; Cheng, X. Dissecting the mechanism of Epac activation via hydrogen-deuterium exchange FT-IR and structural modeling. Biochemistry 2006, 45, 15318–15326. [Google Scholar] [CrossRef] [PubMed]
- VanSchouwen, B.; Selvaratnam, R.; Fogolari, F.; Melacini, G. Role of dynamics in the autoinhibition and activation of the exchange protein directly activated by cyclic AMP (EPAC). J. Biol. Chem. 2011, 286, 42655–42669. [Google Scholar] [CrossRef] [PubMed]
- Fogolari, F.; Corazza, A.; Fortuna, S.; Soler, M.A.; VanSchouwen, B.; Brancolini, G.; Corni, S.; Melacini, G.; Esposito, G. Distance-based configurational entropy of proteins from molecular dynamics simulations. PLoS ONE 2015, 10, e0132356. [Google Scholar] [CrossRef] [PubMed]
- Kornev, A.P.; Taylor, S.S.; Ten Eyck, L.F. A generalized allosteric mechanism for cis-regulated cyclic nucleotide binding domains. PLoS Comput. Biol. 2008, 4, e1000056. [Google Scholar] [CrossRef]
- Rehmann, H.; Prakash, B.; Wolf, E.; Rueppel, A.; de Rooij, J.; Bos, J.L.; Wittinghofer, A. Structure and regulation of the cAMP-binding domains of Epac2. Nat. Struct. Biol. 2003, 10, 26–32. [Google Scholar] [CrossRef]
- Tsalkova, T.; Blumenthal, D.K.; Mei, F.C.; White, M.A.; Cheng, X. Mechanism of Epac activation. J. Biol. Chem. 2009, 284, 23644–23651. [Google Scholar] [CrossRef]
- Selvaratnam, R.; Mazhab-Jafari, M.T.; Das, R.; Melacini, G. The auto-inhibitory role of the EPAC hinge helix as mapped by NMR. PLoS ONE 2012, 7, e48707. [Google Scholar] [CrossRef]
- Enserink, J.M.; Christensen, A.E.; de Rooij, J.; van Triest, M.; Schwede, F.; Genieser, H.G.; Døskeland, S.O.; Blank, J.L.; Bos, J.L. A novel Epac-specific cAMP analogue demonstrates independent regulation of Rap1 and ERK. Nat. Cell Biol. 2002, 4, 901–906. [Google Scholar] [CrossRef]
- Tsalkova, T.; Mei, F.C.; Cheng, X. A fluorescence-based high-throughput assay for the discovery of exchange protein directly activated by cyclic AMP (EPAC) antagonists. PLoS ONE 2012, 7, e30441. [Google Scholar] [CrossRef]
- Chen, H.; Tsalkova, T.; Mei, F.C.; Hu, Y.; Cheng, X.; Zhou, J. 5-Cyano-6-oxo-1,6-dihydro-pyrimidines as potent antagonists targeting exchange proteins directly activated by cAMP. Bioorg. Med. Chem. Lett. 2012, 22, 4038–4043. [Google Scholar] [CrossRef]
- Courilleau, D.; Bisserier, M.; Jullian, J.-C.; Lucas, A.; Bouyssou, P.; Fischmeister, R.; Blondeau, J.-P.; Lezoualc’h, F. Identification of a tetrahydroquinoline analog as a pharmacological inhibitor of the cAMP-binding protein Epac. J. Biol. Chem. 2012, 287, 44192–44202. [Google Scholar] [CrossRef] [PubMed]
- Sonawane, Y.A.; Zhu, Y.; Garrison, J.C.; Ezell, E.L.; Zahid, M.; Cheng, X.; Natarajan, A. Structure–activity relationship studies with tetrahydroquinoline analogs as EPAC inhibitors. ACS Med. Chem. Lett. 2017, 8, 1183–1187. [Google Scholar] [CrossRef] [PubMed]
- Courilleau, D.; Bouyssou, P.; Fischmeister, R.; Lezoualc’h, F.; Blondeau, J.-P. The (R)-enantiomer of CE3F4 is a preferential inhibitor of human exchange protein directly activated by cyclic AMP isoform 1 (Epac1). Biochem. Biophys. Res. Commun. 2013, 440, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.M.; Rogers, K.E.; Aroonsakool, N.; McCammon, J.A.; Insel, P.A. Allosteric inhibition of Epac. J. Biol. Chem. 2014, 289, 29148–29157. [Google Scholar] [CrossRef]
- Brown, L.M.; Rogers, K.E.; McCammon, J.A.; Insel, P.A. Identification and validation of modulators of exchange protein activated by cAMP (Epac) activity. J. Biol. Chem. 2014, 289, 8217–8230. [Google Scholar] [CrossRef]
- Parnell, E.; McElroy, S.P.; Wiejak, J.; Baillie, G.L.; Porter, A.; Adams, D.R.; Rehmann, H.; Smith, B.O.; Yarwood, S.J. Identification of a novel, small molecule partial agonist for the cyclic AMP sensor, EPAC1. Sci. Rep. 2017, 7, 294. [Google Scholar] [CrossRef]
- Gong, B.; Shelite, T.; Mei, F.C.; Ha, T.; Hu, Y.; Xu, G.; Chang, Q.; Wakamiya, M.; Ksiazek, T.G.; Boor, P.J.; et al. Exchange protein directly activated by cAMP plays a critical role in bacterial invasion during fatal rickettsioses. Proc. Natl. Acad. Sci. 2013, 110, 19615–19620. [Google Scholar] [CrossRef]
- Herries, D.G. Principles of enzyme kinetics. Biochem. Educ. 1976, 4, 36. [Google Scholar] [CrossRef]
- Robin, T.; Reuveni, S.; Urbakh, M. Single-molecule theory of enzymatic inhibition. Nat. Commun. 2018, 9, 779. [Google Scholar] [CrossRef]
- Boulton, S.; Melacini, G. Advances in NMR methods to map allosteric sites: From models to translation. Chem. Rev. 2016, 116, 6267–6304. [Google Scholar] [CrossRef]
- Fayos, R.; Melacini, G.; Newlon, M.G.; Burns, L.; Scott, J.D.; Jennings, P.A. Induction of flexibility through protein-protein interactions. J. Biol. Chem. 2003, 278, 18581–18587. [Google Scholar] [CrossRef] [PubMed]
- McNicholl, E.T.; Das, R.; SilDas, S.; Taylor, S.S.; Melacini, G. Communication between tandem cAMP binding domains in the regulatory subunit of protein kinase A-Iα as revealed by domain-silencing mutations. J. Biol. Chem. 2010, 285, 15523–15537. [Google Scholar] [CrossRef] [PubMed]
- Selvaratnam, R.; VanSchouwen, B.; Fogolari, F.; Mazhab-Jafari, M.T.; Das, R.; Melacini, G. The projection analysis of NMR chemical shifts reveals extended EPAC autoinhibition determinants. Biophys. J. 2012, 102, 630–639. [Google Scholar] [CrossRef] [PubMed]
- Moleschi, K.J.; Akimoto, M.; Melacini, G. Measurement of state-specific association constants in allosteric sensors through molecular stapling and NMR. J. Am. Chem. Soc. 2015, 137, 10777–10785. [Google Scholar] [CrossRef] [PubMed]
- Akimoto, M.; Zhang, Z.; Boulton, S.; Selvaratnam, R.; VanSchouwen, B.; Gloyd, M.; Accili, E.A.; Lange, O.F.; Melacini, G. A mechanism for the auto-inhibition of hyperpolarization-activated cyclic nucleotide-gated (HCN) channel opening and its relief by cAMP. J. Biol. Chem. 2014, 289, 22205–22220. [Google Scholar] [CrossRef] [PubMed]
- Boulton, S.; Akimoto, M.; Akbarizadeh, S.; Melacini, G. Free energy landscape remodeling of the cardiac pacemaker channel explains the molecular basis of familial sinus bradycardia. J. Biol. Chem. 2017, 292, 6414–6428. [Google Scholar] [CrossRef]
- Badireddy, S.; Yunfeng, G.; Ritchie, M.; Akamine, P.; Wu, J.; Kim, C.W.; Taylor, S.S.; Qingsong, L.; Swaminathan, K.; Anand, G.S. Cyclic AMP analog blocks kinase activation by stabilizing inactive conformation: Conformational selection highlights a new concept in allosteric inhibitor design. Mol. Cell. Proteomics 2011, 10, M110-004390. [Google Scholar] [CrossRef]
- VanSchouwen, B.; Selvaratnam, R.; Giri, R.; Lorenz, R.; Herberg, F.W.; Kim, C.; Melacini, G. Mechanism of cAMP partial agonism in protein kinase G (PKG). J. Biol. Chem. 2015, 290, 28631–28641. [Google Scholar] [CrossRef]
- Huang, G.Y.; Kim, J.J.; Reger, A.S.; Lorenz, R.; Moon, E.-W.; Zhao, C.; Casteel, D.E.; Bertinetti, D.; VanSchouwen, B.; Selvaratnam, R.; et al. Structural basis for cyclic-nucleotide selectivity and cGMP-selective activation of PKG I. Structure 2014, 22, 116–124. [Google Scholar] [CrossRef]
- Ruschak, A.M.; Kay, L.E. Proteasome allostery as a population shift between interchanging conformers. Proc. Natl. Acad. Sci. 2012, 109, E3454–E3462. [Google Scholar] [CrossRef]
- Jahnke, W. Spin labels as a tool to identify and characterize protein-ligand interactions by NMR spectroscopy. ChemBioChem 2002, 3, 167–173. [Google Scholar] [CrossRef]
- Jahnke, W.; Rüdisser, S.; Zurini, M. Spin label enhanced NMR screening. J. Am. Chem. Soc. 2001, 123, 3149–3150. [Google Scholar] [CrossRef] [PubMed]
- Owen, S.C.; Doak, A.K.; Ganesh, A.N.; Nedyalkova, L.; McLaughlin, C.K.; Shoichet, B.K.; Shoichet, M.S. Colloidal drug formulations can explain “bell-shaped” concentration-response curves. ACS Chem. Biol. 2014, 9, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J.; Baldwin, L.A. The frequency of U-shaped dose responses in the toxicological literature. Toxicol. Sci. 2001, 62, 330–338. [Google Scholar] [CrossRef]
- Tsalkova, T.; Mei, F.C.; Li, S.; Chepurny, O.G.; Leech, C.A.; Liu, T.; Holz, G.G.; Woods, V.L.; Cheng, X. Isoform-specific antagonists of exchange proteins directly activated by cAMP. Proc. Natl. Acad. Sci. 2012, 109, 18613–18618. [Google Scholar] [CrossRef]
- Almahariq, M.; Mei, F.C.; Wang, H.; Cao, A.T.; Yao, S.; Soong, L.; Sun, J.; Cong, Y.; Chen, J.; Cheng, X. Exchange protein directly activated by cAMP modulates regulatory T-cell-mediated immunosuppression. Biochem. J. 2015, 465, 295–303. [Google Scholar] [CrossRef]
- Rehmann, H. Epac-inhibitors: Facts and artefacts. Sci. Rep. 2013, 3, 3032. [Google Scholar] [CrossRef]
- Ding, C.; Zhang, Y.; Chen, H.; Yang, Z.; Wild, C.; Chu, L.; Liu, H.; Shen, Q.; Zhou, J. Novel nitrogen-enriched oridonin analogues with thiazole-fused A-ring: Protecting group-free synthesis, enhanced anticancer profile, and improved aqueous solubility. J. Med. Chem. 2013, 56, 5048–5058. [Google Scholar] [CrossRef]
- LaPlante, S.R.; Carson, R.; Gillard, J.; Aubry, N.; Coulombe, R.; Bordeleau, S.; Bonneau, P.; Little, M.; O’Meara, J.; Beaulieu, P.L. Compound aggregation in drug discovery: Implementing a practical NMR assay for medicinal chemists. J. Med. Chem. 2013, 56, 5142–5150. [Google Scholar] [CrossRef]
- Ryan, A.J.; Gray, N.M.; Lowe, P.N.; Chung, C. Effect of detergent on “promiscuous” inhibitors. J. Med. Chem. 2003, 46, 3448–3451. [Google Scholar] [CrossRef]
- Feng, B.Y.; Simeonov, A.; Jadhav, A.; Babaoglu, K.; Inglese, J.; Shoichet, B.K.; Austin, C.P. A high-throughput screen for aggregation-based inhibition in a large compound library. J. Med. Chem. 2007, 50, 2385–2390. [Google Scholar] [CrossRef] [PubMed]
- Peña, I.; Domínguez, J.M. Thermally denatured BSA, a surrogate additive to replace BSA in buffers for high-throughput screening. J. Biomol. Screen. 2010, 15, 1281–1286. [Google Scholar] [CrossRef] [PubMed]
- Dockal, M.; Carter, D.C.; Rüker, F. The three recombinant domains of human serum albumin. J. Biol. Chem. 1999, 274, 29303–29310. [Google Scholar] [CrossRef] [PubMed]
- Tiller, G.E.; Mueller, T.J.; Dockter, M.E.; Struve, W.G. Hydrogenation of triton X-100 eliminates its fluorescence and ultraviolet light absorption while preserving its detergent properties. Anal. Biochem. 1984, 141, 262–266. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, A.; Boulton, S.; Shao, H.; Akimoto, M.; Natarajan, A.; Cheng, X.; Melacini, G. Recent Advances in EPAC-Targeted Therapies: A Biophysical Perspective. Cells 2019, 8, 1462. https://doi.org/10.3390/cells8111462
Ahmed A, Boulton S, Shao H, Akimoto M, Natarajan A, Cheng X, Melacini G. Recent Advances in EPAC-Targeted Therapies: A Biophysical Perspective. Cells. 2019; 8(11):1462. https://doi.org/10.3390/cells8111462
Chicago/Turabian StyleAhmed, Alveena, Stephen Boulton, Hongzhao Shao, Madoka Akimoto, Amarnath Natarajan, Xiaodong Cheng, and Giuseppe Melacini. 2019. "Recent Advances in EPAC-Targeted Therapies: A Biophysical Perspective" Cells 8, no. 11: 1462. https://doi.org/10.3390/cells8111462
APA StyleAhmed, A., Boulton, S., Shao, H., Akimoto, M., Natarajan, A., Cheng, X., & Melacini, G. (2019). Recent Advances in EPAC-Targeted Therapies: A Biophysical Perspective. Cells, 8(11), 1462. https://doi.org/10.3390/cells8111462




