The Potential of Different Origin Stem Cells in Modulating Oral Bone Regeneration Processes
Abstract
1. Introduction
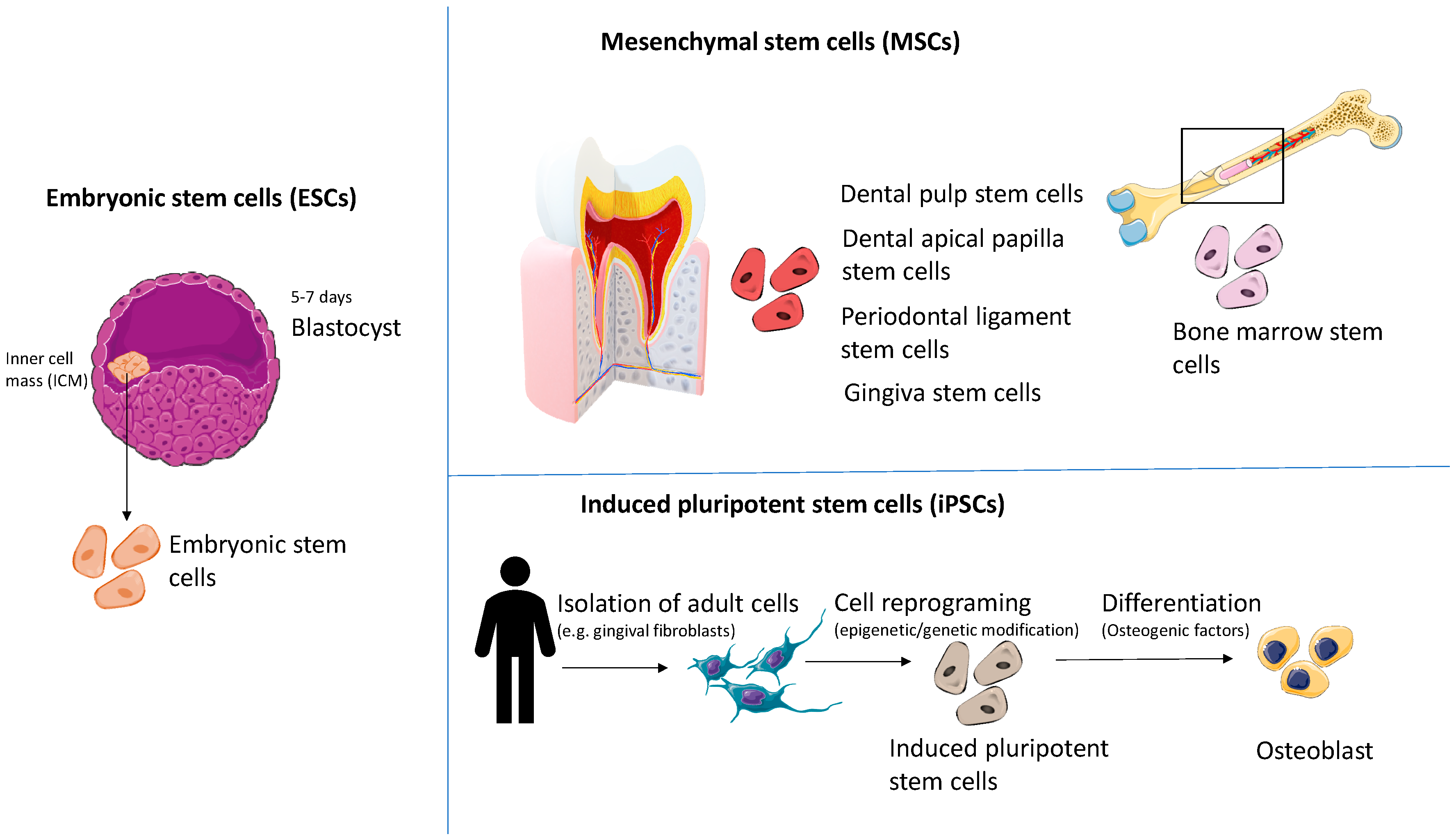
2. Types of Stem Cells Used in Regenerative Medicine
2.1. Embryonic Stem Cells (ESCs)
2.2. Mesenchymal Stem Cells (MSCs)
2.3. Induced Pluripotent Stem Cells (iPSCs)
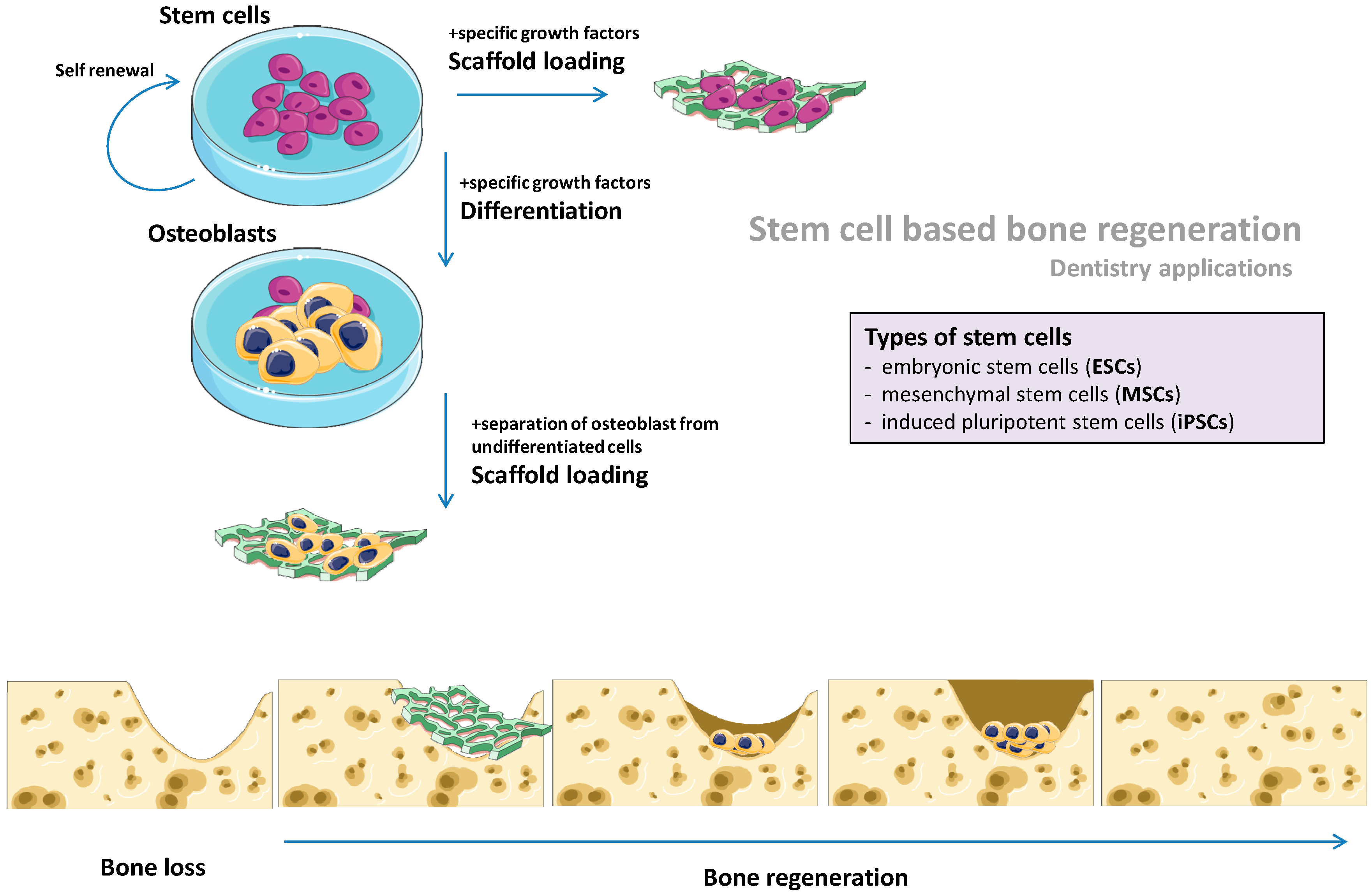
3. Stem Cells-Based Scaffold-Free Bone Regeneration
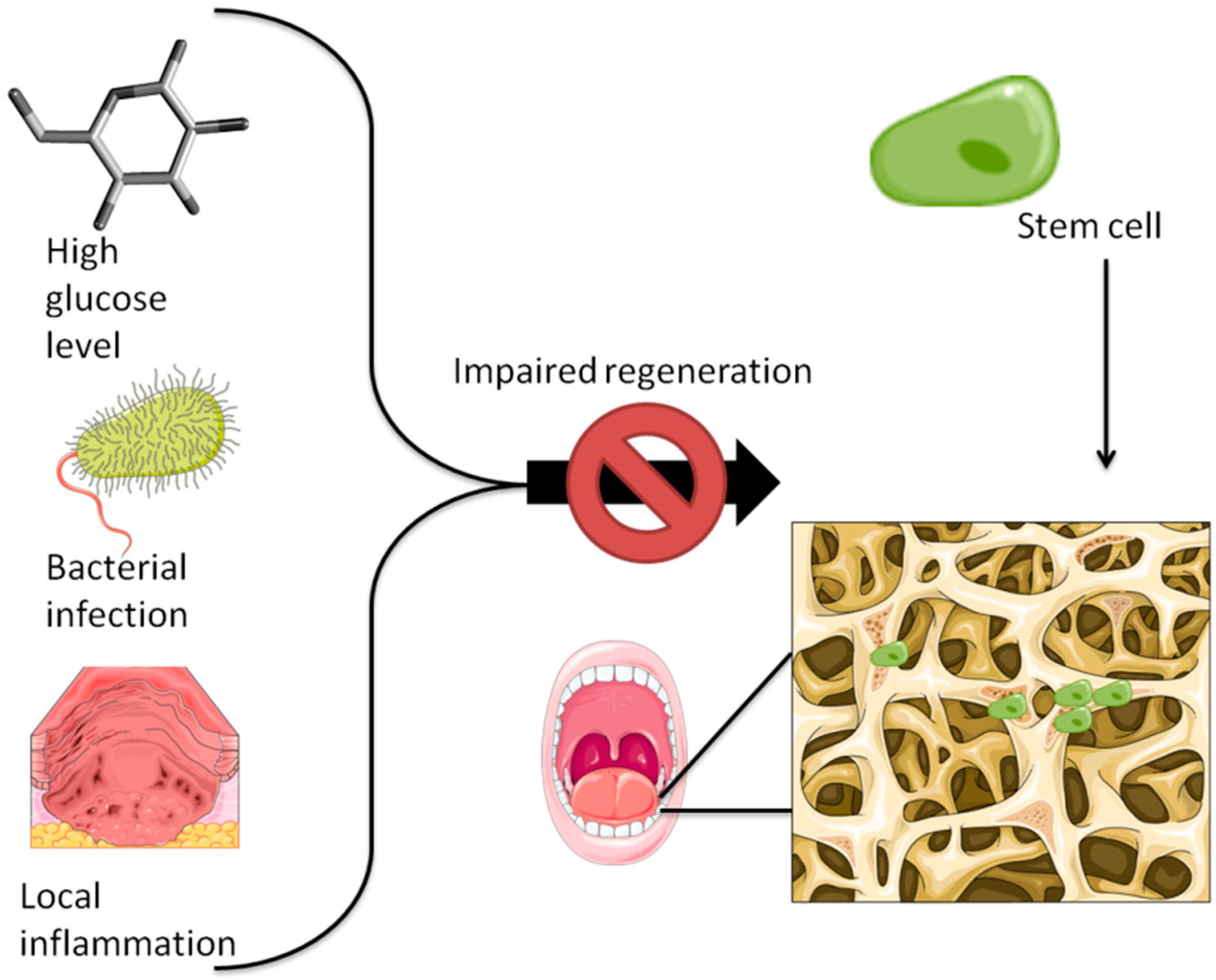
4. The Osteogenic Potential of Stem Cells and Its Correlation with Various Pathologies—Focus on Mesenchymal Stem Cells
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Intini, G.; Katsuragi, Y.; Kirkwood, K.L.; Yang, S. Alveolar bone loss: Mechanisms, potential therapeutic targets, and interventions. Adv. Dent. Res. 2014, 26, 38–46. [Google Scholar] [CrossRef]
- Sakkas, A.; Wilde, F.; Heufelder, M.; Winter, K.; Schramm, A. Autogenous bone grafts in oral implantology-is it still a “gold standard”? A consecutive review of 279 patients with 456 clinical procedures. Int. J. Implant Dent. 2017, 3, 23. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, Z.; Hamdan, N.; Ikeda, Y.; Grynpas, M.; Ganss, B.; Glogauer, M. Natural graft tissues and synthetic biomaterials for periodontal and alveolar bone reconstructive applications: A review. Biomater. Res. 2017, 21, 9. [Google Scholar] [CrossRef] [PubMed]
- Walmsley, G.G.; Ransom, R.C.; Zielins, E.R.; Leavitt, T.; Flacco, J.S.; Hu, M.S.; Lee, A.S.; Longaker, M.T.; Wan, D.C. Stem Cells in Bone Regeneration. Stem Cell Rev. 2016, 12, 524–529. [Google Scholar] [CrossRef]
- Howard, D.; Buttery, L.D.; Shakesheff, K.M.; Roberts, S.J. Tissue engineering: Strategies, stem cells and scaffolds. J. Anat. 2008, 213, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, S.; Jakt, L.M.; Era, T. Embryonic stem-cell culture as a tool for developmental cell biology. Nat. Rev. Mol. Cell Biol. 2007, 8, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Adewumi, O.; Aflatoonian, B.; Ahrlund-Richter, L.; Amit, M.; Andrews, P.W.; Beighton, G.; Bello, P.A.; Benvenisty, N.; Berry, L.S.; Bevan, S.; et al. Characterization of human embryonic stem cell lines by the International Stem Cell Initiative. Nat. Biotechnol. 2007, 25, 803–816. [Google Scholar] [CrossRef] [PubMed]
- Choo, A.B.; Tan, H.L.; Ang, S.N.; Fong, W.J.; Chin, A.; Lo, J.; Zheng, L.; Hentze, H.; Philp, R.J.; Oh, S.K.; et al. Selection against undifferentiated human embryonic stem cells by a cytotoxic antibody recognizing podocalyxin-like protein-1. Stem Cells 2008, 26, 1454–1463. [Google Scholar] [CrossRef] [PubMed]
- Mohseni, R.; Hamidieh, A.A.; Verdi, J.; Shoae Hassani, A. Safe Transplantation Of Pluripotent Stem Cell By Preventing Teratoma Formation. Stem Cell Res. Ther. 2014, 4, 1000212. [Google Scholar] [CrossRef]
- English, K.; Wood, K.J. Immunogenicity of embryonic stem cell-derived progenitors after transplantation. Curr. Opin. Organ Transplant. 2011, 16, 90–95. [Google Scholar] [CrossRef]
- Pearl, J.I.; Lee, A.S.; Leveson-Gower, D.B.; Sun, N.; Ghosh, Z.; Lan, F.; Ransohoff, J.; Negrin, R.S.; Davis, M.M.; Wu, J.C. Short-term immunosuppression promotes engraftment of embryonic and induced pluripotent stem cells. Cell Stem Cell 2011, 8, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Yeo, G.S.; Lim, M.L. Maternal and fetal best interests in day-to-day obstetrics. Ann. Acad. Med. Singap. 2011, 40, 43–49. [Google Scholar] [PubMed]
- Bielby, R.C.; Boccaccini, A.R.; Polak, J.M.; Buttery, L.D. In vitro differentiation and in vivo mineralization of osteogenic cells derived from human embryonic stem cells. Tissue Eng. 2004, 10, 1518–1525. [Google Scholar] [CrossRef] [PubMed]
- Jukes, J.M.; Both, S.K.; Blitterswijk, C.A.v.; Boer, J.d. Potential of embryonic stem cells for in vivo bone regeneration. Regen. Med. 2008, 3, 783–785. [Google Scholar] [CrossRef] [PubMed]
- Jukes, J.M.; Both, S.K.; Leusink, A.; Sterk, L.M.T.; van Blitterswijk, C.A.; de Boer, J. Endochondral bone tissue engineering using embryonic stem cells. Proc. Natl. Acad. Sci. USA 2008, 105, 6840–6845. [Google Scholar] [CrossRef] [PubMed]
- Marolt, D.; Campos, I.M.; Bhumiratana, S.; Koren, A.; Petridis, P.; Zhang, G.; Spitalnik, P.F.; Grayson, W.L.; Vunjak-Novakovic, G. Engineering bone tissue from human embryonic stem cells. Proc. Natl. Acad. Sci. USA 2012, 109, 8705–8709. [Google Scholar] [CrossRef]
- Liu, X.; Wang, P.; Chen, W.; Weir, M.D.; Bao, C.; Xu, H.H. Human embryonic stem cells and macroporous calcium phosphate construct for bone regeneration in cranial defects in rats. Acta Biomater. 2014, 10, 4484–4493. [Google Scholar] [CrossRef]
- Estrela, C.; Alencar, A.H.; Kitten, G.T.; Vencio, E.F.; Gava, E. Mesenchymal stem cells in the dental tissues: Perspectives for tissue regeneration. Braz. Dent. J. 2011, 22, 91–98. [Google Scholar] [CrossRef]
- Yang, H.; Gao, L.N.; An, Y.; Hu, C.H.; Jin, F.; Zhou, J.; Jin, Y.; Chen, F.M. Comparison of mesenchymal stem cells derived from gingival tissue and periodontal ligament in different incubation conditions. Biomaterials 2013, 34, 7033–7047. [Google Scholar] [CrossRef]
- Cantore, S.; Ballini, A.; De Vito, D.; Martelli, F.S.; Georgakopoulos, I.; Almasri, M.; Dibello, V.; Altini, V.; Farronato, G.; Dipalma, G.; et al. Characterization of human apical papilla-derived stem cells. J. Biol. Regul. Homeost. Agents 2017, 31, 901–910. [Google Scholar]
- Kunimatsu, R.; Nakajima, K.; Awada, T.; Tsuka, Y.; Abe, T.; Ando, K.; Hiraki, T.; Kimura, A.; Tanimoto, K. Comparative characterization of stem cells from human exfoliated deciduous teeth, dental pulp, and bone marrow-derived mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2018, 501, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Aghajani, F.; Hooshmand, T.; Khanmohammadi, M.; Khanjani, S.; Edalatkhah, H.; Zarnani, A.H.; Kazemnejad, S. Comparative Immunophenotypic Characteristics, Proliferative Features, and Osteogenic Differentiation of Stem Cells Isolated from Human Permanent and Deciduous Teeth with Bone Marrow. Mol. Biotechnol. 2016, 58, 415–427. [Google Scholar] [CrossRef] [PubMed]
- Isobe, Y.; Koyama, N.; Nakao, K.; Osawa, K.; Ikeno, M.; Yamanaka, S.; Okubo, Y.; Fujimura, K.; Bessho, K. Comparison of human mesenchymal stem cells derived from bone marrow, synovial fluid, adult dental pulp, and exfoliated deciduous tooth pulp. Int. J. Oral Maxillofac. Surg. 2016, 45, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Al-Sharabi, N.; Xue, Y.; Fujio, M.; Ueda, M.; Gjerde, C.; Mustafa, K.; Fristad, I. Bone marrow stromal cell paracrine factors direct osteo/odontogenic differentiation of dental pulp cells. Tissue Eng. Part A 2014, 20, 3063–3072. [Google Scholar] [CrossRef] [PubMed]
- Ichiyanagi, T.; Anabuki, K.; Nishijima, Y.; Ono, H. Isolation of mesenchymal stem cells from bone marrow wastes of spinal fusion procedure (TLIF) for low back pain patients and preparation of bone dusts for transplantable autologous bone graft with a serum glue. Biosci. Trends 2010, 4, 110–118. [Google Scholar] [PubMed]
- Massa, A.; Perut, F.; Chano, T.; Woloszyk, A.; Mitsiadis, T.A.; Avnet, S.; Baldini, N. The effect of extracellular acidosis on the behaviour of mesenchymal stem cells in vitro. Eur. Cells Mater. 2017, 33, 252–267. [Google Scholar] [CrossRef]
- Han, N.; Zhang, F.; Li, G.; Zhang, X.; Lin, X.; Yang, H.; Wang, L.; Cao, Y.; Du, J.; Fan, Z. Local application of IGFBP5 protein enhanced periodontal tissue regeneration via increasing the migration, cell proliferation and osteo/dentinogenic differentiation of mesenchymal stem cells in an inflammatory niche. Stem Cell Res. Ther. 2017, 8, 210. [Google Scholar] [CrossRef]
- Liu, W.; Konermann, A.; Guo, T.; Jager, A.; Zhang, L.; Jin, Y. Canonical Wnt signaling differently modulates osteogenic differentiation of mesenchymal stem cells derived from bone marrow and from periodontal ligament under inflammatory conditions. Biochim. Biophys. Acta 2014, 1840, 1125–1134. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Z.G.; Si, Y.M.; Chen, B.; Meng, J. The difference on the osteogenic differentiation between periodontal ligament stem cells and bone marrow mesenchymal stem cells under inflammatory microenviroments. Differ. Res. Biol. Divers. 2014, 88, 97–105. [Google Scholar] [CrossRef]
- Li, C.; Li, B.; Dong, Z.; Gao, L.; He, X.; Liao, L.; Hu, C.; Wang, Q.; Jin, Y. Lipopolysaccharide differentially affects the osteogenic differentiation of periodontal ligament stem cells and bone marrow mesenchymal stem cells through Toll-like receptor 4 mediated nuclear factor kappaB pathway. Stem Cell Res. Ther. 2014, 5, 67. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, S.; Zhu, B.; Xu, Q.; Ding, Y.; Jin, Y. Composite cell sheet for periodontal regeneration: Crosstalk between different types of MSCs in cell sheet facilitates complex periodontal-like tissue regeneration. Stem Cell Res. Ther. 2016, 7, 168. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Feng, Y.; Liu, H. Conditioned media from differentiating craniofacial bone marrow stromal cells influence mineralization and proliferation in periodontal ligament stem cells. Hum. Cell 2016, 29, 162–175. [Google Scholar] [CrossRef] [PubMed]
- Yusa, K.; Yamamoto, O.; Iino, M.; Takano, H.; Fukuda, M.; Qiao, Z.; Sugiyama, T. Eluted zinc ions stimulate osteoblast differentiation and mineralization in human dental pulp stem cells for bone tissue engineering. Arch. Oral Biol. 2016, 71, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Shen, R.; Komasa, S.; Xue, Y.; Jin, B.; Hou, Y.; Okazaki, J.; Gao, J. Drug-Loadable Calcium Alginate Hydrogel System for Use in Oral Bone Tissue Repair. Int. J. Mol. Sci. 2017, 18, 989. [Google Scholar] [CrossRef] [PubMed]
- Zorin, V.L.; Komlev, V.S.; Zorina, A.I.; Khromova, N.V.; Solovieva, E.V.; Fedotov, A.Y.; Eremin, I.I.; Kopnin, P.B. Octacalcium phosphate ceramics combined with gingiva-derived stromal cells for engineered functional bone grafts. Biomed. Mater. 2014, 9, 055005. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.M.; Chiu, H.C.; Chin, Y.T.; Lin, H.Y.; Chiang, C.Y.; Tu, H.P.; Fu, M.M.; Fu, E. Effects of enamel matrix derivative on the proliferation and osteogenic differentiation of human gingival mesenchymal stem cells. Stem Cell Res. Ther. 2014, 5, 52. [Google Scholar] [CrossRef] [PubMed]
- Tansriratanawong, K.; Tamaki, Y.; Ishikawa, H.; Sato, S. Co-culture with periodontal ligament stem cells enhances osteogenic gene expression in de-differentiated fat cells. Hum. Cell 2014, 27, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Mason, S.; Tarle, S.A.; Osibin, W.; Kinfu, Y.; Kaigler, D. Standardization and safety of alveolar bone-derived stem cell isolation. J. Dent. Res. 2014, 93, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Yang, F.; Yan, X.; Yang, W.; Yu, N.; Oortgiesen, D.A.; Wang, Y.; Jansen, J.A.; Walboomers, X.F. Influence of bone marrow-derived mesenchymal stem cells pre-implantation differentiation approach on periodontal regeneration in vivo. J. Clin. Periodontol. 2015, 42, 380–389. [Google Scholar] [CrossRef]
- Miguita, L.; Mantesso, A.; Pannuti, C.M.; Deboni, M.C.Z. Can stem cells enhance bone formation in the human edentulous alveolar ridge? A systematic review and meta-analysis. Cell Tissue Bank. 2017, 18, 217–228. [Google Scholar] [CrossRef]
- Hong, J.W.; Lim, J.H.; Chung, C.J.; Kang, T.J.; Kim, T.Y.; Kim, Y.S.; Roh, T.S.; Lew, D.H. Immune Tolerance of Human Dental Pulp-Derived Mesenchymal Stem Cells Mediated by CD4(+)CD25(+)FoxP3(+) Regulatory T-Cells and Induced by TGF-beta1 and IL-10. Yonsei Med. J. 2017, 58, 1031–1039. [Google Scholar] [CrossRef] [PubMed]
- Volarevic, V.; Markovic, B.S.; Gazdic, M.; Volarevic, A.; Jovicic, N.; Arsenijevic, N.; Armstrong, L.; Djonov, V.; Lako, M.; Stojkovic, M. Ethical and Safety Issues of Stem Cell-Based Therapy. Int. J. Med. Sci 2018, 15, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, P.; Weir, M.D.; Reynolds, M.A.; Zhao, L.; Xu, H.H. Hydrogel fibers encapsulating human stem cells in an injectable calcium phosphate scaffold for bone tissue engineering. Biomed. Mater. 2016, 11, 065008. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Li, P.; Li, Y.; Cai, Y.; Wen, J.; Luan, Q. Growth/differentiation factor-5 promotes in vitro/vivo periodontal specific differentiation of induced pluripotent stem cell-derived mesenchymal stem cells. Exp. Ther. Med. 2017, 14, 4111–4117. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K. Cellular reprogramming. Cold Spring Harb. Perspect. Biol. 2014, 6. [Google Scholar] [CrossRef]
- Krishnan, V.; Bryant, H.U.; Macdougald, O.A. Regulation of bone mass by Wnt signaling. J. Clin. Investig. 2006, 116, 1202–1209. [Google Scholar] [CrossRef] [PubMed]
- Osathanon, T.; Manokawinchoke, J.; Egusa, H.; Pavasant, P. Notch signaling partly regulates the osteogenic differentiation of retinoic acid-treated murine induced pluripotent stem cells. J. Oral Sci. 2017, 59, 405–413. [Google Scholar] [CrossRef]
- Choi, H.; Park, K.H.; Lee, A.R.; Mun, C.H.; Shin, Y.D.; Park, Y.B.; Park, Y.B. Control of dental-derived induced pluripotent stem cells through modified surfaces for dental application. Acta Odontol. Scand. 2017, 75, 309–318. [Google Scholar] [CrossRef]
- Vo, T.N.; Kasper, F.K.; Mikos, A.G. Strategies for controlled delivery of growth factors and cells for bone regeneration. Adv. Drug Deliv. Rev. 2012, 64, 1292–1309. [Google Scholar] [CrossRef]
- Chien, K.H.; Chang, Y.L.; Wang, M.L.; Chuang, J.H.; Yang, Y.C.; Tai, M.C.; Wang, C.Y.; Liu, Y.Y.; Li, H.Y.; Chen, J.T.; et al. Promoting Induced Pluripotent Stem Cell-driven Biomineralization and Periodontal Regeneration in Rats with Maxillary-Molar Defects using Injectable BMP-6 Hydrogel. Sci. Rep. 2018, 8, 114. [Google Scholar] [CrossRef]
- Liu, J.; Chen, W.; Zhao, Z.; Xu, H.H. Reprogramming of mesenchymal stem cells derived from iPSCs seeded on biofunctionalized calcium phosphate scaffold for bone engineering. Biomaterials 2013, 34, 7862–7872. [Google Scholar] [CrossRef]
- Hosseini, F.S.; Soleimanifar, F.; Khojasteh, A.; Ardeshirylajimi, A. Promoting osteogenic differentiation of human-induced pluripotent stem cells by releasing Wnt/beta-catenin signaling activator from the nanofibers. J. Cell. Biochem. 2018. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Hu, K.; Liu, X.; Reynolds, M.A.; Bao, C.; Wang, P.; Zhao, L.; Xu, H.H.K. Novel hiPSC-based tri-culture for pre-vascularization of calcium phosphate scaffold to enhance bone and vessel formation. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 79, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Okawa, H.; Okita, K.; Kamano, Y.; Wang, F.; Saeki, M.; Yatani, H.; Egusa, H. Gingival Fibroblasts as Autologous Feeders for Induced Pluripotent Stem Cells. J. Dent. Res. 2016, 95, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Jamal, M.; Lewandowski, S.L.; Lawton, M.L.; Huang, G.T.J.; Ikonomou, L. Derivation and characterization of putative craniofacial mesenchymal progenitor cells from human induced pluripotent stem cells. Stem Cell Res. 2018, 33, 100–109. [Google Scholar] [CrossRef]
- Duan, X.; Tu, Q.; Zhang, J.; Ye, J.; Sommer, C.; Mostoslavsky, G.; Kaplan, D.; Yang, P.; Chen, J. Application of induced pluripotent stem (iPS) cells in periodontal tissue regeneration. J. Cell. Physiol. 2011, 226, 150–157. [Google Scholar] [CrossRef]
- Hynes, K.; Bright, R.; Marino, V.; Ng, J.; Verma, P.J.; Gronthos, S.; Bartold, P.M. Potential of iPSC-Derived Mesenchymal Stromal Cells for Treating Periodontal Disease. Stem Cells Int. 2018, 2018, 2601945. [Google Scholar] [CrossRef] [PubMed]
- Hamano, S.; Tomokiyo, A.; Hasegawa, D.; Yoshida, S.; Sugii, H.; Mitarai, H.; Fujino, S.; Wada, N.; Maeda, H. Extracellular Matrix from Periodontal Ligament Cells Could Induce the Differentiation of Induced Pluripotent Stem Cells to Periodontal Ligament Stem Cell-Like Cells. Stem Cells Dev. 2018, 27, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Hisanaga, Y.; Suzuki, E.; Aoki, H.; Sato, M.; Saito, A.; Saito, A.; Azuma, T. Effect of the combined use of enamel matrix derivative and atelocollagen sponge scaffold on osteoblastic differentiation of mouse induced pluripotent stem cells in vitro. J. Periodontal Res. 2018, 53, 240–249. [Google Scholar] [CrossRef]
- Chen, W.; Liu, X.; Chen, Q.; Bao, C.; Zhao, L.; Zhu, Z.; Xu, H.H.K. Angiogenic and osteogenic regeneration in rats via calcium phosphate scaffold and endothelial cell co-culture with human bone marrow mesenchymal stem cells (MSCs), human umbilical cord MSCs, human induced pluripotent stem cell-derived MSCs and human embryonic stem cell-derived MSCs. J. Tissue Eng. Regen. Med. 2018, 12, 191–203. [Google Scholar] [CrossRef]
- Kidwai, F.; Edwards, J.; Zou, L.; Kaufman, D.S. Fibrinogen Induces RUNX2 Activity and Osteogenic Development from Human Pluripotent Stem Cells. Stem Cells 2016, 34, 2079–2089. [Google Scholar] [CrossRef] [PubMed]
- Okawa, H.; Kayashima, H.; Sasaki, J.-I.; Miura, J.; Kamano, Y.; Kosaka, Y.; Imazato, S.; Yatani, H.; Matsumoto, T.; Egusa, H. Scaffold-Free Fabrication of Osteoinductive Cellular Constructs Using Mouse Gingiva-Derived Induced Pluripotent Stem Cells. Stem Cells Int. 2016, 2016, 6240794. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Ochiai-Shino, H.; Shiga, T.; Onodera, S.; Saito, A.; Shibahara, T.; Azuma, T. Transplantation of human-induced pluripotent stem cells carried by self-assembling peptide nanofiber hydrogel improves bone regeneration in rat calvarial bone defects. BDJ Open 2016, 2, 15007. [Google Scholar] [CrossRef] [PubMed]
- Redis, R.S.; Berindan-Neagoe, I.; Pop, V.I.; Calin, G.A. Non-coding RNAs as theranostics in human cancers. J. Cell. Biochem. 2012, 113, 1451–1459. [Google Scholar] [CrossRef] [PubMed]
- Anokye-Danso, F.; Trivedi, C.M.; Juhr, D.; Gupta, M.; Cui, Z.; Tian, Y.; Zhang, Y.; Yang, W.; Gruber, P.J.; Epstein, J.A.; et al. Highly efficient miRNA-mediated reprogramming of mouse and human somatic cells to pluripotency. Cell Stem Cell 2011, 8, 376–388. [Google Scholar] [CrossRef] [PubMed]
- Chira, S.; Gulei, D.; Hajitou, A.; Zimta, A.A.; Cordelier, P.; Berindan-Neagoe, I. CRISPR/Cas9: Transcending the Reality of Genome Editing. Mol. Ther. Nucleic Acids 2017, 7, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Ben Jehuda, R.; Shemer, Y.; Binah, O. Genome Editing in Induced Pluripotent Stem Cells using CRISPR/Cas9. Stem Cell Rev. 2018, 14, 323–336. [Google Scholar] [CrossRef]
- Brunger, J.M.; Zutshi, A.; Willard, V.P.; Gersbach, C.A.; Guilak, F. CRISPR/Cas9 Editing of Murine Induced Pluripotent Stem Cells for Engineering Inflammation-Resistant Tissues. Arthritis Rheumatol. 2017, 69, 1111–1121. [Google Scholar] [CrossRef]
- Chen, Y.L.; Chen, P.K.; Jeng, L.B.; Huang, C.S.; Yang, L.C.; Chung, H.Y.; Chang, S.C. Periodontal regeneration using ex vivo autologous stem cells engineered to express the BMP-2 gene: An alternative to alveolaplasty. Gene Ther. 2008, 15, 1469–1477. [Google Scholar] [CrossRef]
- Mahla, R.S. Stem Cells Applications in Regenerative Medicine and Disease Therapeutics. Int. J. Cell Biol. 2016, 2016, 6940283. [Google Scholar] [CrossRef]
- Halevy, T.; Urbach, A. Comparing ESC and iPSC-Based Models for Human Genetic Disorders. J. Clin. Med. 2014, 3, 1146–1162. [Google Scholar] [CrossRef]
- Schögler, A.; De Sutter, P. Advantages and disadvantages of human embryonic or induced pluripotent stem cells for stem cell therapy. Tijdschr. Geneeskd. 2012, 68, 764–767. [Google Scholar] [CrossRef]
- Kim, H.J.; Park, J.S. Usage of Human Mesenchymal Stem Cells in Cell-based Therapy: Advantages and Disadvantages. Dev. Reprod. 2017, 21, 1–10. [Google Scholar] [CrossRef]
- Shimauchi, H.; Nemoto, E.; Ishihata, H.; Shimomura, M. Possible functional scaffolds for periodontal regeneration. Jpn. Dent. Sci. Rev. 2013, 49, 118–130. [Google Scholar] [CrossRef]
- Wada, N.; Menicanin, D.; Shi, S.; Bartold, P.M.; Gronthos, S. Immunomodulatory properties of human periodontal ligament stem cells. J. Cell. Physiol. 2009, 219, 667–676. [Google Scholar] [CrossRef]
- Karamzadeh, R. Dental-Related Stem Cells and Their Potential in Regenerative Medicine. In Regenerative Medicine and Tissue Engineering; Andrades, J.A., Ed.; Intech Open: London, UK, 2013. [Google Scholar] [CrossRef]
- Chalisserry, E.P.; Nam, S.Y.; Park, S.H.; Anil, S. Therapeutic potential of dental stem cells. J. Tissue Eng. 2017, 8, 2041731417702531. [Google Scholar] [CrossRef]
- Diniz, I.M.A.; Chen, C.; Ansari, S.; Zadeh, H.H.; Moshaverinia, M.; Chee, D.; Marques, M.M.; Shi, S.; Moshaverinia, A. Gingival Mesenchymal Stem Cell (GMSC) Delivery System Based on RGD-Coupled Alginate Hydrogel with Antimicrobial Properties: A Novel Treatment Modality for Peri-Implantitis. J. Prosthodont. 2016, 25, 105–115. [Google Scholar] [CrossRef]
- Singh, V.K.; Kalsan, M.; Kumar, N.; Saini, A.; Chandra, R. Induced pluripotent stem cells: Applications in regenerative medicine, disease modeling, and drug discovery. Front. Cell Dev. Biol. 2015, 3. [Google Scholar] [CrossRef]
- Medvedev, S.P.; Shevchenko, A.I.; Zakian, S.M. Induced Pluripotent Stem Cells: Problems and Advantages when Applying them in Regenerative Medicine. Acta Nat. 2010, 2, 18–28. [Google Scholar]
- Yamato, M.; Okano, T. Cell sheet engineering. Mater. Today 2004, 7, 42–47. [Google Scholar] [CrossRef]
- Pedroni, A.C.F.; Sarra, G.; de Oliveira, N.K.; Moreira, M.S.; Deboni, M.C.Z.; Marques, M.M. Cell sheets of human dental pulp stem cells for future application in bone replacement. Clin. Oral Investig. 2018. [Google Scholar] [CrossRef]
- Yan, J.; Chang, B.; Hu, X.; Cao, C.; Zhao, L.; Zhang, Y. Titanium implant functionalized with antimiR-138 delivered cell sheet for enhanced peri-implant bone formation and vascularization. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 89, 52–64. [Google Scholar] [CrossRef]
- Mu, S.; Tee, B.C.; Emam, H.; Zhou, Y.; Sun, Z. Culture-expanded mesenchymal stem cell sheets enhance extraction-site alveolar bone growth: An animal study. J. Periodontal Res. 2018, 53, 514–524. [Google Scholar] [CrossRef]
- Shao, D.; Wang, C.; Sun, Y.; Cui, L. Effects of oral implants with miR-122-modified cell sheets on rat bone marrow mesenchymal stem cells. Mol. Med. Rep. 2018, 17, 1537–1544. [Google Scholar] [CrossRef]
- Wen, Y.; Yang, H.; Liu, Y.; Liu, Q.; Wang, A.; Ding, Y.; Jin, Z. Evaluation of BMMSCs-EPCs sheets for repairing alveolar bone defects in ovariectomized rats. Sci. Rep. 2017, 7, 16568. [Google Scholar] [CrossRef]
- Washio, K.; Tsutsumi, Y.; Tsumanuma, Y.; Yano, K.; Srithanyarat, S.S.; Takagi, R.; Ichinose, S.; Meinzer, W.; Yamato, M.; Okano, T.; et al. In Vivo Periodontium Formation Around Titanium Implants Using Periodontal Ligament Cell Sheet. Tissue Eng. Part A 2018, 24, 1273–1282. [Google Scholar] [CrossRef]
- Farag, A.; Hashimi, S.M.; Vaquette, C.; Bartold, P.M.; Hutmacher, D.W.; Ivanovski, S. The effect of decellularized tissue engineered constructs on periodontal regeneration. J. Clin. Periodontol. 2018, 45, 586–596. [Google Scholar] [CrossRef]
- Tatsuhiro, F.; Seiko, T.; Yusuke, T.; Reiko, T.-T.; Kazuhito, S. Dental Pulp Stem Cell-Derived, Scaffold-Free Constructs for Bone Regeneration. Int. J. Mol. Sci. 2018, 19, 1846. [Google Scholar] [CrossRef]
- Syed-Picard, F.N.; Ray, H.L., Jr.; Kumta, P.N.; Sfeir, C. Scaffoldless tissue-engineered dental pulp cell constructs for endodontic therapy. J. Dent. Res. 2014, 93, 250–255. [Google Scholar] [CrossRef]
- Wang, Z.-S.; Feng, Z.-H.; Wu, G.-F.; Bai, S.-Z.; Dong, Y.; Chen, F.-M.; Zhao, Y.-M. The use of platelet-rich fibrin combined with periodontal ligament and jaw bone mesenchymal stem cell sheets for periodontal tissue engineering. Sci. Rep. 2016, 6, 28126. [Google Scholar] [CrossRef]
- Fujii, Y.; Kawase-Koga, Y.; Hojo, H.; Yano, F.; Sato, M.; Chung, U.I.; Ohba, S.; Chikazu, D. Bone regeneration by human dental pulp stem cells using a helioxanthin derivative and cell-sheet technology. Stem Cell Res. Ther. 2018, 9, 24. [Google Scholar] [CrossRef]
- Ueyama, Y.; Yagyuu, T.; Maeda, M.; Imada, M.; Akahane, M.; Kawate, K.; Tanaka, Y.; Kirita, T. Maxillofacial bone regeneration with osteogenic matrix cell sheets: An experimental study in rats. Arch. Oral Biol. 2016, 72, 138–145. [Google Scholar] [CrossRef]
- Ding, L.; Tang, S.; Liang, P.; Wang, C.; Zhou, P.F.; Zheng, L. Bone Regeneration of Canine Peri-implant Defects Using Cell Sheets of Adipose-Derived Mesenchymal Stem Cells and Platelet-Rich Fibrin Membranes. J. Oral Maxillofac. Surg. 2018. [Google Scholar] [CrossRef]
- Lee, H.Y.; Hong, I.S. Double-edged sword of mesenchymal stem cells: Cancer-promoting versus therapeutic potential. Cancer Sci. 2017, 108, 1939–1946. [Google Scholar] [CrossRef]
- Cai, C.; Hou, L.; Zhang, J.; Zhao, D.; Wang, Z.; Hu, H.; He, J.; Guan, W.; Ma, Y. The Inhibitory Effect of Mesenchymal Stem Cells with rAd-NK4 on Liver Cancer. Appl. Biochem. Biotechnol. 2017, 183, 444–459. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, Y.; Cheng, L.; Yue, D.; Ma, J.; Zhao, D.; Hou, X.; Xiang, R.; Cheng, P. Mesenchymal Stem Cells Engineered to Secrete Pigment Epithelium-Derived Factor Inhibit Tumor Metastasis and the Formation of Malignant Ascites in a Murine Colorectal Peritoneal Carcinomatosis Model. Hum. Gene Ther. 2016, 27, 267–277. [Google Scholar] [CrossRef]
- Clarke, M.R.; Imhoff, F.M.; Baird, S.K. Mesenchymal stem cells inhibit breast cancer cell migration and invasion through secretion of tissue inhibitor of metalloproteinase-1 and -2. Mol. Carcinog. 2015, 54, 1214–1219. [Google Scholar] [CrossRef]
- Kidd, S.; Spaeth, E.; Dembinski, J.L.; Dietrich, M.; Watson, K.; Klopp, A.; Battula, V.L.; Weil, M.; Andreeff, M.; Marini, F.C. Direct evidence of mesenchymal stem cell tropism for tumor and wounding microenvironments using in vivo bioluminescent imaging. Stem Cells 2009, 27, 2614–2623. [Google Scholar] [CrossRef]
- Beckermann, B.M.; Kallifatidis, G.; Groth, A.; Frommhold, D.; Apel, A.; Mattern, J.; Salnikov, A.V.; Moldenhauer, G.; Wagner, W.; Diehlmann, A.; et al. VEGF expression by mesenchymal stem cells contributes to angiogenesis in pancreatic carcinoma. Br. J. Cancer 2008, 99, 622–631. [Google Scholar] [CrossRef]
- Reagan, M.R.; Kaplan, D.L. Concise review: Mesenchymal stem cell tumor-homing: Detection methods in disease model systems. Stem Cells 2011, 29, 920–927. [Google Scholar] [CrossRef]
- Liotta, F.; Querci, V.; Mannelli, G.; Santarlasci, V.; Maggi, L.; Capone, M.; Rossi, M.C.; Mazzoni, A.; Cosmi, L.; Romagnani, S.; et al. Mesenchymal stem cells are enriched in head neck squamous cell carcinoma, correlates with tumour size and inhibit T-cell proliferation. Br. J. Cancer 2015, 112, 745–754. [Google Scholar] [CrossRef]
- Takeda, D.; Hasegawa, T.; Ueha, T.; Iwata, E.; Harada, R.; Sakakibara, A.; Kawamoto, T.; Minamikawa, T.; Sakai, Y.; Komori, T. Induced Pluripotent-stem-cell Related Genes Contribute to De-differentiation in Oral Squamous Cell Carcinoma. Anticancer Res. 2017, 37, 1075–1082. [Google Scholar] [CrossRef]
- Liang, Y.; Zhang, H.; Feng, Q.-S.; Cai, M.-B.; Deng, W.; Qin, D.; Yun, J.-P.; Tsao, G.S.W.; Kang, T.; Esteban, M.A.; et al. The propensity for tumorigenesis in human induced pluripotent stem cells is related with genomic instability. Chin. J. Cancer 2013, 32, 205–212. [Google Scholar] [CrossRef]
- Ji, X.; Zhang, Z.; Han, Y.; Song, J.; Xu, X.; Jin, J.; Su, S.; Mu, D.; Liu, X.; Xu, S.; et al. Mesenchymal stem cells derived from normal gingival tissue inhibit the proliferation of oral cancer cells in vitro and in vivo. Int. J. Oncol. 2016, 49, 2011–2022. [Google Scholar] [CrossRef]
- Katagiri, W.; Osugi, M.; Kawai, T.; Hibi, H. First-in-human study and clinical case reports of the alveolar bone regeneration with the secretome from human mesenchymal stem cells. Head Face Med. 2016, 12, 5. [Google Scholar] [CrossRef]
- Tomasello, L.; Mauceri, R.; Coppola, A.; Pitrone, M.; Pizzo, G.; Campisi, G.; Pizzolanti, G.; Giordano, C. Mesenchymal stem cells derived from inflamed dental pulpal and gingival tissue: A potential application for bone formation. Stem Cell Res. Ther. 2017, 8, 179. [Google Scholar] [CrossRef]
- Katagiri, W.; Sakaguchi, K.; Kawai, T.; Wakayama, Y.; Osugi, M.; Hibi, H. A defined mix of cytokines mimics conditioned medium from cultures of bone marrow-derived mesenchymal stem cells and elicits bone regeneration. Cell Prolif. 2017, 50, e12333. [Google Scholar] [CrossRef]
- Kato, H.; Taguchi, Y.; Tominaga, K.; Kimura, D.; Yamawaki, I.; Noguchi, M.; Yamauchi, N.; Tamura, I.; Tanaka, A.; Umeda, M. High Glucose Concentrations Suppress the Proliferation of Human Periodontal Ligament Stem Cells and Their Differentiation Into Osteoblasts. J. Periodontol. 2016, 87, e44–e51. [Google Scholar] [CrossRef]
- Yan, L.; Sun, S.; Qu, L. Insulin-like growth factor-1 promotes the proliferation and odontoblastic differentiation of human dental pulp cells under high glucose conditions. Int. J. Mol. Med. 2017, 40, 1253–1260. [Google Scholar] [CrossRef]
- Yamawaki, I.; Taguchi, Y.; Komasa, S.; Tanaka, A.; Umeda, M. Effects of glucose concentration on osteogenic differentiation of type II diabetes mellitus rat bone marrow-derived mesenchymal stromal cells on a nano-scale modified titanium. J. Periodontal Res. 2017, 52, 761–771. [Google Scholar] [CrossRef]
- Hozhabri, N.S.; Benson, M.D.; Vu, M.D.; Patel, R.H.; Martinez, R.M.; Nakhaie, F.N.; Kim, H.K.; Varanasi, V.G. Decreasing NF-kappaB expression enhances odontoblastic differentiation and collagen expression in dental pulp stem cells exposed to inflammatory cytokines. PLoS ONE 2015, 10, e0113334. [Google Scholar] [CrossRef]
- Kato, H.; Taguchi, Y.; Tominaga, K.; Umeda, M.; Tanaka, A. Porphyromonas gingivalis LPS inhibits osteoblastic differentiation and promotes pro-inflammatory cytokine production in human periodontal ligament stem cells. Arch. Oral Biol. 2014, 59, 167–175. [Google Scholar] [CrossRef]
- Albiero, M.L.; Amorim, B.R.; Casati, M.Z.; Sallum, E.A.; Nociti, F.H.J.; Silverio, K.G. Osteogenic potential of periodontal ligament stem cells are unaffected after exposure to lipopolysaccharides. Braz. Oral Res. 2017, 31, e17. [Google Scholar] [CrossRef]
- Ma, L.; Aijima, R.; Hoshino, Y.; Yamaza, H.; Tomoda, E.; Tanaka, Y.; Sonoda, S.; Song, G.; Zhao, W.; Nonaka, K.; et al. Transplantation of mesenchymal stem cells ameliorates secondary osteoporosis through interleukin-17-impaired functions of recipient bone marrow mesenchymal stem cells in MRL/lpr mice. Stem Cell Res. Ther. 2015, 6, 104. [Google Scholar] [CrossRef]
- Yamaza, T.; Kentaro, A.; Chen, C.; Liu, Y.; Shi, Y.; Gronthos, S.; Wang, S.; Shi, S. Immunomodulatory properties of stem cells from human exfoliated deciduous teeth. Stem Cell Res. Ther. 2010, 1, 5. [Google Scholar] [CrossRef]
- Makino, Y.; Yamaza, H.; Akiyama, K.; Ma, L.; Hoshino, Y.; Nonaka, K.; Terada, Y.; Kukita, T.; Shi, S.; Yamaza, T. Immune therapeutic potential of stem cells from human supernumerary teeth. J. Dent. Res. 2013, 92, 609–615. [Google Scholar] [CrossRef]
| Type of Stem Cells | Source | Harvesting Discomfort | Standardization—Study Level | Osteogenic Potential | Self-Renewal Capacity | Costs | Ethical Conflict | Availability | Translational Level | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| ESC | Blastocyst | N/A | + | +++ | +++ | +++ | +++ | + | + | [70,71,72] |
| BM-MSC | Bone marrow from iliac crest, jaw, maxilla | +++ | +++ | ++ | + | ++ | ++ | + | ++ | [73] |
| PDLSC | Periodontal ligaments (wisdom teeth) | ++ | + | + | +++ | + | + | + | + | [74,75,76] |
| DPMSC | Dental pulp from primary or permanent teeth | + | ++ | + | + | + | ++ | + | +++ | [76,77] |
| GMSC | Connective tissue from gingiva | + | + | + | ++ | + | +++ | + | ++ | [77,78] |
| iPSC | Adult cells, especially gingival fibroblasts | + | + | ++ | + | +++ | ++ | +++ | + | [70,71,72,79,80] |
| Condition | Type of Stem Cell | Effect | Ref |
|---|---|---|---|
| High glucose | PDL-MSC | Suppressed proliferation and differentiation into osteoblasts | [109] |
| High glucose | DPSCs | Impaired proliferation and differentiation | [110] |
| Inflammation | BM-MSCs | Enhanced capacity | [28] |
| Inflammatory conditions | PDLSCs | Impaired osteogenic differentiation | |
| Inflammatory conditions | BM-MSCs | Normal osteogenic capacity | |
| Inflammation | BM-MSCs coupled with titanium implants | Stimulated bone formation but disorganized tissue | [111] |
| Inflammation | DPSCs | Anti-inflammatory effect | [41] |
| Inflammation—NFkB expression | DPSCs | Down-regulated NfKB signalling lead to increased osteogenic potential | [112] |
| Infection with Porphyromonas gingivalis | PDL-MSC | Osteoblastic differentiation and promotion of pro-inflammatory cytokine production | [113] |
| Exposure to lipopolysaccharides | PDL-MSC | Does not affect stem cell markers | [114] |
| Bone loss in lupus erythematosus | BMMSCs | Systemic administration reduced Il-17 level and recovered bone loss | [115] |
| DPSCs | Recovered bone loss | ||
| Autoimmunity | DPSCs | Increased Tregs and decreased TH17; are capable of osteogenic differentiation | [116,117] |
| BM-MSCs | Osteogenesis |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buduru, S.D.; Gulei, D.; Zimta, A.-A.; Tigu, A.B.; Cenariu, D.; Berindan-Neagoe, I. The Potential of Different Origin Stem Cells in Modulating Oral Bone Regeneration Processes. Cells 2019, 8, 29. https://doi.org/10.3390/cells8010029
Buduru SD, Gulei D, Zimta A-A, Tigu AB, Cenariu D, Berindan-Neagoe I. The Potential of Different Origin Stem Cells in Modulating Oral Bone Regeneration Processes. Cells. 2019; 8(1):29. https://doi.org/10.3390/cells8010029
Chicago/Turabian StyleBuduru, Smaranda Dana, Diana Gulei, Alina-Andreea Zimta, Adrian Bogdan Tigu, Diana Cenariu, and Ioana Berindan-Neagoe. 2019. "The Potential of Different Origin Stem Cells in Modulating Oral Bone Regeneration Processes" Cells 8, no. 1: 29. https://doi.org/10.3390/cells8010029
APA StyleBuduru, S. D., Gulei, D., Zimta, A.-A., Tigu, A. B., Cenariu, D., & Berindan-Neagoe, I. (2019). The Potential of Different Origin Stem Cells in Modulating Oral Bone Regeneration Processes. Cells, 8(1), 29. https://doi.org/10.3390/cells8010029







