Assessment of the Immunosuppressive Potential of INF-γ Licensed Adipose Mesenchymal Stem Cells, Their Secretome and Extracellular Vesicles
Abstract
:1. Introduction
2. Materials and Methods
2.1. AMSCs Obtention, Culture and Characterization
2.2. AMSCs Licensing
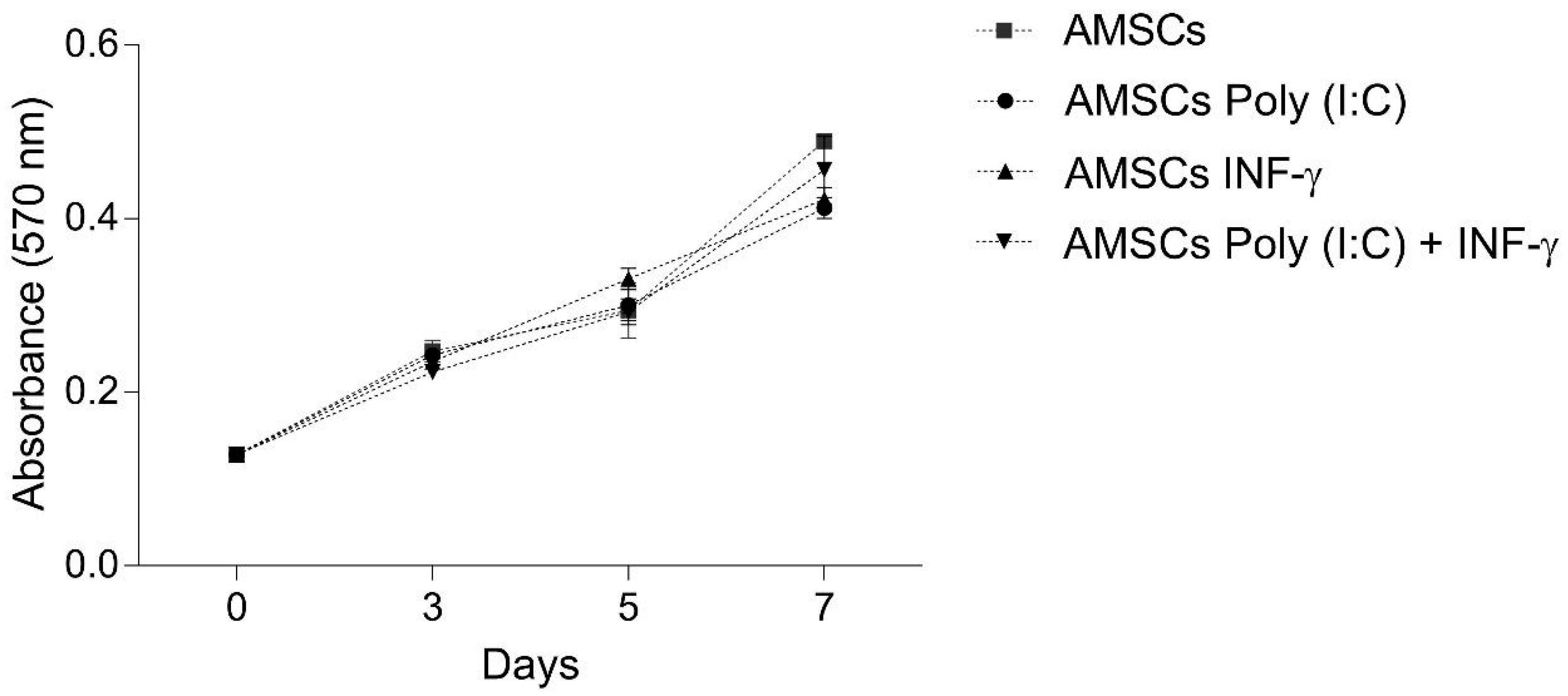
2.3. AMSCs Viability and Proliferation
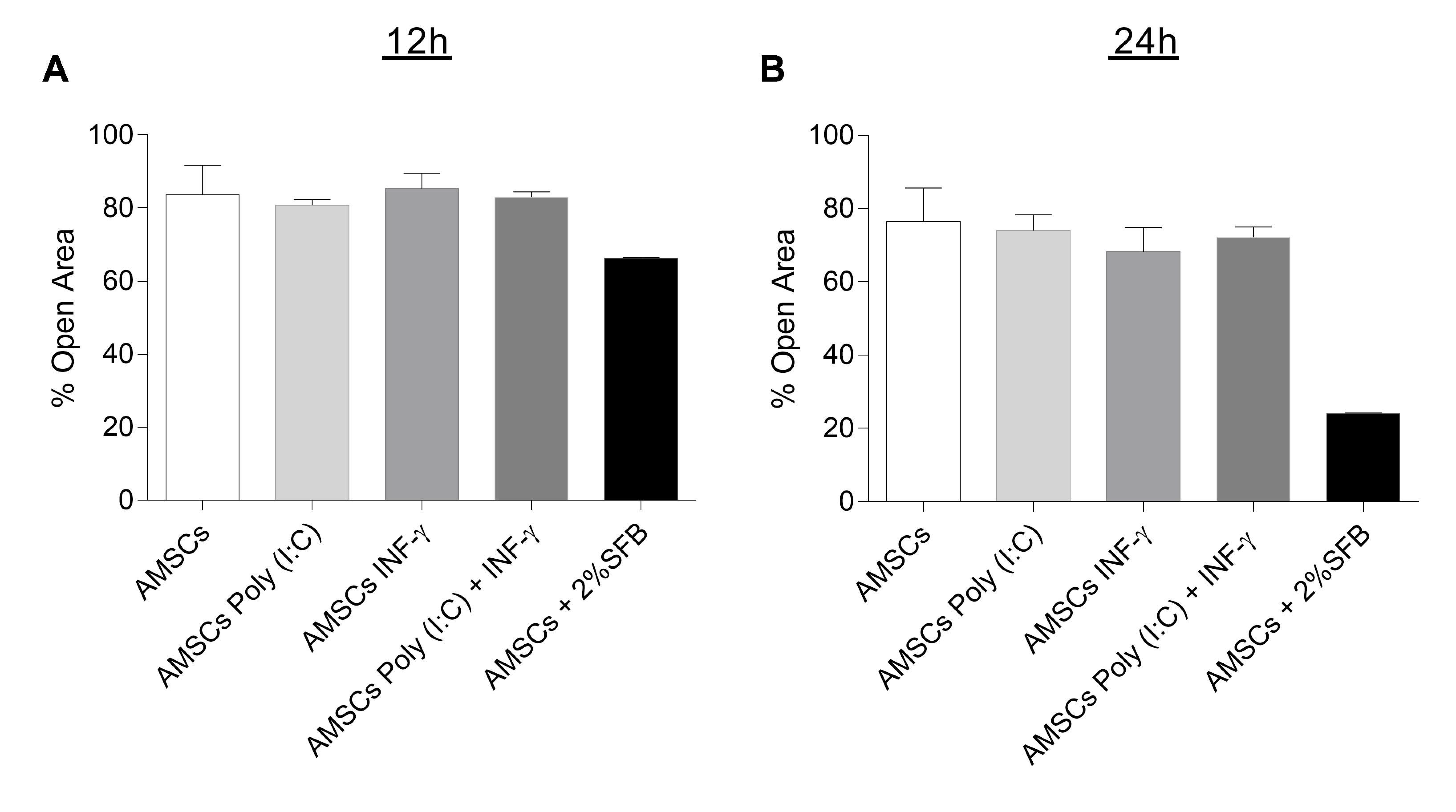
2.4. AMSCs Migration
2.5. Isolation and Activation of Peripheral Blood Mononuclear Cells (PBMCs)
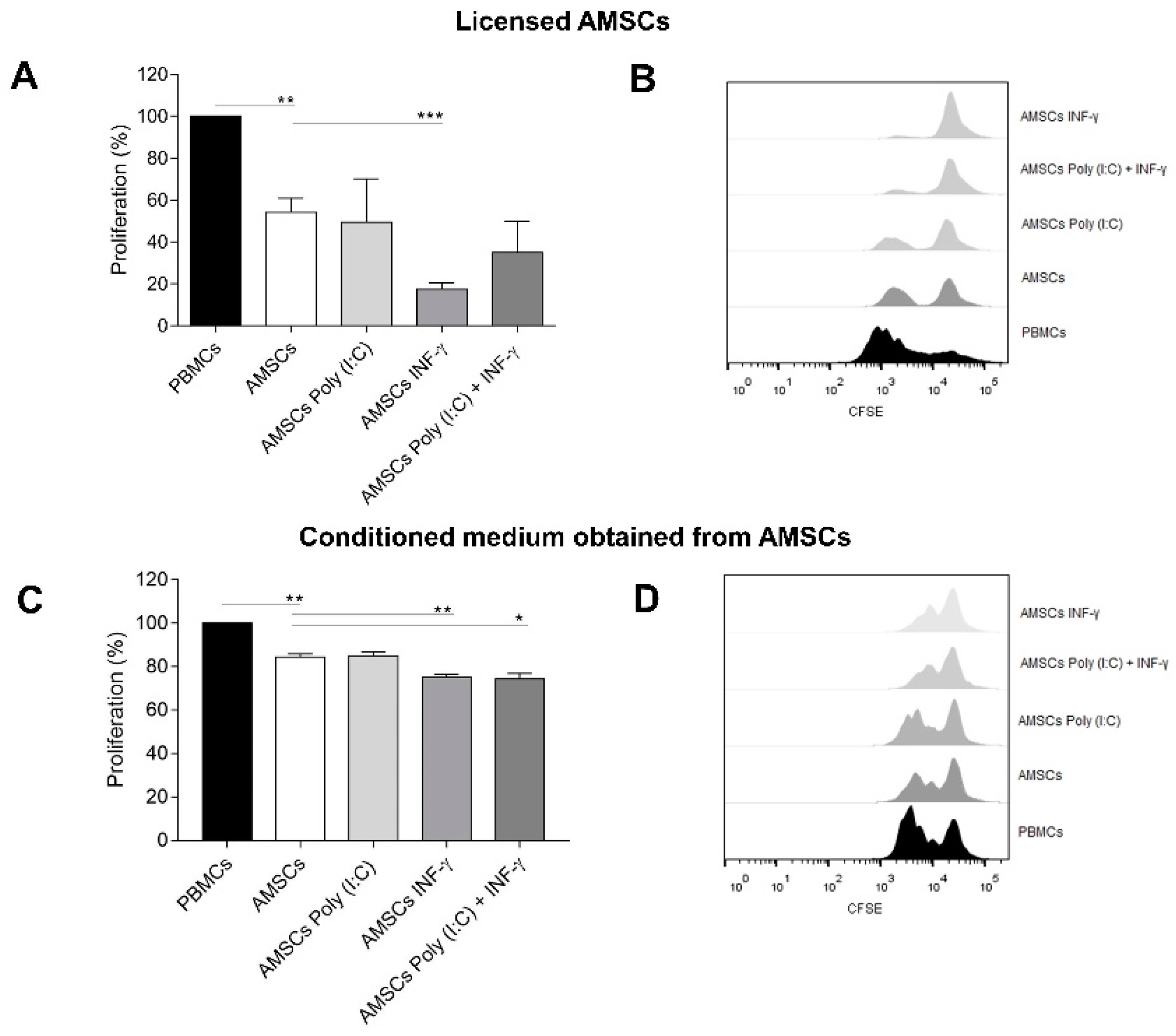
2.6. AMSCs Co-Culture with PBMCs
2.7. PBMCs Culture with AMSCs-Derived Conditioned Medium
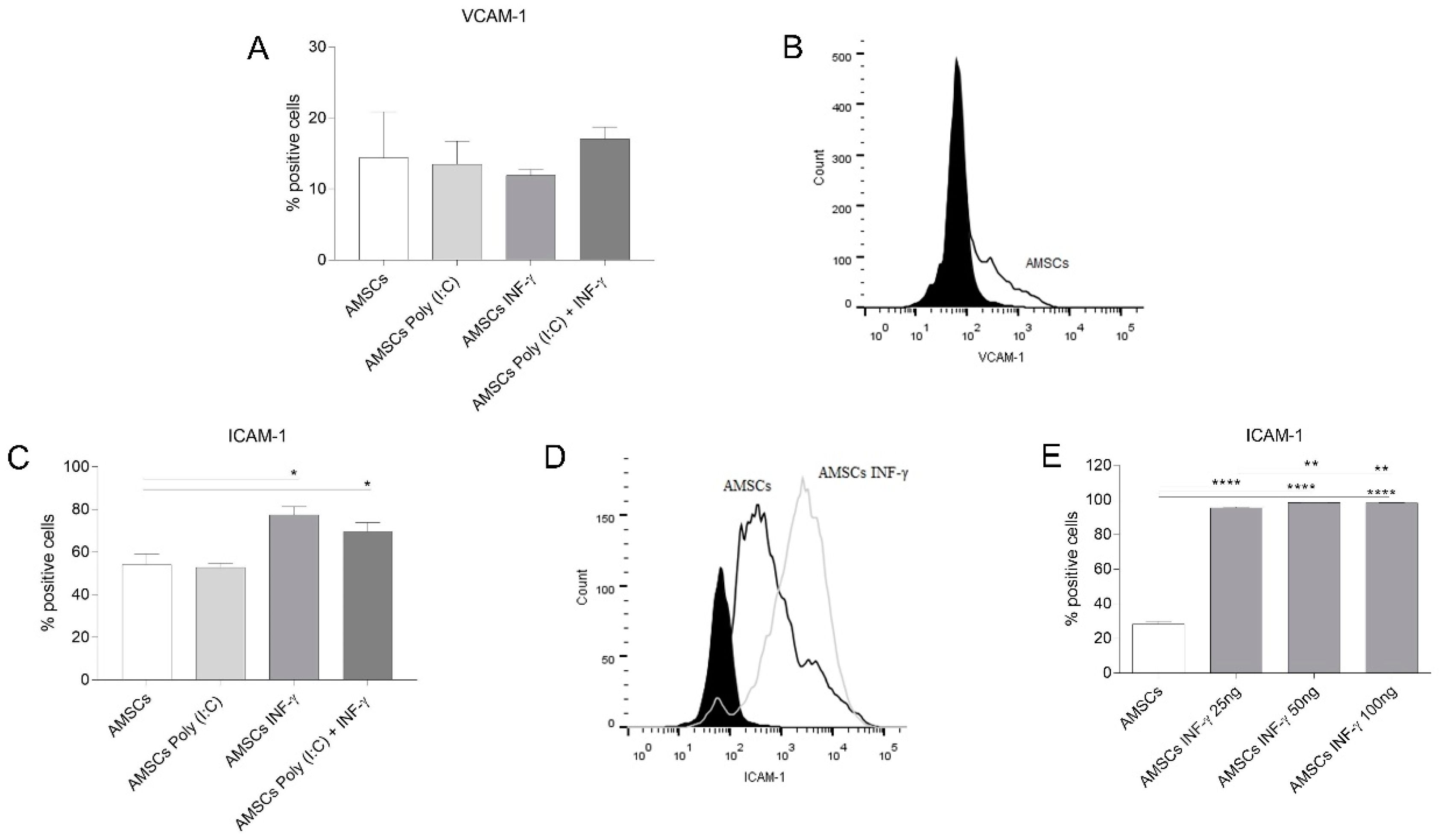
2.8. Vascular Cell Adhesion Protein 1 (VCAM-1) and Intercellular Adhesion Molecule 1 (ICAM-1) Expression on AMSCs
2.9. EVs Isolation and Characterization
2.10. Immunosuppressive Effects of AMSCs-Derived EVs
2.11. RNA Isolation and Real-Time PCR
2.12. Statistical Analysis
3. Results
3.1. INF-γ and/or Poly (I:C) Licensing Maintain AMSCs Phenotype
3.2. INF-γ and/or Poly (I:C) Licensing did not Influence AMSCs Proliferation
3.3. INF-γ and/or Poly (I:C) Licensing Did not Alter AMSCs Migration
3.4. INF-γ Enhances AMSCs-Mediated Immunomodulation
3.5. Conditioned Medium from INF-γ Licensed AMSCs Has Increased Capacity to Control the T-Cell Response
3.6. INF-γ Enhances ICAM-1 Expression on AMSCs
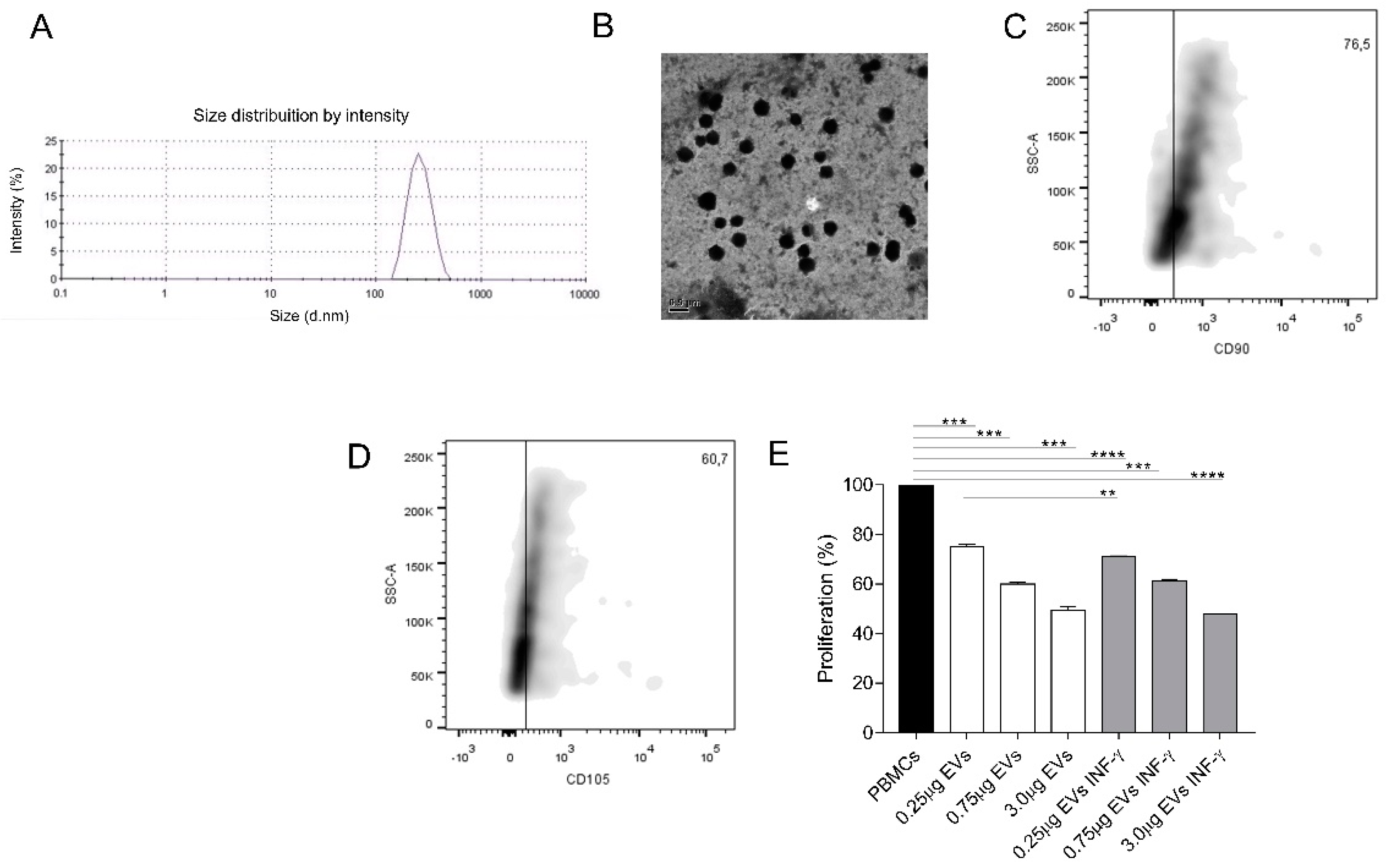
3.7. EVs Characterization
3.8. AMSCs-Derived EVs Present Immunosuppressive Potential
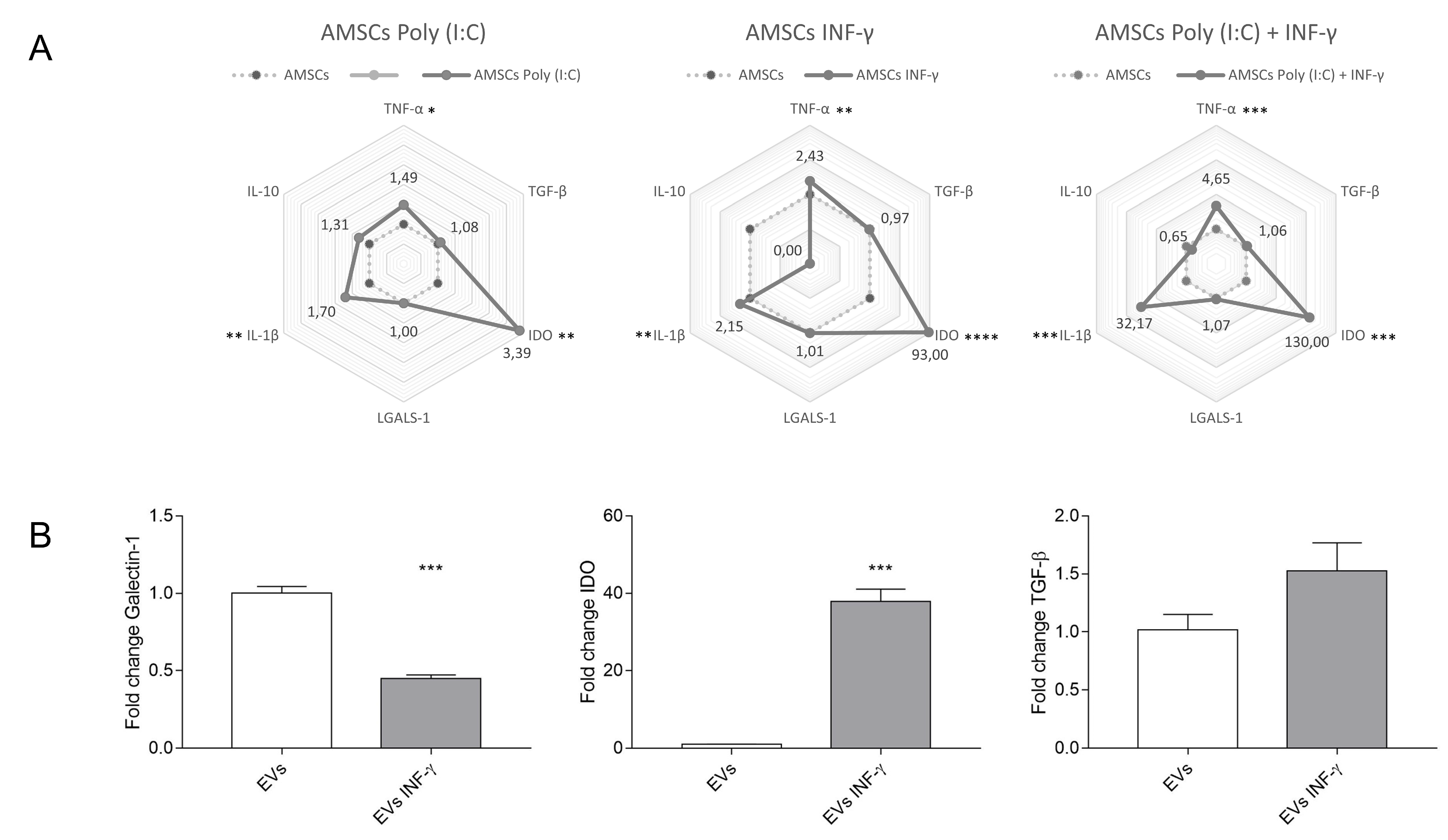
3.9. Expression of Inflammatory Transcripts in Licensed and Unlicensed AMSCs and in Their EVs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Haddad, R.; Saldanha-Araujo, F. Mechanisms of T-cell immunosuppression by mesenchymal stromal cells: What do we know so far? Biomed. Res. Int. 2014, 2014, 216806. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.R.; Pollock, K.; Hubel, A.; McKenna, D. Mesenchymal stem or stromal cells: A review of clinical applications and manufacturing practices. Transfusion 2014, 54, 1418–1437. [Google Scholar] [CrossRef] [PubMed]
- Munneke, J.M.; Spruit, M.J.A.; Cornelissen, A.S.; van Hoeven, V.; Voermans, C.; Hazenberg, M.D. The potential of mesenchymal stromal cells as treatment for severe steroid-refractory acute graft-versus-host disease: A critical review of the literature. Transplantation 2016, 100, 2309–2314. [Google Scholar] [CrossRef] [PubMed]
- Rizk, M.; Monaghan, M.; Shorr, R.; Kekre, N.; Bredeson, C.N.; Allan, D.S. Heterogeneity in studies of mesenchymal stromal cells to treat or prevent graft-versus-host disease: A scoping review of the evidence. Biol. Blood Marrow Transplant. 2016, 22, 1416–1423. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Chiu, S.M.; Motan, D.A.L.; Zhang, Z.; Chen, L.; Ji, H.-L.; Tse, H.-F.; Fu, Q.-L.; Lian, Q. Mesenchymal stem cells and immunomodulation: Current status and future prospects. Cell Death Dis. 2016, 7, e2062. [Google Scholar] [CrossRef] [PubMed]
- Najar, M.; Krayem, M.; Merimi, M.; Burny, A.; Meuleman, N.; Bron, D.; Raicevic, G.; Lagneaux, L. Insights into inflammatory priming of mesenchymal stromal cells: Functional biological impacts. Inflamm. Res. 2018, 67, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Chinnadurai, R.; Copland, I.B.; Patel, S.R.; Galipeau, J. IDO-Independent Suppression of T Cell Effector Function by IFN- -Licensed Human Mesenchymal Stromal Cells. J. Immunol. 2014, 192, 1491–1501. [Google Scholar] [CrossRef] [Green Version]
- Liang, C.; Jiang, E.; Yao, J.; Wang, M.; Chen, S.; Zhou, Z.; Zhai, W.; Ma, Q.; Feng, S.; Han, M. Interferon-γ mediates the immunosuppression of bone marrow mesenchymal stem cells on T-lymphocytes in vitro. Hematology 2018, 23, 44–49. [Google Scholar] [CrossRef]
- Sangiorgi, B.; Panepucci, R.A. Modulation of immunoregulatory properties of mesenchymal stromal cells by Toll-like receptors: Potential applications on GVHD. Stem Cells Int. 2016, 2016, 9434250. [Google Scholar] [CrossRef]
- Najar, M.; Krayem, M.; Meuleman, N.; Bron, D.; Lagneaux, L. Mesenchymal stromal cells and Toll-like receptor priming: A critical review. Immune Netw. 2017, 17, 89–102. [Google Scholar] [CrossRef]
- Konala, V.B.R.; Mamidi, M.K.; Bhonde, R.; Das, A.K.; Pochampally, R.; Pal, R. The current landscape of the mesenchymal stromal cell secretome: A new paradigm for cell-free regeneration. Cytotherapy 2016, 18, 13–24. [Google Scholar] [CrossRef] [Green Version]
- Fatima, F.; Ekstrom, K.; Nazarenko, I.; Maugeri, M.; Valadi, H.; Hill, A.F.; Camussi, G.; Nawaz, M. Non-coding RNAs in mesenchymal stem cell-derived extracellular vesicles: Deciphering regulatory roles in stem cell potency, inflammatory resolve and tissue regeneration. Front. Genet. 2017, 8. [Google Scholar] [CrossRef]
- Klinker, M.W.; Marklein, R.A.; Lo Surdo, J.L.; Wei, C.-H.; Bauer, S.R. Morphological features of IFN-γ–stimulated mesenchymal stromal cells predict overall immunosuppressive capacity. Proc. Natl. Acad. Sci. USA 2017, 114, E2598–E2607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sangiorgi, B.; De Freitas, H.T.; Schiavinato, J.L.D.S.; Leão, V.; Haddad, R.; Orellana, M.D.; Faça, V.M.; Ferreira, G.A.; Covas, D.T.; Zago, M.A.; et al. DSP30 enhances the immunosuppressive properties of mesenchymal stromal cells and protects their suppressive potential from lipopolysaccharide effects: A potential role of adenosine. Cytotherapy 2016, 18, 846–859. [Google Scholar] [CrossRef] [PubMed]
- Oliveira-Bravo, M.; Sangiorgi, B.B.; Schiavinato, J.L.D.S.; Carvalho, J.L.; Covas, D.T.; Panepucci, R.A.; Neves, F.d.A.R.; Franco, O.L.; Pereira, R.W.; Saldanha-Araujo, F. LL-37 boosts immunosuppressive function of placenta-derived mesenchymal stromal cells. Stem Cell Res. Ther. 2016, 7. [Google Scholar] [CrossRef]
- Liang, C.-C.; Park, A.Y.; Guan, J.-L. In vitro scratch assay: A convenient and inexpensive method for analysis of cell migration in vitro. Nat. Protoc. 2007, 2, 329–333. [Google Scholar] [CrossRef]
- Saldanha-Araujo, F.; Ferreira, F.I.S.; Palma, P.V.; Araujo, A.G.; Queiroz, R.H.C.; Covas, D.T.; Zago, M.A.; Panepucci, R.A. Mesenchymal stromal cells up-regulate CD39 and increase adenosine production to suppress activated T-lymphocytes. Stem Cell Res. 2011, 7, 66–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saldanha-Araujo, F.; Haddad, R.; Farias, K.C.; Souza, A.P.; Palma, P.V.; Araujo, A.G.; Orellana, M.D.; Voltarelli, J.C.; Covas, D.T.; Zago, M.A.; et al. Mesenchymal stem cells promote the sustained expression of CD69 on activated T lymphocytes: Roles of canonical and non-canonical NF-κB signalling. J. Cell. Mol. Med. 2012, 16, 1232–1244. [Google Scholar] [CrossRef] [PubMed]
- Vellasamy, S.; Tong, C.K.; Azhar, N.A.; Kodiappan, R.; Chan, S.C.; Veerakumarasivam, A.; Ramasamy, R. Human mesenchymal stromal cells modulate T-cell immune response via transcriptomic regulation. Cytotherapy 2016, 18, 1270–1283. [Google Scholar] [CrossRef]
- Kruger, N.J. The Bradford method for protein quantitation. Methods Mol. Biol. 1994, 32, 9–15. [Google Scholar] [PubMed]
- Blazquez, R.; Sanchez-Margallo, F.M.; de la Rosa, O.; Dalemans, W.; Alvarez, V.; Tarazona, R.; Casado, J.G. Immunomodulatory potential of human adipose mesenchymal stem cells derived exosomes on in vitro stimulated T cells. Front. Immunol. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Quaedackers, M.E.; Baan, C.C.; Weimar, W.; Hoogduijn, M.J. Cell contact interaction between adipose-derived stromal cells and allo-activated T lymphocytes. Eur. J. Immunol. 2009, 39, 3436–3446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Becker, A.; Riet, I.V. Homing and migration of mesenchymal stromal cells: How to improve the efficacy of cell therapy? World J. Stem Cells 2016, 8, 73–87. [Google Scholar] [CrossRef] [PubMed]
- Maijenburg, M.W.; van der Schoot, C.E.; Voermans, C. Mesenchymal stromal cell migration: Possibilities to improve cellular therapy. Stem Cells Dev. 2012, 21, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Hemeda, H.; Jakob, M.; Ludwig, A.-K.; Giebel, B.; Lang, S.; Brandau, S. Interferon-γ and tumor necrosis factor-α differentially affect cytokine expression and migration properties of mesenchymal stem cells. Stem Cells Dev. 2010, 19, 693–706. [Google Scholar] [CrossRef] [PubMed]
- Tomchuck, S.L.; Zwezdaryk, K.J.; Coffelt, S.B.; Waterman, R.S.; Danka, E.S.; Scandurro, A.B. Toll-like receptors on human mesenchymal stem cells drive their migration and immunomodulating responses. Stem Cells 2008, 26, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Shin, J.M.; Jeon, Y.J.; Chung, H.M.; Chae, J.-I. Proteomic validation of multifunctional molecules in mesenchymal stem cells derived from human bone marrow, umbilical cord blood and peripheral blood. PLoS ONE 2012, 7, e32350. [Google Scholar] [CrossRef]
- He, Q.; Wan, C.; Li, G. Concise review: Multipotent mesenchymal stromal cells in blood. Stem Cells 2006, 25, 69–77. [Google Scholar] [CrossRef]
- Xu, L.; Li, G. Circulating mesenchymal stem cells and their clinical implications. J. Orthop. Transl. 2014, 2, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Di Nicola, M. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 2002, 99, 3838–3843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, H.H.; Bae, Y.C.; Jung, J.S. Role of Toll-like receptors on human adipose-derived stromal cells. Stem Cells 2006, 24, 2744–2752. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, E.; DelaRosa, O.; Mancheño-Corvo, P.; Menta, R.; Ramírez, C.; Büscher, D. Toll-like receptor–mediated signaling in human adipose-derived stem cells: Implications for immunogenicity and immunosuppressive potential. Tissue Eng. Part A 2009, 15, 1579–1589. [Google Scholar] [CrossRef] [PubMed]
- Croitoru-Lamoury, J.; Lamoury, F.M.J.; Caristo, M.; Suzuki, K.; Walker, D.; Takikawa, O.; Taylor, R.; Brew, B.J. Interferon-γ regulates the proliferation and differentiation of mesenchymal stem cells via activation of indoleamine 2,3 dioxygenase (IDO). PLoS ONE 2011, 6, e14698. [Google Scholar] [CrossRef] [PubMed]
- Vigo, T.; Procaccini, C.; Ferrara, G.; Baranzini, S.; Oksenberg, J.R.; Matarese, G.; Diaspro, A.; Kerlero de Rosbo, N.; Uccelli, A. IFN-γ orchestrates mesenchymal stem cell plasticity through the signal transducer and activator of transcription 1 and 3 and mammalian target of rapamycin pathways. J. Allergy Clin. Immunol. 2017, 139, 1667–1676. [Google Scholar] [CrossRef] [PubMed]
- Polchert, D.; Sobinsky, J.; Douglas, G.W.; Kidd, M.; Moadsiri, A.; Reina, E.; Genrich, K.; Mehrotra, S.; Setty, S.; Smith, B.; et al. IFN-γ activation of mesenchymal stem cells for treatment and prevention of graft versus host disease. Eur. J. Immunol. 2008, 38, 1745–1755. [Google Scholar] [CrossRef] [Green Version]
- Kronsteiner, B.; Wolbank, S.; Peterbauer, A.; Hackl, C.; Redl, H.; van Griensven, M.; Gabriel, C. Human mesenchymal stem cells from adipose tissue and amnion influence T-cells depending on stimulation method and presence of other immune cells. Stem Cells Dev. 2011, 20, 2115–2126. [Google Scholar] [CrossRef]
- Rubtsov, Y.; Goryunov, K.; Romanov, A.; Suzdaltseva, Y.; Sharonov, G.; Tkachuk, V. Molecular mechanisms of immunomodulation properties of mesenchymal stromal cells: A new insight into the role of ICAM-1. Stem Cells Int. 2017, 2017. [Google Scholar] [CrossRef]
- Chen, D.; Ma, F.; Xu, S.; Yang, S.; Chen, F.; Rong, L.; Chi, Y.; Zhao, Q.; Lu, S.; Han, Z.; et al. Expression and role of Toll-like receptors on human umbilical cord mesenchymal stromal cells. Cytotherapy 2013, 15, 423–433. [Google Scholar] [CrossRef]
- Opitz, C.A.; Litzenburger, U.M.; Lutz, C.; Lanz, T.V.; Tritschler, I.; Köppel, A.; Tolosa, E.; Hoberg, M.; Anderl, J.; Aicher, W.K.; et al. Toll-like receptor engagement enhances the immunosuppressive properties of human bone marrow-derived mesenchymal stem cells by inducing indoleamine-2,3-dioxygenase-1 via interferon-beta and protein kinase R. Stem Cells 2009, 27, 909–919. [Google Scholar] [CrossRef]
- Sims, J.E.; Smith, D.E. The IL-1 family: Regulators of immunity. Nat. Rev. Immunol. 2010, 10, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Kalliolias, G.D.; Ivashkiv, L.B. TNF biology, pathogenic mechanisms and emerging therapeutic strategies. Nat. Rev. Rheumatol. 2016, 12, 49–62. [Google Scholar] [CrossRef]
- Hsu, P.; Santner-Nanan, B.; Hu, M.; Skarratt, K.; Lee, C.H.; Stormon, M.; Wong, M.; Fuller, S.J.; Nanan, R. IL-10 potentiates differentiation of human induced regulatory T cells via STAT3 and Foxo1. J. Immunol. 2015, 195, 3665–3674. [Google Scholar] [CrossRef] [PubMed]
- English, K.; Ryan, J.M.; Tobin, L.; Murphy, M.J.; Barry, F.P.; Mahon, B.P. Cell contact, prostaglandin E(2) and transforming growth factor beta 1 play non-redundant roles in human mesenchymal stem cell induction of CD4 + CD25 (High) forkhead box P3+ regulatory T cells. Clin. Exp. Immunol. 2009, 156, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Selmani, Z.; Naji, A.; Zidi, I.; Favier, B.; Gaiffe, E.; Obert, L.; Borg, C.; Saas, P.; Tiberghien, P.; Rouas-Freiss, N.; et al. Human leukocyte antigen-G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4 + CD25highFOXP3+ regulatory T cells. Stem Cells 2008, 26, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Miyagawa, I.; Nakayamada, S.; Nakano, K.; Yamagata, K.; Sakata, K.; Yamaoka, K.; Tanaka, Y. Induction of regulatory T cells and its regulation with insulin-like growth factor/insulin-like growth factor binding protein-4 by human mesenchymal stem cells. J. Immunol. 2017, 199, 1616–1625. [Google Scholar] [CrossRef]
- Matula, Z.; Németh, A.; Lőrincz, P.; Szepesi, Á.; Brózik, A.; Buzás, E.I.; Lőw, P.; Német, K.; Uher, F.; Urbán, V.S. The Role of extracellular vesicle and tunneling nanotube-mediated intercellular cross-talk between mesenchymal stem cells and human peripheral T cells. Stem Cells Dev. 2016, 25, 1818–1832. [Google Scholar] [CrossRef]
- Phinney, D.G.; Pittenger, M.F. Concise review: MSC-derived exosomes for cell-free therapy. Stem Cells 2017, 35, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Cosenza, S.; Toupet, K.; Maumus, M.; Luz-Crawford, P.; Blanc-Brude, O.; Jorgensen, C.; Noël, D. Mesenchymal stem cells-derived exosomes are more immunosuppressive than microparticles in inflammatory arthritis. Theranostics 2018, 8, 1399–1410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gieseke, F.; Böhringer, J.; Bussolari, R.; Dominici, M.; Handgretinger, R.; Müller, I. Human multipotent mesenchymal stromal cells use galectin-1 to inhibit immune effector cells. Blood 2010, 116, 3770–3779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serejo, T.R.T.; Silva-Carvalho, A.É.; Braga, L.D.d.C.F.; Neves, F.d.A.R.; Pereira, R.W.; Carvalho, J.L.d.; Saldanha-Araujo, F. Assessment of the Immunosuppressive Potential of INF-γ Licensed Adipose Mesenchymal Stem Cells, Their Secretome and Extracellular Vesicles. Cells 2019, 8, 22. https://doi.org/10.3390/cells8010022
Serejo TRT, Silva-Carvalho AÉ, Braga LDdCF, Neves FdAR, Pereira RW, Carvalho JLd, Saldanha-Araujo F. Assessment of the Immunosuppressive Potential of INF-γ Licensed Adipose Mesenchymal Stem Cells, Their Secretome and Extracellular Vesicles. Cells. 2019; 8(1):22. https://doi.org/10.3390/cells8010022
Chicago/Turabian StyleSerejo, Teresa Raquel Tavares, Amandda Évelin Silva-Carvalho, Luma Dayane de Carvalho Filiú Braga, Francisco de Assis Rocha Neves, Rinaldo Wellerson Pereira, Juliana Lott de Carvalho, and Felipe Saldanha-Araujo. 2019. "Assessment of the Immunosuppressive Potential of INF-γ Licensed Adipose Mesenchymal Stem Cells, Their Secretome and Extracellular Vesicles" Cells 8, no. 1: 22. https://doi.org/10.3390/cells8010022
APA StyleSerejo, T. R. T., Silva-Carvalho, A. É., Braga, L. D. d. C. F., Neves, F. d. A. R., Pereira, R. W., Carvalho, J. L. d., & Saldanha-Araujo, F. (2019). Assessment of the Immunosuppressive Potential of INF-γ Licensed Adipose Mesenchymal Stem Cells, Their Secretome and Extracellular Vesicles. Cells, 8(1), 22. https://doi.org/10.3390/cells8010022



