The Expanding Role of Vesicles Containing Aquaporins
Abstract
1. Introduction
2. Aquaporins Activity in Vesicles
3. Aquaporins Trafficking
3.1. Protein–Protein Interaction
3.2. Diacidic and other Motifs
3.3. Phosphorylation and Ubiquitination
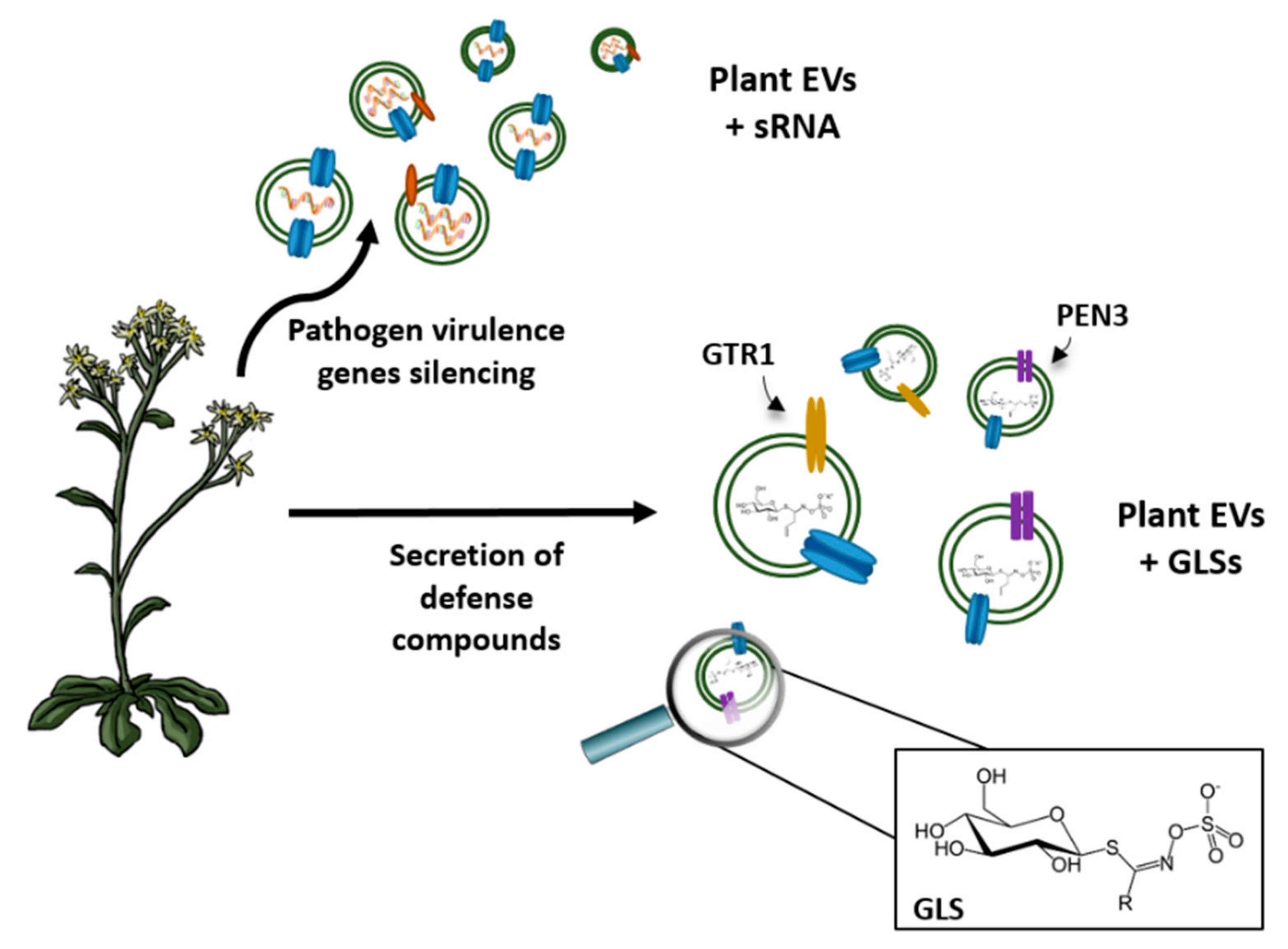
4. Vesicles Containing Aquaporins and Communication between Cells
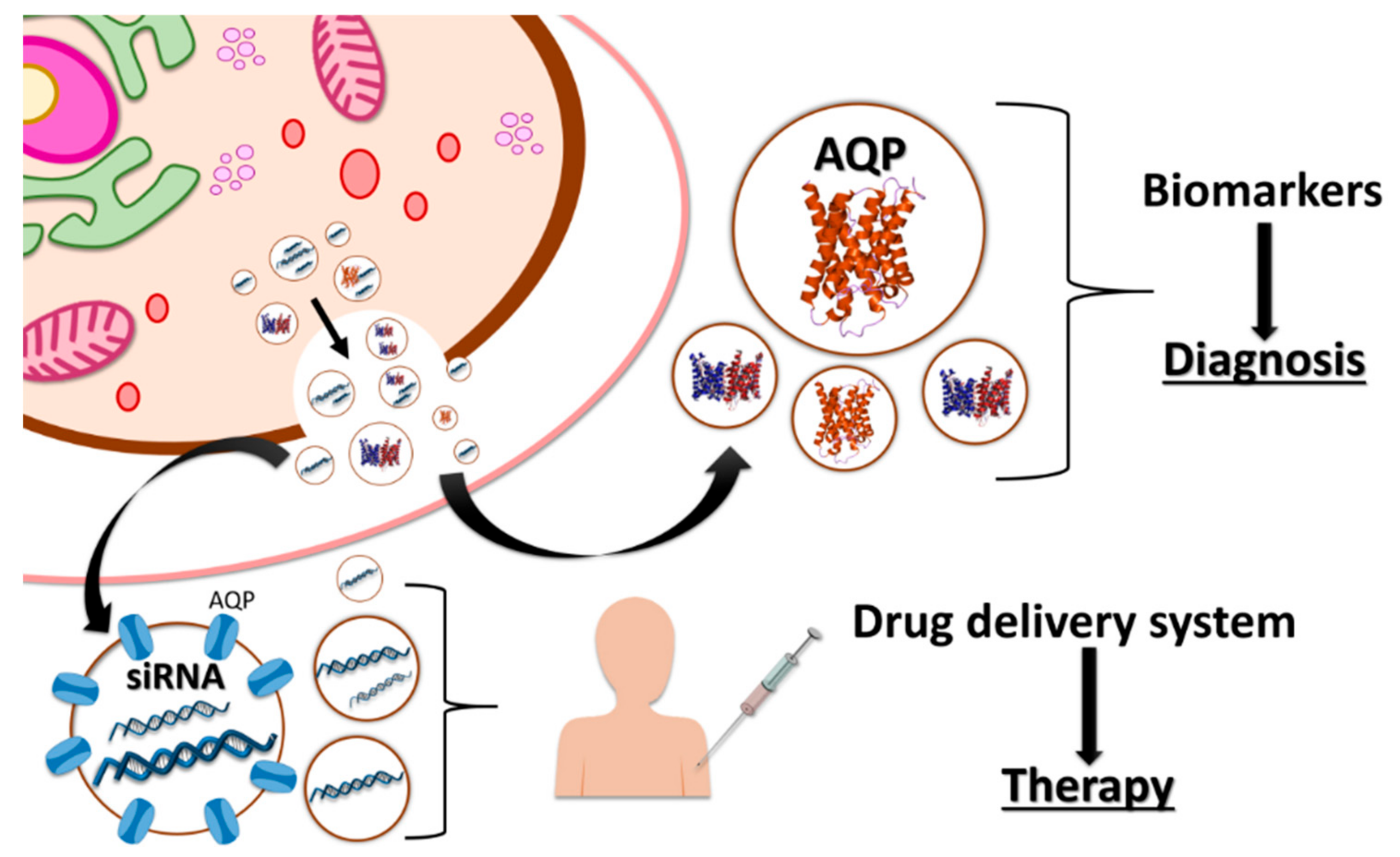
5. Industrial Application of Vesicles in Biomedicine
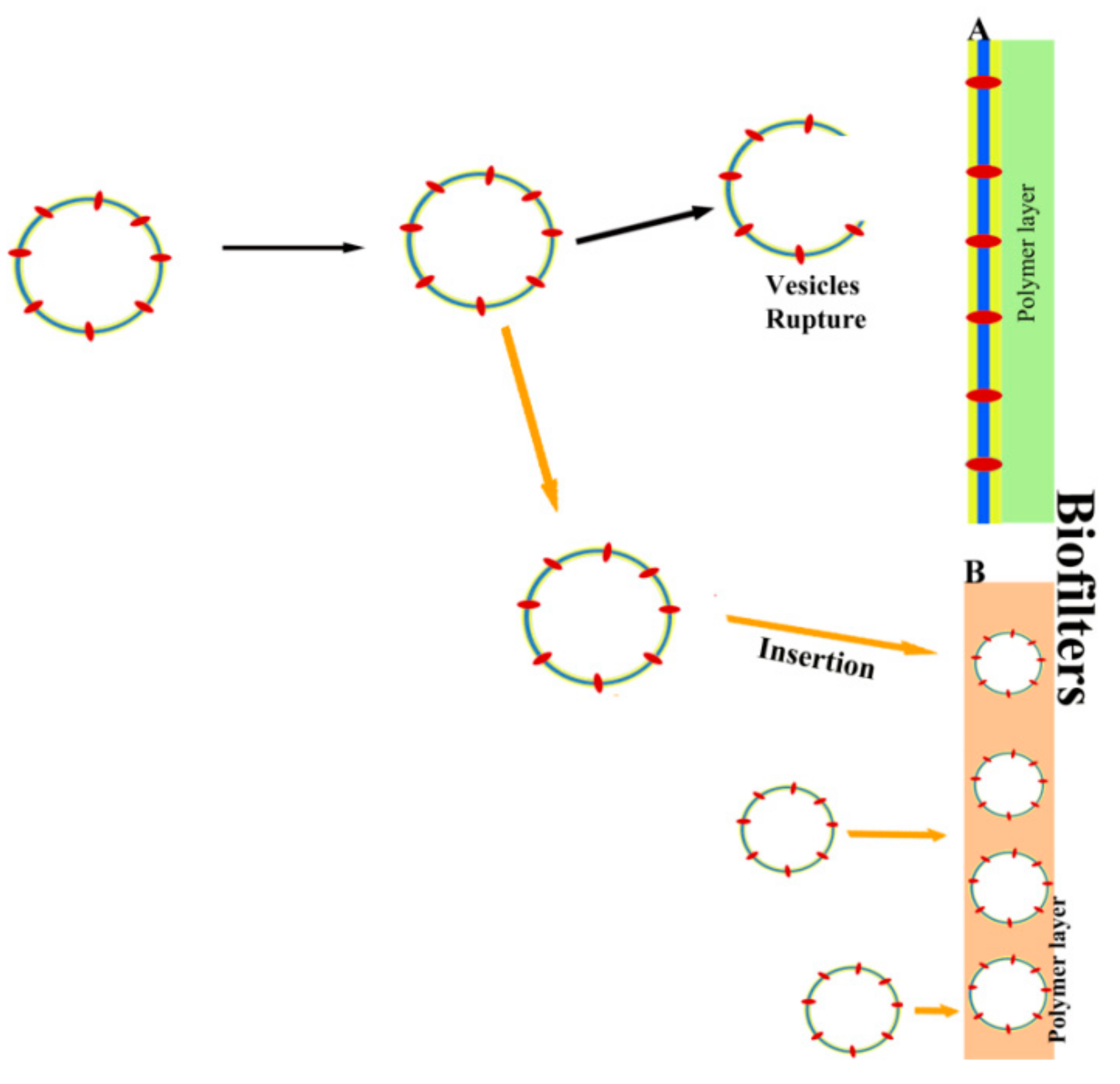
6. Other Biotechnological Applications of Vesicles Containing Aquaporins
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Horsefield, R.; Norden, K.; Fellert, M.; Backmark, A.; Tornroth-Horsefield, S.; van Scheltinga, A.C.T.; Kvassman, J.; Kjellbom, P.; Johanson, U.; Neutze, R. High-resolution X-ray structure of human aquaporin 5. Proc. Natl. Acad. Sci. USA 2008, 105, 13327–13332. [Google Scholar] [CrossRef] [PubMed]
- Walz, T.; Fujiyoshi, Y.; Engel, A. The AQP structure and functional implications. Handb. Exp. Pharmacol. 2009, 190, 31–56. [Google Scholar]
- Verkman, A.S.; Anderson, M.O.; Papadopoulos, M.C. Aquaporins: Important but elusive drug targets. Nat. Rev. Drug. Discov. 2014, 13, 259–277. [Google Scholar] [CrossRef] [PubMed]
- Johanson, U.; Karlsson, M.; Gustavsson, S.; Sjovall, S.; Fraysse, L.; Weig, A.R.; Kjellbom, P. The complete set of genes encoding major intrinsic proteins in Arabidopsis provides a framework for a new nomenclature for major intrinsic proteins in plants. Plant Physiol. 2001, 126, 1358–1369. [Google Scholar] [CrossRef] [PubMed]
- Quigley, F.; Rosenberg, J.M.; Shachar-Hill, Y.; Bohnert, H.J. From genome to function: The Arabidopsis aquaporins. Genome Biol. 2001, 3. [Google Scholar] [CrossRef]
- Gupta, A.B.; Sankararamakrishnan, R. Genome-wide analysis of major intrinsic proteins in the tree plant Populus Trichocarpa: Characterization of XIP subfamily of aquaporins from evolutionary perspective. BMC Plant. Biol. 2009, 9, 134. [Google Scholar] [CrossRef] [PubMed]
- Park, W.; Scheffler, B.E.; Bauer, P.J.; Campbell, B.T. Identification of the family of aquaporin genes and their expression in upland cotton (Gossypium hirsutum L.). BMC Plant Biol. 2010, 10, 142. [Google Scholar] [CrossRef] [PubMed]
- Maurel, C.; Boursiac, Y.; Luu, D.T.; Santoni, V.; Shahzad, Z.; Verdoucq, L. Aquaporins in plants. Physiol. Rev. 2015, 95, 1321–1358. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.N.; Chauvigné, F.; Hlidberg, J.B.; Cutler, C.P.; Cerdà, J. The lineage-specific evolution of aquaporin gene clusters facilitated tetrapod terrestrial adaptation. PLoS ONE 2014, 9, e113686. [Google Scholar] [CrossRef] [PubMed]
- Verkman, A.S. Aquaporins: Translating bench research to human disease. J. Exp. Biol. 2009, 212, 1707–1715. [Google Scholar] [CrossRef] [PubMed]
- Benga, O.; Huber, V.J. Brain water channel proteins in health and disease. Mol. Aspects Med. 2012, 33, 562–578. [Google Scholar] [CrossRef] [PubMed]
- Sutka, M.; Amodeo, G.; Ozu, M. Plant and animal aquaporins crosstalk: What can be revealed from distinct perspectives. Biophys. Rev. 2017, 9, 545562. [Google Scholar] [CrossRef] [PubMed]
- Laloux, T.; Junqueira, B.; Maistriaux, L.C.; Ahmed, J.; Jurkiewicz, A.; Chaumont, F. Plant and mammal aqupaorins: Same but different. Int. J. Mol. Sci. 2018, 19, 521. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Ballesta, M.; García-Gomez, P.; Yepes-Molina, L.; Guarnizo, A.L.; Teruel, J.A.; Carvajal, M. Plasma membrane aquaporins mediates vesicle stability in broccoli. PLoS ONE. 2018, 13, e0192422. [Google Scholar] [CrossRef] [PubMed]
- Madeira, A.; Moura, T.F.; Soveral, G. Detecting aquaporin function and regulation. Front. Chem. 2016, 4, 3. [Google Scholar] [CrossRef] [PubMed]
- Solenov, E.I.; Baturina, G.S.; Katkova, L.E.; Zarogiannis, S.G. Methods to measure water permeability (Aquaporins). Adv. Exp. Med. Bio. Book Ser. 2017, 969, 263–276. [Google Scholar]
- Eto, K.; Noda, Y.; Horikawa, S.; Uchida, S.; Sasaki, S. Phosphorylation of aquaporin-2 regulates its water permeability. J. Biol. Chem. 2010, 285, 40777–40784. [Google Scholar] [CrossRef] [PubMed]
- Dobbs, L.; Gonzalez, R.; Matthay, M.A.; Carter, E.P.; Allen, L.; Verkman, A.S. Highly water-permeable type I alveolar epithelial cells confer high water permeability between the airspace and vasculature in rat lung. Proc. Natl. Acad. Sci. USA 1998, 95, 2991–2996. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Laforge, F.O.; Abeyweera, T.P.; Rotenberg, S.A.; Carpino, J.; Mirkin, M.V. Nanoelectrochemistry of mammalian cells. Proc. Natl. Acad. Sci. USA 2008, 105, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Yakata, K.; Hiroaki, Y.; Ishibashi, K.; Sohara, E.; Sasaki, S.; Mitsuoka, K.; Fujiyoshi, Y. Aquaporin-11 containing a divergent NPA motif has normal water channel activity. Biochim. Biophys. Acta 2007, 1768, 688–693. [Google Scholar] [CrossRef] [PubMed]
- Gorelick, D.A.; Praetorius, J.; Tsunenari, T.; Nielsen, S.; Agre, P. Aquaporin-11: A channel protein lacking apparent transport function expressed in brain. BMC Biochem. 2006, 7, 14. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Morishita, Y.; Matsuzaki, T.; Hara-chikuma, M.; Andoo, A.; Shimono, M.; Matsuki, A.; Kobayashi, K.; Ikeda, M.; Yamamoto, T.; Verkman, A.; et al. Disruption of aquaporin-11 produces polycystic kidneys following vacuolization of the proximal tubule. Mol. Cell Biol. 2005, 25, 7770–7779. [Google Scholar] [CrossRef] [PubMed]
- Yakata, K.; Tani, K.; Fujiyoshi, Y. Water permeability and characterization of aquaporin-11. J. Struct. Biol. 2011, 174, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Hachez, C.; Besserer, A.; Chelavier, A.S.; Chaumont, F. Insights into plasma membrane aquaporin trafficking. Trends Plant Sci. 2013, 18, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Vukicevic, T.; Schulz, M.; Faust, F.; Klussmann, E. The trafficking of the Water channel aquaporin-2 in renal principal cells—A potential target for pharmacological intervention in cardiovascular diseases. Front. Pharmacol. 2016, l7, 1–27. [Google Scholar]
- Langeberg, L.K.; Scott, J.D. Signalling scaffolds and local organization of cellular behaviour. Nat. Rev. Mol. Cell Biol. 2015, 16, 232–244. [Google Scholar] [CrossRef] [PubMed]
- Noda, Y.; Sasaki, S. Regulation of aquaporin-2 trafficking and its binding protein complex. Biochim. Biophys. Act. Biomembr. 2006, 1758, 1117–1125. [Google Scholar] [CrossRef] [PubMed]
- Noda, Y.; Horikawa, S.; Kanda, E.; Yamashita, M.; Meng, H.; Eto, K.; Li, Y.; Kuwahara, M.; Pack, C.; Kinjo, M.; et al. Reciprocal interaction with G-actin and tropomyosinis essential for aquaporin- 2 trafficking. J. Biol. Chem. 2008, 182, 587–601. [Google Scholar]
- Van Balkom, B.W.; Boone, M.; Hendriks, G.; Kamsteeg, E.J.; Robben, J.H.; Stronks, H.C.; van der Voorde, A.; van Herp, F.; van der Sluijs, P.; Deen, P.M. LIP5 interacts with aquaporin 2 and facilitates its lysosomal degradation. J. Am. Soc. Nephrol. 2009, 20, 990–1001. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.A.; Sun, T.X.; Matsuzaki, T.; Yi, X.H.; Eswara, J.; Bouley, R.; McKee, M.; Brown, D. Heat shock protein 70 interacts with aquaporin-2 and regulates its trafficking. J. Biol. Chem. 2007, 282, 28721–28732. [Google Scholar] [CrossRef] [PubMed]
- Zwang, N.A.; Hoffert, J.D.; Pisitkun, T.; Moeller, H.B.; Fenton, R.A.; Knepper, M.A. Identification of phosphorylation-dependent binding partners of aquaporin-2 using protein mass spectrometry. J. Proteome Res. 2009, 8, 1540–1554. [Google Scholar] [CrossRef] [PubMed]
- Park, E.J.; Lim, J.S.; Jung, H.J.; Kim, E.; Han, K.H.; Kwon, T.H. The role of 70-kDa heat shock protein in dDAVP-induced AQP2 trafficking in kidney collecting duct cells. Am. J. Physiol. Renal Physiol. 2013, 304, F958–F971. [Google Scholar] [CrossRef] [PubMed]
- Kitchen, P.; Oberg, F.; Sjohamn, J.; Hedfalk, K.; Bill, R.M.; Conner, A.C.; Conner, M.T.; Tornroth-Horsefield, S. Plasma membrane abundance of human aquaporin 5 is dynamically regulated by multiple pathways. PLoS ONE. 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Roche, J.V.; Törnroth-Horsefield, S. Aquaporin Protein-Protein Interactions. Int. J. Mol. Sci. 2017, 18, 2255. [Google Scholar] [CrossRef] [PubMed]
- Valenti, G.; Procino, G.; Tamma, G.; Carmosino, M.; Svelto, M. Minireview: Aquaporin 2 Trafficking. Endocrinology 2005, 146, 5063–5070. [Google Scholar] [CrossRef] [PubMed]
- Besserer, A.; Bumotte, E.; Bienert, G.P.; Chelavier, A.S.; Errachid, A.; Grefen, C.; Blatt, M.R.; Chaumont, F. Selective Regulation of Maize Plasma Membrane Aquaporin Trafficking and Activity by the SNARE SYP121. Plant Cell 2012, 24, 3463–3481. [Google Scholar] [CrossRef] [PubMed]
- Hachez, C.; Laloux, T.; Reinhardt, H.; Cavez, D.; Degand, H.; Grefen, C.; De Rycke, R.; Inzé, D.; Blatt, M.R.; Russinova, E.; et al. Arabidopsis SNAREs SYP61 and SYP121 coordinate the trafficking of plasma membrane aquaporin PIP2;7 to modulate the cell membrane water Permeability. Plant Cell 2014, 26, 3132–3147. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Ballesta, M.C.; Carvajal, M. Mutual interactions between aquaporins and membrane components. Front. Plant Sci. 2016, 7, 1322. [Google Scholar] [CrossRef] [PubMed]
- Yaneff, A.; Sigaut, L.; Marquez, M.; Alleva, K.; Pietrasanta, L.I.; Amodeo, G. Heteromerization of PIP aquaporins affect their intrinsic permeability. Proc. Natl. Acad. Sci. USA 2014, 111, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Fetter, K.; Van Wilder, V.; Moshelion, M.; Chaumont, F. Interactions between plasma membrane aquaporins modulate their water channel activity. Plant Cell 2004, 16, 215–228. [Google Scholar] [CrossRef] [PubMed]
- Zelazny, E.; Borst, J.W.; Muylaert, M.; Batoko, H.; Hemminga, M.A.; Chaumont, F. Fret imaging in living maize cells reveals that plasma membrane aquaporins interact to regulate their subcellular localization. Proc. Natl. Acad. Sci. USA 2007, 104, 12359–12364. [Google Scholar] [CrossRef] [PubMed]
- Sorieul, M.; Santoni, V.; Maurel, C.; Luu, D.T. Mechanisms and effects of retention of over-expressed aquaporin AtPIP2;1 in the endoplasmic reticulum. Traffic 2011, 12, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Jozefkowicz, C.; Rosi, P.; Sigaut, L.; Soto, G.; Pietrasanta, L.I.; Amodeo, G.; Alleva, K. Loop a is critical for the functional interaction of two Beta Vulgaris PIPAquaporins. PLoS ONE 2013, 8, e57993. [Google Scholar] [CrossRef] [PubMed]
- Neely, J.D.; Christensen, B.M.; Nielsen, S.; Agre, P. Heterotetrameric composition of aquaporin-4 water channels. Biochemistry 1999, 38, 11156–11163. [Google Scholar] [CrossRef] [PubMed]
- Bichet, D.G.; El Tarazi, A.; Matar, J.; Lussier, Y.; Arthus, M.F.; Lonergan, M.; Bockenhauer, D.; Bissonnette, P. Aquaporin-2: New mutations responsible for autosomal-recessive nephrogenic diabetes insipidus-update and epidemiology. Clin. Kidney J. 2012, 5, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Beitz, E.; Liu, K.; Ikeda, M.; Guggino, W.B.; Agre, P.; Yasui, M. Determinants of AQP6 trafficking to intracellular sites versus the plasma membrane in transfected mammalian cells. Biol. Cell 2006, 98, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Itoh, T.; Rai, T.; Kuwahara, M.; Ko, S.B.H.; Uchida, S.; Sasaki, S.; Ishibashiet, K. Identification of a novel aquaporin, AQP12, expressed in pancreatic acinar cells. Biochem. Biophys. Res. Commun. 2005, 330, 832–838. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.G.; Su, W.H.; Yi, F.; Zhang, D.; Hao, F.; Zhang, H.G.; Liu, Y.J.; Feng, X.C.; Ma, T.H. NPA motifs play a key role in plasma membrane targeting of Aquaporin-4. IUBMB Life. 2010, 62, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Zelazny, E.; Miecielica, U.; Borst, J.W.; Hemminga, M.A.; Chaumont, F. An N-terminal diacidic motif is required for the trafficking of maize aquaporins ZmPIP2;4 and ZmPIP2;5 to the plasma membrane. Plant J. 2009, 57, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Procino, G.; Carmosino, M.; Marin, O.; Brunati, A.M.; Contri, A.; Pinna, L.A.; Mannucci, R.; Nielsen, S.; Kwon, T.H.; Svelto, M.; et al. Ser-256 phosphorylation dynamics of aquaporin 2 during maturation from the ER to the vesicular compartment in renal cells. FASEB J. 2003, 17, 1886–1888. [Google Scholar] [CrossRef] [PubMed]
- Hoffert, J.D.; Nielsen, J.; Yu, M.J.; Pisitkun, T.; Schleicher, S.M.; Nielsen, S.; Knepper, M.A. Dynamics of aquaporin-2 serine-261 phosphorylation in response to short-term vasopressin treatment in collecting duct. Am. J. Physiol. Renal Physiol. 2007, 292, F691–F700. [Google Scholar] [CrossRef] [PubMed]
- Fenton, R.A.; Moeller, H.B.; Hoffert, J.D.; Yu, M.J.; Nielsen, S.; Knepper, M.A. Acute regulation of aquaporin-2 phosphorylation at ser-264 by vasopressin. Proc. Natl. Acad. Sci. USA 2008, 105, 3134–3139. [Google Scholar] [CrossRef] [PubMed]
- Kamsteeg, E.J.; Heijnen, I.; van Os, C.H.; Deen, P.M. The subcellular localization of an aquaporin-2 tetramer depends on the stoichiometry of phosphorylated and non-phosphorylated monomers. J. Cell Biol. 2000, 151, 919–930. [Google Scholar] [CrossRef] [PubMed]
- Nejsum, L.N.; Zelenina, M.; Aperia, A.; Frokiaer, J.; Nielsen, S. Bidirectional regulation of AQP2 trafficking and recycling: Involvement of AQP2-S256 phosphorylation. Am. J. Physiol. Renal Physiol. 2005, 288, 930–938. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Sun, T.X.; Bouley, R.; Blackburn, K.; McLaughlin, M.; Brown, D. Inhibition of endocytosis causes phosphorylation (S256)-independent plasma membrane accumulation of AQP2. Am. J. Physiol. Renal Physiol. 2004, 286, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Valenti, G.; Procino, G.; Carmosino, M.; Frigeri, A.; Mannucci, R.; Nicoletti, I.; Svelto, M. The phosphatase inhibitor okadaic acid induces AQP2 translocation independently from AQP2 phosphorylation in renal collecting duct cells. J. Cell Sci. 2000, 113, 1985–1992. [Google Scholar] [PubMed]
- Kitchen, P.; Day, R.E.; Taylor, L.H.; Salman, M.M.; Bill, R.M.; Conner, M.T.; Conner, A.C. Identification and molecular mechanisms of the rapid tonicity-induced Relocalization of the aquaporin 4 channel. J. Biol. Chem. 2015, 290, 16873–16881. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.L.; Kwon, T.H. Ubiquitination of Aquaporin-2 in the Kidney. Electrolytes Blood Press. 2009, 7, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Zelenina, M.; Christensen, B.M.; Palmer, J.; Nairn, A.C.; Nielsen, S.; Aperia, A. Prostaglandin E(2) interaction with AVP: Effects on AQP2 phosphorylation and distribution. Am. J. Physiol. Renal Physiol. 2000, 278, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.K.; Cho, S.K.; Son, O.; Xu, Z.; Hwang, I.; Kimb, W.T. Drought stress-induced Rma1H1, a RING membrane-anchor E3 ubiquitin ligase homolog, Regulates aquaporin levels via ubiquitination in transgenic Arabidopsis plants. Plant Cell 2009, 21, 622–641. [Google Scholar] [CrossRef] [PubMed]
- Bloemendal, S.; Kück, U. Cell-to-cell communication in plants, animals, and fungi: A comparative review. Naturwissenschaften 2013, 100, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.; Raposo, G.; Théry, C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef] [PubMed]
- Maas, S.L.; Breakefield, X.O.; Weaver, A.M. Extracellular vesicles: Unique intercellular delivery vehicles. Trends Cell Biol. 2017, 27, 172–188. [Google Scholar] [CrossRef] [PubMed]
- Yanez-Mo, M.; Siljander, P.R.; Andreu, Z.; Zavec, A.B.; Borras, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles. 2015, 4, 270066. [Google Scholar] [CrossRef] [PubMed]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Kowal, J.; Arras, G.; Colombo, M.; Jouve, M.; Morath, J.P.; Primdal-Bengtson, B.; Dingli, F.; Loew, D.; Tkach, M.; Thery, C. Proteomic comparison defines novel markers to characterize heterogeneous population of extracellular vesicle subtypes. Proc. Natl. Acad. Sci. USA 2016, 113, E968–E977. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.M.; Abdelmohsen, K.; Mustapic, M.; Kapogiannis, D.; Gorospe, M. RNA in extracellular vesicles. Wiley Interdiscip. Rev. RNA 2017, 8, e1413. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, B. Extracellular vesicle microRNAs mediate skeletal muscle myogenesis and disease. Biomed. Rep. 2016, 5, 296–300. [Google Scholar] [CrossRef] [PubMed]
- Halperin, W.; Jensen, W.A. Ultrastructural changes during growth and embryogenesis in carrot cell cultures. J. Ultrastruct. Res. 1967, 18, 428–443. [Google Scholar] [CrossRef]
- Palanisamy, V.; Sharma, S.; Deshpande, A.; Zhou, H.; Gimzewski, J.; Wong, D.T. Nanostructural and transcriptomic analyses of human saliva derived exosomes. PLoS ONE 2010, 5, e8577. [Google Scholar] [CrossRef] [PubMed]
- Street, J.M.; Barran, P.E.; Mackay, C.L.; Weidt, S.; Balmforth, C.; Walsh, T.S.; Chalmers, T.A.; Webb, D.J.; Dear, J.W. Identification and proteomic profiling of exosomes in human cereprospinal fluid. J. Transl. Med. 2012, 10, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Lasser, C.; Alikhani, V.S.; Ekström, K.; Eldh, M.; Paredes, P.T.; Bossios, A.; Sjöstrand, M.; Gabrielsson, S.; Lötvall, J.; Valadi, H. Human saliva, plasma and breast milk exosomes contain RNA: Uptake by macrophages. J. Transl. Med. 2011, 9, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, R.M. Revisiting the road to the discovery of exosomes. Blood Cells Mol. Dis. 2005, 34, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Chasis, J.A.; Mohandas, N. Erythroblastic islands: Niches for erythropoiesis. Blood. 2008, 112, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Gronowicz, G.; Swift, H.; Steck, T.L. Maturation of the reticulocyte in vitro. J. Cell Sci. 1984, 71, 177–197. [Google Scholar] [PubMed]
- Leitch, V.; Agre, P.; King, L.S. Altered ubiquitination and stability of aquaporin-1 in hypertonic stress. Proc. Natl. Acad. Sci. USA 2001, 98, 2894–2898. [Google Scholar] [CrossRef] [PubMed]
- Blanc, L.; Liu, J.; Vidal, M.; Chasis, J.A.; An, X.; Mohandas, N. The water channel aquaporin-1 partitions into exosomes during reticulocyte maturation: Implication for the regulation of cell volume. Blood 2009, 14, 3928–3934. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, Y.; Mikami, S.; Yamamoto, K.; Sakai, M.; Saito, T.; Yamamoto, T.; Ishibashi, K.; Sasaki, S. AQP2 in human urine is predominantly localized to exosomes with preserved water channel activities. J. Clin. Exp. Nephrol. 2018, 22, 782–788. [Google Scholar] [CrossRef] [PubMed]
- Street, J.M.; Birkhoff, W.; Menzies, R.I.; Webb, D.J.; Bailey, M.A.; Dear, J.W. Exosomal transmission of functional aquaporin 2 in kidney cortical collecting duct cells. J. Physiol. 2011, 589, 6119–6127. [Google Scholar] [CrossRef] [PubMed]
- Kelly, M.L.; Cho, W.J.; Jeremic, A.; Abu-Hamdah, R.; Jena, B.P. Vesicle swelling regulates content expulsion during secretion. Cell Biol. Int. 2004, 28, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Jeremic, A.; Cho, W.J.; Jena, B.P. Involvement of water channels in synaptic vesicle swelling. Exp. Biol. Med. 2005, 230, 674–680. [Google Scholar] [CrossRef]
- Shin, L.; Basi, N.; Jeremic, A.; Lee, J.S.; Cho, W.J.; Chen, Z.; Abu-Hamdah, R.; Oupicky, D.; Jena, B.P. Involvement of vH+-ATPase in synaptic vesicle swelling. J. Neurosci. Res. 2010, 88, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Abu-Hamdah, R.; Cho, W.J.; Cho, S.J.; Jeremic, A.; Kelly, M.; Ilie, A.E.; Jena, B.P. Regulation of the water channel aquaporin-1: Isolation and reconstitution of the regulatory complex. Cell Biol. Int. 2004, 28, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Qiao, L.; Wang, M.; He, B.; Lin, F.M.; Palmquist, J.; Huang, S.D.; Jin, H. Plants send smalls RNAs in extracellular vesicles to fungal pathogen to silence virulence genes. Science 2018, 360, 1126–1129. [Google Scholar] [CrossRef] [PubMed]
- Regente, M.; Pinedo, M.; San Clemente, H.; Balliau, T.; Jamet, E.; De la Canal, L. Plant extracellular vesicles are incorporated by a fungal pathogen and inhibit its growth. J. Exp. Bot. 2017, 68, 5485–5495. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Weiberg, A.; Lin, F.M.; Thomma, B.P.; Huang, H.D.; Jin, H. Bidirectional cross-kingdom RNAi and fungal uptake of external RNAs confer plant protection. Nat. Plants 2016, 2, 16151–16161. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, M.E.; Nour-Eldin, H.H.; Halkier, B.A. Transport of defense compounds from source to sink: Lessons learned from glucosinolates. Trends Plant. Sci. 2015, 2, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Underwood, W.; Somerviller, S.C. Perception of conserved pathogen elicitors at the plasma membrane leads to relocalization of the Arabidopsis PEN3 transporter. Proc. Natl. Acad. Sci. USA 2013, 110, 12492–12497. [Google Scholar] [CrossRef] [PubMed]
- Bednarek, P.; Pislewska-Bednarek, M.; Svatos, A.; Schneider, B.; Doubsky, J.; Mansurova, M.; Humphry, M.; Consonni, C.; Panstruga, R.; Sanchez-Vallet, A.; et al. A glucosinolate metabolism pathway in living plant cells mediates broad-spectrum antifungal defense. Science 2009, 323, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Rutter, B.D.; Innes, R.W. Extracellular vesicles isolated from the leaf apoplast carry stress-response proteins. Plant Physiol. 2017, 173, 728–741. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Xiao, S. Lipids in salicylic acid-mediated defense in plants: Focusing on the roles of phosphatidic acid and phosphatidylinositol 4-phosphate. Front. Plant Sci. 2015, 6, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.; Wolf, J.M.; Prados-Rosales, R.; Casadevall, A. Through the wall: Extracellular vesicles in Gram-positive bacteria, mycobacteria and fungi. Nat. Rev. Microbiol. 2015, 13, 620–630. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.Y.; Choi, D.; Kim, D.K.; Kim, J.W.; Park, J.O.; Kim, S.; Kim, S.H.; Desiderio, D.M.; Kim, J.K.; Kim, K.P.; et al. Gram-positive bacteria produce membrane vesicles: Proteomics-based characterization of Staphylococcus aureus-derived membrane vesicles. Proteomics 2009, 9, 5425–5436. [Google Scholar] [CrossRef] [PubMed]
- Casadevall, A.; Nosanchuk, J.D.; Williamson, P.; Rodrigues, M.L. Vesicular transport across the fungal cell wall. Trends Microbiol. 2009, 17, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Jonhanson, U.; Gustavsson, S. A new subfamily of major intrinsic proteins in plants. Mol. Biol. Evol. 2002, 19, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Santoni, V.; Maurel, C. Plant aquaporins: Roles in plant physiology. Biochim. Biophys. Acta 2014, 1848, 1574–1582. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Ballesta, M.; Pérez-Sánchez, H.; Moreno, D.A.; Carvajal, M. Plant plasma membrane aquaporins in natural vesicles as potential stabilizers and carrier of glucosinolates. Colloids Surf. B 2016, 143, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Viennois, E.; Xu, C.; Merlin, D. Plant derived edible nanoparticles as a new therapeutic approach against diseases. Tissue Barriers 2016, 4, e1134415. [Google Scholar] [CrossRef] [PubMed]
- Pegtel, D.M.; Peferoen, L.; Amor, S. Extracellular vesicles as modulators of cell-to-cell communication in the healthy and diseased brain. Philos. Trans. R. Soc. B 2014, 369, 20130516. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, C.; Zhou, T.; Liu, X.; Liu, X.; Li, X.; Chen, D. Role of exosomal proteins in cancer diagnosis. Mol. Cancer. 2017, 16, 14. [Google Scholar] [CrossRef] [PubMed]
- Andre, F.; Schartz, N.E.C.N.E.C.; Movassagh, M.; Flament, C.; Pautier, P.; Morice, P.; Pomel, C.; Lhomme, C.; Escudier, B.; Le Chevalier, T.; et al. Malignant effusions and immunogenic tumour-derived exosomes. Lancet 2002, 360, 295–305. [Google Scholar] [CrossRef]
- Batrakova, E.V.; Kim, M.S. Using exosomes, naturally-equipped nanocarriers, for drug delivery. J. Control Release 2015, 219, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Skog, J.; Wurdinger, T.; Rijn, S. Van; Meijer, D.; Gainche, L.; Sena-esteves, M.; Jr, W.T.C.; Carter, R.S.; Krichevsky, A.M.; Breakefield, X.O. Glioblastoma microvesicles transport RNA and protein that promote tumor growth and provide diagnostic biomarkers. Johan. Nat. Cell Biol. 2012, 10, 1470–1476. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Contreras, M.; Brooks, R.W.; Boccuzzi, L.; Robbins, P.D.; Ricordi, C. Exosomes as biomarkers and therapeutic tools for type 1 diabetes mellitus. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 2940–2956. [Google Scholar] [PubMed]
- Soung, Y.H.; Ford, S.; Zhang, V.; Chung, J. Exosomes in cancer diagnostics. Cancers 2017, 9, 8. [Google Scholar] [CrossRef] [PubMed]
- Erdbrugger, U.; Le, T.H. Extracellular Vesicles in Renal Diseases: More than Novel Biomarkers? J. Am. Soc. Nephrol. 2016, 27, 12–26. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Shen, Y.; Chen, T.; Xu, F.; Chen, X.; Zheng, S. The functions and clinical applications of tumor-derived exosomes. Oncotarget 2016, 7, 60736–60751. [Google Scholar] [CrossRef] [PubMed]
- Eichelser, C.; Stückrath, I.; Müller, V.; Milde-Langosch, K.; Wikman, H.; Pantel, K.; Schwarzenbach, H. Increased serum levels of circulating exosomal microRNA-373 in receptor-negative breast cancer patients. Oncotarget 2014, 5, 9650–9663. [Google Scholar] [CrossRef] [PubMed]
- Pocsfalvi, G.; Turiák, L.; Ambrosone, A.; Del Gaudio, P.; Puska, G.; Fiume, I.; Silvestre, T.; Vékey, K. Protein biocargo of citrus fruit-derived vesicles reveals heterogeneous transport and extracellular vesicle populations. J. Plant. Physiol. 2018, 229, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Verkman, A.S. Aquaporins in clinical medicine. Annu. Rev. Med. 2012, 63, 303–316. [Google Scholar] [CrossRef] [PubMed]
- Fossdal, G.; Vik-Mo, E.O.; Sandberg, C.; Varghese, M.; Kaarb, M.; Telmo, E.; Langmoen, I.A.; Murrell, W. Aqp 9 and brain tumour stem cells. Sci. World J. 2012, 2012, 915176. [Google Scholar] [CrossRef] [PubMed]
- Maugeri, R.; Schiera, G.; di Liegro, C.M.; Fricano, A.; Iacopino, D.G.; Di Liegro, I. Aquaporins and brain tumors. Int. J. Mol. Sci. 2016, 17, 1029. [Google Scholar] [CrossRef] [PubMed]
- McCoy, E.; Sontheimer, H. Expression and function of water channels (aquaporins) in migrating malignant astrocytes. Glia 2007, 55, 1034–1043. [Google Scholar] [CrossRef] [PubMed]
- Sonoda, H.; Yokota-Ikeda, N.; Oshikawa, S.; Kanno, Y.; Yoshinaga, K.; Uchida, K.; Ueda, Y.; Kimiya, K.; Uezono, S.; Ueda, A.; et al. Decreased abundance of urinary exosomal aquaporin-1 in renal ischemia-reperfusion injury. Am. J. Physiol. Renal Physiol. 2009, 297, F1006–F1016. [Google Scholar] [CrossRef] [PubMed]
- Oshikawa, S.; Sonoda, H.; Ikeda, M. Aquaporins in urinary extracellular vesicles (Exosomes). Int. J. Mol. Sci. 2016, 17, 957. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Hewitt, S.M.; Yuen, P.S.T.P.S.T.; Star, R.A. Acute Kidney Injury Biomarkers—Needs, Present Status, and Future Promise. Nephrol. Self. Assess. Prog. 2006, 5, 63–71. [Google Scholar]
- Li, Z.Z.; Zhao, Z.Z.; Wen, J.G.; Xing, L.; Zhang, H.; Zhang, Y. Early alteration of urinary exosomal aquaporin 1 and transforming growth factor β1after release of unilateral pelviureteral junction obstruction. J. Pediatr. Surg. 2012, 47, 1581–1586. [Google Scholar] [CrossRef] [PubMed]
- Abdeen, A.; Sonoda, H.; El-Shawarby, R.; Takahashi, S.; Ikeda, M. Urinary excretion pattern of exosomal aquaporin-2 in rats that received gentamicin. Am. J. Physiol. Renal Physiol. 2014, 307, F1227–F1237. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, R.A.; Diniz, L.F.B.; Teotônio, L.O.; Lima, C.G.; Mota, R.M.S.; Martins, A.; Sanches, T.R.; Seguro, A.C.; Andrade, L.; Silva, G.B., Jr.; et al. Renal tubular dysfunction in patients with American cutaneous leishmaniasis. Kidney Int. 2011, 80, 1099–1106. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Erviti, L.; Seow, Y.; Yin, H.; Betts, C.; Lakhal, S.; Wood, M.J.A.M.J.A. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 2011, 29, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Cloutier, N.; Paré, A.; Farndale, R.W.; Schumacher, H.R.; Nigrovic, P.A.; Lacroix, S.; Boilard, E. Platelets can enhance vascular permeability. Blood 2012, 120, 1334–1343. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Zhuang, X.; Xiang, X.; Liu, Y.; Zhang, S.; Liu, C.; Barnes, S.; Grizzle, W.; Miller, D.; Zhang, H.G. A novel nanoparticle drug delivery system: The anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol. Ther. 2010, 18, 1606–1614. [Google Scholar] [CrossRef] [PubMed]
- Badaut, J.; Ashwal, S.; Obenaus, A. Aquaporins in cerebrovascular disease: A target for treatment of brain edema? Cerebrovasc. Dis. 2011, 31, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, A.M.; Adami, A.; Pop, V.; Bellone, J.A.; Coats, J.S.; Hartman, R.E.; Ashwal, S.; Obenaus, A.; Badaut, J. Posttraumatic reduction of edema with aquaporin-4 RNA interference improves acute and chronic functional recovery. J. Cereb. Blood Flow Metab. 2013, 33, 1621–1632. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Tanugi, D.; McGovern, R.K.; Dave, S.H.; Lienhard, J.H.; Grossman, J.C. Quantifying the potential of ultrapermeable membranes for water desalination. Energy Environ. Sci. 2014, 7, 1134–1141. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Z.; Wang, X.; Wang, S.; Ding, W.; Gao, C. Layer-by-Layer Assembly of Aquaporin Z-Incorporated Biomimetic Membranes for Water Purification. Environ. Sci. Technol. 2015, 49, 3761–3768. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Ma, X.; Tang, C.Y. Recent development of novel membranes for desalination. Desalination 2018, 434, 37–59. [Google Scholar] [CrossRef]
- Kumar, M.; Grzelawoski, M.; Zilles, J.; Clark, M.; Meier, W. Highly permeable polymeric membranes based on the incorporation of the functional water channel protein AquaporinZ. Proc. Natl. Acad. Sci. USA 2007, 104, 20719–20724. [Google Scholar] [CrossRef] [PubMed]
- Borgnia, M.J.; Kozono, D.; Calamita, G.; Maloney, P.C.; Agre, P. Functional reconstitution and characterization of AqpZ, the E. coli water channel protein. J. Mol. Biol. 1999, 291, 1169–1179. [Google Scholar] [CrossRef] [PubMed]
- Stoenescu, R.; Graff, A.; Meier, W. Asymmetric ABC-Triblock Copolymer Membranes Induce a Directed Insertion of Membrane Proteins. Macromol. Biosci. 2004, 4, 930–935. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, R.; Tang, C.; Vararattanavech, A.; Zhao, Y.; Torres, J.; Fane, T. Preparation of supported lipid membranes for aquaporin Z. incorporation. Colloids Surf. B 2012, 94, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Van Hoek, A.N.; Verkman, A.S. Functional reconstruction of the isolated erythrocyted water channel CHIP28. J. Biol. Chem. 1992, 267, 18267–18629. [Google Scholar] [PubMed]
- Van Hoek, A.N.; Wiener, M.; Bicknese, S.; Miercke, L.; Biwersi, J.; Verkman, A.S. Secondary structure analysis of purified functional CHIP28 water channel by CD and FTIR spectrometry. Biochemistry 1993, 32, 11847–11856. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Wang, Z.; Petrinic, I.; Fane, A.G.; Hélix-Nielsen, C. Biometic aquaporin membranes coming of age. Desalination 2015, 368, 89–105. [Google Scholar] [CrossRef]
- Altamura, N.; Calamita, G. Systems for production of proteins for biomimetic membrane devices. In Biomimetic Membranes for Sensor and Separation Applications; Springer: Dordrecht, The Netherlands, 2012; pp. 233–250. [Google Scholar]
- Tang, C.Y.; Zhao, Y.; Wang, R.; Hélix-Nielsen, C.; Fane, A.G. Desalination by biometric aquaporin membrane: Review of status and prospects. Desalination 2013, 308, 34–40. [Google Scholar] [CrossRef]
- Xu, Z.; Lian, J.; Cai, J. Efficient expression of aquaporin Z in Echerichia Coli cell-free system using different fusion vectors. Protein Pept. Lett. 2010, 17, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Shwarz, D.; Junge, F.; Durst, F.; Frolich, N.; Schneider, B.; Reckel, S.; Sobhanifar, S.; Dotsch, S.; Bernhard, F. Preparative scale expression of membrane proteins in Echerichia coli based continuous exchange cell-free system. Nat. Protocols 2007, 2, 2945–2957. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, Y.; Grinberg, S.; Linder, C.; Heldman, E.; Gilron, J.; Shen, Y.X.; Kumar, M.; Lammertink, R.G.H.; Freger, V. Towards supported bolaamphiphile membranes for water filtration: Roles of lipid and substrates. J. Membr. Sci. 2014, 457, 50–61. [Google Scholar] [CrossRef]
- Wang, H.L.; Chung, T.S.; Tong, Y.W.; Jeyaseelan, K.; Armugam, A.; Hoang Hanh Phuoc, D.; Fu, F.; Seah, H.; Yang, J.; Hong, M. Mechanically robust and highly permeable AquaporinZ biomimetic membranes. J. Membr. Sci. 2013, 434, 130–136. [Google Scholar] [CrossRef]
- Zhao, Y.; Qui, C.; Li, X.; Vararattanavech, A.; Shen, W.; Torres, J.; Helix-Nielsen, C.; Wang, R.; Hu, X.; Fane, A.G.; et al. Synyhesis of robust and high-performance aquaporin-based biomimetic membranes by interfacial polymerization-membrane preparation and RO performance characterization. J. Membr. Sci. 2012, 423, 422–428. [Google Scholar] [CrossRef]
- Li, X.; Wang, R.; Wicaksan, F.; Tang, C.Y.; Torres, J.; Fane, A.G. Preparation of high performance nanofiltration (NF) membranes incorporated with aquaporin Z. J. Membr. Sci. 2014, 450, 181–184. [Google Scholar] [CrossRef]
- Sun, G.; Chung, T.S.; Chen, N.; Lu, X.; Zhao, Q. Highly permeable aquiaporin-embedded biomimetic membrane featuring a magnetic-aided approach. RSC Adv. 2013, 3, 9178–9184. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinez-Ballesta, M.C.; Garcia-Ibañez, P.; Yepes-Molina, L.; Rios, J.J.; Carvajal, M. The Expanding Role of Vesicles Containing Aquaporins. Cells 2018, 7, 179. https://doi.org/10.3390/cells7100179
Martinez-Ballesta MC, Garcia-Ibañez P, Yepes-Molina L, Rios JJ, Carvajal M. The Expanding Role of Vesicles Containing Aquaporins. Cells. 2018; 7(10):179. https://doi.org/10.3390/cells7100179
Chicago/Turabian StyleMartinez-Ballesta, M Carmen, Paula Garcia-Ibañez, Lucía Yepes-Molina, Juan José Rios, and Micaela Carvajal. 2018. "The Expanding Role of Vesicles Containing Aquaporins" Cells 7, no. 10: 179. https://doi.org/10.3390/cells7100179
APA StyleMartinez-Ballesta, M. C., Garcia-Ibañez, P., Yepes-Molina, L., Rios, J. J., & Carvajal, M. (2018). The Expanding Role of Vesicles Containing Aquaporins. Cells, 7(10), 179. https://doi.org/10.3390/cells7100179






