Abstract
Following unfavorable environmental cues, cells reprogram pathways that govern transcription, translation, and protein degradation systems. This reprogramming is essential to restore homeostasis or commit to cell death. This review focuses on the secondary roles of two nuclear transcriptional regulators, cyclin C and Med13, which play key roles in this decision process. Both proteins are members of the Mediator kinase module (MKM) of the Mediator complex, which, under normal physiological conditions, positively and negatively regulates a subset of stress response genes. However, cyclin C and Med13 translocate to the cytoplasm following cell death or cell survival cues, interacting with a host of cell death and cell survival proteins, respectively. In the cytoplasm, cyclin C is required for stress-induced mitochondrial hyperfission and promotes regulated cell death pathways. Cytoplasmic Med13 stimulates the stress-induced assembly of processing bodies (P-bodies) and is required for the autophagic degradation of a subset of P-body assembly factors by cargo hitchhiking autophagy. This review focuses on these secondary, a.k.a. “night jobs” of cyclin C and Med13, outlining the importance of these secondary functions in maintaining cellular homeostasis following stress.
1. Introduction
Under normal physiological conditions, cells attain homeostasis through complex regulatory events, including balancing the transcription and translation of proteins with their degradation. Transcriptional control is mediated through the activities of DNA binding transcription factors, RNA polymerase II (RNAPII), RNAPII associating factors, and chromatin remodeling enzymes. Protein degradation is mediated by the ubiquitin proteasome system (UPS) and many macroautophagy (hereafter autophagy) pathways. Understanding the molecular details of how cells balance these events is crucial, as disrupting these pathways results in a homeostatic disruption. Moreover, as the efficiency of degradation pathways deteriorates with age, such imbalances are linked with age-related pathologies, including cancer and proteinopathies [1,2,3,4,5,6,7]. For example, dysfunctional degradative pathways allow misfolded proteins to accumulate, eventually forming aggregates that are characteristic of neurodegenerative diseases, including Alzheimer’s and amyloid lateral sclerosis (ALS) [8,9].
Cells constantly encounter environmental or physiological stress, including osmotic, oxidative, thermal, and nutritional stressors [10]. After exposure, cells face a critical decision: do they adapt and survive or initiate regulated cell death pathways such as necrosis or apoptosis? Adaptation involves reprogramming transcription, translation, and protein degradation pathways. Due to the high degree of conservation among these processes, the budding yeast Saccharomyces cerevisiae has been an excellent model for understanding the molecular stress response. Studies on this single-celled eukaryote have provided valuable insights into many aspects of mammalian biology. For instance, autophagy was first identified in yeast and later found to be highly conserved across species [11]. Furthermore, yeast has been instrumental in characterizing genetic pathways linked to neurodegenerative diseases such as ALS, Parkinson’s, or Alzheimer’s [12,13,14,15,16,17].
Regardless of the chosen survival or death pathway, cells must coordinate various activities to achieve the desired outcome. For example, a survival response upregulates chaperones to help protein refolding, induces autophagy to recycle macromolecules, and increases mitochondrial fusion to enhance ATP production. Conversely, the cell death response stimulates pro-death gene transcription, induces mitochondrial fission, and coopts autophagy genes for cell death functions [18]. However, the connection between survival and cell death pathways is complicated with shared regulators and seemingly shared responses [18].
This review focuses on the activities of the Mediator kinase module (MKM), previously called the Cdk8 kinase module, a complex whose components directly coordinate gene transcription with cytoplasmic events to coordinate survival and death cell fate decisions (Section 2 and Section 3). In short, cyclin C and Med13 (two members of the MKM) have secondary cytoplasmic roles following cell death and cell survival cues, respectively (Figure 1). In the cytoplasm, they interact with a host of cell death and cell survival proteins, including the newly defined cargo hitchhiking autophagy pathways (Section 4, Section 5, Section 6 and Section 7) [19]. This review outlines the importance of these secondary functions of cyclin C and Med13 in maintaining cellular homeostasis following stress and diseases associated with misregulation (Section 5). Throughout, yeast proteins are written with a capital followed by lowercase letters (e.g., Cdk8), whereas all capitals are used to describe mammalian proteins (e.g., CDK8).
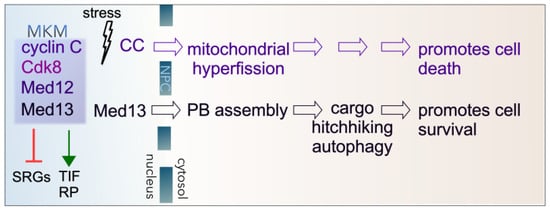
Figure 1.
Cytoplasmic roles of cyclin C (CC) and Med13 following stress in yeast. The Mediator kinase module (MKM) predominantly represses stress response genes (SRGs) under normal physiological conditions. It also positively regulates a subset of genes encoding translation initiation factors (TIFs) and ribosomal proteins (RPs). Following ROS stress, cyclin C translocates to the cytoplasm, which is required for mitochondrial hyperfission. Before destruction by the UPS, cyclin C also promotes regulated cell death by unknown mechanisms. Following starvation stress, Med13 translocates to the cytoplasm, playing a role in P-body (PB) assembly. It is also required for the autophagic degradation of a subset of P-body assembly factors by cargo hitchhiking autophagy. The translocation of Med13 following starvation stress is necessary for cell survival.
2. The Mediator Kinase Module
2.1. Structure and Function of the MKM
2.1.1. MKM Structure
The Mediator comprises a large core 26-subunit Mediator complex (cMED) and a dissociable Mediator kinase module (MKM), which functions as a critical coregulator of RNA polymerase II (RNAPII) transcription (Figure 2) [20,21,22,23,24,25]. As such, the Mediator complex exists as two distinct entities, depending on whether it is bound to the MKM. The MKM is a highly conserved complex consisting of four proteins: cyclin C, its cognate kinase CDK8, and two structural proteins, MED12 and MED13, in a 1:1:1:1 stoichiometric ratio [26]. In mammals, the MKM contains paralogues of its members, except for cyclin C, namely MED12L, MED13L, and CDK19 [27]. Unlike the founding members of the cyclin-dependent kinase family, cyclin C–CDK8 does not mediate cell cycle progression [22,28].
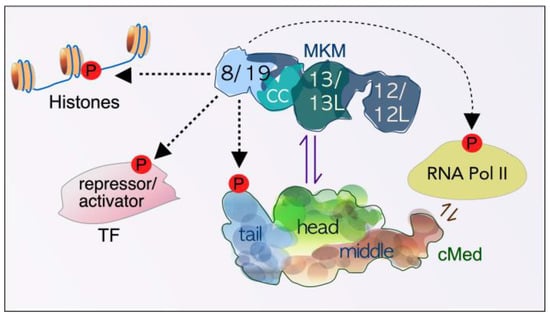
Figure 2.
Schematic overview depicting the relationship between the dissociable Mediator kinase module (MKM), the core Mediator (cMed), and other transcription-associated factors. Under normal physiological conditions, the MKM alone, or attached to cMed, phosphorylates many targets to positively and negatively regulate transcription. Paralogs exist for all subunit components except for cyclin C (CC). 8—CDK8, 19—CDK19, 12—Med12, 12L—Med12L, 13—Med13, 13L—Med13L TF—transcription factor, P—phosphorylation event.
CDKs are typically activated by cyclin association and phosphorylation in the T-loop domain by the CDK-activating kinase (CAK) [29]. However, CDK8 and CDK19 lack this canonical phosphorylation residue suggesting a different mechanism is in place. Previous studies found a role for MED12 in stabilizing the CDK8 or CDK19 T-loop in an active conformation [26,30]. In addition, a recent cryo-electron microscopy structure of the yeast MKM also revealed that Med13’s bilobal architecture resembles that of argonaut proteins [31]. While this would imply a new role for Med13 in nucleic acid binding, the lack of several critical conserved catalytic residues found in other argonaut proteins adds complexity to Med13’s role in transcription and mRNA metabolism.
2.1.2. Function of the MKM in Yeast
The initial identification of the MKM components in yeast was conducted through genetic screens, which searched for mutations that allowed aberrant upregulation of a variety of genes. This formally indicated a role for the MKM in gene repression. Subsequent molecular analyses confirmed this model with the MKM repressing many genes during normal growth that are induced in response to oxidative stress, sucrose utilization, autophagy, and meiotic development [21,32,33,34,35,36,37,38,39,40,41,42,43,44]. Genomic studies confirmed that the MKM played a predominantly negative role in transcription [36,39]. Precise mechanistic details of how the MKM-Mediator regulates gene expression are still emerging [45]. Cdk8 represses transcription through phosphorylating and subsequently inhibiting transactivators. For example, Cdk8 phosphorylation prevents nuclear accumulation of Msn2 and destabilizes Ste12 and Gcn4 [46,47]. In addition, the MKM inhibits pseudohyphal growth by preventing H3K4 trimethylation at the FLO11 promoter [48]. Finally, Cdk8 modification of the great wall protein kinase Rim15 increases its nuclear export [43]. These studies highlight an overall strategy for MKM-dependent phosphorylation stimulating relocalization or degradation of transcription factors to enforce repression. This theme is repeated in mammalian cells.
Although it is a relatively minor role, the MKM is also involved in transcriptional activation. Early studies revealed a positive role for the yeast MKM in phosphorylating TFs responsible for changes in carbon and nitrogen utilization [42,47]. Similar to its repression function, the activated transcription factors, Gal4 and Ste12, are required when cells shift to an alternative carbon source coupled with reduced nitrogen. However, we recently discovered that the MKM also positively regulates a subset of genes encoding ribosomal proteins (RPs) and translation initiation factors (TIFs) under normal physiological conditions [49]. This mechanism demonstrates the conserved nature of MKM activity, as cyclin C is necessary for maintaining steady-state levels of a subset of ribosomal genes in mouse and human cell lines [49]. The repression of this subset of translation-associated genes occurs in response to stress and is dependent on MKM disassembly [49]. This process makes sense as ribosome biogenesis consumes over 60% of cellular energy and resources [50]. Reducing ribosome biosynthesis helps conserve energy to enhance cellular survival during stress [51].
2.1.3. Function of the MKM in Mammalian Cells
Similar to yeast, the MKM both positively and negatively regulates genes involved in the stress response, differentiation, and metabolism [42,52,53,54,55,56,57,58]. However, in mammalian cells, the split between repressed and activated genes is approximately 50:50 [52]. Several models have been put forward to explain the repressor function of the mammalian MKM. For example, biochemical reconstitution experiments and molecular modeling have suggested that the MKM sterically inhibits the Mediator from interacting with RNAPII at promoters [20,22,26,59,60,61,62,63,64,65]. Other methods exist for MKM-dependent repression of gene transcription. For example, CDK8 phosphorylates cyclin H, inhibiting TFIIH activity [66]. In addition, CDK8 phosphorylation induces destruction of the Notch receptor intracellular domain (ICD), inhibiting activation of this pathway [67]. Similar to yeast studies, the mammalian MKM has also been implicated in repression through regulating chromatin modifications. For example, CDK8 is required for Xist-dependent X chromosome silencing in mice [68]. Finally, unbound MKM has been reported to regulate transcription independently of the cMed [26,69]. However, it remains unclear if this function is mediated by the MKM–Mediator complex or the MKM acting as an independent entity [70,71,72].
The role of the MKM in directing transcription in response to external cues has been the subject of several recent reviews [65,72,73] and therefore will not be discussed in detail. Briefly, cyclin C and its cognate kinase are constitutively active in normal unstressed conditions, where they phosphorylate several factors to both positively and negatively regulate transcription [42,53,54,70,71,72,74,75,76,77,78,79,80,81,82]. Key among these targets are many transcription factors (TFs), including STAT-1 and SREBP-1 [53,54,55,56,57]. In addition, the MKM kinase phosphorylates histone H3 at serine 10 (H3S10) [26]. This modification is associated with transcriptional activity [83] as it prevents a repressive methylation mark [84].
In mammals, the MKM controls transcription through both the proximal promoter region and by modifying proteins at super-enhancer sites. Super-enhancers are regions further upstream of the proximal promoter, containing an array of TF binding sites [72,85]. Although the exact contribution of each of these individual sites to gene expression is not clear, it is proposed that they directly interact with RNAPII holoenzyme components through the looping of the chromatin. The specific role of the MKM at the super-enhancers again focuses on modifying TF and/or chromatin [73]. For example, inhibiting CDK8 and CDK19 results in both upregulation and downregulation in super-enhancer-controlled genes in acute myeloid leukemia cells [86]. In addition, chromatin immunoprecipitation (ChIP) studies confirmed that the MKM was resident at these control regions. Formally, these results argue that the MKM either represses or stimulates transcription, depending on the locus. In this scenario, MKM regulatory specificity would be derived from TFs or other chromatin modifying enzymes present. A recent study found that genes induced by the interferon response in cells derived from Down’s syndrome patients are suppressed by treatment with a potent CDK8/CDK19 inhibitor [87]. Although not mutually exclusive, another potential role for the MKM is employing the IDR domains of MED12 and MED13 to establish a liquid–liquid phase separation (LLPS) or biomolecular condensate that increases substrate and enzyme concentrations around promoters [88]. A role for MKM components in establishing such a specialized environment has already been observed in the cytoplasm (Section 3 and Section 4). In summary, the MKM influences important cell fate decisions through modifying a myriad of transcription-related targets that direct cellular and developmental homeostasis.
2.2. Regulation of the MKM
2.2.1. Dynamic MKM Promoter Recruitment and Expulsion
Structural and biochemical studies found that the MKM–Mediator association is a dynamic, reversible process. The MKM concentration is reported to be 10-fold lower than the cMed, consistent with the finding that the Mediator is often found at promoters without the MKM. This dynamic aspect of the MKM was underscored using ChIP experiments to examine promoter occupancy before and after oxidative stress. In these studies, when MKM repressor function needs to be relieved, it is removed from the promoter. Conversely, the MKM is recruited to promoters in which it plays a transcriptional activator role following stress [52]. However, a small subset of promoters show disparity in MKM component association, with cyclin C being absent while CDK8, MED13, and MED13L remained. This may provide a mechanism that allows ~20% of cyclin C to leave the nucleus in response to stress.
In yeast, the situation is different. Rather than the MKM moving as a unit to different promoters, this complex is completely disassembled in response to oxidative or nutritional stress. As detailed below, this disruption allows MKM components to perform their “night jobs” in the cytoplasm. Currently, the underlying mechanism directing recruitment or expulsion of the MKM from promoters is only partially understood (see [73] for a recent review). Although it remains unclear how the MKM–Mediator interaction is controlled in yeast, it has been suggested that Cdk8 phosphorylation of cMed components reduces MKM binding [60,73,89]. Given the seemingly constitutive nature of Cdk8 activation, this suggests that either the MKM is always unstably associated with the cMed or there are other mechanisms for maintaining this interaction when necessary. Finally, the stability of the MKM components is also the subject of regulation. For example, MED13 and MED13L are targeted for degradation by the SCF-Fbs7 E3 ubiquitin ligase in mammals [62]. In addition, loss of Cdk8 results in destabilization of cyclin C in yeast [81,90].
2.2.2. MKM Disassembly Following Cell Death Cues Triggered by ROS
In response to oxidative stress, cyclin C, but not Cdk8, translocates to the cytoplasm, resulting from MKM disassembly [91]. This event is conserved in mammals [92,93]. In yeast, the molecular details of MKM disassembly in response to ROS involve an intertwined network of phosphorylation and ubiquitination events. The stress signal is transmitted by the conserved MAPK of the Cell Wall Integrity (CWI) pathway to Slt2 (Mpk1, (ERK5 ortholog, [94,95]) that directly phosphorylates cyclin C and Med13 [96,97,98,99]. Intriguingly, Kdx1 (Mlp1), the pseudokinase of the CWI pathway, is also required for cyclin C release [98]. Kdx1 interacts with the transcription factor Ask10 and likely induces cyclin C translocation to the cytoplasm indirectly [97,99]. CWI-triggered phosphorylation of Med13 results in its destruction by UPS mediated by the E3 ligase complex SCFGrr1 [99,100]. In addition, cyclin C is directly phosphorylated at a single site (S266). The net outcome is MKM disassembly and cyclin C nuclear release [91,101]. In yeast, the UPS destroys cytoplasmic cyclin C following its role in mitochondrial hyperfission [33,34,101].
Genetic studies showed that Med13 destruction is required for cyclin C nuclear release. In addition, Med13 destruction requires a priming event mediated by Cdk8 in unstressed cells [99]. Thus, recognition of the Med13 degron uses two phosphorylation marks, one to prime the degron, the second for its recognition by ubiquitin ligases [102,103]. This ensures that the nuclear release of cyclin C is the correct response to the environmental input. Additionally, direct phosphorylation of Med13 by Snf1, a highly conserved adenosine monophosphate-activated protein kinase (AMPK) that is activated in response to a variety of stresses, is required for cyclin C’s translocation to the cytoplasm [104,105].
Taken together, the studies discussed above suggest that Med13 acts as a physical tether, keeping cyclin C in the nucleus. Consistent with this, in the absence of Med13, cyclin C is aberrantly released into the cytoplasm, where it induces mitochondrial fission in the absence of stress [100]. Furthermore, deletion of the holoenzyme associating domain (HAD), the domain on cyclin C that interacts with Med13 [106], also results in the precocious release of cyclin C from the MKM. Loss of Med13 interaction leads to constitutive cytoplasmic localization of cyclin C and mitochondrial fission in the absence of stress [100]. The HAD is highly conserved, being required for the cyclin C–Med13 interaction in mammalian cells [107]. Based upon these studies, we designed a peptide mimetic of this domain (S-HAD) which stimulates cyclin C nuclear release in mammalian cells in the absence of stress, highlighting the conservation of both the domain and function [107].
2.2.3. MKM Disassembly Following Cell Survival Cues Triggered by Nitrogen Starvation
In the yeast model system, nitrogen starvation activates both bulk and cargo hitchhiking autophagy pathways. Bulk autophagy is essential for recycling cellular components, producing energy, and ensuring survival [108]. In contrast, cargo hitchhiking autophagy is a selective pathway that degrades specific substrates, including Med13, certain ribosomal proteins, and components involved in P-body assembly [19]. Given that the MKM represses many crucial autophagy genes, it is not surprising that effective induction of autophagy requires its disassembly [44,109]. Following nitrogen starvation cyclin C does not translocate to the cytoplasm. Instead, the ubiquitin–proteasome system (UPS) targets and degrades cyclin C in the nucleus via an unidentified E3 ligase [19,44]. This response is vital for survival, as it prevents cyclin C from inducing mitochondrial hyperfission and regulated cell death [109]. Thus, the mitochondria remain hyperfused, a morphology associated with maximal ATP production [4,110]. MKM disassembly following nitrogen starvation also results in Med13 translocating to the cytoplasm [44,111]. Here, it is required for P-body assembly, and the autophagic degradation of a subset of P-body assembly factors by cargo hitchhiking autophagy [19,112].
How the stress signal is transmitted to the MKM following nitrogen starvation remains unknown. However, it is known that in yeast that the activity of Slt2 is activated in response to the Target of Rapamycin Complex 1 (TORC1) inhibition [113]. However, phosphorylation of cyclin C by Slt2 is not required for its degradation following nitrogen starvation [109]. This suggests that a different protein, possibly Med13, is the target of Slt2 activity following starvation stress. These studies highlight how the MKM responds to cell death and cell survival cues, even though the CWI is activated in both cases. Interpretation of the input signal is critical as expressing a fusion protein in which cyclin C is tethered to the outer mitochondrial membrane results in mitochondrial fission and the execution of cell death pathways following cell survival cues [109].
3. Roles of Cyclin C in the Cytoplasm
3.1. The Role of Cyclin C Stress-Induced Mitochondrial Hyperfission
It is well documented that mitochondria are dynamic organelles with distinct morphologies within and among cell types and tissues (see [114] for a recent comprehensive review). Furthermore, mitochondrial function and morphology are intricately linked, with dynamics playing a role in many processes, including ATP production, calcium homeostasis, and RCD [115]. The remodeling of mitochondria morphology occurs through the regulation of two opposing processes, mitochondrial fission and fusion, both of which are highly dependent on cell type and the functional state of mitochondria [116,117].
3.1.1. Two Classes of Mitochondrial Fission
From yeast to metazoans, mitochondrial fusion and fission often occur simultaneously and in a balanced manner within a cell, preserving homeostasis [118]. Under normal physiological conditions, mitochondrial fission is required for organelle distribution during mitotic cell division [119]. In post-mitotic neurons, fission promotes transport and distribution to distal neurites [120]. Also, mitochondrial fission is used to eliminate impaired or dysfunctional mitochondria during mitophagy, the autophagic process by which defective mitochondria are selectively degraded [121,122,123] (Figure 3).
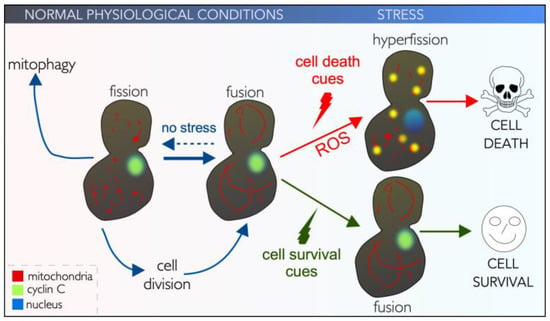
Figure 3.
Mitochondrial morphology is linked to cell survival and cell death. See text for details.
Mitochondrial dynamics are finely tuned by conserved fusion and fission proteins, whose actions are GTP-dependent. The central players are GTPases, members of the dynamin-related protein (DRP) superfamily that promote fusion (Mitofusin 1 and 2, Optic atrophy protein 1, MFN1, MFN2 and OPA1) or fission (DRP1). Here, cytosolic DRP1 is recruited by adaptors present on the outer mitochondrial membrane (OMM) called mitochondrial fission factor (MFF), human mitochondrial dynamics proteins 49 and 51 (MID49, MID51), and mitochondrial fission 1 protein (FIS1). Recruited DRP1 forms a multimeric ring complex around mitochondria, initiating separation of the inner and outer mitochondrial membranes [124,125]. Following GTP hydrolysis, the complexes constrict, resulting in mitochondrial fission and eventual division of the organelle into two [126,127,128]. This process is essentially conserved in yeast [129].
3.1.2. Cyclin C Is Required for Stress-Induced and Mitochondrial Hyperfission in Mammals
The differences between mitochondrial fission used to maintain homeostasis and that used for hyperfission are not well understood. We and others have demonstrated that the presence of cytoplasmic cyclin C following ROS stress drives cells into this hyper-fragmented state by interacting with DRP1 [92,93,130,131]. This is different to mitochondrial fission during G2 phase, in which cyclin B-CDK1 phosphorylation stimulates DRP1 activity [132,133]. Moreover, the activating phosphorylation of DRP1 does not occur following ROS stress [92]. In vitro studies revealed that cyclin C directly interacts with the GTPase domain of DRP1 [130]. Like many cyclins, cyclin C contains two cyclin boxes, a conserved structural motif of ~150 amino acid residues, which are organized into 5-helical regions [134]. The amino terminal cyclin box interfaces with CDK8/19, whereas the carboxy terminal box interacts with DRP1 [130]. This ROS stress-induced interaction triggers structural changes in DRP1, transforming it from oligomers with low GTPase activity to dimers capable of forming high-GTPase activity filaments [130]. In other words, the cyclin C–DRP1 interaction facilitates a confirmational switch that allows DRP1 to more readily bind GTP (Figure 4). Importantly, cyclin C–DRP1 interaction is both necessary and sufficient to induce mitochondrial hyperfission, as Escherichia coli purified GST-cyclin C introduced into permeabilized mouse embryonic fibroblasts induces mitochondrial fission in the absence of stress [92].
3.1.3. Cyclin C Is Required for Stress-Induced Mitochondrial Hyperfission in Yeast
Exactly how yeast cyclin C triggers mitochondrial hyperfission complex following ROS stress is less clear. Cytoplasmic cyclin C interacts with Mitochondrial division protein 1 (Mdv1) [101], which, alongside a second adaptor (CCR4-associated factor 4, Caf4), recruits the yeast orthologue of DRP1, Dynamin 1(Dnm1), to sites of mitochondrial fission by interacting with Fis1, a conserved OMM protein [135,136]. This has led to the model in which cyclin C most likely acts early in the stress response to promote the formation of productive Fis1-Mdv1-Dnm1 complexes, leading to fission [101]. Other observations strongly support this model: firstly, artificially placing cyclin C at the mitochondria via fusion of the OMM-associating domain of Fis1 to cyclin C’s C-terminus is sufficient to induce fission even in unstressed cells [109]. Secondly, cyclin C-mediated hyperfission is not dependent upon its transcriptional duties [91,101]. Lastly, retaining cyclin C in the nucleus following ROS stress significantly reduces hyperfission.
3.2. Cytoplasmic Cyclin C Promotes Regulated Cell Death
3.2.1. Mitochondrial Hyperfission and Intrinsic Regulated Cell Death (iRCD)
Mitochondrial hyperfission occurs in response to various acute stressors including ROS (Figure 3). This is coupled with decreased ATP production, decreased oxygen consumption, and increased mitochondrial ROS production. The outcome is decreased mitochondrial membrane potential, disrupted mitophagy, resulting in accumulation of damaged mitochondria and increased susceptibility to intrinsic regulated cell death cascades (iRCD) [137,138]. Whether mitochondrial hyperfission drives mitochondrial outer membrane permeabilization (MOMP) and iRCD remains unclear [139]. In support of this model, the absence of cyclin C, or in cis and trans mutants that keep cyclin C in the nucleus, stress-induced hyperfission and cell death is significantly reduced in both yeast and mammals alike [101]. Likewise, DRP1 GTPase activity with a dominant negative protein (DRP1K38A) prevents mitochondrial fragmentation and delays iRCD [140,141]. Moreover, BAX, a pro-apoptotic BCL-2 family member, which creates mitochondrial pores, moves to fission sites [142]. Together with the observation that cyclin C interacts with BAX and fission sites in mammalian cells [107], this suggests that mitochondrial hyperfission is a required step in iRCD. However, other studies have disputed this model, as cells deficient in DRP1 also undergo cell death, albeit with altered kinetics, suggesting that mitochondrial fission is not crucial for MOMP and iRCD [143]. However, more recent studies show that BAX and DRP1 directly interact, and their coupling enhances the membrane activity of both proteins [144]. Together these studies all agree that mitochondrial hyperfission occurs following ROS stress and is associated with early cellular changes observed in iRCD pathways.
3.2.2. Cyclin C and Cell Death in Mammals
BAX and other members of the BCL-2 family are important regulators of iRCD in mammalian cells [145]. In healthy cells, BAX is inactive and constantly retro-translocates between cytosol and mitochondria [146]. Following ROS stress, BAX interacts with BH3 domain proteins (like tBID and BIM), which are recruited to the OMM, where they trigger a confirmational change, promoting BAX dimerization and extensive interaction with the OMM (Figure 4). Activated BAX further assembles into multiple oligomeric species, which form supramolecular structures within growing membrane pores at the MOM [147]. This results in MOMP, ultimately releasing of cytochrome c and other factors into the cytoplasm [148,149,150].
So, how do cyclin C and DRP1 fit into this model? Cytoplasmic cyclin C is required for both the activation and mitochondrial localization of BAX in mammalian cells [107]. It directly interacts with active BAX, and this interaction is dependent on the presence of DRP1 [107]. Cyclin C also interacts with DRP1 dimers, although the domains on DRP1 which interact with the second cyclin box in cyclin C remain unknown. More recently, BAX and DRP1 have been shown to directly interact [144], and future studies are needed to determine how DRP1 interacts with both cyclin C and BAX. Together, these data suggest a model where cyclin C, DRP1, BAX, and tBID form a complex essential for apoptosis. (Figure 4). Artificially stimulating cyclin C nuclear release in the absence of stress still leads to BAX–mitochondrial association, however, but does not trigger BAX oligomerization, which most likely requires tBID interaction [107]. Therefore, release of cyclin C from the nucleus is insufficient to promote cell death pathways, making cyclin C necessary but not sufficient for iRCD.
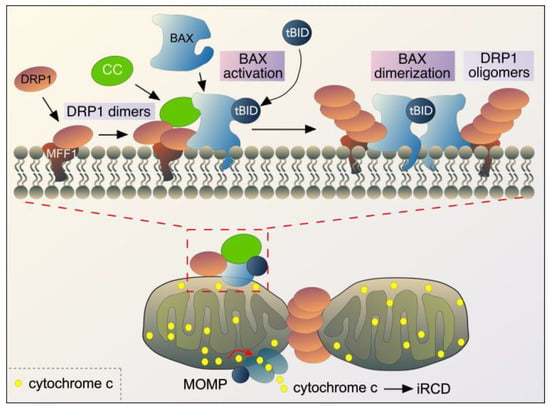
Figure 4.
Outline of the roles of DRP1, cyclin C (CC), BAX, and tBID during intrinsic regulated cell death (iRCD) following ROS stress in mammals. The top half of the figure is an enlarged image of the box in the bottom image, depicting the interaction of key proteins at the OMM. See text for details. BAX also forms oligomers at the OMM, but this is not shown for clarity. MOMP-mitochondrial outer membrane permeabilization. Adapted from [144].
3.2.3. Cyclin C and Cell Death in Yeast
S. cerevisiae does not encode for any known homologs of the BCL-2 family. However, expression of murine BAX in yeast induces cell death, and its cytotoxicity is dependent on its homodimerization and mitochondrial localization [149,151]. Despite this, molecular details of how yeast cells die under these circumstances remain elusive [152]. Similar to mammals, the F0F1-ATPase proton pump is essential for BAX-induced cell death in yeast [153,154]. Likewise, BAX toxicity can be rescued by co-expressing antiapoptotic members of the BCL-2 family, such as BCL-2 or BCL-xL [155]. Although BAX expression in yeast triggers cytochrome c release from mitochondria, this event is not required for cell death [156,157]. This is likely because yeast does not possess the signaling cascade of initiator and executioner caspases, which execute iRCD in mammals [158]. Moreover, the sole caspase-like protein, Yca1, is not essential for BAX-mediated cell death in yeast [159,160,161,162]. Two proteins, Uth1 and Rgl1, which are involved in cell wall strengthening and iron metabolism, respectively, do promote BAX-induced cell death, but their mechanism of action remains unknown [163,164,165].
Numerous key features of iRCD observed in mammalian cells are also conserved in yeast [166]. Interestingly, cells lacking cyclin C display remarkable resistance to stress-induced cell death, a phenomenon observed in both yeast and mammalian systems [92,167]. Although the precise role of cyclin C in this context is not fully understood, simply enhancing cyclin C-mediated mitochondrial fission through overexpression alone is insufficient to trigger RCD [101]. This suggests that an additional signal(s) is necessary to initiate RCD, which might come from another protein localized to the mitochondria during the stress response or through a stress response pathway that modifies cyclin C post-translationally. This important distinction sets cyclin C apart from other known inducers of mitochondrial RCD. For example, ectopic targeting of p53 or the BH3 family member Bax to the mitochondria can effectively induce cell death in non-stressed mammalian cells. In contrast, there is conflicting evidence for the role of Ybh3, the sole BH3 protein in yeast, in inducing regulated cell death [168,169,170,171]. In conclusion, while cyclin C plays an integral role in RCD in yeast, the exact mechanisms underlying its function are still not fully elucidated.
4. Roles of Med13 in the Cytoplasm
4.1. Role of Med13 in P-Body Assembly Following Starvation in Yeast
In the yeast model system, Med13 translocates to the cytoplasm following TOR1 inhibition triggered by either nitrogen starvation or treatment with rapamycin [44,111]. Here, it is required for processing body (P-body) assembly and the autophagic degradation of a subset of P-body assembly factors (Figure 5) [112]. P-body assembly is a conserved mechanism used to store and protect mRNA during stress (reviewed in [172,173,174]). However, the biological significance of P-body formation and stress survival is unclear and a key area of interest in this research field. In yeast, mutants defective in P-body assembly factors are less able to survive extended periods of quiescence [175], suggesting that their formation is a survival response. Likewise, med13∆ cells are less able to survive extended periods of nitrogen starvation [44]. It remains unknown if mammalian MED13 or MED13L are required for P-body formation. Given the conservation of cyclin C’s secondary job, it is highly likely that mammalian MED13 will follow suit.
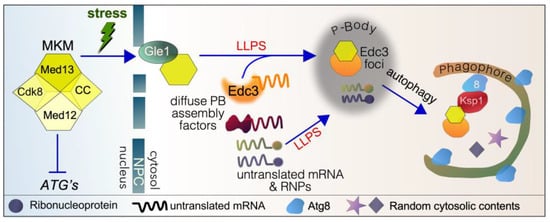
Figure 5.
Cartoon outlining the proteins used to transport Med13 to P-bodies and then to phagophores by cargo hitchhiking autophagy in yeast. See text for details. ATG’s—autophagy-related genes, LLPS—liquid–liquid phase separation, 8—Atg8, NPC—nuclear pore complex, MKM—Mediator kinase model.
4.1.1. P-Body Assembly Following Stress
Inhibiting mRNA translation is a critical strategy cells employ to manage protein levels effectively. Under stress, cells strategically downshift the production of proteins vital for growth while ramping up those necessary for adaptation. A pivotal mechanism for blocking mRNA translation is the formation of P-bodies. These highly conserved, membrane-less structures within the cytoplasm are reservoirs that contain non-translating mRNA-protein complexes (mRNPs) [176,177]. Initially, it was believed that mRNAs within P-bodies were simply stored for future translation or subjected to degradation [173,178,179,180]. However, subsequent studies have revealed that P-bodies are not crucial for mRNA degradation [181]. Instead, they are increasingly recognized as being temporary storage sites for translationally repressed mRNAs that remain ready for activation when conditions improve [182].
The assembly of P-bodies occurs through a process known as liquid–liquid phase separation (LLPS), creating dynamic droplets that are distinctly separate from the surrounding cytoplasm [183]. Their assembly benefits cells, as P-body formation occurs more rapidly than changes in the transcriptional or translational programs. Moreover, the rapid disassembly of P-bodies after the stress abates rapidly provides cells with ready-to-go translation components. The proteins that reside within P-bodies are predominantly RNA-binding proteins or factors with low sequence complexity that contain prion-like domains (PLDs). These PLDs possess the remarkable ability to undergo spontaneous conversion into aggregated states that serve as templates for the recruitment of additional proteins.
4.1.2. Med13’s Role in P-Body Assembly in Yeast
In yeast, P-bodies are consistently present under normal physiological conditions, but their size and number notably increase in response to various stressors, such as nitrogen and carbon starvation [183]. The assembly of P-bodies is fundamentally dependent on several conserved decapping proteins, including Dcp1/Dcp2, Edc3, and Dhh1, along with the Pat1–Lsm1–7 complex and Xrn1 [173]. Edc3 serves as a crucial scaffold for P-body assembly, by leveraging its multivalent interactions [184,185,186,187,188]. Med13 is essential for P-body assembly following nitrogen starvation; the stark reduction in Edc3 foci in med13Δ cells underscores its importance [112]. The mechanistic details of why Med13 is required for P-body formation remain to be completely elucidated; however, it is clear that the PLD domain of Med13 is indispensable for this process [112]. The exact function of these polyQ/N tracts is not well understood, but it has been suggested that the domain can interact with itself or with other polyQ/N regions to promote the aggregation of mRNPs [188].
These studies indicate that Med13 plays a significant role in promoting LLPS of P-body proteins. Furthermore, Med13 contains two motifs strongly linked with LLPS: an RNA recognition motif (RRM) and a large intrinsically disordered region (IDR). IDRs are recognized as key drivers of LLPS, as they can simultaneously create multiple interactions with other components [189]. Additionally, the amino acids within IDRs are more exposed and accessible to post-translational modifications, which are critical regulators of biomolecular phase separation [190,191,192]. This suggests that Med13 itself may undergo LLPS, a direction future studies should pursue.
4.2. Med13’s Role in Cargo Hitchhiking Autophagy in Yeast
Cytoplasmic Med13 is a cargo of cargo hitchhiking autophagy (CHA) in yeast [44,111]. This hybrid autophagy pathway was only discovered in 2024 [19], and the only reports to date are for S. cerevisiae. It remains to be seen whether similar pathways exist in higher eukaryotes. Given the conserved nature of many of the players, it seems highly likely that a similar mechanism exists.
4.2.1. Outline of Autophagy Pathways
Autophagy is a highly conserved core molecular pathway used to maintain homeostasis by intracellular degradation of random cargos, dysfunctional proteins, and organelles. Details of the processes involved have been discussed in many recent excellent reviews by experts in the field [5,193,194]. In short, double-membrane vesicles called autophagosomes engulf proteins and organelles. Cargo-laden autophagosomes fuse with vacuoles (in yeast and plants) or lysosomes (in metazoans), resulting in the degradation of captured contents by resident hydrolases [195,196]. From yeast to humans, ordered steps define the process of autophagy. The first stage is initiation, followed by the double-membrane phagosome’s nucleation, expansion, and elongation. Thereafter, the phagosome closes, forming an autophagosome, which fuses with lysosomes. Here, resident proteases degrade autophagosomes and their cargo, providing fuel for cells [5,197,198,199].
Mechanistically, all autophagy pathways are classified according to cargo type and the lysosomal delivery system employed, resulting in three major groups: macroautophagy, microautophagy, and chaperone-mediated autophagy (CMA). Macroautophagy and microautophagy are conserved pathways [200] with macroautophagy being further classified into selective and non-selective pathways [201,202,203]. Importantly, these pathways play crucial roles in cellular physiology and are protective against a wide variety of diseases, including neurodegeneration, cancer, infections, and cardiovascular disorders [194,204,205,206].
Given the conservation of autophagy, S. cerevisiae is a leading model organism for deciphering molecular details that define macroautophagy pathways. In this system, autophagic pathways fall into two subclasses: selective and non-selective (bulk) autophagy. Bulk autophagy is predominantly upregulated following Target of Rapamycin complex 1 (TORC1) inhibition, triggered by nutrient stress, and degrades superfluous random cytosolic proteins and organelles. In contrast, selective autophagy pathways maintain cellular homeostasis when TORC1 is active by degrading damaged organelles and dysfunctional proteins. Here, selective autophagy receptors mediate cargo recognition by Atg8, a conserved protein required for phagophore expansion.
4.2.2. Med13 Is Degraded by Cargo Hitchhiking Autophagy in Yeast
Very recently, we and others have discovered a new hybrid autophagy mechanism, coined cargo hitchhiking autophagy (CHA) in yeast [44,111,207,208] (reviewed in [19]). This mechanism is induced during starvation and utilizes autophagy components from both bulk and selective autophagy pathways. CHA uses receptor proteins to deliver selected cargos, including a subset of ribosomal proteins, TFs, and TIFs, to phagophores. In CHA, various cargo hitchhiking receptors link their cargos to lipidated Atg8, located on growing phagophores. These phagophores are built to capture random cytosolic content during starvation (bulk autophagy), and the prevailing model is that CHA receptors hitchhike onto Atg8 in these preformed phagophores.
Med13 is one of the cargos degraded by CHA following nitrogen starvation using Ksp1 as a receptor [44,111]. The known players used to mediate Med13 from the nucleus to awaiting phagophores are outlined in Figure 5 and discussed in the sections below. More recently, we discovered that Med13 is also required for the autophagic degradation of both Edc3 and Dhh1 following nitrogen starvation. Since Med13 does not direct the autophagic degradation of Xrn1, this suggests that Med13 serves as an exclusivity factor that differentiates among various P-body components [112]. Given the conserved nature of P-body assembly factors and autophagy pathways, it will be exciting to determine whether this mechanism is conserved in higher eukaryotes.
4.2.3. Snx4 Promotes CHA in Yeast
CHA was previously named Snx4-assisted autophagy, as this conserved sorting nexin is required for the degradation of all identified CHA cargos to date [19,44,207,208,209]. Sorting nexins are a family of conserved phosphoinositide-binding proteins that play fundamental roles in orchestrating cargo sorting through the endosomal network. As such, human SNX4 is implicated in a variety of synaptic processes [210,211]. Significantly, SNX4 has already been linked with the etiology of AD, with SNX4 protein levels being decreased by 70% in the brains of severe Alzheimer’s disease (AD) cases [212].
Sorting nexins are recruited to endosomal membrane domains by a phox homology (PX) domain that recognizes phosphatidylinositol-3-phosphate (PtdIns3P) [209]. Snx4 also contains two Bin–Amphiphysin–Rvs (BAR) domains that sense membrane curvature and tubulate vesicles [213]. The molecular role of Snx4 (Atg24) in autophagy is also related to membrane bending as it stabilizes and drives the opening of the inner membrane rim of non-selective phagophores in yeast [214]. The net effect is a phagophore membrane with a wide enough opening to accommodate large cargos, including ribosome and proteasome subunits [214,215,216,217].
4.2.4. CHA Uses Phagophores Built by the Atg17 Scaffold Complex
In CHA, specific cargos, including Med13, are recruited to growing phagophores that are primarily used to degrade random cytosolic contents by non-selective (a.k.a. bulk) autophagy. These phagophores are triggered by starvation stress. Their formation is dependent on the Atg17 scaffold complex, which consists of the Atg17 scaffold and two regulatory proteins, Atg29 and Atg31 [218]. This process differs from selective autophagy, where phagophores are constructed using the Atg11 scaffold [219]. Selective autophagy pathways play crucial roles in maintaining homeostasis under physiological conditions. They are responsible for removing dysfunctional proteins (such as in mitophagy, ER-phagy, and ribophagy), protein aggregates (aggrephagy), and pathogens like viruses and bacteria (xenophagy) in higher eukaryotes [220]. It is likely that Med13-Edc3 is not the only cargo captured in Atg17-built phagophores. Instead, these phagophores probably contain random cytosolic contents alongside other cargo that may hitch a ride, including a subset of ribosomal proteins [208].
The formation of the phagophore assembly site (PAS) for both selective and non-selective autophagy depends on LLPS [221,222]. During starvation, the Atg1 complex comprising Atg1, Atg13, and a trimeric Atg17 scaffold undergoes LLPS [223]. This fluidity is vital for recruiting downstream components necessary for autophagosome formation. As mentioned above, P-body assembly occurs via LLPS. How these two biomolecular condensates (BMC) interact remains unknown. One intriguing possibility is that Med13 could play a role, possibly acting as a conduit between these two BMCs.
4.2.5. Ksp1 Is the Selective Autophagy Receptor Proteins for Med13 in CHA
Although CHA uses phagophores built for the degradation of random cytoplasmic contents, cargo recognition is dependent upon an autophagic receptor protein [19]. The autophagic receptor for the selective degradation of Med13 is Ksp1. Typical of autophagic receptors, Ksp1 interacts with the phagophore-bound protein Atg8 via its conserved Atg8 interaction motif (AIM) [111,224]. Ksp1 is a casein-like kinase that negatively regulates autophagy in replete media [225,226,227,228,229]. However, its role as a receptor protein is kinase-independent, illustrating its dual and opposing roles in autophagy. These studies support the emerging concept that proteins can perform two distinct functions, referred to as “day and night jobs” [230,231], which various external or intrinsic stimuli can trigger. Ksp1 also associates with ribosomal and PB proteins [232,233], though the function of Ksp1 here remains unknown. Interestingly, Ksp1 does not directly interact with Edc3. Instead, Med13 performs this role, suggesting that it may act as a conduit between Edc3 and Ksp1 [111].
4.2.6. The Nucleoporin Gle1 Is Required for CHA of Med13
Cyclin C is a small 33 kDa protein that can diffuse through the nuclear pore complex (NPC) without the assistance of active transport by exportins. In contrast, Med13 is a larger scaffold protein, approximately 150 kDa in size, which requires active transport to cross the nuclear–cytoplasmic barrier. However, our studies have shown that β karyopherin exportins are not needed for Med13’s transit through the NPC. Similarly, Dpb5 and the Mex67-Mtr2 heterodimer, which can transport cargo across the NPC, do not play a role in this process. Instead, Gle1, a conserved component of cytoplasmic NPC fibrils, is essential for Med13’s passage across the NPC [44].
GLE1 is a mobile nucleoporin in mammalian cells [234], suggesting that it could play a similar role in the nuclear export of Med13. Interestingly, in yeast Med13 remains in the nucleus following nitrogen starvation in snx4∆ mutants [44]. This observation implies that Snx4 may communicate with the NPC, potentially by interacting with Gle1 during the transfer of Med13 as it exits the NPC. Future experiments will be necessary to investigate this idea further.
5. Diseases Associated with the MKM
5.1. Tumor-Suppressive Roles of Cyclin C–CDK8/19
5.1.1. Notch Signaling
The cyclin C locus (CCNC) maps to 6q16.2 [235]. Deletions of 6q16.2 are frequently found in blood cancers and solid tumors, hinting at a tumor suppressor role for cyclin C [236,237,238,239]. Mechanistic details have started to emerge on how cyclin C acts as a tumor suppressor in T-cell acute lymphoblastic leukemia (T-ALL) [240] Here, aberrant NOTCH1 expression is a causative factor in T-ALL development [241]. In the canonical NOTCH signaling pathway, ligand-activated Notch1 receptors are cleaved, releasing a Notch intracellular domain (ICN1) which migrates to the nucleus. Here, ICN1 initiates transcription of downstream target genes by interacting with the DNA-bound CSL–co-repressor complex (CBF-1, Suppressor of Hairless, and LAG-1). By displacement of co-repressors and recruitment of the transcriptional co-activator protein Mastermind-like (MAML), this transforms CSL into a co-activator complex, resulting in transcription of Notch target genes [242,243].
Post-translational modifications are well known to regulate Notch activity [244]. These include ubiquitination and UPS-mediated destruction of the ICN1. Although the exact role of ICN1 destruction is not clear, it has been suggested that the Notch degradation complex is recruited to activated promoters and may function to disassemble active enhancer complexes [67]. Importantly, the cyclin C–CDK8 kinase phosphorylates ICN1, marking it for degradation. In the hematopoietic lineage, the major rate-limiting function of cyclin C is to suppress ICN1. Loss or heterozygosity of cyclin C disrupts this regulatory mechanism, enhancing Notch1 activity, which promotes T-ALL progression [240].
Notch1 activation is present in many cancers (acute lymphoblastic leukemia, non-Hodgkin’s lymphoma, and prostate cancer, amongst others). As 6q16.2 deletion is associated with these cancers, this suggests that the cyclin C–CDK8-mediated degradation of ICN1 likely plays a role [239,245,246,247,248,249]. It should also be noted that in some cellular contexts, Notch1 functions as a tumor suppressor [250]. The role of cyclin C in these contexts, especially with regard to ICN1 degradation, remains unknown [240,251,252]. This is important to understand, given the use of CDK8/19 inhibitors that are currently in clinical trials (see below).
5.1.2. JAK-STAT Signaling
Cyclin C also plays a tumor suppressor role in anaplastic thyroid tumors [253]. This is maybe not surprising as 6q16.2 is lost in 33% of poorly differentiated thyroid tumors and 27% of anaplastic malignancies [254]. In mouse models of thyroid neoplasia [255], deletion of cyclin C alone in the thyroid only stimulates a modest increase in hyperplastic growth. However, in combination with ablation of the PTEN tumor suppressor [256], the thyroid size increases dramatically, eventually killing the animal [253]. Despite the increase in ROS production throughout thyroid cancer development, it is not the mitochondrial role of cyclin C that is involved. Instead, alterations in cyclin C–CDK8-mediated phosphorylation of STAT-3 result in misregulated JAK-STAT signaling, which in turn destabilizes the tumor suppressors p53 and p21 [107,257].
5.2. Oncogene Roles of Cyclin C–CDK8/19
CDK8 was first reported as a putative oncogene in colorectal cancer in 2012 [258]. In this context, CDK8 mediates the aberrant activation of the Wnt/β–catenin signaling pathway. Additionally, the phosphorylation of various transcription factors, such as Smads, STAT1, and NFκB, by CDK8 is frequently dysregulated in many other types of cancer [53,77,259]. This suggests that cancers associated with CDK8 are predominantly driven by transcriptional dysregulation [260]. Currently, mutations in both CDK8 and CDK19 have been linked to over 100 malignancies, including cancers of the colon [258,260], breast [261], prostate [262], pancreas [263], skin [264], and blood [86]. It comes as no surprise then that modulating the transcriptional activity of the MKM has emerged as an attractive therapeutic strategy for treating cancer. Consequently, specific inhibitors targeting CDK8/CDK19 are currently being tested in various clinical trials [259].
Cyclin C–CDK8/19 is not alone in having dual roles with seemingly contradictory functions. Extensive research has determined that other tumor suppressors, including Rb, PTEN, and FOXO, also act as double agents with oncogenic roles [265]. These roles can be dependent upon cell type and/or post-translational modification events. A classic example in this case is Notch (described above), which has an oncogenic role in T-ALL and a tumor suppressor role in squamous epithelial cells [266,267]. Interestingly, a recent survey identified 73 unique tumor suppressors with oncogenic potential. Like cyclin C–CDK8/19, many of these double agents are transcription factors, whose oncogenic potential is realized by interaction with different proteins [268]. It is therefore critical to determine the context-dependent role of these double agents before treatment.
5.3. Role of Cyclin C in TDP-43-Mediated Cell Death
5.3.1. Role of TDP-43 in Maintaining Homeostasis
Transactive Response Binding Protein 43 (TARDBP, hereafter referred to as TDP-43) is a ubiquitously expressed protein well conserved among mammals and vertebrates [269]. It contains two RNA-recognition motifs (RRMs) that can bind UG/TG-rich single-stranded or double-stranded DNA/RNA. As such, TDP-43 performs various functions in transcriptional repression, pre-mRNA, and alternative splicing [270,271]. The C-terminus is essential for solubility and regulates protein–protein interactions. It also contains a prion-like domain where many disease-associated mutations are located [272]. TDP-43 also contains a nuclear localization sequence and as such is predominantly located in the nucleus. However, in non-pathogenic states it also shuttles to the cytoplasm to carry out additional functions, including mRNA stability and transport, translation, the stress response, and autophagy regulation (see [273] for a recent review). Here, it interacts with many subcellular compartments, including the endoplasmic reticulum and stress granules. Also, a small amount of TDP-43 is transported into mitochondria via the TOM20 and TIM22 complex [274] to stabilize mitochondrial transcript intermediates [275]. Therefore, the normal physiological functions of TDP-43 are particularly important for cell survival. Importantly, under normal physiological conditions, the low cytoplasmic levels of TDP-43 are finely tuned by a negative-feedback mechanism [276]. In the mitochondria, this includes TDP-43 degradation by mitochondrial proteases [277]. There is still much debate in the field on whether TDP-43 is internalized into the mitochondria, but it is clear that mitochondria play a role in response to aberrant TDP-43 [278].
5.3.2. Aberrant Cytoplasmic Roles of TDP-43
A hallmark of ALS and other proteinopathies is the aberrant localization of TDP-43 into cytoplasmic aggregates (Figure 6, [279,280]). The diseased form of TDP-43 is predominantly found in the cytoplasm, where it is hyperphosphorylated, ubiquitinated, and proteolytically cleaved into C-terminal fragments (CTFs) that are devoid of a functional nuclear localization signal [281,282,283]. Cytoplasmic accumulation of CFTs results in nuclear depletion and aggregate formation sequestering full-length TDP-43 and increasing cellular reactive oxygen species (ROS) [284,285]. Aggregate accumulation in TDP-43 toxicity has been associated with autophagy impairment [286,287,288,289,290].
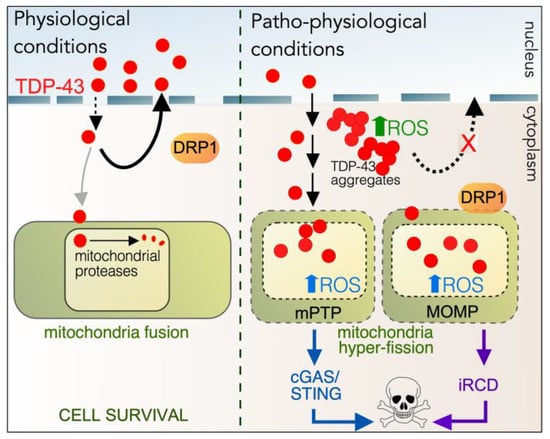
Figure 6.
Proposed roles of TDP-43 in physiological and patho-physiological conditions. Left hand panel: In unstressed cells, TDP-43 is predominantly a nuclear protein, but it also shuttles to the cytoplasm to play various roles, including translation. Most TDP-43 is shunted back into the nucleus mediated by its nuclear localization signal. Some TDP-43 also enters the matrix of mitochondria, where it regulates mitochondrial translation before being destroyed by resident proteases. DRP1 does not co-localize at the OMM, and mitochondrial dynamics are normal, with most cells exhibiting a fused morphology. Right-hand panel: Mutant TDP-43 is not well retained in the nucleus, resulting in cytoplasmic aggregates, increasing intracellular ROS. More TDP-43 is also found in mitochondria, which also trigger increase in ROS, resulting in cell death via the cGAS-STING and/or iRCD pathways. DRP1 co-localizes to the OMM inducing hyperfission and MOMP.
The mitochondrial accumulation of TDP-43 is also observed in TDP-43 toxicity. This results in increased DRP1-mediated mitochondrial fragmentation. Consistent with this are several observations: inhibiting TDP-43 localization to mitochondria or preventing fission blocks ALS-associated pathologies [277,291,292,293,294]; decreased MFF1 and 2 and increased DRP1 expression occur in motor neurons in a mouse model of ALS [295]; mitochondrial hyperfission has also been observed in the post-mortem neurons of patients diagnosed with ALS and other proteinopathies, as well as in cell cultures [284,285,291,292,294].
The accumulation of mitochondrial TDP-43 also triggers cell death by pathways that converge at the mitochondria (Figure 6, [275,292]). TDP-43 mitochondrial overload induces mitochondrial outer membrane permeabilization (MOMP), allowing iRCD). Alternatively, the mitochondrial permeability transition pore (mPTP) opens releasing mitochondrial DNA (mtDNA) and mtRNA:DNA into the cytoplasm. This stimulates the cyclic GMP-AMP synthase (cGAS)/Stimulator of Interferon Genes (STING) cell death pathway [294]. Therefore, understanding the molecular details underlying the mitochondrial invasion by TDP-43 represents an important step in devising therapeutic treatments for TDP-43 proteinopathies.
5.3.3. Cyclin C and TDP-43
Using the established yeast model of TDP-43 pathology, it has been shown that cyclin C, but not Med13, promotes TDP-43-mediated cell death [296]. Likewise, the deletion of Dnm1 rescues TDP-43 toxicity, pointing to the potential mitochondrial fission role of cyclin C in TDP-43 toxicity. Furthermore, cyclin C translocates to the cytoplasm following TDP-43 overexpression. The free radical scavenger, N-acetyl-cysteine (NAC), inhibits cyclin C cytoplasmic relocalization. These studies suggest the possibility that cytoplasmic cyclin C may promote mitochondrial hyperfission and cell death upon expression of mutant TDP-43. This is exciting as cyclin C is a new player in TDP-43 biology and could represent a new target for drug therapies. This is important as currently there are no effective cures for ALS, and patient survival upon diagnosis is around 3–5 years [297].
5.4. Diseases Associated with MED13 Biology
In mammals, the MKM contains paralogues of its members, except cyclin C, namely MED12L, MED13L, and CDK19 [27]. The biological roles of these paralogues remain poorly understood, but they appear to be functionally distinct [298]. Many mutations in MED13L result in MED13L syndrome, a disease characterized by a range of symptoms that vary in severity. These include intellectual disability, facial dysmorphism, hypotonia, congenital heart disease, and speech and motor delays [299,300,301,302]. In MED13L, mutant fibroblast cyclin C is aberrantly released into the cytoplasm, leading to mitochondrial fragmentation and increased mitochondrial dysfunction [303]. Variants in CDK8, MED13, MED12, and MED12L are also associated with neurodevelopmental disorders [73,304,305,306,307,308], with cyclin C’s role here being unknown. In addition, mutations in MED12 are linked with uterine leiomyomas [309]. Interestingly, all the MKM neurodevelopmental disorders are phenotypically similar, indicating a probable overlap in pathogenic mechanisms.
6. Conclusions
The MKM has evolved in the last 20 years from being a transcriptional regulator to mediating mitochondrial hyperfission, regulated cell death, P-body assembly, and cargo hitchhiking autophagy. These stress-dependent secondary roles trigger the nuclear release of either cyclin C or Med13, following ROS or starvation stress, respectively. This enables cells to quickly respond to unfavorable environmental cues, facilitating communication and regulatory functions between cellular compartments.
The ability of transcriptional regulators to shuttle between the nucleus and cytoplasm in response to cellular cues is well established. For example, upon activation the transcription factor NF-κB moves into the nucleus, where it triggers transcription of genes involved in survival, inflammation, and the immune response [310]. More unusual though are proteins like cyclin C and Med13 that play different roles in different compartments. A great example is the nuclear transcription factor TAF7 that chaperones its target RNAs from the nucleus to polysomes in the cytoplasm, where it contributes to the regulation of translation [311]. Another dual-role transcription factor is p53, which also regulates translation primarily through its interaction with components of the translation machinery [312]. p53 also migrates to the mitochondria, mediating iRCD primarily through direct protein–protein interactions with BCL-2 family proteins [313]. TDP-43, as discussed above, plays roles in splicing in the nucleus and translation in the cytoplasm. Likewise, alpha-synuclein, the protein long associated with Parkinson’s disease and Lewy body dementia, activates a calcium pump in cell membranes [314] and modulates P-bodies in the cytoplasm [315]. These examples suggest that other proteins may have dual roles that are currently unknown. It is important to investigate, not only from the point of view of unravelling complex molecular pathways but also for targeted drug design, which has been dominated by the “one target, one drug” concept.
The conservation of the roles of cytoplasmic cyclin C are remarkable, with minor differences being observed between yeast and mammalian cells. Maybe the largest difference is the observation that in yeast most of cyclin C shuttles to the cytoplasm [91,101], whereas in mammalian cells only 10% of cyclin C is observed at this new subcellular address after stress [92]. The most logical explanation for these differences is that in mammals, cyclin C positively and negatively regulates an equal number of SRGs, whereas in yeast it is mainly a negative regulator [316].
7. Future Prospectives
The most important gaps in our knowledge of MKM biology following stress are related to the determination of whether the cytoplasmic role of Med13 found in yeast is conserved. Yeast Med13 and MED13/13L in mammals share structural features, including large IRDs and prion-like domains. As these motifs are characteristic of proteins able to undergo LLPS, this suggests a strong possibility that Med13 may also contribute to BMC formation after stress. Consistent with this, the PrLD of yeast Med13 is required for P-body formation [112]. Future experiments need to address whether this cytoplasmic role of Med13 is conserved. If MED13 and MED13L do relocate to the cytoplasm in mammals, then it would be important to ask whether they also undergo autophagy. This avenue of research could potentially lead to the discovery of CHA in mammals. As autophagy is a key regulator of homeostasis, including the removal of aggregate proteins, [317] this line of research could provide valuable insights into the molecular basis of proteinopathies.
Lastly, in addition to guiding studies in higher eukaryotes, understanding the stress response in yeast is important in its own right. In the US, the wine industry generates billions of dollars of revenue annually, and craft beer brewing alone has recently emerged as a multibillion-dollar industry [318,319]. S. cerevisiae encounters different stresses during brewing, such as hyperosmotic, ethanol, and thermal stresses [320,321]. In addition, in fed-batch-operated industrial bioreactors, pockets of yeast can face glucose starvation, resulting in negative consequences for production performance [322]. Understanding and manipulating the yeast stress response could spur industrial advancements and increase fermentation yield efficiency [323,324,325]. Ultimately, the MKM, particularly cyclin C and Med13, plays a critical role in a variety of diseases, emphasizing the need for further investigations of both the mechanistical aspects and the therapeutic potential.
Author Contributions
All authors contributed to the writing and editing of this review article. All authors have read and agreed to the published version of the manuscript.
Funding
K.F.C is supported in part by N. I. H. grant #1R21AG080666-01A1.
Data Availability Statement
No new data were created or analyzed in this study.
Acknowledgments
We thank members of the Cooper and Strich labs for carefully reading this review.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the writing of this review.
Abbreviations
The following abbreviations are used in this manuscript:
| AMPK | Adenosine Monophosphate-activated Protein Kinase |
| ALS | Amyloid Lateral Sclerosis |
| AIM | Atg8 Interacting Motif |
| BAR | Bin–Amphiphysin–Rvs |
| BMC | Biomolecular Condensate |
| cGAS | Second messenger cyclic GMP–AMP |
| ChIP | Chromatin Immunoprecipitation |
| CWI | Cell Wall Integrity pathway |
| cMED | Core Mediator Complex |
| HAD | Holoenzyme-associating Domain |
| IMM | Inner Mitochondrial Membrane |
| IDR | Intrinsically Disordered Region |
| iRCD | Intrinsic Regulated Cell Death |
| LLPS | Liquid–liquid Phase Separation |
| MKM | Mediator Kinase Module |
| OMM | Outer Mitochondrial Membrane |
| MOMP | Mitochondrial Outer Membrane permeabilization |
| MEFs | Mouse Embryonic Fibroblasts |
| NAC | N-acetyl-cysteine |
| mPTP | Mitochondrial Permeability Transition Pore |
| mRNPs | Non-translating mRNA-protein complexes |
| NPC | Nuclear Pore Complex |
| PI(3)P | Phosphatidylinositol-3-phosphate |
| PX | Phox Homology |
| PrLD | Prion-Like Domains |
| P-Body | Processing Body |
| RCD | Regulated Cell Death |
| RNPs | Ribonucleoproteins |
| RNAPII | RNA Polymerase II |
| RRM | RNA Recognition Motif |
| STING | Cyclic GMP–AMP receptor stimulator of interferon genes |
| SRGs | Stress Response Genes |
| T-ALL | T-Cell Acute Lymphoblastic Leukemia |
| TFs | Transcription Factors |
| TIFs | Translation Initiation Factors |
| UPS | Ubiquitin–Proteasome System |
References
- Koyuncu, S.; Loureiro, R.; Lee, H.J.; Wagle, P.; Krueger, M.; Vilchez, D. Rewiring of the ubiquitinated proteome determines ageing in C. elegans. Nature 2021, 596, 285–290. [Google Scholar] [CrossRef]
- Kevei, É.; Hoppe, T. Ubiquitin sets the timer: Impacts on aging and longevity. Nat. Struct. Mol. Biol. 2014, 21, 290–292. [Google Scholar] [CrossRef]
- Tramutola, A.; Di Domenico, F.; Barone, E.; Perluigi, M.; Butterfield, D.A. It Is All about (U)biquitin: Role of Altered Ubiquitin-Proteasome System and UCHL1 in Alzheimer Disease. Oxid. Med. Cell. Longev. 2016, 2016, 2756068. [Google Scholar] [CrossRef]
- Abdullah, M.O.; Zeng, R.X.; Margerum, C.L.; Papadopoli, D.; Monnin, C.; Punter, K.B.; Chu, C.; Al-Rofaidi, M.; Al-Tannak, N.F.; Berardi, D.; et al. Mitochondrial hyperfusion via metabolic sensing of regulatory amino acids. Cell Rep. 2022, 40, 111198. [Google Scholar] [CrossRef]
- Aman, Y.; Schmauck-Medina, T.; Hansen, M.; Morimoto, R.I.; Simon, A.K.; Bjedov, I.; Palikaras, K.; Simonsen, A.; Johansen, T.; Tavernarakis, N.; et al. Autophagy in healthy aging and disease. Nat. Aging 2021, 1, 634–650. [Google Scholar] [CrossRef]
- Leidal, A.M.; Levine, B.; Debnath, J. Autophagy and the cell biology of age-related disease. Nat. Cell Biol. 2018, 20, 1338–1348. [Google Scholar] [CrossRef]
- Kim, Y.J.; Lee, Y.; Shin, H.; Hwang, S.; Park, J.; Song, E.J. Ubiquitin–proteasome system as a target for anticancer treatment—An update. Arch. Pharmacal Res. 2023, 46, 573–597. [Google Scholar] [CrossRef]
- Ajmal, M.R. Protein Misfolding and Aggregation in Proteinopathies: Causes, Mechanism and Cellular Response. Diseases 2023, 11, 30. [Google Scholar] [CrossRef]
- Louros, N.; Schymkowitz, J.; Rousseau, F. Mechanisms and pathology of protein misfolding and aggregation. Nat. Rev. Mol. Cell Biol. 2023, 24, 912–933. [Google Scholar] [CrossRef] [PubMed]
- Dawes, I.W.; Perrone, G.G. Stress and ageing in yeast. FEMS Yeast Res. 2019, 20, foz085. [Google Scholar] [CrossRef] [PubMed]
- Ohsumi, Y. Historical landmarks of autophagy research. Cell Res. 2014, 24, 9–23. [Google Scholar] [CrossRef]
- Cazzanelli, G.; Pereira, F.; Alves, S.; Francisco, R.; Azevedo, L.; Dias Carvalho, P.; Almeida, A.; Côrte-Real, M.; Oliveira, M.J.; Lucas, C.; et al. The Yeast Saccharomyces cerevisiae as a Model for Understanding RAS Proteins and their Role in Human Tumorigenesis. Cells 2018, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira, F.; Medeiros, R.; Lopes, I.; Campos, C.; Ferraz, M.P.; Silva, F.; Alves, L.G.; Pinto, E. A Cyclam Salt as an Antifungal Agent: Interference with Candida spp. and Cryptococcus neoformans Mechanisms of Virulence. Antibiotics 2024, 13, 222. [Google Scholar] [CrossRef]
- Stieber, H.; Junghanns, L.; Wilhelm, H.; Batliner, M.; Aldejohann, A.M.; Kurzai, O.; Martin, R. The sphingolipid inhibitor myriocin increases Candida auris susceptibility to amphotericin B. Mycoses 2024, 67, e13723. [Google Scholar] [CrossRef] [PubMed]
- Schneider, K.L.; Hao, X.; Keuenhof, K.S.; Berglund, L.L.; Fischbach, A.; Ahmadpour, D.; Chawla, S.; Gómez, P.; Höög, J.L.; Widlund, P.O.; et al. Elimination of virus-like particles reduces protein aggregation and extends replicative lifespan in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 2024, 121, e2313538121. [Google Scholar] [CrossRef]
- Zhou, L.; Xu, R. Invertebrate genetic models of amyotrophic lateral sclerosis. Front. Mol. Neurosci. 2024, 17, 1328578. [Google Scholar] [CrossRef] [PubMed]
- Rencus-Lazar, S.; DeRowe, Y.; Adsi, H.; Gazit, E.; Laor, D. Yeast Models for the Study of Amyloid-Associated Disorders and Development of Future Therapy. Front. Mol. Biosci. 2019, 6, 15. [Google Scholar] [CrossRef]
- Cooper, K.F. Till Death Do Us Part: The Marriage of Autophagy and Apoptosis. Oxid. Med. Cell. Longev. 2018, 2018, 4701275. [Google Scholar] [CrossRef]
- Cooper, K.F. Cargo hitchhiking autophagy—A hybrid autophagy pathway utilized in yeast. Autophagy 2025, 21, 500–512. [Google Scholar] [CrossRef]
- Tsai, K.L.; Sato, S.; Tomomori-Sato, C.; Conaway, R.C.; Conaway, J.W.; Asturias, F.J. A conserved Mediator-CDK8 kinase module association regulates Mediator-RNA polymerase II interaction. Nat. Struct. Mol. Biol. 2013, 20, 611–619. [Google Scholar] [CrossRef]
- Allen, B.L.; Taatjes, D.J. The Mediator complex: A central integrator of transcription. Nat. Rev. Mol. Cell Biol. 2015, 16, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Fant, C.B.; Taatjes, D.J. Regulatory functions of the Mediator kinases CDK8 and CDK19. Transcription 2019, 10, 76–90. [Google Scholar] [CrossRef] [PubMed]
- Robinson, P.J.; Trnka, M.J.; Pellarin, R.; Greenberg, C.H.; Bushnell, D.A.; Davis, R.; Burlingame, A.L.; Sali, A.; Kornberg, R.D. Molecular architecture of the yeast Mediator complex. eLife 2015, 4, e08719. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Chao, T.C.; Tsai, K.L. Structures and compositional dynamics of Mediator in transcription regulation. Curr. Opin. Struct. Biol. 2024, 88, 102892. [Google Scholar] [CrossRef]
- Chao, T.C.; Chen, S.F.; Kim, H.J.; Tang, H.C.; Tseng, H.C.; Xu, A.; Palao, L., 3rd; Khadka, S.; Li, T.; Huang, M.F.; et al. Structural basis of the human transcriptional Mediator regulated by its dissociable kinase module. Mol. Cell 2024, 84, 3932–3949.e3910. [Google Scholar] [CrossRef]
- Knuesel, M.T.; Meyer, K.D.; Donner, A.J.; Espinosa, J.M.; Taatjes, D.J. The human CDK8 subcomplex is a histone kinase that requires Med12 for activity and can function independently of mediator. Mol. Cell. Biol. 2009, 29, 650–661. [Google Scholar] [CrossRef]
- Soutourina, J. Transcription regulation by the Mediator complex. Nat. Rev. Mol. Cell Biol. 2018, 19, 262–274. [Google Scholar] [CrossRef]
- Galbraith, M.D.; Donner, A.J.; Espinosa, J.M. CDK8: A positive regulator of transcription. Transcription 2010, 1, 4–12. [Google Scholar] [CrossRef]
- Echalier, A.; Endicott, J.A.; Noble, M.E. Recent developments in cyclin-dependent kinase biochemical and structural studies. Biochim. Biophys. Acta 2010, 1804, 511–519. [Google Scholar] [CrossRef]
- Schneider, E.V.; Bottcher, J.; Blaesse, M.; Neumann, L.; Huber, R.; Maskos, K. The structure of CDK8/CycC implicates specificity in the CDK/cyclin family and reveals interaction with a deep pocket binder. J. Mol. Biol. 2011, 412, 251–266. [Google Scholar] [CrossRef]
- Li, Y.C.; Chao, T.C.; Kim, H.J.; Cholko, T.; Chen, S.F.; Li, G.; Snyder, L.; Nakanishi, K.; Chang, C.E.; Murakami, K.; et al. Structure and noncanonical Cdk8 activation mechanism within an Argonaute-containing Mediator kinase module. Sci. Adv. 2021, 7, eabd4484. [Google Scholar] [CrossRef]
- Kuchin, S.; Yeghiayan, P.; Carlson, M. Cyclin-dependent protein kinase and cyclin homologs SSN3 and SSN8 contribute to transcriptional control in yeast. Proc. Natl. Acad. Sci. USA 1995, 92, 4006–4010. [Google Scholar] [CrossRef] [PubMed]
- Cooper, K.F.; Mallory, M.J.; Smith, J.B.; Strich, R. Stress and developmental regulation of the yeast C-type cyclin Ume3p (Srb11p/Ssn8p). EMBO J. 1997, 16, 4665–4675. [Google Scholar] [CrossRef]
- Cooper, K.F.; Mallory, M.J.; Strich, R. Oxidative stress-induced destruction of the yeast C-type cyclin Ume3p requires phosphatidylinositol-specific phospholipase C and the 26S proteasome. Mol. Cell. Biol. 1999, 19, 3338–3348. [Google Scholar] [CrossRef] [PubMed]
- Cooper, K.F.; Strich, R. Saccharomyces cerevisiae C-type cyclin Ume3p/Srb11p is required for efficient induction and execution of meiotic development. Eukaryot. Cell 2002, 1, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Holstege, F.C.; Jennings, E.G.; Wyrick, J.J.; Lee, T.I.; Hengartner, C.J.; Green, M.R.; Golub, T.R.; Lander, E.S.; Young, R.A. Dissecting the regulatory circuitry of a eukaryotic genome. Cell 1998, 95, 717–728. [Google Scholar] [CrossRef]
- Wahi, M.; Johnson, A.D. Identification of genes required for alpha 2 repression in Saccharomyces cerevisiae. Genetics 1995, 140, 79–90. [Google Scholar] [CrossRef]
- Bjorklund, S.; Gustafsson, C.M. The yeast Mediator complex and its regulation. Trends Biochem. Sci. 2005, 30, 240–244. [Google Scholar] [CrossRef]
- van de Peppel, J.; Kettelarij, N.; van Bakel, H.; Kockelkorn, T.T.; van Leenen, D.; Holstege, F.C. Mediator expression profiling epistasis reveals a signal transduction pathway with antagonistic submodules and highly specific downstream targets. Mol. Cell 2005, 19, 511–522. [Google Scholar] [CrossRef]
- Surosky, R.T.; Strich, R.; Esposito, R.E. The yeast UME5 gene regulates the stability of meiotic mRNAs in response to glucose. Mol. Cell. Biol. 1994, 14, 3446–3458. [Google Scholar] [CrossRef]
- Strich, R.; Slater, M.R.; Esposito, R.E. Identification of negative regulatory genes that govern the expression of early meiotic genes in yeast. Proc. Natl. Acad. Sci. USA 1989, 86, 10018–10022. [Google Scholar] [CrossRef] [PubMed]
- Hirst, M.; Kobor, M.S.; Kuriakose, N.; Greenblatt, J.; Sadowski, I. GAL4 is regulated by the RNA polymerase II holoenzyme-associated cyclin-dependent protein kinase SRB10/CDK8. Mol. Cell 1999, 3, 673–678. [Google Scholar] [CrossRef] [PubMed]
- Willis, S.D.; Hanley, S.E.; Doyle, S.J.; Beluch, K.; Strich, R.; Cooper, K.F. Cyclin C-Cdk8 Kinase Phosphorylation of Rim15 Prevents the Aberrant Activation of Stress Response Genes. Front. Cell Dev. Biol. 2022, 10, 867257. [Google Scholar] [CrossRef]
- Hanley, S.E.; Willis, S.D.; Cooper, K.F. Snx4-assisted vacuolar targeting of transcription factors defines a new autophagy pathway for controlling ATG expression. Autophagy 2021, 17, 3547–3565. [Google Scholar] [CrossRef]
- Jeronimo, C.; Robert, F. The Mediator Complex: At the Nexus of RNA Polymerase II Transcription. Trends Cell Biol. 2017, 27, 765–783. [Google Scholar] [CrossRef]
- Chi, Y.; Huddleston, M.J.; Zhang, X.; Young, R.A.; Annan, R.S.; Carr, S.A.; Deshaies, R.J. Negative regulation of Gcn4 and Msn2 transcription factors by Srb10 cyclin-dependent kinase. Genes Dev. 2001, 15, 1078–1092. [Google Scholar] [CrossRef]
- Nelson, C.; Goto, S.; Lund, K.; Hung, W.; Sadowski, I. Srb10/Cdk8 regulates yeast filamentous growth by phosphorylating the transcription factor Ste12. Nature 2003, 421, 187–190. [Google Scholar] [CrossRef]
- Law, M.J.; Ciccaglione, K. Fine-Tuning of Histone H3 Lys4 Methylation During Pseudohyphal Differentiation by the CDK Submodule of RNA Polymerase II. Genetics 2015, 199, 435–453. [Google Scholar] [CrossRef] [PubMed]
- Friedson, B.; Willis, S.D.; Shcherbik, N.; Campbell, A.N.; Cooper, K.F. The CDK8 kinase module: A novel player in the transcription of translation initiation and ribosomal genes. Mol. Biol. Cell 2025, 36, ar2. [Google Scholar] [CrossRef]
- Shore, D.; Albert, B. Ribosome biogenesis and the cellular energy economy. Curr. Biol. 2022, 32, R611–R617. [Google Scholar] [CrossRef]
- Hirai, H.; Ohta, K. Comparative Research: Regulatory Mechanisms of Ribosomal Gene Transcription in Saccharomyces cerevisiae and Schizosaccharomyces pombe. Biomolecules 2023, 13, 288. [Google Scholar] [CrossRef] [PubMed]
- Stieg, D.C.; Cooper, K.F.; Strich, R. The extent of cyclin C promoter occupancy directs changes in stress-dependent transcription. J. Biol. Chem. 2020, 295, 16280–16291. [Google Scholar] [CrossRef]
- Bancerek, J.; Poss, Z.C.; Steinparzer, I.; Sedlyarov, V.; Pfaffenwimmer, T.; Mikulic, I.; Dolken, L.; Strobl, B.; Muller, M.; Taatjes, D.J.; et al. CDK8 kinase phosphorylates transcription factor STAT1 to selectively regulate the interferon response. Immunity 2013, 38, 250–262. [Google Scholar] [CrossRef]
- Steinparzer, I.; Sedlyarov, V.; Rubin, J.D.; Eislmayr, K.; Galbraith, M.D.; Levandowski, C.B.; Vcelkova, T.; Sneezum, L.; Wascher, F.; Amman, F.; et al. Transcriptional Responses to IFN-γ Require Mediator Kinase-Dependent Pause Release and Mechanistically Distinct CDK8 and CDK19 Functions. Mol. Cell 2019, 76, 485–499.e488. [Google Scholar] [CrossRef] [PubMed]
- Alarcon, C.; Zaromytidou, A.I.; Xi, Q.; Gao, S.; Yu, J.; Fujisawa, S.; Barlas, A.; Miller, A.N.; Manova-Todorova, K.; Macias, M.J.; et al. Nuclear CDKs drive Smad transcriptional activation and turnover in BMP and TGF-beta pathways. Cell 2009, 139, 757–769. [Google Scholar] [CrossRef]
- Morris, E.J.; Ji, J.-Y.; Yang, F.; Di Stefano, L.; Herr, A.; Moon, N.-S.; Kwon, E.-J.; Haigis, K.M.; Näär, A.M.; Dyson, N.J. E2F1 represses β-catenin transcription and is antagonized by both pRB and CDK8. Nature 2008, 455, 552–556. [Google Scholar] [CrossRef]
- Zhao, X.; Feng, D.; Wang, Q.; Abdulla, A.; Xie, X.J.; Zhou, J.; Sun, Y.; Yang, E.S.; Liu, L.P.; Vaitheesvaran, B.; et al. Regulation of lipogenesis by cyclin-dependent kinase 8-mediated control of SREBP-1. J. Clin. Investig. 2012, 122, 2417–2427. [Google Scholar] [CrossRef]
- Vincent, O.; Kuchin, S.; Hong, S.P.; Townley, R.; Vyas, V.K.; Carlson, M. Interaction of the Srb10 kinase with Sip4, a transcriptional activator of gluconeogenic genes in Saccharomyces cerevisiae. Mol. Cell. Biol. 2001, 21, 5790–5796. [Google Scholar] [CrossRef] [PubMed]
- Elmlund, H.; Baraznenok, V.; Lindahl, M.; Samuelsen, C.O.; Koeck, P.J.; Holmberg, S.; Hebert, H.; Gustafsson, C.M. The cyclin-dependent kinase 8 module sterically blocks Mediator interactions with RNA polymerase II. Proc. Natl. Acad. Sci. USA 2006, 103, 15788–15793. [Google Scholar] [CrossRef]
- Osman, S.; Mohammad, E.; Lidschreiber, M.; Stuetzer, A.; Bazso, F.L.; Maier, K.C.; Urlaub, H.; Cramer, P. The Cdk8 kinase module regulates interaction of the mediator complex with RNA polymerase II. J. Biol. Chem. 2021, 296, 100734. [Google Scholar] [CrossRef]
- Petrenko, N.; Jin, Y.; Wong, K.H.; Struhl, K. Mediator Undergoes a Compositional Change during Transcriptional Activation. Mol. Cell 2016, 64, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.A.; Larimore, E.A.; Fissel, B.M.; Swanger, J.; Taatjes, D.J.; Clurman, B.E. The SCF-Fbw7 ubiquitin ligase degrades MED13 and MED13L and regulates CDK8 module association with Mediator. Genes Dev. 2013, 27, 151–156. [Google Scholar] [CrossRef]
- Mo, X.; Kowenz-Leutz, E.; Xu, H.; Leutz, A. Ras induces mediator complex exchange on C/EBP beta. Mol. Cell 2004, 13, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Pavri, R.; Lewis, B.; Kim, T.K.; Dilworth, F.J.; Erdjument-Bromage, H.; Tempst, P.; de Murcia, G.; Evans, R.; Chambon, P.; Reinberg, D. PARP-1 determines specificity in a retinoid signaling pathway via direct modulation of mediator. Mol. Cell 2005, 18, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Lambert, E.; Puwakdandawa, K.; Tao, Y.F.; Robert, F. From structure to molecular condensates: Emerging mechanisms for Mediator function. FEBS J. 2023, 290, 286–309. [Google Scholar] [CrossRef]
- Akoulitchev, S.; Chuikov, S.; Reinberg, D. TFIIH is negatively regulated by cdk8-containing mediator complexes. Nature 2000, 407, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Fryer, C.J.; White, J.B.; Jones, K.A. Mastermind recruits CycC:CDK8 to phosphorylate the Notch ICD and coordinate activation with turnover. Mol. Cell 2004, 16, 509–520. [Google Scholar] [CrossRef]
- Postlmayr, A.; Dumeau, C.E.; Wutz, A. Cdk8 is required for establishment of H3K27me3 and gene repression by Xist and mouse development. Development 2020, 147, dev175141. [Google Scholar] [CrossRef]
- Anandhakumar, J.; Moustafa, Y.W.; Chowdhary, S.; Kainth, A.S.; Gross, D.S. Evidence for Multiple Mediator Complexes in Yeast Independently Recruited by Activated Heat Shock Factor. Mol. Cell. Biol. 2016, 36, 1943–1960. [Google Scholar] [CrossRef]
- Donner, A.J.; Ebmeier, C.C.; Taatjes, D.J.; Espinosa, J.M. CDK8 is a positive regulator of transcriptional elongation within the serum response network. Nat. Struct. Mol. Biol. 2010, 17, 194–201. [Google Scholar] [CrossRef]
- Galbraith, M.D.; Allen, M.A.; Bensard, C.L.; Wang, X.; Schwinn, M.K.; Qin, B.; Long, H.W.; Daniels, D.L.; Hahn, W.C.; Dowell, R.D.; et al. HIF1A employs CDK8-mediator to stimulate RNAPII elongation in response to hypoxia. Cell 2013, 153, 1327–1339. [Google Scholar] [CrossRef] [PubMed]
- Richter, W.F.; Nayak, S.; Iwasa, J.; Taatjes, D.J. The Mediator complex as a master regulator of transcription by RNA polymerase II. Nat. Rev. Mol. Cell Biol. 2022, 23, 732–749. [Google Scholar] [CrossRef] [PubMed]
- Luyties, O.; Taatjes, D.J. The Mediator kinase module: An interface between cell signaling and transcription. Trends Biochem. Sci. 2022, 47, 314–327. [Google Scholar] [CrossRef]
- Gonzalez, D.; Hamidi, N.; Del Sol, R.; Benschop, J.J.; Nancy, T.; Li, C.; Francis, L.; Tzouros, M.; Krijgsveld, J.; Holstege, F.C.; et al. Suppression of Mediator is regulated by Cdk8-dependent Grr1 turnover of the Med3 coactivator. Proc. Natl. Acad. Sci. USA 2014, 111, 2500–2505. [Google Scholar] [CrossRef] [PubMed]
- Alber, F.; Dokudovskaya, S.; Veenhoff, L.M.; Zhang, W.; Kipper, J.; Devos, D.; Suprapto, A.; Karni-Schmidt, O.; Williams, R.; Chait, B.T.; et al. The molecular architecture of the nuclear pore complex. Nature 2007, 450, 695–701. [Google Scholar] [CrossRef]
- Adler, A.S.; McCleland, M.L.; Truong, T.; Lau, S.; Modrusan, Z.; Soukup, T.M.; Roose-Girma, M.; Blackwood, E.M.; Firestein, R. CDK8 maintains tumor dedifferentiation and embryonic stem cell pluripotency. Cancer Res. 2012, 72, 2129–2139. [Google Scholar] [CrossRef]
- Chen, M.; Liang, J.; Ji, H.; Yang, Z.; Altilia, S.; Hu, B.; Schronce, A.; McDermott, M.S.J.; Schools, G.P.; Lim, C.U.; et al. CDK8/19 Mediator kinases potentiate induction of transcription by NFkappaB. Proc. Natl. Acad. Sci. USA 2017, 114, 10208–10213. [Google Scholar] [CrossRef]
- Hirst, K.; Fisher, F.; McAndrew, P.C.; Goding, C.R. The transcription factor, the Cdk, its cyclin and their regulator: Directing the transcriptional response to a nutritional signal. EMBO J. 1994, 13, 5410–5420. [Google Scholar] [CrossRef]
- Lenssen, E.; Azzouz, N.; Michel, A.; Landrieux, E.; Collart, M.A. The Ccr4-not complex regulates Skn7 through Srb10 kinase. Eukaryot. Cell 2007, 6, 2251–2259. [Google Scholar] [CrossRef]
- Freitas, K.A.; Belk, J.A.; Sotillo, E.; Quinn, P.J.; Ramello, M.C.; Malipatlolla, M.; Daniel, B.; Sandor, K.; Klysz, D.; Bjelajac, J.; et al. Enhanced T cell effector activity by targeting the Mediator kinase module. Science 2022, 378, eabn5647. [Google Scholar] [CrossRef]
- Chen, M.; Li, J.; Zhang, L.; Wang, L.; Cheng, C.; Ji, H.; Altilia, S.; Ding, X.; Cai, G.; Altomare, D.; et al. CDK8 and CDK19: Positive regulators of signal-induced transcription and negative regulators of Mediator complex proteins. Nucleic Acids Res. 2023, 51, 7288–7313. [Google Scholar] [CrossRef] [PubMed]
- Stieg, D.C.; Chang, K.T.; Cooper, K.F.; Strich, R. Cyclin C Regulated Oxidative Stress Responsive Transcriptome in Mus musculus Embryonic Fibroblasts. G3 2019, 9, 1901–1908. [Google Scholar] [CrossRef]
- Nowak, S.J.; Corces, V.G. Phosphorylation of histone H3: A balancing act between chromosome condensation and transcriptional activation. Trends Genet. 2004, 20, 214–220. [Google Scholar] [CrossRef]
- Fischle, W.; Tseng, B.S.; Dormann, H.L.; Ueberheide, B.M.; Garcia, B.A.; Shabanowitz, J.; Hunt, D.F.; Funabiki, H.; Allis, C.D. Regulation of HP1-chromatin binding by histone H3 methylation and phosphorylation. Nature 2005, 438, 1116–1122. [Google Scholar] [CrossRef]
- Kim, T.W.; Kwon, Y.J.; Kim, J.M.; Song, Y.H.; Kim, S.N.; Kim, Y.J. MED16 and MED23 of Mediator are coactivators of lipopolysaccharide- and heat-shock-induced transcriptional activators. Proc. Natl. Acad. Sci. USA 2004, 101, 12153–12158. [Google Scholar] [CrossRef] [PubMed]
- Pelish, H.E.; Liau, B.B.; Nitulescu, I.I.; Tangpeerachaikul, A.; Poss, Z.C.; Da Silva, D.H.; Caruso, B.T.; Arefolov, A.; Fadeyi, O.; Christie, A.L.; et al. Mediator kinase inhibition further activates super-enhancer-associated genes in AML. Nature 2015, 526, 273–276. [Google Scholar] [CrossRef]
- Cozzolino, K.A.; Sanford, L.; Hunter, S.; Molison, K.; Erickson, B.; Courvan, M.C.S.; Jones, T.; Ajit, D.; Galbraith, M.D.; Espinosa, J.M.; et al. Mediator kinase inhibition suppresses hyperactive interferon signaling in Down syndrome. eLife 2025, 13, RP100197. [Google Scholar] [CrossRef] [PubMed]
- Sabari, B.R.; Dall’Agnese, A.; Boija, A.; Klein, I.A.; Coffey, E.L.; Shrinivas, K.; Abraham, B.J.; Hannett, N.M.; Zamudio, A.V.; Manteiga, J.C.; et al. Coactivator condensation at super-enhancers links phase separation and gene control. Science 2018, 361, eaar3958. [Google Scholar] [CrossRef]
- Jeronimo, C.; Langelier, M.F.; Bataille, A.R.; Pascal, J.M.; Pugh, B.F.; Robert, F. Tail and Kinase Modules Differently Regulate Core Mediator Recruitment and Function In Vivo. Mol. Cell 2016, 64, 455–466. [Google Scholar] [CrossRef]
- Barette, C.; Jariel-Encontre, I.; Piechaczyk, M.; Piette, J. Human cyclin C protein is stabilized by its associated kinase cdk8, independently of its catalytic activity. Oncogene 2001, 20, 551–562. [Google Scholar] [CrossRef]
- Cooper, K.F.; Scarnati, M.S.; Krasley, E.; Mallory, M.J.; Jin, C.; Law, M.J.; Strich, R. Oxidative-stress-induced nuclear to cytoplasmic relocalization is required for Not4-dependent cyclin C destruction. J. Cell Sci. 2012, 125, 1015–1026. [Google Scholar] [CrossRef]
- Wang, K.; Yan, R.; Cooper, K.F.; Strich, R. Cyclin C mediates stress-induced mitochondrial fission and apoptosis. Mol. Biol. Cell 2015, 26, 1030–1043. [Google Scholar] [CrossRef] [PubMed]
- Ponce, J.M.; Coen, G.; Spitler, K.M.; Dragisic, N.; Martins, I.; Hinton, A.; Mungai, M.; Tadinada, S.M.; Zhang, H.; Oudit, G.Y.; et al. Stress-Induced Cyclin C Translocation Regulates Cardiac Mitochondrial Dynamics. J. Am. Heart Assoc. 2020, 9, e014366. [Google Scholar] [CrossRef] [PubMed]
- Truman, A.W.; Millson, S.H.; Nuttall, J.M.; King, V.; Mollapour, M.; Prodromou, C.; Pearl, L.H.; Piper, P.W. Expressed in the yeast Saccharomyces cerevisiae, human ERK5 is a client of the Hsp90 chaperone that complements loss of the Slt2p (Mpk1p) cell integrity stress-activated protein kinase. Eukaryot. Cell 2006, 5, 1914–1924. [Google Scholar] [CrossRef] [PubMed]
- Levin, D.E. Regulation of cell wall biogenesis in Saccharomyces cerevisiae: The cell wall integrity signaling pathway. Genetics 2011, 189, 1145–1175. [Google Scholar] [CrossRef]
- Krasley, E.; Cooper, K.F.; Mallory, M.J.; Dunbrack, R.; Strich, R. Regulation of the oxidative stress response through Slt2p-dependent destruction of cyclin C in Saccharomyces cerevisiae. Genetics 2006, 172, 1477–1486. [Google Scholar] [CrossRef]
- Jin, C.; Parshin, A.V.; Daly, I.; Strich, R.; Cooper, K.F. The cell wall sensors Mtl1, Wsc1, and Mid2 are required for stress-induced nuclear to cytoplasmic translocation of cyclin C and programmed cell death in yeast. Oxid. Med. Cell. Longev. 2013, 2013, 320823. [Google Scholar] [CrossRef]
- Jin, C.; Strich, R.; Cooper, K.F. Slt2p phosphorylation induces cyclin C nuclear-to-cytoplasmic translocation in response to oxidative stress. Mol. Biol. Cell 2014, 25, 1396–1407. [Google Scholar] [CrossRef]
- Stieg, D.C.; Willis, S.D.; Ganesan, V.; Ong, K.L.; Scuorzo, J.; Song, M.; Grose, J.; Strich, R.; Cooper, K.F. A complex molecular switch directs stress-induced cyclin C nuclear release through SCF(Grr1)-mediated degradation of Med13. Mol. Biol. Cell 2018, 29, 363–375. [Google Scholar] [CrossRef]
- Khakhina, S.; Cooper, K.F.; Strich, R. Med13p prevents mitochondrial fission and programmed cell death in yeast through nuclear retention of cyclin C. Mol. Biol. Cell 2014, 25, 2807–2816. [Google Scholar] [CrossRef]
- Cooper, K.F.; Khakhina, S.; Kim, S.K.; Strich, R. Stress-induced nuclear-to-cytoplasmic translocation of cyclin C promotes mitochondrial fission in yeast. Dev. Cell 2014, 28, 161–173. [Google Scholar] [CrossRef]
- Nash, P.; Tang, X.; Orlicky, S.; Chen, Q.; Gertler, F.B.; Mendenhall, M.D.; Sicheri, F.; Pawson, T.; Tyers, M. Multisite phosphorylation of a CDK inhibitor sets a threshold for the onset of DNA replication. Nature 2001, 414, 514–521. [Google Scholar] [CrossRef]
- Ang, X.L.; Wade Harper, J. SCF-mediated protein degradation and cell cycle control. Oncogene 2005, 24, 2860–2870. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.P.; Carlson, M. Regulation of snf1 protein kinase in response to environmental stress. J. Biol. Chem. 2007, 282, 16838–16845. [Google Scholar] [CrossRef]
- Willis, S.D.; Stieg, D.C.; Ong, K.L.; Shah, R.; Strich, A.K.; Grose, J.H.; Cooper, K.F. Snf1 cooperates with the CWI MAPK pathway to mediate the degradation of Med13 following oxidative stress. Microb. Cell 2018, 5, 357–370. [Google Scholar] [CrossRef] [PubMed]
- Cooper, K.F.; Strich, R. Functional analysis of the Ume3p/Srb11p-RNA polymerase II holoenzyme interaction. Gene Expr. 1999, 8, 43–57. [Google Scholar] [PubMed]
- Jezek, J.; Chang, K.T.; Joshi, A.M.; Strich, R. Mitochondrial translocation of cyclin C stimulates intrinsic apoptosis through Bax recruitment. EMBO Rep. 2019, 20, e47425. [Google Scholar] [CrossRef]
- Feng, Y.; Chen, Y.; Wu, X.; Chen, J.; Zhou, Q.; Liu, B.; Zhang, L.; Yi, C. Interplay of energy metabolism and autophagy. Autophagy 2024, 20, 4–14. [Google Scholar] [CrossRef]
- Willis, S.D.; Hanley, S.E.; Beishke, T.; Tati, P.D.; Cooper, K.F. Ubiquitin-proteasome-mediated cyclin C degradation promotes cell survival following nitrogen starvation. Mol. Biol. Cell 2020, 31, 1015–1031. [Google Scholar] [CrossRef]
- Rambold, A.S.; Kostelecky, B.; Elia, N.; Lippincott-Schwartz, J. Tubular network formation protects mitochondria from autophagosomal degradation during nutrient starvation. Proc. Natl. Acad. Sci. USA 2011, 108, 10190–10195. [Google Scholar] [CrossRef]
- Hanley, S.E.; Willis, S.D.; Doyle, S.J.; Strich, R.; Cooper, K.F. Ksp1 is an autophagic receptor protein for the Snx4-assisted autophagy of Ssn2/Med13. Autophagy 2024, 20, 397–415. [Google Scholar] [CrossRef]
- Hanley, S.E.; Willis, S.D.; Friedson, B.; Cooper, K.F. Med13 is required for efficient P-body recruitment and autophagic degradation of Edc3 following nitrogen starvation. Mol. Biol. Cell 2024, 35, ar142. [Google Scholar] [CrossRef]
- Torres, J.; Di Como, C.J.; Herrero, E.; De La Torre-Ruiz, M.A. Regulation of the cell integrity pathway by rapamycin-sensitive TOR function in budding yeast. J. Biol. Chem. 2002, 277, 43495–43504. [Google Scholar] [CrossRef]
- Kondadi, A.K.; Reichert, A.S. Mitochondrial Dynamics at Different Levels: From Cristae Dynamics to Interorganellar Cross Talk. Annu. Rev. Biophys. 2024, 53, 147–168. [Google Scholar] [CrossRef] [PubMed]
- Glancy, B.; Kim, Y.; Katti, P.; Willingham, T.B. The Functional Impact of Mitochondrial Structure Across Subcellular Scales. Front. Physiol. 2020, 11, 541040. [Google Scholar] [CrossRef] [PubMed]
- Westrate, L.M.; Drocco, J.A.; Martin, K.R.; Hlavacek, W.S.; MacKeigan, J.P. Mitochondrial morphological features are associated with fission and fusion events. PLoS ONE 2014, 9, e95265. [Google Scholar] [CrossRef] [PubMed]
- Pagliuso, A.; Cossart, P.; Stavru, F. The ever-growing complexity of the mitochondrial fission machinery. Cell. Mol. Life Sci. 2018, 75, 355–374. [Google Scholar] [CrossRef]
- Nunnari, J.; Suomalainen, A. Mitochondria: In sickness and in health. Cell 2012, 148, 1145–1159. [Google Scholar] [CrossRef]
- Horbay, R.; Bilyy, R. Mitochondrial dynamics during cell cycling. Apoptosis 2016, 21, 1327–1335. [Google Scholar] [CrossRef]
- Lewis, T.L., Jr.; Kwon, S.K.; Lee, A.; Shaw, R.; Polleux, F. MFF-dependent mitochondrial fission regulates presynaptic release and axon branching by limiting axonal mitochondria size. Nat. Commun. 2018, 9, 5008. [Google Scholar] [CrossRef]
- Twig, G.; Elorza, A.; Molina, A.J.; Mohamed, H.; Wikstrom, J.D.; Walzer, G.; Stiles, L.; Haigh, S.E.; Katz, S.; Las, G.; et al. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J. 2008, 27, 433–446. [Google Scholar] [CrossRef] [PubMed]
- Narendra, D.; Tanaka, A.; Suen, D.F.; Youle, R.J. Parkin-induced mitophagy in the pathogenesis of Parkinson disease. Autophagy 2009, 5, 706–708. [Google Scholar] [CrossRef]
- Chen, W.; Zhao, H.; Li, Y. Mitochondrial dynamics in health and disease: Mechanisms and potential targets. Signal Transduct. Target. Ther. 2023, 8, 333. [Google Scholar] [CrossRef]
- Zou, W.; Yang, L.; Lu, H.; Li, M.; Ji, D.; Slone, J.; Huang, T. Application of super-resolution microscopy in mitochondria-dynamic diseases. Adv. Drug Deliv. Rev. 2023, 200, 115043. [Google Scholar] [CrossRef] [PubMed]
- Ingerman, E.; Perkins, E.M.; Marino, M.; Mears, J.A.; McCaffery, J.M.; Hinshaw, J.E.; Nunnari, J. Dnm1 forms spirals that are structurally tailored to fit mitochondria. J. Cell Biol. 2005, 170, 1021–1027. [Google Scholar] [CrossRef]
- Mishra, P.; Chan, D.C. Mitochondrial dynamics and inheritance during cell division, development and disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 634–646. [Google Scholar] [CrossRef] [PubMed]
- Tilokani, L.; Nagashima, S.; Paupe, V.; Prudent, J. Mitochondrial dynamics: Overview of molecular mechanisms. Essays Biochem. 2018, 62, 341–360. [Google Scholar] [CrossRef]
- Mears, J.A.; Lackner, L.L.; Fang, S.; Ingerman, E.; Nunnari, J.; Hinshaw, J.E. Conformational changes in Dnm1 support a contractile mechanism for mitochondrial fission. Nat. Struct. Mol. Biol. 2011, 18, 20–26. [Google Scholar] [CrossRef]
- Westermann, B. Mitochondrial fusion and fission in cell life and death. Nat. Rev. Mol. Cell Biol. 2010, 11, 872–884. [Google Scholar] [CrossRef]
- Ganesan, V.; Willis, S.D.; Chang, K.T.; Beluch, S.; Cooper, K.F.; Strich, R. Cyclin C directly stimulates Drp1 GTP affinity to mediate stress-induced mitochondrial hyperfission. Mol. Biol. Cell 2019, 30, 302–311. [Google Scholar] [CrossRef]
- Ježek, J.; Smethurst, D.G.J.; Stieg, D.C.; Kiss, Z.A.C.; Hanley, S.E.; Ganesan, V.; Chang, K.T.; Cooper, K.F.; Strich, R. Cyclin C: The Story of a Non-Cycling Cyclin. Biology 2019, 8, 3. [Google Scholar] [CrossRef]
- Taguchi, N.; Ishihara, N.; Jofuku, A.; Oka, T.; Mihara, K. Mitotic phosphorylation of dynamin-related GTPase Drp1 participates in mitochondrial fission. J. Biol. Chem. 2007, 282, 11521–11529. [Google Scholar] [CrossRef] [PubMed]
- Liesa, M.; Palacin, M.; Zorzano, A. Mitochondrial dynamics in mammalian health and disease. Physiol. Rev. 2009, 89, 799–845. [Google Scholar] [CrossRef]
- Hoeppner, S.; Baumli, S.; Cramer, P. Structure of the mediator subunit cyclin C and its implications for CDK8 function. J. Mol. Biol. 2005, 350, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Bui, H.T.; Karren, M.A.; Bhar, D.; Shaw, J.M. A novel motif in the yeast mitochondrial dynamin Dnm1 is essential for adaptor binding and membrane recruitment. J. Cell Biol. 2012, 199, 613–622. [Google Scholar] [CrossRef]
- Guo, Q.; Koirala, S.; Perkins, E.M.; McCaffery, J.M.; Shaw, J.M. The mitochondrial fission adaptors Caf4 and Mdv1 are not functionally equivalent. PLoS ONE 2012, 7, e53523. [Google Scholar] [CrossRef] [PubMed]
- Youle, R.J.; Karbowski, M. Mitochondrial fission in apoptosis. Nat. Rev. Mol. Cell Biol. 2005, 6, 657–663. [Google Scholar] [CrossRef]
- Cho, D.H.; Nakamura, T.; Lipton, S.A. Mitochondrial dynamics in cell death and neurodegeneration. Cell. Mol. Life Sci. 2010, 67, 3435–3447. [Google Scholar] [CrossRef]
- Sheridan, C.; Martin, S.J. Mitochondrial fission/fusion dynamics and apoptosis. Mitochondrion 2010, 10, 640–648. [Google Scholar] [CrossRef]
- Frank, S.; Gaume, B.; Bergmann-Leitner, E.S.; Leitner, W.W.; Robert, E.G.; Catez, F.; Smith, C.L.; Youle, R.J. The role of dynamin-related protein 1, a mediator of mitochondrial fission, in apoptosis. Dev. Cell 2001, 1, 515–525. [Google Scholar] [CrossRef]
- Arnoult, D.; Rismanchi, N.; Grodet, A.; Roberts, R.G.; Seeburg, D.P.; Estaquier, J.; Sheng, M.; Blackstone, C. Bax/Bak-dependent release of DDP/TIMM8a promotes Drp1-mediated mitochondrial fission and mitoptosis during programmed cell death. Curr. Biol. 2005, 15, 2112–2118. [Google Scholar] [CrossRef]
- Karbowski, M.; Lee, Y.J.; Gaume, B.; Jeong, S.Y.; Frank, S.; Nechushtan, A.; Santel, A.; Fuller, M.; Smith, C.L.; Youle, R.J. Spatial and temporal association of Bax with mitochondrial fission sites, Drp1, and Mfn2 during apoptosis. J. Cell Biol. 2002, 159, 931–938. [Google Scholar] [CrossRef]
- Sheridan, C.; Delivani, P.; Cullen, S.P.; Martin, S.J. Bax- or Bak-induced mitochondrial fission can be uncoupled from cytochrome C release. Mol. Cell 2008, 31, 570–585. [Google Scholar] [CrossRef] [PubMed]
- Jenner, A.; Peña-Blanco, A.; Salvador-Gallego, R.; Ugarte-Uribe, B.; Zollo, C.; Ganief, T.; Bierlmeier, J.; Mund, M.; Lee, J.E.; Ries, J.; et al. DRP1 interacts directly with BAX to induce its activation and apoptosis. EMBO J. 2022, 41, e108587. [Google Scholar] [CrossRef] [PubMed]
- Qian, S.; Wei, Z.; Yang, W.; Huang, J.; Yang, Y.; Wang, J. The role of BCL-2 family proteins in regulating apoptosis and cancer therapy. Front. Oncol. 2022, 12, 985363. [Google Scholar] [CrossRef]
- Schellenberg, B.; Wang, P.; Keeble, J.A.; Rodriguez-Enriquez, R.; Walker, S.; Owens, T.W.; Foster, F.; Tanianis-Hughes, J.; Brennan, K.; Streuli, C.H.; et al. Bax exists in a dynamic equilibrium between the cytosol and mitochondria to control apoptotic priming. Mol. Cell 2013, 49, 959–971. [Google Scholar] [CrossRef] [PubMed]
- Subburaj, Y.; Cosentino, K.; Axmann, M.; Pedrueza-Villalmanzo, E.; Hermann, E.; Bleicken, S.; Spatz, J.; García-Sáez, A.J. Bax monomers form dimer units in the membrane that further self-assemble into multiple oligomeric species. Nat. Commun. 2015, 6, 8042. [Google Scholar] [CrossRef]
- Pena-Blanco, A.; Garcia-Saez, A.J. Bax, Bak and beyond—Mitochondrial performance in apoptosis. FEBS J. 2018, 285, 416–431. [Google Scholar] [CrossRef]
- Manon, S.; Chaudhuri, B.; Guerin, M. Release of cytochrome c and decrease of cytochrome c oxidase in Bax-expressing yeast cells, and prevention of these effects by coexpression of Bcl-xL. FEBS Lett. 1997, 415, 29–32. [Google Scholar] [CrossRef]
- McArthur, K.; Whitehead, L.W.; Heddleston, J.M.; Li, L.; Padman, B.S.; Oorschot, V.; Geoghegan, N.D.; Chappaz, S.; Davidson, S.; San Chin, H.; et al. BAK/BAX macropores facilitate mitochondrial herniation and mtDNA efflux during apoptosis. Science 2018, 359, aao6047. [Google Scholar] [CrossRef]
- Zha, H.; Fisk, H.A.; Yaffe, M.P.; Mahajan, N.; Herman, B.; Reed, J.C. Structure-function comparisons of the proapoptotic protein Bax in yeast and mammalian cells. Mol. Cell. Biol. 1996, 16, 6494–6508. [Google Scholar] [CrossRef]
- Polcic, P.; Jaka, P.; Mentel, M. Yeast as a tool for studying proteins of the Bcl-2 family. Microb. Cell 2015, 2, 74–87. [Google Scholar] [CrossRef] [PubMed]
- Lhuissier, C.; Desquiret-Dumas, V.; Girona, A.; Alban, J.; Faure, J.; Cassereau, J.; Codron, P.; Lenaers, G.; Baris, O.R.; Gueguen, N.; et al. Mitochondrial F0F1-ATP synthase governs the induction of mitochondrial fission. iScience 2024, 27, 109808. [Google Scholar] [CrossRef]
- Matsuyama, S.; Xu, Q.; Velours, J.; Reed, J.C. The Mitochondrial F0F1-ATPase Proton Pump Is Required for Function of the Proapoptotic Protein Bax in Yeast and Mammalian Cells. Mol. Cell 1998, 1, 327–336. [Google Scholar] [CrossRef]
- Sato, T.; Hanada, M.; Bodrug, S.; Irie, S.; Iwama, N.; Boise, L.H.; Thompson, C.B.; Golemis, E.; Fong, L.; Wang, H.G.; et al. Interactions among members of the Bcl-2 protein family analyzed with a yeast two-hybrid system. Proc. Natl. Acad. Sci. USA 1994, 91, 9238–9242. [Google Scholar] [CrossRef] [PubMed]
- Légiot, A.; Céré, C.; Dupoiron, T.; Kaabouni, M.; Camougrand, N.; Manon, S. Mitochondria-Associated Membranes (MAMs) are involved in Bax mitochondrial localization and cytochrome c release. Microb. Cell 2019, 6, 257–266. [Google Scholar] [CrossRef]
- Toth, A.; Aufschnaiter, A.; Fedotovskaya, O.; Dawitz, H.; Ädelroth, P.; Büttner, S.; Ott, M. Membrane-tethering of cytochrome c accelerates regulated cell death in yeast. Cell Death Dis. 2020, 11, 722. [Google Scholar] [CrossRef]
- Sun, G. Death and survival from executioner caspase activation. Semin. Cell Dev. Biol. 2024, 156, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Madeo, F.; Herker, E.; Maldener, C.; Wissing, S.; Lachelt, S.; Herlan, M.; Fehr, M.; Lauber, K.; Sigrist, S.J.; Wesselborg, S.; et al. A caspase-related protease regulates apoptosis in yeast. Mol. Cell 2002, 9, 911–917. [Google Scholar] [CrossRef]
- Shrestha, A.; Brunette, S.; Stanford, W.L.; Megeney, L.A. The metacaspase Yca1 maintains proteostasis through multiple interactions with the ubiquitin system. Cell Discov. 2019, 5, 6. [Google Scholar] [CrossRef]
- Lee, R.E.; Brunette, S.; Puente, L.G.; Megeney, L.A. Metacaspase Yca1 is required for clearance of insoluble protein aggregates. Proc. Natl. Acad. Sci. USA 2010, 107, 13348–13353. [Google Scholar] [CrossRef] [PubMed]
- Lam, D.K.; Sherlock, G. Yca1 metacaspase: Diverse functions determine how yeast live and let die. FEMS Yeast Res. 2023, 23, foad022. [Google Scholar] [CrossRef] [PubMed]
- Camougrand, N.; Grelaud-Coq, A.; Marza, E.; Priault, M.; Bessoule, J.J.; Manon, S. The product of the UTH1 gene, required for Bax-induced cell death in yeast, is involved in the response to rapamycin. Mol. Microbiol. 2003, 47, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Ritch, J.J.; Davidson, S.M.; Sheehan, J.J.; Austriaco, N. The Saccharomyces SUN gene, UTH1, is involved in cell wall biogenesis. FEMS Yeast Res. 2010, 10, 168–176. [Google Scholar] [CrossRef]
- Eid, R.; Boucher, E.; Gharib, N.; Khoury, C.; Arab, N.T.; Murray, A.; Young, P.G.; Mandato, C.A.; Greenwood, M.T. Identification of human ferritin, heavy polypeptide 1 (FTH1) and yeast RGI1 (YER067W) as pro-survival sequences that counteract the effects of Bax and copper in Saccharomyces cerevisiae. Exp. Cell Res. 2016, 342, 52–61. [Google Scholar] [CrossRef]
- Carmona-Gutierrez, D.; Bauer, M.A.; Zimmermann, A.; Aguilera, A.; Austriaco, N.; Ayscough, K.; Balzan, R.; Bar-Nun, S.; Barrientos, A.; Belenky, P.; et al. Guidelines and recommendations on yeast cell death nomenclature. Microb. Cell 2018, 5, 4–31. [Google Scholar] [CrossRef]
- Strich, R.; Cooper, K.F. The dual role of cyclin C connects stress regulated gene expression to mitochondrial dynamics. Microb. Cell 2014, 1, 318–324. [Google Scholar] [CrossRef]
- Moll, U.M.; Wolff, S.; Speidel, D.; Deppert, W. Transcription-independent pro-apoptotic functions of p53. Curr. Opin. Cell Biol. 2005, 17, 631–636. [Google Scholar] [CrossRef]
- Karbowski, M.; Norris, K.L.; Cleland, M.M.; Jeong, S.Y.; Youle, R.J. Role of Bax and Bak in mitochondrial morphogenesis. Nature 2006, 443, 658–662. [Google Scholar] [CrossRef]
- Wilkinson, D.; Ramsdale, M. Proteases and caspase-like activity in the yeast Saccharomyces cerevisiae. Biochem. Soc. Trans. 2011, 39, 1502–1508. [Google Scholar] [CrossRef]
- Mentel, M.; Illová, M.; Krajčovičová, V.; Kroupová, G.; Mannová, Z.; Chovančíková, P.; Polčic, P. Yeast Bax Inhibitor (Bxi1p/Ybh3p) Is Not Required for the Action of Bcl-2 Family Proteins on Cell Viability. Int. J. Mol. Sci. 2023, 24, 12011. [Google Scholar] [CrossRef]
- Buchan, J.R. Stress granule and P-body clearance: Seeking coherence in acts of disappearance. Semin. Cell Dev. Biol. 2024, 159–160, 10–26. [Google Scholar] [CrossRef]
- Luo, Y.; Na, Z.; Slavoff, S.A. P-Bodies: Composition, Properties, and Functions. Biochemistry 2018, 57, 2424–2431. [Google Scholar] [CrossRef]
- Nostramo, R.; Xing, S.; Zhang, B.; Herman, P.K. Insights into the Role of P-Bodies and Stress Granules in Protein Quality Control. Genetics 2019, 213, 251–265. [Google Scholar] [CrossRef]
- Ramachandran, V.; Shah, K.H.; Herman, P.K. The cAMP-dependent protein kinase signaling pathway is a key regulator of P body foci formation. Mol. Cell 2011, 43, 973–981. [Google Scholar] [CrossRef] [PubMed]
- Anderson, P.; Kedersha, N. RNA granules: Post-transcriptional and epigenetic modulators of gene expression. Nat. Rev. Mol. Cell Biol. 2009, 10, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Brangwynne, C.P. Phase transitions and size scaling of membrane-less organelles. J. Cell Biol. 2013, 203, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Sheth, U.; Parker, R. Decapping and decay of messenger RNA occur in cytoplasmic processing bodies. Science 2003, 300, 805–808. [Google Scholar] [CrossRef]
- Wang, C.; Schmich, F.; Srivatsa, S.; Weidner, J.; Beerenwinkel, N.; Spang, A. Context-dependent deposition and regulation of mRNAs in P-bodies. eLife 2018, 7, e29815. [Google Scholar] [CrossRef]
- Standart, N.; Weil, D. P-Bodies: Cytosolic Droplets for Coordinated mRNA Storage. Trends Genet. 2018, 34, 612–626. [Google Scholar] [CrossRef]
- Horvathova, I.; Voigt, F.; Kotrys, A.V.; Zhan, Y.; Artus-Revel, C.G.; Eglinger, J.; Stadler, M.B.; Giorgetti, L.; Chao, J.A. The Dynamics of mRNA Turnover Revealed by Single-Molecule Imaging in Single Cells. Mol. Cell 2017, 68, 615–625 e619. [Google Scholar] [CrossRef] [PubMed]
- Hubstenberger, A.; Courel, M.; Bénard, M.; Souquere, S.; Ernoult-Lange, M.; Chouaib, R.; Yi, Z.; Morlot, J.B.; Munier, A.; Fradet, M.; et al. P-Body Purification Reveals the Condensation of Repressed mRNA Regulons. Mol. Cell 2017, 68, 144–157.e145. [Google Scholar] [CrossRef]
- Kroschwald, S.; Maharana, S.; Mateju, D.; Malinovska, L.; Nüske, E.; Poser, I.; Richter, D.; Alberti, S. Promiscuous interactions and protein disaggregases determine the material state of stress-inducible RNP granules. eLife 2015, 4, e06807. [Google Scholar] [CrossRef]
- Fromm, S.A.; Truffault, V.; Kamenz, J.; Braun, J.E.; Hoffmann, N.A.; Izaurralde, E.; Sprangers, R. The structural basis of Edc3- and Scd6-mediated activation of the Dcp1:Dcp2 mRNA decapping complex. EMBO J. 2012, 31, 279–290. [Google Scholar] [CrossRef]
- Fromm, S.A.; Kamenz, J.; Noldeke, E.R.; Neu, A.; Zocher, G.; Sprangers, R. In vitro reconstitution of a cellular phase-transition process that involves the mRNA decapping machinery. Angew. Chem. Int. Ed. Engl. 2014, 53, 7354–7359. [Google Scholar] [CrossRef] [PubMed]
- Sharif, H.; Ozgur, S.; Sharma, K.; Basquin, C.; Urlaub, H.; Conti, E. Structural analysis of the yeast Dhh1–Pat1 complex reveals how Dhh1 engages Pat1, Edc3 and RNA in mutually exclusive interactions. Nucleic Acids Res. 2013, 41, 8377–8390. [Google Scholar] [CrossRef]
- Schütz, S.; Nöldeke, E.R.; Sprangers, R. A synergistic network of interactions promotes the formation of in vitro processing bodies and protects mRNA against decapping. Nucleic Acids Res. 2017, 45, 6911–6922. [Google Scholar] [CrossRef] [PubMed]
- Decker, C.J.; Teixeira, D.; Parker, R. Edc3p and a glutamine/asparagine-rich domain of Lsm4p function in processing body assembly in Saccharomyces cerevisiae. J. Cell Biol. 2007, 179, 437–449. [Google Scholar] [CrossRef]
- Uversky, V.N.; Kuznetsova, I.M.; Turoverov, K.K.; Zaslavsky, B. Intrinsically disordered proteins as crucial constituents of cellular aqueous two phase systems and coacervates. FEBS Lett. 2015, 589, 15–22. [Google Scholar] [CrossRef]
- Galea, C.A.; Wang, Y.; Sivakolundu, S.G.; Kriwacki, R.W. Regulation of cell division by intrinsically unstructured proteins: Intrinsic flexibility, modularity, and signaling conduits. Biochemistry 2008, 47, 7598–7609. [Google Scholar] [CrossRef]
- Wang, J.T.; Smith, J.; Chen, B.C.; Schmidt, H.; Rasoloson, D.; Paix, A.; Lambrus, B.G.; Calidas, D.; Betzig, E.; Seydoux, G. Regulation of RNA granule dynamics by phosphorylation of serine-rich, intrinsically disordered proteins in C. elegans. eLife 2014, 3, e04591. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Hess, D.; Eglinger, J.; Fritsch, A.W.; Kreysing, M.; Weinert, B.T.; Choudhary, C.; Matthias, P. Acetylation of intrinsically disordered regions regulates phase separation. Nat. Chem. Biol. 2019, 15, 51–61. [Google Scholar] [CrossRef]
- Galluzzi, L.; Baehrecke, E.H.; Ballabio, A.; Boya, P.; Bravo-San Pedro, J.M.; Cecconi, F.; Choi, A.M.; Chu, C.T.; Codogno, P.; Colombo, M.I.; et al. Molecular definitions of autophagy and related processes. EMBO J. 2017, 36, 1811–1836. [Google Scholar] [CrossRef]
- Klionsky, D.J.; Petroni, G.; Amaravadi, R.K.; Baehrecke, E.H.; Ballabio, A.; Boya, P.; Bravo-San Pedro, J.M.; Cadwell, K.; Cecconi, F.; Choi, A.M.K.; et al. Autophagy in major human diseases. EMBO J. 2021, 40, e108863. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, R.E.; Zoncu, R. The lysosome as a cellular centre for signalling, metabolism and quality control. Nat. Cell Biol. 2019, 21, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Dikic, I.; Elazar, Z. Mechanism and medical implications of mammalian autophagy. Nat. Rev. Mol. Cell Biol. 2018, 19, 349–364. [Google Scholar] [CrossRef]
- Ichimiya, T.; Yamakawa, T.; Hirano, T.; Yokoyama, Y.; Hayashi, Y.; Hirayama, D.; Wagatsuma, K.; Itoi, T.; Nakase, H. Autophagy and Autophagy-Related Diseases: A Review. Int. J. Mol. Sci. 2020, 21, 8974. [Google Scholar] [CrossRef]
- Yin, Z.; Pascual, C.; Klionsky, D.J. Autophagy: Machinery and regulation. Microb. Cell 2016, 3, 588–596. [Google Scholar] [CrossRef]
- Wen, X.; Klionsky, D.J. An overview of macroautophagy in yeast. J. Mol. Biol. 2016, 428, 1681–1699. [Google Scholar] [CrossRef]
- Jafari, M.; McCabe, M.; Cuervo, A.M. Chaperone-mediated autophagy: Mechanisms and physiological relevance. Curr. Opin. Physiol. 2022, 30, 100597. [Google Scholar] [CrossRef]
- Vargas, J.N.S.; Hamasaki, M.; Kawabata, T.; Youle, R.J.; Yoshimori, T. The mechanisms and roles of selective autophagy in mammals. Nat. Rev. Mol. Cell Biol. 2023, 24, 167–185. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Klionsky, D.J.; Shen, H.-M. The emerging mechanisms and functions of microautophagy. Nat. Rev. Mol. Cell Biol. 2023, 24, 186–203. [Google Scholar] [CrossRef]
- Kaushik, S.; Cuervo, A.M. The coming of age of chaperone-mediated autophagy. Nat. Rev. Mol. Cell Biol. 2018, 19, 365–381. [Google Scholar] [CrossRef]
- Levine, B.; Kroemer, G. Biological Functions of Autophagy Genes: A Disease Perspective. Cell 2019, 176, 11–42. [Google Scholar] [CrossRef] [PubMed]
- Fleming, A.; Bourdenx, M.; Fujimaki, M.; Karabiyik, C.; Krause, G.J.; Lopez, A.; Martín-Segura, A.; Puri, C.; Scrivo, A.; Skidmore, J.; et al. The different autophagy degradation pathways and neurodegeneration. Neuron 2022, 110, 935–966. [Google Scholar] [CrossRef] [PubMed]
- Mulcahy Levy, J.M.; Thorburn, A. Autophagy in cancer: Moving from understanding mechanism to improving therapy responses in patients. Cell Death Differ. 2020, 27, 843–857. [Google Scholar] [CrossRef]
- Isoda, T.; Takeda, E.; Hosokawa, S.; Hotta-Ren, S.; Ohsumi, Y. Atg45 is an autophagy receptor for glycogen, a non-preferred cargo of bulk autophagy in yeast. iScience 2024, 27, 109810. [Google Scholar] [CrossRef]
- Takeda, E.; Isoda, T.; Hosokawa, S.; Oikawa, Y.; Hotta-Ren, S.; May, A.I.; Ohsumi, Y. Receptor-mediated cargo hitchhiking on bulk autophagy. EMBO J. 2024, 43, 3116–3140. [Google Scholar] [CrossRef] [PubMed]
- Hanley, S.E.; Cooper, K.F. Sorting Nexins in Protein Homeostasis. Cells 2020, 10, 17. [Google Scholar] [CrossRef]
- Vazquez-Sanchez, S.; Gonzalez-Lozano, M.A.; Walfenzao, A.; Li, K.W.; van Weering, J.R.T. The endosomal protein sorting nexin 4 is a synaptic protein. Sci. Rep. 2020, 10, 18239. [Google Scholar] [CrossRef]
- Poppinga, J.; Barret, N.J.; Cornelisse, L.N.; Verhage, M.; van Weering, J.R.T. Endosomal sorting protein SNX4 limits synaptic vesicle docking and release. eLife 2024, 13, RP97910. [Google Scholar] [CrossRef]
- Kim, N.Y.; Cho, M.H.; Won, S.H.; Kang, H.J.; Yoon, S.Y.; Kim, D.H. Sorting nexin-4 regulates β-amyloid production by modulating β-site-activating cleavage enzyme-1. Alzheimers Res. Ther. 2017, 9, 4. [Google Scholar] [CrossRef]
- Cullen, P.J.; Steinberg, F. To degrade or not to degrade: Mechanisms and significance of endocytic recycling. Nat. Rev. Mol. Cell Biol. 2018, 19, 679–696. [Google Scholar] [CrossRef] [PubMed]
- Kotani, T.; Sakai, Y.; Kirisako, H.; Kakuta, C.; Kakuta, S.; Ohsumi, Y.; Nakatogawa, H. A mechanism that ensures non-selective cytoplasm degradation by autophagy. Nat. Commun. 2023, 14, 5815. [Google Scholar] [CrossRef]
- Nemec, A.A.; Howell, L.A.; Peterson, A.K.; Murray, M.A.; Tomko, R.J., Jr. Autophagic clearance of proteasomes in yeast requires the conserved sorting nexin Snx4. J. Biol. Chem. 2017, 292, 21466–21480. [Google Scholar] [CrossRef]
- Makino, S.; Kawamata, T.; Iwasaki, S.; Ohsumi, Y. Selectivity of mRNA degradation by autophagy in yeast. Nat. Commun. 2021, 12, 2316. [Google Scholar] [CrossRef]
- Shpilka, T.; Welter, E.; Borovsky, N.; Amar, N.; Shimron, F.; Peleg, Y.; Elazar, Z. Fatty acid synthase is preferentially degraded by autophagy upon nitrogen starvation in yeast. Proc. Natl. Acad. Sci. USA 2015, 112, 1434–1439. [Google Scholar] [CrossRef]
- Liu, X.; Mao, K.; Yu, A.Y.H.; Omairi-Nasser, A.; Austin, J., 2nd; Glick, B.S.; Yip, C.K.; Klionsky, D.J. The Atg17-Atg31-Atg29 Complex Coordinates with Atg11 to Recruit the Vam7 SNARE and Mediate Autophagosome-Vacuole Fusion. Curr. Biol. 2016, 26, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Noda, N.N. Biophysical characterization of Atg11, a scaffold protein essential for selective autophagy in yeast. FEBS Open Bio 2018, 8, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Lamark, T.; Johansen, T. Mechanisms of Selective Autophagy. Annu. Rev. Cell. Dev. Biol. 2021, 37, 143–169. [Google Scholar] [CrossRef]
- Fujioka, Y.; Alam, J.M.; Noshiro, D.; Mouri, K.; Ando, T.; Okada, Y.; May, A.I.; Knorr, R.L.; Suzuki, K.; Ohsumi, Y.; et al. Phase separation organizes the site of autophagosome formation. Nature 2020, 578, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Noda, N.N.; Wang, Z.; Zhang, H. Liquid–liquid phase separation in autophagy. J. Cell Biol. 2020, 219, e202004062. [Google Scholar] [CrossRef]
- Yamamoto, H.; Fujioka, Y.; Suzuki, S.W.; Noshiro, D.; Suzuki, H.; Kondo-Kakuta, C.; Kimura, Y.; Hirano, H.; Ando, T.; Noda, N.N.; et al. The Intrinsically Disordered Protein Atg13 Mediates Supramolecular Assembly of Autophagy Initiation Complexes. Dev. Cell 2016, 38, 86–99. [Google Scholar] [CrossRef] [PubMed]
- Rogov, V.V.; Nezis, I.P.; Tsapras, P.; Zhang, H.; Dagdas, Y.; Noda, N.N.; Nakatogawa, H.; Wirth, M.; Mouilleron, S.; McEwan, D.G.; et al. Atg8 family proteins, LIR/AIM motifs and other interaction modes. Autophagy Rep. 2023, 2, 2188523. [Google Scholar] [CrossRef]
- Laxman, S.; Tu, B.P. Multiple TORC1-associated proteins regulate nitrogen starvation-dependent cellular differentiation in Saccharomyces cerevisiae. PLoS ONE 2011, 6, e26081. [Google Scholar] [CrossRef] [PubMed]
- Umekawa, M.; Klionsky, D.J. Ksp1 kinase regulates autophagy via the target of rapamycin complex 1 (TORC1) pathway. J. Biol. Chem. 2012, 287, 16300–16310. [Google Scholar] [CrossRef] [PubMed]
- Huber, A.; Bodenmiller, B.; Uotila, A.; Stahl, M.; Wanka, S.; Gerrits, B.; Aebersold, R.; Loewith, R. Characterization of the rapamycin-sensitive phosphoproteome reveals that Sch9 is a central coordinator of protein synthesis. Genes Dev. 2009, 23, 1929–1943. [Google Scholar] [CrossRef]
- Oliveira, A.P.; Ludwig, C.; Zampieri, M.; Weisser, H.; Aebersold, R.; Sauer, U. Dynamic phosphoproteomics reveals TORC1-dependent regulation of yeast nucleotide and amino acid biosynthesis. Sci. Signal 2015, 8, rs4. [Google Scholar] [CrossRef] [PubMed]
- Soulard, A.; Cremonesi, A.; Moes, S.; Schütz, F.; Jenö, P.; Hall, M.N. The rapamycin-sensitive phosphoproteome reveals that TOR controls protein kinase A toward some but not all substrates. Mol. Biol. Cell 2010, 21, 3475–3486. [Google Scholar] [CrossRef] [PubMed]
- Shamas-Din, A.; Kale, J.; Leber, B.; Andrews, D.W. Mechanisms of action of Bcl-2 family proteins. Cold Spring Harb. Perspect. Biol. 2013, 5, a008714. [Google Scholar] [CrossRef]
- Gross, A.; Katz, S.G. Non-apoptotic functions of BCL-2 family proteins. Cell Death Differ. 2017, 24, 1348–1358. [Google Scholar] [CrossRef] [PubMed]
- Buchan, J.R.; Muhlrad, D.; Parker, R. P bodies promote stress granule assembly in Saccharomyces cerevisiae. J. Cell Biol. 2008, 183, 441–455. [Google Scholar] [CrossRef]
- Mitchell, S.F.; Jain, S.; She, M.; Parker, R. Global analysis of yeast mRNPs. Nat. Struct. Mol. Biol. 2013, 20, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Mason, A.C.; Wente, S.R. Functions of Gle1 are governed by two distinct modes of self-association. J. Biol. Chem. 2020, 295, 16813–16825. [Google Scholar] [CrossRef] [PubMed]
- Demetrick, D.J.; Matsumoto, S.; Hannon, G.J.; Okamoto, K.; Xiong, Y.; Zhang, H.; Beach, D.H. Chromosomal mapping of the genes for the human cell cycle proteins cyclin C (CCNC), cyclin E (CCNE), p21 (CDKN1) and KAP (CDKN3). Cytogenet. Cell Genet. 1995, 69, 190–192. [Google Scholar] [CrossRef]
- Offit, K.; Parsa, N.Z.; Gaidano, G.; Filippa, D.A.; Louie, D.; Pan, D.; Jhanwar, S.C.; Dalla-Favera, R.; Chaganti, R.S.K. 6q deletions define distinct clinico-pathologic subsets of non-Hodgkin’s lympoma. Blood 1993, 82, 2157–2162. [Google Scholar] [CrossRef]
- Ohata, N.; Ito, S.; Yoshida, A.; Kunisada, T.; Numoto, K.; Jitsumori, Y.; Kanzaki, H.; Ozaki, T.; Shimizu, K.; Ouchida, M. Highly frequent allelic loss of chromosome 6q16-23 in osteosarcoma: Involvement of cyclin C in osteosarcoma. Int. J. Mol. Med. 2006, 18, 1153–1158. [Google Scholar] [CrossRef]
- Clark, A.D.; Oldenbroek, M.; Boyer, T.G. Mediator kinase module and human tumorigenesis. Crit. Rev. Biochem. Mol. Biol. 2015, 50, 393–426. [Google Scholar] [CrossRef]
- Li, H.; Lahti, J.M.; Valentine, M.; Saito, M.; Reed, S.I.; Look, A.T.; Kidd, V.J. Molecular cloning and chromosomal localization of the human cyclin C (CCNC) and cyclin E (CCNE) genes: Deletion of the CCNC gene in human tumors. Genomics 1996, 32, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Fassl, A.; Chick, J.; Inuzuka, H.; Li, X.; Mansour, M.R.; Liu, L.; Wang, H.; King, B.; Shaik, S.; et al. Cyclin C is a haploinsufficient tumour suppressor. Nat. Cell Biol. 2014, 16, 1080–1091. [Google Scholar] [CrossRef]
- Ellisen, L.W.; Bird, J.; West, D.C.; Soreng, A.L.; Reynolds, T.C.; Smith, S.D.; Sklar, J. TAN-1, the human homolog of the Drosophila Notch gene, is broken by chromosomal translocations in T lymphoblastic neoplasms. Cell 1991, 66, 649–661. [Google Scholar] [CrossRef]
- Schroeter, E.H.; Kisslinger, J.A.; Kopan, R. Notch-1 signalling requires ligand-induced proteolytic release of intracellular domain. Nature 1998, 393, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Kopan, R.; Ilagan, M.X. The canonical Notch signaling pathway: Unfolding the activation mechanism. Cell 2009, 137, 216–233. [Google Scholar] [CrossRef]
- Andersson, E.R.; Lendahl, U. Therapeutic modulation of Notch signalling—Are we there yet? Nat. Rev. Drug Discov. 2014, 13, 357–378. [Google Scholar] [CrossRef] [PubMed]
- Gharaibeh, L.; Elmadany, N.; Alwosaibai, K.; Alshaer, W. Notch1 in Cancer Therapy: Possible Clinical Implications and Challenges. Mol. Pharmacol. 2020, 98, 559–576. [Google Scholar] [CrossRef] [PubMed]
- Koch, U.; Radtke, F. Notch signaling in solid tumors. Curr. Top. Dev. Biol. 2010, 92, 411–455. [Google Scholar] [CrossRef]
- Hyytinen, E.R.; Saadut, R.; Chen, C.; Paull, L.; Koivisto, P.A.; Vessella, R.L.; Frierson, H.F., Jr.; Dong, J.T. Defining the region(s) of deletion at 6q16-q22 in human prostate cancer. Genes. Chromosomes Cancer 2002, 34, 306–312. [Google Scholar] [CrossRef]
- Jackson, A.; Carrara, P.; Duke, V.; Sinclair, P.; Papaioannou, M.; Harrison, C.J.; Foroni, L. Deletion of 6q16-q21 in human lymphoid malignancies: A mapping and deletion analysis. Cancer Res. 2000, 60, 2775–2779. [Google Scholar]
- Sinclair, P.B.; Sorour, A.; Martineau, M.; Harrison, C.J.; Mitchell, W.A.; O’Neill, E.; Foroni, L. A fluorescence in situ hybridization map of 6q deletions in acute lymphocytic leukemia: Identification and analysis of a candidate tumor suppressor gene. Cancer Res. 2004, 64, 4089–4098. [Google Scholar] [CrossRef]
- Lobry, C.; Oh, P.; Mansour, M.R.; Look, A.T.; Aifantis, I. Notch signaling: Switching an oncogene to a tumor suppressor. Blood 2014, 123, 2451–2459. [Google Scholar] [CrossRef]
- Parmigiani, E.; Taylor, V.; Giachino, C. Oncogenic and Tumor-Suppressive Functions of NOTCH Signaling in Glioma. Cells 2020, 9, 2304. [Google Scholar] [CrossRef] [PubMed]
- Choschzick, M.; Stergiou, C.; Gut, A.; Zoche, M.; Ross, J.S.; Moch, H. NOTCH1 and PIK3CA mutation are related to HPV-associated vulvar squamous cell carcinoma. Pathol. Res. Pract. 2023, 251, 154877. [Google Scholar] [CrossRef]
- Jezek, J.; Wang, K.; Yan, R.; Di Cristofano, A.; Cooper, K.F.; Strich, R. Synergistic repression of thyroid hyperplasia by cyclin C and Pten. J. Cell Sci. 2019, 132, jcs230029. [Google Scholar] [CrossRef]
- Wreesmann, V.B.; Ghossein, R.A.; Patel, S.G.; Harris, C.P.; Schnaser, E.A.; Shaha, A.R.; Tuttle, R.M.; Shah, J.P.; Rao, P.H.; Singh, B. Genome-wide appraisal of thyroid cancer progression. Am. J. Pathol. 2002, 161, 1549–1556. [Google Scholar] [CrossRef]
- Antico Arciuch, V.G.; Russo, M.A.; Dima, M.; Kang, K.S.; Dasrath, F.; Liao, X.H.; Refetoff, S.; Montagna, C.; Di Cristofano, A. Thyrocyte-specific inactivation of p53 and Pten results in anaplastic thyroid carcinomas faithfully recapitulating human tumors. Oncotarget 2011, 2, 1109–1126. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yen, C.; Liaw, D.; Podsypanina, K.; Bose, S.; Wang, S.I.; Puc, J.; Miliaresis, C.; Rodgers, L.; McCombie, R.; et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science 1997, 275, 1943–1947. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; li, J.; Fu, M.; Zhao, X.; Wang, W. The JAK/STAT signaling pathway: From bench to clinic. Signal Transduct. Target. Ther. 2021, 6, 402. [Google Scholar] [CrossRef]
- Firestein, R.; Bass, A.J.; Kim, S.Y.; Dunn, I.F.; Silver, S.J.; Guney, I.; Freed, E.; Ligon, A.H.; Vena, N.; Ogino, S.; et al. CDK8 is a colorectal cancer oncogene that regulates beta-catenin activity. Nature 2008, 455, 547–551. [Google Scholar] [CrossRef]
- Ho, T.-Y.; Sung, T.-Y.; Pan, S.-L.; Huang, W.-J.; Hsu, K.-C.; Hsu, J.-Y.; Lin, T.E.; Hsu, C.-M.; Yang, C.-R. The study of a novel CDK8 inhibitor E966-0530–45418 that inhibits prostate cancer metastasis in vitro and in vivo. Biomed. Pharmacother. 2023, 162, 114667. [Google Scholar] [CrossRef]
- Roninson, I.B.; Győrffy, B.; Mack, Z.T.; Shtil, A.A.; Shtutman, M.S.; Chen, M.; Broude, E.V. Identifying Cancers Impacted by CDK8/19. Cells 2019, 8, 821. [Google Scholar] [CrossRef]
- Porter, D.C.; Farmaki, E.; Altilia, S.; Schools, G.P.; West, D.K.; Chen, M.; Chang, B.D.; Puzyrev, A.T.; Lim, C.U.; Rokow-Kittell, R.; et al. Cyclin-dependent kinase 8 mediates chemotherapy-induced tumor-promoting paracrine activities. Proc. Natl. Acad. Sci. USA 2012, 109, 13799–13804. [Google Scholar] [CrossRef] [PubMed]
- Brägelmann, J.; Klümper, N.; Offermann, A.; von Mässenhausen, A.; Böhm, D.; Deng, M.; Queisser, A.; Sanders, C.; Syring, I.; Merseburger, A.S.; et al. Pan-Cancer Analysis of the Mediator Complex Transcriptome Identifies CDK19 and CDK8 as Therapeutic Targets in Advanced Prostate Cancer. Clin. Cancer Res. 2017, 23, 1829–1840. [Google Scholar] [CrossRef]
- Menzl, I.; Witalisz-Siepracka, A.; Sexl, V. CDK8-Novel Therapeutic Opportunities. Pharmaceuticals 2019, 12, 92. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, A.; Goldberg, M.S.; Cumberland, L.K.; Ratnakumar, K.; Segura, M.F.; Emanuel, P.O.; Menendez, S.; Vardabasso, C.; Leroy, G.; Vidal, C.I.; et al. The histone variant macroH2A suppresses melanoma progression through regulation of CDK8. Nature 2010, 468, 1105–1109. [Google Scholar] [CrossRef]
- Datta, N.; Chakraborty, S.; Basu, M.; Ghosh, M.K. Tumor Suppressors Having Oncogenic Functions: The Double Agents. Cells 2020, 10, 46. [Google Scholar] [CrossRef] [PubMed]
- Grabher, C.; von Boehmer, H.; Look, A.T. Notch 1 activation in the molecular pathogenesis of T-cell acute lymphoblastic leukaemia. Nat. Rev. Cancer 2006, 6, 347–359. [Google Scholar] [CrossRef]
- Dotto, G.P. Crosstalk of Notch with p53 and p63 in cancer growth control. Nat. Rev. Cancer 2009, 9, 587–595. [Google Scholar] [CrossRef]
- Shen, L.; Shi, Q.; Wang, W. Double agents: Genes with both oncogenic and tumor-suppressor functions. Oncogenesis 2018, 7, 25. [Google Scholar] [CrossRef]
- Ayala, Y.M.; Pantano, S.; D’Ambrogio, A.; Buratti, E.; Brindisi, A.; Marchetti, C.; Romano, M.; Baralle, F.E. Human, Drosophila, and C.elegans TDP43: Nucleic acid binding properties and splicing regulatory function. J. Mol. Biol. 2005, 348, 575–588. [Google Scholar] [CrossRef]
- Kuo, P.H.; Doudeva, L.G.; Wang, Y.T.; Shen, C.K.; Yuan, H.S. Structural insights into TDP-43 in nucleic-acid binding and domain interactions. Nucleic Acids Res. 2009, 37, 1799–1808. [Google Scholar] [CrossRef]
- Ayala, Y.M.; Zago, P.; D’Ambrogio, A.; Xu, Y.F.; Petrucelli, L.; Buratti, E.; Baralle, F.E. Structural determinants of the cellular localization and shuttling of TDP-43. J. Cell Sci. 2008, 121, 3778–3785. [Google Scholar] [CrossRef]
- Sreedharan, J.; Blair, I.P.; Tripathi, V.B.; Hu, X.; Vance, C.; Rogelj, B.; Ackerley, S.; Durnall, J.C.; Williams, K.L.; Buratti, E.; et al. TDP-43 mutations in familial and sporadic amyotrophic lateral sclerosis. Science 2008, 319, 1668–1672. [Google Scholar] [CrossRef]
- Birsa, N.; Bentham, M.P.; Fratta, P. Cytoplasmic functions of TDP-43 and FUS and their role in ALS. Semin. Cell Dev. Biol. 2020, 99, 193–201. [Google Scholar] [CrossRef]
- Gottschalk, W.K.; Lutz, M.W.; He, Y.T.; Saunders, A.M.; Burns, D.K.; Roses, A.D.; Chiba-Falek, O. The Broad Impact of TOM40 on Neurodegenerative Diseases in Aging. J. Park. Dis. Alzheimers Dis. 2014, 1, 12. [Google Scholar] [CrossRef]
- Izumikawa, K.; Nobe, Y.; Yoshikawa, H.; Ishikawa, H.; Miura, Y.; Nakayama, H.; Nonaka, T.; Hasegawa, M.; Egawa, N.; Inoue, H.; et al. TDP-43 stabilises the processing intermediates of mitochondrial transcripts. Sci. Rep. 2017, 7, 7709. [Google Scholar] [CrossRef]
- Ayala, Y.M.; De Conti, L.; Avendaño-Vázquez, S.E.; Dhir, A.; Romano, M.; D’Ambrogio, A.; Tollervey, J.; Ule, J.; Baralle, M.; Buratti, E.; et al. TDP-43 regulates its mRNA levels through a negative feedback loop. EMBO J. 2011, 30, 277–288. [Google Scholar] [CrossRef]
- Wang, P.; Deng, J.; Dong, J.; Liu, J.; Bigio, E.H.; Mesulam, M.; Wang, T.; Sun, L.; Wang, L.; Lee, A.Y.; et al. TDP-43 induces mitochondrial damage and activates the mitochondrial unfolded protein response. PLoS Genet. 2019, 15, e1007947. [Google Scholar] [CrossRef] [PubMed]
- Lucini, C.B.; Braun, R.J. Mitochondrion-Dependent Cell Death in TDP-43 Proteinopathies. Biomedicines 2021, 9, 376. [Google Scholar] [CrossRef] [PubMed]
- Hardiman, O.; Al-Chalabi, A.; Chio, A.; Corr, E.M.; Logroscino, G.; Robberecht, W.; Shaw, P.J.; Simmons, Z.; van den Berg, L.H. Amyotrophic lateral sclerosis. Nat. Rev. Dis. Primers 2017, 3, 17071. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.; Bharathi, V.; Sivalingam, V.; Girdhar, A.; Patel, B.K. Molecular Mechanisms of TDP-43 Misfolding and Pathology in Amyotrophic Lateral Sclerosis. Front. Mol. Neurosci. 2019, 12, 25. [Google Scholar] [CrossRef]
- Wang, Y.T.; Kuo, P.H.; Chiang, C.H.; Liang, J.R.; Chen, Y.R.; Wang, S.; Shen, J.C.; Yuan, H.S. The truncated C-terminal RNA recognition motif of TDP-43 protein plays a key role in forming proteinaceous aggregates. J. Biol. Chem. 2013, 288, 9049–9057. [Google Scholar] [CrossRef] [PubMed]
- Winton, M.J.; Igaz, L.M.; Wong, M.M.; Kwong, L.K.; Trojanowski, J.Q.; Lee, V.M. Disturbance of nuclear and cytoplasmic TAR DNA-binding protein (TDP-43) induces disease-like redistribution, sequestration, and aggregate formation. J. Biol. Chem. 2008, 283, 13302–13309. [Google Scholar] [CrossRef]
- Berning, B.A.; Walker, A.K. The Pathobiology of TDP-43 C-Terminal Fragments in ALS and FTLD. Front. Neurosci. 2019, 13, 335. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Ngo, S.T. Altered TDP-43 Structure and Function: Key Insights into Aberrant RNA, Mitochondrial, and Cellular and Systemic Metabolism in Amyotrophic Lateral Sclerosis. Metabolites 2022, 12, 709. [Google Scholar] [CrossRef]
- Liu, Y.J.; McIntyre, R.L.; Janssens, G.E.; Houtkooper, R.H. Mitochondrial fission and fusion: A dynamic role in aging and potential target for age-related disease. Mech. Ageing Dev. 2020, 186, 111212. [Google Scholar] [CrossRef]
- Schwenk, B.M.; Hartmann, H.; Serdaroglu, A.; Schludi, M.H.; Hornburg, D.; Meissner, F.; Orozco, D.; Colombo, A.; Tahirovic, S.; Michaelsen, M.; et al. TDP-43 loss of function inhibits endosomal trafficking and alters trophic signaling in neurons. EMBO J. 2016, 35, 2350–2370. [Google Scholar] [CrossRef]
- McDonald, K.K.; Aulas, A.; Destroismaisons, L.; Pickles, S.; Beleac, E.; Camu, W.; Rouleau, G.A.; Vande Velde, C. TAR DNA-binding protein 43 (TDP-43) regulates stress granule dynamics via differential regulation of G3BP and TIA-1. Hum. Mol. Genet. 2011, 20, 1400–1410. [Google Scholar] [CrossRef]
- Osaka, M.; Ito, D.; Suzuki, N. Disturbance of proteasomal and autophagic protein degradation pathways by amyotrophic lateral sclerosis-linked mutations in ubiquilin 2. Biochem. Biophys. Res. Commun. 2016, 472, 324–331. [Google Scholar] [CrossRef]
- Bose, J.K.; Huang, C.C.; Shen, C.K. Regulation of autophagy by neuropathological protein TDP-43. J. Biol. Chem. 2011, 286, 44441–44448. [Google Scholar] [CrossRef]
- Joshi, A.U.; Saw, N.L.; Vogel, H.; Cunnigham, A.D.; Shamloo, M.; Mochly-Rosen, D. Inhibition of Drp1/Fis1 interaction slows progression of amyotrophic lateral sclerosis. EMBO Mol. Med. 2018, 10, e8166. [Google Scholar] [CrossRef]
- Wang, W.; Wang, L.; Lu, J.; Siedlak, S.L.; Fujioka, H.; Liang, J.; Jiang, S.; Ma, X.; Jiang, Z.; da Rocha, E.L.; et al. The inhibition of TDP-43 mitochondrial localization blocks its neuronal toxicity. Nat. Med. 2016, 22, 869–878. [Google Scholar] [CrossRef]
- Kang, Y.; Stroud, D.A.; Baker, M.J.; De Souza, D.P.; Frazier, A.E.; Liem, M.; Tull, D.; Mathivanan, S.; McConville, M.J.; Thorburn, D.R.; et al. Sengers Syndrome-Associated Mitochondrial Acylglycerol Kinase Is a Subunit of the Human TIM22 Protein Import Complex. Mol. Cell 2017, 67, 457–470.e455. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.H.; Davidson, S.; Harapas, C.R.; Hilton, J.B.; Mlodzianoski, M.J.; Laohamonthonkul, P.; Louis, C.; Low, R.R.J.; Moecking, J.; De Nardo, D.; et al. TDP-43 Triggers Mitochondrial DNA Release via mPTP to Activate cGAS/STING in ALS. Cell 2020, 183, 636–649 e618. [Google Scholar] [CrossRef]
- Liu, W.; Yamashita, T.; Tian, F.; Morimoto, N.; Ikeda, Y.; Deguchi, K.; Abe, K. Mitochondrial fusion and fission proteins expression dynamically change in a murine model of amyotrophic lateral sclerosis. Curr. Neurovascular Res. 2013, 10, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Bharathi, V.; Girdhar, A.; Patel, B.K. Role of CNC1 gene in TDP-43 aggregation-induced oxidative stress-mediated cell death in S. cerevisiae model of ALS. Biochim. Biophys. Acta Mol. Cell Res. 2021, 1868, 118993. [Google Scholar] [CrossRef] [PubMed]
- Riva, N.; Domi, T.; Pozzi, L.; Lunetta, C.; Schito, P.; Spinelli, E.G.; Cabras, S.; Matteoni, E.; Consonni, M.; Bella, E.D.; et al. Update on recent advances in amyotrophic lateral sclerosis. J. Neurol. 2024, 271, 4693–4723. [Google Scholar] [CrossRef]
- Daniels, D.L.; Ford, M.J.; Schwinn, M.K.; Benink, H.A.; Galbraith, M.D.; Amunugama, R.; Jones, R.J.; Allen, D.; Okazaki, N.; Yamakawa, H.; et al. Mutual Exclusivity of MED12/MED12L, MED13/13L, and CDK8/19Paralogs Revealed within the CDK-Mediator Kinase Module. J. Proteom. Bioinform. 2013, 2013, 1–7. [Google Scholar]
- Bessenyei, B.; Balogh, I.; Mokánszki, A.; Ujfalusi, A.; Pfundt, R.; Szakszon, K. MED13L-related intellectual disability due to paternal germinal mosaicism. Cold Spring Harb. Mol. Case Stud. 2022, 8, a006124. [Google Scholar] [CrossRef]
- Hamada, N.; Iwamoto, I.; Nagata, K.I. MED13L and its disease-associated variants influence the dendritic development of cerebral cortical neurons in the mammalian brain. J. Neurochem. 2023, 165, 334–347. [Google Scholar] [CrossRef]
- Siavrienė, E.; Petraitytė, G.; Mikštienė, V.; Maldžienė, Ž.; Sasnauskienė, A.; Žitkutė, V.; Ambrozaitytė, L.; Rančelis, T.; Utkus, A.; Kučinskas, V.; et al. Molecular and Functional Characterisation of a Novel Intragenic 12q24.21 Deletion Resulting in MED13L Haploinsufficiency Syndrome. Medicina 2023, 59, 1225. [Google Scholar] [CrossRef] [PubMed]
- Heilmann, R.; Pfalzer, A.; Bichell, T.J.; Terala, A.; Campbell, A.; Taatjes, D.; Ghoumid, J.; Grueter, C.; Bain, J.; Strich, R.; et al. The MED13L Foundation strategic research plan: A roadmap to the future. Ther. Adv. Rare Dis. 2024, 5, 26330040241290252. [Google Scholar] [CrossRef]
- Chang, K.T.; Jezek, J.; Campbell, A.N.; Stieg, D.C.; Kiss, Z.A.; Kemper, K.; Jiang, P.; Lee, H.O.; Kruger, W.D.; van Hasselt, P.M.; et al. Aberrant cyclin C nuclear release induces mitochondrial fragmentation and dysfunction in MED13L syndrome fibroblasts. iScience 2022, 25, 103823. [Google Scholar] [CrossRef]
- Snijders Blok, L.; Hiatt, S.M.; Bowling, K.M.; Prokop, J.W.; Engel, K.L.; Cochran, J.N.; Bebin, E.M.; Bijlsma, E.K.; Ruivenkamp, C.A.L.; Terhal, P.; et al. De novo mutations in MED13, a component of the Mediator complex, are associated with a novel neurodevelopmental disorder. Hum. Genet. 2018, 137, 375–388. [Google Scholar] [CrossRef] [PubMed]
- Polla, D.L.; Bhoj, E.J.; Verheij, J.B.G.M.; Wassink-Ruiter, J.S.K.; Reis, A.; Deshpande, C.; Gregor, A.; Hill-Karfe, K.; Silfhout, A.T.V.-v.; Pfundt, R.; et al. De novo variants in MED12 cause X-linked syndromic neurodevelopmental disorders in 18 females. Genet. Med. 2021, 23, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Plassche, S.V.; Brouwer, A.P. MED12-Related (Neuro)Developmental Disorders: A Question of Causality. Genes 2021, 12, 663. [Google Scholar] [CrossRef]
- Calpena, E.; Hervieu, A.; Kaserer, T.; Swagemakers, S.M.A.; Goos, J.A.C.; Popoola, O.; Ortiz-Ruiz, M.J.; Barbaro-Dieber, T.; Bownass, L.; Brilstra, E.H.; et al. De Novo Missense Substitutions in the Gene Encoding CDK8, a Regulator of the Mediator Complex, Cause a Syndromic Developmental Disorder. Am. J. Hum. Genet. 2019, 104, 709–720. [Google Scholar] [CrossRef]
- Uehara, T.; Abe, K.; Oginuma, M.; Ishitani, S.; Yoshihashi, H.; Okamoto, N.; Takenouchi, T.; Kosaki, K.; Ishitani, T. Pathogenesis of CDK8-associated disorder: Two patients with novel CDK8 variants and in vitro and in vivo functional analyses of the variants. Sci. Rep. 2020, 10, 17575. [Google Scholar] [CrossRef]
- Lee, M.; Cheon, K.; Chae, B.; Hwang, H.; Kim, H.K.; Chung, Y.J.; Song, J.Y.; Cho, H.H.; Kim, J.H.; Kim, M.R. Analysis of MED12 Mutation in Multiple Uterine Leiomyomas in South Korean patients. Int. J. Med. Sci. 2018, 15, 124–128. [Google Scholar] [CrossRef]
- Guo, Q.; Jin, Y.; Chen, X.; Ye, X.; Shen, X.; Lin, M.; Zeng, C.; Zhou, T.; Zhang, J. NF-κB in biology and targeted therapy: New insights and translational implications. Signal Transduct. Target. Ther. 2024, 9, 53. [Google Scholar] [CrossRef]
- Cheng, D.; Semmens, K.; McManus, E.; Chen, Q.; Meerzaman, D.; Wang, X.; Hafner, M.; Lewis, B.A.; Takahashi, H.; Devaiah, B.N.; et al. The nuclear transcription factor, TAF7, is a cytoplasmic regulator of protein synthesis. Sci. Adv. 2021, 7, eabi5751. [Google Scholar] [CrossRef] [PubMed]
- Marcel, V.; Catez, F.; Diaz, J.J. p53, a translational regulator: Contribution to its tumour-suppressor activity. Oncogene 2015, 34, 5513–5523. [Google Scholar] [CrossRef] [PubMed]
- Leu, J.I.; Dumont, P.; Hafey, M.; Murphy, M.E.; George, D.L. Mitochondrial p53 activates Bak and causes disruption of a Bak-Mcl1 complex. Nat. Cell Biol. 2004, 6, 443–450. [Google Scholar] [CrossRef]
- Kowalski, A.; Betzer, C.; Larsen, S.T.; Gregersen, E.; Newcombe, E.A.; Bermejo, M.C.; Bendtsen, V.W.; Diemer, J.; Ernstsen, C.V.; Jain, S.; et al. Monomeric alpha-synuclein activates the plasma membrane calcium pump. EMBO J. 2023, 42, e111122. [Google Scholar] [CrossRef]
- Hallacli, E.; Kayatekin, C.; Nazeen, S.; Wang, X.H.; Sheinkopf, Z.; Sathyakumar, S.; Sarkar, S.; Jiang, X.; Dong, X.; Di Maio, R.; et al. The Parkinson’s disease protein alpha-synuclein is a modulator of processing bodies and mRNA stability. Cell 2022, 185, 2035–2056.e2033. [Google Scholar] [CrossRef]
- Friedson, B.; Cooper, K.F. Cdk8 Kinase Module: A Mediator of Life and Death Decisions in Times of Stress. Microorganisms 2021, 9, 2152. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Virgilio, L.; Silva-Lucero, M.D.; Flores-Morelos, D.S.; Gallardo-Nieto, J.; Lopez-Toledo, G.; Abarca-Fernandez, A.M.; Zacapala-Gomez, A.E.; Luna-Munoz, J.; Montiel-Sosa, F.; Soto-Rojas, L.O.; et al. Autophagy: A Key Regulator of Homeostasis and Disease: An Overview of Molecular Mechanisms and Modulators. Cells 2022, 11, 2262. [Google Scholar] [CrossRef]
- Koller, H.; Perkins, L.B. Brewing and the Chemical Composition of Amine-Containing Compounds in Beer: A Review. Foods 2022, 11, 257. [Google Scholar] [CrossRef]
- Reiter, T.; Montpetit, R.; Byer, S.; Frias, I.; Leon, E.; Viano, R.; McLoughlin, M.; Halligan, T.; Hernandez, D.; Figueroa-Balderas, R.; et al. Transcriptomics Provides a Genetic Signature of Vineyard Site and Offers Insight into Vintage-Independent Inoculated Fermentation Outcomes. mSystems 2021, 6, e00033-21. [Google Scholar] [CrossRef]
- Liu, Y.; Lin, Y.; Guo, Y.; Wu, F.; Zhang, Y.; Qi, X.; Wang, Z.; Wang, Q. Stress tolerance enhancement via SPT15 base editing in Saccharomyces cerevisiae. Biotechnol. Biofuels 2021, 14, 155. [Google Scholar] [CrossRef]
- Postaru, M.; Tucaliuc, A.; Cascaval, D.; Galaction, A.I. Cellular Stress Impact on Yeast Activity in Biotechnological Processes—A Short Overview. Microorganisms 2023, 11, 2522. [Google Scholar] [CrossRef] [PubMed]
- Minden, S.; Aniolek, M.; Noorman, H.; Takors, R. Performing in spite of starvation: How Saccharomyces cerevisiae maintains robust growth when facing famine zones in industrial bioreactors. Microb. Biotechnol. 2023, 16, 148–168. [Google Scholar] [CrossRef] [PubMed]
- Iorizzo, M.; Coppola, F.; Letizia, F.; Testa, B.; Sorrentino, E. Role of Yeasts in the Brewing Process: Tradition and Innovation. Processes 2021, 9, 839. [Google Scholar] [CrossRef]
- Yang, T.; Zhang, S.; Pan, Y.; Li, X.; Liu, G.; Sun, H.; Zhang, R.; Zhang, C. Breeding of high-tolerance yeast by adaptive evolution and high-gravity brewing of mutant. J. Sci. Food Agric. 2024, 104, 686–697. [Google Scholar] [CrossRef]
- Hou, D.; Xu, X.; Wang, J.; Liu, C.; Niu, C.; Zheng, F.; Li, Q. Effect of environmental stresses during fermentation on brewing yeast and exploration on the novel flocculation-associated function of RIM15 gene. Bioresour. Technol. 2023, 379, 129004. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).