Abstract
The endocannabinoid system (ECS) plays a crucial role in maintaining homeostasis by regulating immune response, energy metabolism, cognitive functions, and neuronal activity. It consists of endocannabinoids (eCBs), cannabinoid receptors (CBRs), and enzymes involved in eCB biosynthesis and degradation. Increasing evidence highlights the involvement of the ECS under several pathological conditions, making it a promising therapeutic target. Recent research efforts have focused on modulating endogenous eCB levels, particularly through the inhibition of fatty acid amide hydrolase (FAAH), the main catabolic enzyme of the major eCB anandamide. Natural substances, including plant extracts and purified compounds, can inhibit FAAH and represent a promising area of pharmacological research. Natural FAAH inhibitors are particularly attractive due to their potentially lower toxicity compared to synthetic compounds, making them safer candidates for therapeutic applications. Phytocannabinoids, flavonoids, and flavolignans have been shown to efficiently inhibit FAAH. The structural diversity and bioactivity of these natural substances provide a valuable alternative to synthetic inhibitors, and may open new avenues for developing innovative pharmacological tools.
1. Introduction
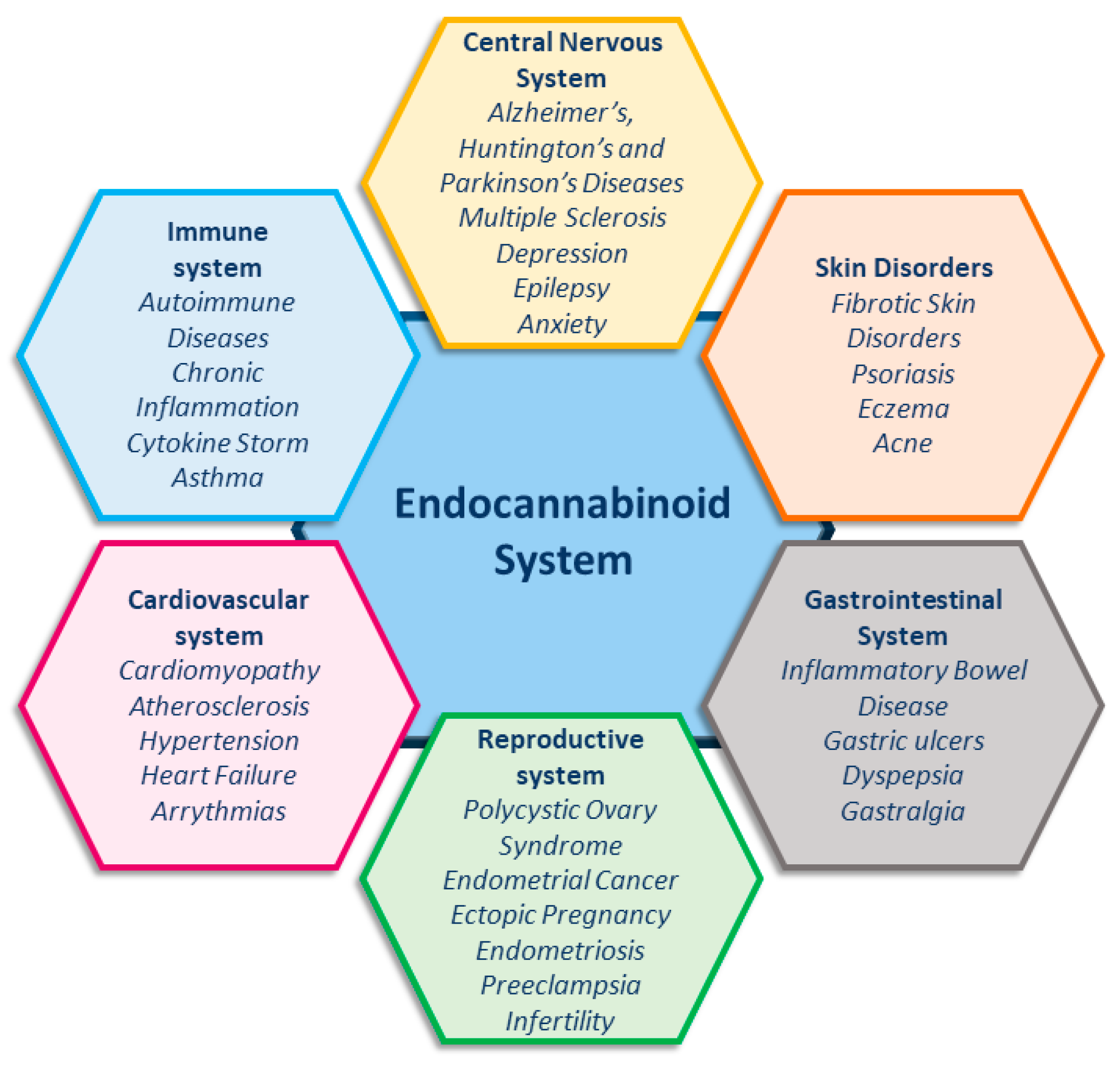
The endocannabinoid system (ECS) is essential for maintaining homeostasis, as it regulates the immune system, energy metabolism, and cognitive functions, and also modulates neuronal activity [1,2]. Unsurprisingly, increasing evidence shows the involvement of the ECS in an ever-growing number of pathological conditions (Figure 1) [1,2,3,4,5,6,7,8,9], making this system an important target for developing new therapies in medicine.
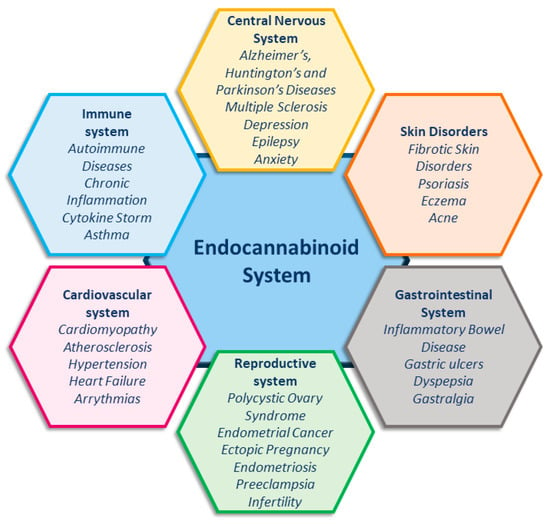
Figure 1.
Endocannabinoid system and related pathologies.
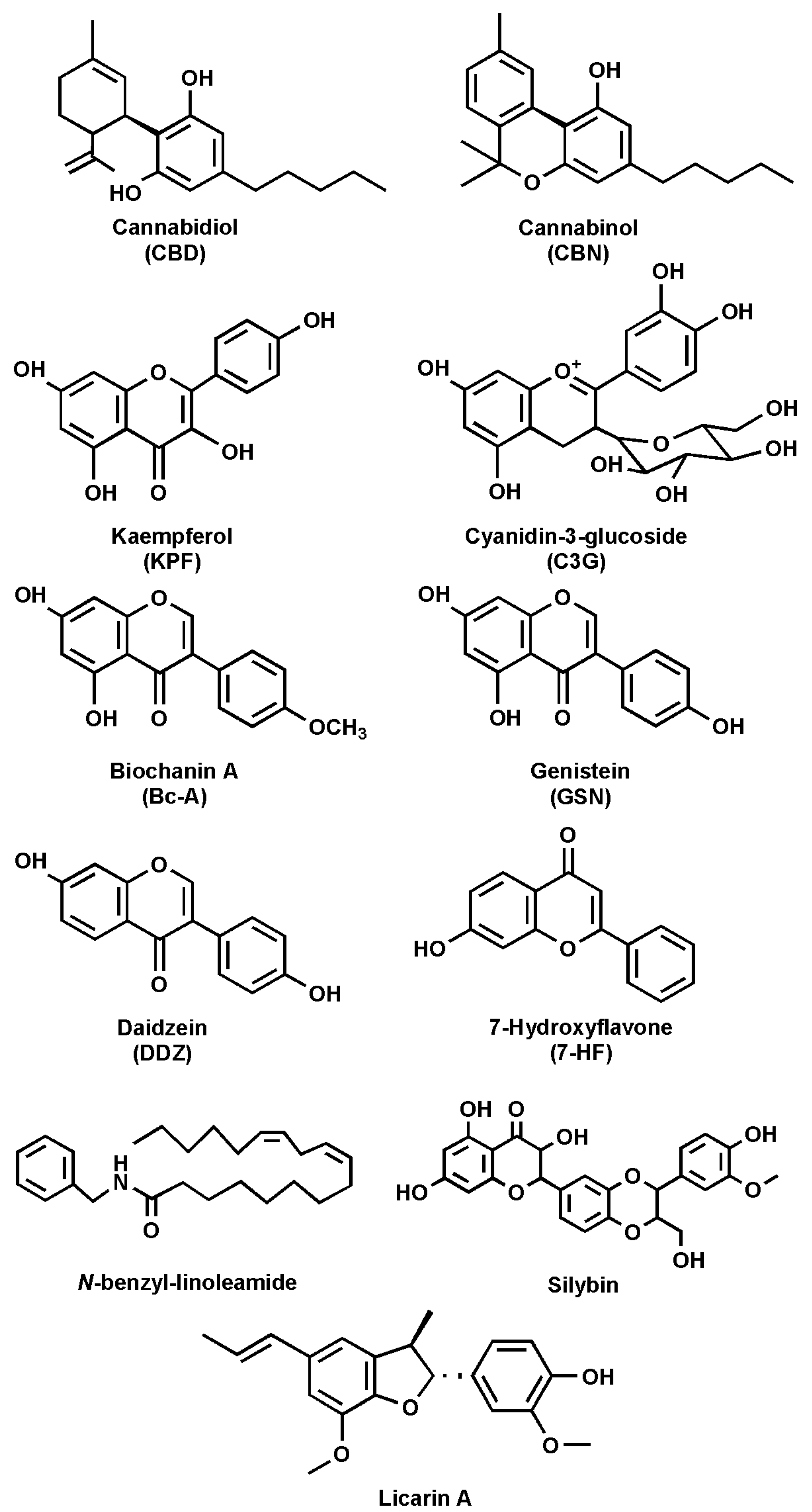
The ECS comprises endocannabinoids (eCBs), cannabinoid receptors 1 and 2 (CB1R and CB2R), and the enzymes responsible for eCB synthesis and degradation [10,11]. The main eCBs, N-arachidonoylethanolamine (anandamide, AEA) and 2-arachidonoylglycerol (2-AG), are derived from C20:4 ω-6 arachidonic acid (AA) and belong to the N-acylethanolamines (NAEs) and 2-monoacylglycerols (MAG) families, respectively [12,13]. Besides AEA and 2-AG, other derivatives of ω-6 and ω-3 polyunsaturated fatty acids (PUFAs) have also been identified, including N-linoleoylethanolamine, N-eicosapentaenoyl ethanolamine, and N-docosahexaenoylethanolamine [14]. The main receptors of eCBs (CB1R and CB2R) belong to the family of G protein-coupled receptors (GPCRs) [3,15]. Indeed, it was the interest in the pharmacological and psychotropic effects of trans-Δ9-tetrahydrocannabinol (THC), one of the two main plant-derived phytocannabinoids (pCBs), that led to the discovery of CB1R and, a few years later, of CB2R [1,16]. It seems noteworthy that many pCBs, including cannabidiol (CBD) (Figure 2), have low binding affinity for both CBRs [17,18].

Figure 2.
Chemical structures of the main natural FAAH inhibitors.
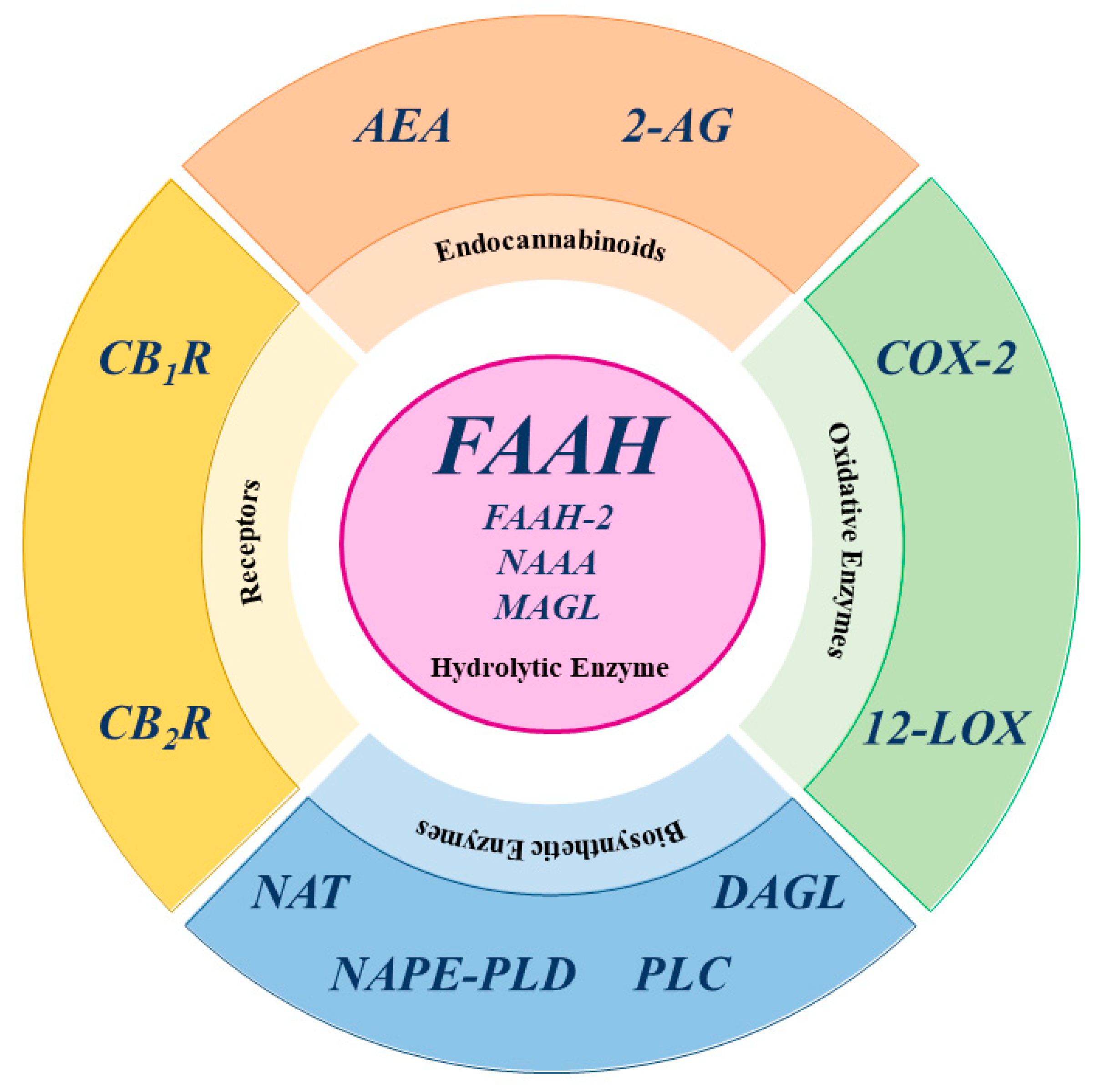
In fact, CBD is a negative allosteric modulator with low affinity for both CB1R and CB2R, yet it also acts on non-CBR molecular targets, such as transient receptor potential vanilloid type 1, 2, 3, and 4; peroxisome proliferator-activated receptor γ; serotonin receptor type 1 and 2, G-protein receptor 55; and adenosine A1 and A2 receptor [2,9,19,20,21,22,23,24]. The third component of the ECS consists of enzymes responsible for the biosynthesis and degradation of eCBs. The biosynthesis of AEA and 2-AG occurs in two main steps. For AEA, the first step involves N-acyltransferase (NAT) enzymes, which transfer an acyl group to phosphatidylethanolamine, forming N-acylphosphatidylethanolamine (NAPE). The second step is catalyzed by N-acyl phosphatidylethanolamine-specific phospholipase D (NAPE-PLD), which hydrolyzes NAPE to produce AEA and phosphatidic acid [8,12,25]. Similar to AEA, the biosynthetic pathway of 2-AG can be divided into two distinct steps. First, phospholipase C (PLC) hydrolyzes phosphoinositides like 2-arachidonoyl-phosphatidylinositol, generating 2-arachidonoyl-diacylglycerol (2-Ar-DAG) [26]. Next, diacylglycerol lipase (DAGL) converts 2-Ar-DAG into 2-AG [8,27,28]. Notably, the biosynthesis of 2-AG is tissue-specific due to the selective recognition of different phosphoinositides by PLC [12]. Additionally, there are alternative biosynthetic pathways for both AEA and 2-AG [12]. The primary catabolic enzyme responsible for the degradation of AEA is fatty acid amide hydrolase (FAAH), which produces AA and ethanolamine [28], but also appears to play a catabolic role for 2-AG [12,26]. Several hydrolases are currently known: FAAH or FAAH-1, predominantly expressed in the brain; FAAH-2, found in human adipocytes; and the N-acylethanolamine acid amide (NAAA) hydrolase, which is localized in lysosomes [12]. Additionally, AEA is also catabolized by oxidative enzymes, including 12-lipoxygenase (12-LOX), cyclooxygenase-2 (COX-2), and cytochrome P450 [12].
In contrast, 2-AG is primarily catabolized by monoacylglycerol lipase (MAGL), but also by other enzymes, such as COX-2, 12-LOX, α/β-hydrolase domain-containing 6 (ABHD6), and α/β-hydrolase domain-containing 12 (ABHD12), with FAAH playing a minor role [26,28]. It is noteworthy that MAGL, ABHD6, and ABHD12 exhibit specific distribution within the central nervous system (CNS), suggesting distinct physiological roles in the regulation of 2-AG signaling [12]. A schematic representation of the main ECS elements is shown in Figure 3.
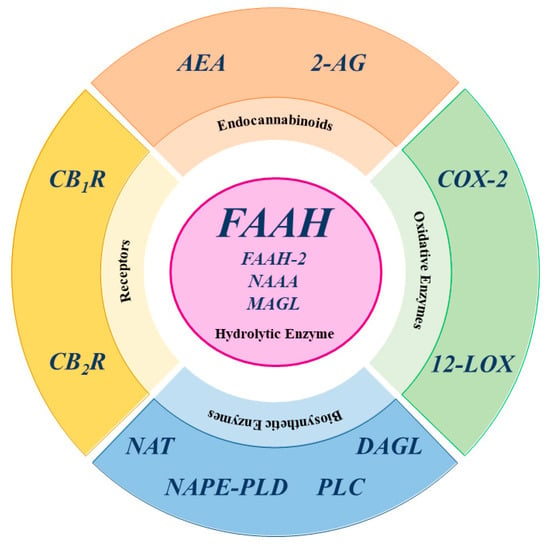
Figure 3.
Schematic representation of the main ECS elements. AEA: anandamide; 2-AG: 2-arachidonoylglycerol; COX-2: cyclooxygenase-2; CB1R cannabinoid receptor type 1; CB2R cannabinoid receptor type 2; DAGL: diacylglycerol lipase; FAAH: fatty acid amide hydrolase; FAAH-2: fatty acid amide hydrolase-2; 12-LOX: 12-lipoxygenase; MAGL: monoacylglycerol lipase; NAAA: N-acylethanolamine acid amide hydrolase; NAPE-PLD: N-acyl phosphatidylethanolamine-specific phospholipase D; NAT: N-acyltransferase enzymes; PLC: phospholipase C.
The manipulation of the ECS began through the use of compounds capable of activating the cannabinoid receptor, leading to changes in brain signaling, including undesirable psychotropic side effects [17]. These complicating factors have hijacked research towards agents capable of manipulating endogenous endocannabinoid levels to avoid unwanted adverse effects. Among the various enzymes involved in endocannabinoid degradation, one of the most studied and that has attracted research, also by the pharmaceutical industry, is FAAH.
FAAH Structure
FAAH is a dimeric enzyme, composed of two identical monomers anchored to the endoplasmic reticulum (ER) membranes, and able to regulate the hydrolysis and biological activity of AEA, as well as of other NAEs and, to some extent, of 2-AG [29]. Each monomer consists of two transversal α-helical structures (α-18 and α-19) and an N-terminal transmembrane domain of 20 amino acids (residues 9–29), allowing the substrate to reach the active site though a membrane channel [29,30]. The parallel orientation of the monomers allows both subunits to simultaneously bind and hydrolyze substrates [31]. FAAH is a serine hydrolase situated on the membrane of ER [32]. The binding site consists of three functional channels: the membrane access channel (MAC), acyl binding pocket (ABP), and cytosolic port (CP). Within the active site, the substrate is surrounded by the catalytic triad consisting of Ser241, Ser217, and Lys142, which are essential for the enzymatic reaction. Furthermore, the residues Gly240, Gly239, and Ile238 play crucial roles in substrate stabilization [29,33,34].
FAAH belongs to a large class of hydrolytic enzymes known as the amidase signature (AS) family. However, this family has diverged significantly in terms of substrate specificity and function [35]. Unlike most other AS enzymes, which are primarily soluble proteins, FAAH is an integral membrane enzyme that preferentially binds and hydrolyzes hydrophobic substrates, including amides and esters [29].
Remarkably, FAAH is an allosteric enzyme and represents an interesting case of communication between two enzyme subunits, controlled by a single amino acid at the dimer interface [31]. Additionally, as previously reported for CB1R [36,37], the important connection between steroids and the ECS has also been demonstrated for rat FAAH (rFAAH). In particular, the molecular interaction of different steroids with rFAAH appears to be able to modulate the enzyme’s membrane-binding properties, impacting the biological activity of eCBs [38]. Notably, cholesterol, a key sterol in membrane composition, has also been shown to influence FAAH activity by stabilizing its interaction with lipid bilayers and facilitating substrate accessibility to the active site [39].
Based on the current knowledge of rFAAH and human FAAH (hFAAH), a high homology has been identified, with a difference of six amino acids between the two FAAH isoforms [33,40]. Additionally, the secondary and tertiary structures of hFAAH and rFAAH are not identical, showing specific differences around the side chain of the aromatic residues [41].
2. FAAH Modulators
FAAH modulators are molecules that can regulate FAAH activity in either a positive or a negative manner; among them, inhibitors play a central role. By blocking enzyme activity, inhibitors increase levels of NAEs, and particularly of AEA, N-palmitoylethanolamine, and N-oleoylethanolamine, in both the CNS and at the periphery.
Initially, the first inhibitors discovered, such as phenylmethylsulfonylfluoride, trifluoromethyl ketones, and fluorophosphonates, exhibited good inhibitory activity but showed poor selectivity for FAAH [42,43]. In fact, they also interacted with various serine hydrolases (e.g., MAGL), as well as CB1R, neuropathy target esterase, acetylcholinesterase, and butyrylcholinesterase [43].
Then, more selective inhibitors were identified and subsequently classified based on their action mechanism. Specifically, the three main categories include irreversible covalent inhibitors, reversible covalent inhibitors and reversible non-covalent inhibitors (Table 1) [42].

Table 1.
Main synthetic FAAH inhibitors and their associated clinical trials.
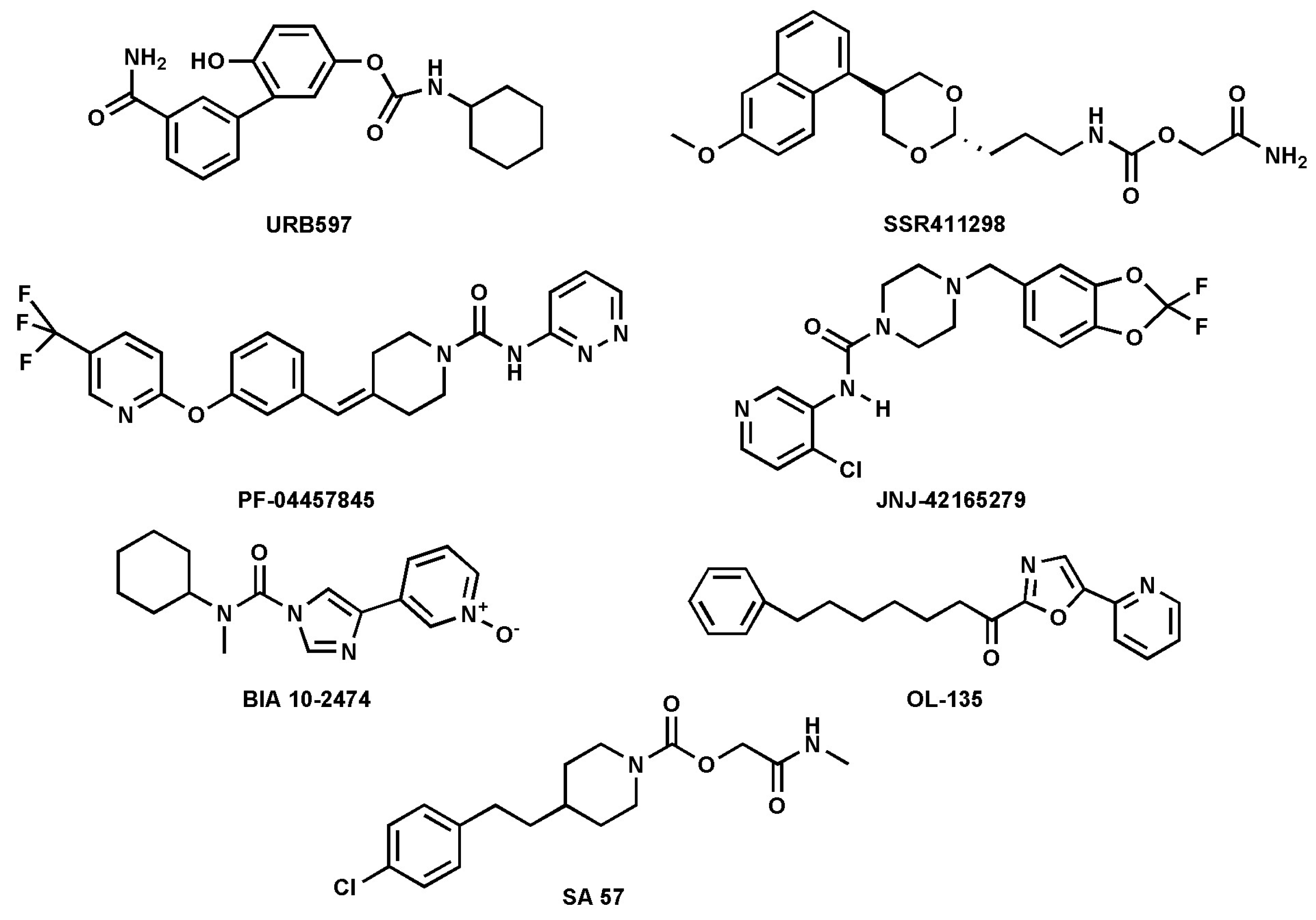
FAAH inhibitors contain electrophilic groups, such as carbamates, urea, and ketoheterocycles, which react with the catalytic site, forming new bonds with the enzyme (Table 1 and Figure 4 [44,53].

Figure 4.
Chemical structures of the synthetic FAAH inhibitors listed in Table 1.
Carbamates are potent FAAH inhibitors in vivo, due to their potential irreversible mechanism of action. Indeed, they inactivate FAAH through covalent carbamylation of nucleophile Ser241 [54]. Currently, cyclohexylcarbamic acid 3′-carbamoyl-biphenyl-3-yl ester (URB-597), a compound that belongs to the class of carbamates, is considered one of the most potent FAAH inhibitors with an IC50 of 4.6 nM [34]. The main synthetic FAAH inhibitors that are being tested in clinical trials are listed in Table 1. Many of these inhibitors have passed the pre-clinical stage but have not progressed to phase 1, as reported on ClinicalTrials.gov—the official website of the U.S. Department of Health and Human Services (HHS), National Institutes of Health (NIH), National Library of Medicine (NLM), and National Center for Biotechnology Information (NCBI). An outstanding example of an inhibitor that did not pass clinical phase 1 is BIA10-2474, which, during the phase 1 trial, caused the death of one volunteer and led to neurological events ranging from mild to severe to four other volunteers. The causes of these adverse effects were found to be due to relevant off-target activity [50,55]. In general, as mentioned previously, FAAH inhibition leads to an increase in NAEs, such as AEA, which can result in anxiolytic and analgesic effects mediated by the activation of CB1R [40,56]. To date, synthetic inhibitors of FAAH have been extensively addressed in the literature [8,29,57]; thus, here the focus was on natural inhibitors only.
2.1. Natural FAAH Inhibitors
Natural FAAH inhibitors may include plants, extracts, and isolated compounds, representing a fascinating area of pharmacological research. Furthermore, due to their therapeutic applications, natural compounds are often claimed to act as nutraceuticals [58,59] and, starting from their chemical structures, they were grouped in different classes, as reported in Table 2 [49].

Table 2.
Structural classification and potency of major natural FAAH inhibitors.
Several natural compounds have been identified as FAAH inhibitors (Table 2), and among them, some are pCBs [15,62]. Furthermore, natural FAAH inhibitors include kaempferol and galangin, two flavonoids; cyanidin-3-glucoside, an anthocyanin belonging to the polyphenol class; biochanin-A, an isoflavone; genistein, also an isoflavone; and daidzein, closely related to genistein (Figure 2 and Table 2).
Other flavonoids with FAAH inhibitory activity include 7-hydroxyflavone, apigenin and taxifolin. Additionally, macamide, a long-chain fatty acid N-benzylamide, has been studied for its potential role in FAAH modulation. Moreover, silymarin, a flavolignan extracted from milk thistle, along with its components silychristin, silydianin, silybin, and isosilybin, has been identified as a possible FAAH modulator. Lastly, 5′-methoxylicarin A and licarin A, two neolignans, and malabaricone C, a diarylnonanoid extracted from nutmeg, have been reported to interact with FAAH (Table 2).
These natural compounds, due to their structural diversity and ability to influence FAAH activity, represent an interesting alternative to synthetic inhibitors and may open new avenues in pharmacological research.
Interestingly, many of these FAAH-interacting compounds are currently undergoing clinical trials for diverse illnesses, such as epilepsy, inflammation, and menopause-related complications, as well as liver diseases (Table 3).

Table 3.
Main natural FAAH inhibitors and their associated clinical trials.
2.2. Phytocannabinoids
These is a group of more than 100 natural compounds isolated from Cannabis sativa that have been extensively studied for their pharmacological effects [9,16,62]. The most abundant pCBs, besides THC and CBD, are cannabinol (CBN) (Figure 2 and Table 2), cannabigerol (CBG), cannabichromene (CBC), and Δ9-tetrahydrocannabivarin (THCV) [89].
Among pCBs, CBD was found to be the most effective FAAH inhibitor, with IC50 values of ~10 µM for the rat enzyme [62,63]. Interestingly, CBD has been found to inhibit tumor growth in both in vivo and in vitro studies by modulating LOX activity, and hence the ECS [90]. These findings suggest a possible interaction between the oxidation and hydrolysis pathways involved in the regulation of the endogenous tone of AEA.
A dose-dependent increase in FAAH activity by CBD has also been documented in both in vitro and in vivo models [90]. Instead, CBN had IC50 values higher than 50 µM, and pCBs such as CBC, CBG, and THCV showed IC50 values higher than 100 µM towards rat FAAH [62]. In line with this, a recent study by our group investigated the ability of various pCBs to inhibit FAAH, using both in silico simulations and in vitro activity assays [31]. This study further highlighted the differences between hFAAH and rFAAH inhibition, emphasizing how enzyme structural variations may influence the efficacy of different natural compounds in modulating FAAH activity [33]. The analysis of binding free energy for various pCBs interacting with hFAAH and rFAAH revealed variations in binding affinity between the two enzymes [33]. Of note, the in silico data were confirmed by the in vitro activity assays. For instance, it was found that CBD was the most potent pCB for rFAAH inhibition, with an IC50 of 43.5 ± 1.5 µM, followed by CBN, with an IC50 of 60.0 ± 10 µM. Instead, CBC and CBG were weaker inhibitors (IC50 ~ 100 µM), and THCV was ineffective (IC50 > 100 µM). For hFAAH, all tested pCBs showed an IC50 > 100 µM, except for CBN, which acted as a weak inhibitor, with an IC50 ~ 100 µM [33].
Currently, there are 474 published clinical trials on CBD, 17 of which have reached phase 4. In contrast, only one phase 3 clinical trial on CBN is currently under investigation (Table 3).
2.3. Kaempferol
Kaempferol (3,5,7-trihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one, KPF) (Figure 2 and Table 2) is a flavonoid found in Camellia sinensis, the tea plant, and is commonly present in various other plant species such as tea, spinach, broccoli, tomatoes, and grapes [67,91]. Numerous studies have highlighted the beneficial effects of KPF, including the reduction in chronic conditions like cancer, liver injury, obesity, and diabetes [67]. Additionally, it is associated with significant anti-inflammatory activity, showing potential to improve inflammation-related disorders such as intervertebral disc degeneration, colitis, and postmenopausal bone loss [67].
Notably, several studies have reported that KPF can inhibit FAAH activity [68,70]. Specifically, FAAH inhibition by KPF was evaluated at concentrations ranging from 0.1 µM to 200 µM, with the highest concentration showing an inhibition comparable to JZL195, a potent synthetic FAAH inhibitor, with an IC50 value of 1.064 µM on human recombinant FAAH [68]. An in silico study revealed that KPF interacts with the catalytic amino acids (Ser241, Phe192, Phe381, and Thr377) of the FAAH enzyme [68]. Furthermore, treatment with KPF (compared to URB597) has been linked to anxiolytic effects in fear-conditioned rats in a dose-dependent manner. This effect was abolished when co-administered with the CB1 receptor antagonist rimonabant [68].
Currently, a clinical study on KPF focused on female sexual dysfunctions, published on ClinicalTrials.gov, has completed phase 1 (Table 3).
2.4. Cyanidin-3-Glucoside
Cyanidin-3-glucoside (C3G) (Figure 2 and Table 2), also known as kuromanin, asterin, and chrysanthemin, is a hydrophilic compound belonging to the anthocyanin class, an important flavonoid subclass [75,76]. Anthocyanins are secondary metabolites classified within the polyphenol family and are distinguished from anthocyanidins by the presence of a glycosidic bond. In particular, C3G is characterized by a glycosylation at the C3 position [92]. Both anthocyanins and anthocyanidins have attracted scientific interest due to their role in coloring fruits, vegetables, and flowers from red to blue, as well as for health benefits such as antioxidant properties [75,76,92]. Notably, C3G has been identified as an FAAH inhibitor, with an IC50 value of 152.1 µM on human recombinant FAAH [75].
A clinical study is presently investigating the effects of C3G (Table 3).
2.5. Biochanin-A
Biochanin-A (5,7-dihydroxy-4′-methoxyisoflavone, Bc-A) (Figure 2 and Table 2) is a phytoestrogen that can be found in many plants, such as chickpeas, peanuts, and soybean [77]. Bc-A has demonstrated several beneficial effects, including slowing the progression of nonalcoholic fatty liver disease (NAFLD) by regulating cholesterol metabolism and blood lipid levels [77,93]. It also exhibits antimicrobial effects, as it appears to inhibit the growth of Clostridium, a bacterium responsible for intestinal infections, without affecting the growth of beneficial bacteria [77,94]. Furthermore, antitumoral properties have been reported, involving the downregulation of biosignaling processes related to p38, mitogen-activated protein kinase (MAPK), nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB), and protein kinase B (PKB or Akt) [77,95]. Lastly, Bc-A demonstrates significant anti-inflammatory and antioxidant effects by reducing levels of tumor necrosis factor-α (TNF-α), nitric oxide (NO), and superoxide anion, which are molecules produced in response to lipopolysaccharide (LPS) administration [77,96]. Several studies have reported that Bc-A is able to inhibit FAAH activity [79,80]. In particular, an in silico study revealed that it is capable of binding to Ser241 within the active site, forming a stable ligand–receptor complex with a docking score of –6.9290 [79]. To validate the results obtained in silico, the authors tested Bc-A in an in vitro assay, obtaining an IC50 of 2.1 ± 0.24 µM on human recombinant FAAH [79].
In keeping with the previous study, Bc-A was reported to inhibit FAAH activity in mouse brain, rat liver, and cells transfected with hFAAH, with IC50 values of 1.8, 1.4, and 2.4 µM, respectively [80]. Instead, Bc-A had modest effects on CB1 and CB2 receptors [80], and it was found to be selective for FAAH versus FAAH-2 [80]. Moreover, in an animal model of depression, treatment with Bc-A led to a significant increase in AEA levels in the prefrontal cortex, along with a significant reduction in immobility time compared to vehicle-treated controls [79]. Unfortunately, Bc-A has not yet reached the clinical phase.
2.6. Genistein and Daidzein
Genistein (4′,5,7-trihydroxyisoflavone, GSN) (Figure 2 and Table 2) acts as a phytoestrogen and belongs, along with biochanin-A, to the class of isoflavones [78]. Furthermore, as with many flavonoids, GSN is the aglycone form, which is less common in nature than genistin, which is the glycoside form at position 7 [97]. GSN can be found in soy-based foods, such as soy drinks and soy cheese [78]. In addition, GSN can be endogenously produced in the gut through demethylation of the methoxy group present in biochanin-A [98]. It has been reported that GSN is a strong tyrosine-specific protein kinase inhibitor of the epidermal growth factor receptor kinase [99], and this may be related to the prevention, in Asia, of the incidence of cancer diseases [97,100]. In contrast, a study on the MCF-7 cell line, which is associated with breast cancer, demonstrated that GSN has a pro-proliferative effect at low concentrations (ranging from 1.56 to 13.06 µM), whereas at higher concentrations (between 13.06 and 100 µM), it exhibits an anti-proliferative effect [101].
Furthermore, GSN, as with many isoflavonoids, shows significant antioxidant activity that can be associated with a reduction in occurrence of chronic diseases such as cardiovascular diseases by preventing the low-density lipoprotein cholesterol oxidation [102].
A recent in silico study revealed that GSN interacts with Ser241, achieving a docking score of −6.5791 [79]. The same authors conducted an in vitro FAAH inhibitory assay, which showed that GSN inhibits FAAH in a dose-dependent manner, with an IC50 of 1.3 ± 0.13 µM against human recombinant FAAH [79]. As reported for Bc-A, this flavone has been shown to increase AEA levels and to influence immobility time in mice [79].
According to the previous study, as reported above to Bc-A, GSN has been shown to inhibit FAAH activity in mice, rats, and humans, with IC50 values of 2.7, 3.1, and 4.8 µM, respectively [80].
Currently, 77 clinical trials on isoflavonoids are listed on ClinicalTrials.gov, including those categorized as recruiting, active but not recruiting, withdrawn, unknown status, terminated, and completed. Among these, five have reached phase 4 of pharmacovigilance, and four have been completed (Table 3).
Daidzein (7-hydroxy-3-(4-hydroxyphenyl)-4H-1-benzopyran-4-one, DDZ) (Figure 2 and Table 2), a derivative lacking a hydroxyl group comparable to genistein, has also been shown to inhibit FAAH activity [103,104]. DDZ is found, alongside genistein, in soy-derived foods and legumes in two different forms: daidzin, the 7-glycosilated form, releases daidzein, the aglycone form, through hydrolysis. Both can be considered analogous to human estrogens exerting protective effects against osteoporosis and cardiovascular and cerebrovascular diseases [105].
DDZ exhibits a binding affinity of −11.77 kcal/mol and a docking score of −6.2772 and it is able to interact with Ser-241 [104], while in vitro, DDZ has been shown to inhibit FAAH activity in mouse, rat, and human, with IC50 values of 4.4, 2.5, and 14 µM, respectively [80]. Furthermore, DDZ significantly reduced immobility time in the forced swim test, which is an indicator of an anti-depressive effect [104].
Notably, in Asian countries, where soy consumption is significantly higher than in Western countries, serum concentrations of genistein and daidzein can reach 2–4 μM [80,106], suggesting that the levels required to inhibit FAAH might be attainable in vivo, at least in theory.
Currently, there are three clinical trials that have completed phase 4 and are published on ClinicalTrials.gov) (Table 3).
2.7. 7-Hydroxyflavone
7-Hydroxyflavone (7-HF) (Figure 2 and Table 2) is a flavonoid found in Dracaena cochinchinensis, Clerodendron phlomoidis, and Platymiscium praecox Mart and is known to exhibit anti-nociceptive and anti-inflammatory effects [107]. An inhibitory effect of 7-HF has been demonstrated on rat FAAH, with an IC50 of 0.99 µM in dimethyl sulfoxide and 0.48 µM in ethanol [70]. Of note, the flavone, without or with six hydroxyl groups, does not exhibit any FAAH inhibitory activity [70]. Shifting the hydroxyl group from position 7 of 7-HF to position 5 results in the complete loss of FAAH inhibitory activity. However, adding a second hydroxyl group to 7-HF, as in the case of 3,7-dihydroxyflavone or 7,4′-dihydroxyflavone, increases the IC50 to 2.2 and 5.6–8.3 µM, respectively, while the addition of a hydroxyl group in positions 5 and 7, as in the case of 5,7-dihydroxyflavone (chrysin), completely abolishes the inhibitory activity. Finally, compounds such as 5,7,4′-trihydroxyflavone (apigenin) and 3,5,7-trihydroxyflavone (galangin) exhibit an IC50 of 35 and 31 µM, respectively, on rat FAAH [70]. Moreover, more recently, 7-HF has been reported to inhibit recombinant human FAAH, with an IC50 of 2.04 ± 0.19 μM [79].
Much like Bc-A and GSN, 7-HF is also able to reduce immobility time and to modulate AEA levels in animal models [79], but it has not yet reached the clinical phase.
2.8. Macamide
Macamides (MACs) are derivatives of non-polar and long-chain fatty acid of N-benzylamides [66] that can be found in maca (Lepidium meyenii), a central Peruvian plant [108]. MACs exhibit various pharmacological effects, such as neuroprotective, anti-fatigue, and fertility-enhancing effects [109].
The inhibitory activity of four MACs was reported, with IC50 values of 7.9, 7.2, and 8.5 µM for N-benzyl-oleamide, N-benzyl-linoleamide (Figure 2 and Table 2), and N-benzyl-linolenamide, respectively [65]. In contrast, the derivative containing a saturated fatty acid, N-benzyl-stearamide, exhibited lower inhibitory activity, with an IC50 of 43.7 µM [65]. Notably, the presence of unsaturation in the fatty acid moiety resulted in greater FAAH inhibitory activity. Moreover, mass spectrometry analysis indicates that N-benzyl-linoleamide is also a slow substrate for FAAH [65].
A very recent work evaluated the interaction between some MACs and rFAAH using various in silico techniques, finding a high affinity for two of them [108]. These latter compounds showed a reduction, albeit at high concentrations, of epileptic seizures, as well as mortality in a rat model of epilepsy [108]. It was hypothesized that this effect may be due to an interaction with FAAH.
Currently, there are 16 clinical trials published, of which 3 have reached clinical phase 3 (Table 3).
2.9. Silymarin
Silymarin, derived from Silybum marianum (L.) Gaertn (milk thistle), is a mixture of flavonolignans such as silychristins, silydianins, silybins, and isosilybins as well as the flavonoid taxifolin [71,74]. It is a nutritional supplement known for its hepatoprotective and regenerative properties. The literature also reports other effects, including antioxidant, anti-inflammatory, anticancer, antiviral, antidiabetic, immunomodulatory, and anti-arthritic activities [110,111]. Furthermore, milk thistle is used in various liver diseases, such as alcoholic liver disease, cirrhosis, hepatitis, liver fibrosis, and liver tissue regeneration [110].
A molecular docking study provides insight into the binding affinities of silybin A and B, silydianin, silychristin, isosilybin A and B, and taxifolin to FAAH, reporting the following binding free energies: −66.08, −66.67, −42.16, −57.44, −64.15, −65.68, and −42.33 kcal/mol againest rFAAH, respectively [74]. They identified that silybin A exhibits the best-predicted affinity with rFAAH and has the highest binding affinity compared to the other compounds. In fact, Silybin A penetrates deeply into the catalytic triad and interacts directly with Ser241 [74]. Moreover, the authors report the IC50 values of the tested inhibitors on hFAAH as follows: taxifolin, 7.20 ± 0.31 µM; silychristin, 6.11 ± 1.03 µM; silydianin, 5.38 ± 0.31 µM; silybin (Figure 2 and Table 2), 5.08 ± 0.08 µM; and isosilybin, 6.18 ± 0.38 µM [74].
The treatment with a gel formulation containing AEA and silybin against peripheral neuropathic pain is particularly interesting. Indeed, the AEA–silybin gel showed a maximum antinociceptive activity > 50% higher than AEA alone and higher than the positive control (gabapentin gel). These results indicate that FAAH inhibition improves the analgesic efficacy of AEA in peripheral neuropathic pain conditions [74].
Currently, there are 76 studies on ClinicalTrials.gov, and 13 have reached phase 4 (Table 3).
2.10. Nutmeg Phenols
Nutmeg is the seed of the aromatic evergreen Myristica fragrans, native to Indonesia, India, South Africa, and United States. This seed is widely used as a spice and in traditional medicine [85,112,113,114,115]. The significant increase in the use of nutmeg has stimulated research interest in its bioactive compounds. To date, 35 lignans and 91 neolignans, 8 diphenylalkanes, 17 phenylpropanoids, and some terpenes have been isolated from Myristica fragrans [115,116]. These compounds have been shown to possess antioxidant, anti-inflammatory, antibacterial, and analgesic properties [85,112,113,114,115,116]. Also, a potent hepatoprotective effect was reported after treatment with nutmeg extracts [116]. Specifically, under conditions of acute liver injury, plasma levels of alanine transaminase and aspartate transaminase were reduced 24 h after treatment with nutmeg extract (250 mg/kg), suggesting that this extract may help mitigate this condition in mice [116]. Conversely, exposure to high doses (1 and 4 g/kg) of nutmeg powder for 7 and 14 days induced an increase in the aminotransferases in mice in a dose and time-dependent manner [117]. These results suggest that nutmeg has protective effects at low concentrations, while it becomes toxic at high ones. Moreover, concentrations higher than 4 g/kg led to hypoactivity, unstable gait, and dizziness, while the LD50 in mice was 5.1 g/kg [113].
Nutmeg has been linked also to various neurological activities. Currently, the use of this seed has expanded beyond its traditional role as a spice, and now it is used as a recreational drug due to the hallucinogenic effect of some of its compounds [118,119]. Because of these psychoactive effects, nutmeg has also been associated with cases of overdose [119,120], as illustrated by the case of a 20-year-old man who was hospitalized after ingesting 75 g of nutmeg powder [119].
In keeping with these data, the neuropharmacological effects of different nutmeg extracts, administered orally and intraperitoneally, were compared to those of THC, amphetamine, and morphine [121]. The observed activities were not cannabinoid-like in nature and varied depending on the type of extract and the route of administration [121]. Furthermore, no binding activity was found for different nutmeg fractions relative to various CNS receptors, including CB1R e CB2R [118]. Conversely, the same fractions exerted significant inhibition of FAAH and MAGL [118]. Subsequently, 13 compounds were isolated from nutmeg [85], including myristicin, which is potentially toxic [122]. The compounds that most strongly inhibited hFAAH activity are 5′-methoxylicarin A, licarin A, and malabaricone C, with IC50 values of 4.57 ± 0.66, 7.02 ± 2.02, and 38.29 ± 6.18 μM, respectively [85]. The same compounds were also tested as MAGL inhibitors, without significant effects [85]. Furthermore, a significant anxiolytic effect induced by 5′-methoxylicarin A was also observed at a concentration of 120 mg/kg in mice, with no interactions with the locomotor system [85]. Taken together, these results show an indirect modulation of the ECS through FAAH inhibition, along with a notable anxiolytic effect in vivo [85].
No clinical trials have been yet reported on nutmeg components.
3. Discussion
Due to the relevance of the eCB signaling in various physio-pathological conditions [1,2,3,4,5,6,7,8,9], and to the growing interest towards natural substances (particularly those of plant origin), many compounds are being tested on FAAH, one of the best-studied ECS components.
It is important to emphasize that signaling driven by eCBs—AEA included—can be modulated through various mechanisms, such as blocking its cellular transport or modulating FAAH activity and expression [123,124].
Although a significant body of literature exists regarding the effects of compounds that activate cannabinoid receptors (CBRs) and their associated psychotropic effects, the transition from cannabis prohibition to medical acceptance hinges on the complex interaction of scientific progress, societal views, and the regulatory environment surrounding legalization.
To overcome this issue, FAAH has emerged as a promising target for modulating eCBs signaling. Thus, numerous synthetic inhibitors have been developed, many of which demonstrated potent in vivo effects, including anti-inflammatory and analgesic properties [29]. Unfortunately, in 2016, a synthetic FAAH inhibitor, BIA 10-2474, caused the most serious incident ever recorded during a clinical trial targeting the ECS [50,55].
It is necessary to emphasize that this episode is unique and unfortunately has represented a setback in the research, for a period, of compounds capable of interacting with FAAH. To highlight, this episode led the EMA to make changes to its guidelines (https://www.ema.europa.eu/en/news/revised-guideline-first-human-clinical-trials (accessed on 25 July 2017); https://www.fda.gov/drugs/drug-safety-and-availability/fda-finds-drugs-under-investigation-us-related-french-bia-10-2474-drug-do-not-pose-similar-safety (accessed on 12 August 2016)) [125]. Furthermore, separately, the Food and Drug Administration (FDA) determined that BIA 10-2474 exhibits a unique toxicity that does not apply to other drugs within the same class [125].
Given the potential benefits and safety profile of natural compounds, research has shifted to studying these substances as a promising strategy to modulate FAAH activity.
The completion of phase 1 clinical trials indicates a level of safety for most of the compounds discussed in this review (Table 3). Moreover, in line with this, as shown in Table 3, 35 studies have reached Phase 4, a crucial stage of a clinical trial that facilitates the collection of data on market-approved drugs, enhancing the understanding of their benefits, previously unidentified risks, and optimal use for long-term patient treatment. In this review, several natural compounds capable of inhibiting FAAH are described, whose biological effects may be indeed linked to their inhibitory power. In particular, various non-psychoactive pCBs have been shown to inhibit FAAH, but with significant species-specific selectivity [33,41].
Notably, an increase in AEA levels has been reported in the plasma and serum of CBD-treated schizophrenic patients [63,126]. Specifically, CBD has demonstrated the ability to alleviate psychotic symptoms associated with schizophrenia when compared to current medications, indicating that the capacity of CBD to inhibit FAAH activity and augment intrinsic AEA signaling may represent a functionally relevant component of its antipsychotic properties [63]. Moreover, CBD has proven effective for the treatment of cannabis use disorder compared to placebo [126].
Furthermore, several isoflavones, such as GSN, DDZ, and Bc-A, have been shown to exert an anti-depressant effect by inhibiting the FAAH-mediated degradation of AEA [79,104].
Of note, structural analysis highlights that compounds containing a rigid ring and an aromatic system have a greater ability to interact with FAAH (Figure 2). These structural remarks can also be observed in synthetic inhibitors [29]. Moreover, by analyzing the potency of the natural inhibitors presented here—excluding pCBs and C3G—it emerges that they can inhibit FAAH within a range of 1 to 15 µM. CBD and CBN exhibit greater affinity for rFAAH compared to hFAAH [33]. In particular, the in silico analysis showed that, despite the two isoforms being similar, they display some structural differences in the catalytic site region, making hFAAH less sensitive to the inhibitory action of these pCBs [33]. For C3G, its lower potency may be due to the ability of FAAH to primarily hydrolyze lipophilic compounds. Consequently, a compound containing the polar saccharinic residue could negatively interact with the residues present in its three functional channels and with the catalytic triad.
The research on the potential of natural compounds as inhibitors towards different targets arises from a combination of factors. One of the most significant and intriguing aspects is that, in several cases, their use has been documented through sometimes millennia-old practices, although often not according to the regulations or standards required by today’s control systems. This makes these substances particularly interesting regarding the safety of their use. Moreover, the accumulation of scientific literature over the past few decades supports the notion that regular consumption of fruits and vegetables, rich in natural substances, can exert a protective effect against the development and progression of various disorders [127,128].
Additionally, it is important to point out that even naturally derived compounds can present side effects, meaning “natural” is not equivalent to “safe” and that bioavailability studies are necessary to assess their therapeutic impact from “natural” exposure through diet.
This aspect, together with the use of new techniques (such as computational analysis), enables the screening of compound libraries at a very low cost, similar to what already happens for synthetic inhibitors [129,130]. Moreover, by analyzing the interactions and chemical structures of natural compounds, a structure–activity relationship (SAR) can be developed, providing a valuable tool for future drug design.
An additional benefit to consider is that using natural sources can reduce the environmental impact of research.
This review supports the use of natural compounds as therapeutic agents to modulate ECS. Notably, as a further advantage, FAAH inhibition represents an emerging physiological target for the treatment of several pathological conditions, including neurodegenerative diseases, inflammation, and reproductive system disorders (Figure 1) [1,2,3,4,5,6,7]. Consequently, remarkable efforts have been made both by academia and pharmaceutical companies into developing potent, selective, and safe FAAH inhibitors.
Author Contributions
Conceptualization, F.F. and M.M.; writing—original draft preparation, C.N. and F.F.; writing—review and editing, C.N., F.F. and M.M.; visualization, C.N. and F.F.; supervision, M.M. All authors have read and agreed to the published version of the manuscript.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Maia, J.; Fonseca, B.; Teixeira, N.; Correia-da-Silva, G. The Fundamental Role of the Endocannabinoid System in Endometrium and Placenta: Implications in Pathophysiological Aspects of Uterine and Pregnancy Disorders. Hum. Reprod. Update 2020, 26, 586–602. [Google Scholar] [CrossRef]
- Pagano, C.; Navarra, G.; Coppola, L.; Avilia, G.; Bifulco, M.; Laezza, C. Cannabinoids: Therapeutic Use in Clinical Practice. Int. J. Mol. Sci. 2022, 23, 3344. [Google Scholar] [CrossRef] [PubMed]
- Walker, O.S.; Holloway, A.C.; Raha, S. The Role of the Endocannabinoid System in Female Reproductive Tissues. J. Ovarian Res. 2019, 12, 3. [Google Scholar] [CrossRef] [PubMed]
- Lowe, H.; Toyang, N.; Steele, B.; Bryant, J.; Ngwa, W. The Endocannabinoid System: A Potential Target for the Treatment of Various Diseases. Int. J. Mol. Sci. 2021, 22, 9472. [Google Scholar] [CrossRef]
- Rabino, M.; Mallia, S.; Castiglioni, E.; Rovina, D.; Pompilio, G.; Gowran, A. The Endocannabinoid System and Cannabidiol: Past, Present, and Prospective for Cardiovascular Diseases. Pharmaceuticals 2021, 14, 936. [Google Scholar] [CrossRef]
- Scipioni, L.; Ciaramellano, F.; Carnicelli, V.; Leuti, A.; Lizzi, A.R.; De Dominicis, N.; Oddi, S.; Maccarrone, M. Microglial Endocannabinoid Signalling in AD. Cells 2022, 11, 1237. [Google Scholar] [CrossRef] [PubMed]
- Chiurchiù, V.; Battistini, L.; Maccarrone, M. Endocannabinoid Signalling in Innate and Adaptive Immunity. Immunology 2015, 144, 352–364. [Google Scholar] [CrossRef]
- Maccarrone, M.; Bab, I.; Bíró, T.; Cabral, G.A.; Dey, S.K.; Di Marzo, V.; Konje, J.C.; Kunos, G.; Mechoulam, R.; Pacher, P.; et al. Endocannabinoid Signaling at the Periphery: 50 Years after THC. Trends Pharmacol. Sci. 2015, 36, 277–296. [Google Scholar] [CrossRef]
- Friedman, D.; French, J.A.; Maccarrone, M. Safety, Efficacy, and Mechanisms of Action of Cannabinoids in Neurological Disorders. Lancet Neurol. 2019, 18, 504–512. [Google Scholar] [CrossRef]
- Oddi, S.; Scipioni, L.; Maccarrone, M. Endocannabinoid System and Adult Neurogenesis: A Focused Review. Curr. Opin. Pharmacol. 2020, 50, 25–32. [Google Scholar] [CrossRef]
- Joshi, N.; Onaivi, E.S. Endocannabinoid System Components: Overview and Tissue Distribution. In Recent Advances in Cannabinoid Physiology and Pathology; Bukiya, A.N., Ed.; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2019; Volume 1162, pp. 1–12. ISBN 978-3-030-21736-5. [Google Scholar]
- Fezza, F.; Bari, M.; Florio, R.; Talamonti, E.; Feole, M.; Maccarrone, M. Endocannabinoids, Related Compounds and Their Metabolic Routes. Molecules 2014, 19, 17078–17106. [Google Scholar] [CrossRef] [PubMed]
- Biringer, R.G. The Rise and Fall of Anandamide: Processes That Control Synthesis, Degradation, and Storage. Mol. Cell. Biochem. 2021, 476, 2753–2775. [Google Scholar] [CrossRef]
- Aydin, E.; Cebo, M.; Mielnik, J.; Richter, H.; Schüle, R.; Sievers-Engler, A.; Młynarz, P.; Lämmerhofer, M. UHPLC-ESI-MS/MS Assay for Quantification of Endocannabinoids in Cerebrospinal Fluid Using Surrogate Calibrant and Surrogate Matrix Approaches. J. Pharm. Biomed. Anal. 2023, 222, 115090. [Google Scholar] [CrossRef]
- Criscuolo, E.; De Sciscio, M.L.; De Cristofaro, A.; Nicoara, C.; Maccarrone, M.; Fezza, F. Computational and Experimental Drug Repurposing of FDA-Approved Compounds Targeting the Cannabinoid Receptor CB1. Pharmaceuticals 2023, 16, 1678. [Google Scholar] [CrossRef] [PubMed]
- Crocq, M.-A. History of Cannabis and the Endocannabinoid System. Dialogues Clin. Neurosci. 2020, 22, 223–228. [Google Scholar] [CrossRef]
- Maccarrone, M.; Di Marzo, V.; Gertsch, J.; Grether, U.; Howlett, A.C.; Hua, T.; Makriyannis, A.; Piomelli, D.; Ueda, N.; Van Der Stelt, M. Goods and Bads of the Endocannabinoid System as a Therapeutic Target: Lessons Learned after 30 Years. Pharmacol. Rev. 2023, 75, 885–958. [Google Scholar] [CrossRef]
- Di Meo, C.; Tortolani, D.; Standoli, S.; Ciaramellano, F.; Angelucci, B.C.; Tisi, A.; Kadhim, S.; Hsu, E.; Rapino, C.; Maccarrone, M. Cannabinol Modulates the Endocannabinoid System and Shows TRPV1-mediated Anti-inflammatory Properties in Human Keratinocytes. BioFactors 2025, 51, e2122. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, D.L.; Devi, L.A. Diversity of Molecular Targets and Signaling Pathways for CBD. Pharmacol. Res. Perspect. 2020, 8, e00682. [Google Scholar] [CrossRef]
- Castillo-Arellano, J.; Canseco-Alba, A.; Cutler, S.J.; León, F. The Polypharmacological Effects of Cannabidiol. Molecules 2023, 28, 3271. [Google Scholar] [CrossRef]
- Britch, S.C.; Babalonis, S.; Walsh, S.L. Cannabidiol: Pharmacology and Therapeutic Targets. Psychopharmacology 2021, 238, 9–28. [Google Scholar] [CrossRef]
- Kisková, T.; Mungenast, F.; Suváková, M.; Jäger, W.; Thalhammer, T. Future Aspects for Cannabinoids in Breast Cancer Therapy. Int. J. Mol. Sci. 2019, 20, 1673. [Google Scholar] [CrossRef] [PubMed]
- Raïch, I.; Lillo, J.; Rivas-Santisteban, R.; Rebassa, J.B.; Capó, T.; Santandreu, M.; Cubeles-Juberias, E.; Reyes-Resina, I.; Navarro, G. Potential of CBD Acting on Cannabinoid Receptors CB1 and CB2 in Ischemic Stroke. Int. J. Mol. Sci. 2024, 25, 6708. [Google Scholar] [CrossRef]
- Khosropoor, S.; Alavi, M.S.; Etemad, L.; Roohbakhsh, A. Cannabidiol Goes Nuclear: The Role of PPARγ. Phytomedicine 2023, 114, 154771. [Google Scholar] [CrossRef] [PubMed]
- Ueda, N.; Tsuboi, K.; Uyama, T. Enzymological Studies on the Biosynthesis of N-Acylethanolamines. Biochim. Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2010, 1801, 1274–1285. [Google Scholar] [CrossRef]
- Tsuboi, K.; Uyama, T.; Okamoto, Y.; Ueda, N. Endocannabinoids and Related N-Acylethanolamines: Biological Activities and Metabolism. Inflamm. Regen. 2018, 38, 28. [Google Scholar] [CrossRef] [PubMed]
- Bisogno, T.; Howell, F.; Williams, G.; Minassi, A.; Cascio, M.G.; Ligresti, A.; Matias, I.; Schiano-Moriello, A.; Paul, P.; Williams, E.-J.; et al. Cloning of the First Sn1-DAG Lipases Points to the Spatial and Temporal Regulation of Endocannabinoid Signaling in the Brain. J. Cell Biol. 2003, 163, 463–468. [Google Scholar] [CrossRef]
- Bagues, A.; Goicoechea, C. Cannabinoid Agonists. In Neuropathology of Drug Addictions and Substance Misuse; Elsevier: Amsterdam, The Netherlands, 2016; pp. 702–712. ISBN 978-0-12-800213-1. [Google Scholar]
- Fazio, D.; Criscuolo, E.; Piccoli, A.; Barboni, B.; Fezza, F.; Maccarrone, M. Advances in the Discovery of Fatty Acid Amide Hydrolase Inhibitors: What Does the Future Hold? Expert Opin. Drug Discov. 2020, 15, 765–778. [Google Scholar] [CrossRef]
- Palermo, G.; Campomanes, P.; Neri, M.; Piomelli, D.; Cavalli, A.; Rothlisberger, U.; De Vivo, M. Wagging the Tail: Essential Role of Substrate Flexibility in FAAH Catalysis. J. Chem. Theory Comput. 2013, 9, 1202–1213. [Google Scholar] [CrossRef]
- Dainese, E.; Oddi, S.; Simonetti, M.; Sabatucci, A.; Angelucci, C.B.; Ballone, A.; Dufrusine, B.; Fezza, F.; De Fabritiis, G.; Maccarrone, M. The Endocannabinoid Hydrolase FAAH Is an Allosteric Enzyme. Sci. Rep. 2020, 10, 2292. [Google Scholar] [CrossRef]
- Leuci, R.; Brunetti, L.; Laghezza, A.; Piemontese, L.; Carrieri, A.; Pisani, L.; Tortorella, P.; Catto, M.; Loiodice, F. A New Series of Aryloxyacetic Acids Endowed with Multi-Target Activity towards Peroxisome Proliferator-Activated Receptors (PPARs), Fatty Acid Amide Hydrolase (FAAH), and Acetylcholinesterase (AChE). Molecules 2022, 27, 958. [Google Scholar] [CrossRef]
- Criscuolo, E.; De Sciscio, M.L.; Fezza, F.; Maccarrone, M. In Silico and In Vitro Analysis of Major Cannabis-Derived Compounds as Fatty Acid Amide Hydrolase Inhibitors. Molecules 2020, 26, 48. [Google Scholar] [CrossRef]
- Tuo, W.; Leleu-Chavain, N.; Barczyk, A.; Renault, N.; Lemaire, L.; Chavatte, P.; Millet, R. Design, Synthesis and Biological Evaluation of Potent FAAH Inhibitors. Bioorg. Med. Chem. Lett. 2016, 26, 2701–2705. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Liu, C.; Zhang, Z.; Zheng, R.; Zheng, Y. Amidase as a Versatile Tool in Amide-Bond Cleavage: From Molecular Features to Biotechnological Applications. Biotechnol. Adv. 2020, 43, 107574. [Google Scholar] [CrossRef] [PubMed]
- Bari, M.; Battista, N.; Fezza, F.; Finazzi-Agrò, A.; Maccarrone, M. Lipid Rafts Control Signaling of Type-1 Cannabinoid Receptors in Neuronal Cells. J. Biol. Chem. 2005, 280, 12212–12220. [Google Scholar] [CrossRef] [PubMed]
- Sarnataro, D.; Pisanti, S.; Santoro, A.; Gazzerro, P.; Malfitano, A.M.; Laezza, C.; Bifulco, M. The Cannabinoid CB1 Receptor Antagonist Rimonabant (SR141716) Inhibits Human Breast Cancer Cell Proliferation through a Lipid Raft-Mediated Mechanism. Mol. Pharmacol. 2006, 70, 1298–1306. [Google Scholar] [CrossRef]
- Sabatucci, A.; Simonetti, M.; Tortolani, D.; Angelucci, C.B.; Dainese, E.; Maccarrone, M. Role of Steroids on the Membrane Binding Ability of Fatty Acid Amide Hydrolase. Cannabis Cannabinoid Res. 2019, 4, 42–50. [Google Scholar] [CrossRef]
- Dainese, E.; De Fabritiis, G.; Sabatucci, A.; Oddi, S.; Angelucci, C.B.; Di Pancrazio, C.; Giorgino, T.; Stanley, N.; Del Carlo, M.; Cravatt, B.F.; et al. Membrane Lipids Are Key Modulators of the Endocannabinoid-Hydrolase FAAH. Biochem. J. 2014, 457, 463–472. [Google Scholar] [CrossRef]
- Tripathi, R.K.P. A Perspective Review on Fatty Acid Amide Hydrolase (FAAH) Inhibitors as Potential Therapeutic Agents. Eur. J. Med. Chem. 2020, 188, 111953. [Google Scholar] [CrossRef]
- Di Venere, A.; Dainese, E.; Fezza, F.; Angelucci, B.C.; Rosato, N.; Cravatt, B.F.; Finazzi-Agrò, A.; Mei, G.; Maccarrone, M. Rat and Human Fatty Acid Amide Hydrolases: Overt Similarities and Hidden Differences. Biochim. Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2012, 1821, 1425–1433. [Google Scholar] [CrossRef]
- Tuo, W.; Leleu-Chavain, N.; Spencer, J.; Sansook, S.; Millet, R.; Chavatte, P. Therapeutic Potential of Fatty Acid Amide Hydrolase, Monoacylglycerol Lipase, and N -Acylethanolamine Acid Amidase Inhibitors. J. Med. Chem. 2017, 60, 4–46. [Google Scholar] [CrossRef]
- Lambert, D.M.; Fowler, C.J. The Endocannabinoid System: Drug Targets, Lead Compounds, and Potential Therapeutic Applications. J. Med. Chem. 2005, 48, 5059–5087. [Google Scholar] [CrossRef] [PubMed]
- Seierstad, M.; Breitenbucher, J.G. Discovery and Development of Fatty Acid Amide Hydrolase (FAAH) Inhibitors. J. Med. Chem. 2008, 51, 7327–7343. [Google Scholar] [CrossRef]
- Piomelli, D.; Tarzia, G.; Duranti, A.; Tontini, A.; Mor, M.; Compton, T.R.; Dasse, O.; Monaghan, E.P.; Parrott, J.A.; Putman, D. Pharmacological Profile of the Selective FAAH Inhibitor KDS-4103 (URB597). CNS Drug Rev. 2006, 12, 21–38. [Google Scholar] [CrossRef] [PubMed]
- Griebel, G.; Stemmelin, J.; Lopez-Grancha, M.; Fauchey, V.; Slowinski, F.; Pichat, P.; Dargazanli, G.; Abouabdellah, A.; Cohen, C.; Bergis, O.E. The Selective Reversible FAAH Inhibitor, SSR411298, Restores the Development of Maladaptive Behaviors to Acute and Chronic Stress in Rodents. Sci. Rep. 2018, 8, 2416. [Google Scholar] [CrossRef]
- Keith, J.M.; Jones, W.M.; Tichenor, M.; Liu, J.; Seierstad, M.; Palmer, J.A.; Webb, M.; Karbarz, M.; Scott, B.P.; Wilson, S.J.; et al. Preclinical Characterization of the FAAH Inhibitor JNJ-42165279. ACS Med. Chem. Lett. 2015, 6, 1204–1208. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.S.; Stiff, C.; Lazerwith, S.E.; Kesten, S.R.; Fay, L.K.; Morris, M.; Beidler, D.; Liimatta, M.B.; Smith, S.E.; Dudley, D.T.; et al. Discovery of PF-04457845: A Highly Potent, Orally Bioavailable, and Selective Urea FAAH Inhibitor. ACS Med. Chem. Lett. 2011, 2, 91–96. [Google Scholar] [CrossRef]
- Mangiatordi, G.F.; Cavalluzzi, M.M.; Delre, P.; Lamanna, G.; Lumuscio, M.C.; Saviano, M.; Majoral, J.-P.; Mignani, S.; Duranti, A.; Lentini, G. Endocannabinoid Degradation Enzyme Inhibitors as Potential Antipsychotics: A Medicinal Chemistry Perspective. Biomedicines 2023, 11, 469. [Google Scholar] [CrossRef]
- Van Esbroeck, A.C.M.; Janssen, A.P.A.; Cognetta, A.B.; Ogasawara, D.; Shpak, G.; Van Der Kroeg, M.; Kantae, V.; Baggelaar, M.P.; De Vrij, F.M.S.; Deng, H.; et al. Activity-Based Protein Profiling Reveals off-Target Proteins of the FAAH Inhibitor BIA 10-2474. Science 2017, 356, 1084–1087. [Google Scholar] [CrossRef]
- Timmons, A.; Seierstad, M.; Apodaca, R.; Epperson, M.; Pippel, D.; Brown, S.; Chang, L.; Scott, B.; Webb, M.; Chaplan, S.R.; et al. Novel Ketooxazole Based Inhibitors of Fatty Acid Amide Hydrolase (FAAH). Bioorg. Med. Chem. Lett. 2008, 18, 2109–2113. [Google Scholar] [CrossRef]
- Niphakis, M.J.; Johnson, D.S.; Ballard, T.E.; Stiff, C.; Cravatt, B.F. O -Hydroxyacetamide Carbamates as a Highly Potent and Selective Class of Endocannabinoid Hydrolase Inhibitors. ACS Chem. Neurosci. 2012, 3, 418–426. [Google Scholar] [CrossRef]
- Gowlugari, S.; DeFalco, J.; Nguyen, M.T.; Kaub, C.; Chi, C.; Duncton, M.A.J.; Emerling, D.E.; Kelly, M.G.; Kincaid, J.; Vincent, F. Discovery of Potent, Non-Carbonyl Inhibitors of Fatty Acid Amide Hydrolase (FAAH). Med. Chem. Commun. 2012, 3, 1258. [Google Scholar] [CrossRef]
- Alexander, J.P.; Cravatt, B.F. Mechanism of Carbamate Inactivation of FAAH: Implications for the Design of Covalent Inhibitors and In Vivo Functional Probes for Enzymes. Chem. Biol. 2005, 12, 1179–1187. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rocha, J.; Santos, A.; Gama, H.; Moser, P.; Falcão, A.; Pressman, P.; Wallace Hayes, A.; Soares-da-Silva, P. Safety, Tolerability, and Pharmacokinetics of FAAH Inhibitor BIA 10-2474: A Double-Blind, Randomized, Placebo-Controlled Study in Healthy Volunteers. Clin. Pharmacol. Ther. 2022, 111, 391–403. [Google Scholar] [CrossRef]
- Mallet, C.; Dubray, C.; Dualé, C. FAAH Inhibitors in the Limelight, but Regrettably. Int. J. Clin. Pharmacol. Ther. 2016, 54, 498–501. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, Y.; Cai, N.; Liao, X.; Tang, L.; Wang, Y. Endocannabinoid Hydrolase Inhibitors: Potential Novel Anxiolytic Drugs. Drug Des. Dev. Ther. 2024, 18, 2143–2167. [Google Scholar] [CrossRef]
- Chandra, S.; Saklani, S.; Kumar, P.; Kim, B.; Coutinho, H.D.M. Nutraceuticals: Pharmacologically Active Potent Dietary Supplements. BioMed Res. Int. 2022, 2022, 2051017. [Google Scholar] [CrossRef] [PubMed]
- Watkins, B.A.; Mitchell, A.E.; Shin, A.C.; Dehghani, F.; Shen, C.-L. Dietary Flavonoid Actions on Senescence, Aging, and Applications for Health. J. Nutr. Biochem. 2025, 139, 109862. [Google Scholar] [CrossRef]
- Fu, Z.; Zhao, P.-Y.; Yang, X.-P.; Li, H.; Hu, S.-D.; Xu, Y.-X.; Du, X.-H. Cannabidiol Regulates Apoptosis and Autophagy in Inflammation and Cancer: A Review. Front. Pharmacol. 2023, 14, 1094020. [Google Scholar] [CrossRef]
- Capasso, R.; Borrelli, F.; Aviello, G.; Romano, B.; Scalisi, C.; Capasso, F.; Izzo, A.A. Cannabidiol, Extracted from Cannabis sativa, Selectively Inhibits Inflammatory Hypermotility in Mice. Br. J. Pharmacol. 2008, 154, 1001–1008. [Google Scholar] [CrossRef]
- De Petrocellis, L.; Ligresti, A.; Moriello, A.S.; Allarà, M.; Bisogno, T.; Petrosino, S.; Stott, C.G.; Di Marzo, V. Effects of Cannabinoids and Cannabinoid-enriched Cannabis Extracts on TRP Channels and Endocannabinoid Metabolic Enzymes. Br. J Pharmacol. 2011, 163, 1479–1494. [Google Scholar] [CrossRef]
- Leweke, F.M.; Piomelli, D.; Pahlisch, F.; Muhl, D.; Gerth, C.W.; Hoyer, C.; Klosterkötter, J.; Hellmich, M.; Koethe, D. Cannabidiol Enhances Anandamide Signaling and Alleviates Psychotic Symptoms of Schizophrenia. Transl. Psychiatry 2012, 2, e94. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Peng, Z.; Lai, W.; Shao, Y.; Gao, Q.; He, M.; Zhou, W.; Guo, L.; Kang, J.; Jin, X.; et al. The Efficient Synthesis and Anti-Fatigue Activity Evaluation of Macamides: The Unique Bioactive Compounds in Maca. Molecules 2023, 28, 3943. [Google Scholar] [CrossRef]
- Alasmari, M.; Bӧhlke, M.; Kelley, C.; Maher, T.; Pino-Figueroa, A. Inhibition of Fatty Acid Amide Hydrolase (FAAH) by Macamides. Mol. Neurobiol. 2019, 56, 1770–1781. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Jin, W.; Cui, Y.; Ao, M.; Liu, H.; Xu, H.; Yu, L. Protective Effects of Macamides from Lepidium meyenii Walp. against Corticosterone-Induced Neurotoxicity in PC12 Cells. RSC Adv. 2019, 9, 23096–23108. [Google Scholar] [CrossRef]
- Kim, J.K.; Park, S.U. Recent Studies on Kaempferol and Its Biological and Pharmacological Activities. EXCLI J. 2020, 19, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, H.; Rauf, K.; Zada, W.; McCarthy, M.; Abbas, G.; Anwar, F.; Shah, A.J. Kaempferol Facilitated Extinction Learning in Contextual Fear Conditioned Rats via Inhibition of Fatty-Acid Amide Hydrolase. Molecules 2020, 25, 4683. [Google Scholar] [CrossRef]
- Thapa, R.; Afzal, O.; Alfawaz Altamimi, A.S.; Goyal, A.; Almalki, W.H.; Alzarea, S.I.; Kazmi, I.; Jakhmola, V.; Singh, S.K.; Dua, K.; et al. Galangin as an Inflammatory Response Modulator: An Updated Overview and Therapeutic Potential. Chem.-Biol. Interact. 2023, 378, 110482. [Google Scholar] [CrossRef]
- Thors, L.; Belghiti, M.; Fowler, C.J. Inhibition of Fatty Acid Amide Hydrolase by Kaempferol and Related Naturally Occurring Flavonoids. Br. J Pharmacol. 2008, 155, 244–252. [Google Scholar] [CrossRef]
- Zugravu, G.S.; Pintilescu, C.; Cumpat, C.-M.; Miron, S.D.; Miron, A. Silymarin Supplementation in Active Rheumatoid Arthritis: Outcomes of a Pilot Randomized Controlled Clinical Study. Medicina 2024, 60, 999. [Google Scholar] [CrossRef]
- Yang, R.; Yang, X.; Zhang, F. New Perspectives of Taxifolin in Neurodegenerative Diseases. Curr. Neuropharmacol. 2023, 21, 2097–2109. [Google Scholar] [CrossRef]
- Yao, W.; Gong, H.; Mei, H.; Shi, L.; Yu, J.; Hu, Y. Taxifolin Targets PI3K and mTOR and Inhibits Glioblastoma Multiforme. J. Oncol. 2021, 2021, 5560915. [Google Scholar] [CrossRef]
- Police, A.; Shankar, V.K.; Pandey, P.; Rangappa, S.; Doerksen, R.J.; Narasimha Murthy, S. Novel Topical Anandamide Formulation for Alleviating Peripheral Neuropathic Pain. Int. J. Pharm. 2023, 641, 123085. [Google Scholar] [CrossRef]
- Cásedas, G.; Les, F.; González-Burgos, E.; Gómez-Serranillos, M.P.; Smith, C.; López, V. Cyanidin-3-O-Glucoside Inhibits Different Enzymes Involved in Central Nervous System Pathologies and Type-2 Diabetes. S. Afr. J. Bot. 2019, 120, 241–246. [Google Scholar] [CrossRef]
- Zannou, O.; Oussou, K.F.; Chabi, I.B.; Awad, N.M.H.; Aïssi, M.V.; Goksen, G.; Mortas, M.; Oz, F.; Proestos, C.; Kayodé, A.P.P. Nanoencapsulation of Cyanidin 3-O-Glucoside: Purpose, Technique, Bioavailability, and Stability. Nanomaterials 2023, 13, 617. [Google Scholar] [CrossRef]
- Feng, Z.-J.; Lai, W.-F. Chemical and Biological Properties of Biochanin A and Its Pharmaceutical Applications. Pharmaceutics 2023, 15, 1105. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, N.; Akhtar, J.; Singh, S.P.; Badruddeen; Ahsan, F. An Overview on Genistein and Its Various Formulations. Drug Res. 2019, 69, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Zada, W.; VanRyzin, J.W.; Perez-Pouchoulen, M.; Baglot, S.L.; Hill, M.N.; Abbas, G.; Clark, S.M.; Rashid, U.; McCarthy, M.M.; Mannan, A. Fatty Acid Amide Hydrolase Inhibition and N-arachidonoylethanolamine Modulation by Isoflavonoids: A Novel Target for Upcoming Antidepressants. Pharmacol. Res. Perspect. 2022, 10, e00999. [Google Scholar] [CrossRef]
- Thors, L.; Burston, J.; Alter, B.; McKinney, M.; Cravatt, B.; Ross, R.; Pertwee, R.; Gereau 4Th, R.; Wiley, J.; Fowler, C. Biochanin A, a Naturally Occurring Inhibitor of Fatty Acid Amide Hydrolase. Br. J Pharmacol. 2010, 160, 549–560. [Google Scholar] [CrossRef]
- Křížová, L.; Dadáková, K.; Kašparovská, J.; Kašparovský, T. Isoflavones. Molecules 2019, 24, 1076. [Google Scholar] [CrossRef]
- Espíndola, C.; Correa, A.J.; López-López, M.; López-Cornejo, P.; Bernal, E.; Lebrón, J.A.; Ostos, F.J.; Benhnia, M.R.-E.-I.; Moyá, M.L. Single -and Multi-Walled Carbon Nanotubes as Nanocarriers for the Delivery of 7-Hydroxyflavone. Pharmaceutics 2022, 14, 2806. [Google Scholar] [CrossRef]
- Salehi, B.; Venditti, A.; Sharifi-Rad, M.; Kręgiel, D.; Sharifi-Rad, J.; Durazzo, A.; Lucarini, M.; Santini, A.; Souto, E.B.; Novellino, E.; et al. The Therapeutic Potential of Apigenin. Int. J. Mol. Sci. 2019, 20, 1305. [Google Scholar] [CrossRef] [PubMed]
- Křenek, K.; Marhol, P.; Peikerová, Ž.; Křen, V.; Biedermann, D. Preparatory Separation of the Silymarin Flavonolignans by Sephadex LH-20 Gel. Food Res. Int. 2014, 65, 115–120. [Google Scholar] [CrossRef]
- El-Alfy, A.T.; Abourashed, E.A.; Patel, C.; Mazhari, N.; An, H.; Jeon, A. Phenolic Compounds from Nutmeg (Myristica fragrans Houtt.) Inhibit the Endocannabinoid-Modulating Enzyme Fatty Acid Amide Hydrolase. J. Pharm. Pharmacol. 2019, 71, 1879–1889. [Google Scholar] [CrossRef] [PubMed]
- Blanco Carcache, P.J.; Castro-Dionicio, I.Y.; Mirtallo Ezzone, N.P.; Salinas-Arrellano, E.D.; Bahar, J.; Clinton, S.K.; Kinghorn, A.D. Molecular Networking, Docking, and Biological Evaluation of Licarin A from Myristica Fragrans as a Potential Cancer Chemopreventive Agent. Molecules 2024, 29, 4919. [Google Scholar] [CrossRef]
- Mengarda, A.C.; Silva, M.P.; Cirino, M.E.; Morais, T.R.; Conserva, G.A.A.; Lago, J.H.G.; De Moraes, J. Licarin A, a neolignan isolated from Nectandra oppositifolia Nees & Mart. (Lauraceae), exhibited moderate preclinical efficacy against Schistosoma mansoni infection. Phytother. Res. 2021, 35, 5154–5162. [Google Scholar] [CrossRef]
- Jeong, S.-H.; Park, J.-Y.; Ryu, Y.B.; Kim, W.S.; Lee, I.-C.; Kim, J.-H.; Kim, D.; Ha, J.-H.; Lee, B.-W.; Nam, J.; et al. Myristica Fragrans Extract Inhibits Platelet Desialylation and Activation to Ameliorate Sepsis-Associated Thrombocytopenia in a Murine CLP-Induced Sepsis Model. Int. J. Mol. Sci. 2023, 24, 8863. [Google Scholar] [CrossRef]
- Morales, P.; Hurst, D.P.; Reggio, P.H. Molecular Targets of the Phytocannabinoids: A Complex Picture. In Phytocannabinoids; Kinghorn, A.D., Falk, H., Gibbons, S., Kobayashi, J., Eds.; Progress in the Chemistry of Organic Natural Products; Springer International Publishing: Cham, Switzerland, 2017; Volume 103, pp. 103–131. ISBN 978-3-319-45539-6. [Google Scholar]
- Massi, P.; Valenti, M.; Vaccani, A.; Gasperi, V.; Perletti, G.; Marras, E.; Fezza, F.; Maccarrone, M.; Parolaro, D. 5-Lipoxygenase and Anandamide Hydrolase (FAAH) Mediate the Antitumor Activity of Cannabidiol, a Non-psychoactive Cannabinoid. J. Neurochem. 2008, 104, 1091–1100. [Google Scholar] [CrossRef]
- Periferakis, A.; Periferakis, K.; Badarau, I.A.; Petran, E.M.; Popa, D.C.; Caruntu, A.; Costache, R.S.; Scheau, C.; Caruntu, C.; Costache, D.O. Kaempferol: Antimicrobial Properties, Sources, Clinical, and Traditional Applications. Int. J. Mol. Sci. 2022, 23, 15054. [Google Scholar] [CrossRef]
- Sinopoli, A.; Calogero, G.; Bartolotta, A. Computational Aspects of Anthocyanidins and Anthocyanins: A Review. Food Chem. 2019, 297, 124898. [Google Scholar] [CrossRef]
- Fan, Y.; Yan, L.-T.; Yao, Z.; Xiong, G.-Y. Biochanin A Regulates Cholesterol Metabolism Further Delays the Progression of Nonalcoholic Fatty Liver Disease. Diabetes Metab. Syndr. Obes. 2021, 14, 3161–3172. [Google Scholar] [CrossRef]
- Sklenickova, O.; Flesar, J.; Kokoska, L.; Vlkova, E.; Halamova, K.; Malik, J. Selective Growth Inhibitory Effect of Biochanin A Against Intestinal Tract Colonizing Bacteria. Molecules 2010, 15, 1270–1279. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.-N.; Shyu, H.-W.; Hu, T.-W.; Yeh, J.-P.; Lin, Y.-W.; Lee, L.-Y.; Yeh, Y.-T.; Dai, H.-Y.; Perng, D.-S.; Su, S.-H.; et al. Anti-Proliferative Activity of Biochanin A in Human Osteosarcoma Cells via Mitochondrial-Involved Apoptosis. Food Chem. Toxicol. 2018, 112, 194–204. [Google Scholar] [CrossRef]
- Cao, Y.; Tan, Y.J.; Huang, D. Anti-Inflammation Mechanisms of Flavones Are Highly Sensitive to the Position Isomers of Flavonoids: Acacetin vs. Biochanin A. J. Agric. Food Chem. 2024, 72, 22939–22951. [Google Scholar] [CrossRef]
- Choi, E.J.; Kim, T.; Lee, M.-S. Pro-Apoptotic Effect and Cytotoxicity of Genistein and Genistin in Human Ovarian Cancer SK-OV-3 Cells. Life Sci. 2007, 80, 1403–1408. [Google Scholar] [CrossRef]
- Steensma, A. Bioavailability of Genistein and Its Glycoside Genistin. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 2006. [Google Scholar]
- Akiyama, T.; Ishida, J.; Nakagawa, S.; Ogawara, H.; Watanabe, S.; Itoh, N.; Shibuya, M.; Fukami, Y. Genistein, a Specific Inhibitor of Tyrosine-Specific Protein Kinases. J. Biol. Chem. 1987, 262, 5592–5595. [Google Scholar] [CrossRef]
- Bhat, S.S.; Prasad, S.K.; Shivamallu, C.; Prasad, K.S.; Syed, A.; Reddy, P.; Cull, C.A.; Amachawadi, R.G. Genistein: A Potent Anti-Breast Cancer Agent. Curr. Issues Mol. Biol. 2021, 43, 1502–1517. [Google Scholar] [CrossRef] [PubMed]
- Uifălean, A.; Schneider, S.; Gierok, P.; Ionescu, C.; Iuga, C.; Lalk, M. The Impact of Soy Isoflavones on MCF-7 and MDA-MB-231 Breast Cancer Cells Using a Global Metabolomic Approach. Int. J. Mol. Sci. 2016, 17, 1443. [Google Scholar] [CrossRef] [PubMed]
- Miadoková, E. Isoflavonoids—An Overview of Their Biological Activities and Potential Health Benefits. Interdiscip. Toxicol. 2009, 2, 211–218. [Google Scholar] [CrossRef]
- Alshehri, M.M.; Sharifi-Rad, J.; Herrera-Bravo, J.; Jara, E.L.; Salazar, L.A.; Kregiel, D.; Uprety, Y.; Akram, M.; Iqbal, M.; Martorell, M.; et al. Therapeutic Potential of Isoflavones with an Emphasis on Daidzein. Oxidative Med. Cell. Longev. 2021, 2021, 6331630. [Google Scholar] [CrossRef]
- Zada, W.; Murtaza, G.; Iqbal, G.; Abbas, G.; Ali Khan, S.; Abdul Mannan, A.M. Antidepressant Potential of Daidzein Through Modulation of Endocannabinoid System by Targeting Fatty Acid Amide Hydrolase. Sains Malays. 2022, 51, 3383–3399. [Google Scholar] [CrossRef]
- Tan, Y.; Zhang, X.; Cheang, W.S. Isoflavones Daidzin and Daidzein Inhibit Lipopolysaccharide-Induced Inflammation in RAW264.7 Macrophages. Chin. Med. 2022, 17, 95. [Google Scholar] [CrossRef] [PubMed]
- Morton, M.S.; Arisaka, O.; Miyake, N.; Morgan, L.D.; Evans, B.A.J. Phytoestrogen Concentrations in Serum from Japanese Men and Women over Forty Years of Age. J. Nutr. 2002, 132, 3168–3171. [Google Scholar] [CrossRef] [PubMed]
- Ullah, R.; Ali, G.; Rasheed, A.; Subhan, F.; Khan, A.; Ahsan Halim, S.; Al-Harrasi, A. The 7-Hydroxyflavone Attenuates Chemotherapy-Induced Neuropathic Pain by Targeting Inflammatory Pathway. Int. Immunopharmacol. 2022, 107, 108674. [Google Scholar] [CrossRef] [PubMed]
- Vera-López, K.J.; Davila-Del-Carpio, G.; Nieto-Montesinos, R. Macamides as Potential Therapeutic Agents in Neurological Disorders. Neurol. Int. 2024, 16, 1611–1625. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, H.; Guo, F.; Si, N.; Brantner, A.; Yang, J.; Han, L.; Wei, X.; Zhao, H.; Bian, B. Integrated Proteomics and Lipidomics Investigation of the Mechanism Underlying the Neuroprotective Effect of N-Benzylhexadecanamide. Molecules 2018, 23, 2929. [Google Scholar] [CrossRef]
- Bhattacharya, S. Milk Thistle (Silybum marianum L. Gaert.) Seeds in Health. In Nuts and Seeds in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2011; pp. 759–766. ISBN 978-0-12-375688-6. [Google Scholar]
- Younas, M.; Drouet, S.; Nadeem, M.; Giglioli-Guivarc’h, N.; Hano, C.; Abbasi, B.H. Differential Accumulation of Silymarin Induced by Exposure of Silybum marianum L. Callus Cultures to Several Spectres of Monochromatic Lights. J. Photochem. Photobiol. B Biol. 2018, 184, 61–70. [Google Scholar] [CrossRef]
- Shen, R.-S.; Cao, D.; Chen, F.-L.; Wu, X.-J.; Gao, J.; Bai, L.-P.; Zhang, W.; Jiang, Z.-H.; Zhu, G.-Y. New Monoterpene-Conjugated Phenolic Constituents from Nutmeg and Their Autophagy Modulating Activities. J. Agric. Food Chem. 2022, 70, 9684–9693. [Google Scholar] [CrossRef]
- Hayfaa, A.A.S.; Sahar, A.M.A.S.; Awatif, M.A.S. Evaluation of Analgesic Activity and Toxicity of Alkaloids in Myristica Fragrans Seeds in Mice. J. Pain Res. 2013, 6, 611–615. [Google Scholar] [CrossRef]
- Lv, W.; Zhang, X.; Zhang, H.; Alitanhua; Xiao, Y. Safety of Nutmeg Powder by Oral Exposure: Toxicity Prediction and in Vivo Evaluation. Food Chem. Toxicol. 2025, 200, 115364. [Google Scholar] [CrossRef]
- Ha, M.T.; Vu, N.K.; Tran, T.H.; Kim, J.A.; Woo, M.H.; Min, B.S. Phytochemical and Pharmacological Properties of Myristica Fragrans Houtt.: An Updated Review. Arch. Pharm. Res. 2020, 43, 1067–1092. [Google Scholar] [CrossRef]
- Yang, X.-N.; Liu, X.-M.; Fang, J.-H.; Zhu, X.; Yang, X.-W.; Xiao, X.-R.; Huang, J.-F.; Gonzalez, F.J.; Li, F. PPARα Mediates the Hepatoprotective Effects of Nutmeg. J. Proteome Res. 2018, 17, 1887–1897. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Xia, W.; Zhang, X.; Yuan, H.; Guan, D.; Gao, L. Hepatotoxicity of Nutmeg: A Pilot Study Based on Metabolomics. Biomed. Pharmacother. 2020, 131, 110780. [Google Scholar] [CrossRef]
- El-Alfy, A.T.; Joseph, S.; Brahmbhatt, A.; Akati, S.; Abourashed, E.A. Indirect Modulation of the Endocannabinoid System by Specific Fractions of Nutmeg Total Extract. Pharm. Biol. 2016, 54, 2933–2938. [Google Scholar] [CrossRef] [PubMed]
- Usui, K.; Kubota, E.; Kobayashi, H.; Fujita, Y.; Hatanaka, K.; Kamijo, Y.; Funayama, M.; Mimasaka, S. Detection of Major Psychoactive Compounds (Safrole, Myristicin, and Elemicin) of Nutmeg in Human Serum via GC–MS/MS Using MonoSpin® Extraction: Application in a Nutmeg Poisoning Case. J. Pharm. Biomed. Anal. 2023, 234, 115565. [Google Scholar] [CrossRef]
- Sangalli, B.C.; Sangalli, B.; Chiang, W. Toxicology of Nutmeg Abuse. J. Toxicol. Clin. Toxicol. 2000, 38, 671–678. [Google Scholar] [CrossRef] [PubMed]
- El-Alfy, A.T.; Wilson, L.; ElSohly, M.A.; Abourashed, E.A. Towards a Better Understanding of the Psychopharmacology of Nutmeg: Activities in the Mouse Tetrad Assay. J. Ethnopharmacol. 2009, 126, 280–286. [Google Scholar] [CrossRef]
- Götz, M.E.; Sachse, B.; Schäfer, B.; Eisenreich, A. Myristicin and Elemicin: Potentially Toxic Alkenylbenzenes in Food. Foods 2022, 11, 1988. [Google Scholar] [CrossRef]
- Toczek, M.; Malinowska, B. Enhanced Endocannabinoid Tone as a Potential Target of Pharmacotherapy. Life Sci. 2018, 204, 20–45. [Google Scholar] [CrossRef]
- Paredes-Ruiz, K.J.; Chavira-Ramos, K.; Orozco-Morales, M.; Karasu, C.; Tinkov, A.A.; Aschner, M.; Santamaría, A.; Colín-González, A.L. On the Biomedical Properties of Endocannabinoid Degradation and Reuptake Inhibitors: Pre-Clinical and Clinical Evidence. Neurotox. Res. 2021, 39, 2072–2097. [Google Scholar] [CrossRef]
- Mullard, A. EMA Rewrites Phase I Guidelines in Aftermath of FAAH Tragedy. Nat. Rev. Drug Discov. 2016, 15, 595. [Google Scholar] [CrossRef]
- Hua, D.Y.-H.; Hindocha, C.; Baio, G.; Lees, R.; Shaban, N.; Morgan, C.J.; Mofeez, A.; Curran, H.V.; Freeman, T.P. Effects of Cannabidiol on Anandamide Levels in Individuals with Cannabis Use Disorder: Findings from a Randomised Clinical Trial for the Treatment of Cannabis Use Disorder. Transl. Psychiatry 2023, 13, 131. [Google Scholar] [CrossRef] [PubMed]
- Poulia, K.-A.; Bakaloudi, D.R.; Alevizou, M.; Papakonstantinou, E.; Zampelas, A.; Chourdakis, M. Impact of Organic Foods on Chronic Diseases and Health Perception: A Systematic Review of the Evidence. Eur. J. Clin. Nutr. 2025, 79, 90–103. [Google Scholar] [CrossRef] [PubMed]
- Pagidipati, N.J.; Taub, P.R.; Ostfeld, R.J.; Kirkpatrick, C.F. Dietary Patterns to Promote Cardiometabolic Health. Nat. Rev. Cardiol. 2025, 22, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; the International Natural Product Sciences Taskforce; Supuran, C.T. Natural Products in Drug Discovery: Advances and Opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Hentsch, A.; Guberman, M.; Radetzki, S.; Kaushik, S.; Huizenga, M.; He, Y.; Contzen, J.; Kuhn, B.; Benz, J.; Schippers, M.; et al. Highly Specific Miniaturized Fluorescent Monoacylglycerol Lipase Probes Enable Translational Research. J. Am. Chem. Soc. 2025, 147, 10188–10202. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).