Subcellular Compartmentalization of Glucose Mediated Insulin Secretion
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells
2.2. Animals
2.3. Mouse Single Islet Cells
2.4. Molecular Biology and Generation of Adenoviruses
2.5. FLIM Imaging
2.6. Organelle Live Staining
2.7. Glucose Stimulation
2.8. ATP and Calcium Live Imaging
2.9. Quantification and Statistical Analysis
3. Results
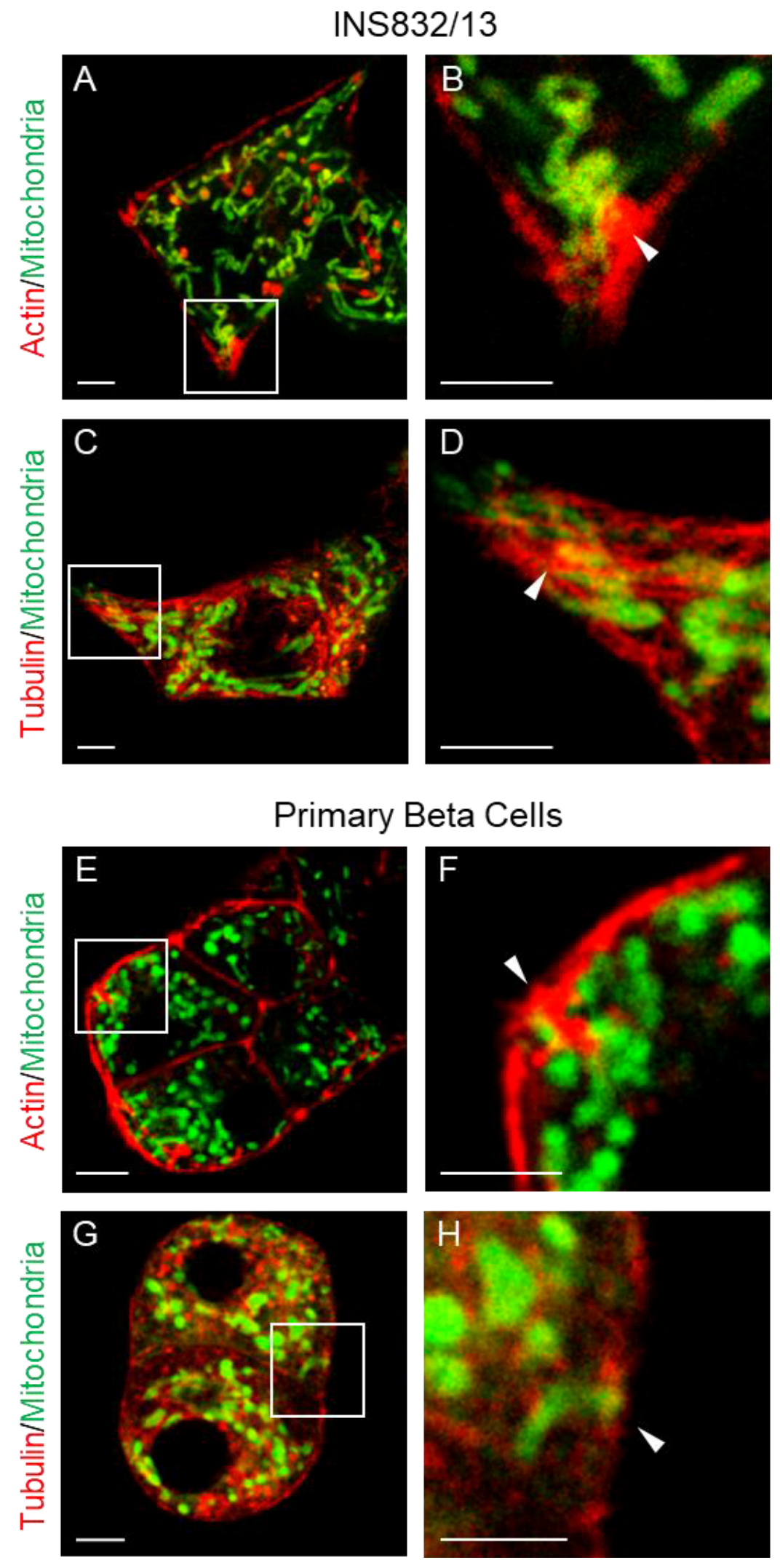
3.1. Mitochondria Are Present in the Submembrane Zone of Beta Cells
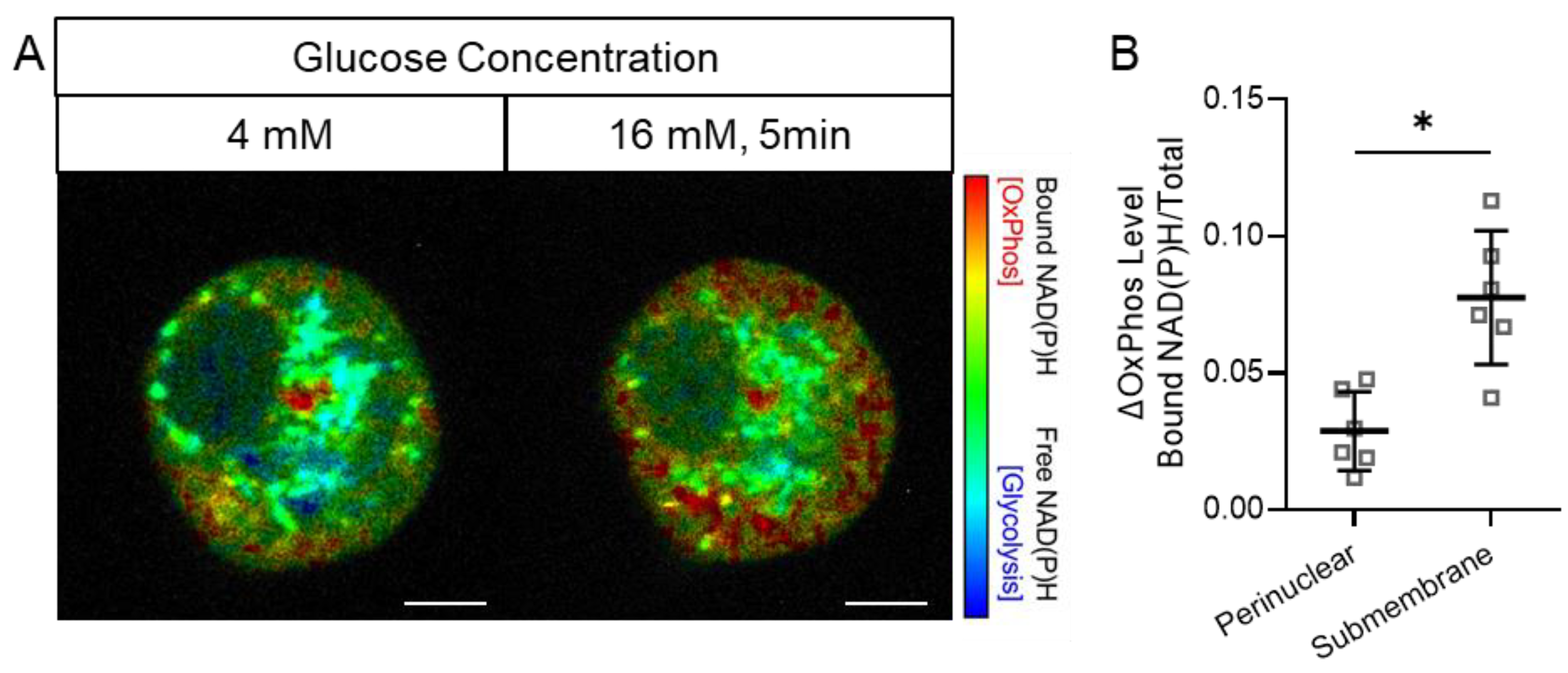
3.2. Preferential Early Activation of Mitochondrial OxPhos in the Submembrane Zone of Beta Cells in Response to a Glucose Stimulus
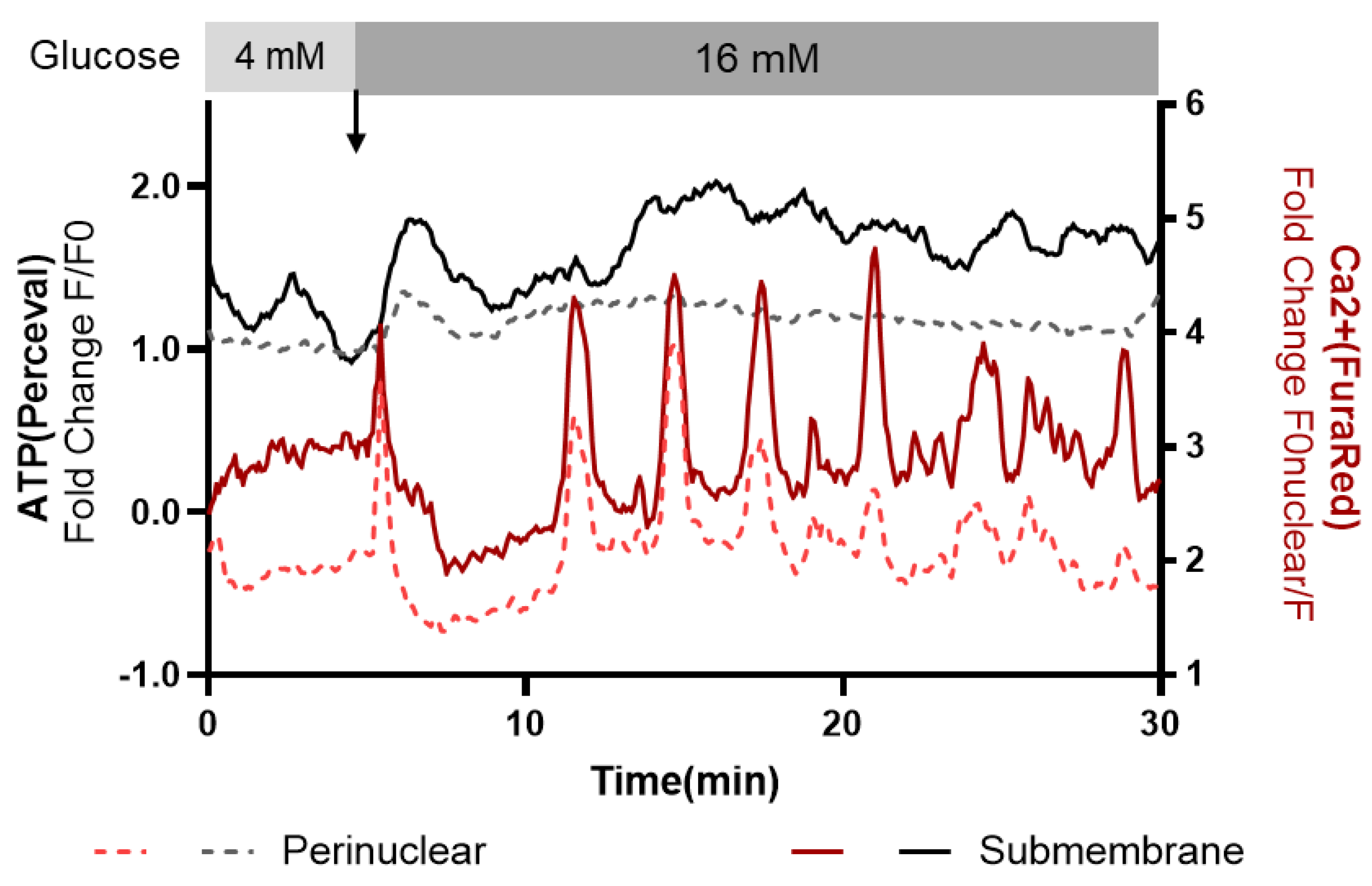
3.3. Subcellular Ca2+ and ATP Responses to a Glucose Stimulus
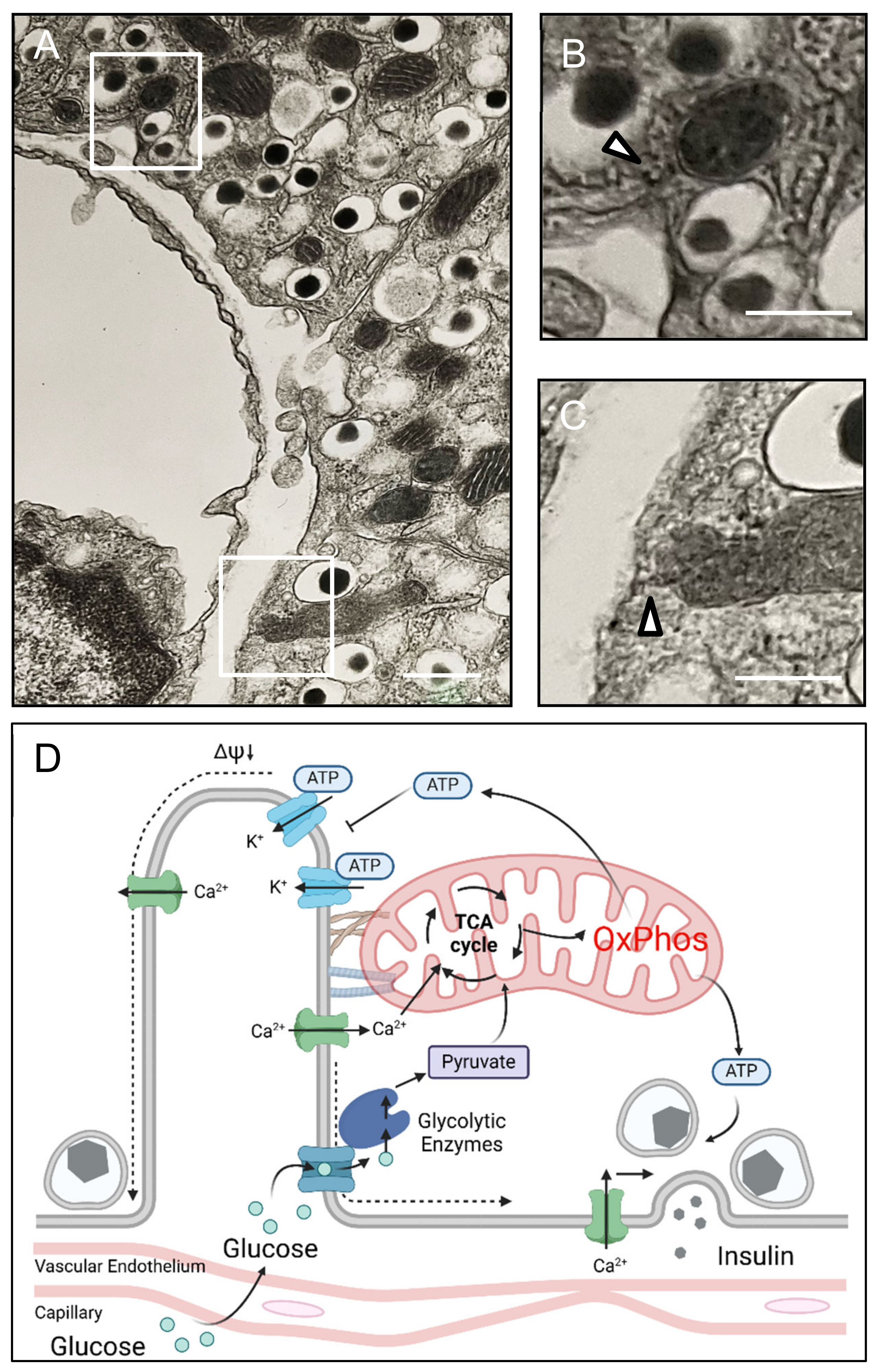
3.4. Beta Cell Mitochondria Are Proximate to the Islet Capillary Interface
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bedoya, F.J.; Wilson, J.M.; Ghosh, A.K.; Finegold, D.; Matschinsky, F.M. The glucokinase glucose sensor in human pancreatic islet tissue. Diabetes 1986, 35, 61–67. [Google Scholar] [CrossRef]
- Rorsman, P.; Ramracheya, R.; Rorsman, N.J.; Zhang, Q. ATP-regulated potassium channels and voltage-gated calcium channels in pancreatic alpha and beta cells: Similar functions but reciprocal effects on secretion. Diabetologia 2014, 57, 1749–1761. [Google Scholar] [CrossRef] [PubMed]
- Ashcroft, F.M.; Rorsman, P. K(ATP) channels and islet hormone secretion: New insights and controversies. Nat. Rev. Endocrinol. 2013, 9, 660–669. [Google Scholar] [CrossRef] [PubMed]
- Sanchez Caballero, L.; Gorgogietas, V.; Arroyo, M.N.; Igoillo-Esteve, M. Molecular mechanisms of β-cell dysfunction and death in monogenic forms of diabetes. Int. Rev. Cell Mol. Biol. 2021, 359, 139–256. [Google Scholar] [CrossRef]
- Wang, Z.; Gurlo, T.; Matveyenko, A.V.; Elashoff, D.; Wang, P.; Rosenberger, M.; Junge, J.A.; Stevens, R.C.; White, K.L.; Fraser, S.E. Live-cell imaging of glucose-induced metabolic coupling of β and α cell metabolism in health and type 2 diabetes. Commun. Biol. 2021, 4, 594. [Google Scholar] [CrossRef]
- Cottle, L.; Gan, W.J.; Gilroy, I.; Samra, J.S.; Gill, A.J.; Loudovaris, T.; Thomas, H.E.; Hawthorne, W.J.; Kebede, M.A.; Thorn, P. Structural and functional polarisation of human pancreatic beta cells in islets from organ donors with and without type 2 diabetes. Diabetologia 2021, 64, 618–629. [Google Scholar] [CrossRef]
- Kennedy, H.J.; Pouli, A.E.; Ainscow, E.K.; Jouaville, L.S.; Rizzuto, R.; Rutter, G.A. Glucose generates sub-plasma membrane ATP microdomains in single islet beta-cells. Potential role for strategically located mitochondria. J. Biol. Chem. 1999, 274, 13281–13291. [Google Scholar] [CrossRef]
- Gan, W.J.; Zavortink, M.; Ludick, C.; Templin, R.; Webb, R.; Ma, W.; Poronnik, P.; Parton, R.G.; Gaisano, H.Y.; Shewan, A.M.; et al. Cell polarity defines three distinct domains in pancreatic β-cells. J. Cell Sci. 2017, 130, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.; Potapenko, E.; Davis, D.B.; Merrins, M.J. A plasma membrane-associated glycolytic metabolon is functionally coupled to K(ATP) channels in pancreatic α and β cells from humans and mice. Cell Rep. 2023, 42, 112394. [Google Scholar] [CrossRef]
- Sherman, A. Contributions of modeling to understanding stimulus-secretion coupling in pancreatic beta-cells. Am. J. Physiol. 1996, 271, E362–E372. [Google Scholar] [CrossRef]
- Zhang, M.; Goforth, P.; Bertram, R.; Sherman, A.; Satin, L. The Ca2+ dynamics of isolated mouse beta-cells and islets: Implications for mathematical models. Biophys. J. 2003, 84, 2852–2870. [Google Scholar] [CrossRef] [PubMed]
- Hohmeier, H.E.; Mulder, H.; Chen, G.; Henkel-Rieger, R.; Prentki, M.; Newgard, C.B. Isolation of INS-1-derived cell lines with robust ATP-sensitive K+ channel-dependent and -independent glucose-stimulated insulin secretion. Diabetes 2000, 49, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Tarasov, A.I.; Semplici, F.; Ravier, M.A.; Bellomo, E.A.; Pullen, T.J.; Gilon, P.; Sekler, I.; Rizzuto, R.; Rutter, G.A. The mitochondrial Ca2+ uniporter MCU is essential for glucose-induced ATP increases in pancreatic β-cells. PLoS ONE 2012, 7, e39722. [Google Scholar] [CrossRef] [PubMed]
- Perkins, G.A.; Tjong, J.; Brown, J.M.; Poquiz, P.H.; Scott, R.T.; Kolson, D.R.; Ellisman, M.H.; Spirou, G.A. The micro-architecture of mitochondria at active zones: Electron tomography reveals novel anchoring scaffolds and cristae structured for high-rate metabolism. J. Neurosci. 2010, 30, 1015–1026. [Google Scholar] [CrossRef] [PubMed]
- Montes de Oca Balderas, P. Mitochondria-plasma membrane interactions and communication. J. Biol. Chem. 2021, 297, 101164. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, A.; Shi, R.; Luciani, D.S. A pipeline for multidimensional confocal analysis of mitochondrial morphology, function, and dynamics in pancreatic β-cells. Am. J. Physiol. Endocrinol. Metab. 2020, 318, E87–E101. [Google Scholar] [CrossRef]
- Li, J.; Shuai, H.Y.; Gylfe, E.; Tengholm, A. Oscillations of sub-membrane ATP in glucose-stimulated beta cells depend on negative feedback from Ca(2+). Diabetologia 2013, 56, 1577–1586. [Google Scholar] [CrossRef] [PubMed]
- Pørksen, N.; Munn, S.; Steers, J.; Veldhuis, J.D.; Butler, P.C. Effects of glucose ingestion versus infusion on pulsatile insulin secretion. The incretin effect is achieved by amplification of insulin secretory burst mass. Diabetes 1996, 45, 1317–1323. [Google Scholar] [CrossRef] [PubMed]
- Song, S.H.; McIntyre, S.S.; Shah, H.; Veldhuis, J.D.; Hayes, P.C.; Butler, P.C. Direct measurement of pulsatile insulin secretion from the portal vein in human subjects. J. Clin. Endocrinol. Metab. 2000, 85, 4491–4499. [Google Scholar] [CrossRef] [PubMed]
- Bertram, R.; Satin, L.; Zhang, M.; Smolen, P.; Sherman, A. Calcium and glycolysis mediate multiple bursting modes in pancreatic islets. Biophys. J. 2004, 87, 3074–3087. [Google Scholar] [CrossRef]
- Henquin, J.C. Triggering and amplifying pathways of regulation of insulin secretion by glucose. Diabetes 2000, 49, 1751–1760. [Google Scholar] [CrossRef]
- Griesche, N.; Sanchez, G.; Hermans, C.; Idevall-Hagren, O. Cortical mitochondria regulate insulin secretion by local Ca(2+) buffering in rodent beta cells. J. Cell Sci. 2019, 132, jcs228544. [Google Scholar] [CrossRef] [PubMed]
- Mawla, A.M.; Huising, M.O. Navigating the Depths and Avoiding the Shallows of Pancreatic Islet Cell Transcriptomes. Diabetes 2019, 68, 1380–1393. [Google Scholar] [CrossRef]
- Lewandowski, S.L.; Cardone, R.L.; Foster, H.R.; Ho, T.; Potapenko, E.; Poudel, C.; VanDeusen, H.R.; Sdao, S.M.; Alves, T.C.; Zhao, X.; et al. Pyruvate Kinase Controls Signal Strength in the Insulin Secretory Pathway. Cell Metab. 2020, 32, 736–750.e735. [Google Scholar] [CrossRef] [PubMed]
- Jeyarajan, S.; Zhang, I.X.; Arvan, P.; Lentz, S.I.; Satin, L.S. Simultaneous Measurement of Changes in Mitochondrial and Endoplasmic Reticulum Free Calcium in Pancreatic Beta Cells. Biosensors 2023, 13, 382. [Google Scholar] [CrossRef]
- Bingley, P.J.; Matthews, D.R.; Williams, A.J.; Bottazzo, G.F.; Gale, E.A. Loss of regular oscillatory insulin secretion in islet cell antibody positive non-diabetic subjects. Diabetologia 1992, 35, 32–38. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Satin, L.S.; Butler, P.C.; Ha, J.; Sherman, A.S. Pulsatile insulin secretion, impaired glucose tolerance and type 2 diabetes. Mol. Aspects Med. 2015, 42, 61–77. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Dominguez, J.R.; Melton, D.A. Cell maturation: Hallmarks, triggers, and manipulation. Cell 2022, 185, 235–249. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Gurlo, T.; Satin, L.S.; Fraser, S.E.; Butler, P.C. Subcellular Compartmentalization of Glucose Mediated Insulin Secretion. Cells 2025, 14, 198. https://doi.org/10.3390/cells14030198
Wang Z, Gurlo T, Satin LS, Fraser SE, Butler PC. Subcellular Compartmentalization of Glucose Mediated Insulin Secretion. Cells. 2025; 14(3):198. https://doi.org/10.3390/cells14030198
Chicago/Turabian StyleWang, Zhongying, Tatyana Gurlo, Leslie S. Satin, Scott E. Fraser, and Peter C. Butler. 2025. "Subcellular Compartmentalization of Glucose Mediated Insulin Secretion" Cells 14, no. 3: 198. https://doi.org/10.3390/cells14030198
APA StyleWang, Z., Gurlo, T., Satin, L. S., Fraser, S. E., & Butler, P. C. (2025). Subcellular Compartmentalization of Glucose Mediated Insulin Secretion. Cells, 14(3), 198. https://doi.org/10.3390/cells14030198




