Vaccinia Virus Converts Microglia into Potent Oncolytic Agent for Glioblastoma and Neuroblastoma
Highlights
- Microglia infected with vaccinia virus (VV) efficiently deliver viruses to neuroblastoma and glioblastoma cells.
- Infected microglia promote oncolysis and viral spread in both 2D and 3D tumor models.
- Microglia act as effective carriers for VV.
- This platform can enhance oncolytic virotherapy and potentially integrate with immuno- or radiotherapy.
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines and Media
2.2. Virus Strain
2.3. Viral Replication and Plaque Assay
2.4. MTT Cell Viability Assay
2.5. Co-Culture Assays
2.6. Cell Invasion Assay
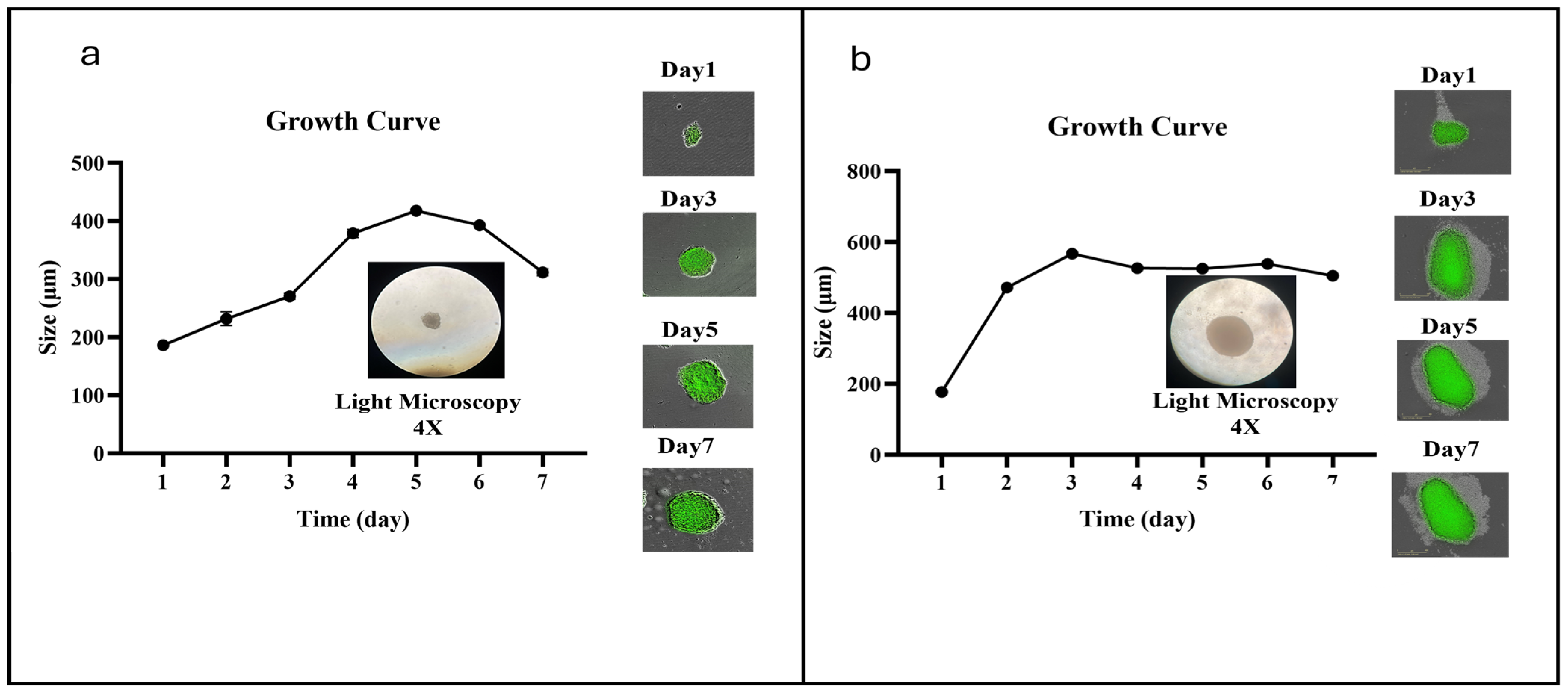
2.7. Scaffold-Free 3D Culture
2.8. Statistical Analysis
3. Results
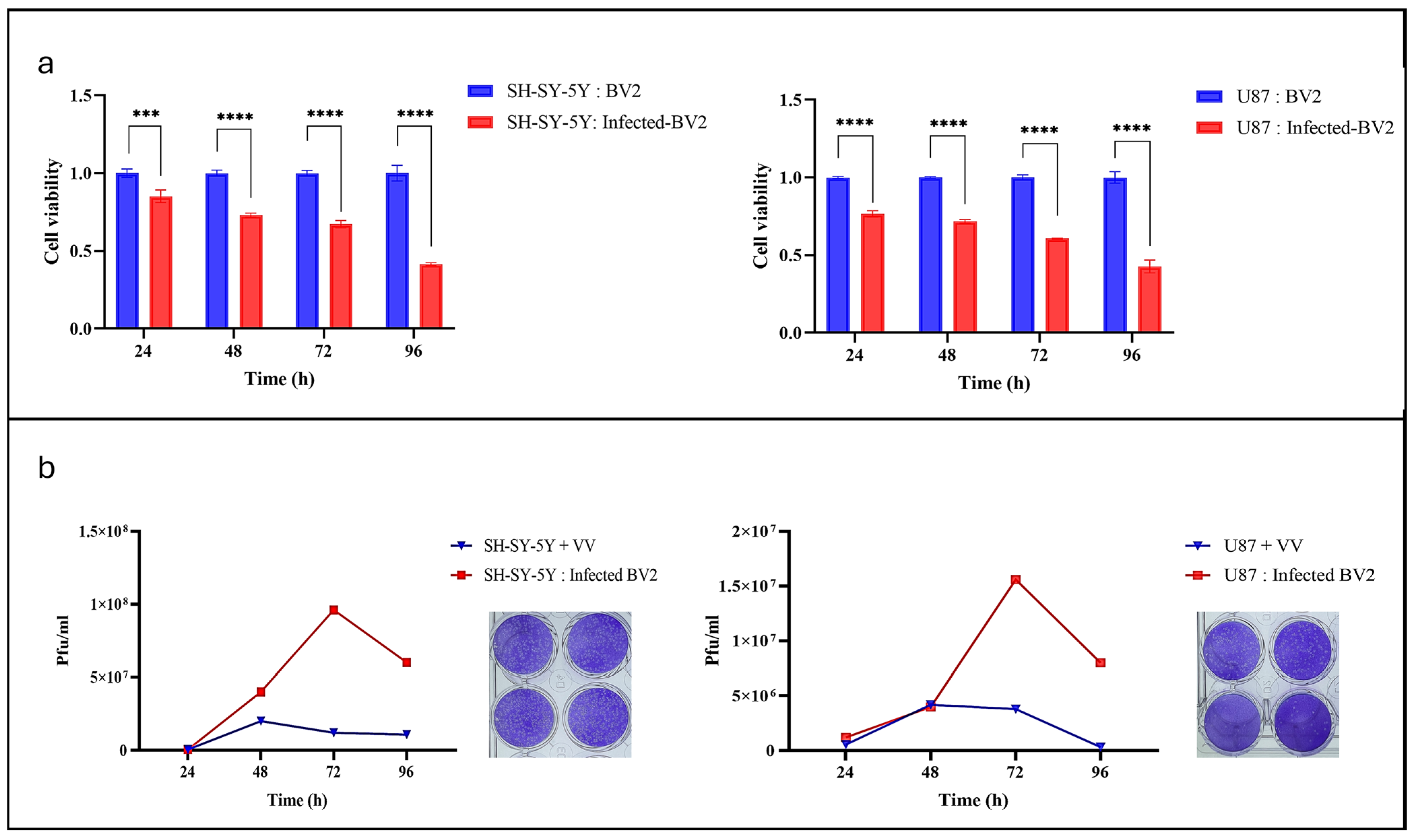
3.1. VV Efficiently Replicates in BV2 Microglia
3.2. VV-Infected Microglia Induce Cytotoxicity in Neuroblastoma and Glioblastoma Tumor Cell Lines
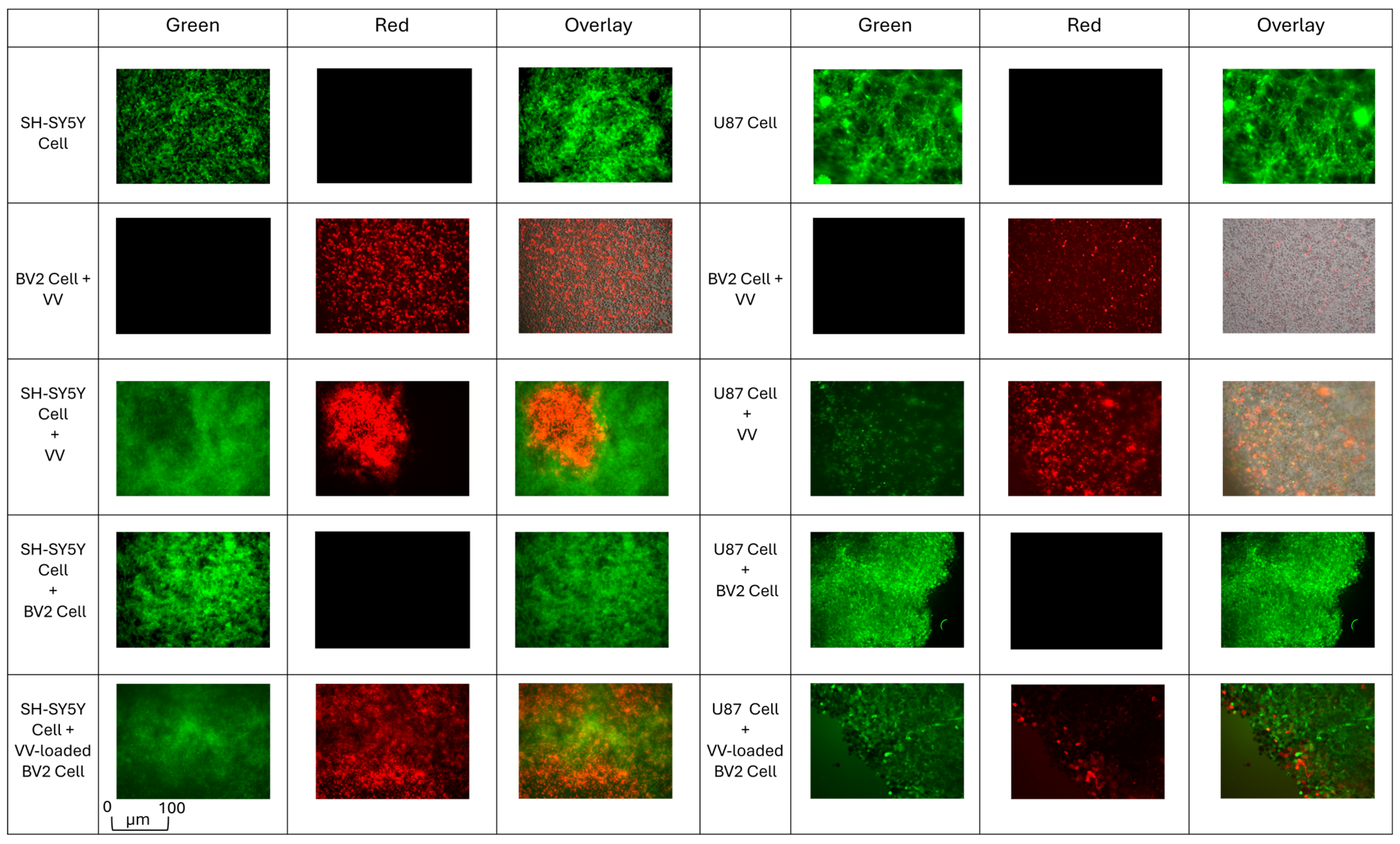
3.3. Post-Coculture VV-Infected Microglia Enhance Viral Spread and Cancer Cell Death
3.4. VV-Infected Microglia Suppress Tumor Cell Invasion and Promote Post-Coculture Viral Transfer
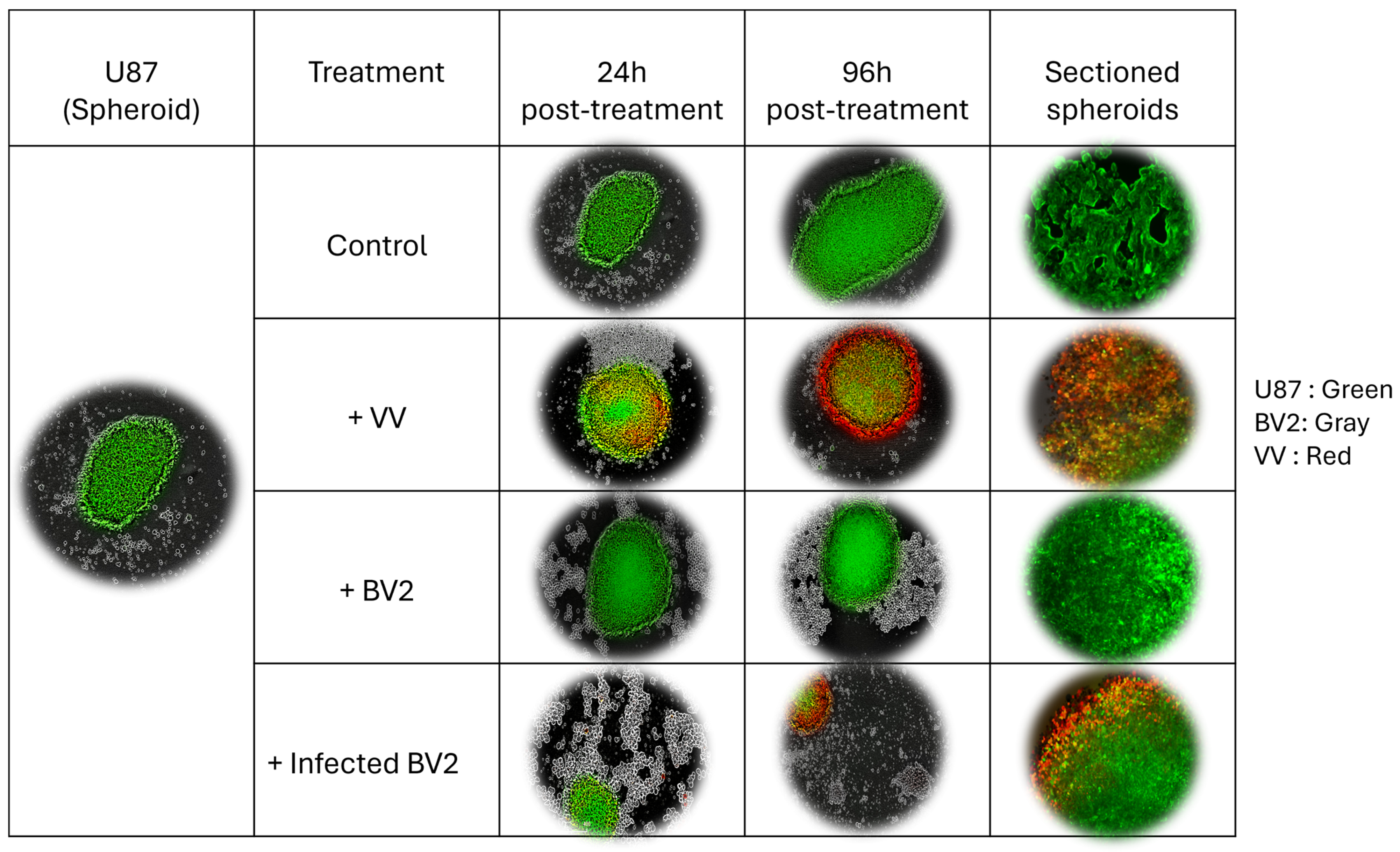
3.5. VV-Infected Microglia Disrupt 3D Tumor Spheroid Structure and Cell Viability
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of Variance |
| ATCC | American Type Culture Collection |
| CAR | Chimeric Antigen Receptor |
| CNS | Central Nervous System |
| CV-1 | African Green Monkey Kidney Fibroblasts (cell line) |
| CXCR4 | C-X-C Motif Chemokine Receptor 4 |
| DC | Dendritic Cell |
| DMEM | Dulbecco’s Modified Eagle Medium |
| EV | Extracellular Virus |
| FBS | Fetal Bovine Serum |
| GBM | Glioblastoma |
| GFP | Green Fluorescent Protein |
| GM-CSF | Granulocyte-Macrophage Colony-Stimulating Factor |
| IFNγ | Interferon Gamma |
| IL-15/IL-21 | Interleukin-15/Interleukin-21 |
| LIVP | Lister Institute of Viral Preparations (Vaccinia virus strain) |
| LPS | Lipopolysaccharide |
| MMP | Matrix Metalloprotease |
| MOI | Multiplicity of Infection |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| nAbs | Neutralizing Antibodies |
| NAP | Neutrophil-Activating Protein |
| OCT | Optimal Cutting Temperature (compound) |
| OV | Oncolytic Virus |
| PBS | Phosphate-Buffered Saline |
| PFU | Plaque-Forming Unit |
| poly(I:C) | Polyinosinic-polycytidylic acid |
| rAAV2 | Recombinant Adeno-Associated Virus serotype 2 |
| SEM | Standard Error of the Mean |
| TLRTLR9 | Toll-like Receptor 9 |
| VV | Vaccinia Virus |
| 2D | Two-Dimensional |
| 3D | Three-Dimensional |
References
- Fukuhara, H.; Ino, Y.; Todo, T. Oncolytic virus therapy: A new era of cancer treatment at dawn. Cancer Sci. 2016, 107, 1373–1379. [Google Scholar] [CrossRef] [PubMed]
- Fenner, F. Global eradication of smallpox. Rev. Infect. Dis. 1982, 4, 916–930. [Google Scholar] [CrossRef] [PubMed]
- Dales, S. The uptake and development of vaccinia virus in strain L cells followed with labeled viral deoxyribonucleic acid. J. Cell Biol. 1963, 18, 51–72. [Google Scholar] [CrossRef] [PubMed]
- Moss, B. Poxvirus cell entry: How many proteins does it take? Viruses 2012, 4, 688–707. [Google Scholar] [CrossRef]
- Boucher, Y.; Jain, R.K. Microvascular pressure is the principal driving force for interstitial hypertension in solid tumors: Implications for vascular collapse. Cancer Res. 1992, 52, 5110–5114. [Google Scholar]
- Jain, R.K.; Stylianopoulos, T. Delivering nanomedicine to solid tumors. Nat. Rev. Clin. Oncol. 2010, 7, 653–664. [Google Scholar] [CrossRef]
- Zhang, X.; He, J.; Shao, Y. Research progress and development potential of oncolytic vaccinia virus. Chin. Med. J. 2025, 138, 777–791. [Google Scholar] [CrossRef]
- Grovas, A.; Fremgen, A.; Rauck, A.; Ruymann, F.B.; Hutchinson, C.L.; Winchester, D.P.; Menck, H.R. The National Cancer Data Base report on patterns of childhood cancers in the United States. Cancer Interdiscip. Int. J. Am. Cancer Soc. 1997, 80, 2321–2332. [Google Scholar] [CrossRef]
- Gurney, J.G.; Ross, J.A.; Wall, D.A.; Bleyer, W.A.; Severson, R.K.; Robison, L.L. Infant cancer in the US: Histology-specific incidence and trends, 1973 to 1992. J. Pediatr. Hematol. Oncol. 1997, 19, 428–432. [Google Scholar] [CrossRef]
- Cleveland Clinic. Glioblastoma (GBM). 23 April 2025. Available online: https://my.clevelandclinic.org/health/diseases/17032-glioblastoma (accessed on 7 August 2025).
- Davis, M.E. Glioblastoma: Overview of disease and treatment. Clin. J. Oncol. Nurs. 2016, 20, S2–S8. [Google Scholar] [CrossRef]
- Wu, S.-Y.; Watabe, K. The roles of microglia/macrophages in tumor progression of brain cancer and metastatic disease. Front. Biosci. (Landmark Ed.) 2017, 22, 1805. [Google Scholar] [CrossRef] [PubMed]
- Bo, L.; Tianming, L.; Fengliang, F.; Wenping, L.; Jinzuo, H.; Dongbo, X.; Biao, M.; Haijun, S. Global trends of Vaccinia oncolytic virus therapy over the past two decades: Bibliometric and visual analysis. Front. Immunol. 2023, 14, 1063548. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Huang, J.; Tong, A.; Yang, H. Oncolytic viruses for cancer therapy: Barriers and recent advances. Mol. Ther.-Oncolytics 2019, 15, 234–247. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-T.; Dai, S.-Y.; Zhan, Y.; Yang, R.; Chen, D.-Q.; Li, Y.; Zhou, E.-Q.; Dong, R. Progress of oncolytic virotherapy for neuroblastoma. Front. Pediatr. 2022, 10, 1055729. [Google Scholar] [CrossRef]
- Gross, E.G.; Hamo, M.A.; Estevez-Ordonez, D.; Laskay, N.M.; Atchley, T.J.; Johnston, J.M.; Markert, J.M. Oncolytic virotherapies for pediatric tumors. Expert Opin. Biol. Ther. 2023, 23, 987–1003. [Google Scholar] [CrossRef]
- Kim, J.; Thorne, S.; Oh, J.; Park, B.; Lee, D.; Kim, J.; Je, J.; Hwang, T.-H. Systemic armed oncolytic and immunologic therapy for cancer with JX-594, a targeted poxvirus expressing GM-CSF. Mol. Ther. 2006, 14, 361–370. [Google Scholar] [CrossRef]
- Parato, K.A.; Breitbach, C.J.; Le Boeuf, F.; Wang, J.; Storbeck, C.; Ilkow, C.; Diallo, J.-S.; Falls, T.; Burns, J.; Garcia, V.; et al. The oncolytic poxvirus JX-594 selectively replicates in and destroys cancer cells driven by genetic pathways commonly activated in cancers. Mol. Ther. 2012, 20, 749–758. [Google Scholar] [CrossRef]
- Cripe, T.P.; Ngo, M.C.; Geller, J.I.; Louis, C.U.; Currier, M.A.; Racadio, J.M.; Towbin, A.J.; Rooney, C.M.; Pelusio, A.; Moon, A.; et al. Phase 1 study of intratumoral Pexa-Vec (JX-594), an oncolytic and immunotherapeutic vaccinia virus, in pediatric cancer patients. Mol. Ther. 2015, 23, 602–608. [Google Scholar] [CrossRef]
- Garzon-Muvdi, T.; Theodros, D.; Luksik, A.S.; Maxwell, R.; Kim, E.; Jackson, C.M.; Belcaid, Z.; Ganguly, S.; Tyler, B.; Brem, H.; et al. Dendritic cell activation enhances anti-PD-1 mediated immunotherapy against glioblastoma. Oncotarget 2018, 9, 20681. [Google Scholar] [CrossRef]
- Polychronopoulos, P.A.; Bedoya-Reina, O.C.; Johnsen, J.I. The neuroblastoma microenvironment, heterogeneity and immunotherapeutic approaches. Cancers 2024, 16, 1863. [Google Scholar] [CrossRef]
- Webb, M.J.; Sener, U.; Vile, R.G. Current status and challenges of oncolytic virotherapy for the treatment of glioblastoma. Pharmaceuticals 2023, 16, 793. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, Z.; Zhang, C.; Zhang, N.; Wang, P.; Chu, Y.; Dunmall, L.S.C.; Lemoine, N.R.; Wang, Y. An effective therapeutic regime for treatment of glioma using oncolytic vaccinia virus expressing IL-21 in combination with immune checkpoint inhibition. Mol. Ther.-Oncolytics 2022, 26, 105–119. [Google Scholar] [CrossRef] [PubMed]
- Storozynsky, Q.T.; Agopsowicz, K.C.; Noyce, R.S.; Bukhari, A.B.; Han, X.; Snyder, N.; Umer, B.A.; Gamper, A.M.; Godbout, R.; Evans, D.H.; et al. Radiation combined with oncolytic vaccinia virus provides pronounced antitumor efficacy and induces immune protection in an aggressive glioblastoma model. Cancer Lett. 2023, 562, 216169. [Google Scholar] [CrossRef] [PubMed]
- Vasileva, N.; Ageenko, A.; Byvakina, A.; Sen’kova, A.; Kochneva, G.; Mishinov, S.; Richter, V.; Kuligina, E. The recombinant oncolytic virus VV-GMCSF-lact and chemotherapy drugs against human glioma. Int. J. Mol. Sci. 2024, 25, 4244. [Google Scholar] [CrossRef]
- Luo, E.Y.; Sugimura, R.R. Taming microglia: The promise of engineered microglia in treating neurological diseases. J. Neuroinflamm. 2024, 21, 19. [Google Scholar] [CrossRef]
- Hwang, S.-Y.; Yoo, B.-C.; Jung, J.-W.; Oh, E.-S.; Hwang, J.-S.; Shin, J.-A.; Kim, S.-Y.; Cha, S.-H.; Han, I.-O. Induction of glioma apoptosis by microglia-secreted molecules: The role of nitric oxide and cathepsin B. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2009, 1793, 1656–1668. [Google Scholar] [CrossRef]
- Chen, J.-W.E.; Lumibao, J.; Leary, S.; Sarkaria, J.N.; Steelman, A.J.; Gaskins, H.R.; Harley, B.A.C. Crosstalk between microglia and patient-derived glioblastoma cells inhibit invasion in a three-dimensional gelatin hydrogel model. J. Neuroinflamm. 2020, 17, 346. [Google Scholar] [CrossRef]
- Chadarevian, J.P.; Davtyan, H.; Chadarevian, A.L.; Nguyen, J.; Capocchi, J.K.; Le, L.; Escobar, A.; Chadarevian, T.; Mansour, K.; Deynega, E.; et al. Harnessing human iPSC-microglia for CNS-wide delivery of disease-modifying proteins. Cell Stem Cell 2025, 32, 914–934.e8. [Google Scholar] [CrossRef]
- Zha, X.; Zheng, G.; Skutella, T.; Kiening, K.; Unterberg, A.; Younsi, A. Microglia: A promising therapeutic target in spinal cord injury. Neural Regen. Res. 2025, 20, 454–463. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ekrami, E.; Ghanbari, P.; Othman, E.M.; Szalay, A.A. Vaccinia Virus Converts Microglia into Potent Oncolytic Agent for Glioblastoma and Neuroblastoma. Cells 2025, 14, 1943. https://doi.org/10.3390/cells14241943
Ekrami E, Ghanbari P, Othman EM, Szalay AA. Vaccinia Virus Converts Microglia into Potent Oncolytic Agent for Glioblastoma and Neuroblastoma. Cells. 2025; 14(24):1943. https://doi.org/10.3390/cells14241943
Chicago/Turabian StyleEkrami, Elena, Parisa Ghanbari, Eman M. Othman, and Aladar A. Szalay. 2025. "Vaccinia Virus Converts Microglia into Potent Oncolytic Agent for Glioblastoma and Neuroblastoma" Cells 14, no. 24: 1943. https://doi.org/10.3390/cells14241943
APA StyleEkrami, E., Ghanbari, P., Othman, E. M., & Szalay, A. A. (2025). Vaccinia Virus Converts Microglia into Potent Oncolytic Agent for Glioblastoma and Neuroblastoma. Cells, 14(24), 1943. https://doi.org/10.3390/cells14241943





