Association Between Complete Blood Count and the Lipoxygenase Pathway in Hashimoto’s Thyroiditis
Highlights
- Pro-inflammatory arachidonic acid (AA) derivatives may play a vital role in the development of Hashimoto’s Thyroiditis (HT) and the production of anti-thyroid peroxidase antibodies (ATPO) and anti-thyroglobulin antibodies (ATG).
- Excessive activation of lipoxygenases (LOX) in HT may lead to increased pro-inflammatory hydroxyeico-satetraenoic acids (5S-HETE, 12S-HETE and 15S-HETE) and 5-oxo-eicosatetraenoic acid (5-oxo-ETE).
- It may be associated with the development of the most wide-spread endocrine cancer in the world, thyroid cancer.
- This study may contribute to developing new guidelines for diagnosing and treating HT and other autoimmune diseases.
Abstract
1. Introduction
1.1. Hashimoto’s Thyroiditis—Epidemiology, Etiology, and Pathogenesis
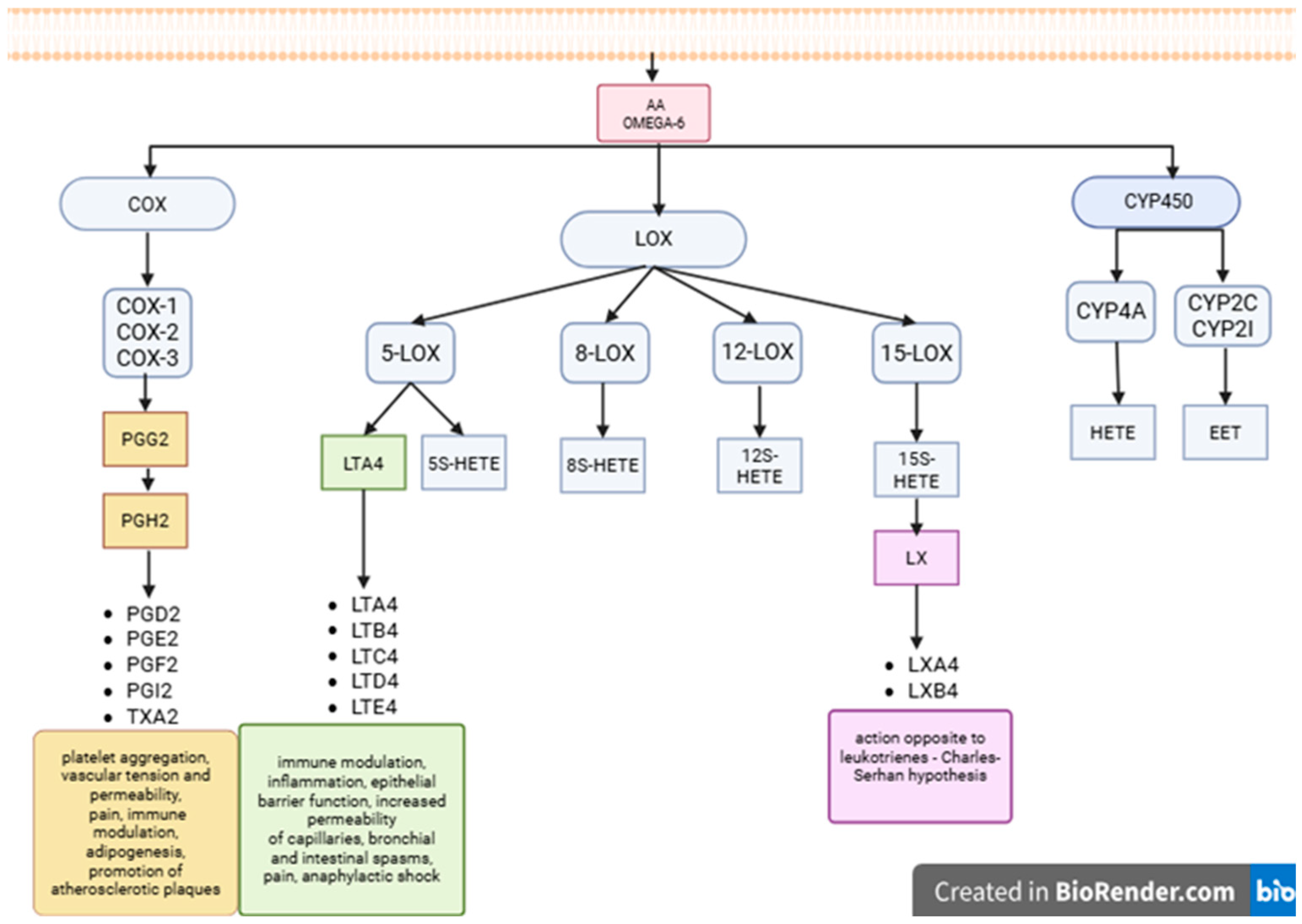
1.2. Lipoxygenase-Derived Products
2. Materials and Methods
2.1. Characteristics of the Study Group
2.2. Sample Collection
2.3. Eicosanoid Extraction
2.4. HPLC Operating Parameters
2.5. Statistical Analysis
3. Results
3.1. Analysis of the Study Group
3.2. Analysis of Complete Blood Count and C-Reactive Protein in the Study Group
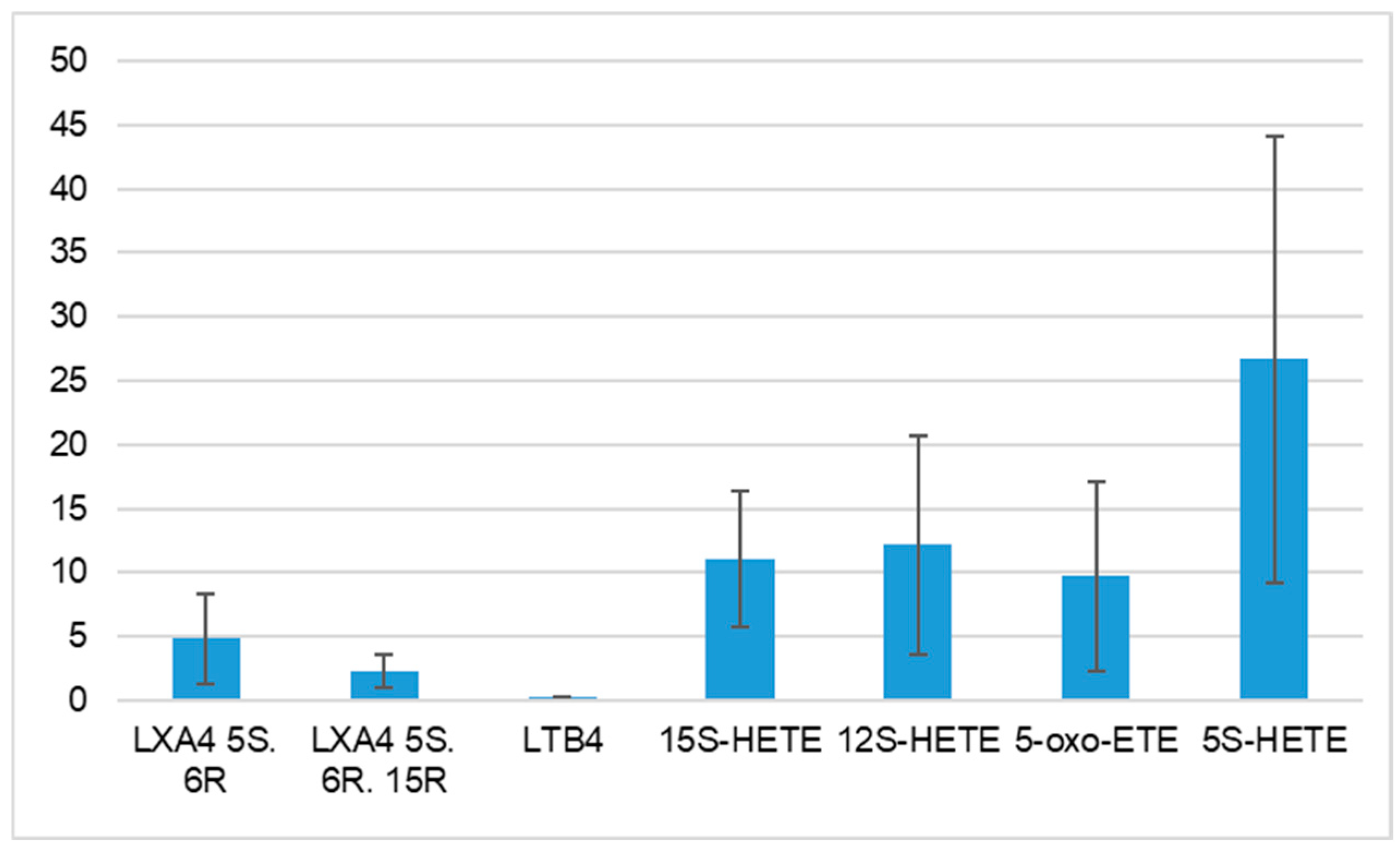
3.3. Characteristics of Arachidonic Acids Derivatives in HT Patients
- -
- LTB4 and fat tissue mass (r = 0.352; p = 0.035)
- -
- LXA4 5S. 6R. 15R and body weight (r = 0.488; p = 0.002)
- -
- LXA4 5S. 6R. 15R and BMI (r = 0.484; p = 0.003)
- -
- LXA4 5S. 6R. 15R and fat tissue mass (r = 0.545; p = 0.000)
- -
- LXA4 5S. 6R. 15R and % body fat content (r = 0.525; p = 0.001)
- -
- 5S-HETE and body weight (r = 0.399; p = 0.016)
- -
- 5S-HETE and BMI (r = 0.346; p = 0.039)
- -
- 5S-HETE and fat tissue mass (r = 0.415; p = 0.012)
- -
- 5S-HETE and % body fat content (r = 0.369; p = 0.027)
- -
- 5S-HETE and soft lean mass (r = 0.337; p = 0.044)
- -
- 15S-HETE and fat tissue mass (r = 0.307; p = 0.068)
- -
- 5-oxo-ETE and fat tissue mass (r = 0.297; p = 0.078)
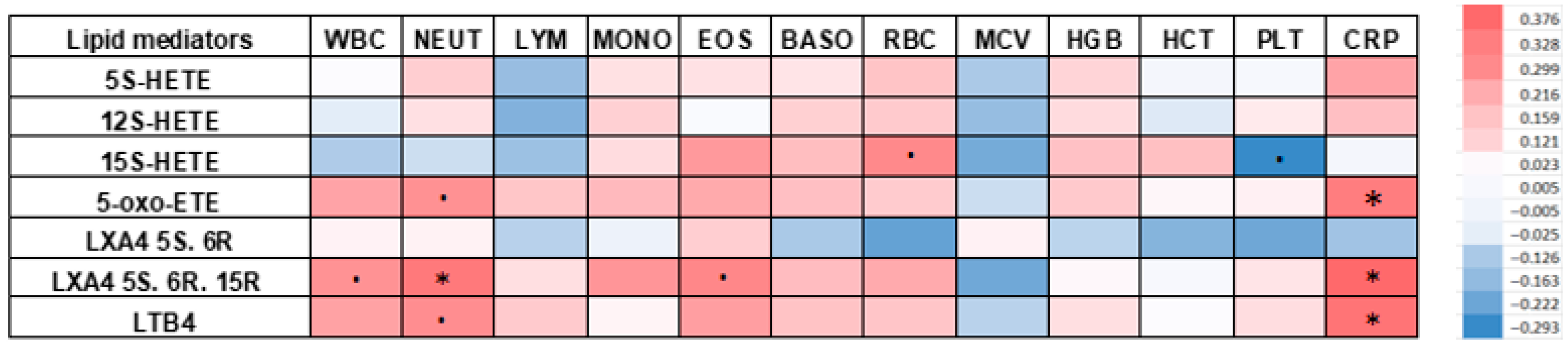
3.4. Correlations Between Complete Blood Count and C-Reactive Protein with Arachidonic Acids Derivatives in HT Patients
- -
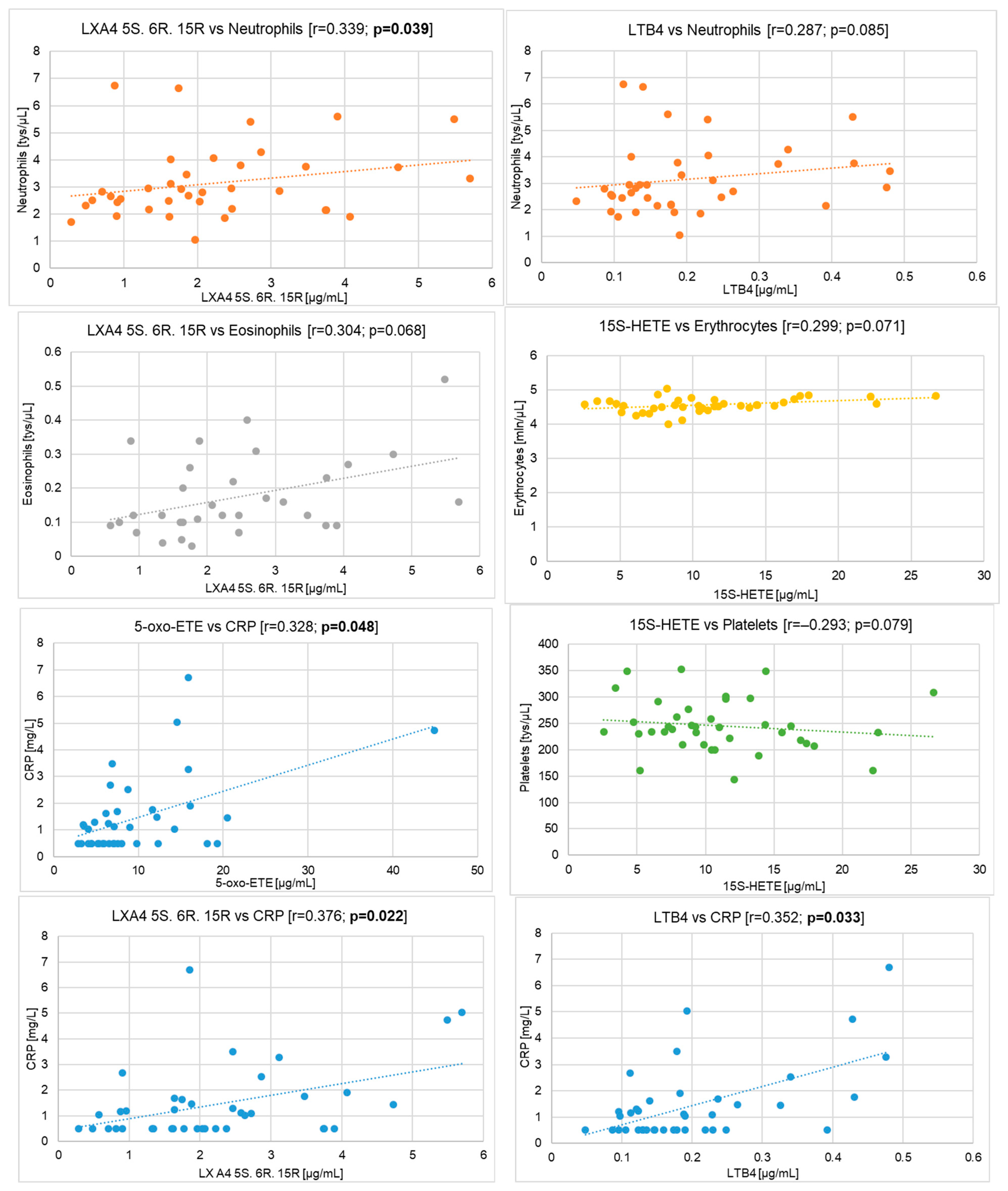
- neutrophils and LXA4 5S. 6R. 15R (r = 0.339; p = 0.039)
- -
- eosinophils and LXA4 5S. 6R. 15R (r = 0.304; p = 0.068)
- -
- erythrocytes and 15S-HETE (r = 0.299; p = 0.071)
- -
- neutrophils and LTB4 (r = 0.287; p = 0.085)
- -
- neutrophils and 5-oxo-ETE (r = 0.277; p = 0.097)
- -
- leukocytes and LXA4 5S. 6R. 15R (r = 0.275; p = 0.099)
- -
- monocytes and LXA4 5S. 6R. 15R (r = 0.267; p = 0.111)
- -
- eosinophils and 15S-HETE (r = 0.257; p = 0.124)
- -
- eosinophils and LTB4 (r = 0.244; p = 0.145)
- -
- leukocytes and LTB4 (r = 0.237; p = 0.158)
- -
- leukocytes and 5-oxo-ETE (r = 0.231; p = 0.169)
- -
- eosinophils and 5-oxo-ETE (r = 0.216; p = 0.201)
- -
- erythrocytes and LXA4 5S. 6R. 15R (r = 0.215; p = 0.202)
- -
- platelets and 15S-HETE (r = −0.293; p = 0.079)
- -
- erythrocytes and LXA4 5S. 6R (r = −0.236; p = 0.161)
- -
- platelets and LXA4 5S. 6R (r = −0.222; p = 0.187)
- -
- mean red blood cell volume and LXA4 5S. 6R. 15R (r = −0.219; p = 0.191)
- -
- mean red blood cell volume and 15S-HETE (r = −0.212; p = 0.208)
- -
- lymphocytes and 12S-HETE (r = −0.194; p = 0.249)
- -
- mean red blood cell volume and 12S-HETE (r = −0.163; p = 0.334)
4. Discussion
4.1. Pro–Inflammatory Lipid Mediators in the Course of Autoimmune Diseases
4.2. The Association Between Hashimoto’s Thyroiditis and Complete Blood Count
4.3. The Association Between Products of LOX and Complete Blood Count and CRP in Hashimoto’s Thyroiditis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AITD | Autoimmune Thyroid Diseases |
| AA | Arachidonic Acid |
| ATG | Anti–thyroglobulin Antibodies |
| ATPO | Anti–thyroid Peroxidase Antibodies |
References
- Ruggeri, R.M.; Barbalace, M.C.; Croce, L.; Malaguti, M.; Campennì, A.; Rotondi, M.; Cannavò, S.; Hrelia, S. Autoimmune Thyroid Disorders: The Mediterranean Diet as a Protective Choice. Nutrients 2023, 15, 3953. [Google Scholar] [CrossRef]
- Ihnatowicz, P.; Drywień, M.; Wątor, P.; Wojsiat, J. The Importance of Nutritional Factors and Dietary Management of Hashimoto’s Thyroiditis. Ann. Agric. Environ. Med. 2020, 27, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Klubo-Gwiezdzinska, J.; Wartofsky, L. Hashimoto Thyroiditis: An Evidence-Based Guide to Etiology, Diagnosis and Treatment. Pol. Arch. Intern. Med. 2022, 132, 16222. [Google Scholar] [CrossRef] [PubMed]
- Mikosch, P.; Aistleitner, A.; Oehrlein, M.; Trifina-Mikosch, E. Hashimoto’s Thyroiditis and Coexisting Disorders in Correlation with HLA Status—An Overview. Wien. Med. Wochenschr. 2023, 173, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Dobiecka, D.; Moskwa, J.; Markiewicz-Żukowska, R.; Socha, K.; Naliwajko, S. Warzywa krzyżowe w diecie pacjentów z chorobą Hashimoto. Postęp. Biochem. 2024, 70, 413–419. [Google Scholar] [CrossRef]
- Shulhai, A.-M.; Rotondo, R.; Petraroli, M.; Patianna, V.; Predieri, B.; Iughetti, L.; Esposito, S.; Street, M.E. The Role of Nutrition on Thyroid Function. Nutrients 2024, 16, 2496. [Google Scholar] [CrossRef]
- Wrońska, K.; Hałasa, M.; Szczuko, M. The Role of the Immune System in the Course of Hashimoto’s Thyroiditis: The Current State of Knowledge. Int. J. Mol. Sci. 2024, 25, 6883. [Google Scholar] [CrossRef]
- Mikoś, H.; Mikoś, M.; Obara-Moszyńska, M.; Niedziela, M. The Role of the Immune System and Cytokines Involved in the Pathogenesis of Autoimmune Thyroid Disease (AITD). Endokrynol. Pol. 2014, 65, 150–155. [Google Scholar] [CrossRef]
- Song, K.; Wang, X.; Yao, W.; Wang, Y.; Zhang, Q.; Tang, Y.; Mou, Y.; Song, X.; Zhou, J. Construction of the Single-Cell Landscape of Hashimoto’s Thyroiditis Tissue and Peripheral Blood by Single-Cell RNA Sequencing. Immun. Inflamm. Dis. 2025, 13, e70153. [Google Scholar] [CrossRef]
- Khan, F.A.; Al-Jameil, N.; Khan, M.F.; Al-Rashid, M.; Tabassum, H. Thyroid Dysfunction: An Autoimmune Aspect. Int. J. Clin. Exp. Med. 2015, 8, 6677–6681. [Google Scholar]
- Stramazzo, I.; Mangino, G.; Capriello, S.; Romeo, G.; Ferrari, S.M.; Fallahi, P.; Bagaglini, M.F.; Centanni, M.; Virili, C. CD20 + T Lymphocytes in Isolated Hashimoto’s Thyroiditis and Type 3 Autoimmune Polyendocrine Syndrome: A Pilot Study. J. Endocrinol. Investig. 2024, 47, 2865–2871. [Google Scholar] [CrossRef]
- Szczuko, M.; Zawadzka, K.; Szczuko, U.; Rudak, L.; Pobłocki, J. The Significance and Process of Inflammation Involving Eicosapentaenoic and Docosahexaenoic Derivatives in Hashimoto’s Disease. Nutrients 2025, 17, 1715. [Google Scholar] [CrossRef]
- Dakal, T.C.; Xiao, F.; Bhusal, C.K.; Sabapathy, P.C.; Segal, R.; Chen, J.; Bai, X. Lipids Dysregulation in Diseases: Core Concepts, Targets and Treatment Strategies. Lipids Health Dis. 2025, 24, 61. [Google Scholar] [CrossRef]
- Zhu, Y.; Wan, F.; Liu, J.; Jia, Z.; Song, T. The Critical Role of Lipid Metabolism in Health and Diseases. Nutrients 2024, 16, 4414. [Google Scholar] [CrossRef]
- de Carvalho, C.C.C.R.; Caramujo, M.J. The Various Roles of Fatty Acids. Molecules 2018, 23, 2583. [Google Scholar] [CrossRef]
- Yoon, H.; Shaw, J.L.; Haigis, M.C.; Greka, A. Lipid Metabolism in Sickness and in Health: Emerging Regulators of Lipotoxicity. Mol. Cell 2021, 81, 3708–3730. [Google Scholar] [CrossRef] [PubMed]
- Visioli, F.; Poli, A. Omega 6 Fatty Acids: Helpful, Harmless or Harmful? Curr. Opin. Clin. Nutr. Metab. Care 2025, 28, 114–120. [Google Scholar] [CrossRef]
- Lee, J.M.; Lee, H.; Kang, S.; Park, W.J. Fatty Acid Desaturases, Polyunsaturated Fatty Acid Regulation, and Biotechnological Advances. Nutrients 2016, 8, 23. [Google Scholar] [CrossRef] [PubMed]
- Qian, C.; Wang, Q.; Qiao, Y.; Xu, Z.; Zhang, L.; Xiao, H.; Lin, Z.; Wu, M.; Xia, W.; Yang, H.; et al. Arachidonic Acid in Aging: New Roles for Old Players. J. Adv. Res. 2024, 70, 79–101. [Google Scholar] [CrossRef]
- Djuricic, I.; Calder, P.C. Beneficial Outcomes of Omega-6 and Omega-3 Polyunsaturated Fatty Acids on Human Health: An Update for 2021. Nutrients 2021, 13, 2421. [Google Scholar] [CrossRef]
- D’Orazio, S.; Mattoscio, D. Dysregulation of the Arachidonic Acid Pathway in Cystic Fibrosis: Implications for Chronic Inflammation and Disease Progression. Pharmaceuticals 2024, 17, 1185. [Google Scholar] [CrossRef]
- Dicks, L.M.T. How Important Are Fatty Acids in Human Health and Can They Be Used in Treating Diseases? Gut Microbes 2024, 16, 2420765. [Google Scholar] [CrossRef]
- Brouwers, H.; Jónasdóttir, H.S.; Kuipers, M.E.; Kwekkeboom, J.C.; Auger, J.L.; Gonzalez-Torres, M.; López-Vicario, C.; Clària, J.; Freysdottir, J.; Hardardottir, A.I.; et al. Anti-Inflammatory and Proresolving Effects of the Omega-6 Polyunsaturated Fatty Acid Adrenic Acid. J. Immunol. 2020, 205, 2840–2849. [Google Scholar] [CrossRef]
- Yui, K.; Imataka, G.; Nakamura, H.; Ohara, N.; Naito, Y. Eicosanoids Derived From Arachidonic Acid and Their Family Prostaglandins and Cyclooxygenase in Psychiatric Disorders. Curr. Neuropharmacol. 2015, 13, 776–785. [Google Scholar] [CrossRef] [PubMed]
- Leuti, A.; Fazio, D.; Fava, M.; Piccoli, A.; Oddi, S.; Maccarrone, M. Bioactive Lipids, Inflammation and Chronic Diseases. Adv. Drug Deliv. Rev. 2020, 159, 133–169. [Google Scholar] [CrossRef] [PubMed]
- Tański, W.; Świątoniowska-Lonc, N.; Tabin, M.; Jankowska-Polańska, B. The Relationship between Fatty Acids and the Development, Course and Treatment of Rheumatoid Arthritis. Nutrients 2022, 14, 1030. [Google Scholar] [CrossRef] [PubMed]
- Biernacki, M.; Skrzydlewska, E. Metabolic Pathways of Eicosanoids—Derivatives of Arachidonic Acid and Their Significance in Skin. Cell. Mol. Biol. Lett. 2025, 30, 7. [Google Scholar] [CrossRef]
- Maciejewska, D.; Drozd, A.; Ossowski, P.; Ryterska, K.; Jamioł-Milc, D.; Banaszczak, M.; Raszeja-Wyszomirska, J.; Kaczorowska, M.; Sabinicz, A.; Stachowska, E. Fatty Acid Changes Help to Better Understand Regression of Nonalcoholic Fatty Liver Disease. World J. Gastroenterol. 2015, 21, 301–310. [Google Scholar] [CrossRef]
- Grzesiak, K.; Rył, A.; Stachowska, E.; Słojewski, M.; Rotter, I.; Ratajczak, W.; Sipak, O.; Piasecka, M.; Dołęgowska, B.; Laszczyńska, M. The Relationship between Eicosanoid Levels and Serum Levels of Metabolic and Hormonal Parameters Depending on the Presence of Metabolic Syndrome in Patients with Benign Prostatic Hyperplasia. Int. J. Environ. Res. Public Health 2019, 16, 1006. [Google Scholar] [CrossRef]
- Hou, M.; Sun, Y. Integrative Analysis of Arachidonic Acid Metabolism in the Pathogenesis and Immune Dysregulation of Psoriasis. Clin. Cosmet. Investig. Dermatol. 2025, 18, 601–615. [Google Scholar] [CrossRef]
- Korbecki, J.; Rębacz-Maron, E.; Kupnicka, P.; Chlubek, D.; Baranowska-Bosiacka, I. Synthesis and Significance of Arachidonic Acid, a Substrate for Cyclooxygenases, Lipoxygenases, and Cytochrome P450 Pathways in the Tumorigenesis of Glioblastoma Multiforme, Including a Pan-Cancer Comparative Analysis. Cancers 2023, 15, 946. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Sun, J.; Zhang, W.; Guo, Z.; Ma, Q. Arachidonic Acid Metabolism in Health and Disease. MedComm 2023, 4, e363. [Google Scholar] [CrossRef]
- Kalyvianaki, K.; Salampasi, E.M.; Katsoulieris, E.N.; Boukla, E.; Vogiatzoglou, A.P.; Notas, G.; Castanas, E.; Kampa, M. 5-Oxo-ETE/OXER1: A Link between Tumor Cells and Macrophages Leading to Regulation of Migration. Molecules 2023, 29, 224. [Google Scholar] [CrossRef]
- Wang, T.; Fu, X.; Chen, Q.; Patra, J.K.; Wang, D.; Wang, Z.; Gai, Z. Arachidonic Acid Metabolism and Kidney Inflammation. Int. J. Mol. Sci. 2019, 20, 3683. [Google Scholar] [CrossRef]
- Szczuko, M.; Kacprzak, J.; Przybylska, A.; Szczuko, U.; Pobłocki, J.; Syrenicz, A.; Drozd, A. The Influence of an Anti-Inflammatory Gluten-Free Diet with EPA and DHA on the Involvement of Maresin and Resolvins in Hashimoto’s Disease. Int. J. Mol. Sci. 2024, 25, 11692. [Google Scholar] [CrossRef] [PubMed]
- Szczuko, M.; Kotlęga, D.; Palma, J.; Zembroń-Łacny, A.; Tylutka, A.; Gołąb-Janowska, M.; Drozd, A. Lipoxins, RevD1 and 9, 13 HODE as the Most Important Derivatives after an Early Incident of Ischemic Stroke. Sci. Rep. 2020, 10, 12849. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Huang, M.; Ge, W.; Feng, J.; Zhang, X.; Li, C.; Wang, L. Identifying Serum Metabolite Biomarkers for Autoimmune Diseases: A Two-Sample Mendelian Randomization and Meta-Analysis. Front. Immunol. 2024, 15, 1300457. [Google Scholar] [CrossRef]
- Robinson, G.; Pineda-Torra, I.; Ciurtin, C.; Jury, E.C. Lipid Metabolism in Autoimmune Rheumatic Disease: Implications for Modern and Conventional Therapies. J. Clin. Investig. 2022, 132, e148552. [Google Scholar] [CrossRef]
- Czapiewska, M.; Hellmann, A.; Zwara, A.; Weryszko, O.; Korczyńska, J.; Taciak, A.; Śledziński, T.; Mika, A. Rearrangements of Tissue Fatty Acid Profile in Papillary Thyroid Cancer with Hashimoto’s Thyroid. Eur. J. Transl. Clin. Med. 2023, 6 (Suppl. 4), 146. [Google Scholar]
- Lukasiewicz, M.; Zwara, A.; Kowalski, J.; Mika, A.; Hellmann, A. The Role of Lipid Metabolism Disorders in the Development of Thyroid Cancer. Int. J. Mol. Sci. 2024, 25, 7129. [Google Scholar] [CrossRef] [PubMed]
- Broos, J.Y.; van der Burgt, R.T.M.; Konings, J.; Rijnsburger, M.; Werz, O.; de Vries, H.E.; Giera, M.; Kooij, G. Arachidonic Acid-Derived Lipid Mediators in Multiple Sclerosis Pathogenesis: Fueling or Dampening Disease Progression? J. Neuroinflamm. 2024, 21, 21. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, A.; Nadler, J.L.; Mirmira, R.G.; Casimiro, I. Regulation of Tissue Inflammation by 12-Lipoxygenases. Biomolecules 2021, 11, 717. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, B.; Xu, J.; Zhao, M.; Wang, Z.; Song, Y.; Zhang, H.; Gao, L.; Zhang, Q.; Zhao, J. Thyroxine Therapy Ameliorates Serum Levels of Eicosanoids in Chinese Subclinical Hypothyroidism Patients. Acta Pharmacol. Sin. 2016, 37, 656–663. [Google Scholar] [CrossRef]
- Haynes, R.L.; van Leyen, K. 12/15-Lipoxygenase Expression Is Increased in Oligodendrocytes and Microglia of Periventricular Leukomalacia. Dev. Neurosci. 2013, 35, 140–154. [Google Scholar] [CrossRef] [PubMed]
- Kahnt, A.S.; Häfner, A.-K.; Steinhilber, D. The Role of Human 5-Lipoxygenase (5-LO) in Carcinogenesis—A Question of Canonical and Non-Canonical Functions. Oncogene 2024, 43, 1319–1327. [Google Scholar] [CrossRef]
- Yao, X.; Sa, R.; Ye, C.; Zhang, D.; Zhang, S.; Xia, H.; Wang, Y.; Jiang, J.; Yin, H.; Ying, H. Effects of Thyroid Hormone Status on Metabolic Pathways of Arachidonic Acid in Mice and Humans: A Targeted Metabolomic Approach. Prostaglandins Other Lipid Mediat. 2015, 118–119, 11–18. [Google Scholar] [CrossRef]
- Ahmed, S.S.; Mohammed, A.A. Effects of Thyroid Dysfunction on Hematological Parameters: Case Controlled Study. Ann. Med. Surg. 2020, 57, 52–55. [Google Scholar] [CrossRef]
- Xue, H.; Xu, R. The Lymphocyte Levels of Hashimoto Thyroiditis Patients Were Significantly Lower than That of Healthy Population. Front. Endocrinol. 2025, 16, 1472856. [Google Scholar] [CrossRef]
- Tomczyńska, M.; Salata, I.; Bijak, M.; Saluk-Bijak, J. The Potential Contribution and Role of a Blood Platelets in Autoimmune Thyroid Diseases. J. Cell. Mol. Med. 2018, 22, 6386–6390. [Google Scholar] [CrossRef]
- Onalan, E.; Dönder, E. Neutrophil and Platelet to Lymphocyte Ratio in Patients with Hypothyroid Hashimoto’s Thyroiditis. Acta Biomed. 2020, 91, 310–314. [Google Scholar] [CrossRef]
- Kyritsi, E.M.A.; Yiakoumis, X.; Pangalis, G.A.; Pontikoglou, C.; Pyrovolaki, K.; Kalpadakis, C.; Mavroudi, I.; Koutala, H.; Mastrodemou, S.; Vassilakopoulos, T.P.; et al. High Frequency of Thyroid Disorders in Patients Presenting With Neutropenia to an Outpatient Hematology Clinic STROBE-Compliant Article. Medicine 2015, 94, e886. [Google Scholar] [CrossRef]
- Arpaci, D.; Gurol, G.; Ergenc, H.; Yazar, H.; Tocoglu, A.G.; Ciftci, I.H.; Tamer, A. A Controversial New Approach to Address Hematological Parameters in Hashimoto’s Thyroiditis. Clin. Lab. 2016, 62, 1225–1231. [Google Scholar] [CrossRef]
- Saqib, U.; Pandey, M.; Vyas, A.; Patidar, P.; Hajela, S.; Ali, A.; Tiwari, M.; Sarkar, S.; Yadav, N.; Patel, S.; et al. Lipoxins as Modulators of Diseases. Cells 2025, 14, 1244. [Google Scholar] [CrossRef]
- Chandrasekharan, J.A.; Sharma-Walia, N. Lipoxins: Nature’s Way to Resolve Inflammation. J. Inflamm. Res. 2015, 8, 181–192. [Google Scholar] [CrossRef]
- Kooij, G.; Troletti, C.D.; Leuti, A.; Norris, P.C.; Riley, I.; Albanese, M.; Ruggieri, S.; Libreros, S.; van der Pol, S.M.A.; van het Hof, B.; et al. Specialized Pro-Resolving Lipid Mediators Are Differentially Altered in Peripheral Blood of Patients with Multiple Sclerosis and Attenuate Monocyte and Blood-Brain Barrier Dysfunction. Haematologica 2020, 105, 2056–2070. [Google Scholar] [CrossRef]
- Powell, W.S.; Rokach, J. The Eosinophil Chemoattractant 5-Oxo-ETE and the OXE Receptor. Prog. Lipid Res. 2013, 52, 651–665. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Dai, F.; Wei, J.; Chen, Z. Biological Roles of 5-Oxo-6,8,11,14-Eicosatetraenoic Acid and the OXE Receptor in Allergic Diseases: Collegium Internationale Allergologicum Update 2024. Int. Arch. Allergy Immunol. 2024, 185, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Schrimpe, A.C.; Wright, D.W. Differential Gene Expression Mediated by 15-Hydroxyeicosatetraenoic Acid in LPS-Stimulated RAW 264.7 Cells. Malar. J. 2009, 8, 195. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.; Cong, Y.; Meng, J.; Qian, H.; Ye, W.; Sun, W.-S.; Zhao, J.-N.; Bao, N.-R. Arachidonic Acid Causes Hidden Blood Loss–like Red Blood Cell Damage through Oxidative Stress Reactions. J. Surg. Res. 2017, 211, 14–20. [Google Scholar] [CrossRef]
- Guijas, C.; Bermúdez, M.A.; Meana, C.; Astudillo, A.M.; Pereira, L.; Fernández-Caballero, L.; Balboa, M.A.; Balsinde, J. Neutral Lipids Are Not a Source of Arachidonic Acid for Lipid Mediator Signaling in Human Foamy Monocytes. Cells 2019, 8, 941. [Google Scholar] [CrossRef]
- Yamaguchi, A.; van Hoorebeke, C.; Tourdot, B.E.; Perry, S.C.; Lee, G.; Rhoads, N.; Rickenberg, A.; Green, A.R.; Sorrentino, J.; Yeung, J.; et al. Fatty Acids Negatively Regulate Platelet Function through Formation of Noncanonical 15-lipoxygenase-derived Eicosanoids. Pharmacol. Res. Perspect. 2023, 11, e01056. [Google Scholar] [CrossRef] [PubMed]
- AlOmar, S.; Mitchell, J.L.; AlZahrani, E. Lipoxin A4 Analogue, BML-111, Reduces Platelet Activation and Protects from Thrombosis. Thromb. J. 2024, 22, 39. [Google Scholar] [CrossRef] [PubMed]
- Szczuko, M.; Kwiatkowska, L.; Szczuko, U.; Rudak, L.; Ryterska, K.; Syrenicz, A.; Pobłocki, J.; Drozd, A. Dietary Gluten-Free Regimen Does Not Affect the Suppression of the Inflammatory Response in the Arachidonic Acid Cascade in Hashimoto’s Disease. Int. J. Mol. Sci. 2025, 26, 6507. [Google Scholar] [CrossRef] [PubMed]

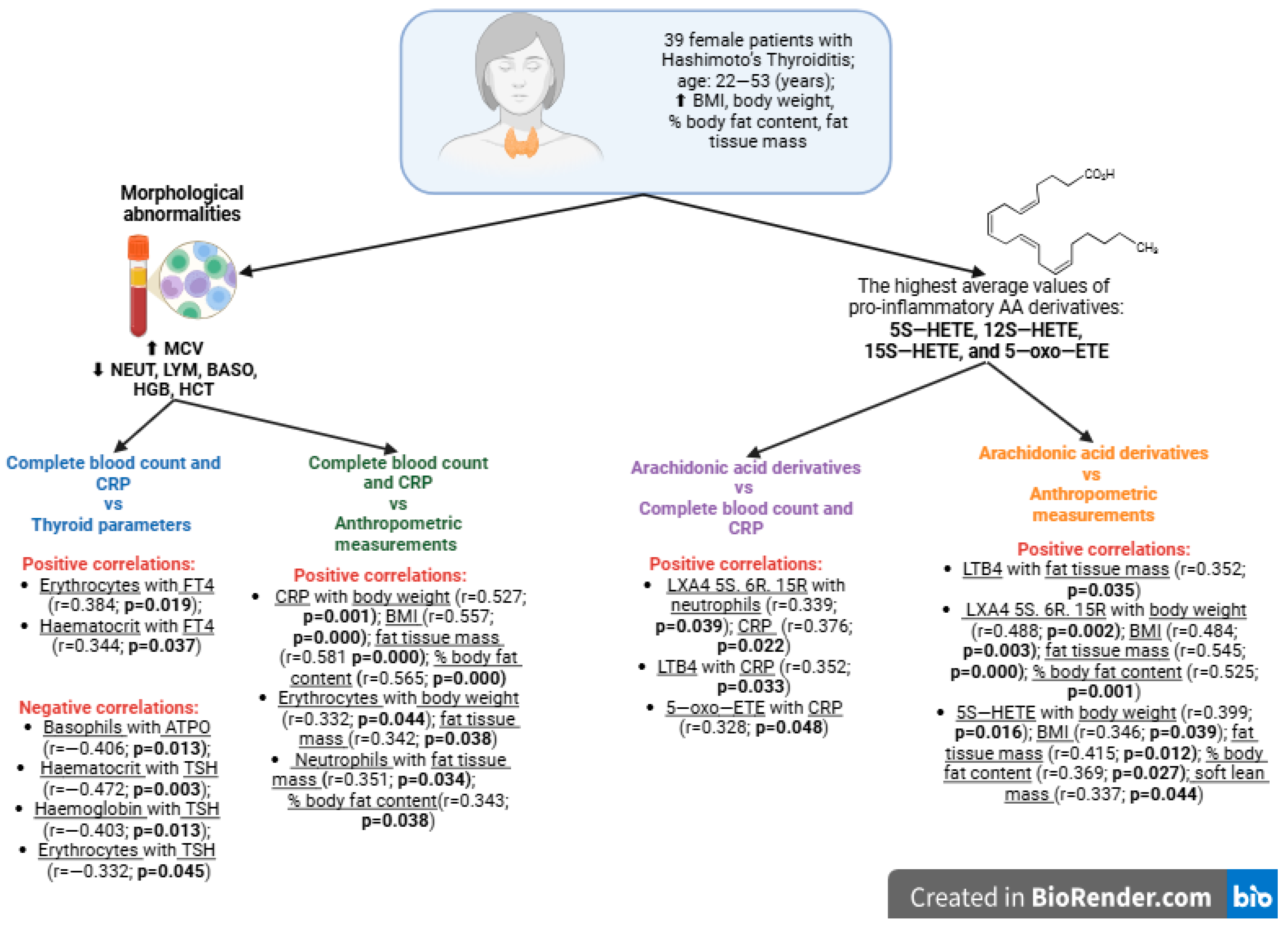
| Parameters | M ± SD [Min–Max] |
|---|---|
| Age [years] | 37.395 ± 8.959 [22–53] |
| Height [cm] | 166.615 ± 5.628 [150–178] |
| Body weight [kg] | 71.521 ± 13.117 [51.6–110.2] |
| BMI [kg/m2] | 25.739 ± 4.417 [18.28–37.25] |
| Fat tissue mass [g] | 26.391.41 ± 9421.325 [1.954–54.316] |
| Body fat content [%] | 35.888 ± 6.972 [20.12–49.30] |
| Soft lean mass [g] | 42.686.051 ± 4749.222 [34.546–55.415] |
| ATPO [0–34 IU/mL] | 228.581 ± 290.014 [9.34–1767] |
| ATG [0–115 IU/mL] | 319.631 ± 546.504 [16.79–3423] |
| TSH [0.270–4.200 µIU/mL] | 3.041 ± 2.748 [0.01–13.92] |
| FT3 [2.00–4.40 pg/mL] | 2.985 ± 0.565 [1.78–4.87] |
| FT4 [0.93–1.70 ng/dL] | 1.284 ± 0.196 [0.8–1.72] |
| Levothyroxine dose [µg] | 68.792 ± 33.938 [25–150] |
| Parameters [Reference Range] | M ± SD [Min–Max] |
|---|---|
| CRP [0.0–5.0 mg/L] | 1.449 ± 1.439 [<1–6.7] |
| WBC [3.8–10.00 tys/µL] | 5.87 ± 1.691 [2.86–9.89] |
| NEUT [2.5–5.4 tys/µL] | 3.153 ± 1.337 [1.05–6.74] |
| LYM [1.5–3.5 tys/µL] | 2.007 ± 0.434 [1.02–2.83] |
| MONO [0.2–1.00 tys/µL] | 0.528 ± 0.14 [0.27–0.87] |
| EOS [0.04–0.40 tys/µL] | 0.173 ± 0.112 [0.03–0.52] |
| BASO [0.02– 0.10 tys/µL] | 0.028 ± 0.015 [0.01–0.07] |
| RBC [3.7–5.10 mln/µL] | 4.558 ± 0.207 [3.99–5.03] |
| HGB [12.0–16.0 g/dL] | 13.346 ± 0.897 [10.3–15.1] |
| HCT [37.0–47.0%] | 38.987 ± 2.264 [32.7–44] |
| MCV [80.0–90.0 fL] | 85.564 ± 4.208 [71.9–92.7] |
| PLT [150–450 tys/µL] | 245.769 ± 49.794 [143–353] |
| 5S–HETE | 12S–HETE | 15S–HETE | 5–oxo–ETE | LXA4 5S. 6R | LXA4 5S. 6R. 15R | LTB4 | |
|---|---|---|---|---|---|---|---|
| [μg/mL] | [μg/mL] | [μg/mL] | [μg/mL] | [μg/mL] | [μg/mL] | [μg/mL] | |
| WBC [tys/µL] | r = 0.005 | r = −0.040 | r = −0.126 | r = 0.231 | r = 0.026 | r = 0.275 | r = 0.237 |
| p = 0.978 | p = 0.813 | p = 0.457 | p = 0.169 | p = 0.879 | p = 0.099 | p = 0.158 | |
| NEUT [tys/µL] | r = 0.121 | r = 0.076 | r = −0.078 | r = 0.277 | r = 0.028 | r = 0.339 | r = 0.287 |
| p = 0.475 | p = 0.655 | p = 0.648 | p = 0.097 | p = 0.870 | p = 0.039 | p = 0.085 | |
| LYM [tys/µL] | r = −0.162 | r = −0.194 | r = −0.154 | r = 0.143 | r = −0.111 | r = 0.079 | r = 0.132 |
| p = 0.338 | p = 0.249 | p = 0.362 | p = 0.397 | p = 0.513 | p = 0.642 | p = 0.435 | |
| MONO [tys/µL] | r = 0.074 | r = 0.116 | r = 0.085 | r = 0.175 | r = −0.025 | r = 0.267 | r = 0.023 |
| p = 0.662 | p = 0.494 | p = 0.616 | p = 0.299 | p = 0.884 | p = 0.111 | p = 0.895 | |
| EOS [tys/µL] | r = 0.074 | r = −0.005 | r = 0.257 | r = 0.216 | r = 0.121 | r = 0.304 | r = 0.244 |
| p = 0.665 | p = 0.978 | p = 0.124 | p = 0.201 | p = 0.475 | p = 0.068 | p = 0.145 | |
| BASO [tys/µL] | r = 0.066 | r = 0.117 | r = 0.163 | r = 0.160 | r = −0.133 | r = 0.167 | r = 0.150 |
| p = 0.697 | p = 0.492 | p = 0.335 | p = 0.343 | p = 0.432 | p = 0.323 | p = 0.376 | |
| RBC [mln/µL] | r = 0.148 | r = 0.131 | r = 0.299 | r = 0.128 | r = −0.236 | r = 0.215 | r = 0.145 |
| p = 0.381 | p = 0.440 | p = 0.071 | p = 0.449 | p = 0.161 | p = 0.202 | p = 0.393 | |
| MCV [fL] | r = −0.132 | r = −0.163 | r = −0.212 | r = −0.080 | r = 0.029 | r = −0.219 | r = −0.109 |
| p = 0.437 | p = 0.334 | p = 0.208 | p = 0.636 | p = 0.864 | p = 0.191 | p = 0.521 | |
| HGB [g/dL] | r = 0.106 | r = 0.087 | r = 0.154 | r = 0.134 | r = −0.105 | r = 0.014 | r = 0.079 |
| p = 0.533 | p = 0.607 | p = 0.362 | p = 0.429 | p = 0.537 | p = 0.933 | p = 0.644 | |
| HCT [%] | r = −0.013 | r = −0.049 | r = 0.158 | r = 0.016 | r = −0.189 | r = −0.009 | r = 0.004 |
| p = 0.941 | p = 0.776 | p = 0.351 | p = 0.925 | p = 0.262 | p = 0.956 | p = 0.981 | |
| PLT [tys/µL] | r = −0.010 | r = 0.051 | r = −0.293 | r = 0.031 | r = −0.222 | r = 0.066 | r = 0.084 |
| p = 0.951 | p = 0.762 | p = 0.079 | p = 0.855 | p = 0.187 | p = 0.698 | p = 0.622 | |
| CRP [mg/L] | r = 0.231 | r = 0.159 | r = −0.014 | r = 0.328 | r = −0.148 | r = 0.376 | r = 0.352 |
| p = 0.169 | p = 0.346 | p = 0.935 | p = 0.048 | p = 0.382 | p = 0.022 | p = 0.033 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wrońska, K.; Ziętek, M.; Machałowski, T.; Szczuko, M. Association Between Complete Blood Count and the Lipoxygenase Pathway in Hashimoto’s Thyroiditis. Cells 2025, 14, 1933. https://doi.org/10.3390/cells14241933
Wrońska K, Ziętek M, Machałowski T, Szczuko M. Association Between Complete Blood Count and the Lipoxygenase Pathway in Hashimoto’s Thyroiditis. Cells. 2025; 14(24):1933. https://doi.org/10.3390/cells14241933
Chicago/Turabian StyleWrońska, Karolina, Maciej Ziętek, Tomasz Machałowski, and Małgorzata Szczuko. 2025. "Association Between Complete Blood Count and the Lipoxygenase Pathway in Hashimoto’s Thyroiditis" Cells 14, no. 24: 1933. https://doi.org/10.3390/cells14241933
APA StyleWrońska, K., Ziętek, M., Machałowski, T., & Szczuko, M. (2025). Association Between Complete Blood Count and the Lipoxygenase Pathway in Hashimoto’s Thyroiditis. Cells, 14(24), 1933. https://doi.org/10.3390/cells14241933






