Mucosal Melanoma: Mechanisms of Its Etiology, Progression, Resistance and Therapy
Highlights
- Both cutaneous melanoma (CM) and mucosal melanomas (MM) differ significantly in their epidemiology, genetic profile, and clinical presentation, while their treatment options are similar.
- The response rates to treatment and consequently the survival rate are significantly lower in MM than in CM.
- MM exhibits unique clinical and molecular characteristics that can lead to resistance mechanisms not observed in CM.
- Compared to available anticancer agents, immunotherapy is the best treatment option for MM.
Abstract
1. Introduction
2. Epidemiology
3. Common Sites for the Occurrence of Mucosal Melanomas
4. Biology of Melanocytes and Their Function
5. Mechanisms of Mucosal Melanoma Development and Progression
6. Therapeutic Option of Mucosal Melanoma
6.1. Immune Therapy
6.2. Radiotherapy
6.3. Targeted Therapies
7. Resistance Mechanisms of Mucosal Melanoma
8. Mechanisms of Intrinsic Resistance in Melanoma
9. Mechanisms of Extrinsic Resistance in Melanoma
10. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Santeufemia, D.A.; Palmieri, G.; Miolo, G.; Colombino, M.; Doro, M.G.; Frogheri, L.; Paliogiannis, P.; Capobianco, G.; Madonia, M.; Cossu, A.; et al. Current Trends in Mucosal Melanomas: An Overview. Cancers 2023, 15, 1356. [Google Scholar] [CrossRef]
- Sergi, M.C.; Filoni, E.; Triggiano, G.; Cazzato, G.; Interno, V.; Porta, C.; Tucci, M. Mucosal Melanoma: Epidemiology, Clinical Features, and Treatment. Curr. Oncol. Rep. 2023, 25, 1247–1258. [Google Scholar] [CrossRef]
- Ortega, C.A.; Stevens, M.N.; Lewis, J.S.; Topf, M.C. Nasal Mucosal Desmoplastic Melanoma: A Case Report with Review of the Literature. Head Neck Pathol. 2022, 16, 942–946. [Google Scholar] [CrossRef]
- Shah, N.J.; Aloysius, M.M.; Bhanat, E.; Gupta, S.; Aswath, G.; John, S.; Tang, S.J.; Goyal, H. Epidemiology and outcomes of gastrointestinal mucosal melanomas: A national database analysis. BMC Gastroenterol. 2022, 22, 178. [Google Scholar] [CrossRef]
- Al-Haseni, A.; Vrable, A.; Qureshi, M.M.; Mathews, S.; Pollock, S.; Truong, M.T.; Sahni, D. Survival outcomes of mucosal melanoma in the USA. Future Oncol. 2019, 15, 3977–3986. [Google Scholar] [CrossRef]
- Cichorek, M.; Wachulska, M.; Stasiewicz, A.; Tyminska, A. Skin melanocytes: Biology and development. Postepy Dermatol. Alergol. 2013, 30, 30–41. [Google Scholar] [CrossRef]
- Seetharamu, N.; Ott, P.A.; Pavlick, A.C. Mucosal melanomas: A case-based review of the literature. Oncologist 2010, 15, 772–781. [Google Scholar] [CrossRef]
- Vos, J.L.; Traets, J.J.; Qiao, X.; Seignette, I.M.; Peters, D.; Wouters, M.W.; Hooijberg, E.; Broeks, A.; van der Wal, J.E.; Karakullukcu, M.B.; et al. Diversity of the immune microenvironment and response to checkpoint inhibitor immunotherapy in mucosal melanoma. JCI Insight 2024, 9, e179982. [Google Scholar] [CrossRef] [PubMed]
- Yentz, S.; Lao, C.D. Immunotherapy for mucosal melanoma. Ann. Transl. Med. 2019, 7 (Suppl. S3), S118. [Google Scholar] [CrossRef] [PubMed]
- Nassar, K.W.; Tan, A.C. The mutational landscape of mucosal melanoma. Semin. Cancer Biol. 2020, 61, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Feller, L.; Masilana, A.; Khammissa, R.A.; Altini, M.; Jadwat, Y.; Lemmer, J. Melanin: The biophysiology of oral melanocytes and physiological oral pigmentation. Head Face Med. 2014, 10, 8. [Google Scholar] [CrossRef] [PubMed]
- D’Mello, S.A.; Finlay, G.J.; Baguley, B.C.; Askarian-Amiri, M.E. Signaling Pathways in Melanogenesis. Int. J. Mol. Sci. 2016, 17, 1144. [Google Scholar] [CrossRef]
- Babu, S.; Chen, J.; Baron, C.S.; Sun, K.; Robitschek, E.; McConnell, A.M.; Wu, C.; Dedeilia, A.; Sade-Feldman, M.; Modhurima, R.; et al. Specific oncogene activation of the cell of origin in mucosal melanoma. Nat. Commun. 2025, 16, 6750. [Google Scholar] [CrossRef]
- Garrido, M.C.; Bastian, B.C. KIT as a therapeutic target in melanoma. J. Investig. Dermatol. 2010, 130, 20–27. [Google Scholar] [CrossRef]
- Babu, S.; Chen, J.; Robitschek, E.; Baron, C.S.; McConnell, A.; Wu, C.; Dedeilia, A.; Sade-Feldman, M.; Modhurima, R.; Manos, M.P.; et al. Specific oncogene activation of the cell of origin in mucosal melanoma. bioRxiv 2024. [Google Scholar] [CrossRef]
- Carvajal, R.D.; Antonescu, C.R.; Wolchok, J.D.; Chapman, P.B.; Roman, R.A.; Teitcher, J.; Panageas, K.S.; Busam, K.J.; Chmielowski, B.; Lutzky, J.; et al. KIT as a therapeutic target in metastatic melanoma. JAMA 2011, 305, 2327–2334. [Google Scholar] [CrossRef] [PubMed]
- Altieri, L.; Eguchi, M.; Peng, D.H.; Cockburn, M. Predictors of mucosal melanoma survival in a population-based setting. J. Am. Acad. Dermatol. 2019, 81, 136–142.e2. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Huang, X.; Gao, L.; Zhang, Y.; Luo, J.; Zhang, S.; Wang, K.; Qu, Y.; Wu, R.; Liu, Q.; et al. Long-term treatment outcomes and prognosis of mucosal melanoma of the head and neck: 161 cases from a single institution. Oral Oncol. 2017, 74, 115–122. [Google Scholar] [CrossRef]
- Jethanamest, D.; Vila, P.M.; Sikora, A.G.; Morris, L.G. Predictors of survival in mucosal melanoma of the head and neck. Ann. Surg. Oncol. 2011, 18, 2748–2756. [Google Scholar] [CrossRef]
- Rojas-Lechuga, M.J.; Jubes, S.; Molina-Garcia, M.; da Silva-Junior, R.M.P.; Sampieri, C.; Langdon, C.; Gras-Cabrerizo, J.R.; Bernal-Sprekelsen, M.; Puig, S.; Alobid, I. Survival Outcomes in Sinonasal Mucosal Melanoma: Systematic Review and Meta-Analysis. J. Pers. Med. 2024, 14, 1120. [Google Scholar] [CrossRef]
- Ma, Y.; Xia, R.; Ma, X.; Judson-Torres, R.L.; Zeng, H. Mucosal Melanoma: Pathological Evolution, Pathway Dependency and Targeted Therapy. Front. Oncol. 2021, 11, 702287. [Google Scholar] [CrossRef]
- Kerr, E.H.; Hameed, O.; Lewis, J.S., Jr.; Bartolucci, A.A.; Wang, D.; Said-Al-Naief, N. Head and neck mucosal malignant melanoma: Clinicopathologic correlation with contemporary review of prognostic indicators. Int. J. Surg. Pathol. 2012, 20, 37–46. [Google Scholar] [CrossRef]
- Kumar, V.; Vishnoi, J.R.; Kori, C.G.; Gupta, S.; Misra, S.; Akhtar, N. Primary malignant melanoma of oral cavity: A tertiary care center experience. Natl. J. Maxillofac. Surg. 2015, 6, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Mondal, K.; Mandal, R. Oral Mucosal Melanoma and Sinonasal Amelanotic Melanoma: A Summary of Two Unusual Cases. Iran. J. Pathol. 2024, 19, 4474–4476. [Google Scholar] [CrossRef] [PubMed]
- Ottaviano, M.; Giunta, E.F.; Marandino, L.; Tortora, M.; Attademo, L.; Bosso, D.; Cardalesi, C.; Fabbrocini, A.; Rosanova, M.; Silvestri, A.; et al. Anorectal and Genital Mucosal Melanoma: Diagnostic Challenges, Current Knowledge and Therapeutic Opportunities of Rare Melanomas. Biomedicines 2022, 10, 150. [Google Scholar] [CrossRef] [PubMed]
- Mastoraki, E.; Kravvas, G.; Dear, K.; Sim, S.; James, M.; Watchorn, R.; Haider, A.; Ellery, P.; Freeman, A.; Basha, M.; et al. Primary vulval melanoma and genital lichen sclerosus. Ski. Health Dis. 2024, 4, e411. [Google Scholar] [CrossRef] [PubMed]
- Merkel, E.A.; Gerami, P. Malignant melanoma of sun-protected sites: A review of clinical, histological, and molecular features. Lab. Investig. 2017, 97, 630–635. [Google Scholar] [CrossRef]
- Damsky, W.E.; Bosenberg, M. Melanocytic nevi and melanoma: Unraveling a complex relationship. Oncogene 2017, 36, 5771–5792. [Google Scholar] [CrossRef]
- Ferrara, G.; Argenziano, G. The WHO 2018 Classification of Cutaneous Melanocytic Neoplasms: Suggestions from Routine Practice. Front. Oncol. 2021, 11, 675296. [Google Scholar] [CrossRef] [PubMed]
- Khan, B.; Qahwaji, R.M.; Alfaifi, M.S.; Mobashir, M. Nivolumab and Ipilimumab Acting as Tormentors of Advanced Tumors by Unleashing Immune Cells and Associated Collateral Damage. Pharmaceutics 2024, 16, 732. [Google Scholar] [CrossRef] [PubMed]
- Belinky, P.A.; Aviram, M.; Mahmood, S.; Vaya, J. Structural aspects of the inhibitory effect of glabridin on LDL oxidation. Free Radic. Biol. Med. 1998, 24, 1419–1429. [Google Scholar] [CrossRef]
- Jeong, Y.J.; Thompson, J.F.; Ch’ng, S. Epidemiology, staging and management of mucosal melanoma of the head and neck: A narrative review. Chin. Clin. Oncol. 2023, 12, 28. [Google Scholar] [CrossRef]
- Ali, Z.; Yousaf, N.; Larkin, J. Melanoma epidemiology, biology and prognosis. Eur. J. Cancer Suppl. 2013, 11, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Gilain, L.; Houette, A.; Montalban, A.; Mom, T.; Saroul, N. Mucosal melanoma of the nasal cavity and paranasal sinuses. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2014, 131, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Conforti, C.; Zalaudek, I. Epidemiology and Risk Factors of Melanoma: A Review. Dermatol. Pract. Concept. 2021, 11 (Suppl. S1), e2021161S. [Google Scholar] [CrossRef]
- Ferguson, S.D.; Bindal, S.; Bassett, R.L., Jr.; Haydu, L.E.; McCutcheon, I.E.; Heimberger, A.B.; Li, J.; O’Brien, B.J.; Guha-Thakurta, N.; Tetzlaff, M.T.; et al. Predictors of survival in metastatic melanoma patients with leptomeningeal disease (LMD). J. Neuro-Oncol. 2019, 142, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Clavero-Rovira, L.; Gomez-Tomas, A.; Bassas-Freixas, P.; Bodet, D.; Ferrer, B.; Hernandez-Losa, J.; Munoz-Couselo, E.; Perez-Benavente, A.; Garcia-Patos, V.; Ferrandiz-Pulido, C. Mucosal Melanoma Clinical Management and Prognostic Implications: A Retrospective Cohort Study. Cancers 2024, 16, 227. [Google Scholar] [CrossRef]
- Lamichhane, N.S.; An, J.; Liu, Q.; Zhang, W. Primary malignant mucosal melanoma of the upper lip: A case report and review of the literature. BMC Res. Notes 2015, 8, 499. [Google Scholar] [CrossRef]
- Svedman, F.C.; Pillas, D.; Taylor, A.; Kaur, M.; Linder, R.; Hansson, J. Stage-specific survival and recurrence in patients with cutaneous malignant melanoma in Europe—A systematic review of the literature. Clin. Epidemiol. 2016, 8, 109–122. [Google Scholar] [CrossRef]
- Tyrrell, H.; Payne, M. Combatting mucosal melanoma: Recent advances and future perspectives. Melanoma Manag. 2018, 5, MMT11. [Google Scholar] [CrossRef]
- Ionescu, S.; Nicolescu, A.C.; Madge, O.L.; Simion, L.; Marincas, M.; Ceausu, M. Intra-Abdominal Malignant Melanoma: Challenging Aspects of Epidemiology, Clinical and Paraclinical Diagnosis and Optimal Treatment—A Literature Review. Diagnostics 2022, 12, 2054. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yang, L.; Lai, Y.; Wang, X.; Han, X.; Liu, S.; Wang, D.; Li, X.; Hu, N.; Kong, Y.; et al. Genetic alteration of Chinese patients with rectal mucosal melanoma. BMC Cancer 2021, 21, 623. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, C.C.; Wu, X.C.; Jemal, A.; Martin, H.J.; Roche, L.M.; Chen, V.W. Incidence of noncutaneous melanomas in the U.S. Cancer 2005, 103, 1000–1007. [Google Scholar] [CrossRef]
- Amabebe, E.; Anumba, D.O.C. Female Gut and Genital Tract Microbiota-Induced Crosstalk and Differential Effects of Short-Chain Fatty Acids on Immune Sequelae. Front. Immunol. 2020, 11, 2184. [Google Scholar] [CrossRef]
- Pandey, M.; Mathew, A.; Abraham, E.K.; Ahamed, I.M.; Nair, K.M. Primary malignant melanoma of the mucous membranes. Eur. J. Surg. Oncol. 1998, 24, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Qi, Z.; Zhang, L.; Guo, J.; Si, L. Immunotherapy in Acral and Mucosal Melanoma: Current Status and Future Directions. Front. Immunol. 2021, 12, 680407. [Google Scholar] [CrossRef]
- Wong, V.K.; Lubner, M.G.; Menias, C.O.; Mellnick, V.M.; Kennedy, T.A.; Bhalla, S.; Pickhardt, P.J. Clinical and Imaging Features of Noncutaneous Melanoma. Am. J. Roentgenol. 2017, 208, 942–959. [Google Scholar] [CrossRef]
- DePalo, D.K.; Elleson, K.M.; Carr, M.J.; Spiess, P.E.; Zager, J.S. Genitourinary melanoma: An overview for the clinician. Asian J. Urol. 2022, 9, 407–422. [Google Scholar] [CrossRef]
- Todorovic, N.; Djurkovic, P.; Krstic, A.; Tomanovic, N.; Milanovic, P.; Kablar, D.; Rajkovic Pavlovic, Z.; Stevanovic, M.; Milanovic, J.; Arnaut, A.; et al. Primary Sinonasal Mucosal Melanoma: A Narrative Review. Diagnostics 2025, 15, 496. [Google Scholar] [CrossRef]
- Nenclares, P.; Ap Dafydd, D.; Bagwan, I.; Begg, D.; Kerawala, C.; King, E.; Lingley, K.; Paleri, V.; Paterson, G.; Payne, M.; et al. Head and neck mucosal melanoma: The United Kingdom national guidelines. Eur. J. Cancer 2020, 138, 11–18. [Google Scholar] [CrossRef]
- Foreman, R.K.; Duncan, L.M. Sinonasal Mucosal Melanoma: A Contemporary Review. Surg. Pathol. Clin. 2024, 17, 667–682. [Google Scholar] [CrossRef]
- Tlholoe, M.M.; Khammissa, R.A.; Bouckaert, M.; Altini, M.; Lemmer, J.; Feller, L. Oral mucosal melanoma: Some pathobiological considerations and an illustrative report of a case. Head Neck Pathol. 2015, 9, 127–134. [Google Scholar] [CrossRef]
- Prasad, M.L.; Busam, K.J.; Patel, S.G.; Hoshaw-Woodard, S.; Shah, J.P.; Huvos, A.G. Clinicopathologic differences in malignant melanoma arising in oral squamous and sinonasal respiratory mucosa of the upper aerodigestive tract. Arch. Pathol. Lab. Med. 2003, 127, 997–1002. [Google Scholar] [CrossRef] [PubMed]
- Sortino-Rachou, A.M.; Cancela, M.d.C.; Voti, L.; Curado, M.P. Primary oral melanoma: Population-based incidence. Oral Oncol. 2009, 45, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Groeger, S.; Meyle, J. Oral Mucosal Epithelial Cells. Front. Immunol. 2019, 10, 208. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Ni, Y.; Liang, X.; Lin, Y.; An, B.; He, X.; Zhao, X. Mechanisms of tumor resistance to immune checkpoint blockade and combination strategies to overcome resistance. Front. Immunol. 2022, 13, 915094. [Google Scholar] [CrossRef]
- Allam, J.P.; Peng, W.M.; Appel, T.; Wenghoefer, M.; Niederhagen, B.; Bieber, T.; Berge, S.; Novak, N. Toll-like receptor 4 ligation enforces tolerogenic properties of oral mucosal Langerhans cells. J. Allergy Clin. Immunol. 2008, 121, 368–374.e1. [Google Scholar] [CrossRef]
- Huang, L.; Guo, Y.; Liu, S.; Wang, H.; Zhu, J.; Ou, L.; Xu, X. Targeting regulatory T cells for immunotherapy in melanoma. Mol. Biomed. 2021, 2, 11. [Google Scholar] [CrossRef]
- Wang, D.; Xu, T.; Zhu, H.; Dong, J.; Fu, L. Primary malignant melanomas of the female lower genital tract: Clinicopathological characteristics and management. Am. J. Cancer Res. 2020, 10, 4017–4037. [Google Scholar]
- Dobrica, E.C.; Vajaitu, C.; Condrat, C.E.; Cretoiu, D.; Popa, I.; Gaspar, B.S.; Suciu, N.; Cretoiu, S.M.; Varlas, V.N. Vulvar and Vaginal Melanomas-The Darker Shades of Gynecological Cancers. Biomedicines 2021, 9, 758. [Google Scholar] [CrossRef]
- Falcicchio, G.; Vinci, L.; Cicinelli, E.; Loizzi, V.; Arezzo, F.; Silvestris, E.; Resta, L.; Serio, G.; Cazzato, G.; Mastronardi, M.; et al. Vulvar Malignant Melanoma: A Narrative Review. Cancers 2022, 14, 5217. [Google Scholar] [CrossRef]
- Barrios De Tomasi, J.; Opata, M.M.; Mowa, C.N. Immunity in the Cervix: Interphase between Immune and Cervical Epithelial Cells. J. Immunol. Res. 2019, 2019, 7693183. [Google Scholar] [CrossRef]
- Ramanathan, R.; Woodrow, K. Engineering immunity in the mucosal niche against sexually transmitted infections. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2016, 8, 107–122. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.A.; Nelson, W.J.; Chavez, N. Cell-Cell Junctions Organize Structural and Signaling Networks. Cold Spring Harb. Perspect. Biol. 2018, 10, a029181. [Google Scholar] [CrossRef]
- Row, D.; Weiser, M.R. Anorectal melanoma. Clin. Colon Rectal Surg. 2009, 22, 120–126. [Google Scholar] [CrossRef]
- Lei, X.; Qingqing, L.; Weijie, Y.; Li, P.; Huang, C.; Kexun, Y.; Zihua, C. Effect of surgical treatment for anorectal melanoma: A propensity score-matched analysis of the Surveillance, Epidemiology, and End Results programme data. BMJ Open 2022, 12, e053339. [Google Scholar] [CrossRef]
- Chen, H.; Cai, Y.; Liu, Y.; He, J.; Hu, Y.; Xiao, Q.; Hu, W.; Ding, K. Incidence, Surgical Treatment, and Prognosis of Anorectal Melanoma from 1973 to 2011: A Population-Based SEER Analysis. Medicine 2016, 95, e2770. [Google Scholar] [CrossRef] [PubMed]
- Weinstock, M.A. Epidemiology and prognosis of anorectal melanoma. Gastroenterology 1993, 104, 174–178. [Google Scholar] [CrossRef]
- Barleben, A.; Mills, S. Anorectal anatomy and physiology. Surg. Clin. N. Am. 2010, 90, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Gabriel, H.; Awiwi, M.O.; Maheshwari, E.; Lopes Vendrami, C.; Konishi, T.; Taggart, M.W.; Magnetta, M.; Kelahan, L.C.; Lee, S. Anatomic Basis of Rectal Cancer Staging: Clarifying Controversies and Misconceptions. Radiographics 2024, 44, e230203. [Google Scholar] [CrossRef]
- Brenner, M.; Hearing, V.J. The protective role of melanin against UV damage in human skin. Photochem. Photobiol. 2008, 84, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Slominski, R.M.; Sarna, T.; Plonka, P.M.; Raman, C.; Brozyna, A.A.; Slominski, A.T. Melanoma, Melanin, and Melanogenesis: The Yin and Yang Relationship. Front. Oncol. 2022, 12, 842496. [Google Scholar] [CrossRef]
- Janjetovic, Z.; Nahmias, Z.P.; Hanna, S.; Jarrett, S.G.; Kim, T.K.; Reiter, R.J.; Slominski, A.T. Melatonin and its metabolites ameliorate ultraviolet B-induced damage in human epidermal keratinocytes. J. Pineal Res. 2014, 57, 90–102. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, M.; Nguyen, T.; Gundre, E.; Ogunlusi, O.; El-Sobky, M.; Giri, B.; Sarkar, T.R. Cancer-associated fibroblasts: The chief architect in the tumor microenvironment. Front. Cell Dev. Biol. 2023, 11, 1089068. [Google Scholar] [CrossRef]
- Mort, R.L.; Jackson, I.J.; Patton, E.E. The melanocyte lineage in development and disease. Development 2015, 142, 620–632. [Google Scholar] [CrossRef]
- Coutant, K.; Magne, B.; Ferland, K.; Fuentes-Rodriguez, A.; Chancy, O.; Mitchell, A.; Germain, L.; Landreville, S. Melanocytes in regenerative medicine applications and disease modeling. J. Transl. Med. 2024, 22, 336. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Liu, L.P.; Zhou, H.; Zheng, Y.W.; Li, Y.M. Recognition of Melanocytes in Immuno-Neuroendocrinology and Circadian Rhythms: Beyond the Conventional Melanin Synthesis. Cells 2022, 11, 2082. [Google Scholar] [CrossRef] [PubMed]
- Bi, O.; Caballero-Lima, D.; Sikkink, S.; Westgate, G.; Kauser, S.; Elies, J.; Thornton, M.J. Do Melanocytes Have a Role in Controlling Epidermal Bacterial Colonisation and the Skin Microbiome? Exp. Dermatol. 2025, 34, e70071. [Google Scholar] [CrossRef]
- Mackintosh, J.A. The antimicrobial properties of melanocytes, melanosomes and melanin and the evolution of black skin. J. Theor. Biol. 2001, 211, 101–113. [Google Scholar] [CrossRef]
- Koike, S.; Yamasaki, K. Melanogenesis Connection with Innate Immunity and Toll-Like Receptors. Int. J. Mol. Sci. 2020, 21, 9769. [Google Scholar] [CrossRef]
- Yu, N.; Zhang, S.; Zuo, F.; Kang, K.; Guan, M.; Xiang, L. Cultured human melanocytes express functional toll-like receptors 2-4, 7 and 9. J. Dermatol. Sci. 2009, 56, 113–120. [Google Scholar] [CrossRef]
- Berg, S.Z.; Berg, J. Microbes, macrophages, and melanin: A unifying theory of disease as exemplified by cancer. Front. Immunol. 2024, 15, 1493978. [Google Scholar] [CrossRef] [PubMed]
- Nosanchuk, J.D.; Casadevall, A. The contribution of melanin to microbial pathogenesis. Cell. Microbiol. 2003, 5, 203–223. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.H.; Park, T.J.; Jin, S.H.; Kang, H.Y. Human melanocytes express functional Toll-like receptor 4. Exp. Dermatol. 2008, 17, 412–417. [Google Scholar] [CrossRef]
- Bjorgen, H.; Koppang, E.O. The melano-macrophage: The black leukocyte of fish immunity. Fish Shellfish Immunol. 2024, 148, 109523. [Google Scholar] [CrossRef]
- Speeckaert, R.; Belpaire, A.; Speeckaert, M.; van Geel, N. The delicate relation between melanocytes and skin immunity: A game of hide and seek. Pigment Cell Melanoma Res. 2022, 35, 392–407. [Google Scholar] [CrossRef]
- Hong, Y.; Song, B.; Chen, H.D.; Gao, X.H. Melanocytes and Skin Immunity. J. Investig. Dermatol. Symp. Proc. 2015, 17, 37–39. [Google Scholar] [CrossRef]
- Brady, M.S.; Eckels, D.D.; Ree, S.Y.; Schultheiss, K.E.; Lee, J.S. MHC class II-mediated antigen presentation by melanoma cells. J. Immunother. Emphasis Tumor Immunol. 1996, 19, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.B.; Estrada, M.V.; Salgado, R.; Sanchez, V.; Doxie, D.B.; Opalenik, S.R.; Vilgelm, A.E.; Feld, E.; Johnson, A.S.; Greenplate, A.R.; et al. Melanoma-specific MHC-II expression represents a tumour-autonomous phenotype and predicts response to anti-PD-1/PD-L1 therapy. Nat. Commun. 2016, 7, 10582. [Google Scholar] [CrossRef]
- Goldstein, A.M.; Tucker, M.A. Dysplastic nevi and melanoma. Cancer Epidemiol. Biomarkers Prev. 2013, 22, 528–532. [Google Scholar] [CrossRef]
- Elder, D.E. Precursors to melanoma and their mimics: Nevi of special sites. Mod. Pathol. 2006, 19 (Suppl. S2), S4–S20. [Google Scholar] [CrossRef] [PubMed]
- Hafner, C.; Stoehr, R.; van Oers, J.M.; Zwarthoff, E.C.; Hofstaedter, F.; Klein, C.; Landthaler, M.; Hartmann, A.; Vogt, T. The absence of BRAF, FGFR3, and PIK3CA mutations differentiates lentigo simplex from melanocytic nevus and solar lentigo. J. Investig. Dermatol. 2009, 129, 2730–2735. [Google Scholar] [CrossRef] [PubMed]
- Abati, S.; Sandri, G.F.; Finotello, L.; Polizzi, E. Differential Diagnosis of Pigmented Lesions in the Oral Mucosa: A Clinical Based Overview and Narrative Review. Cancers 2024, 16, 2487. [Google Scholar] [CrossRef]
- Di Altobrando, A.; Misciali, C.; Baraldi, C.; Patrizi, A.; Savoia, F. Genital pigmented lesion in a Caucasian woman. Int. J. Dermatol. 2021, 60, 309–310. [Google Scholar] [CrossRef]
- Wohlmuth, C.; Wohlmuth-Wieser, I. Vulvar Melanoma: Molecular Characteristics, Diagnosis, Surgical Management, and Medical Treatment. Am. J. Clin. Dermatol. 2021, 22, 639–651. [Google Scholar] [CrossRef]
- Dummett, C.O. Physiologic pigmentation of the oral and cutaneous tissues in the Negro. J. Dent. Res. 1946, 25, 421–432. [Google Scholar] [CrossRef]
- Kucher, C.; Zhang, P.J.; Pasha, T.; Elenitsas, R.; Wu, H.; Ming, M.E.; Elder, D.E.; Xu, X. Expression of Melan-A and Ki-67 in desmoplastic melanoma and desmoplastic nevi. Am. J. Dermatopathol. 2004, 26, 452–457. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.Y.; Lee, J.W.; Papaccio, F.; Bellei, B.; Picardo, M. Alterations of the pigmentation system in the aging process. Pigment Cell Melanoma Res. 2021, 34, 800–813. [Google Scholar] [CrossRef]
- Zembowicz, A.; Phadke, P.A. Blue nevi and variants: An update. Arch. Pathol. Lab. Med. 2011, 135, 327–336. [Google Scholar] [CrossRef]
- Frischhut, N.; Zelger, B.; Andre, F.; Zelger, B.G. The spectrum of melanocytic nevi and their clinical implications. J. Dtsch. Dermatol. Ges. 2022, 20, 483–504. [Google Scholar] [CrossRef]
- Sil, S.; Pyne, R.; Ghosh, S.; Saha, N. Can a black pigmented lesion of the oral cavity predict future development of melanoma-Report of a case and review of literature. J. Family Med. Prim. Care 2025, 14, 1147–1151. [Google Scholar] [CrossRef]
- Rahmanipour, E.; Sadeghi, E.; Arora, S.; Ghorbani, M.; Maalhagh, M.; Venkatesh, R.; Chhablani, J. Choroidal hypopigmented lesions: A review. Surv. Ophthalmol. 2025, 70, 1111–1143. [Google Scholar] [CrossRef]
- Wong, T.; Yap, T.; Wiesenfeld, D. Common benign and malignant oral mucosal disease. Aust. J. Gen. Pract. 2020, 49, 568–573. [Google Scholar] [CrossRef]
- Ferreira, L.; Jham, B.; Assi, R.; Readinger, A.; Kessler, H.P. Oral melanocytic nevi: A clinicopathologic study of 100 cases. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2015, 120, 358–367. [Google Scholar] [CrossRef]
- Tziveleka, S.; Georgaki, M.; Pettas, E.; Savva, V.; Papadopoulou, E.; Katafygiotis, P.; Vardas, E.; Piperi, E.; Nikitakis, N.G. Acquired Compound Melanocytic Nevus on the Palate of a Child: Report of a Case. J. Oral Maxillofac. Res. 2022, 13, e5. [Google Scholar] [CrossRef] [PubMed]
- Izzetti, R.; Minuti, F.; Pucci, A.; Cinquini, C.; Barone, A.; Nisi, M. Amelanotic Melanocytic Nevus of the Oral Cavity: A Case Report and Literature Review. Diagnostics 2025, 15, 1554. [Google Scholar] [CrossRef]
- Hicks, M.J.; Flaitz, C.M. Oral mucosal melanoma: Epidemiology and pathobiology. Oral Oncol. 2000, 36, 152–169. [Google Scholar] [CrossRef]
- Rohilla, K.; Ramesh, V.; Balamurali, P.; Singh, N. Oral melanoacanthoma of a rare intraoral site: Case report and review of literature. Int. J. Clin. Pediatr. Dent. 2013, 6, 40–43. [Google Scholar] [CrossRef]
- Sebastian, S.; Yuwanati, M.; Ramani, P. Prognostic Predictors of Oral Mucosal Melanoma—A Systematic Review and Meta-Analysis. Oral Dis. 2025. early view. [Google Scholar] [CrossRef]
- Aloua, R.; Kaouani, A.; Kerdoud, O.; Salissou, I.; Slimani, F. Melanoma of the oral cavity: A silent killer. Ann. Med. Surg. 2021, 62, 182–185. [Google Scholar] [CrossRef] [PubMed]
- Cazzato, G.; Mangialardi, K.; Falcicchio, G.; Colagrande, A.; Ingravallo, G.; Arezzo, F.; Giliberti, G.; Trilli, I.; Loizzi, V.; Lettini, T.; et al. Preferentially Expressed Antigen in Melanoma (PRAME) and Human Malignant Melanoma: A Retrospective Study. Genes 2022, 13, 545. [Google Scholar] [CrossRef] [PubMed]
- Cascardi, E.; Cazzato, G.; Ingravallo, G.; Dellino, M.; Lupo, C.; Casatta, N.; Ballini, A.; Pacifici, A.; Marenzi, G.; Sammartino, G.; et al. PReferentially Expressed Antigen in MElanoma (PRAME): Preliminary communication on a translational tool able to early detect Oral Malignant Melanoma (OMM). J. Cancer 2023, 14, 628–633. [Google Scholar] [CrossRef]
- Ricci, C.; Altavilla, M.V.; de Biase, D.; Corti, B.; Pasquini, E.; Molteni, G.; Tarsitano, A.; Baietti, A.M.; Amorosa, L.; Ambrosi, F.; et al. Unveiling the molecular landscape and clinically relevant molecular heterogeneity of mucosal melanoma of the head and neck region. Histopathology 2025, 87, 270–283. [Google Scholar] [CrossRef] [PubMed]
- Mokos, M.; Prkacin, I.; Gacina, K.; Brkic, A.; Pondeljak, N.; Situm, M. Therapeutic Opportunities in Melanoma Through PRAME Expression. Biomedicines 2025, 13, 1988. [Google Scholar] [CrossRef] [PubMed]
- Scheurleer, W.F.J.; Braunius, W.W.; Tijink, B.M.; Suijkerbuijk, K.P.M.; Dierselhuis, M.P.; Meijers, R.W.J.; Blokx, W.A.M.; de Bree, R.; Breimer, G.E.; Rijken, J.A. PRAME Staining in Sinonasal Mucosal Melanoma: A Single-Center Experience. Head Neck Pathol. 2023, 17, 401–408. [Google Scholar] [CrossRef]
- Mundra, P.A.; Dhomen, N.; Rodrigues, M.; Mikkelsen, L.H.; Cassoux, N.; Brooks, K.; Valpione, S.; Reis-Filho, J.S.; Heegaard, S.; Stern, M.H.; et al. Ultraviolet radiation drives mutations in a subset of mucosal melanomas. Nat. Commun. 2021, 12, 259. [Google Scholar] [CrossRef]
- Lian, B.; Guo, J. Therapeutic Approaches to Mucosal Melanoma. Am. Soc. Clin. Oncol. Educ. Book 2025, 45, e4733858. [Google Scholar] [CrossRef] [PubMed]
- Patrick, R.J.; Fenske, N.A.; Messina, J.L. Primary mucosal melanoma. J. Am. Acad. Dermatol. 2007, 56, 828–834. [Google Scholar] [CrossRef]
- Griffin, Z.; Mattei, J. Mucosal Melanoma: An Overview of Recent Therapies. Curr. Pharm. Des. 2025. online ahead of print. [Google Scholar] [CrossRef]
- Azem, O.; Nabulsi, O.; Jelinek, M.; Joshi, N. Radiation Therapy in the Management of Head and Neck Mucosal Melanoma. Cancers 2024, 16, 3304. [Google Scholar] [CrossRef]
- Arosio, A.D.; Bernasconi, D.P.; Valsecchi, M.G.; Pacifico, C.; Battaglia, P.; Bignami, M.; Ferrari, M.; Mattavelli, D.; Rampinelli, V.; Tomasoni, M.; et al. Patterns of recurrences in sinonasal cancers undergoing an endoscopic surgery-based treatment: Results of the MUSES* on 940 patients: *MUlti-institutional collaborative Study on Endoscopically treated Sinonasal cancers. Oral Oncol. 2022, 134, 106123. [Google Scholar] [CrossRef]
- Podlinska, K.; Monist, M.; Slawinska, M.; Popowski, W. Multifocal Oral Mucosal Melanoma with an Atypical Clinical Presentation. Dent. J. 2025, 13, 432. [Google Scholar] [CrossRef]
- Chlopek, M.; Lasota, J.; Thompson, L.D.R.; Szczepaniak, M.; Kuzniacka, A.; Hincza, K.; Kubicka, K.; Kaczorowski, M.; Newford, M.; Liu, Y.; et al. Alterations in key signaling pathways in sinonasal tract melanoma. A molecular genetics and immunohistochemical study of 90 cases and comprehensive review of the literature. Mod. Pathol. 2022, 35, 1609–1617. [Google Scholar] [CrossRef] [PubMed]
- Cosgarea, I.; Ugurel, S.; Sucker, A.; Livingstone, E.; Zimmer, L.; Ziemer, M.; Utikal, J.; Mohr, P.; Pfeiffer, C.; Pfohler, C.; et al. Targeted next generation sequencing of mucosal melanomas identifies frequent NF1 and RAS mutations. Oncotarget 2017, 8, 40683–40692. [Google Scholar] [CrossRef] [PubMed]
- Mano, R.; Hoeh, B.; DiNatale, R.G.; Sanchez, A.; Benfante, N.E.; Reznik, E.; Leitao, M.M.; Shoushtari, A.N.; Goh, A.; Donat, S.M.; et al. Urethral Melanoma—Clinical, Pathological and Molecular Characteristics. Bladder Cancer 2022, 8, 291–301. [Google Scholar] [CrossRef]
- Yelamos, O.; Merkel, E.A.; Sholl, L.M.; Zhang, B.; Amin, S.M.; Lee, C.Y.; Guitart, G.E.; Yang, J.; Wenzel, A.T.; Bunick, C.G.; et al. Nonoverlapping Clinical and Mutational Patterns in Melanomas from the Female Genital Tract and Atypical Genital Nevi. J. Investig. Dermatol. 2016, 136, 1858–1865. [Google Scholar] [CrossRef]
- Gutierrez-Castaneda, L.D.; Gamboa, M.; Nova, J.A.; Pulido, L.; Tovar-Parra, J.D. Mutations in the BRAF, NRAS, and C-KIT Genes of Patients Diagnosed with Melanoma in Colombia Population. Biomed. Res. Int. 2020, 2020, 2046947. [Google Scholar] [CrossRef]
- Castellani, G.; Buccarelli, M.; Arasi, M.B.; Rossi, S.; Pisanu, M.E.; Bellenghi, M.; Lintas, C.; Tabolacci, C. BRAF Mutations in Melanoma: Biological Aspects, Therapeutic Implications, and Circulating Biomarkers. Cancers 2023, 15, 4026. [Google Scholar] [CrossRef] [PubMed]
- Heidrich, I.; Rautmann, C.; Ly, C.; Khatri, R.; Kott, J.; Geidel, G.; Runger, A.; Andreas, A.; Hansen-Abeck, I.; Abeck, F.; et al. In-depth assessment of BRAF, NRAS, KRAS, EGFR, and PIK3CA mutations on cell-free DNA in the blood of melanoma patients receiving immune checkpoint inhibition. J. Exp. Clin. Cancer Res. 2025, 44, 202. [Google Scholar] [CrossRef]
- Dumaz, N.; Jouenne, F.; Delyon, J.; Mourah, S.; Bensussan, A.; Lebbe, C. Atypical BRAF and NRAS Mutations in Mucosal Melanoma. Cancers 2019, 11, 1133. [Google Scholar] [CrossRef]
- Si, L.; Wang, X.; Guo, J. Genotyping of mucosal melanoma. Chin. Clin. Oncol. 2014, 3, 34. [Google Scholar] [CrossRef]
- Molina-Garcia, M.; Rojas-Lechuga, M.J.; Torres Moral, T.; Bague, J.; Mateu, J.; Langdon, C.; Lop, J.; Goncalves de Souza, V.; Alos, L.; Lopez-Chacon, M.; et al. Distinct Transcriptomic and Tumor Microenvironment Profiles in Sinonasal Mucosal Melanoma and Aggressive Cutaneous Melanomas. Cancers 2024, 16, 4172. [Google Scholar] [CrossRef]
- Newell, F.; Kong, Y.; Wilmott, J.S.; Johansson, P.A.; Ferguson, P.M.; Cui, C.; Li, Z.; Kazakoff, S.H.; Burke, H.; Dodds, T.J.; et al. Whole-genome landscape of mucosal melanoma reveals diverse drivers and therapeutic targets. Nat. Commun. 2019, 10, 3163. [Google Scholar] [CrossRef]
- Beaudoux, O.; Oudart, J.B.; Riffaud, L.; Visseaux, L.; Marchal, A.; Lebre, A.S.; Grange, F. Mutational Characteristics of Primary Mucosal Melanoma: A Systematic Review. Mol. Diagn. Ther. 2022, 26, 189–202. [Google Scholar] [CrossRef]
- Millan-Esteban, D.; Garcia-Casado, Z.; Manrique-Silva, E.; Viros, A.; Kumar, R.; Furney, S.; Lopez-Guerrero, J.A.; Requena, C.; Banuls, J.; Traves, V.; et al. Distribution and clinical role of KIT gene mutations in melanoma according to subtype: A study of 492 Spanish patients. Eur. J. Dermatol. 2021, 31, 830–838. [Google Scholar] [CrossRef]
- Toscano de Mendonca, U.B.; Cernea, C.R.; Matos, L.L.; Monteiro de Araujo Lima, R.R. Analysis of KIT gene mutations in patients with melanoma of the head and neck mucosa: A retrospective clinical report. Oncotarget 2018, 9, 22886–22894. [Google Scholar] [CrossRef] [PubMed]
- Jutten, E.; van Kempen, L.; Diercks, G.F.H.; van Leeuwen, B.L.; Kruijff, S.; Wevers, K.P. Real-World Evidence of the Prevalence of Driver Mutations in Anorectal Melanoma. Mol. Diagn. Ther. 2025, 29, 229–238. [Google Scholar] [CrossRef]
- Yang, H.M.; Hsiao, S.J.; Schaeffer, D.F.; Lai, C.; Remotti, H.E.; Horst, D.; Mansukhani, M.M.; Horst, B.A. Identification of recurrent mutational events in anorectal melanoma. Mod. Pathol. 2017, 30, 286–296. [Google Scholar] [CrossRef]
- Colombino, M.; Casula, M.; Paliogiannis, P.; Manca, A.; Sini, M.C.; Pisano, M.; Santeufemia, D.A.; Cossu, A.; Palmieri, G. Heterogeneous pathogenesis of melanoma: BRAF mutations and beyond. Crit. Rev. Oncol. Hematol. 2024, 201, 104435. [Google Scholar] [CrossRef]
- Silva-Rodriguez, P.; Fernandez-Diaz, D.; Bande, M.; Pardo, M.; Loidi, L.; Blanco-Teijeiro, M.J. GNAQ and GNA11 Genes: A Comprehensive Review on Oncogenesis, Prognosis and Therapeutic Opportunities in Uveal Melanoma. Cancers 2022, 14, 3066. [Google Scholar] [CrossRef] [PubMed]
- Pilch, J.; Mizera, J.; Tota, M.; Donizy, P. GNAQ/GNA11-Related Benign and Malignant Entities—A Common Histoembriologic Origin or a Tissue-Dependent Coincidence. Cancers 2024, 16, 3672. [Google Scholar] [CrossRef]
- Dika, E.; Lambertini, M.; Pellegrini, C.; Veronesi, G.; Melotti, B.; Riefolo, M.; Sperandi, F.; Patrizi, A.; Ricci, C.; Mussi, M.; et al. Cutaneous and Mucosal Melanomas of Uncommon Sites: Where Do We Stand Now? J. Clin. Med. 2021, 10, 478. [Google Scholar] [CrossRef] [PubMed]
- Maphutha, J.; Twilley, D.; Lall, N. The Role of the PTEN Tumor Suppressor Gene and Its Anti-Angiogenic Activity in Melanoma and Other Cancers. Molecules 2024, 29, 721. [Google Scholar] [CrossRef] [PubMed]
- Mirmohammadsadegh, A.; Marini, A.; Nambiar, S.; Hassan, M.; Tannapfel, A.; Ruzicka, T.; Hengge, U.R. Epigenetic silencing of the PTEN gene in melanoma. Cancer Res. 2006, 66, 6546–6552. [Google Scholar] [CrossRef] [PubMed]
- Ichimura, N.; Urata, Y.; Kobayashi, T.; Hibi, H. Mutational landscape of oral mucosal melanoma based on comprehensive cancer genomic profiling tests in a Japanese cohort. Oral Oncol. 2024, 152, 106807. [Google Scholar] [CrossRef]
- Xu, L.; Cheng, Z.; Cui, C.; Wu, X.; Yu, H.; Guo, J.; Kong, Y. Frequent genetic aberrations in the cell cycle related genes in mucosal melanoma indicate the potential for targeted therapy. J. Transl. Med. 2019, 17, 245. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.J.; Xu, S.M.; Han, Y.; Zhou, R.; Zhang, Z.Y. Targeting cyclin-dependent kinase 4/6 as a therapeutic approach for mucosal melanoma. Melanoma Res. 2021, 31, 495–503. [Google Scholar] [CrossRef]
- Fortuna, A.; Amaral, T. Multidisciplinary approach and treatment of acral and mucosal melanoma. Front. Oncol. 2024, 14, 1340408. [Google Scholar] [CrossRef]
- Bak-Gordon, P.; Manley, J.L. SF3B1: From core splicing factor to oncogenic driver. RNA 2025, 31, 314–332. [Google Scholar] [CrossRef]
- Zhang, Q.; Ai, Y.; Abdel-Wahab, O. Molecular impact of mutations in RNA splicing factors in cancer. Mol. Cell 2024, 84, 3667–3680. [Google Scholar] [CrossRef]
- Rambow, F.; Marine, J.C.; Goding, C.R. Melanoma plasticity and phenotypic diversity: Therapeutic barriers and opportunities. Genes Dev. 2019, 33, 1295–1318. [Google Scholar] [CrossRef]
- Beigi, Y.Z.; Lanjanian, H.; Fayazi, R.; Salimi, M.; Hoseyni, B.H.M.; Noroozizadeh, M.H.; Masoudi-Nejad, A. Heterogeneity and molecular landscape of melanoma: Implications for targeted therapy. Mol. Biomed. 2024, 5, 17. [Google Scholar] [CrossRef]
- Curioni-Fontecedro, A.; Pitocco, R.; Schoenewolf, N.L.; Holzmann, D.; Soldini, D.; Dummer, R.; Calvieri, S.; Moch, H.; Mihic-Probst, D.; Fitsche, A. Intratumoral Heterogeneity of MAGE-C1/CT7 and MAGE-C2/CT10 Expression in Mucosal Melanoma. Biomed. Res. Int. 2015, 2015, 432479. [Google Scholar] [CrossRef]
- Nachankar, A.; Pelak, M.; Schafasand, M.; Martino, G.; Tubin, S.; Hug, E.; Carlino, A.; Lutgendorf-Caucig, C.; Stock, M.; Fossati, P. Carbon-Ion Radiotherapy for Head and Neck Mucosal Melanoma: Preliminary Clinical Outcomes and the MedAustron Approach for Reporting RBE-Weighted Dose with 2 Models. Int. J. Part. Ther. 2025, 15, 100738. [Google Scholar] [CrossRef]
- Lazarev, S.; Gupta, V.; Hu, K.; Harrison, L.B.; Bakst, R. Mucosal melanoma of the head and neck: A systematic review of the literature. Int. J. Radiat. Oncol. Biol. Phys. 2014, 90, 1108–1118. [Google Scholar] [CrossRef]
- Restaino, S.; Pellecchia, G.; Arcieri, M.; Bogani, G.; Taliento, C.; Greco, P.; Driul, L.; Chiantera, V.; De Vincenzo, R.P.; Garganese, G.; et al. Management of Patients with Vulvar Cancers: A Systematic Comparison of International Guidelines (NCCN-ASCO-ESGO-BGCS-IGCS-FIGO-French Guidelines-RCOG). Cancers 2025, 17, 186. [Google Scholar] [CrossRef]
- Trivedi, S.D.; Shukla, S.; Pandya, S.V.; Mehta, J.S.; Pandya, S.J.; Sharma, M.; Patel, S.; Warikoo, V.; Rathod, P.; Puj, K.S.; et al. Mucosal Malignant Melanoma of Head and Neck: A Case Series from a Single Institute and Review of Literature Abstract. Indian J. Otolaryngol. Head Neck Surg. 2023, 75, 3415–3420. [Google Scholar] [CrossRef]
- Witt, R.G.; Erstad, D.J.; Wargo, J.A. Neoadjuvant therapy for melanoma: Rationale for neoadjuvant therapy and pivotal clinical trials. Ther. Adv. Med. Oncol. 2022, 14, 17588359221083052. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.P.; Othus, M.; Chen, Y.; Wright, G.P., Jr.; Yost, K.J.; Hyngstrom, J.R.; Hu-Lieskovan, S.; Lao, C.D.; Fecher, L.A.; Truong, T.G.; et al. Neoadjuvant-Adjuvant or Adjuvant-Only Pembrolizumab in Advanced Melanoma. N. Engl. J. Med. 2023, 388, 813–823. [Google Scholar] [CrossRef]
- Boutros, A.; Croce, E.; Ferrari, M.; Gili, R.; Massaro, G.; Marconcini, R.; Arecco, L.; Tanda, E.T.; Spagnolo, F. The treatment of advanced melanoma: Current approaches and new challenges. Crit. Rev. Oncol. Hematol. 2024, 196, 104276. [Google Scholar] [CrossRef]
- Dobosz, P.; Dzieciatkowski, T. The Intriguing History of Cancer Immunotherapy. Front. Immunol. 2019, 10, 2965. [Google Scholar] [CrossRef] [PubMed]
- Shan, Z.; Liu, F. Advances in immunotherapy for mucosal melanoma: Harnessing immune checkpoint inhibitors for improved treatment outcomes. Front. Immunol. 2024, 15, 1441410. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Kan, H.; Zhao, L.; Sun, Z.; Bai, C. Immune checkpoint inhibitors in advanced or metastatic mucosal melanoma: A systematic review. Ther. Adv. Med. Oncol. 2020, 12, 1758835920922028. [Google Scholar] [CrossRef]
- Khaddour, K.; Maahs, L.; Avila-Rodriguez, A.M.; Maamar, Y.; Samaan, S.; Ansstas, G. Melanoma Targeted Therapies beyond BRAF-Mutant Melanoma: Potential Druggable Mutations and Novel Treatment Approaches. Cancers 2021, 13, 5847. [Google Scholar] [CrossRef]
- Chao, T.N.; Kuan, E.C.; Tong, C.C.L.; Kohanski, M.A.; Grady, M.S.; Palmer, J.N.; Adappa, N.D.; O’Malley, B.W., Jr. Surgical Treatment of Sinonasal Mucosal Melanoma in Patients Treated with Systemic Immunotherapy. J. Neurol. Surg. B Skull Base 2021, 82 (Suppl. S3), e148–e154. [Google Scholar] [CrossRef] [PubMed]
- Owens, J.M.; Roberts, D.B.; Myers, J.N. The role of postoperative adjuvant radiation therapy in the treatment of mucosal melanomas of the head and neck region. Arch. Otolaryngol. Head Neck Surg. 2003, 129, 864–868. [Google Scholar] [CrossRef]
- Gupta, A.; Gomes, F.; Lorigan, P. The role for chemotherapy in the modern management of melanoma. Melanoma Manag. 2017, 4, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Luke, J.J.; Schwartz, G.K. Chemotherapy in the management of advanced cutaneous malignant melanoma. Clin. Dermatol. 2013, 31, 290–297. [Google Scholar] [CrossRef] [PubMed]
- van Akkooi, A.C.; Hauschild, A.; Long, G.V.; Mandala, M.; Kicinski, M.; Govaerts, A.S.; Klauck, I.; Ouali, M.; Lorigan, P.C.; Eggermont, A.M. COLUMBUS-AD: Phase III study of adjuvant encorafenib + binimetinib in resected stage IIB/IIC BRAF V600-mutated melanoma. Future Oncol. 2023, 19, 2017–2027. [Google Scholar] [CrossRef]
- Dudnichenko, O.; Penkov, K.; McKean, M.; Mandala, M.; Kukushkina, M.; Panella, T.; Csoszi, T.; Gerletti, P.; Thakur, M.; Polli, A.; et al. First-line encorafenib plus binimetinib and pembrolizumab for advanced BRAF V600-mutant melanoma: Safety lead-in results from the randomized phase III STARBOARD study. Eur. J. Cancer 2024, 213, 115070. [Google Scholar] [CrossRef]
- Teo, A.Y.T.; Yau, C.E.; Low, C.E.; Pereira, J.V.; Ng, J.Y.X.; Soong, T.K.; Lo, J.Y.T.; Yang, V.S. Effectiveness of immune checkpoint inhibitors and other treatment modalities in patients with advanced mucosal melanomas: A systematic review and individual patient data meta-analysis. EClinicalMedicine 2024, 77, 102870. [Google Scholar] [CrossRef]
- Cai, W.; Nguyen, M.Q.; Wilski, N.A.; Purwin, T.J.; Vernon, M.; Tiago, M.; Aplin, A.E. A Genome-Wide Screen Identifies PDPK1 as a Target to Enhance the Efficacy of MEK1/2 Inhibitors in NRAS Mutant Melanoma. Cancer Res. 2022, 82, 2625–2639. [Google Scholar] [CrossRef]
- Shtivelman, E.; Davies, M.Q.; Hwu, P.; Yang, J.; Lotem, M.; Oren, M.; Flaherty, K.T.; Fisher, D.E. Pathways and therapeutic targets in melanoma. Oncotarget 2014, 5, 1701–1752. [Google Scholar] [CrossRef]
- Randic, T.; Kozar, I.; Margue, C.; Utikal, J.; Kreis, S. NRAS mutant melanoma: Towards better therapies. Cancer Treat. Rev. 2021, 99, 102238. [Google Scholar] [CrossRef]
- Muto, Y.; Fujimura, T.; Asano, Y. Recent Advances in Immunotherapy for Melanoma: Perspectives on the Development of Novel Treatments: A Mini Review. Cancers 2025, 17, 2265. [Google Scholar] [CrossRef] [PubMed]
- Carlino, M.S.; Larkin, J.; Long, G.V. Immune checkpoint inhibitors in melanoma. Lancet 2021, 398, 1002–1014. [Google Scholar] [CrossRef]
- Caksa, S.; Baqai, U.; Aplin, A.E. The future of targeted kinase inhibitors in melanoma. Pharmacol. Ther. 2022, 239, 108200. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, S.P.; Larkin, J.; Sosman, J.A.; Lebbe, C.; Brady, B.; Neyns, B.; Schmidt, H.; Hassel, J.C.; Hodi, F.S.; Lorigan, P.; et al. Efficacy and Safety of Nivolumab Alone or in Combination with Ipilimumab in Patients with Mucosal Melanoma: A Pooled Analysis. J. Clin. Oncol. 2017, 35, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Horisaki, K.; Yoshikawa, S.; Omata, W.; Tsutsumida, A.; Kiyohara, Y. Comparison of efficacy between anti-PD-1 antibody monotherapy and nivolumab plus ipilimumab therapy as first-line immunotherapy for advanced mucosal melanoma in Japanese patients: A single-center, retrospective cohort study. J. Dermatol. 2024, 51, 1425–1433. [Google Scholar] [CrossRef]
- Wolchok, J.D.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Rutkowski, P.; Lao, C.D.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Long-Term Outcomes with Nivolumab Plus Ipilimumab or Nivolumab Alone Versus Ipilimumab in Patients with Advanced Melanoma. J. Clin. Oncol. 2022, 40, 127–137. [Google Scholar] [CrossRef]
- Sabbatino, F.; Liguori, L.; Pepe, S.; Ferrone, S. Immune checkpoint inhibitors for the treatment of melanoma. Expert Opin. Biol. Ther. 2022, 22, 563–576. [Google Scholar] [CrossRef]
- Tang, B.; Mo, J.; Yan, X.; Duan, R.; Chi, Z.; Cui, C.; Si, L.; Kong, Y.; Mao, L.; Li, S.; et al. Real-world efficacy and safety of axitinib in combination with anti-programmed cell death-1 antibody for advanced mucosal melanoma. Eur. J. Cancer 2021, 156, 83–92. [Google Scholar] [CrossRef]
- Li, S.; Wu, X.; Yan, X.; Zhou, L.; Chi, Z.; Si, L.; Cui, C.; Tang, B.; Mao, L.; Lian, B.; et al. Toripalimab plus axitinib in patients with metastatic mucosal melanoma: 3-year survival update and biomarker analysis. J. Immunother. Cancer 2022, 10, e004036. [Google Scholar] [CrossRef]
- Yi, M.; Zheng, X.; Niu, M.; Zhu, S.; Ge, H.; Wu, K. Combination strategies with PD-1/PD-L1 blockade: Current advances and future directions. Mol. Cancer 2022, 21, 28. [Google Scholar] [CrossRef]
- Kooshkaki, O.; Derakhshani, A.; Hosseinkhani, N.; Torabi, M.; Safaei, S.; Brunetti, O.; Racanelli, V.; Silvestris, N.; Baradaran, B. Combination of Ipilimumab and Nivolumab in Cancers: From Clinical Practice to Ongoing Clinical Trials. Int. J. Mol. Sci. 2020, 21, 4427. [Google Scholar] [CrossRef]
- Clarke, J.M.; Gu, L.; Wang, X.F.; Stinchcombe, T.E.; Stevenson, M.M.; Ramalingam, S.; Shariff, A.; Garst, J.; Nixon, A.B.; Antonia, S.J.; et al. A Phase 2 Clinical Trial of Combination Nivolumab, Ipilimumab, and Paclitaxel in Patients with Untreated Metastatic NSCLC: The OPTIMAL Trial. JTO Clin. Res. Rep. 2022, 3, 100337. [Google Scholar] [CrossRef] [PubMed]
- Asher, N.; Ben-Betzalel, G.; Lev-Ari, S.; Shapira-Frommer, R.; Steinberg-Silman, Y.; Gochman, N.; Schachter, J.; Meirson, T.; Markel, G. Real World Outcomes of Ipilimumab and Nivolumab in Patients with Metastatic Melanoma. Cancers 2020, 12, 2329. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Bian, Y.; Chu, T.; Wang, Y.; Man, S.; Song, Y.; Wang, Z. The role of angiogenesis in melanoma: Clinical treatments and future expectations. Front. Pharmacol. 2022, 13, 1028647. [Google Scholar] [CrossRef] [PubMed]
- Valdez-Salazar, F.; Jimenez-Del Rio, L.A.; Padilla-Gutierrez, J.R.; Valle, Y.; Munoz-Valle, J.F.; Valdes-Alvarado, E. Advances in Melanoma: From Genetic Insights to Therapeutic Innovations. Biomedicines 2024, 12, 1851. [Google Scholar] [CrossRef]
- Zhang, L.; Hao, B.; Geng, Z.; Geng, Q. Toripalimab: The First Domestic Anti-Tumor PD-1 Antibody in China. Front. Immunol. 2021, 12, 730666. [Google Scholar] [CrossRef]
- Lian, B.; Si, L.; Chi, Z.H.; Sheng, X.N.; Kong, Y.; Wang, X.; Tian, H.; Li, K.; Mao, L.L.; Bai, X.; et al. Toripalimab (anti-PD-1) versus high-dose interferon-alpha2b as adjuvant therapy in resected mucosal melanoma: A phase II randomized trial. Ann. Oncol. 2022, 33, 1061–1070. [Google Scholar] [CrossRef]
- Morris, V.K.; Liu, S.; Lin, K.; Zhu, H.; Prasad, S.; Mahvash, A.; Bhosale, P.; Sun, B.; Parra, E.R.; Wistuba, I.; et al. Phase II Trial of Atezolizumab and Bevacizumab for Treatment of HPV-Positive Unresectable or Metastatic Squamous Cell Carcinoma of the Anal Canal. Clin. Cancer Res. 2025, 31, 1657–1666. [Google Scholar] [CrossRef]
- Zhang, J.; Tian, H.; Mao, L.; Li, C.; Wei, X.; Gu, J.; Wang, X.; Zhou, L.; Lian, B.; Tang, B.; et al. Real-World Efficacy and Safety of Anti-PD-1 Antibody Plus Apatinib and Temozolomide for Advanced Acral Melanoma. Cancer Manag. Res. 2025, 17, 905–916. [Google Scholar] [CrossRef]
- Zhou, L.; Wu, X.; Chi, Z.; Si, L.; Sheng, X.; Kong, Y.; Mao, L.; Lian, B.; Tang, B.; Yan, X.; et al. Safety, activity, and pharmacokinetics of camrelizumab in advanced Asian melanoma patients: A phase I study. BMC Cancer 2022, 22, 565. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Wang, X.; Lian, B.; Yan, X.; Si, L.; Chi, Z.; Sheng, X.; Kong, Y.; Mao, L.; Bai, X.; et al. Safety Profile of Immunotherapy Combined with Antiangiogenic Therapy in Patients with Melanoma: Analysis of Three Clinical Studies. Front. Pharmacol. 2021, 12, 747416. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Ren, Y.; Zhang, G.; Zheng, K.; Wang, J.; Sha, H.; Zhao, M.; Huang, R.; Kang, D.; Su, X.; et al. Single-arm study of camrelizumab plus apatinib for patients with advanced mucosal melanoma. J. Immunother. Cancer 2024, 12, e008611. [Google Scholar] [CrossRef]
- Abdelwahed, A.; Ahmed, M. Rare epithelial breast cancer: Surgery and adjuvant therapy. Transl. Cancer Res. 2019, 8 (Suppl. S5), S479–S492. [Google Scholar] [CrossRef]
- King, A.L.O.; Lee, V.; Yu, B.; Mirza, F.N.; Zogg, C.K.; Yang, D.X.; Tran, T.; Leventhal, J.; An, Y. Factors associated with the use of adjuvant radiation therapy in stage III melanoma. Front. Oncol. 2023, 13, 1005930. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Li, H.; Ma, Q.; Liu, J.; Ren, F.; Song, Y.; Wang, T.; Li, K.; Li, N. Synergies between radiotherapy and immunotherapy: A systematic review from mechanism to clinical application. Front. Immunol. 2025, 16, 1554499. [Google Scholar] [CrossRef]
- Spiotto, M.; Fu, Y.X.; Weichselbaum, R.R. The intersection of radiotherapy and immunotherapy: Mechanisms and clinical implications. Sci. Immunol. 2016, 1, eaag1266. [Google Scholar] [CrossRef]
- Brix, N.; Tiefenthaller, A.; Anders, H.; Belka, C.; Lauber, K. Abscopal, immunological effects of radiotherapy: Narrowing the gap between clinical and preclinical experiences. Immunol. Rev. 2017, 280, 249–279. [Google Scholar] [CrossRef]
- Zheng, Y.; Liu, X.; Li, N.; Zhao, A.; Sun, Z.; Wang, M.; Luo, J. Radiotherapy combined with immunotherapy could improve the immune infiltration of melanoma in mice and enhance the abscopal effect. Radiat. Oncol. J. 2023, 41, 129–139. [Google Scholar] [CrossRef]
- Kim, H.J.; Chang, J.S.; Roh, M.R.; Oh, B.H.; Chung, K.Y.; Shin, S.J.; Koom, W.S. Effect of Radiotherapy Combined with Pembrolizumab on Local Tumor Control in Mucosal Melanoma Patients. Front. Oncol. 2019, 9, 835. [Google Scholar] [CrossRef] [PubMed]
- Yin, G.; Guo, W.; Chen, X.; Zhang, Y.; Huang, Z. Efficacy and Mechanism of Hypofractionation Radiotherapy Combined with PD-1 Inhibitors in a Model of Head and Neck Melanoma. Cancers 2024, 16, 675. [Google Scholar] [CrossRef]
- Falcone, R.; Verkhovskaia, S.; Di Pietro, F.R.; Poti, G.; Samela, T.; Carbone, M.L.; Morelli, M.F.; Zappala, A.R.; di Rocco, Z.C.; Morese, R.; et al. Primary Mucosal Melanoma: Clinical Experience from a Single Italian Center. Curr. Oncol. 2024, 31, 588–597. [Google Scholar] [CrossRef]
- Kshirsagar, R.S.; Eide, J.G.; Harris, J.; Abiri, A.; Beswick, D.M.; Chang, E.H.; Fung, N.; Hong, M.; Johnson, B.J.; Kohanski, M.A.; et al. Outcomes of Immunotherapy Treatment in Sinonasal Mucosal Melanoma. Am. J. Rhinol. Allergy 2025, 39, 102–108. [Google Scholar] [CrossRef]
- Pradeep, J.; Win, T.T.; Aye, S.N.; Sreeramareddy, C.T. Efficacy and Safety of Immune Checkpoint Inhibitors for Advanced Malignant Melanoma: A Meta-Analysis on Monotherapy vs Combination Therapy. J. Cancer 2022, 13, 3091–3102. [Google Scholar] [CrossRef]
- Rager, T.; Eckburg, A.; Patel, M.; Qiu, R.; Gantiwala, S.; Dovalovsky, K.; Fan, K.; Lam, K.; Roesler, C.; Rastogi, A.; et al. Treatment of Metastatic Melanoma with a Combination of Immunotherapies and Molecularly Targeted Therapies. Cancers 2022, 14, 3779. [Google Scholar] [CrossRef]
- Hodi, F.S.; Corless, C.L.; Giobbie-Hurder, A.; Fletcher, J.A.; Zhu, M.; Marino-Enriquez, A.; Friedlander, P.; Gonzalez, R.; Weber, J.S.; Gajewski, T.F.; et al. Imatinib for melanomas harboring mutationally activated or amplified KIT arising on mucosal, acral, and chronically sun-damaged skin. J. Clin. Oncol. 2013, 31, 3182–3190. [Google Scholar] [CrossRef]
- Steeb, T.; Wessely, A.; Petzold, A.; Kohl, C.; Erdmann, M.; Berking, C.; Heppt, M.V. c-Kit inhibitors for unresectable or metastatic mucosal, acral or chronically sun-damaged melanoma: A systematic review and one-arm meta-analysis. Eur. J. Cancer 2021, 157, 348–357. [Google Scholar] [CrossRef]
- Vanneman, M.; Dranoff, G. Combining immunotherapy and targeted therapies in cancer treatment. Nat. Rev. Cancer 2012, 12, 237–251. [Google Scholar] [CrossRef] [PubMed]
- Morrissey, K.M.; Yuraszeck, T.M.; Li, C.C.; Zhang, Y.; Kasichayanula, S. Immunotherapy and Novel Combinations in Oncology: Current Landscape, Challenges, and Opportunities. Clin. Transl. Sci. 2016, 9, 89–104. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Chi, Z.; Guo, J. Toripalimab for the treatment of melanoma. Expert Opin. Biol. Ther. 2020, 20, 863–869. [Google Scholar] [CrossRef]
- Indini, A.; Roila, F.; Grossi, F.; Massi, D.; Mandala, M. Molecular Profiling and Novel Therapeutic Strategies for Mucosal Melanoma: A Comprehensive Review. Int. J. Mol. Sci. 2021, 23, 147. [Google Scholar] [CrossRef]
- Yang, J.; Wang, X.; Meng, Y.; Zhu, M.; Kong, F. Combination Immunotherapy for Mucosal Melanoma: Molecular Mechanism, Research Status, and Future Directions. Curr. Treat. Options Oncol. 2025, 26, 431–444. [Google Scholar] [CrossRef]
- Guo, J.; Carvajal, R.D.; Dummer, R.; Hauschild, A.; Daud, A.; Bastian, B.C.; Markovic, S.N.; Queirolo, P.; Arance, A.; Berking, C.; et al. Efficacy and safety of nilotinib in patients with KIT-mutated metastatic or inoperable melanoma: Final results from the global, single-arm, phase II TEAM trial. Ann. Oncol. 2017, 28, 1380–1387. [Google Scholar] [CrossRef]
- Usta, S.Z.; Uchihashi, T.; Kodama, S.; Kurioka, K.; Inubushi, T.; Shimooka, T.; Sugauchi, A.; Seki, S.; Tanaka, S. Current Status and Molecular Mechanisms of Resistance to Immunotherapy in Oral Malignant Melanoma. Int. J. Mol. Sci. 2023, 24, 17282. [Google Scholar] [CrossRef]
- Chacon, M.; Pfluger, Y.; Angel, M.; Waisberg, F.; Enrico, D. Uncommon Subtypes of Malignant Melanomas: A Review Based on Clinical and Molecular Perspectives. Cancers 2020, 12, 2362. [Google Scholar] [CrossRef]
- Leonardi, G.C.; Falzone, L.; Salemi, R.; Zanghi, A.; Spandidos, D.A.; McCubrey, J.A.; Candido, S.; Libra, M. Cutaneous melanoma: From pathogenesis to therapy (Review). Int. J. Oncol. 2018, 52, 1071–1080. [Google Scholar] [CrossRef] [PubMed]
- Al Hmada, Y.; Brodell, R.T.; Kharouf, N.; Flanagan, T.W.; Alamodi, A.A.; Hassan, S.Y.; Shalaby, H.; Hassan, S.L.; Haikel, Y.; Megahed, M.; et al. Mechanisms of Melanoma Progression and Treatment Resistance: Role of Cancer Stem-like Cells. Cancers 2024, 16, 470. [Google Scholar] [CrossRef] [PubMed]
- Hoffmeister, F.; Dycka, J.; Ramsch, K. Intragastric self-administration in the rhesus monkey: A comparison of the reinforcing effects of codeine, phenacetin and paracetamol. J. Pharmacol. Exp. Ther. 1980, 214, 213–218. [Google Scholar] [CrossRef]
- Goyal, Y.; Busch, G.T.; Pillai, M.; Li, J.; Boe, R.H.; Grody, E.I.; Chelvanambi, M.; Dardani, I.P.; Emert, B.; Bodkin, N.; et al. Diverse clonal fates emerge upon drug treatment of homogeneous cancer cells. Nature 2023, 620, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, F.; Shields, B.; Makhoul, I.; Avaritt, N.; Wong, H.K.; Hutchins, L.F.; Shalin, S.; Tackett, A.J. Immune surveillance in melanoma: From immune attack to melanoma escape and even counterattack. Cancer Biol. Ther. 2017, 18, 451–469. [Google Scholar] [CrossRef]
- Timis, T.; Buruiana, S.; Dima, D.; Nistor, M.; Muresan, X.M.; Cenariu, D.; Tigu, A.B.; Tomuleasa, C. Advances in Cell and Immune Therapies for Melanoma. Biomedicines 2025, 13, 98. [Google Scholar] [CrossRef]
- Corica, D.A.; Bell, S.D.; Miller, P.J.; Kasperbauer, D.T.; Lawler, N.J.; Wakefield, M.R.; Fang, Y. Into the Future: Fighting Melanoma with Immunity. Cancers 2024, 16, 4002. [Google Scholar] [CrossRef]
- Khair, D.O.; Bax, H.J.; Mele, S.; Crescioli, S.; Pellizzari, G.; Khiabany, A.; Nakamura, M.; Harris, R.J.; French, E.; Hoffmann, R.M.; et al. Combining Immune Checkpoint Inhibitors: Established and Emerging Targets and Strategies to Improve Outcomes in Melanoma. Front. Immunol. 2019, 10, 453. [Google Scholar] [CrossRef]
- Bai, R.; Chen, N.; Li, L.; Du, N.; Bai, L.; Lv, Z.; Tian, H.; Cui, J. Mechanisms of Cancer Resistance to Immunotherapy. Front. Oncol. 2020, 10, 1290. [Google Scholar] [CrossRef]
- Vu, S.H.; Vetrivel, P.; Kim, J.; Lee, M.S. Cancer Resistance to Immunotherapy: Molecular Mechanisms and Tackling Strategies. Int. J. Mol. Sci. 2022, 23, 10906. [Google Scholar] [CrossRef]
- Said, S.S.; Ibrahim, W.N. Cancer Resistance to Immunotherapy: Comprehensive Insights with Future Perspectives. Pharmaceutics 2023, 15, 1143. [Google Scholar] [CrossRef] [PubMed]
- Kong, S.; Zhang, J.; Wang, L.; Li, W.; Guo, H.; Weng, Q.; He, Q.; Lou, H.; Ding, L.; Yang, B. Mechanisms of low MHC I expression and strategies for targeting MHC I with small molecules in cancer immunotherapy. Cancer Lett. 2024, 611, 217432. [Google Scholar] [CrossRef]
- Alsaafeen, B.H.; Ali, B.R.; Elkord, E. Resistance mechanisms to immune checkpoint inhibitors: Updated insights. Mol. Cancer 2025, 24, 20. [Google Scholar] [CrossRef] [PubMed]
- Mpakali, A.; Stratikos, E. The Role of Antigen Processing and Presentation in Cancer and the Efficacy of Immune Checkpoint Inhibitor Immunotherapy. Cancers 2021, 13, 134. [Google Scholar] [CrossRef] [PubMed]
- Hargadon, K.M. Genetic dysregulation of immunologic and oncogenic signaling pathways associated with tumor-intrinsic immune resistance: A molecular basis for combination targeted therapy-immunotherapy for cancer. Cell Mol. Life Sci. 2023, 80, 40. [Google Scholar] [CrossRef]
- Alam, J.; Cook, J.L. Transcriptional regulation of the heme oxygenase-1 gene via the stress response element pathway. Curr. Pharm. Des. 2003, 9, 2499–2511. [Google Scholar] [CrossRef]
- Medina, M.V.; Sapochnik, D.; Garcia Sola, M.; Coso, O. Regulation of the Expression of Heme Oxygenase-1: Signal Transduction, Gene Promoter Activation, and Beyond. Antioxid. Redox Signal 2020, 32, 1033–1044. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Kensler, T.W.; Motohashi, H. The KEAP1-NRF2 System: A Thiol-Based Sensor-Effector Apparatus for Maintaining Redox Homeostasis. Physiol. Rev. 2018, 98, 1169–1203. [Google Scholar] [CrossRef] [PubMed]
- Buchbinder, E.I.; Desai, A. CTLA-4 and PD-1 Pathways: Similarities, Differences, and Implications of Their Inhibition. Am. J. Clin. Oncol. 2016, 39, 98–106. [Google Scholar] [CrossRef]
- Zabeti Touchaei, A.; Vahidi, S. MicroRNAs as regulators of immune checkpoints in cancer immunotherapy: Targeting PD-1/PD-L1 and CTLA-4 pathways. Cancer Cell Int. 2024, 24, 102. [Google Scholar] [CrossRef] [PubMed]
- Want, M.Y.; Bashir, Z.; Najar, R.A. T Cell Based Immunotherapy for Cancer: Approaches and Strategies. Vaccines 2023, 11, 835. [Google Scholar] [CrossRef]
- Ahmed, H.; Mahmud, A.R.; Siddiquee, M.F.; Shahriar, A.; Biswas, P.; Shimul, M.E.K.; Ahmed, S.Z.; Ema, T.I.; Rahman, N.; Khan, M.A.; et al. Role of T cells in cancer immunotherapy: Opportunities and challenges. Cancer Pathog. Ther. 2023, 1, 116–126. [Google Scholar] [CrossRef]
- de Charette, M.; Marabelle, A.; Houot, R. Turning tumour cells into antigen presenting cells: The next step to improve cancer immunotherapy? Eur. J. Cancer 2016, 68, 134–147. [Google Scholar] [CrossRef]
- Dhatchinamoorthy, K.; Colbert, J.D.; Rock, K.L. Cancer Immune Evasion Through Loss of MHC Class I Antigen Presentation. Front. Immunol. 2021, 12, 636568. [Google Scholar] [CrossRef]
- Hazini, A.; Fisher, K.; Seymour, L. Deregulation of HLA-I in cancer and its central importance for immunotherapy. J. Immunother. Cancer 2021, 9, e002899. [Google Scholar] [CrossRef]
- Almawash, S. Revolutionary Cancer Therapy for Personalization and Improved Efficacy: Strategies to Overcome Resistance to Immune Checkpoint Inhibitor Therapy. Cancers 2025, 17, 880. [Google Scholar] [CrossRef]
- Bernal, M.; Ruiz-Cabello, F.; Concha, A.; Paschen, A.; Garrido, F. Implication of the beta2-microglobulin gene in the generation of tumor escape phenotypes. Cancer Immunol. Immunother. 2012, 61, 1359–1371. [Google Scholar] [CrossRef]
- Le Bouteiller, P.; Solier, C. Is antigen presentation the primary function of HLA-G? Microbes Infect. 2001, 3, 323–332. [Google Scholar] [CrossRef]
- Torrejon, D.Y.; Galvez, M.; Abril-Rodriguez, G.; Campbell, K.M.; Medina, E.; Vega-Crespo, A.; Kalbasi, A.; Comin-Anduix, B.; Ribas, A. Antitumor Immune Responses in B2M-Deficient Cancers. Cancer Immunol. Res. 2023, 11, 1642–1655. [Google Scholar] [CrossRef]
- Lauss, M.; Phung, B.; Borch, T.H.; Harbst, K.; Kaminska, K.; Ebbesson, A.; Hedenfalk, I.; Yuan, J.; Nielsen, K.; Ingvar, C.; et al. Molecular patterns of resistance to immune checkpoint blockade in melanoma. Nat. Commun. 2024, 15, 3075. [Google Scholar] [CrossRef] [PubMed]
- Horowitch, B.; Lee, D.Y.; Ding, M.; Martinez-Morilla, S.; Aung, T.N.; Ouerghi, F.; Wang, X.; Wei, W.; Damsky, W.; Sznol, M.; et al. Subsets of IFN Signaling Predict Response to Immune Checkpoint Blockade in Patients with Melanoma. Clin. Cancer Res. 2023, 29, 2908–2918. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Wu, H.; Wu, J.; Ding, P.; He, J.; Sang, M.; Liu, L. Mechanisms of immune checkpoint inhibitors: Insights into the regulation of circular RNAS involved in cancer hallmarks. Cell Death Dis. 2024, 15, 3. [Google Scholar] [CrossRef] [PubMed]
- Imai, Y.; Chiba, T.; Kondo, T.; Kanzaki, H.; Kanayama, K.; Ao, J.; Kojima, R.; Kusakabe, Y.; Nakamura, M.; Saito, T.; et al. Interferon-gamma induced PD-L1 expression and soluble PD-L1 production in gastric cancer. Oncol. Lett. 2020, 20, 2161–2168. [Google Scholar] [CrossRef]
- Mimura, K.; Teh, J.L.; Okayama, H.; Shiraishi, K.; Kua, L.F.; Koh, V.; Smoot, D.T.; Ashktorab, H.; Oike, T.; Suzuki, Y.; et al. PD-L1 expression is mainly regulated by interferon gamma associated with JAK-STAT pathway in gastric cancer. Cancer Sci. 2018, 109, 43–53. [Google Scholar] [CrossRef]
- Abiko, K.; Matsumura, N.; Hamanishi, J.; Horikawa, N.; Murakami, R.; Yamaguchi, K.; Yoshioka, Y.; Baba, T.; Konishi, I.; Mandai, M. IFN-gamma from lymphocytes induces PD-L1 expression and promotes progression of ovarian cancer. Br. J. Cancer 2015, 112, 1501–1509. [Google Scholar] [CrossRef] [PubMed]
- Gorniak, P.; Wasylecka-Juszczynska, M.; Lugowska, I.; Rutkowski, P.; Polak, A.; Szydlowski, M.; Juszczynski, P. BRAF inhibition curtails IFN-gamma-inducible PD-L1 expression and upregulates the immunoregulatory protein galectin-1 in melanoma cells. Mol. Oncol. 2020, 14, 1817–1832. [Google Scholar] [CrossRef]
- Gao, J.; Shi, L.Z.; Zhao, H.; Chen, J.; Xiong, L.; He, Q.; Chen, T.; Roszik, J.; Bernatchez, C.; Woodman, S.E.; et al. Loss of IFN-gamma Pathway Genes in Tumor Cells as a Mechanism of Resistance to Anti-CTLA-4 Therapy. Cell 2016, 167, 397–404.E9. [Google Scholar] [CrossRef]
- Shen, H.; Huang, F.; Zhang, X.; Ojo, O.A.; Li, Y.; Trummell, H.Q.; Anderson, J.C.; Fiveash, J.; Bredel, M.; Yang, E.S.; et al. Selective suppression of melanoma lacking IFN-gamma pathway by JAK inhibition depends on T cells and host TNF signaling. Nat. Commun. 2022, 13, 5013. [Google Scholar] [CrossRef]
- Benci, J.L.; Xu, B.; Qiu, Y.; Wu, T.J.; Dada, H.; Twyman-Saint Victor, C.; Cucolo, L.; Lee, D.S.M.; Pauken, K.E.; Huang, A.C.; et al. Tumor Interferon Signaling Regulates a Multigenic Resistance Program to Immune Checkpoint Blockade. Cell 2016, 167, 1540–1554.e12. [Google Scholar] [CrossRef]
- Soares-Silva, M.; Diniz, F.F.; Gomes, G.N.; Bahia, D. The Mitogen-Activated Protein Kinase (MAPK) Pathway: Role in Immune Evasion by Trypanosomatids. Front. Microbiol. 2016, 7, 183. [Google Scholar] [CrossRef]
- Bedognetti, D.; Roelands, J.; Decock, J.; Wang, E.; Hendrickx, W. The MAPK hypothesis: Immune-regulatory effects of MAPK-pathway genetic dysregulations and implications for breast cancer immunotherapy. Emerg. Top. Life Sci. 2017, 1, 429–445. [Google Scholar] [CrossRef]
- Yang, J.; Yan, J.; Liu, B. Targeting VEGF/VEGFR to Modulate Antitumor Immunity. Front. Immunol. 2018, 9, 978. [Google Scholar] [CrossRef] [PubMed]
- Atefi, M.; Avramis, E.; Lassen, A.; Wong, D.J.; Robert, L.; Foulad, D.; Cerniglia, M.; Titz, B.; Chodon, T.; Graeber, T.G.; et al. Effects of MAPK and PI3K pathways on PD-L1 expression in melanoma. Clin. Cancer Res. 2014, 20, 3446–3457. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Zhou, J.; Giobbie-Hurder, A.; Wargo, J.; Hodi, F.S. The activation of MAPK in melanoma cells resistant to BRAF inhibition promotes PD-L1 expression that is reversible by MEK and PI3K inhibition. Clin. Cancer Res. 2013, 19, 598–609. [Google Scholar] [CrossRef]
- Peng, W.; Chen, J.Q.; Liu, C.; Malu, S.; Creasy, C.; Tetzlaff, M.T.; Xu, C.; McKenzie, J.A.; Zhang, C.; Liang, X.; et al. Loss of PTEN Promotes Resistance to T Cell-Mediated Immunotherapy. Cancer Discov. 2016, 6, 202–216. [Google Scholar] [CrossRef]
- Trujillo, J.A.; Luke, J.J.; Zha, Y.; Segal, J.P.; Ritterhouse, L.L.; Spranger, S.; Matijevich, K.; Gajewski, T.F. Secondary resistance to immunotherapy associated with beta-catenin pathway activation or PTEN loss in metastatic melanoma. J. Immunother. Cancer 2019, 7, 295. [Google Scholar] [CrossRef]
- Jiang, M.; Zhang, K.; Zhang, Z.; Zeng, X.; Huang, Z.; Qin, P.; Xie, Z.; Cai, X.; Ashrafizadeh, M.; Tian, Y.; et al. PI3K/AKT/mTOR Axis in Cancer: From Pathogenesis to Treatment. MedComm 2025, 6, e70295. [Google Scholar] [CrossRef]
- Zou, Y.; Yaguchi, T. Programmed cell death-1 blockade therapy in melanoma: Resistance mechanisms and combination strategies. Exp. Dermatol. 2023, 32, 264–275. [Google Scholar] [CrossRef]
- Plaschka, M.; Benboubker, V.; Grimont, M.; Berthet, J.; Tonon, L.; Lopez, J.; Le-Bouar, M.; Balme, B.; Tondeur, G.; de la Fouchardiere, A.; et al. ZEB1 transcription factor promotes immune escape in melanoma. J. Immunother. Cancer 2022, 10, e003484. [Google Scholar] [CrossRef] [PubMed]
- Shirley, C.A.; Chhabra, G.; Amiri, D.; Chang, H.; Ahmad, N. Immune escape and metastasis mechanisms in melanoma: Breaking down the dichotomy. Front. Immunol. 2024, 15, 1336023. [Google Scholar] [CrossRef]
- Reschke, R.; Enk, A.H.; Hassel, J.C. Chemokines and Cytokines in Immunotherapy of Melanoma and Other Tumors: From Biomarkers to Therapeutic Targets. Int. J. Mol. Sci. 2024, 25, 6532. [Google Scholar] [CrossRef] [PubMed]
- Sackstein, R.; Schatton, T.; Barthel, S.R. T-lymphocyte homing: An underappreciated yet critical hurdle for successful cancer immunotherapy. Lab. Investig. 2017, 97, 669–697. [Google Scholar] [CrossRef] [PubMed]
- Pitcovski, J.; Shahar, E.; Aizenshtein, E.; Gorodetsky, R. Melanoma antigens and related immunological markers. Crit. Rev. Oncol. Hematol. 2017, 115, 36–49. [Google Scholar] [CrossRef]
- Borgers, J.S.W.; Lenkala, D.; Kohler, V.; Jackson, E.K.; Linssen, M.D.; Hymson, S.; McCarthy, B.; O’Reilly Cosgrove, E.; Balogh, K.N.; Esaulova, E.; et al. Personalized, autologous neoantigen-specific T cell therapy in metastatic melanoma: A phase 1 trial. Nat. Med. 2025, 31, 881–893. [Google Scholar] [CrossRef]
- Haibe, Y.; El Husseini, Z.; El Sayed, R.; Shamseddine, A. Resisting Resistance to Immune Checkpoint Therapy: A Systematic Review. Int. J. Mol. Sci. 2020, 21, 6176. [Google Scholar] [CrossRef]
- Hossain, S.M.; Eccles, M.R. Phenotype Switching and the Melanoma Microenvironment; Impact on Immunotherapy and Drug Resistance. Int. J. Mol. Sci. 2023, 24, 1601. [Google Scholar] [CrossRef]
- Wang, Q.; Shao, X.; Zhang, Y.; Zhu, M.; Wang, F.X.C.; Mu, J.; Li, J.; Yao, H.; Chen, K. Role of tumor microenvironment in cancer progression and therapeutic strategy. Cancer Med. 2023, 12, 11149–11165. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; Rathore, S.; Munshi, A.; Ramesh, R. Extracellular Vesicles in Oncology: From Immune Suppression to Immunotherapy. AAPS J. 2021, 23, 30. [Google Scholar] [CrossRef] [PubMed]
- Oparaugo, N.C.; Ouyang, K.; Nguyen, N.P.N.; Nelson, A.M.; Agak, G.W. Human Regulatory T Cells: Understanding the Role of Tregs in Select Autoimmune Skin Diseases and Post-Transplant Nonmelanoma Skin Cancers. Int. J. Mol. Sci. 2023, 24, 1527. [Google Scholar] [CrossRef] [PubMed]
- Fiyouzi, T.; Pelaez-Prestel, H.F.; Reyes-Manzanas, R.; Lafuente, E.M.; Reche, P.A. Enhancing Regulatory T Cells to Treat Inflammatory and Autoimmune Diseases. Int. J. Mol. Sci. 2023, 24, 7797. [Google Scholar] [CrossRef]
- Asnagli, H.; Martire, D.; Belmonte, N.; Quentin, J.; Bastian, H.; Boucard-Jourdin, M.; Fall, P.B.; Mausset-Bonnefont, A.L.; Mantello-Moreau, A.; Rouquier, S.; et al. Type 1 regulatory T cells specific for collagen type II as an efficient cell-based therapy in arthritis. Arthritis Res. Ther. 2014, 16, R115. [Google Scholar] [CrossRef]
- Rudensky, A.Y.; Campbell, D.J. In vivo sites and cellular mechanisms of T reg cell-mediated suppression. J. Exp. Med. 2006, 203, 489–492. [Google Scholar] [CrossRef]
- John, P.; Pulanco, M.C.; Galbo, P.M., Jr.; Wei, Y.; Ohaegbulam, K.C.; Zheng, D.; Zang, X. The immune checkpoint B7x expands tumor-infiltrating Tregs and promotes resistance to anti-CTLA-4 therapy. Nat. Commun. 2022, 13, 2506. [Google Scholar] [CrossRef]
- Fujimura, T.; Ring, S.; Umansky, V.; Mahnke, K.; Enk, A.H. Regulatory T cells stimulate B7-H1 expression in myeloid-derived suppressor cells in ret melanomas. J. Investig. Dermatol. 2012, 132, 1239–1246. [Google Scholar] [CrossRef]
- Nurieva, R.I.; Liu, X.; Dong, C. Molecular mechanisms of T-cell tolerance. Immunol. Rev. 2011, 241, 133–144. [Google Scholar] [CrossRef]
- Carbone, F.; Russo, C.; Colamatteo, A.; La Rocca, C.; Fusco, C.; Matarese, A.; Procaccini, C.; Matarese, G. Cellular and molecular signaling towards T cell immunological self-tolerance. J. Biol. Chem. 2024, 300, 107134. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, J.; Wu, S.; Chen, Z.; Wu, S.; Chen, L.; Zhu, X.; Li, Z. Why Treg should be the focus of cancer immunotherapy: The latest thought. Biomed. Pharmacother. 2023, 168, 115142. [Google Scholar] [CrossRef]
- Pan, Y.; Zhou, H.; Sun, Z.; Zhu, Y.; Zhang, Z.; Han, J.; Liu, Y.; Wang, Q. Regulatory T cells in solid tumor immunotherapy: Effect, mechanism and clinical application. Cell Death Dis. 2025, 16, 277. [Google Scholar] [CrossRef]
- Wan, Y.Y. Regulatory T cells: Immune suppression and beyond. Cell Mol. Immunol. 2010, 7, 204–210. [Google Scholar] [CrossRef]
- Goldmann, O.; Nwofor, O.V.; Chen, Q.; Medina, E. Mechanisms underlying immunosuppression by regulatory cells. Front. Immunol. 2024, 15, 1328193. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Fan, T.; Liu, Y.; Yu, G.; Li, C.; Jiang, Z. Regulatory T cells in immune checkpoint blockade antitumor therapy. Mol. Cancer 2024, 23, 251. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Li, S.; Zhao, Y.; Cheng, K. Mechanisms of drug resistance to immune checkpoint inhibitors in non-small cell lung cancer. Front. Immunol. 2023, 14, 1127071. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Shi, H.; Zhang, B.; Ou, X.; Ma, Q.; Chen, Y.; Shu, P.; Li, D.; Wang, Y. Myeloid-derived suppressor cells as immunosuppressive regulators and therapeutic targets in cancer. Signal Transduct. Target. Ther. 2021, 6, 362. [Google Scholar] [CrossRef]
- Gabrilovich, D.I.; Nagaraj, S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 2009, 9, 162–174. [Google Scholar] [CrossRef]
- Karadima, E.; Chavakis, T.; Alexaki, V.I. Arginine metabolism in myeloid cells in health and disease. Semin. Immunopathol. 2025, 47, 11. [Google Scholar] [CrossRef] [PubMed]
- Waldron, T.J.; Quatromoni, J.G.; Karakasheva, T.A.; Singhal, S.; Rustgi, A.K. Myeloid derived suppressor cells: Targets for therapy. Oncoimmunology 2013, 2, e24117. [Google Scholar] [CrossRef]
- Hou, A.; Hou, K.; Huang, Q.; Lei, Y.; Chen, W. Targeting Myeloid-Derived Suppressor Cell, a Promising Strategy to Overcome Resistance to Immune Checkpoint Inhibitors. Front. Immunol. 2020, 11, 783. [Google Scholar] [CrossRef]
- Shibata, M.; Nanno, K.; Yoshimori, D.; Nakajima, T.; Takada, M.; Yazawa, T.; Mimura, K.; Inoue, N.; Watanabe, T.; Tachibana, K.; et al. Myeloid-derived suppressor cells: Cancer, autoimmune diseases, and more. Oncotarget 2022, 13, 1273–1285. [Google Scholar] [CrossRef]
- Tobin, R.P.; Jordan, K.R.; Robinson, W.A.; Davis, D.; Borges, V.F.; Gonzalez, R.; Lewis, K.D.; McCarter, M.D. Targeting myeloid-derived suppressor cells using all-trans retinoic acid in melanoma patients treated with Ipilimumab. Int. Immunopharmacol. 2018, 63, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Bjoern, J.; Juul Nitschke, N.; Zeeberg Iversen, T.; Schmidt, H.; Fode, K.; Svane, I.M. Immunological correlates of treatment and response in stage IV malignant melanoma patients treated with Ipilimumab. Oncoimmunology 2016, 5, e1100788. [Google Scholar] [CrossRef] [PubMed]
- Sade-Feldman, M.; Kanterman, J.; Klieger, Y.; Ish-Shalom, E.; Olga, M.; Saragovi, A.; Shtainberg, H.; Lotem, M.; Baniyash, M. Clinical Significance of Circulating CD33+CD11b+HLA-DR− Myeloid Cells in Patients with Stage IV Melanoma Treated with Ipilimumab. Clin. Cancer Res. 2016, 22, 5661–5672. [Google Scholar] [CrossRef] [PubMed]
- de Coana, Y.P.; Wolodarski, M.; Poschke, I.; Yoshimoto, Y.; Yang, Y.; Nystrom, M.; Edback, U.; Brage, S.E.; Lundqvist, A.; Masucci, G.V.; et al. Ipilimumab treatment decreases monocytic MDSCs and increases CD8 effector memory T cells in long-term survivors with advanced melanoma. Oncotarget 2017, 8, 21539–21553. [Google Scholar] [CrossRef]
- de Visser, K.E.; Joyce, J.A. The evolving tumor microenvironment: From cancer initiation to metastatic outgrowth. Cancer Cell 2023, 41, 374–403. [Google Scholar] [CrossRef]
- Zhao, Y.; Shen, M.; Wu, L.; Yang, H.; Yao, Y.; Yang, Q.; Du, J.; Liu, L.; Li, Y.; Bai, Y. Stromal cells in the tumor microenvironment: Accomplices of tumor progression? Cell Death Dis. 2023, 14, 587. [Google Scholar] [CrossRef]
- Baghban, R.; Roshangar, L.; Jahanban-Esfahlan, R.; Seidi, K.; Ebrahimi-Kalan, A.; Jaymand, M.; Kolahian, S.; Javaheri, T.; Zare, P. Tumor microenvironment complexity and therapeutic implications at a glance. Cell Commun. Signal 2020, 18, 59. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, B.; Elkord, E. Regulatory T Cells in the Tumor Microenvironment and Cancer Progression: Role and Therapeutic Targeting. Vaccines 2016, 4, 28. [Google Scholar] [CrossRef]
- Goswami, T.K.; Singh, M.; Dhawan, M.; Mitra, S.; Emran, T.B.; Rabaan, A.A.; Mutair, A.A.; Alawi, Z.A.; Alhumaid, S.; Dhama, K. Regulatory T cells (Tregs) and their therapeutic potential against autoimmune disorders—Advances and challenges. Hum. Vaccin. Immunother. 2022, 18, 2035117. [Google Scholar] [CrossRef]
- Workman, C.J.; Szymczak-Workman, A.L.; Collison, L.W.; Pillai, M.R.; Vignali, D.A. The development and function of regulatory T cells. Cell Mol. Life Sci. 2009, 66, 2603–2622. [Google Scholar] [CrossRef]
- Meng, X.; Layhadi, J.A.; Keane, S.T.; Cartwright, N.J.K.; Durham, S.R.; Shamji, M.H. Immunological mechanisms of tolerance: Central, peripheral and the role of T and B cells. Asia Pac. Allergy 2023, 13, 175–186. [Google Scholar] [CrossRef]
- Thangavelu, G.; Smolarchuk, C.; Anderson, C.C. Co-inhibitory molecules: Controlling the effectors or controlling the controllers? Self Nonself 2010, 1, 77–88. [Google Scholar] [CrossRef]
- Chen, Y.; McAndrews, K.M.; Kalluri, R. Clinical and therapeutic relevance of cancer-associated fibroblasts. Nat. Rev. Clin. Oncol. 2021, 18, 792–804. [Google Scholar] [CrossRef]
- Yang, D.; Liu, J.; Qian, H.; Zhuang, Q. Cancer-associated fibroblasts: From basic science to anticancer therapy. Exp. Mol. Med. 2023, 55, 1322–1332. [Google Scholar] [CrossRef] [PubMed]
- Forsthuber, A.; Aschenbrenner, B.; Korosec, A.; Jacob, T.; Annusver, K.; Krajic, N.; Kholodniuk, D.; Frech, S.; Zhu, S.; Purkhauser, K.; et al. Cancer-associated fibroblast subtypes modulate the tumor-immune microenvironment and are associated with skin cancer malignancy. Nat. Commun. 2024, 15, 9678. [Google Scholar] [CrossRef] [PubMed]
- Wellbrock, C.; Arozarena, I. Microphthalmia-associated transcription factor in melanoma development and MAP-kinase pathway targeted therapy. Pigment Cell Melanoma Res. 2015, 28, 390–406. [Google Scholar] [CrossRef]
- Bianchi-Smiraglia, A.; Bagati, A.; Fink, E.E.; Moparthy, S.; Wawrzyniak, J.A.; Marvin, E.K.; Battaglia, S.; Jowdy, P.; Kolesnikova, M.; Foley, C.E.; et al. Microphthalmia-associated transcription factor suppresses invasion by reducing intracellular GTP pools. Oncogene 2017, 36, 84–96. [Google Scholar] [CrossRef] [PubMed]
- Najem, A.; Soumoy, L.; Sabbah, M.; Krayem, M.; Awada, A.; Journe, F.; Ghanem, G.E. Understanding Molecular Mechanisms of Phenotype Switching and Crosstalk with TME to Reveal New Vulnerabilities of Melanoma. Cells 2022, 11, 1157. [Google Scholar] [CrossRef]
- Hunter, M.V.; Joshi, E.; Bowker, S.; Montal, E.; Ma, Y.; Kim, Y.H.; Yang, Z.; Tuffery, L.; Li, Z.; Rosiek, E.; et al. Mechanical confinement governs phenotypic plasticity in melanoma. Nature 2025, 647, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.Y.; Shklovskaya, E.; Lee, J.H.; Pedersen, B.; Stewart, A.; Ming, Z.; Irvine, M.; Shivalingam, B.; Saw, R.P.M.; Menzies, A.M.; et al. The molecular and functional landscape of resistance to immune checkpoint blockade in melanoma. Nat. Commun. 2023, 14, 1516. [Google Scholar] [CrossRef]
- Ascierto, M.L.; Makohon-Moore, A.; Lipson, E.J.; Taube, J.M.; McMiller, T.L.; Berger, A.E.; Fan, J.; Kaunitz, G.J.; Cottrell, T.R.; Kohutek, Z.A.; et al. Transcriptional Mechanisms of Resistance to Anti-PD-1 Therapy. Clin. Cancer Res. 2017, 23, 3168–3180. [Google Scholar] [CrossRef] [PubMed]
- Segui, E.; Braso-Maristany, F.; Pascual, T.; Sanfeliu, E.; Victoria, I.; Saura, C.; Hierro, C.; Lopez-Gonzalez, A.; Izarzugaza, Y.; Ciruelos, E.; et al. Efficacy of anti-PD1 therapy in PD1-high mRNA tumors across multiple cancer types: Results from cohort 1 and cohort 2 of the phase II SOLTI-1904 ACROPOLI trial. ESMO Open 2025, 10, 105838. [Google Scholar] [CrossRef]
- Schmidt, A.; Oberle, N.; Krammer, P.H. Molecular mechanisms of treg-mediated T cell suppression. Front. Immunol. 2012, 3, 51. [Google Scholar] [CrossRef]
- Li, C.; Jiang, P.; Wei, S.; Xu, X.; Wang, J. Regulatory T cells in tumor microenvironment: New mechanisms, potential therapeutic strategies and future prospects. Mol. Cancer 2020, 19, 116. [Google Scholar] [CrossRef]
- Dallavalasa, S.; Beeraka, N.M.; Basavaraju, C.G.; Tulimilli, S.V.; Sadhu, S.P.; Rajesh, K.; Aliev, G.; Madhunapantula, S.V. The Role of Tumor Associated Macrophages (TAMs) in Cancer Progression, Chemoresistance, Angiogenesis and Metastasis—Current Status. Curr. Med. Chem. 2021, 28, 8203–8236. [Google Scholar] [CrossRef]
- Wang, S.; Wang, J.; Chen, Z.; Luo, J.; Guo, W.; Sun, L.; Lin, L. Targeting M2-like tumor-associated macrophages is a potential therapeutic approach to overcome antitumor drug resistance. NPJ Precis. Oncol. 2024, 8, 31. [Google Scholar] [CrossRef]
- Takahashi, H.; Perez-Villarroel, P.; Falahat, R.; Mule, J.J. Targeting MARCO in combination with anti-CTLA-4 leads to enhanced melanoma regression and immune cell infiltration via macrophage reprogramming. J. Immunother. Cancer 2025, 13, e011030. [Google Scholar] [CrossRef]
- Eisinger, S.; Sarhan, D.; Boura, V.F.; Ibarlucea-Benitez, I.; Tyystjarvi, S.; Oliynyk, G.; Arsenian-Henriksson, M.; Lane, D.; Wikstrom, S.L.; Kiessling, R.; et al. Targeting a scavenger receptor on tumor-associated macrophages activates tumor cell killing by natural killer cells. Proc. Natl. Acad. Sci. USA 2020, 117, 32005–32016. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Zhang, X.; Li, A.; Qiao, X.; Xu, Y. The mechanism of action and therapeutic potential of tumor-associated macrophages in tumor immune evasion. Front. Immunol. 2025, 16, 1545928. [Google Scholar] [CrossRef] [PubMed]
- Kzhyshkowska, J.; Shen, J.; Larionova, I. Targeting of TAMs: Can we be more clever than cancer cells? Cell Mol. Immunol. 2024, 21, 1376–1409. [Google Scholar] [CrossRef]
- Kumai, T.; Oikawa, K.; Aoki, N.; Kimura, S.; Harabuchi, Y.; Celis, E.; Kobayashi, H. Tumor-derived TGF-beta and prostaglandin E2 attenuate anti-tumor immune responses in head and neck squamous cell carcinoma treated with EGFR inhibitor. J. Transl. Med. 2014, 12, 265. [Google Scholar] [CrossRef]
- Bekeredjian-Ding, I.; Schafer, M.; Hartmann, E.; Pries, R.; Parcina, M.; Schneider, P.; Giese, T.; Endres, S.; Wollenberg, B.; Hartmann, G. Tumour-derived prostaglandin E and transforming growth factor-beta synergize to inhibit plasmacytoid dendritic cell-derived interferon-alpha. Immunology 2009, 128, 439–450. [Google Scholar] [CrossRef]
- Malla, R.R.; Vasudevaraju, P.; Vempati, R.K.; Rakshmitha, M.; Merchant, N.; Nagaraju, G.P. Regulatory T cells: Their role in triple-negative breast cancer progression and metastasis. Cancer 2022, 128, 1171–1183. [Google Scholar] [CrossRef]
- Batlle, E.; Massague, J. Transforming Growth Factor-beta Signaling in Immunity and Cancer. Immunity 2019, 50, 924–940. [Google Scholar] [CrossRef]
- Pu, Y.; Ji, Q. Tumor-Associated Macrophages Regulate PD-1/PD-L1 Immunosuppression. Front. Immunol. 2022, 13, 874589. [Google Scholar] [CrossRef]
- Chocarro de Erauso, L.; Zuazo, M.; Arasanz, H.; Bocanegra, A.; Hernandez, C.; Fernandez, G.; Garcia-Granda, M.J.; Blanco, E.; Vera, R.; Kochan, G.; et al. Resistance to PD-L1/PD-1 Blockade Immunotherapy. A Tumor-Intrinsic or Tumor-Extrinsic Phenomenon? Front. Pharmacol. 2020, 11, 441. [Google Scholar] [CrossRef] [PubMed]
- Yerolatsite, M.; Torounidou, N.; Gogadis, A.; Kapoulitsa, F.; Ntellas, P.; Lampri, E.; Tolia, M.; Batistatou, A.; Katsanos, K.; Mauri, D. TAMs and PD-1 Networking in Gastric Cancer: A Review of the Literature. Cancers 2023, 16, 196. [Google Scholar] [CrossRef] [PubMed]

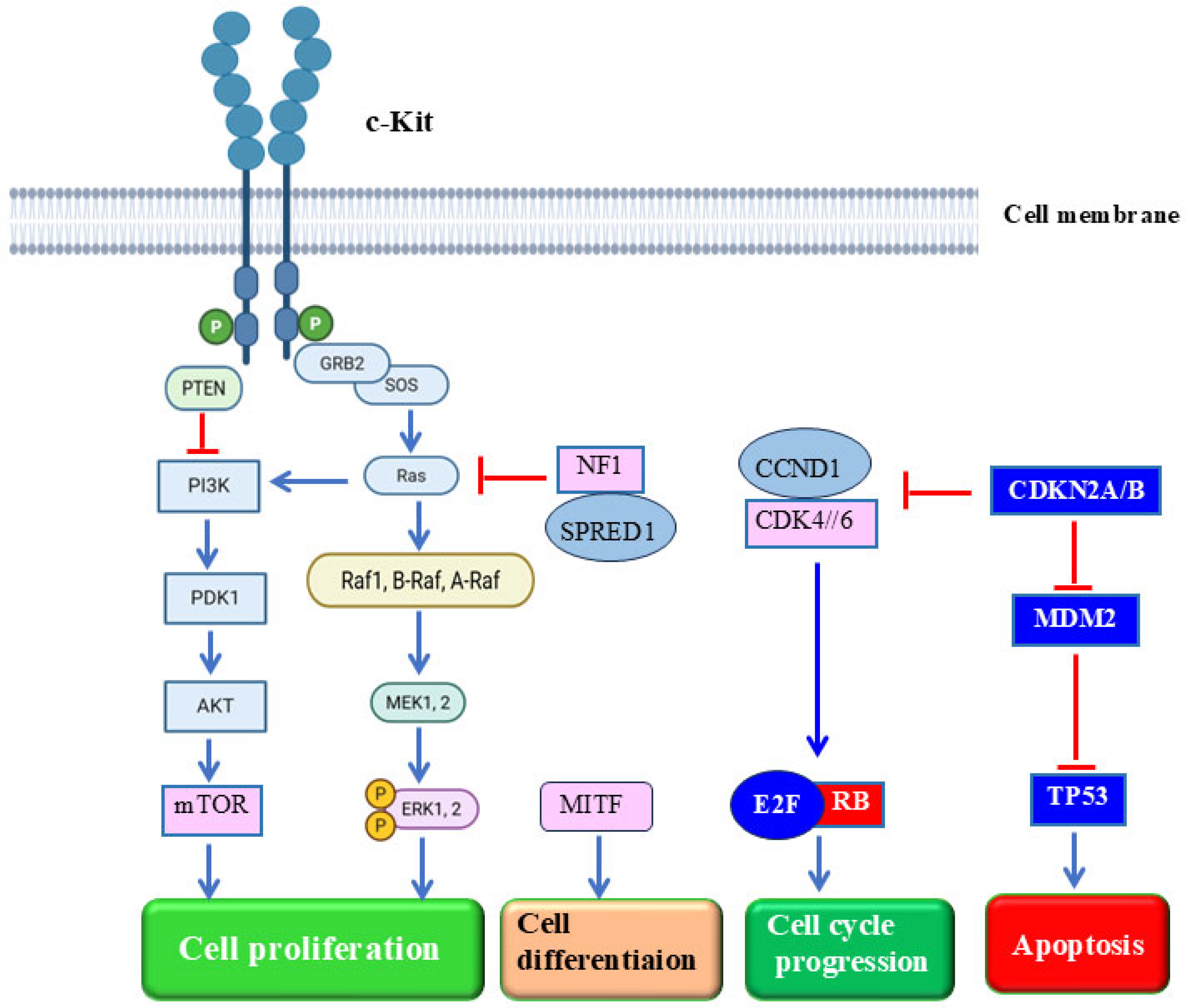




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassan, S.-Y.; Flanagan, T.W.; Hassan, S.-L.; Facca, S.; Haikel, Y.; Hassan, M. Mucosal Melanoma: Mechanisms of Its Etiology, Progression, Resistance and Therapy. Cells 2025, 14, 1884. https://doi.org/10.3390/cells14231884
Hassan S-Y, Flanagan TW, Hassan S-L, Facca S, Haikel Y, Hassan M. Mucosal Melanoma: Mechanisms of Its Etiology, Progression, Resistance and Therapy. Cells. 2025; 14(23):1884. https://doi.org/10.3390/cells14231884
Chicago/Turabian StyleHassan, Sofie-Yasmin, Thomas W. Flanagan, Sarah-Lilly Hassan, Sybille Facca, Youssef Haikel, and Mohamed Hassan. 2025. "Mucosal Melanoma: Mechanisms of Its Etiology, Progression, Resistance and Therapy" Cells 14, no. 23: 1884. https://doi.org/10.3390/cells14231884
APA StyleHassan, S.-Y., Flanagan, T. W., Hassan, S.-L., Facca, S., Haikel, Y., & Hassan, M. (2025). Mucosal Melanoma: Mechanisms of Its Etiology, Progression, Resistance and Therapy. Cells, 14(23), 1884. https://doi.org/10.3390/cells14231884








