Therapeutic Potential of an Anti-CD44v6 Monoclonal Antibody in Xenograft Models of Colorectal and Gastric Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines
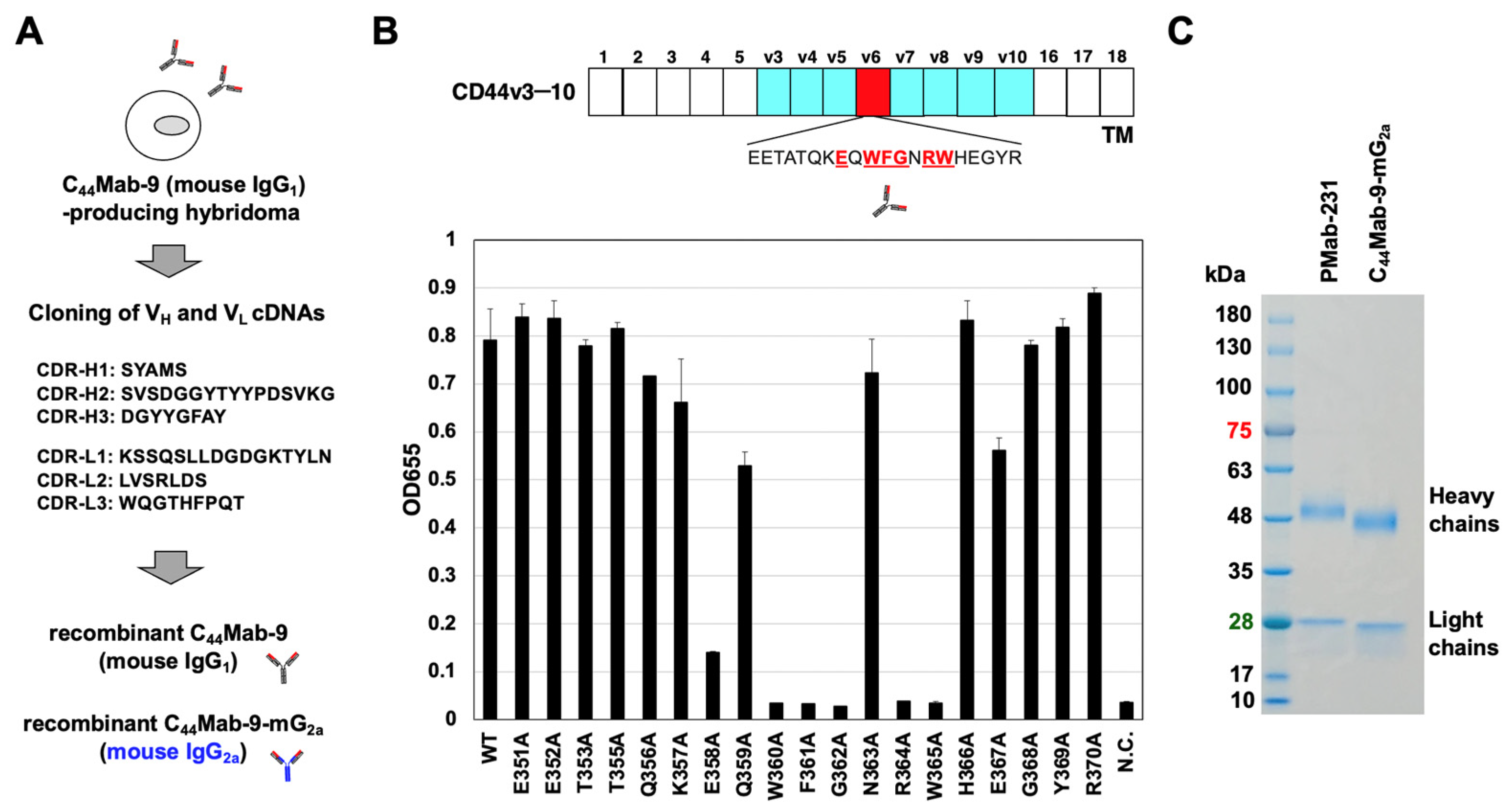
2.2. Recombinant mAb Production
2.3. ELISA
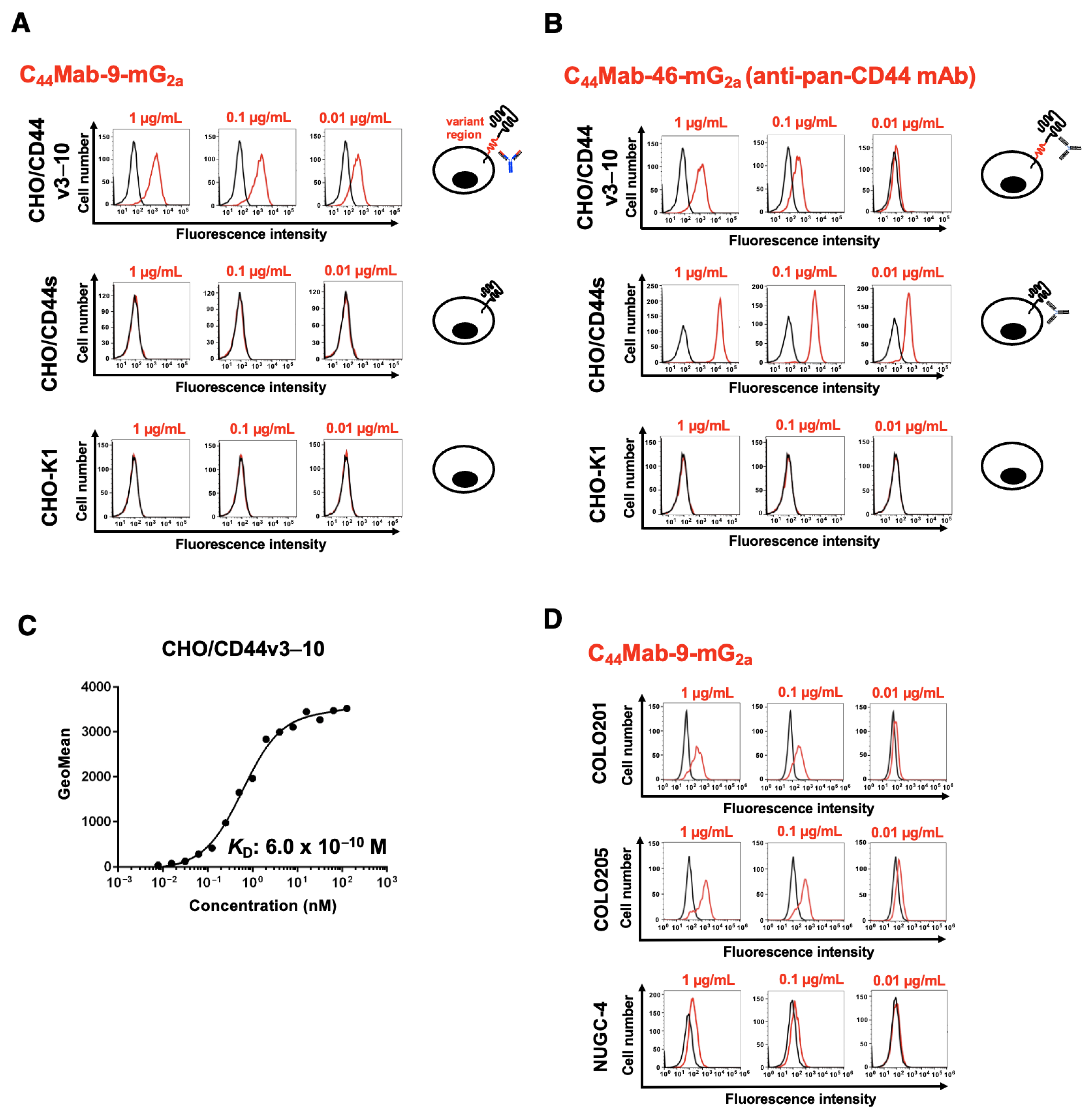
2.4. Flow Cytometry
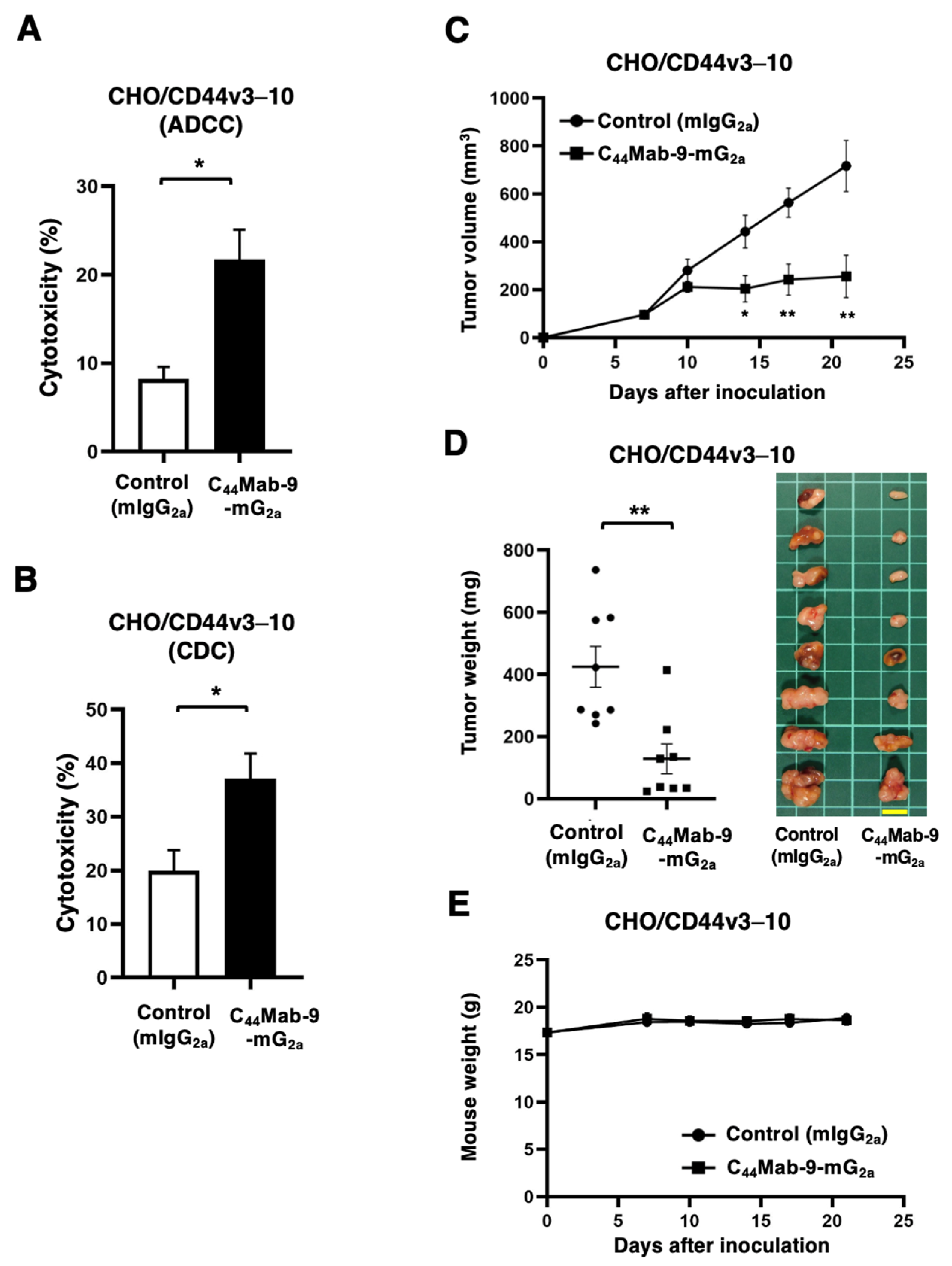
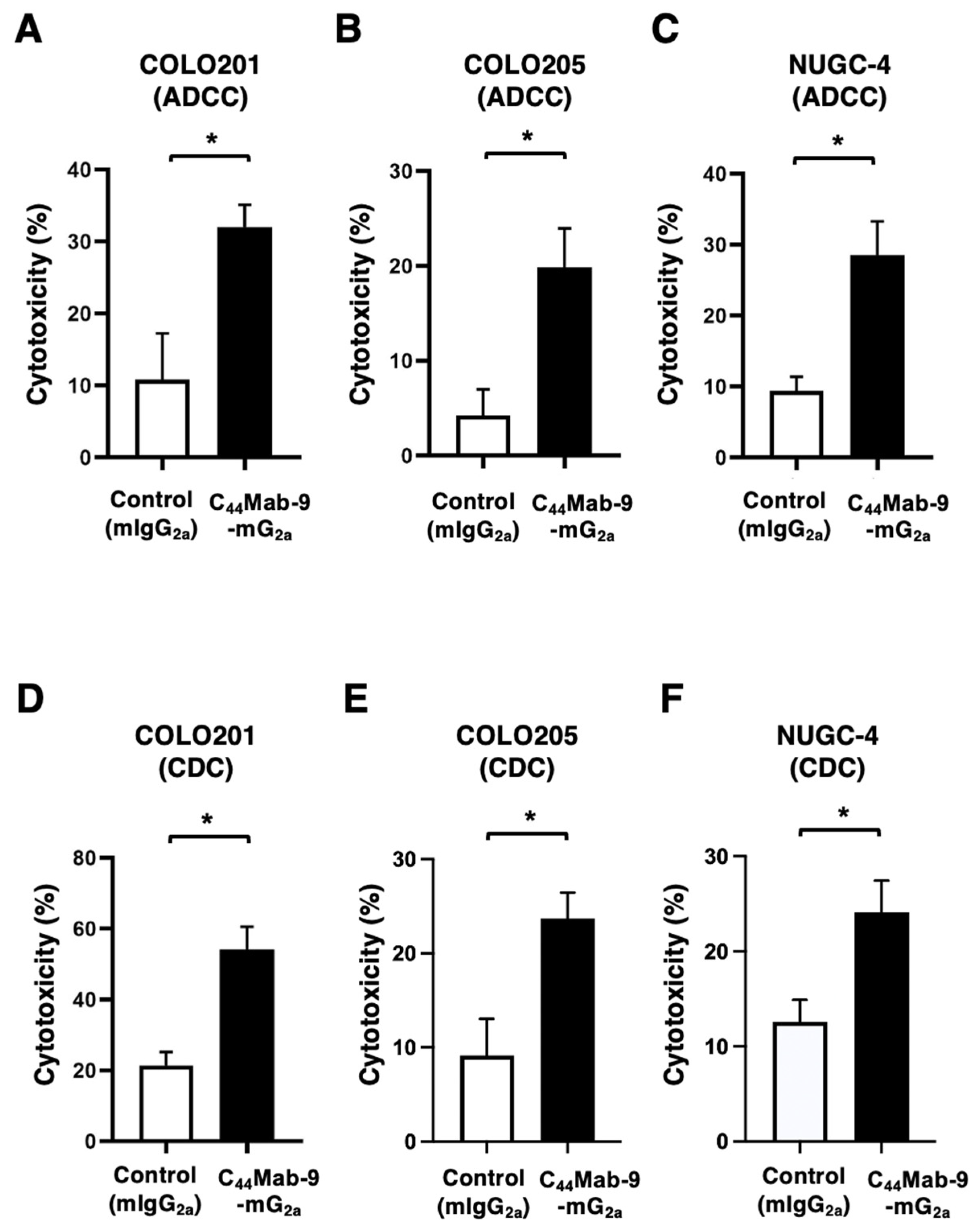
2.5. Measurement of ADCC by C44Mab-9-mG2a
2.6. Measurement of CDC by C44Mab-9-mG2a
2.7. Antitumor Activity of C44Mab-9-mG2a
3. Results
3.1. Production of Recombinant Anti-CD44v6 mAbs
3.2. Flow Cytometry Using C44Mab-9-mG2a
3.3. ADCC, CDC, and Antitumor Effects Against CHO/CD44v3–10 by C44Mab-9-mG2a
3.4. ADCC and CDC Against COLO201, COLO205, and NUGC-4 by C44Mab-9-mG2a
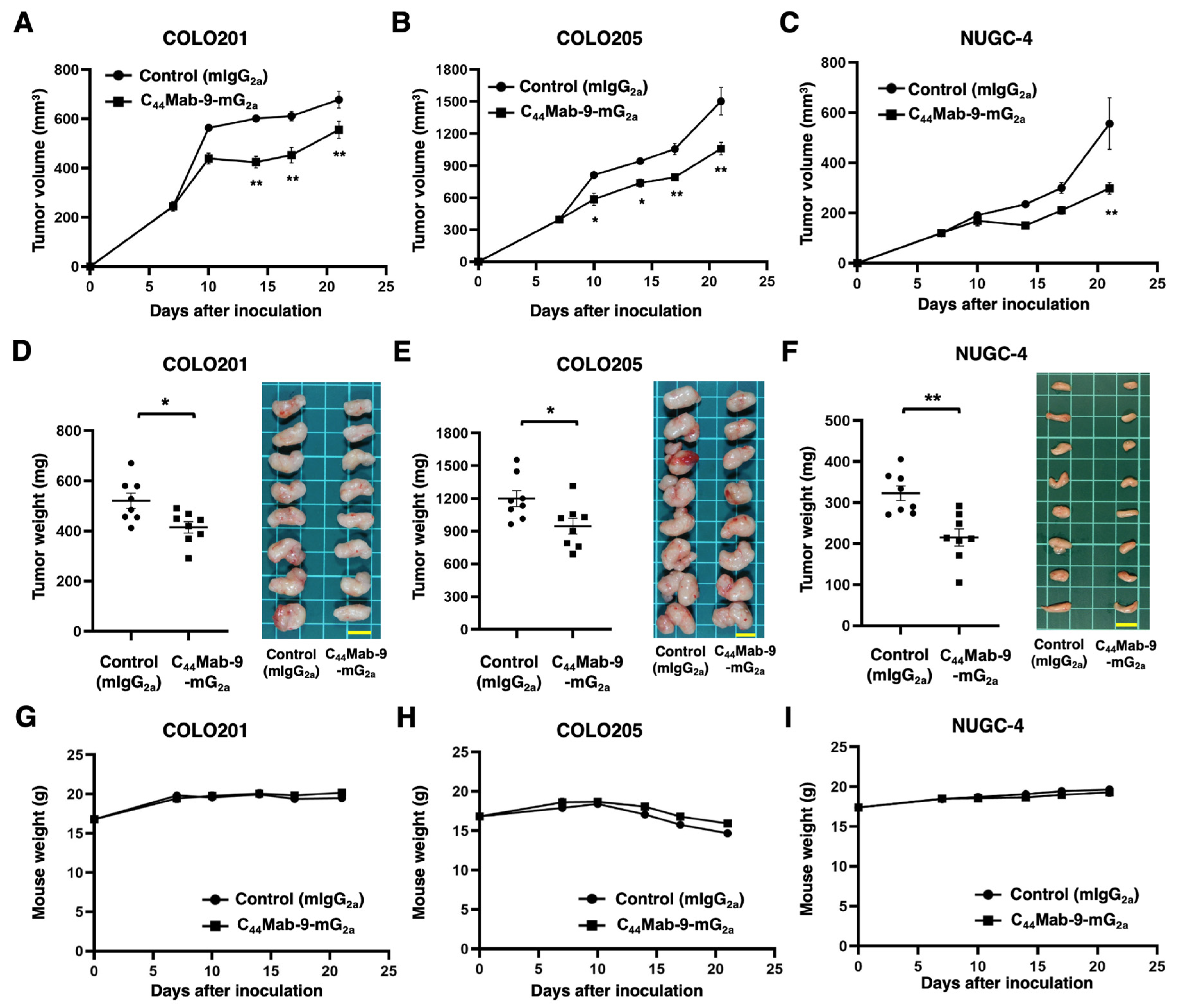
3.5. Antitumor Effects by C44Mab-9-mG2a Against COLO201, COLO205, and NUGC-4 Xenografts
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stamenkovic, I.; Amiot, M.; Pesando, J.M.; Seed, B. A lymphocyte molecule implicated in lymph node homing is a member of the cartilage link protein family. Cell 1989, 56, 1057–1062. [Google Scholar] [CrossRef]
- Ponta, H.; Sherman, L.; Herrlich, P.A. CD44: From adhesion molecules to signalling regulators. Nat. Rev. Mol. Cell Biol. 2003, 4, 33–45. [Google Scholar] [CrossRef]
- Zöller, M. CD44: Can a cancer-initiating cell profit from an abundantly expressed molecule? Nat. Rev. Cancer 2011, 11, 254–267. [Google Scholar] [CrossRef]
- Guo, Q.; Yang, C.; Gao, F. The state of CD44 activation in cancer progression and therapeutic targeting. FEBS J. 2021, 289, 7970–7986. [Google Scholar] [CrossRef]
- Slevin, M.; Krupinski, J.; Gaffney, J.; Matou, S.; West, D.; Delisser, H.; Savani, R.C.; Kumar, S. Hyaluronan-mediated angiogenesis in vascular disease: Uncovering RHAMM and CD44 receptor signaling pathways. Matrix Biol. 2007, 26, 58–68. [Google Scholar] [CrossRef]
- Hassn Mesrati, M.; Syafruddin, S.E.; Mohtar, M.A.; Syahir, A. CD44: A Multifunctional Mediator of Cancer Progression. Biomolecules 2021, 11, 1850. [Google Scholar] [CrossRef]
- Morath, I.; Hartmann, T.N.; Orian-Rousseau, V. CD44: More than a mere stem cell marker. Int. J. Biochem. Cell Biol. 2016, 81, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Bennett, K.L.; Jackson, D.G.; Simon, J.C.; Tanczos, E.; Peach, R.; Modrell, B.; Stamenkovic, I.; Plowman, G.; Aruffo, A. CD44 isoforms containing exon V3 are responsible for the presentation of heparin-binding growth factor. J. Cell Biol. 1995, 128, 687–698. [Google Scholar] [CrossRef]
- Orian-Rousseau, V.; Chen, L.; Sleeman, J.P.; Herrlich, P.; Ponta, H. CD44 is required for two consecutive steps in HGF/c-Met signaling. Genes. Dev. 2002, 16, 3074–3086. [Google Scholar] [CrossRef] [PubMed]
- Ishimoto, T.; Nagano, O.; Yae, T.; Tamada, M.; Motohara, T.; Oshima, H.; Oshima, M.; Ikeda, T.; Asaba, R.; Yagi, H.; et al. CD44 variant regulates redox status in cancer cells by stabilizing the xCT subunit of system xc(-) and thereby promotes tumor growth. Cancer Cell 2011, 19, 387–400. [Google Scholar] [CrossRef] [PubMed]
- Massagué, J.; Ganesh, K. Metastasis-Initiating Cells and Ecosystems. Cancer Discov. 2021, 11, 971–994. [Google Scholar] [CrossRef]
- Ganesh, K.; Massagué, J. Targeting metastatic cancer. Nat. Med. 2021, 27, 34–44. [Google Scholar] [CrossRef]
- Ma, L.; Dong, L.; Chang, P. CD44v6 engages in colorectal cancer progression. Cell Death Dis. 2019, 10, 30. [Google Scholar] [CrossRef]
- Todaro, M.; Gaggianesi, M.; Catalano, V.; Benfante, A.; Iovino, F.; Biffoni, M.; Apuzzo, T.; Sperduti, I.; Volpe, S.; Cocorullo, G.; et al. CD44v6 is a marker of constitutive and reprogrammed cancer stem cells driving colon cancer metastasis. Cell Stem Cell 2014, 14, 342–356. [Google Scholar] [CrossRef]
- Heider, K.H.; Sproll, M.; Susani, S.; Patzelt, E.; Beaumier, P.; Ostermann, E.; Ahorn, H.; Adolf, G.R. Characterization of a high-affinity monoclonal antibody specific for CD44v6 as candidate for immunotherapy of squamous cell carcinomas. Cancer Immunol. Immunother. 1996, 43, 245–253. [Google Scholar] [CrossRef]
- Heider, K.H.; Mulder, J.W.; Ostermann, E.; Susani, S.; Patzelt, E.; Pals, S.T.; Adolf, G.R. Splice variants of the cell surface glycoprotein CD44 associated with metastatic tumour cells are expressed in normal tissues of humans and cynomolgus monkeys. Eur. J. Cancer 1995, 31, 2385–2391. [Google Scholar] [CrossRef]
- Wang, Z.; Tang, Y.; Xie, L.; Huang, A.; Xue, C.; Gu, Z.; Wang, K.; Zong, S. The Prognostic and Clinical Value of CD44 in Colorectal Cancer: A Meta-Analysis. Front. Oncol. 2019, 9, 309. [Google Scholar] [CrossRef]
- Verel, I.; Heider, K.H.; Siegmund, M.; Ostermann, E.; Patzelt, E.; Sproll, M.; Snow, G.B.; Adolf, G.R.; van Dongen, G.A. Tumor targeting properties of monoclonal antibodies with different affinity for target antigen CD44V6 in nude mice bearing head-and-neck cancer xenografts. Int. J. Cancer 2002, 99, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Riechelmann, H.; Sauter, A.; Golze, W.; Hanft, G.; Schroen, C.; Hoermann, K.; Erhardt, T.; Gronau, S. Phase I trial with the CD44v6-targeting immunoconjugate bivatuzumab mertansine in head and neck squamous cell carcinoma. Oral. Oncol. 2008, 44, 823–829. [Google Scholar] [CrossRef] [PubMed]
- Tijink, B.M.; Buter, J.; de Bree, R.; Giaccone, G.; Lang, M.S.; Staab, A.; Leemans, C.R.; van Dongen, G.A. A phase I dose escalation study with anti-CD44v6 bivatuzumab mertansine in patients with incurable squamous cell carcinoma of the head and neck or esophagus. Clin. Cancer Res. 2006, 12, 6064–6072. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Huang, H.; Tang, Y.; Li, Q.; Wang, J.; Li, D.; Zhong, Z.; Zou, P.; You, Y.; Cao, Y.; et al. CD44v6 chimeric antigen receptor T cell specificity towards AML with FLT3 or DNMT3A mutations. Clin. Transl. Med. 2022, 12, e1043. [Google Scholar] [CrossRef]
- Greco, B.; Malacarne, V.; De Girardi, F.; Scotti, G.M.; Manfredi, F.; Angelino, E.; Sirini, C.; Camisa, B.; Falcone, L.; Moresco, M.A.; et al. Disrupting N-glycan expression on tumor cells boosts chimeric antigen receptor T cell efficacy against solid malignancies. Sci. Transl. Med. 2022, 14, eabg3072. [Google Scholar] [CrossRef]
- Porcellini, S.; Asperti, C.; Corna, S.; Cicoria, E.; Valtolina, V.; Stornaiuolo, A.; Valentinis, B.; Bordignon, C.; Traversari, C. CAR T Cells Redirected to CD44v6 Control Tumor Growth in Lung and Ovary Adenocarcinoma Bearing Mice. Front. Immunol. 2020, 11, 99. [Google Scholar] [CrossRef]
- Saeidpour Masouleh, S.; Nasiri, K.; Ostovar Ravari, A.; Saligheh Rad, M.; Kiani, K.; Sharifi Sultani, A.; Nejati, S.T.; Nabi Afjadi, M. Advances and challenges in CAR-T cell therapy for head and neck squamous cell carcinoma. Biomark. Res. 2025, 13, 69. [Google Scholar] [CrossRef]
- Ciulean, I.S.; Fischer, J.; Quaiser, A.; Bach, C.; Abken, H.; Tretbar, U.S.; Fricke, S.; Koehl, U.; Schmiedel, D.; Grunwald, T. CD44v6 specific CAR-NK cells for targeted immunotherapy of head and neck squamous cell carcinoma. Front. Immunol. 2023, 14, 1290488. [Google Scholar] [CrossRef] [PubMed]
- Ejima, R.; Suzuki, H.; Tanaka, T.; Asano, T.; Kaneko, M.K.; Kato, Y. Development of a Novel Anti-CD44 Variant 6 Monoclonal Antibody C(44)Mab-9 for Multiple Applications against Colorectal Carcinomas. Int. J. Mol. Sci. 2023, 24, 4007. [Google Scholar] [CrossRef]
- Li, G.; Suzuki, H.; Ohishi, T.; Asano, T.; Tanaka, T.; Yanaka, M.; Nakamura, T.; Yoshikawa, T.; Kawada, M.; Kaneko, M.K.; et al. Antitumor activities of a defucosylated anti-EpCAM monoclonal antibody in colorectal carcinoma xenograft models. Int. J. Mol. Med. 2023, 51, 18. [Google Scholar] [CrossRef]
- Kaneko, M.K.; Suzuki, H.; Ohishi, T.; Nakamura, T.; Tanaka, T.; Kato, Y. A Cancer-Specific Monoclonal Antibody against HER2 Exerts Antitumor Activities in Human Breast Cancer Xenograft Models. Int. J. Mol. Sci. 2024, 25, 1941. [Google Scholar] [CrossRef] [PubMed]
- Orian-Rousseau, V. CD44, a therapeutic target for metastasising tumours. Eur. J. Cancer 2010, 46, 1271–1277. [Google Scholar] [CrossRef] [PubMed]
- Maurya, S.K.; Jaramillo-Gómez, J.A.; Rehman, A.U.; Gautam, S.K.; Fatima, M.; Khan, M.A.; Zaidi, M.A.A.; Khan, P.; Anwar, L.; Alsafwani, Z.W.; et al. Mucin 5AC Promotes Breast Cancer Brain Metastasis through cMET/CD44v6. Clin. Cancer Res. 2025, 31, 921–935. [Google Scholar] [CrossRef]
- Chen, H.; Ling, R.; Lai, J.; Liu, Z.; Wang, Z.; Yang, H.; Kong, Y. CD44v6-mediated regulation of gastric cancer stem cells: A potential therapeutic target. Clin. Exp. Med. 2025, 25, 80. [Google Scholar] [CrossRef]
- Stornaiuolo, A.; Valentinis, B.; Sirini, C.; Scavullo, C.; Asperti, C.; Zhou, D.; Martinez De La Torre, Y.; Corna, S.; Casucci, M.; Porcellini, S.; et al. Characterization and Functional Analysis of CD44v6.CAR T Cells Endowed with a New Low-Affinity Nerve Growth Factor Receptor-Based Spacer. Hum. Gene Ther. 2021, 32, 744–760. [Google Scholar] [CrossRef] [PubMed]
- Wielenga, V.J.; van der Neut, R.; Offerhaus, G.J.; Pals, S.T. CD44 glycoproteins in colorectal cancer: Expression, function, and prognostic value. Adv. Cancer Res. 2000, 77, 169–187. [Google Scholar] [CrossRef] [PubMed]
- Pan, T.; Zhu, J.; Wang, X.; Tang, D.; Wang, Z.; Yang, Y.; Teng, Y.; Tian, Q.; Dan, G.; Chen, C.; et al. MEN1 Promotes Ferroptosis by Disrupting CD44 Alternative Splicing to Suppress Lung Cancer. Cancer Res. 2025, in press. [Google Scholar] [CrossRef]
- Matzke, A.; Herrlich, P.; Ponta, H.; Orian-Rousseau, V. A five-amino-acid peptide blocks Met- and Ron-dependent cell migration. Cancer Res. 2005, 65, 6105–6110. [Google Scholar] [CrossRef] [PubMed]
- Tremmel, M.; Matzke, A.; Albrecht, I.; Laib, A.M.; Olaku, V.; Ballmer-Hofer, K.; Christofori, G.; Héroult, M.; Augustin, H.G.; Ponta, H.; et al. A CD44v6 peptide reveals a role of CD44 in VEGFR-2 signaling and angiogenesis. Blood 2009, 114, 5236–5244. [Google Scholar] [CrossRef]
- Orian-Rousseau, V.; Ponta, H. Perspectives of CD44 targeting therapies. Arch. Toxicol. 2015, 89, 3–14. [Google Scholar] [CrossRef]
- Vey, N.; Delaunay, J.; Martinelli, G.; Fiedler, W.; Raffoux, E.; Prebet, T.; Gomez-Roca, C.; Papayannidis, C.; Kebenko, M.; Paschka, P.; et al. Phase I clinical study of RG7356, an anti-CD44 humanized antibody, in patients with acute myeloid leukemia. Oncotarget 2016, 7, 32532–32542. [Google Scholar] [CrossRef]
- Menke-van der Houven van Oordt, C.W.; Gomez-Roca, C.; van Herpen, C.; Coveler, A.L.; Mahalingam, D.; Verheul, H.M.; van der Graaf, W.T.; Christen, R.; Rüttinger, D.; Weigand, S.; et al. First-in-human phase I clinical trial of RG7356, an anti-CD44 humanized antibody, in patients with advanced, CD44-expressing solid tumors. Oncotarget 2016, 7, 80046–80058. [Google Scholar] [CrossRef]
- Birzele, F.; Voss, E.; Nopora, A.; Honold, K.; Heil, F.; Lohmann, S.; Verheul, H.; Le Tourneau, C.; Delord, J.P.; van Herpen, C.; et al. CD44 Isoform Status Predicts Response to Treatment with Anti-CD44 Antibody in Cancer Patients. Clin. Cancer Res. 2015, 21, 2753–2762. [Google Scholar] [CrossRef]
- Zhang, S.; Wu, C.C.; Fecteau, J.F.; Cui, B.; Chen, L.; Zhang, L.; Wu, R.; Rassenti, L.; Lao, F.; Weigand, S.; et al. Targeting chronic lymphocytic leukemia cells with a humanized monoclonal antibody specific for CD44. Proc. Natl. Acad. Sci. USA 2013, 110, 6127–6132. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, M.K.; Suzuki, H.; Kato, Y. Establishment of a Novel Cancer-Specific Anti-HER2 Monoclonal Antibody H2Mab-250/H2CasMab-2 for Breast Cancers. Monoclon. Antibodies Immunodiagn. Immunother. 2024, 43, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Hosking, M.P.; Shirinbak, S.; Omilusik, K.; Chandra, S.; Kaneko, M.K.; Gentile, A.; Yamamoto, S.; Shrestha, B.; Grant, J.; Boyett, M.; et al. Preferential tumor targeting of HER2 by iPSC-derived CAR T cells engineered to overcome multiple barriers to solid tumor efficacy. Cell Stem Cell 2025, 32, 1087–1101. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hirayama, A.; Tanaka, T.; Ohishi, T.; Shinoda, K.; Nakamura, T.; Nomura, A.; Kojo, N.; Araki, H.; Suzuki, K.; Kaneko, M.K.; et al. Therapeutic Potential of an Anti-CD44v6 Monoclonal Antibody in Xenograft Models of Colorectal and Gastric Cancer. Cells 2025, 14, 1873. https://doi.org/10.3390/cells14231873
Hirayama A, Tanaka T, Ohishi T, Shinoda K, Nakamura T, Nomura A, Kojo N, Araki H, Suzuki K, Kaneko MK, et al. Therapeutic Potential of an Anti-CD44v6 Monoclonal Antibody in Xenograft Models of Colorectal and Gastric Cancer. Cells. 2025; 14(23):1873. https://doi.org/10.3390/cells14231873
Chicago/Turabian StyleHirayama, Aoi, Tomohiro Tanaka, Tomokazu Ohishi, Keisuke Shinoda, Takuya Nakamura, Airi Nomura, Naoki Kojo, Haruto Araki, Kaito Suzuki, Mika K. Kaneko, and et al. 2025. "Therapeutic Potential of an Anti-CD44v6 Monoclonal Antibody in Xenograft Models of Colorectal and Gastric Cancer" Cells 14, no. 23: 1873. https://doi.org/10.3390/cells14231873
APA StyleHirayama, A., Tanaka, T., Ohishi, T., Shinoda, K., Nakamura, T., Nomura, A., Kojo, N., Araki, H., Suzuki, K., Kaneko, M. K., Suzuki, H., & Kato, Y. (2025). Therapeutic Potential of an Anti-CD44v6 Monoclonal Antibody in Xenograft Models of Colorectal and Gastric Cancer. Cells, 14(23), 1873. https://doi.org/10.3390/cells14231873







